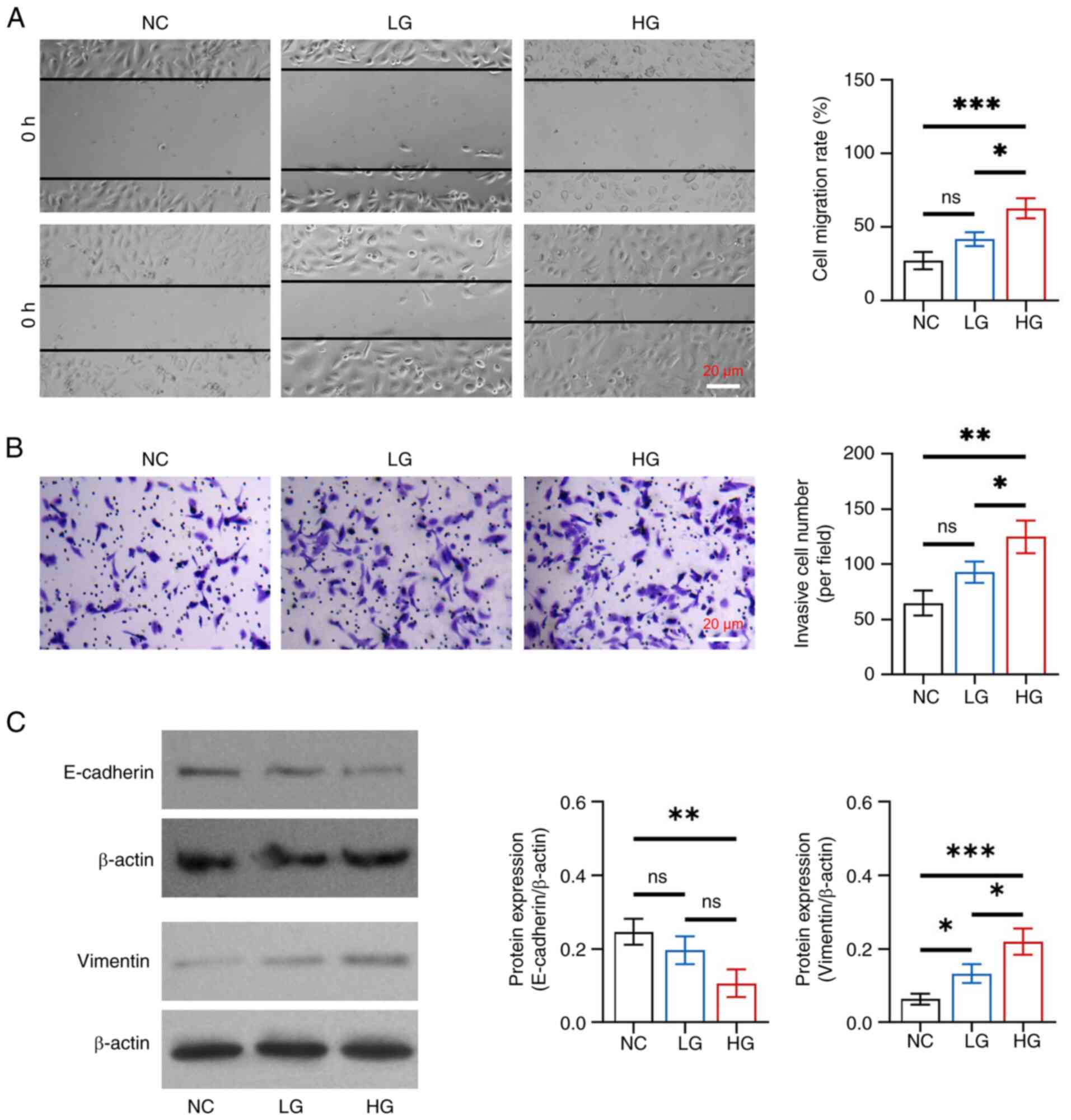
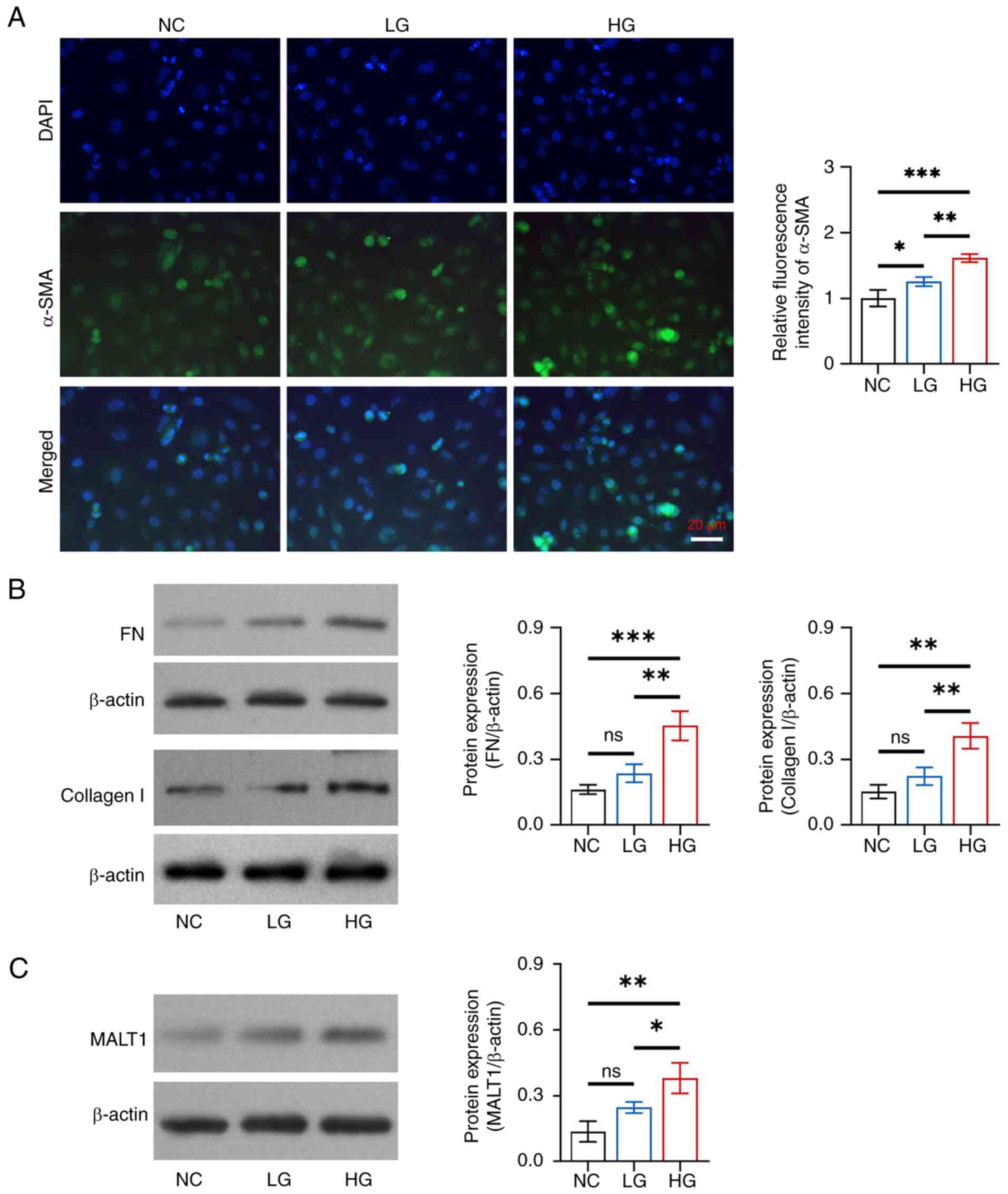
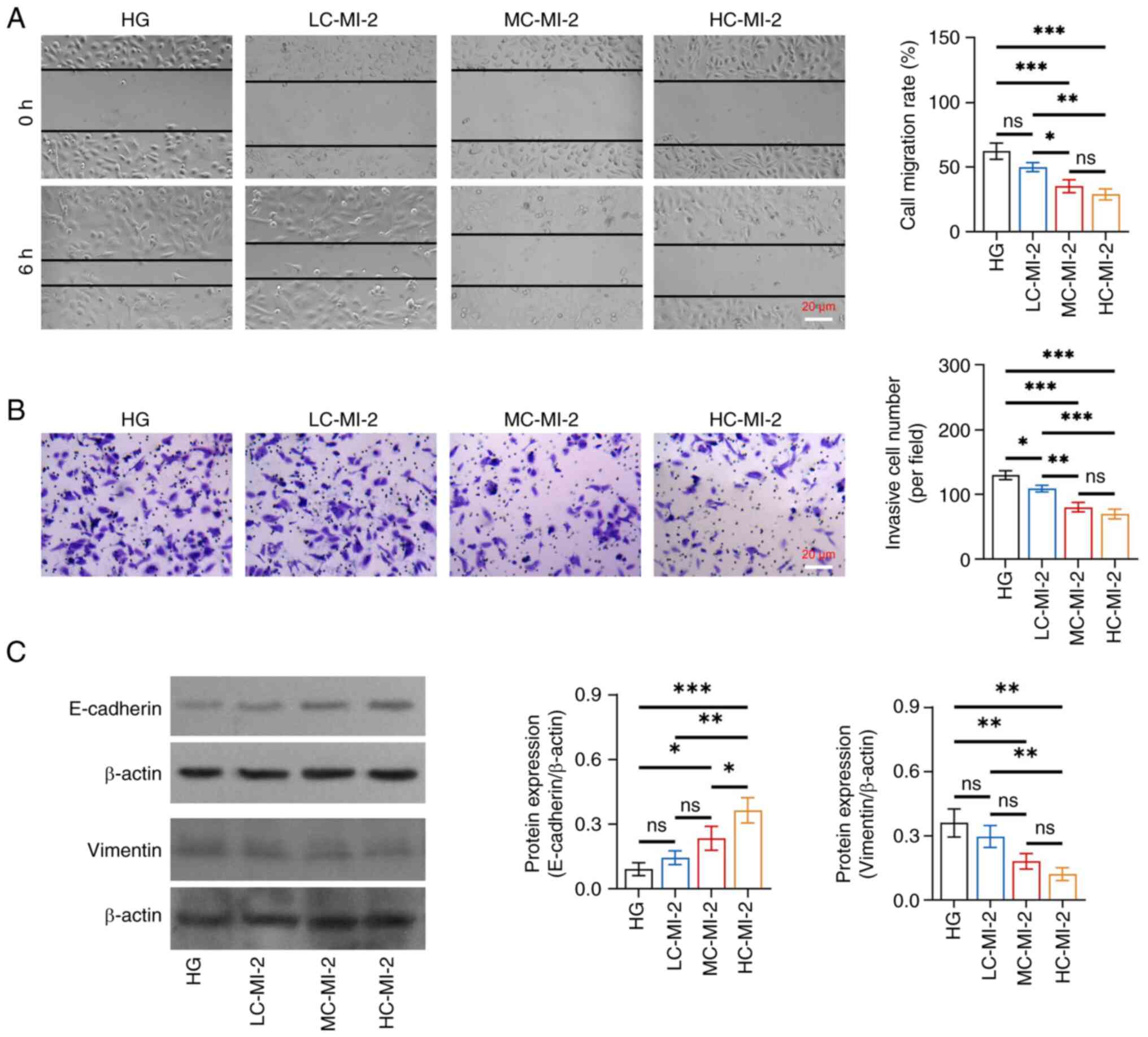
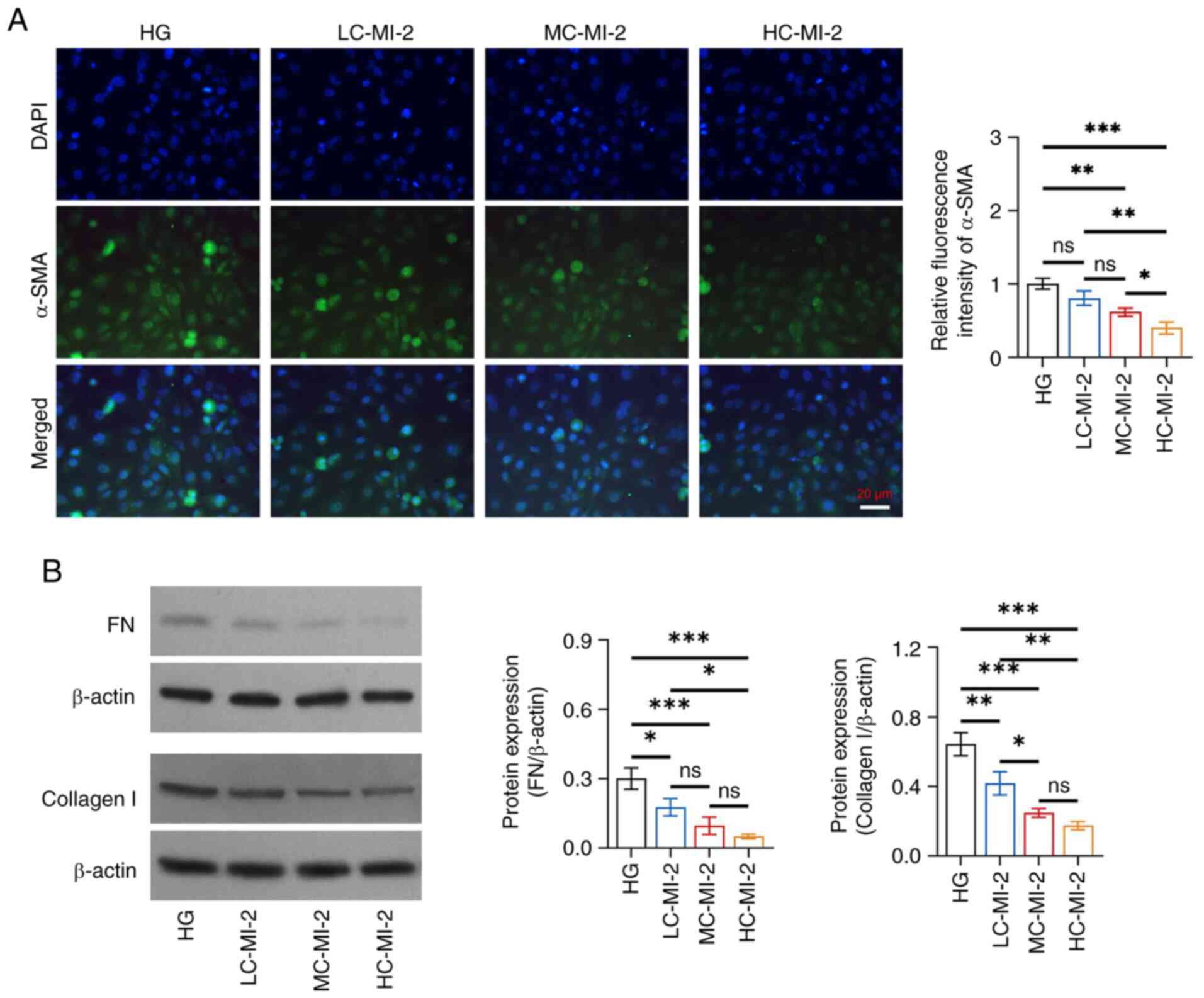
|
1
|
Caramori ML and Rossing P: Diabetic Kidney
Disease. Endotext. Feingold KR, Anawalt B, Blackman MR, Boyce A,
Chrousos G, Corpas E, de Herder WW, Dhatariya K, Dungan K, Hofland
J, Kalra S, Kaltsas G, Kapoor N, Koch C, Kopp P, Korbonits M,
Kovacs CS, Kuohung W, Laferrere B, Levy M, McGee EA, McLachlan R,
New M, Purnell J, Sahay R, Shah AS, Singer F, Sperling MA,
Stratakis CA, Trence DL and Wilson DP: South Dartmouth (MA):
2000
|
|
2
|
Dwivedi S and Sikarwar MS: Diabetic
nephropathy: Pathogenesis, mechanisms, and therapeutic strategies.
Horm Metab Res. 57:7–17. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hu Q, Chen Y, Deng X, Li Y, Ma X, Zeng J
and Zhao Y: Diabetic nephropathy: Focusing on pathological signals,
clinical treatment, and dietary regulation. Biomed Pharmacother.
159:1142522023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zac-Varghese S, Mark P, Bain S, Banerjee
D, Chowdhury TA, Dasgupta I, De P, Fogarty D, Frankel A, Goldet G,
et al: Clinical practice guideline for the management of lipids in
adults with diabetic kidney disease: Abbreviated summary of the
Joint Association of British Clinical Diabetologists and UK Kidney
Association (ABCD-UKKA) Guideline 2024. BMC Nephrol. 25:2162024.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zeng LF, Xiao Y and Sun L: A glimpse of
the mechanisms related to renal fibrosis in diabetic nephropathy.
Adv Exp Med Biol. 1165:49–79. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Watanabe K, Sato E, Mishima E, Miyazaki M
and Tanaka T: What's new in the molecular mechanisms of diabetic
kidney disease: Recent advances. Int J Mol Sci. 24:5702022.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jonckheere S, Adams J, De Groote D,
Campbell K, Berx G and Goossens S: Epithelial-mesenchymal
transition (EMT) as a therapeutic target. Cells Tissues Organs.
211:157–182. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen Y, Zou H, Lu H, Xiang H and Chen S:
Research progress of endothelial-mesenchymal transition in diabetic
kidney disease. J Cell Mol Med. 26:3313–3322. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Balakumar P, Sambathkumar R, Mahadevan N,
Muhsinah AB, Alsayari A, Venkateswaramurthy N and Jagadeesh G: A
potential role of the renin-angiotensin-aldosterone system in
epithelial-to-mesenchymal transition-induced renal abnormalities:
Mechanisms and therapeutic implications. Pharmacol Res.
146:1043142019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hadpech S and Thongboonkerd V:
Epithelial-mesenchymal plasticity in kidney fibrosis. Genesis.
62:e235292024. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang YY, Peng J and Luo XJ:
Post-translational modification of MALT1 and its role in B cell-
and T cell-related diseases. Biochem Pharmacol. 198:1149772022.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Afonina IS, Elton L, Carpentier I and
Beyaert R: MALT1-a universal soldier: Multiple strategies to ensure
NF-ĸB activation and target gene expression. FEBS J. 282:3286–3297.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hachmann J and Salvesen GS: The
Paracaspase MALT1. Biochimie. 122:324–338. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee JL, Ekambaram P, Carleton NM, Hu D,
Klei LR, Cai Z, Myers MI, Hubel NE, Covic L, Agnihotri S, et al:
MALT1 is a targetable driver of epithelial-to-mesenchymal
transition in claudin-low, triple-negative breast cancer. Mol
Cancer Res. 20:373–386. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fusco R, Siracusa R, D'Amico R, Cordaro M,
Genovese T, Gugliandolo E, Peritore AF, Crupi R, Di Paola R,
Cuzzocrea S and Impellizzeri D: Mucosa-associated lymphoid tissue
lymphoma translocation 1 inhibitor as a novel therapeutic tool for
lung injury. Int J Mol Sci. 21:77612020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fontan L, Yang C, Kabaleeswaran V, Volpon
L, Osborne MJ, Beltran E, Garcia M, Cerchietti L, Shaknovich R,
Yang SN, et al: MALT1 small molecule inhibitors specifically
suppress ABC-DLBCL in vitro and in vivo. Cancer Cell. 22:812–824.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Elendu C, John Okah M, Fiemotongha KDJ,
Adeyemo BI, Bassey BN, Omeludike EK and Obidigbo B: Comprehensive
advancements in the prevention and treatment of diabetic
nephropathy: A narrative review. Medicine (Baltimore).
102:e353972023. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Samsu N: Diabetic nephropathy: Challenges
in pathogenesis, diagnosis, and treatment. Biomed Res Int.
2021:14974492021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang R, Fu P and Ma L: Kidney fibrosis:
From mechanisms to therapeutic medicines. Signal Transduct Target
Ther. 8:1292023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cao Y, Lin JH, Hammes HP and Zhang C:
Cellular phenotypic transitions in diabetic nephropathy: An update.
Front Pharmacol. 13:10380732022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang J, Chen G, Wang J, Liu S and Su J:
Platycodin D regulates high glucose-induced ferroptosis of HK-2
cells through glutathione peroxidase 4 (GPX4). Bioengineered.
13:6627–6637. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li J, Shu L, Jiang Q, Feng B, Bi Z, Zhu G,
Zhang Y, Li X and Wu J: Oridonin ameliorates renal fibrosis in
diabetic nephropathy by inhibiting the Wnt/β-catenin signaling
pathway. Ren Fail. 46:23474622024. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zeng J and Bao X: Tanshinone IIA
attenuates high glucose-induced epithelial-to-mesenchymal
transition in HK-2 cells through VDR/Wnt/β-catenin signaling
pathway. Folia Histochem Cytobiol. 59:259–270. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sun Y, Qu H, Song Q, Shen Y, Wang L and
Niu X: High-glucose induced toxicity in HK-2 cells can be
alleviated by inhibition of miRNA-320c. Ren Fail. 44:1388–1398.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Deng J, Zheng C, Hua Z, Ci H, Wang G and
Chen L: Diosmin mitigates high glucose-induced endoplasmic
reticulum stress through PI3K/AKT pathway in HK-2 cells. BMC
Complement Med Ther. 22:1162022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen ST, Chang KS, Lin YH, Hou CP, Lin WY,
Hsu SY, Sung HC, Feng TH, Tsui KH and Juang HH: Glucose upregulates
ChREBP via phosphorylation of AKT and AMPK to modulate MALT1 and
WISP1 expression. J Cell Physiol. 240:e314782025. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang X, Gao Y, Sun H, et al: Mechanism of
Tangluoning for alleviating high glucose-induced inflammatory
reaction of Schwann cells by regulating lncRNA MALAT1. Beijing
Journal of Traditional Chinese Medicine. 41:236–239. 2022.(In
Chinese).
|
|
28
|
Yang J, Antin P, Berx G, Blanpain C,
Brabletz T, Bronner M, Campbell K, Cano A, Casanova J, Christofori
G, et al: Guidelines and definitions for research on
epithelial-mesenchymal transition. Nat Rev Mol Cell Biol.
21:341–352. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Serrano-Gomez SJ, Maziveyi M and Alahari
SK: Regulation of epithelial-mesenchymal transition through
epigenetic and post-translational modifications. Mol Cancer.
15:182016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Meran S and Steadman R: Fibroblasts and
myofibroblasts in renal fibrosis. Int J Exp Pathol. 92:158–167.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang H, Sun G, Li X, Fu Z, Guo C, Cao G,
Wang B, Wang Q, Yang S, Li D, et al: Inhibition of MALT1
paracaspase activity improves lesion recovery following spinal cord
injury. Sci Bull (Beijing). 64:1179–1194. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yan B, Belke D, Gui Y, Chen YX, Jiang ZS
and Zheng XL: Pharmacological inhibition of MALT1
(mucosa-associated lymphoid tissue lymphoma translocation protein
1) induces ferroptosis in vascular smooth muscle cells. Cell Death
Discov. 9:4562023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gu H, Qiu H, Yang H, Deng Z, Zhang S, Du L
and He F: PRRSV utilizes MALT1-regulated autophagy flux to switch
virus spread and reserve. Autophagy. 20:2697–2718. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li Y, Huang X, Huang S, He H, Lei T,
Saaoud F, Yu XQ, Melnick A, Kumar A, Papasian CJ, et al: Central
role of myeloid MCPIP1 in protecting against LPS-induced
inflammation and lung injury. Signal Transduct Target Ther.
2:170662017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jiang VC, Liu Y, Lian J, Huang S, Jordan
A, Cai Q, Lin R, Yan F, McIntosh J, Li Y, et al: Cotargeting of BTK
and MALT1 overcomes resistance to BTK inhibitors in mantle cell
lymphoma. J Clin Invest. 133:e1656942023. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Qian R, Niu X, Wang Y, Guo Z, Deng X, Ding
Z, Zhou M and Deng H: Targeting MALT1 suppresses the malignant
progression of colorectal cancer via miR-375/miR-365a-3p/NF-ĸB
axis. Front Cell Dev Biol. 10:8450482022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yao Y, Yuan M, Shi M, Li W, Sha Y, Zhang
Y, Yuan C, Luo J, Li Z, Liao C, et al: Halting multiple myeloma
with MALT1 inhibition: suppressing BCMA-induced NF-ĸB and inducing
immunogenic cell death. Blood Adv. 8:4003–4016. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lee JH and Massague J: TGF-β in
developmental and fibrogenic EMTs. Semin Cancer Biol. 86:136–145.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hu L, Ding M and He W: Emerging
therapeutic strategies for attenuating tubular EMT and kidney
fibrosis by targeting Wnt/β-catenin signaling. Front Pharmacol.
12:8303402022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lu Q, Wang WW, Zhang MZ, Ma ZX, Qiu XR,
Shen M and Yin XX: ROS induces epithelial-mesenchymal transition
via the TGF-β1/PI3K/Akt/mTOR pathway in diabetic nephropathy. Exp
Ther Med. 17:835–846. 2019.PubMed/NCBI
|
|
41
|
Moud BN, Ober F, O'Neill TJ and Krappmann
D: MALT1 substrate cleavage: What is it good for? Front Immunol.
15:14123472024. View Article : Google Scholar : PubMed/NCBI
|