Introduction
Diabetic nephropathy (DN) is a prevalent and severe
complication of diabetes mellitus, and it is the main cause of
chronic kidney disease worldwide (1,2). Due
to the complex and multifactorial pathogenesis of DN, the treatment
of patients with DN is challenging (3,4).
Notably, renal fibrosis is essential for the pathological process
of DN (5,6), and epithelial-to-mesenchymal
transition (EMT), which refers to the differentiation of epithelial
cells into migratory and invasive mesenchymal cells, is considered
a critical step in the development of renal fibrosis (7–10).
Therefore, inhibiting EMT and renal fibrosis may contribute to
suppressing the progression of DN.
Mucosa-associated lymphoid tissue lymphoma
translocation protein 1 (MALT1) is a scaffold protein and protease
regulating downstream signaling pathways (11–13).
Previous studies have indicated that the inhibition of MALT1 has a
suppressive effect on EMT and organ fibrosis (14,15).
For example, a study suggested that MALT1 could facilitate EMT
through regulation of EMT-transcription factors (14). Another study revealed that the
inhibition of MALT1 reduces fibrosis, and this may be attributed to
suppression of the NF-κB signaling pathway (15). Therefore, the inhibition of MALT1
could be considered a potential treatment target for DN. MI-2 is an
irreversible small molecule inhibitor that combines with MALT1 and
disrupts its protease function (16). However, to the best of our
knowledge, the role of MI-2 in attenuating the EMT of kidney cells
or renal fibrosis to mitigate DN has not yet been demonstrated.
Therefore, the current study aimed to investigate the effect of
MALT1 inhibition by MI-2 on high glucose-induced EMT and fibrosis
in HK-2 cells.
Materials and methods
Cell culture
HK-2 cells were purchased from Xiamen Yimo
Biotechnology Co., Ltd. The cells were cultured in a special HK-2
culture medium (Wuhan Servicebio Technology Co., Ltd.) containing
5.6 mM D-glucose (MedChemExpress), pre-supplemented with 10% fetal
bovine serum (FBS; Wuhan Pricella Biotechnology Co., Ltd.) and 1%
penicillin/streptomycin solution (Wuhan Servicebio Technology Co.,
Ltd.).
Glucose treatment
HK-2 cells were cultured to 80% confluence in
standard medium. Subsequently, the cells were treated with fresh
medium plus either 15 mM D-glucose [low-concentration glucose (LG)
group] or 30 mM D-glucose [high-concentration glucose (HG) group].
The negative control (NC) group consisted of cells cultured only
with the standard medium. After being treated for 48 h at 37°C, the
cells were harvested for wound healing and invasion assays,
immunofluorescence staining and western blotting.
MI-2 treatment
MI-2 (MedChemExpress), a MALT1 inhibitor, was used
to suppress the protease function of MALT1. HK-2 cells were treated
with medium containing 30 mM D-glucose and either 0, 1, 2 or 4 µM
MI-2, and were named as the HG group, the low concentration MI-2
(LC-MI-2) group, the medium concentration MI-2 (MC-MI-2) group and
the high concentration MI-2 (HC-MI-2) group, respectively. The
wound healing and invasion assays, immunofluorescence staining and
western blotting were performed after 48 h of treatment at
37°C.
Cell viability
Cell viability was assessed using the Cell Counting
Kit-8 (CCK-8; Wuhan Servicebio Technology Co., Ltd.). Briefly,
4×103 cells were plated and treated with high glucose
and/or MI-2 as aforementioned for 24 h, followed by incubation with
10% CCK-8 solution for 2 h. Subsequently, the optical density (OD)
value was detected using a microplate reader (Nanjing Huadong
Electronics Information & Technology Co., Ltd.).
Wound healing assay
The cell migration rate following treatment was
assessed using a wound healing assay. Briefly, after treatment, the
cells were plated and cultured to achieve 90% confluence.
Subsequently, a 10 µl sterile pipette tip was used to create a
straight wound across the cell monolayer. After washing, medium
containing 1% FBS was added and images of the wound area were
captured as baseline (0 h). The cells were then cultured for
another 6 h, and images of the wound area were captured again with
a light microscope (Motic Industrial Group Co., Ltd.). The width of
the wound at 0 and 6 h was measured to calculate the cell migration
rate as follows: (Area of wound at 0 h - area of wound at 6 h)/area
of wound at 0 h × 100%.
Cell invasion assay
Cell invasion was measured after treatment using a
Transwell assay. Briefly, following treatment, the HK-2 cells
(2×104) were resuspended in serum-free medium and were
plated in the upper chamber of pre-coated Transwell inserts (0.8
µm, Corning, Inc.). The Transwell inserts were pre-coated with
Matrigel matrix (Corning, Inc.) at 37°C for 1 h. The lower chamber
was filled with the standard medium. After 24 h of incubation at
37°C, the invasive cells were counted after staining with crystal
violet solution (Wuhan Servicebio Technology Co., Ltd.) at room
temperature for 5 min. Images of the invasive cells were captured
with an inverted light microscope (Motic Industrial Group Co.,
Ltd.).
Immunofluorescence staining
After treatment, the HK-2 cells were washed and
fixed in 4% paraformaldehyde solution (Wuhan Servicebio Technology
Co., Ltd.) at room temperature for 20 min to preserve cell
morphology. The cells were then permeabilized using 0.3% Triton
X-100 (Wuhan Servicebio Technology Co., Ltd.) and blocked with
bovine serum albumin (Beyotime Institute of Biotechnology) at 37°C
for 1 h. The anti-α-smooth muscle actin (α-SMA) antibody (1:1,000;
cat. no. GB111364-50; Wuhan Servicebio Technology Co., Ltd.) was
incubated with the cells at 4°C overnight, and then a Alexa Fluor
488 labeled secondary antibody (1:1,000; cat. no. GB25303; Wuhan
Servicebio Technology Co., Ltd.) was incubated with the cells at
37°C for 30 min, followed by staining with DAPI (Wuhan Servicebio
Technology Co., Ltd.) at room temperature for 5 min. An inverted
fluorescence microscope (Motic Industrial Group Co., Ltd.) was used
to collect images. Relative fluorescence intensity was measured
using ImageJ (V1.8.0; National Institutes of Health).
Western blotting
After treatment, the HK-2 cells were washed and the
RIPA Kit (Wuhan Servicebio Technology Co., Ltd.) was added to the
cells for lysis. Subsequently, the cell lysates were harvested and
proteins were isolated after centrifugation at 12,000 × g for 5 min
at 4°C. The protein concentration was quantified using a BCA Kit
(Wuhan Servicebio Technology Co., Ltd.). Subsequently, western
blotting was performed according to standard procedures, including
electrophoresis, electrophoretic transfer, blocking and antibody
incubation. Briefly, 20 µg protein was separated by a 4–20% precast
gel (Shanghai Willget Biotechnology Co., Ltd.) and transferred to a
polyvinylidene difluoride membrane (Merck KGaA). The membrane was
blocked with 5% BSA (Wuhan Servicebio Technology Co., Ltd.) at 37°C
for 1 h. Afterwards, the membrane was incubated with primary
antibodies overnight at 4°C and with secondary antibody at 37°C for
1.5 h. Finally, the signal was detected using an enhanced
chemiluminescence kit (Dalian Meilun Biology Technology Co., Ltd.).
The antibodies used included: Anti-vimentin (1:50,000; cat. no.
10366-1-AP), anti-inhibitor of κB α (IκBα; 1:10,000; cat. no.
10268-1-AP), anti-phosphorylated (p)-p65 (1:5,000; cat. no.
82335-1-RR) and anti-p65 (1:20,000; cat. no. 80979-1-RR) (all from
Wuhan Sanying Biotechnology); anti-E-cadherin (1:2,000; cat. no.
AF0131), anti-fibronectin (FN; 1:1,500; cat. no. AF5335) and
anti-collagen I (1:2,000; cat. no. AF7001) (all from Affinity
Biosciences); and anti-MALT1 (1:1,000; cat. no. GB11790-100),
anti-β-actin (1:5,000; cat. no. GB15003-100) and HRP-linked
secondary antibodies (1:10,000; cat. no. GB23303) (all from Wuhan
Servicebio Technology Co., Ltd.). The intensity of target bands was
assessed using ImageJ software V1.8.0, and the expression levels of
the target proteins were normalized to β-actin.
Statistical analysis
Statistical analyses were performed using GraphPad
(version 9.0; Dotmatics). The Shapiro-Wilk test of normality was
initially performed. Subsequently, group comparisons of normal data
were carried out using one-way analysis of variance, followed by
Tukey's multiple comparisons test. P<0.05 was considered to
indicate a statistically significant difference.
Results
HG facilitates EMT and fibrosis and
enhances MALT1 expression in HK-2 cells
In detail, cell migration rate and invasive cell
number were increased in the HG group compared with those in the NC
and LG groups (all P<0.05), whereas there was no change between
the LG and NC groups (both P>0.05) (Fig. 1A and B). Furthermore, E-cadherin
protein expression levels were reduced in the HG group compared
with those in the NC group (P<0.01), whereas they did not differ
between the HG and LG groups, or between the LG and NC groups (both
P>0.05) (Fig. 1C). By contrast,
elevated vimentin protein expression was observed in the HG group
compared with that in the NC (P<0.001) and LG (P<0.05)
groups, as well as in the LG group compared with that in the NC
group (P<0.05). These results indicated that glucose promoted
EMT with gradient concentration effects in HK-2 cells.
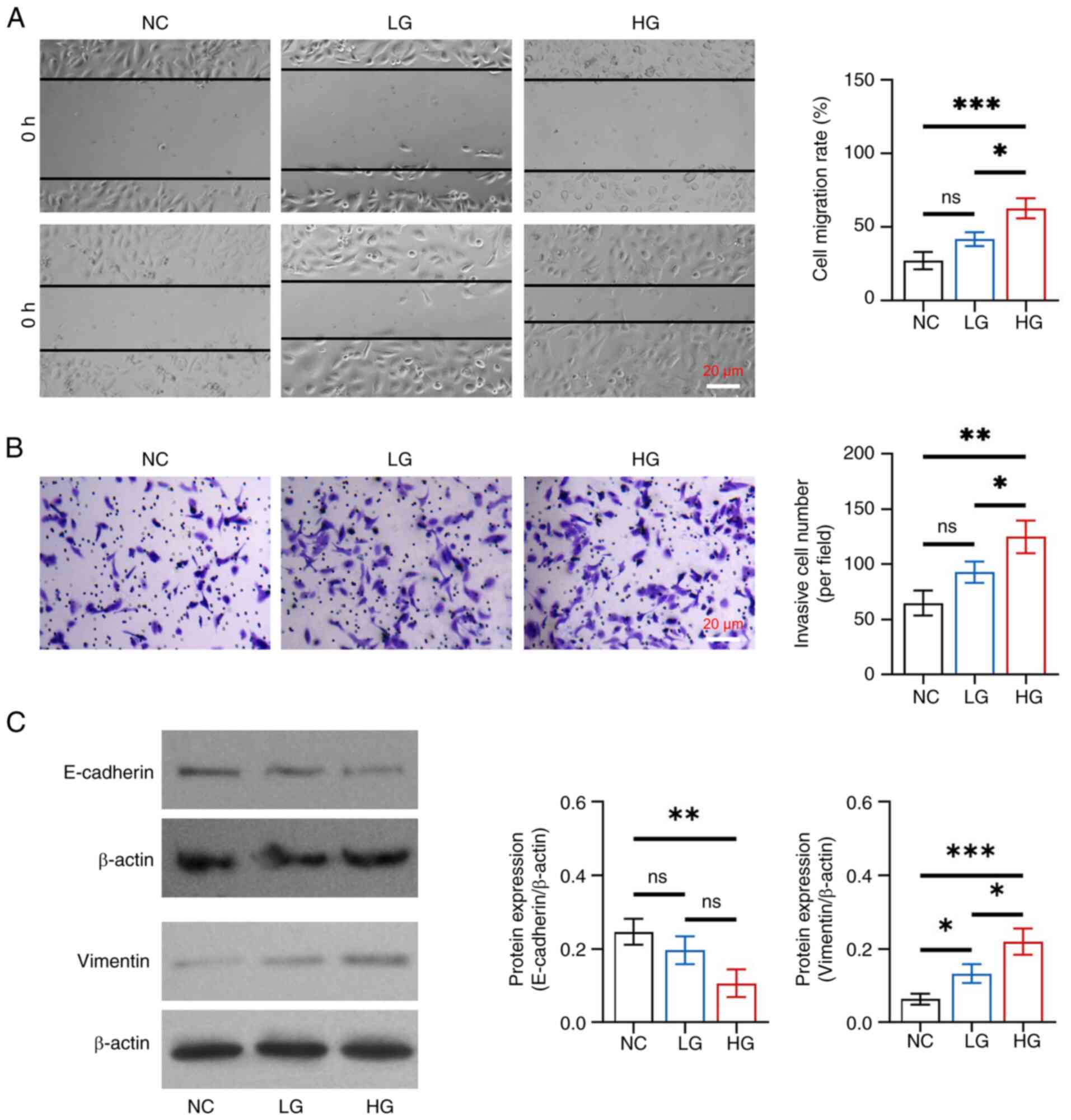 | Figure 1.Effect of different concentrations of
glucose on epithelial-to-mesenchymal transition in HK-2 cells. (A)
Comparison of cell migration rate among the NC, LG and HG groups,
between the NC and LG groups, between the NC and HG groups, and
between the LG and HG groups. (B) Comparison of invasive cell
number among the NC, LG and HG groups, between the NC and LG
groups, between the NC and HG groups, and between the LG and HG
groups. (C) Comparison of the protein expression levels of
E-cadherin among the NC, LG and HG groups, between the NC and LG
groups, between the NC and HG groups, and between the LG and HG
groups; and comparison of the protein expression levels of vimentin
among the NC, LG and HG groups, between the NC and LG groups,
between the NC and HG groups, and between the LG and HG groups.
*P<0.05, **P<0.01, ***P<0.001; ns, not significant;
n=3/group. NC, negative control; LG, low-concentration glucose; HG,
high-concentration glucose. |
Glucose promoted fibrosis in HK-2 cells in a
dose-dependent manner. The relative fluorescence intensity of α-SMA
was increased in the HG group compared with that in the NC
(P<0.001) and LG (P<0.01) groups, as well as in the LG group
compared with in the NC group (P<0.05) (Fig. 2A). In addition, the protein
expression levels of FN and collagen I were increased in the HG
group compared with those in the NC and LG groups (all P<0.05),
but there was no difference in expression between the LG and NC
groups (both P>0.05) (Fig.
2B).
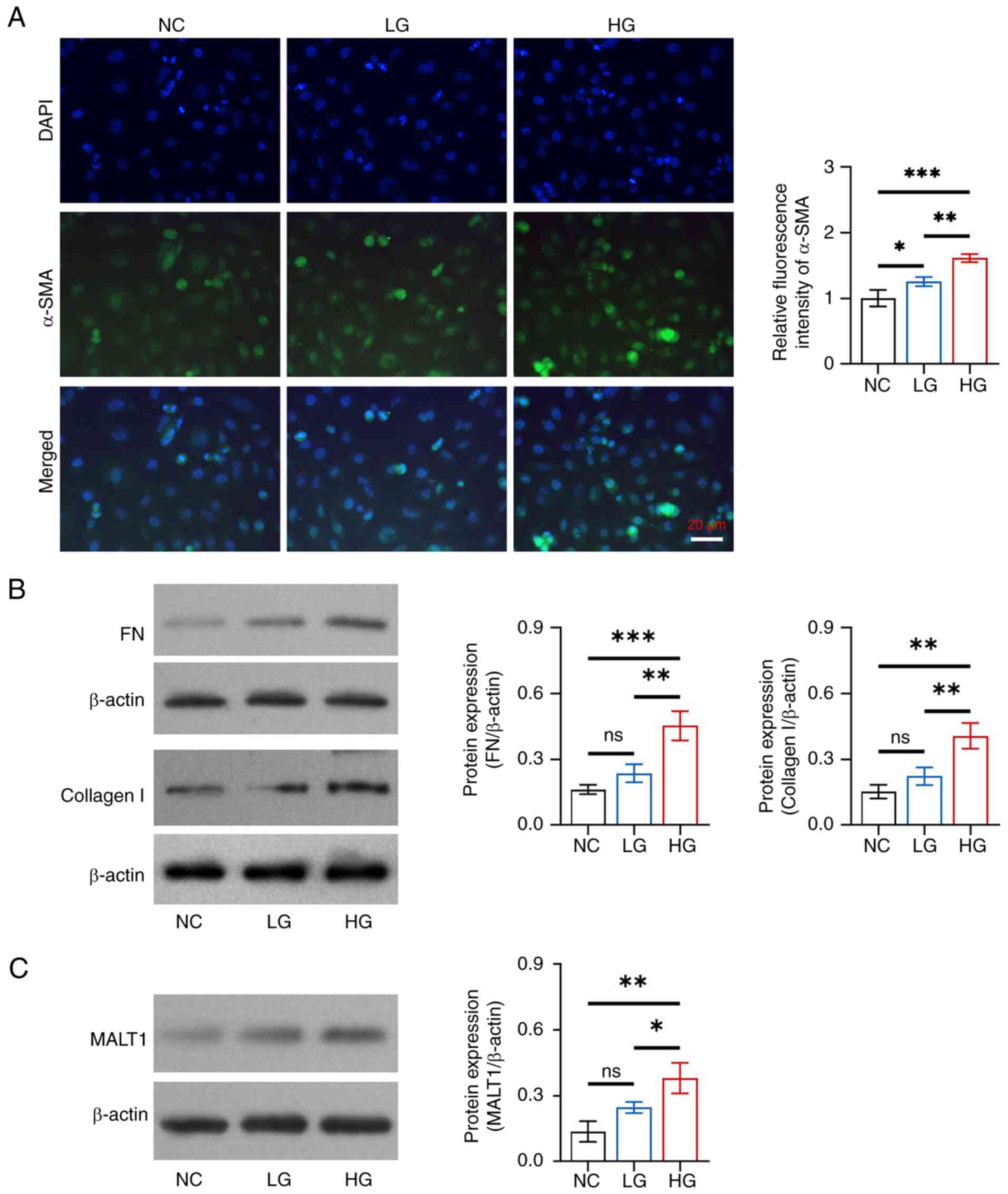 | Figure 2.Effects of different concentrations
of glucose on fibrosis and MALT1 expression in HK-2 cells. (A)
Comparison of the relative fluorescence intensity of α-SMA among
the NC, LG and HG groups, between the NC and LG groups, between the
NC and HG groups, and between the LG and HG groups. (B) Comparison
of the protein expression levels of FN among the NC, LG and HG
groups, between the NC and LG groups, between the NC and HG groups,
and between the LG and HG groups; and comparison of the protein
expression levels of collagen I among the NC, LG and HG groups,
between the NC and LG groups, between the NC and HG groups, and
between the LG and HG groups. (C) Comparison of the protein
expression levels of MALT1 among the NC, LG and HG groups, between
the NC and LG groups, between the NC and HG groups, and between the
LG and HG groups. *P<0.05, **P<0.01, ***P<0.001; ns, not
significant; n=3/group. MALT1, mucosa-associated lymphoid tissue
lymphoma translocation protein 1; α-SMA, α-smooth muscle actin; NC,
negative control; LG, low-concentration glucose; HG,
high-concentration glucose; FN, fibronectin. |
Additionally, glucose facilitated MALT1 expression
in a dose-dependent manner in HK-2 cells. Specifically, the protein
expression levels of MALT1 were increased in the HG group compared
with those in the NC (P<0.01) and LG (P<0.05) groups, whereas
there was no difference between the LG and NC groups (P>0.05)
(Fig. 2C).
MI-2 inhibits cell viability and EMT
in HG-treated HK-2 cells
No difference was found in the protein expression
levels of MALT1 among the HG, LC-MI-2, MC-MI-2 and HC-MI-2 groups
(all P>0.05; Fig. S1),
indicating that MI-2 functions by inhibiting MALT1 activity but not
its direct expression. However, MI-2 dose-dependently reduced cell
viability in HG-treated HK-2 cells, and HC-MI-2 exhibited the best
effect. In detail, the OD value was reduced in the HC-MI-2 group
compared with that in the HG, LC-MI-2, and MC-MI-2 groups (all
P<0.01; Fig. S2). The effect
of MI-2 on the inhibition of EMT was dose-dependent in HG-treated
HK-2 cells and HC-MI-2 showed the best inhibitory effect. In
detail, the cell migration rate and invasive cell number were
reduced in the HC-MI-2 group compared with those in the HG and
LC-MI-2 groups (all P<0.05), whereas there was no significant
difference between the HC-MI-2 group and the MC-MI-2 group (both
P>0.05) (Fig. 3A and B).
Furthermore, the protein expression levels of E-cadherin were
elevated, whereas those of vimentin were reduced in the HC-MI-2
group compared with those in the HG group and the LC-MI-2 group
(all P<0.01) (Fig. 3C).
Moreover, the protein expression of E-cadherin was elevated in the
HC-MI-2 group compared with that in the MC-MI-2 group (P<0.05),
but the reduction in vimentin protein expression between the
HC-MI-2 and MC-MI-2 groups was not statistically significant
(P>0.05).
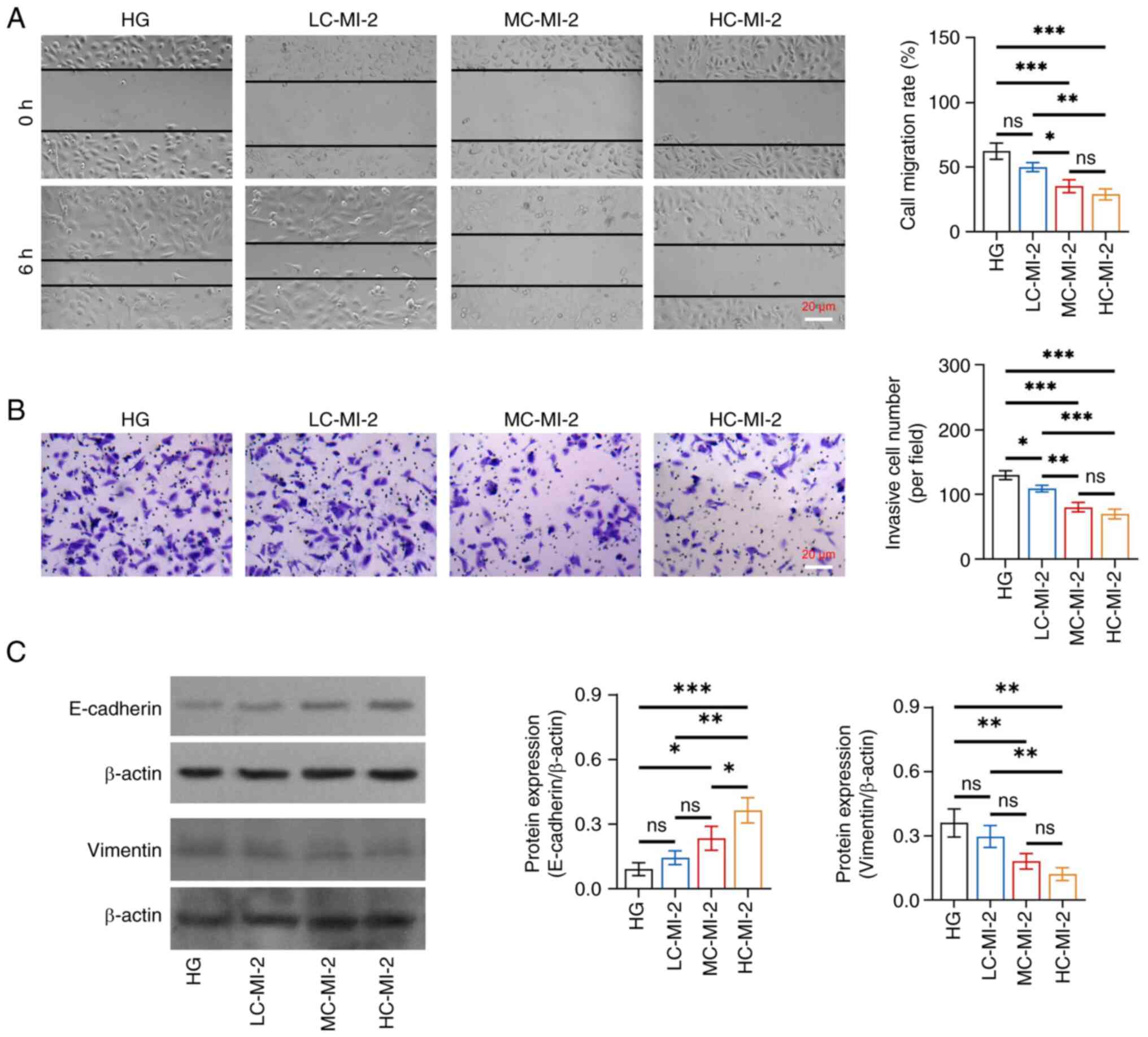 | Figure 3.Effect of different concentrations of
MI-2 on epithelial-to-mesenchymal transition in HG-treated HK-2
cells. (A) Comparison of cell migration rate among the HG, LC-MI-2,
MC-MI-2 and HC-MI-2 groups, between the HG and LC-MI-2 groups,
between the HG and MC-MI-2 groups, between the HG and HC-MI-2
groups, between the LC-MI-2 and MC-MI-2 groups, between the LC-MI-2
and HC-MI-2 groups, and between the MC-MI-2 and HC-MI-2 groups. (B)
Comparison of invasive cell number among the HG, LC-MI-2, MC-MI-2
and HC-MI-2 groups, between the HG and LC-MI-2 groups, between the
HG and MC-MI-2 groups, between the HG and HC-MI-2 groups, between
the LC-MI-2 and MC-MI-2 groups, between the LC-MI-2 and HC-MI-2
groups, and between the MC-MI-2 and HC-MI-2 groups. (C) Comparison
of the protein expression levels of E-cadherin among the HG,
LC-MI-2, MC-MI-2 and HC-MI-2 groups, between the HG and LC-MI-2
groups, between the HG and MC-MI-2 groups, between the HG and
HC-MI-2 groups, between the LC-MI-2 and MC-MI-2 groups, between the
LC-MI-2 and HC-MI-2 groups, and between the MC-MI-2 and HC-MI-2
groups; and comparison of the protein expression levels of vimentin
among the HG, LC-MI-2, MC-MI-2 and HC-MI-2 groups, between the HG
and LC-MI-2 groups, between the HG and MC-MI-2 groups, between the
HG and HC-MI-2 groups, between the LC-MI-2 and MC-MI-2 groups,
between the LC-MI-2 and HC-MI-2 groups, and between the MC-MI-2 and
HC-MI-2 groups. HK-2 cells in the LC-MI-2, MC-MI-2 and HC-MI-2
groups were treated with HG. *P<0.05, **P<0.01,
***P<0.001; ns, not significant; n=3/group. HG,
high-concentration glucose; LC-MI-2, low concentration MI-2;
MC-MI-2, medium concentration MI-2; HC-MI-2; high concentration
MI-2. |
MI-2 inhibits fibrosis in HG-treated
HK-2 cells
MI-2 dose-dependently reduced fibrosis in HG-treated
HK-2 cells and HC-MI-2 exhibited the best effect. Specifically, the
relative fluorescence intensity of α-SMA was reduced in the HC-MI-2
group compared with that in the HG, LC-MI-2 and MC-MI-2 groups (all
P<0.05; Fig. 4A). Furthermore,
the protein expression levels of FN were reduced in the HC-MI-2
group compared with those in the HG and LC-MI-2 groups (both
P<0.05), but there was no significant difference between the
HC-MI-2 group and the MC-MI-2 group (P>0.05) (Fig. 4B). Collagen I protein expression
was also decreased in the HC-MI-2 group compared with that in the
HG (P<0.001) and LC-MI-2 (P<0.01) groups, but it did not
differ statistically between the HC-MI-2 and MC-MI-2 groups
(P>0.05).
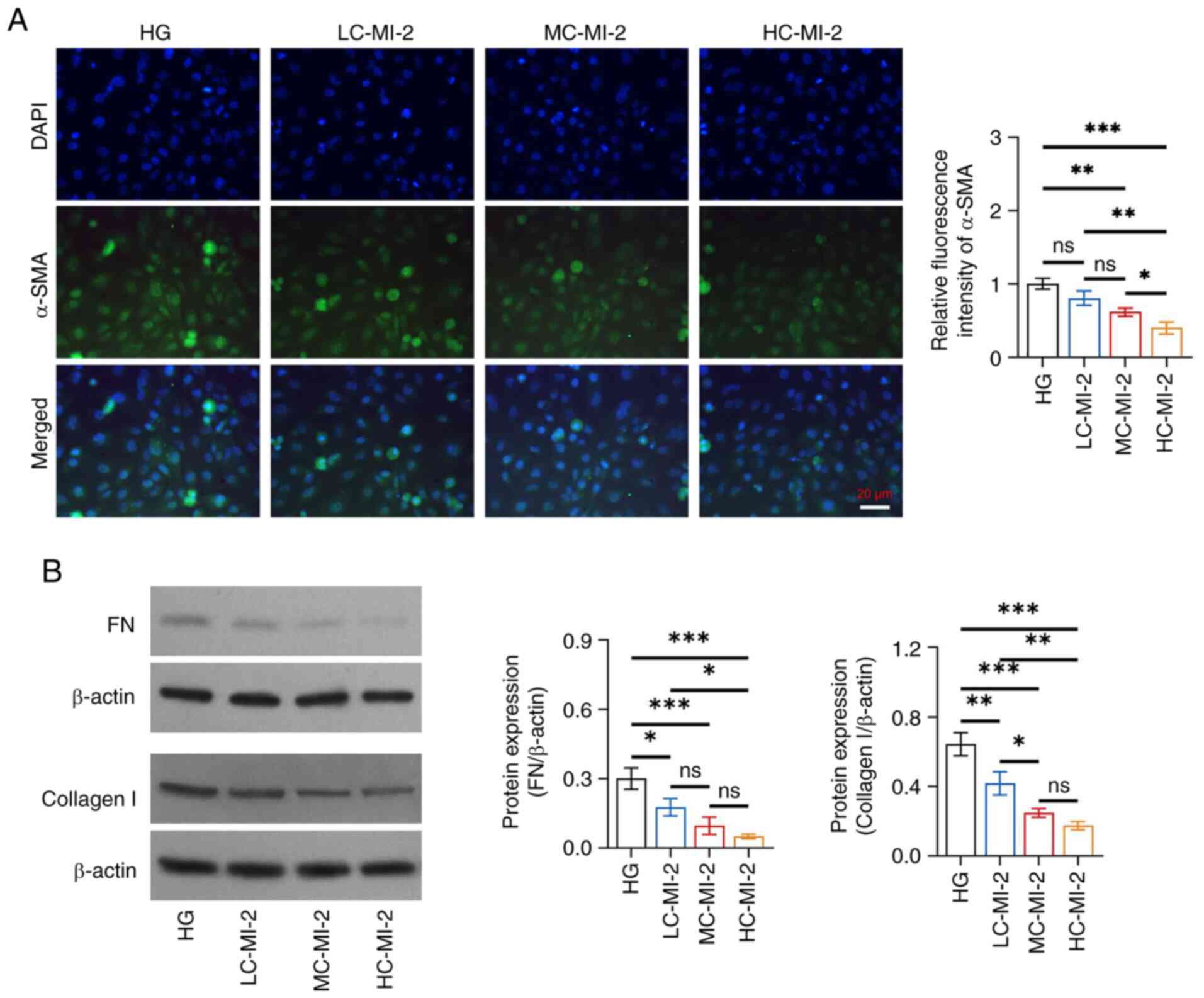 | Figure 4.Effect of different concentrations of
MI-2 on fibrosis in HG-treated HK-2 cells. (A) Comparison of the
relative fluorescence intensity of α-SMA among the HG, LC-MI-2,
MC-MI-2 and HC-MI-2 groups between the HG and LC-MI-2 groups,
between the HG and MC-MI-2 groups, between the HG and HC-MI-2
groups, between the LC-MI-2 and MC-MI-2 groups, between the LC-MI-2
and HC-MI-2 groups, and between the MC-MI-2 and HC-MI-2 groups. (B)
Comparison of the protein expression levels of FN among the HG,
LC-MI-2, MC-MI-2 and HC-MI-2 groups, between the HG and LC-MI-2
groups, between the HG and MC-MI-2 groups, between the HG and
HC-MI-2 groups, between the LC-MI-2 and MC-MI-2 groups, between the
LC-MI-2 and HC-MI-2 groups, and between the MC-MI-2 and HC-MI-2
groups; and comparison of the protein expression levels of collagen
I among the HG, LC-MI-2, MC-MI-2 and HC-MI-2 groups, between the HG
and LC-MI-2 groups, between the HG and MC-MI-2 groups, between the
HG and HC-MI-2 groups, between the LC-MI-2 and MC-MI-2 groups,
between the LC-MI-2 and HC-MI-2 groups, and between the MC-MI-2 and
HC-MI-2 groups. HK-2 cells in the LC-MI-2, MC-MI-2 and HC-MI-2
groups were treated with HG. *P<0.05, **P<0.01,
***P<0.001; ns, not significant; n=3/group. HG,
high-concentration glucose; α-SMA, α-smooth muscle actin; LC-MI-2,
low concentration MI-2; MC-MI-2, medium concentration MI-2;
HC-MI-2; high concentration MI-2; FN, fibronectin. |
MI-2 inhibits the NF-κB pathway in
HG-treated HK-2 cells
MI-2 inhibited the NF-κB pathway in HG-treated HK-2
cells in a dose-dependent manner and HC-MI-2 had the best
inhibitory effect. In particular, IκBα protein expression was
increased in the HC-MI-2 group compared with that in the HG
(P<0.001), LC-MI-2 (P<0.001) and MC-MI-2 (P<0.05) groups
(Fig. 5). By contrast, the protein
expression ratio of p-p65/p65 was descended in the HC-MI-2 group
compared with that in the HG (P<0.001) and LC-MI-2 (P<0.05)
groups; however, no significant difference was found between the
HC-MI-2 and MC-MI-2 groups (P>0.05).
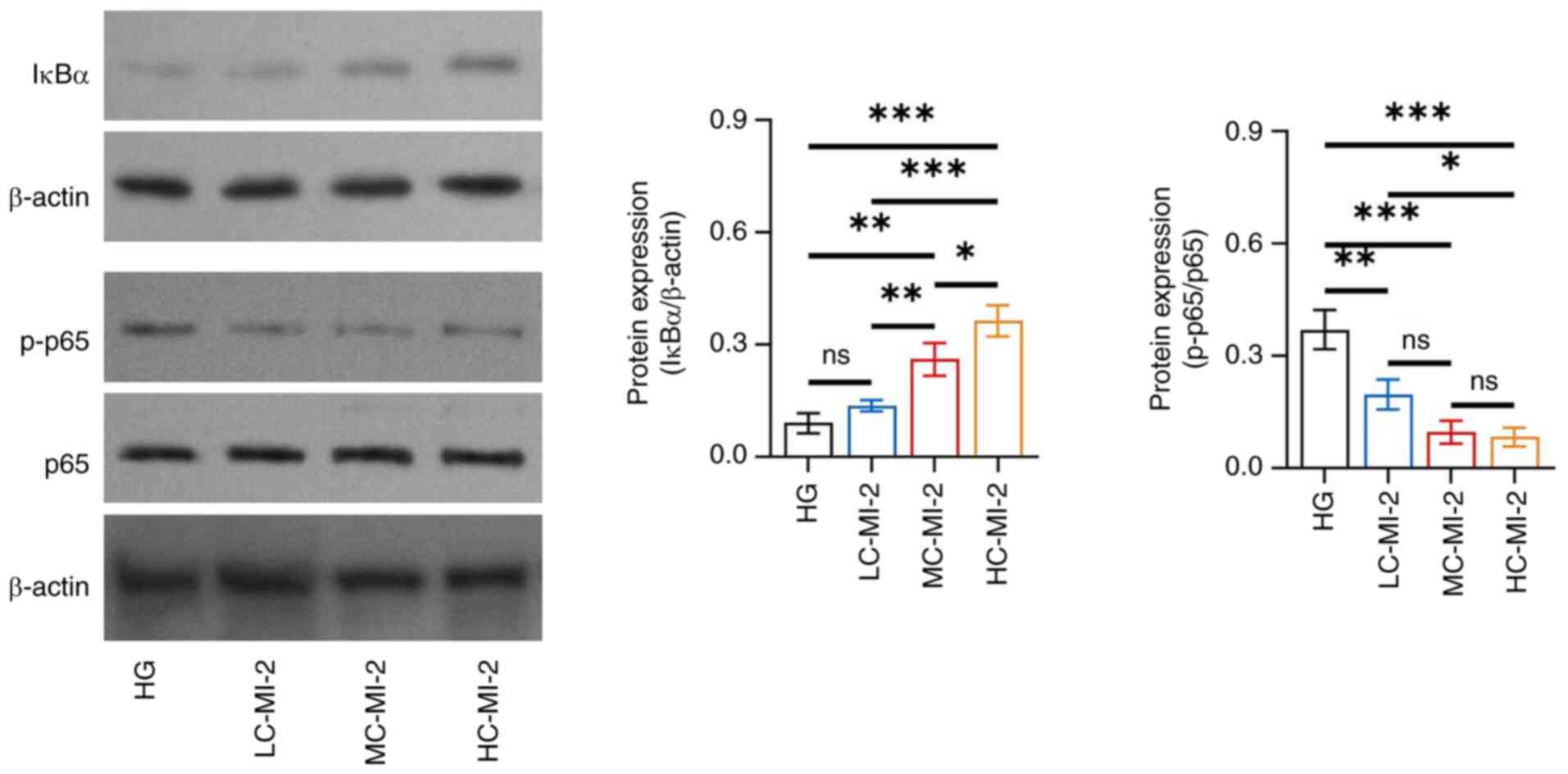 | Figure 5.Effect of different concentrations of
MI-2 on the NF-κB pathway in HG-treated HK-2 cells. Comparison of
the protein expression levels of IκBα among the HG, LC-MI-2,
MC-MI-2 and HC-MI-2 groups, between the HG and LC-MI-2 groups,
between the HG and MC-MI-2 groups, between the HG and HC-MI-2
groups, between the LC-MI-2 and MC-MI-2 groups, between the LC-MI-2
and HC-MI-2 groups, and between the MC-MI-2 and HC-MI-2 groups; and
comparison of p-p65/p65 among the HG, LC-MI-2, MC-MI-2 and HC-MI-2
groups, between the HG and LC-MI-2 groups, between the HG and
MC-MI-2 groups, between the HG and HC-MI-2 groups, between the
LC-MI-2 and MC-MI-2 groups, between the LC-MI-2 and HC-MI-2 groups,
and between the MC-MI-2 and HC-MI-2 groups. HK-2 cells in the
LC-MI-2, MC-MI-2 and HC-MI-2 groups were treated with HG.
*P<0.05, **P<0.01, ***P<0.001; ns, not significant;
n=3/group. HG, high-concentration glucose; IκBα, inhibitor of κB α;
LC-MI-2, low concentration MI-2; MC-MI-2, medium concentration
MI-2; HC-MI-2; high concentration MI-2; p, phosphorylated. |
Discussion
DN is a burden on healthcare systems that affects
~1/3 of patients with diabetes mellitus (3,17,18).
Renal fibrosis, marked by the excessive deposition of extracellular
matrix components, destroys kidney structure and causes loss of
kidney function, which is closely linked to the development and
progression of DN (19). EMT is
one of the major pathogeneses of renal fibrosis (20). In the present study, the HK-2 cell
line was used, a human renal proximal tubule epithelial cell line,
to construct a widely used in vitro model of DN (21–25).
The results revealed that HG promoted EMT and fibrosis in HK-2
cells, which was similar to the findings of previous studies
(22,23). Moreover, previous studies have
revealed that HG can induce the protein expression of MALT1, which
was similar to the findings of the current study (26,27).
In the present study, HG promoted the protein expression levels of
MALT1 in HK-2 cells, which indicated that MALT1 may be a promising
target and a potential biomarker for DN.
Enhanced cell migration and invasion reflect the
development of EMT (28), and the
reduction of epithelial surface markers (such as E-cadherin) and
the elevation of mesenchymal markers (such as vimentin) are the
hallmarks of EMT (29).
Furthermore, the excessive deposition of extracellular matrix
components, such as α-SMA, FN and collagen I are considered
important markers of fibrosis (30). The present study revealed that MI-2
could reverse HG-induced EMT and fibrosis with gradient
concentration effects in HK-2 cells. These findings suggested that
MI-2 might be an effective therapeutic agent for DN. In the wound
healing assay, the choice of time point of cell migration depends
on numerous factors. The main factor is the specific requirements
of the experiment and cell type. In addition, other factors, such
as cell density, scratch width and cell processing methods need to
be taken into account. Due to a comprehensive consideration of
these factors, the present study set multiple evaluation time
points in the preliminary experiments. The time point of 6 h was
eventually selected as the evaluation time point to prevent the
cells from becoming too dense and affecting the migration
evaluation.
The current study suggested that MI-2 did not
significantly affect the protein expression of MALT1 in HG-treated
HK-2 cells. The cause of this phenomenon may be that MI-2 is an
irreversible MALT1 inhibitor, which works by directly combining
with MALT1 and inhibiting MALT1 protease activity (15,31–34);
thus, MI-2 did not directly affect the protein expression of MALT1.
In addition, due to the fact that MALT1 is the key regulator in
activating the NF-κB signaling pathway, the present study further
explored whether the NF-κB signaling pathway was inhibited by MI-2
in HG-treated HK-2 cells. The findings revealed that MI-2 increased
the protein expression levels of IκBα, while reducing the protein
expression ratio of p-p65/p65 in a dose-dependent manner in
HG-treated HK-2 cells. Notably, a previous study showed that MALT1
inhibition by MI-2 can suppress the NF-κB signaling pathway in
mantle cell lymphoma cells (35).
The results of this previous study were similar to the present
results (35), indicating that
MALT1 inhibition by MI-2 may decrease cell viability and migration
via the NF-κB signaling pathway. However, there were some
differences between the previous study and the current study: i)
The previous study focused on mantle cell lymphoma cells, whereas
the present study focused on HK-2 cells (which were used for in
vitro studies of DN) (35).
ii) The previous study highlighted the impact of MI-2 inhibiting
the NF-κB signaling pathway on tumor resistance, while the present
study focused on its impact on EMT and fibrosis (35). iii) The previous study did not
construct MI-2 concentration gradient models; however, the present
study constructed these models, which further indicated that the
inhibitory effect of MI-2 on the NF-κB pathway was dose-dependent
(35). The possible mechanisms
underlying the inhibitory effects of MI-2 on the NF-κB signaling
pathway may be as follows: MI-2 could directly bind to MALT1 and
suppress its protease function, thus disrupting the assembly of the
CARMA1/BCL10/MALT1 (CBM) complex, further inactivating the IκB
kinase complex, inhibiting the phosphorylation of IκB and reducing
NF-κB activation (36,37). However, the current study did not
explore specific molecular interactions or downstream effects,
which require verification in future studies. Overall, the results
indicated that MI-2 inhibited MALT1, further blocking the NF-κB
signaling pathway to suppress HG-induced EMT and fibrosis. Notably,
some other signaling pathways, such as transforming growth
factor-β/SMAD, Wnt/β-catenin and phosphatidylinositol
3-kinase/protein kinase B/mammalian target of the rapamycin, are
also involved in EMT and fibrosis (38–40).
These signaling pathways could be explored in future studies.
Overall, the aforementioned findings highlighted
that MI-2 might be a promising agent, which could meet the current
clinical needs for the management of DN. However, the CCK-8 cell
viability assay showed that MI-2 dose-dependently reduced the
viability of HG-treated HK-2 cells. This result indicated that
although MI-2 showed a favorable effect on the treatment of DN, its
clinical application may be limited by toxicity. Thus, strict dose
optimization is required to avoid exacerbating toxicity in clinical
practice. Moreover, more clinical studies are required to further
validate the clinical application of MI-2, including the efficacy
and safety assessment of MI-2 in patients with DN.
The current study has the following limitations: i)
The mechanism by which HG promoted the protein expression of MALT1
in HK-2 cells remains unknown. Future studies should explore the
signaling pathways or transcription factors involved in MALT1
upregulation under HG conditions, which may provide deeper insights
into the pathogenesis of DN and potential therapeutic targets. ii)
The study only focused on HK-2 cells. The findings may not be
generalizable to other types of kidney cells, such as podocytes and
mesangial cells, which also serve crucial roles in DN. Thus, future
studies should investigate whether MI-2 has similar effects on
these kidney cells. iii) Due to the lack of experimental
conditions, the current study was entirely based on in vitro
experiments. The results might not fully reflect the complex
interactions and responses in a living organism. Future studies
should conduct in vivo studies using animal models of DN to
validate the findings and assess the therapeutic potential of MI-2
in a more physiologically relevant context. iv) The current study
evaluated the effects of MI-2 treatment after 48 h, and the short
duration might not capture the full spectrum of cellular responses
or long-term effects. Future studies should extend the treatment
and observation periods to evaluate the sustained effects and
potential side effects of MI-2. v) The current study lacked
detailed mechanistic studies to elucidate the specific interactions
and downstream effects of MALT1 inhibition by MI-2 on the NF-κB
pathway. On one hand, MALT1 acts as a scaffolding protein, which
mediates the formation of the CBM complex to drive activation of
the NF-κB pathway (41). Thus,
immunoprecipitation is required to evaluate the effect of MALT1 on
the CBM complex, and western blot analysis should be performed to
assess the effect of MI-2 on downstream proteins of the CBM complex
(such as TRAF6 and TAK1). On the other hand, MALT1 acts as a
protease, which cleaves several proteins directly connected to the
NF-κB pathway, such as CYLD, A20 and RelB (41). Thus, western blotting is required
to assess the effect of MI-2 on these related proteins. However,
considering technical and financial limitations, the detailed
molecular studies were not conducted. vi) Due to financial
limitations, the current study prioritized and only focused on the
NF-κB pathway. Future studies may consider investigating other
relevant signaling pathways to provide a more comprehensive
understanding of the molecular mechanisms. vii) The current study
only used western blotting to measure proteins, whereas dual
measurements of proteins using enzyme-linked immunosorbent assay
and western blotting assay may enhance the quality of results.
In conclusion, the present study revealed that MALT1
inhibition by MI-2 may attenuate HG-induced EMT and fibrosis in
HK-2 cells, and this attenuation may be attributed to inhibition of
the NF-κB signaling pathway.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by the Postdoctoral Foundation of
Heilongjiang Province (grant no. LBH-Z23284), Heilongjiang
University of Chinese Medicine Young Researchers Scientific and
Technological Innovation Capacity Cultivation Project (grant nos.
2024XJJ-QNCX023 and 2024-KYYWF-1392), Heilongjiang University of
Traditional Chinese Medicine ‘Double First Class’ Discipline
Development Assistance Fund (grant no. GJJGSPZDXK31003) and the
NSFC Cultivation Support Program, First Affiliated Hospital,
Heilongjiang University of Chinese Medicine (grant no.
PYQN202501008).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
NZ contributed to the conception and the design of
the study. YL and JM were responsible for the acquisition and
analysis of the data. HC and CL contributed to data interpretation.
All authors contributed to manuscript drafting or critical
revisions of the intellectual content. NZ and YL confirm the
authenticity of all the raw data. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Caramori ML and Rossing P: Diabetic Kidney
Disease. Endotext. Feingold KR, Anawalt B, Blackman MR, Boyce A,
Chrousos G, Corpas E, de Herder WW, Dhatariya K, Dungan K, Hofland
J, Kalra S, Kaltsas G, Kapoor N, Koch C, Kopp P, Korbonits M,
Kovacs CS, Kuohung W, Laferrere B, Levy M, McGee EA, McLachlan R,
New M, Purnell J, Sahay R, Shah AS, Singer F, Sperling MA,
Stratakis CA, Trence DL and Wilson DP: South Dartmouth (MA):
2000
|
|
2
|
Dwivedi S and Sikarwar MS: Diabetic
nephropathy: Pathogenesis, mechanisms, and therapeutic strategies.
Horm Metab Res. 57:7–17. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hu Q, Chen Y, Deng X, Li Y, Ma X, Zeng J
and Zhao Y: Diabetic nephropathy: Focusing on pathological signals,
clinical treatment, and dietary regulation. Biomed Pharmacother.
159:1142522023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zac-Varghese S, Mark P, Bain S, Banerjee
D, Chowdhury TA, Dasgupta I, De P, Fogarty D, Frankel A, Goldet G,
et al: Clinical practice guideline for the management of lipids in
adults with diabetic kidney disease: Abbreviated summary of the
Joint Association of British Clinical Diabetologists and UK Kidney
Association (ABCD-UKKA) Guideline 2024. BMC Nephrol. 25:2162024.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zeng LF, Xiao Y and Sun L: A glimpse of
the mechanisms related to renal fibrosis in diabetic nephropathy.
Adv Exp Med Biol. 1165:49–79. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Watanabe K, Sato E, Mishima E, Miyazaki M
and Tanaka T: What's new in the molecular mechanisms of diabetic
kidney disease: Recent advances. Int J Mol Sci. 24:5702022.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jonckheere S, Adams J, De Groote D,
Campbell K, Berx G and Goossens S: Epithelial-mesenchymal
transition (EMT) as a therapeutic target. Cells Tissues Organs.
211:157–182. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen Y, Zou H, Lu H, Xiang H and Chen S:
Research progress of endothelial-mesenchymal transition in diabetic
kidney disease. J Cell Mol Med. 26:3313–3322. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Balakumar P, Sambathkumar R, Mahadevan N,
Muhsinah AB, Alsayari A, Venkateswaramurthy N and Jagadeesh G: A
potential role of the renin-angiotensin-aldosterone system in
epithelial-to-mesenchymal transition-induced renal abnormalities:
Mechanisms and therapeutic implications. Pharmacol Res.
146:1043142019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hadpech S and Thongboonkerd V:
Epithelial-mesenchymal plasticity in kidney fibrosis. Genesis.
62:e235292024. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang YY, Peng J and Luo XJ:
Post-translational modification of MALT1 and its role in B cell-
and T cell-related diseases. Biochem Pharmacol. 198:1149772022.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Afonina IS, Elton L, Carpentier I and
Beyaert R: MALT1-a universal soldier: Multiple strategies to ensure
NF-ĸB activation and target gene expression. FEBS J. 282:3286–3297.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hachmann J and Salvesen GS: The
Paracaspase MALT1. Biochimie. 122:324–338. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee JL, Ekambaram P, Carleton NM, Hu D,
Klei LR, Cai Z, Myers MI, Hubel NE, Covic L, Agnihotri S, et al:
MALT1 is a targetable driver of epithelial-to-mesenchymal
transition in claudin-low, triple-negative breast cancer. Mol
Cancer Res. 20:373–386. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fusco R, Siracusa R, D'Amico R, Cordaro M,
Genovese T, Gugliandolo E, Peritore AF, Crupi R, Di Paola R,
Cuzzocrea S and Impellizzeri D: Mucosa-associated lymphoid tissue
lymphoma translocation 1 inhibitor as a novel therapeutic tool for
lung injury. Int J Mol Sci. 21:77612020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fontan L, Yang C, Kabaleeswaran V, Volpon
L, Osborne MJ, Beltran E, Garcia M, Cerchietti L, Shaknovich R,
Yang SN, et al: MALT1 small molecule inhibitors specifically
suppress ABC-DLBCL in vitro and in vivo. Cancer Cell. 22:812–824.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Elendu C, John Okah M, Fiemotongha KDJ,
Adeyemo BI, Bassey BN, Omeludike EK and Obidigbo B: Comprehensive
advancements in the prevention and treatment of diabetic
nephropathy: A narrative review. Medicine (Baltimore).
102:e353972023. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Samsu N: Diabetic nephropathy: Challenges
in pathogenesis, diagnosis, and treatment. Biomed Res Int.
2021:14974492021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang R, Fu P and Ma L: Kidney fibrosis:
From mechanisms to therapeutic medicines. Signal Transduct Target
Ther. 8:1292023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cao Y, Lin JH, Hammes HP and Zhang C:
Cellular phenotypic transitions in diabetic nephropathy: An update.
Front Pharmacol. 13:10380732022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang J, Chen G, Wang J, Liu S and Su J:
Platycodin D regulates high glucose-induced ferroptosis of HK-2
cells through glutathione peroxidase 4 (GPX4). Bioengineered.
13:6627–6637. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li J, Shu L, Jiang Q, Feng B, Bi Z, Zhu G,
Zhang Y, Li X and Wu J: Oridonin ameliorates renal fibrosis in
diabetic nephropathy by inhibiting the Wnt/β-catenin signaling
pathway. Ren Fail. 46:23474622024. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zeng J and Bao X: Tanshinone IIA
attenuates high glucose-induced epithelial-to-mesenchymal
transition in HK-2 cells through VDR/Wnt/β-catenin signaling
pathway. Folia Histochem Cytobiol. 59:259–270. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sun Y, Qu H, Song Q, Shen Y, Wang L and
Niu X: High-glucose induced toxicity in HK-2 cells can be
alleviated by inhibition of miRNA-320c. Ren Fail. 44:1388–1398.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Deng J, Zheng C, Hua Z, Ci H, Wang G and
Chen L: Diosmin mitigates high glucose-induced endoplasmic
reticulum stress through PI3K/AKT pathway in HK-2 cells. BMC
Complement Med Ther. 22:1162022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen ST, Chang KS, Lin YH, Hou CP, Lin WY,
Hsu SY, Sung HC, Feng TH, Tsui KH and Juang HH: Glucose upregulates
ChREBP via phosphorylation of AKT and AMPK to modulate MALT1 and
WISP1 expression. J Cell Physiol. 240:e314782025. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang X, Gao Y, Sun H, et al: Mechanism of
Tangluoning for alleviating high glucose-induced inflammatory
reaction of Schwann cells by regulating lncRNA MALAT1. Beijing
Journal of Traditional Chinese Medicine. 41:236–239. 2022.(In
Chinese).
|
|
28
|
Yang J, Antin P, Berx G, Blanpain C,
Brabletz T, Bronner M, Campbell K, Cano A, Casanova J, Christofori
G, et al: Guidelines and definitions for research on
epithelial-mesenchymal transition. Nat Rev Mol Cell Biol.
21:341–352. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Serrano-Gomez SJ, Maziveyi M and Alahari
SK: Regulation of epithelial-mesenchymal transition through
epigenetic and post-translational modifications. Mol Cancer.
15:182016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Meran S and Steadman R: Fibroblasts and
myofibroblasts in renal fibrosis. Int J Exp Pathol. 92:158–167.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang H, Sun G, Li X, Fu Z, Guo C, Cao G,
Wang B, Wang Q, Yang S, Li D, et al: Inhibition of MALT1
paracaspase activity improves lesion recovery following spinal cord
injury. Sci Bull (Beijing). 64:1179–1194. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yan B, Belke D, Gui Y, Chen YX, Jiang ZS
and Zheng XL: Pharmacological inhibition of MALT1
(mucosa-associated lymphoid tissue lymphoma translocation protein
1) induces ferroptosis in vascular smooth muscle cells. Cell Death
Discov. 9:4562023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gu H, Qiu H, Yang H, Deng Z, Zhang S, Du L
and He F: PRRSV utilizes MALT1-regulated autophagy flux to switch
virus spread and reserve. Autophagy. 20:2697–2718. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li Y, Huang X, Huang S, He H, Lei T,
Saaoud F, Yu XQ, Melnick A, Kumar A, Papasian CJ, et al: Central
role of myeloid MCPIP1 in protecting against LPS-induced
inflammation and lung injury. Signal Transduct Target Ther.
2:170662017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jiang VC, Liu Y, Lian J, Huang S, Jordan
A, Cai Q, Lin R, Yan F, McIntosh J, Li Y, et al: Cotargeting of BTK
and MALT1 overcomes resistance to BTK inhibitors in mantle cell
lymphoma. J Clin Invest. 133:e1656942023. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Qian R, Niu X, Wang Y, Guo Z, Deng X, Ding
Z, Zhou M and Deng H: Targeting MALT1 suppresses the malignant
progression of colorectal cancer via miR-375/miR-365a-3p/NF-ĸB
axis. Front Cell Dev Biol. 10:8450482022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yao Y, Yuan M, Shi M, Li W, Sha Y, Zhang
Y, Yuan C, Luo J, Li Z, Liao C, et al: Halting multiple myeloma
with MALT1 inhibition: suppressing BCMA-induced NF-ĸB and inducing
immunogenic cell death. Blood Adv. 8:4003–4016. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lee JH and Massague J: TGF-β in
developmental and fibrogenic EMTs. Semin Cancer Biol. 86:136–145.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hu L, Ding M and He W: Emerging
therapeutic strategies for attenuating tubular EMT and kidney
fibrosis by targeting Wnt/β-catenin signaling. Front Pharmacol.
12:8303402022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lu Q, Wang WW, Zhang MZ, Ma ZX, Qiu XR,
Shen M and Yin XX: ROS induces epithelial-mesenchymal transition
via the TGF-β1/PI3K/Akt/mTOR pathway in diabetic nephropathy. Exp
Ther Med. 17:835–846. 2019.PubMed/NCBI
|
|
41
|
Moud BN, Ober F, O'Neill TJ and Krappmann
D: MALT1 substrate cleavage: What is it good for? Front Immunol.
15:14123472024. View Article : Google Scholar : PubMed/NCBI
|



















