Introduction
Endometriosis is a frequent benign gynecological
disease that occurs in 10–15% of women of childbearing age. It is
typically characterized by endometrial cells and stroma implanting
and growing outside the endometrial cavity (1). Endometriosis has been associated with
~50% of women with infertility receiving assisted reproductive
technology (2,3). Moreover, women with endometriosis
treated with in vitro fertilization (IVF) have a lower
pregnancy rate and a higher miscarriage rate relative to those
without the condition (4,5).
The pathogenesis of endometriosis is not well
understood, but several hypotheses have been proposed, including
retrograde menstruation, hematogenous/lymphatic dissemination of
endometrial cells, coelomic metaplasia, migration of endometrial
stem cells, epigenetic modifications promoting inflammation and
endocrine disruption due to environmental toxins (6). A key feature of endometriosis is
elevated reactive oxygen species (ROS) levels in the pelvic cavity,
coupled with reduced antioxidant defenses, suggesting that
oxidative stress serves a critical role in disease progression
(7). ROS, primarily generated as
byproducts of mitochondrial metabolism (8), contributes to cellular senescence,
which is a state of irreversible cell cycle arrest accompanied by
active metabolic activity and the secretion of
senescence-associated secretory phenotypes (SASP). Emerging
evidence, including our unpublished data and other studies
(9,10), has indicated that cellular
senescence is notably associated with endometriotic lesions
(9). Furthermore, previous
research using cellular and murine models reported that excessive
ROS-induced senescence in ovarian granulosa cells contributed to
endometriosis-associated infertility (10). However, the precise molecular
mechanisms by which peritoneal ROS impair folliculogenesis remain
elusive, largely due to ethical constraints limiting functional
ovarian studies in young women with endometriosis.
Given that peritoneal fluid surrounds the ovaries
(11), we hypothesized that
excessive ROS in the pelvic cavity induces ovarian senescence,
disrupting follicular maturation and leading to infertility. A
hallmark of cellular senescence is the activation of the pro-growth
signaling pathway, mammalian target of rapamycin (mTOR), in
non-proliferating cells (12).
Rapamycin, as an mTOR inhibitor, has been theorized to inhibit
cellular senescence, suppress SASP and prolong lifespan (13). Supporting this, a recent clinical
study demonstrated that rapamycin administration prior to IVF in
women with endometriosis reduced senescence markers in follicular
fluid and markedly improved pregnancy and live birth rates
(14).
A growing body of evidence has also reported an
association between proliferator-activated receptor α (PPARα) and
cellular senescence. During aging, PPARα expression or activity was
reported to decrease in tissues such as the kidney, heart and
spleen (15–17). Insulin-like growth factor-binding
protein 2 (IGFBP-2), a target protein of PPARα (18,19),
is dynamically regulated in follicular fluid during follicular
growth and maturation (20).
Notably, IGFBP-2 expression was reported to be reduced in granulosa
cells from polycystic ovaries (21) and was upregulated in response to
gonadotropin stimulation during final oocyte maturation (22).
Therefore, the present study aimed to assess whether
downregulation of the PPARα/IGFBP2 pathway in senescent ovarian
tissue impairs follicular development, and whether rapamycin can
restore fertility in endometriosis by modulating this signaling
axis.
Materials and methods
Animals
A total of 50 8-week-old female 18–22 g BALB/c mice
were purchased from Shanghai JST Laboratory Animal Co., Ltd., of
which 20 were recipient mice, 20 were donor mice and 10 were
control mice. All mice were subjected to controlled conditions
under a 12 h light/dark cycle with a temperature of 22±2°C and
relative humidity of 50±10%, as well as free access to food and
water. All experiments were performed under the guidelines of the
National Research Council Guide for the Care and Use of Laboratory
Animals and were approved by the Institutional Laboratory Animal
Review Board of Shanghai First Maternity and Infant Hospital
(Shanghai, China; approval no. TJBG28625103).
Induction of endometriosis and
rapamycin treatment
A mouse model of endometriosis was established by
intraperitoneal injection of endometrial debris (23). Briefly, after 1 week of adaptation,
20 donor mice were injected intramuscularly with 100 mg/kg
estradiol 2–3 times per week (Hangzhou Animal Pharmaceutical
(Hangzhou) Co., Ltd.). Euthanasia was performed after 1 week by
cervical dislocation. The uteri were removed and collected, and the
uterine tissue was cut up and injected intraperitoneally into the
abdominal cavity of the recipient mice. The uterine horns of one
donor mouse were injected into one recipient mouse. A total of 30
mice (20 recipient mice and 10 control mice) were randomly divided
into three groups: i) Control group (CTL group), in which 10 mice
received an intraperitoneal injection of saline in equal amounts to
the mice in the established mouse endometriosis groups; ii)
endometriosis with rapamycin treatment group (EM-R group), in which
10 mice received an intraperitoneal injection of endometrial debris
and 250 µg rapamycin/mouse once per week at one 1 after
endometriosis induction; and iii) endometriosis group (EM group),
in which 10 mice received an intraperitoneal injection of
endometrial debris only. Rapamycin was purchased from Sigma-Aldrich
(Merck KGaA; cat. no. 553210). The present study referred to the
study by Ren et al (24)
for the dose of rapamycin used in mice. Body weight was measured
and the hotplate test was performed in all mice at 1, 2, 3 and 4
weeks after endometriosis induction, and mice were executed 4 weeks
after endometriosis induction by cervical dislocation.
Hotplate test
The hotplate test was performed using a commercial
hotplate analgesia meter (BME-480; Institute of Biomedical
Engineering, Chinese Academy of Medical Sciences) to assess the
degree of discomfort caused by endometriosis, as previously
described (25). In summary, mice
were given 10 min to acclimate before the test. The mouse had to
hop on the hotplate or shake or lick its rear paws during 1 min of
being placed within the cylinder to assess the withdrawal latencies
to thermal stimulation. Over the course of 1 h, the latency was
measured twice and then averaged by the two test results.
Peritoneal fluid collection and ROS
detection in mice
After the mice were executed, they were placed in a
supine position with their limbs unfolded and fixed on a dissecting
board. The abdomen was disinfected with 75% alcohol, the lower
abdominal skin was lifted with ophthalmic forceps and a 2-cm
incision was made along the abdominal midline with scissors. A
total of 3–5 ml of sterile saline was injected into the abdominal
cavity of the mice and the abdominal cavity of the mice was opened
under aseptic conditions. The abdominal fluid was rinsed three
times and the rinsed fluid was collected into centrifuge tubes for
the detection of oxidative stress-related substances. Superoxide
dismutase (SOD), malondialdehyde (MDA), glutathione peroxidase
(GSH-PX) and 8-hydroxydeoxyguanosine (8-OHdG) were detected as the
oxidative and antioxidant markers in the peritoneal fluid of mice.
The SOD assay kit (cat. no. A001-3-2; WST-1 method), MDA assay kit
(cat. no. A003-1-2; TBA method), GSH-PX assay kit (cat. no.
A005-1-2; colorimetric method) and mouse 8-OHdG ELISA kit (cat. no.
H165-1-2) were purchased from Nanjing Jiancheng Bioengineering
Institute. The peritoneal fluid of PPARα and IGFBP2 in mice were
detected using an ELISA kit (cat. nos. MM-0249M1 and MM-0029H2,
respectively; Meimian Industry; Jiangsu ELISA Industrial Co.,
Ltd.). All operations are strictly in accordance with the kit
instructions.
Immunohistochemistry of mouse ovarian
tissue
Ovarian tissue samples were fixed in 4%
paraformaldehyde for 24 h at 4°C and embedded in paraffin. Sections
(4 µm) were used for subsequent staining. The first section was
stained with hematoxylin and eosin to confirm the pathological
diagnosis, and subsequent sections were stained with
senescence-related markers such as p16, p21 and Lamin B1. Tissue
sections were deparaffinized in xylene and rehydrated through a
graded ethanol series. Antigen retrieval was performed in EDTA
buffer (pH 9.0; Shanghai Sun Biotechnology Co., Ltd.) or citric
acid solution (pH 6.0; Shanghai Sun Biotechnology Co., Ltd.) using
a microwave heating method, then allowed to cool to room
temperature. Following antigen retrieval and cooling, sections were
washed three times with PBS. To block non-specific binding,
sections were incubated in a solution of PBS containing 5% normal
goat serum (cat. no. G1208; Wuhan Servicebio Technology Co., Ltd.)
for 1 h at room temperature. The following antibodies were used in
this experiment: p16 (1:50; cat. no. ab51243), p21 (1:1,000; cat.
no. ab188224) and Lamin B1 (1:1,000; cat. no. ab16048). All
antibodies were purchased from Abcam. All sections were incubated
with the aforementioned primary antibodies overnight at 4°C. The
sections were then incubated with horseradish peroxidase-labeled
secondary antibody detection reagent (1:300; cat. no. A0303;
Beyotime Biotechnology) for 1 h at room temperature. The bound
antibody complexes were stained with diaminobenzidine for 3–5 min
at room temperature, followed by hematoxylin for nuclear staining
at room temperature. The development time was monitored under a
microscope (typically between 3 to 5 min) and stopped immediately
when optimal staining intensity was achieved with minimal
background. Images were captured under a microscope (BX51; Olympus
Corporation) equipped with a digital camera (DP70; Olympus
Corporation). According to the manufacturer's instructions,
distinct tissue slides were utilized for several antibodies as
positive control. For negative controls, tissue samples were
treated with mouse or rabbit serum (Wuhan Servicebio Technology
Co., Ltd.) in lieu of primary antibodies.
Mouse ovarian tissue PCR assay
Mouse ovarian tissues were cut into small pieces
(soybean size), placed in a pre-cooled homogenizer, 1 ml RNA
extract (Wuhan Servicebio Technology Co., Ltd.) was added per 100
mg of tissue, and the tissues were homogenized until no visible
pieces were present. A total of 200 µl chloroform was added per 1
ml RNA extract, and the mixture was vortexed and mixed or shaken
upside down vigorously for 15 sed, let stand at room temperature
for 3–5 min, then centrifuged at 12,000 × g for 15 min at 4°C. The
upper colorless aqueous phase was carefully transferred to a new
centrifuge tube, aspirating 500–550 µl per ml of extract. A total
of 500 µl isopropanol was added to the collected aqueous extract
tube, which was centrifuged at 4°C for 10 min at 12,000 × g. The
white precipitate visible at the bottom of the tube was the total
RNA, and so supernatant was discarded. A total of 1 ml of 75%
ethanol was then added, which was then mixed upside down (until the
white precipitate floats) and centrifuged at 12,000 × g for 5 min
at 4°C. The supernatant was discarded, then centrifugation was
performed briefly at high speed (3–5 sec at 5,000 × g at 4°C) and
the liquid was carefully aspirated with a micropipette. The
centrifuge tube with RNA precipitate was left open for 3–5 min to
allow the RNA to dry slightly, and then 20–50 µl DEPC-treated water
(Wuhan Servicebio Technology Co., Ltd.) was added to fully dissolve
the RNA. The NanoDrop™ Microvolume UV–Vis
spectrophotometer (Thermo Fisher Scientific, Inc.) was used to
assess the quality control and quantification of total RNA. RNA
with good integrity and purity (OD260/OD280 values of 1.8–2.0) was
used for further analysis. Mouse tissue RNA was reverse transcribed
and quantitative PCR (qPCR) was performed using forward and reverse
primers. The cDNA synthesis was performed using a reverse
transcription kit (Tiangen Biotech Co., Ltd.) at 42°C for 30 min.
To evaluate the abundance of mRNA, real-time PCR was conducted
using SYBR Premix Ex Taq (Takara Bio, Inc.). The thermocycling
protocol consisted of an initial denaturation step at 95°C for 30
sec, followed by 40 cycles of 95°C for 5 sec and 60°C for 30 sec.
GAPDH was used as a housekeeping gene. The sequences of
senescence-related markers (p16, p21 and γH2AX), gonadotropin
receptor [follicle-stimulating hormone receptor (FSHR) and
luteinizing hormone receptor (LHR)] and PPARα and IGFBP2 primers
are listed in Table I. All primers
were designed by Sangon Biotech (Shanghai) Co., Ltd. The relative
gene expression levels were calculated as ΔCq values (Cq ‘target
gene’ minus Cq ‘housekeeping gene’) and 2−ΔΔCq ratios
indicated fold changes (26).
 | Table I.Names and sequences of all primers
used in the present study. |
Table I.
Names and sequences of all primers
used in the present study.
| Gene name | Direction | Sequence
(5′-3′) |
|---|
| p16 (human) | Forward |
AGGCCGATCCAGGTCATGATGA |
|
| Reverse |
ACCACCAGCGTGTCCAGGAA |
| p21 (human) | Forward |
GACCTGTCACTGTCTTGTACCCTTG |
|
| Reverse |
TTGGAGTGGTAGAAATCTGTCATG |
|
|
| CTG |
| FSHR (human) | Forward |
TCCCTCCTTGTGCTCAATGTC |
|
| Reverse |
GATGTTGGGGTTCCGCACT |
| LHR (human) | Forward |
TTCTGTCTACACCCTCACCG |
|
| Reverse |
AAAAGAGCCATCCTCCAAGC |
| γH2AX (human) | Forward |
CAGTGCTGGAGTACCTCAC |
|
| Reverse |
GATGATTCGCGTCTTCTTGTTG |
| PPARα (human) | Forward |
TCGGCGAGGATAGTTCTGGAAGC |
|
| Reverse |
ACCACAGGATAAGTCACCGAGGAG |
| IGFBP2 (human) | Forward |
ACAGTGCAAGATGTCTCTGAACGG |
|
| Reverse |
GCCTCCTGCTGCTCATTGTAGAAG |
| GAPDH (human) | Forward |
GCACCGTCAAGGCTGAGAAC |
|
| Reverse |
TGGTGAAGACGCCAGTGGA |
| p16 (mouse) | Forward |
CGCAGGTTCTTGGTCACTGT |
|
| Reverse |
TGTTCACGAAAGCCAGAGCG |
| p21(mouse) | Forward |
CCTGGTGATGTCCGACCTG |
|
| Reverse |
CCATGAGCGCATCGCAATC |
| FSHR (mouse) | Forward |
CCTTGCTCCTGGTCTCCTTG |
|
| Reverse |
CTCGGTCACCTTGCTATCTTG |
| LHR (mouse) | Forward |
CAGCTGCCTTCAAAGTACCC |
|
| Reverse |
TTGGCACAAGAATTGACAGG |
| γH2AX (mouse) | Forward |
GGTGCTCGAGTACCTCACTG |
|
| Reverse |
CTTGTTGAGCTCCTCGTCGT |
| PPARα (mouse) | Forward |
CAAGGCCTCAGGGTACCACT |
|
| Reverse |
TTGCAGCTCCGATCACACTT |
| IGFBP2 (mouse) | Forward |
CCTTGCCAGCAGGAGTTG |
|
| Reverse |
TCCGTTCAGAGACATCTTGC |
| GAPDH (mouse) | Forward |
GGTTGTCTCCTGCGACTTCA |
|
| Reverse |
TGGTCCAGGGTTTCTTACTCC |
Ovarian tissue follicle counting in
mice
A total of 30 mice were sacrificed 4 weeks after
endometriosis induction. After fixation with 4% paraformaldehyde in
phosphate buffer at 4°C for 24 h, the tissues were embedded in
paraffin and sectioned at 4 µm thickness. Ovarian tissue sections
were stained with HE at room temperature and the number of
follicles at different stages was counted for each mouse. The
method of follicle counting involved 5-µm continuous sections, with
1/5 sections stained and counted (27). A total of five types of follicles
were counted: i) Primordial follicles; ii) primary follicles; iii)
secondary follicles; iv) antral follicles; and v) corpus luteum.
Primordial follicles were defined as oocytes surrounded by a layer
of squamous (flattened) granulosa cells; primary follicles were
defined as one follicle containing several or all oocytes
surrounded by a single layer of cuboidal granulosa cells; secondary
follicles were defined as a follicle surrounded by >1 layer of
cuboidal granulosa cells with no visible lumen; a follicle was
identified as a antral follicle if it had a well-defined lumen and
a layer of cumulus granulosa cells; and the corpus luteum was a
post-ovulatory structure, which was filled with lutein cells that
form only after ovulation (28).
Images were captured using a microscope (BX51; Olympus Corporation)
fitted with a digital camera (DP70; Olympus Corporation).
Granulosa cell culture
Mouse primary granulosa cells were extracted and
cultured with reference to the study by Tian et al (29). Mouse ovaries were washed with
sterile PBS and transferred to DMEM/F12 (cat. no. 11330-057;
Invitrogen™; Thermo Fisher Scientific, Inc.). Follicles
were punctured with a 31-gauge needle under a dissecting
microscope, and granulosa cells were released from the follicles,
centrifuged at 1,000 × g for 5 min at room temperature, resuspended
in fresh DMEM/F12 with 10% FBS, and incubated at 37°C and 5%
CO2. Cells were passaged every other day.
The granulosa KGN cell line was purchased from
Shanghai Kanglang Biotechnology Co., Ltd. and cultured in DMEM/F12
supplemented with 10% FBS at 37°C and 5% CO2. Cells were
passaged every 5–6 days, and the number of passages was <15 for
the experiments.
Transient transfection for knockdown
and overexpression of PPARα
Small interfering (si)RNA of the human PPARα gene
(siRNA-PPARα) and non-specific control siRNA (siRNAc) were
purchased from Shanghai GenePharma Co., Ltd. and were transfected
into cells at room temperature using Lipofectamine™ 3000
(Invitrogen; Thermo Fisher Scientific, Inc.) for 48 h at a final
concentration of 30 nM, according to the manufacturer's
instructions. Subsequent experiments were conducted 48 h after cell
transfection. The following siRNA sequences were used: siRNA-PPARα,
5′-GCUUUACGGAAUACCAGUAUU-3′; and siRNAc, (forward)
5′-UUCUCCGAACGUGUCACGUdTdT-3′ and (reverse)
5′-ACGUGACACGUUCGGAGAAdTdT-3′.
The PPARα overexpression plasmid [pCDNA3.1(+)
plasmid vector Asian Vector Biotechnology Co., Ltd.] was
transfected into granulocyte cells for 48 h using Lipofectamine
3000 and an empty plasmid (Asian Vector Biotechnology Co., Ltd.)
was used as a negative control. Cells were transfected with 1.0 µg
of either the PPARα overexpression plasmid or the empty control
plasmid using 2.0 µl of Lipofectamine 3000 reagent per well in a
12-well plate, according to the manufacturer's instructions.
Subsequent experiments were conducted 48 h after cell
transfection.
To demonstrated the effects of PPARα knockdown or
overexpression on cellular senescence, cells were treated with
H2O2 following knockdown or overexpression.
To demonstrated the anti-senescence effects of rapamycin, cells
were treated with (50 nM) rapamycin (Sangon Biotech Co., Ltd.).
Statistical analysis
All statistical analyses were performed using
GraphPad Prism 8 (Dotmatics). For comparisons among ≥3 groups,
one-way analysis of variance (ANOVA) was employed, followed by
Tukey's post hoc test for multiple comparisons when ANOVA indicated
statistical significance (P<0.05). Unless otherwise stated,
continuous variables are presented as mean ± standard deviation for
normally distributed data. All tests were two-tailed, and P<0.05
was considered to indicate a statistically significant
difference.
Results
Rapamycin reduces ectopic lesions
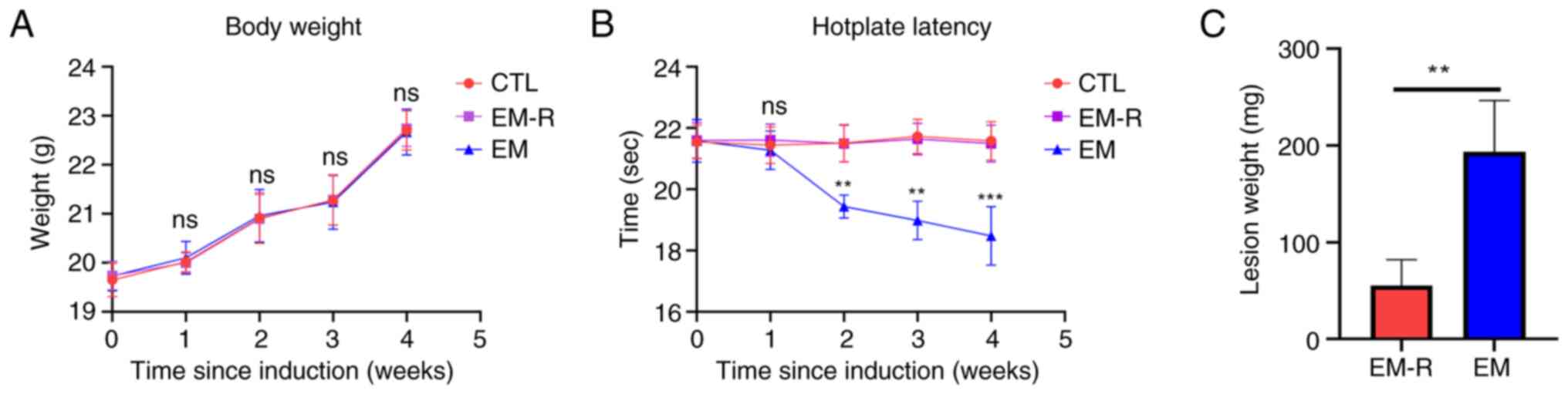
The findings demonstrated that there was no
significant difference in body weight among the 3 groups of mice
before the induction of endometriosis and their death (Fig. 1A). However, the results of the
hotplate test were significantly different between the EM group and
EM-R group, and the hotplate time from week 2 post-endometriosis
induction was significantly lower in the mice of the EM group than
in the EM-R group. There is no difference in hotplate test between
CTL group and EM-R group (Fig.
1B). No lesions of endometriosis were found in the mice of the
CTL group; however, obvious ectopic lesions were observed in the
peritoneal cavity of mice in the EM-R and EM groups. Moreover, the
weight of endometriotic lesions in mice in the EM group were
significantly higher than that in the EM-R group (193.4±53.22 and
55.4±26.69 mg, respectively; P<0.01; Fig. 1C). This indicates that rapamycin
may inhibit endometriosis.
Rapamycin decreases expression of
markers associated with oxidative stress in mouse peritoneal
fluid
There was no significant difference between the CTL
and EM-R groups for the levels of oxidative stress-related
molecules, SOD (8.60±1.71 and 7.93±1.32 U/ml, respectively;
P=0.49), GSH-PX (18.99±2.32 and 17.30±2.25 µmol/l, respectively;
P=0.17), MDA (1.11±0.13 and 1.39±0.32 nmol/ml, respectively;
P=0.06) and 8-OHdG (4.34±2.31 and 5.91±2.68 ng/l, respectively;
P=0.45). The antioxidant molecules, SOD and GSH-PX, in mouse
peritoneal fluid were significantly lower in the EM group compared
with in the EM-R group (SOD, 1.65±0.69 vs. 7.93±1.32 U/ml; GSH-PX,
4.62±1.48 vs. 17.30±2.25 µmol/l; both P<0.01). By contrast, the
markers of increased oxidative capacity, MDA and 8-OHdG, were
significantly higher in the EM group than in EM-R group (MDA,
4.79±0.32 vs. 1.39±0.32 nmol/ml; 8-OHdG, 24.31±3.48 vs. 5.91±2.68
ng/l; both P<0.001) (Fig. 2).
This suggests the presence of oxidative stress, decreased
antioxidant capacity and increased oxidative function in the
peritoneal cavity of endometriosis mice without rapamycin
treatment. Moreover, levels of activation of intraperitoneal
oxidative stress were reduced in rapamycin-treated endometriosis
mice.
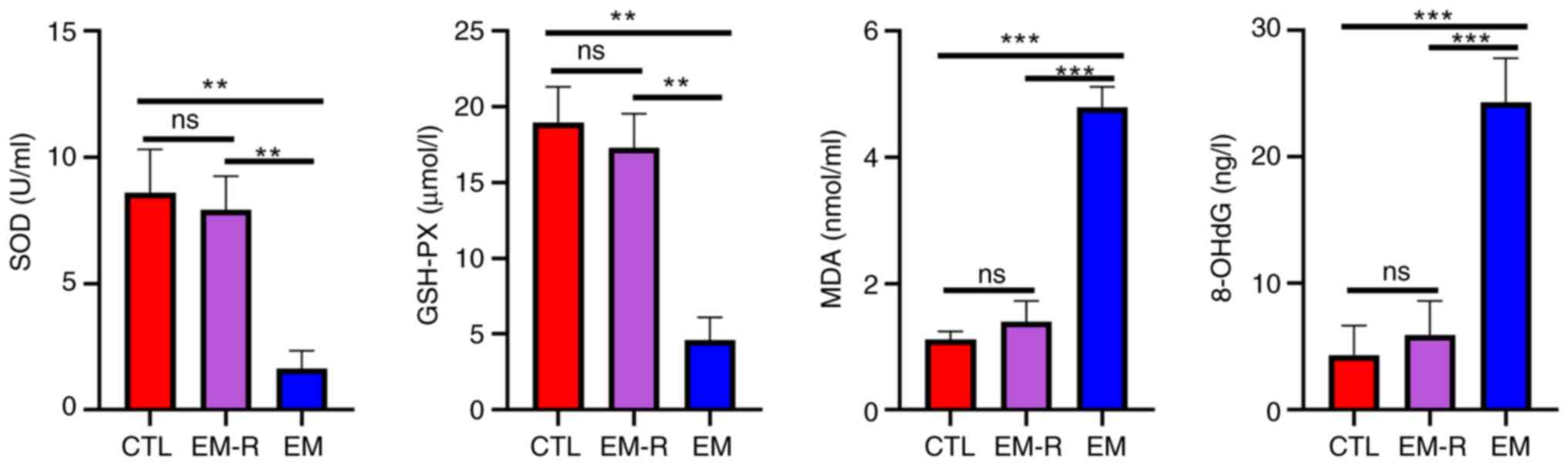 | Figure 2.Detection of oxidative stress-related
markers in peritoneal fluid of mice. Comparison of
antioxidant-related molecules, SOD, GSH-PX and oxidative
capacity-related molecules MDA and 8-OHdG in peritoneal fluid of
three groups. **P<0.01; ***P<0.001. SOD, superoxide
dismutase; GSH-PX, glutathione peroxidase; MDA, malondialdehyde;
8-OHdG, 8-hydroxydeoxyguanosine; CTL, control group; EM-R,
endometriosis + rapamycin group; EM, endometriosis group; ns, not
statistically significant. |
Rapamycin reduces senescence markers
and increases gonadotropin receptors in mouse ovaries
Increased expression of senescence-associated
markers, p16, p21 and γH2AX, and decreased expression of Lamin B1
suggest that tissue or cellular senescence is occurring (30). The immunohistochemical experiments
in the present study demonstrated that senescence-related markers
expression in ovarian granulosa cells and oocyte did not differ
significantly between the CTL and EM-R group. However, the
expression of senescence-related markers, p16 and p21, in ovarian
granulosa cells and oocytes was significantly higher in the EM
group than in the EM-R group (p16, 0.42±0.14 vs. 0.12±0.03; p21,
0.37±0.04 vs. 0.11±0.02; both P<0.01). Another
senescence-related marker, Lamin B1, was significantly lower in
ovarian granulosa cells and oocytes of the EM group compared with
the EM-R group (0.13±0.05 vs. 0.37±0.07; P<0.01) (Fig. 3A). This suggests that ovarian
tissues in endometriosis mice without rapamycin treatment undergo
senescence, and rapamycin could decrease expression of senescence
markers in ovaries. Similarly, ovarian tissue PCR experiments
revealed significantly higher gene expression of p16, p21 and γH2AX
in mice in the EM group than in mice in the EM-R group (p16,
3.57±0.36 vs. 0.94±0.04; p21, 3.80±0.39 vs. 0.93±0.06; γH2AX,
5.63±1.37 vs. 1.61±0.61; all P<0.01). Moreover, gonadotropin
receptor (FSHR and LHR) gene expression was significantly higher in
the EM-R group than the EM group (FSHR, 0.95±0.04 vs. 0.23±0.11;
LHR, 0.98±0.04 vs. 0.25±0.07; both P<0.01) (Fig. 3B).
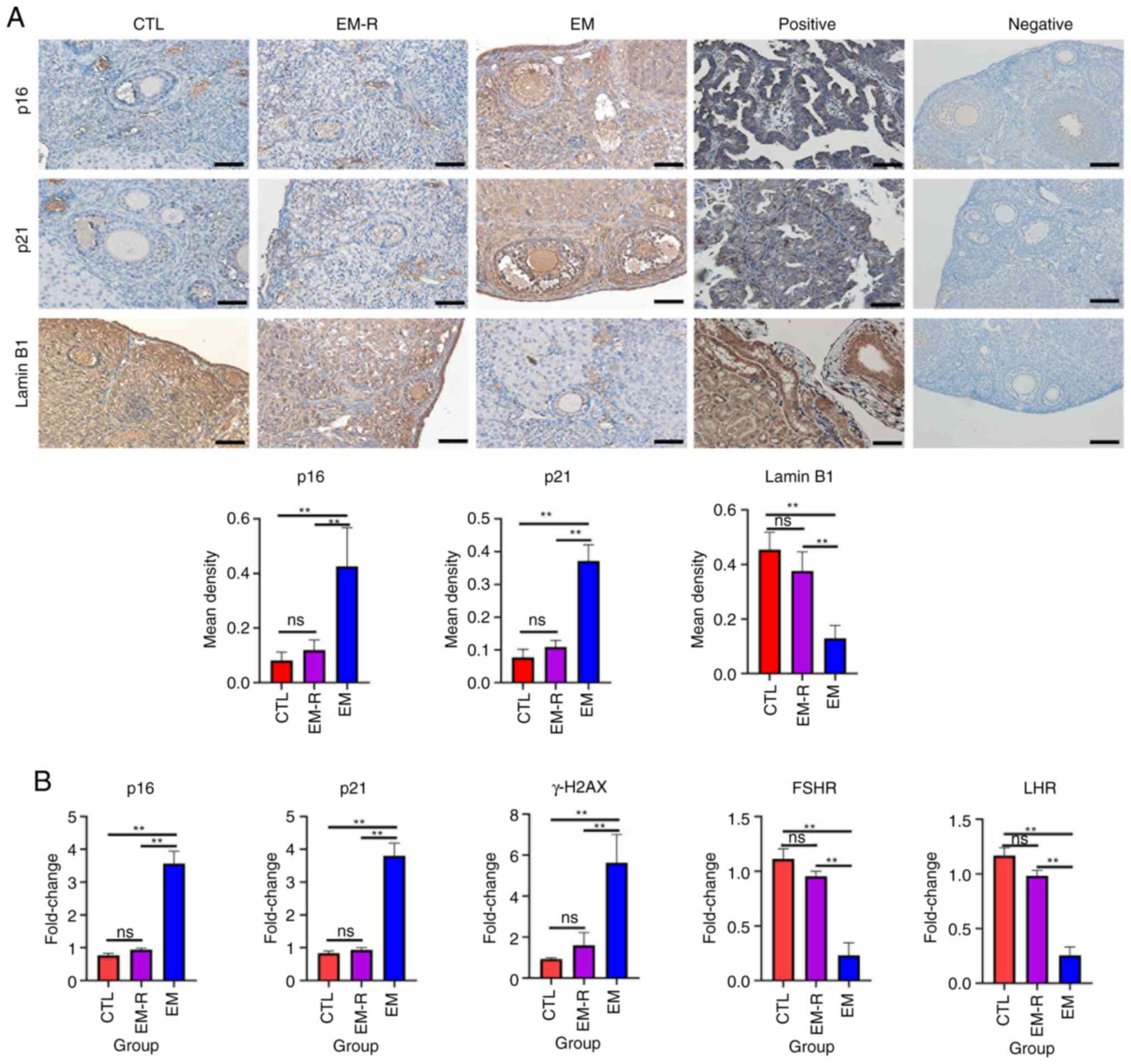 | Figure 3.Detection of senescence-related
markers and gonadotropin receptor in mice ovarian tissues. (A)
Representative immunohistochemical images and the corresponding
statistical results of senescence-related markers, p16, p21 and
Lamin B1 in the ovarian tissues of three groups, as well as
positive and negative controls for immunostaining of p16, p21 and
Lamin B1. For positive controls, human endometrial adenocarcinoma
tissues were used for p16, human lung tissues were used for p21 and
human kidney tissues were used for Lamin B1. p16, p21, Lamin B1
showed positive staining in the nuclei. For negative controls,
mouse ovarian tissues were used. The controls all showed negative
staining. Scale bar, 50 µm. (B) Gene expression of p16, p21, γH2AX,
FSHR and LHR in mice ovarian tissues among the three groups.
**P<0.01. FSHR, follicle-stimulating hormone receptor; LHR,
luteinizing hormone receptor; CTL, control group; EM-R,
endometriosis + rapamycin group; EM, endometriosis group; ns, not
statistically significant. |
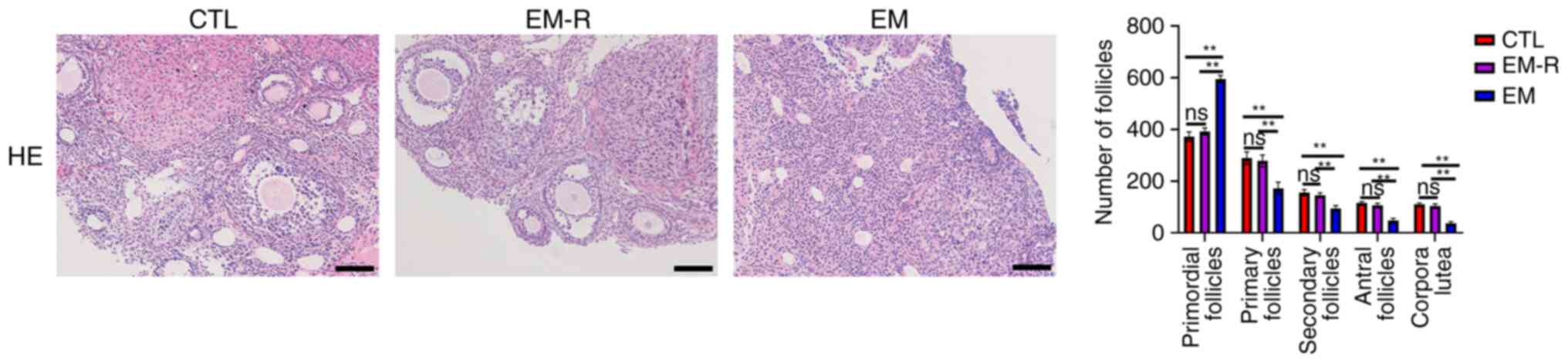
Rapamycin improves follicular
development
The number of follicles at different stages was not
significantly different between the CTL and EM-R groups. However,
primordial follicles of the EM group were significantly higher than
in the EM-R group (595.6±13.66 vs. 392.1±13.89; P<0.01), whilst
primary follicles, secondary follicles, antral follicles and corpus
luteum were significantly lower in the EM group than in the EM-R
group (primary follicles, 172.2±24.15 vs. 278.6±23.08; secondary
follicles, 94.9±10.62 vs. 145.3±9.81; antral follicles, 48±8.56 vs.
106.2±7.88; corpus luteum, 37.8±5.73 vs. 103.6±8.5; All P<0.01).
This suggests that endometriosis mice follicles undergo senescence
in response to oxidative stress, which leads to impaired
development and significant increase in the number of mature
follicles in endometriosis mice treated with rapamycin (Fig. 4).
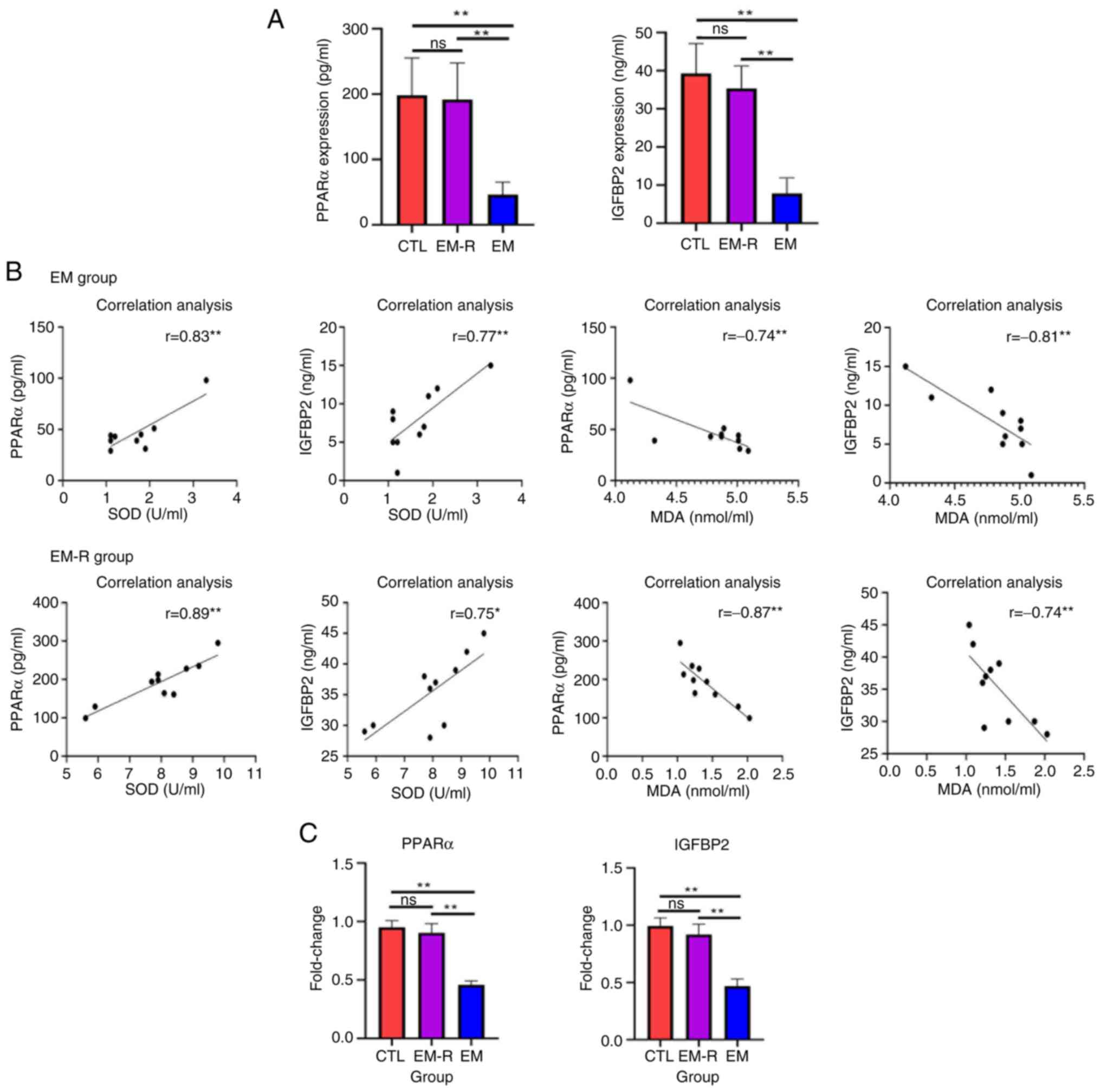
Activation of PPARα and IGFBP2 in the
ovaries of endometriosis mice treated with rapamycin
The concentration of PPARα and IGFBP2 in the
peritoneal fluid of mice in the CTL group were not significantly
different from those of the EM-R group; however, the concentration
of PPARα and IGFBP2 in the EM group were significantly lower than
those in the EM-R group (PPARα, 46.2±19.33 vs. 191.6±56.37 pg/ml;
IGFBP2, 7.90±4.04 vs. 35.4±5.89 ng/ml; both P<0.01) (Fig. 5A). Furthermore, the results
revealed a positive correlation between SOD and PPARα, and between
SOD and IGFBP2, compared with a negative correlation between MDA
and PPARα, and between MDA and IGFBP2 in both the EM-R and EM
groups (Fig. 5B). In addition, the
gene expression of PPARα and IGFBP2 in the ovaries of mice in the
EM-R group was not significantly different from that of mice in the
CTL group; however, the expression of PPARα and IGFBP2 in the
ovaries of mice in the EM group was significantly lower than that
of mice in the EM-R group (PPARα, 0.90±0.07 vs. 0.45±0.03; IGFBP2,
0.91±0.09 vs. 0.46±0.06; both P<0.01). This indicated that the
expression of PPARα and IGFBP2 in the ovaries of endometriosis mice
was significantly decreased, and treatment with rapamycin activated
PPARα and IGFBP2 expression (Fig.
5C).
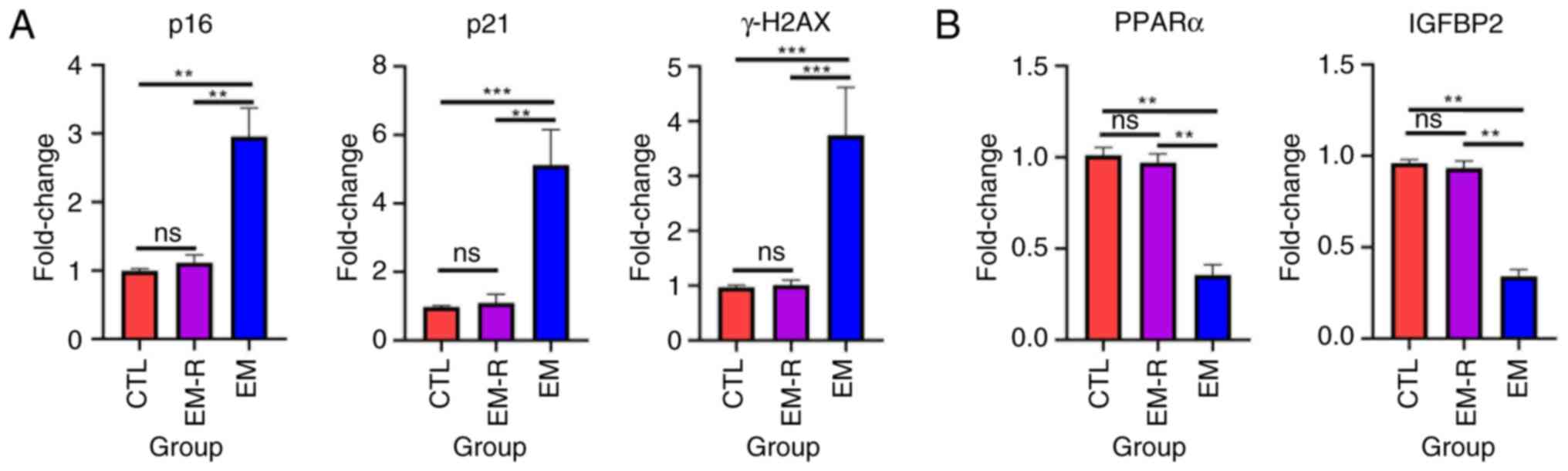
Rapamycin decreases the expression of
senescence markers and increases PPARα and IGFBP2 expression in
primary mouse granulosa cells
Granulosa cells, which closely surround the oocyte,
are essential for supporting oocyte development (31). To assess cellular senescence in
these cells, primary granulosa cells were isolated from three
experimental groups of mice. PCR analysis revealed that granulosa
cells from the EM group exhibited significantly elevated levels of
senescence-associated markers, including p16, p21 and γH2AX,
compared with in the EM-R group (p16, 2.95±0.41 vs. 1.11±0.11; p21,
5.13±1.02 vs. 1.09±0.25; γH2AX, 3.74±0.87 vs. 1.01±0.09; all
P<0.01; Fig. 6A). Notably, the
expression of these markers in the EM-R group was comparable with
that of the CTL group, with no statistically significant
differences observed between them (Fig. 6A). By contrast, the expression
levels of PPARα and IGFBP2 were significantly reduced in the EM
group compared with the EM-R group (PPARα, 0.35±0.55 vs. 0.97±0.04;
IGFBP2, 0.34±0.03 vs. 0.93±0.04; both P<0.01; Fig. 6B).
Rapamycin increases PPARα and IGFBP2
expression and inhibits granulosa cell senescence in vitro
To evaluate the functional relationship between
PPARα and IGFBP2, knockdown and overexpression of PPARα experiments
were performed using KGN cells. First, PCR experiments were
performed to assess the expression of PPARα after transfecting
cells with siRNA and overexpression plasmids. The results
demonstrated that PPARα expression was significantly lower after
siRNA transfection (0.37±0.05 vs. 1.04±0.06; P=0.0002) and
significantly higher after overexpression plasmid transfection
(4.50±0.73 vs. 1.06±0.11; P=0.0013) than in the respective control
groups (Fig. 7A). Notably,
silencing PPARα significantly reduced IGFBP2 expression, whereas
PPARα overexpression significantly increased IGFBP2 levels
(Fig. 7B and C). This indicated
that IGFBP2 is a downstream target of PPARα.
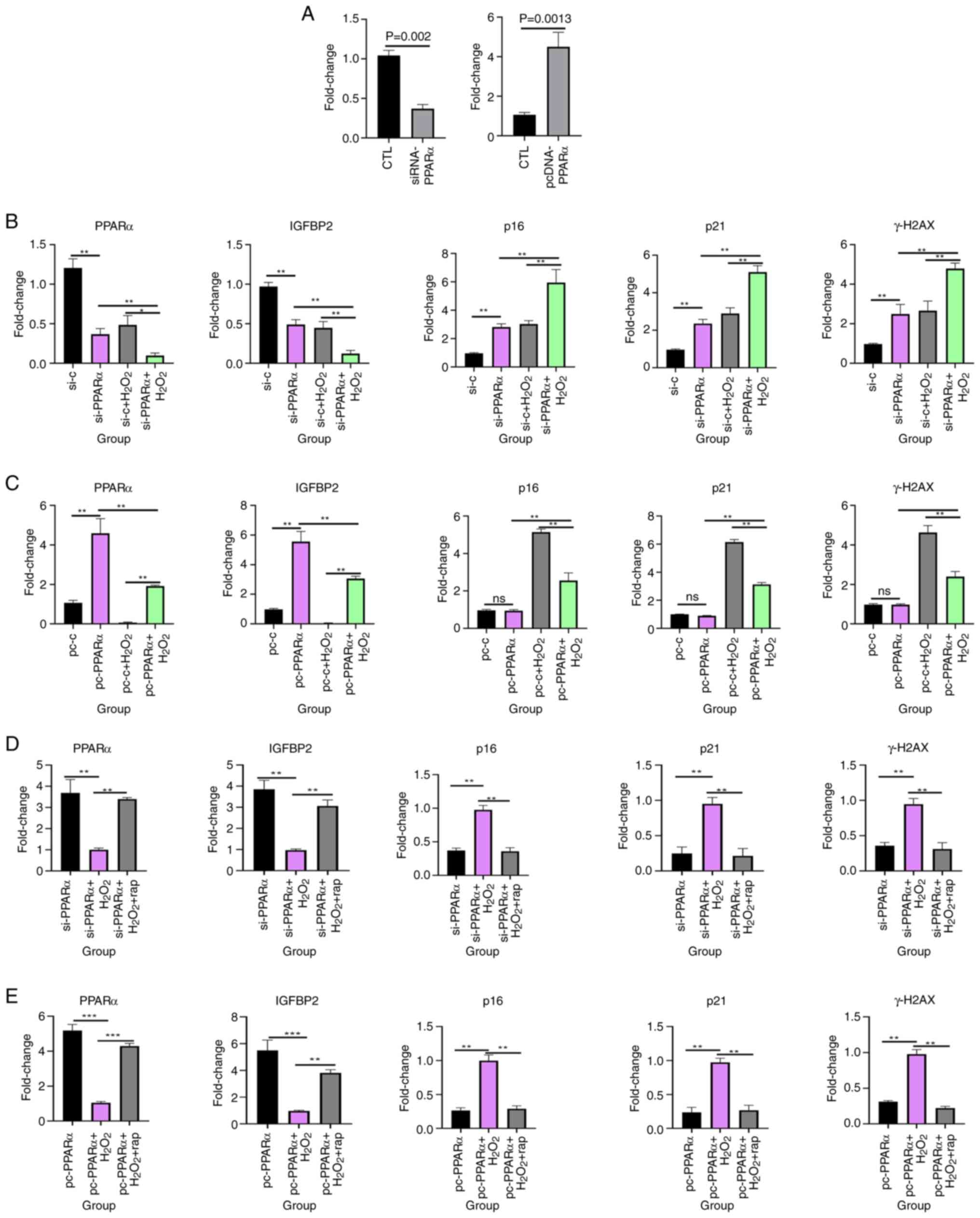 | Figure 7.PPARα modulates oxidative
stress-induced senescence and IGFBP2 expression in KGN cells. (A)
Compared with the blank control group, the PCR results of PPARα
after cell transfection with siRNA and overexpression plasmids.
Gene expression of PPARα, IGFBP2, p16, p21 and γH2AX in KGN cells
treated with H2O2 after (B) knockdown and (C)
overexpression of PPARα. Gene expression of PPARα, IGFBP2, p16, p21
and γH2AX in KGN cells treated with H2O2 and
rapamycin after (D) knockdown and (E) overexpression of PPARα.
**P<0.01; ***P<0.001. PPARα, proliferator-activated receptor
α; IGFBP2, insulin-like growth factor-binding protein 2; si, small
interfering; si-c, non-specific control siRNA; pc, pcDNA; rap,
rapamycin; CTL, control; ns, not statistically significant. |
To assess the role of PPARα in cellular senescence,
granulosa cells were treated with H2O2
following PPARα knockdown or overexpression. The siRNA-PPARα +
H2O2 group exhibited the highest expression
of senescence markers (p16, p21 and γH2AX), significantly exceeding
levels in the siRNA-control + H2O2 group (p16, 5.96±0.90
vs. 3.03±0.23; p21, 5.10±0.34 vs. 2.89±0.30; γH2AX, 4.79±0.27 vs.
2.66±0.48; all P<0.01; Fig.
7B). Conversely, the pcDNA-PPARα + H2O2
group exhibited lower expression of the senescence markers (p16,
p21 and γH2AX) than the pcDNA + H2O2 group,
(p16, 2.56±0.41 vs. 5.13±0.17; p21, 3.13±0.13 vs. 6.15±0.17; γH2AX,
2.40±0.25 vs. 4.63±0.35; all P<0.01; Fig. 7C). PPARα overexpression attenuated
H2O2-induced senescence, further supporting the protective role of
PPARα against cellular aging. Moreover, cells with knockdown or
overexpression of PPARα were treated with
H2O2 for 24 h followed by the addition of 30
µM rapamycin (32) for 48 h,
revealed that rapamycin significantly increased PPARα and IGFBP2
expression and significantly decreased the expression of
senescence-related markers, p16, p21 and γH2AX. The siRNA-PPARα +
H2O2 group exhibited higher expression of the
senescence markers (p16, p21 and γH2AX) than the siRNA-PPARα +
H2O2 + rapamycin group (p16, 0.97±0.06 vs.
0.35±0.05; p21, 0.95±0.09 vs. 0.21±0.10; γH2AX, 0.94±0.08 vs.
0.31±0.09; all P<0.01; Fig.
7D). The pcDNA-PPARα + H2O2 group
exhibited higher expression of the senescence markers (p16, p21 and
γH2AX) than the pcDNA-PPARα + H2O2 +
rapamycin group (p16, 0.99±0.08 vs. 0.29±0.04; p21, 0.97±0.06 vs.
0.26±0.07; γH2AX, 0.98±0.06 vs. 0.22±0.02; all P<0.01; Fig. 7E) These findings suggest that
rapamycin counteracted senescence by upregulating the PPARα-IGFBP2
axis.
Discussion
The present study demonstrated that elevated ROS in
the peritoneal fluid of endometriosis mice contributed to ovarian
senescence and impaired follicular development, whilst rapamycin
treatment mitigated these effects by reducing oxidative stress and
activating the PPARα/IGFBP2 pathway.
Endometriosis is characterized by a pro-oxidative
peritoneal environment (33), with
increased ROS levels adversely affecting ovarian function (34,35).
It is well documented that oxidative stress is a major source of
endogenous and exogenous challenges that promotes senescence and
the senescence phenotype (36). A
previous study reported that excessive oxidative stress in cumulus
granulosa cells triggered cellular senescence which contributed to
endometriosis-related infertility (10). Consistent with previous studies
(9,10), the present study demonstrated that
ROS in peritoneal fluid promoted cellular senescence in ovarian
tissues, as shown by elevated senescence-related markers and
reduced expression of gonadotropin receptors (FSHR and LHR),
critical for follicular maturation (37). This senescence phenotype was
associated with a decline in mature follicles and an accumulation
of primordial follicles, suggesting impaired folliculogenesis.
Rapamycin, an established anti-senescence agent (38), reversed these effects, restoring
follicular development and ovarian function.
The mTOR network is an evolutionarily conserved
signaling hub that detects and integrates environmental and
intracellular nutrients, as well as growth factor signals, thereby
orchestrating fundamental cellular and organismal responses such as
cell growth, proliferation, apoptosis and inflammation. Previous
research supports the notion that mTOR signaling influences
lifespan and senescence (39). At
present, the only known pharmacological approach to extend lifespan
in all studied model organisms, to the best of our knowledge, is
the inhibition of the mTOR complex 1 using rapamycin (39). Treatment of hepatocytes with
pterostilbene inhibits mTOR and promotes the expression of its
downstream molecule, PPARα (40).
PPARα serves a crucial role in metabolic homeostasis and aging,
with its deficiency associated with fibrotic and senescent
phenotypes in several tissues (41). Similarly, IGFBP2 has been
implicated in mitigating senescence in pulmonary fibrosis models
(42). The findings of the present
study revealed for the first time, to the best of our knowledge,
that PPARα and IGFBP2 are downregulated in endometriosis-affected
ovaries and are negatively associated with senescence. In
endometriosis, reduced PPARα/IGFBP2 signaling may exacerbate
oxidative stress-induced ovarian damage. Rapamycin restored their
expression, suggesting that this pathway is a key mediator in
counteracting endometriosis-related ovarian dysfunction.
Whilst the present study characterized
folliculogenesis dysfunction in endometriosis-related infertility,
it is acknowledged that direct fertility assessments (such as
ovulation rates, pregnancy outcomes or litter sizes) were not
assessed. These endpoints would provide critical translational
insights into how observed follicular abnormalities impact
reproductive success. Future studies should integrate longitudinal
fertility metrics in murine models, ideally combining hormonal
profiling with timed mating trials, to establish functional
associations between folliculogenesis defects and infertility
phenotypes. Such data would strengthen the clinical relevance of
the findings of the present study for patients with
endometriosis.
Additionally, although the in vitro
experiments in the present study demonstrated the regulatory role
of the PPARα/IGFBP2 axis in granulosa cells, further in vivo
validation is warranted to confirm its causal involvement in
rapamycin-mediated effects. Pharmacological modulation (such as
PPARα agonists/antagonists) or conditional knockout models could
elucidate whether targeting this pathway rescues ovarian function
in endometriosis. These experiments would not only solidify the
mechanistic link, but also explore therapeutic potential, aligning
with the broader goal of developing targeted interventions for
endometriosis-associated infertility.
In summary, the results of the present study
highlight that ROS-induced ovarian senescence contributed to
endometriosis-related infertility, and rapamycin counteracted this
process by activating the PPARα/IGFBP2 pathway. These findings not
only deepen the understanding of endometriosis pathogenesis but
also suggest that targeting senescence pathways may offer a
promising strategy to improve fertility outcomes in affected women.
Future studies should focus on translating these findings into
clinical applications.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JF and QW were responsible for writing the article,
data statistics and data collection. QS was responsible for data
collection. XH conributed to conceptualization, methodology,
writing the original draft and also reviewing and editing,
supervisionand project administration. XH and JF confirm the
authenticity of all the raw data. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
The present research was approved by the
Institutional Laboratory Animal Review Board of Shanghai First
Maternity and Infant Hospital (approval no. TJBG28625103).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bulun SE, Yilmaz BD, Sison C, Miyazaki K,
Bernardi L, Liu S, Kohlmeier A, Yin P, Milad M and Wei J:
Endometriosis. Endocr Rev. 40:1048–1079. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Harb HM, Gallos ID, Chu J, Harb M and
Coomarasamy A: The effect of endometriosis on in vitro
fertilisation outcome: A systematic review and meta-analysis. BJOG.
120:1308–1320. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sanchez AM, Vanni VS, Bartiromo L, Papaleo
E, Zilberberg E, Candiani M, Orvieto R and Viganò P: Is the oocyte
quality affected by endometriosis? A review of the literature. J
Ovarian Res. 10:432017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
De Hondt A, Peeraer K, Meuleman C, Meeuwis
L, De Loecker P and D'Hooghe TM: Endometriosis and subfertility
treatment: A review. Minerva Ginecol. 57:257–267. 2005.PubMed/NCBI
|
|
5
|
Barnhart K, Dunsmoor-Su R and Coutifaris
C: Effect of endometriosis on in vitro fertilization. Fertil
Steril. 77:1148–1155. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Burney RO and Giudice LC: Pathogenesis and
pathophysiology of endometriosis. Fertil Steril. 98:511–519. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ansariniya H, Yavari A, Javaheri A and
Zare F: Oxidative stress-related effects on various aspects of
endometriosis. Am J Reprod Immunol. 88:e135932022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schieber M and Chandel NS: ROS function in
redox signaling and oxidative stress. Curr Biol. 24:R453–R462.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Malvezzi H, Dobo C, Filippi RZ, Mendes do
Nascimento H, Palmieri da Silva E Sousa L, Meola J, Piccinato CA
and Podgaec S: Altered p16Ink4a, IL-1β, and lamin b1
protein expression suggest cellular senescence in deep
endometriotic lesions. Int J Mol Sci. 23:24762022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lin X, Dai Y, Tong X, Xu W, Huang Q, Jin
X, Li C, Zhou F, Zhou H, Lin X, et al: Excessive oxidative stress
in cumulus granulosa cells induced cell senescence contributes to
endometriosis-associated infertility. Redox Biol. 30:1014312020.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Duval C, Wyse BA, Tsang BK and Librach CL:
Extracellular vesicles and their content in the context of
polycystic ovarian syndrome and endometriosis: A review. J Ovarian
Res. 17:1602024. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Blagosklonny MV: Cell senescence,
rapamycin and hyperfunction theory of aging. Cell Cycle.
21:1456–1467. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Selvarani R, Mohammed S and Richardson A:
Effect of rapamycin on aging and age-related diseases-past and
future. Geroscience. 43:1135–1158. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fan J, Chen C and Zhong Y: A cohort study
on IVF outcomes in infertile endometriosis patients: The effects of
rapamycin treatment. Reprod Biomed Online. 48:1033192024.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sung B, Park S, Yu BP and Chung HY:
Modulation of PPAR in aging, inflammation, and calorie restriction.
J Gerontol A Biol Sci Med Sci. 59:997–1006. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Iemitsu M, Miyauchi T, Maeda S, Tanabe T,
Takanashi M, Irukayama-Tomobe Y, Sakai S, Ohmori H, Matsuda M and
Yamaguchi I: Aging-induced decrease in the PPAR-alpha level in
hearts is improved by exercise training. Am J Physiol Heart Circ
Physiol. 283:H1750–H1760. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sanguino E, Roglans N, Alegret M, Sánchez
RM, Vázquez-Carrera M and Laguna JC: Atorvastatin reverses
age-related reduction in rat hepatic PPARalpha and HNF-4. Br J
Pharmacol. 145:853–861. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kang HS, Cho HC, Lee JH, Oh GT, Koo SH,
Park BH, Lee IK, Choi HS, Song DK and Im SS: Metformin stimulates
IGFBP-2 gene expression through PPARalpha in diabetic states. Sci
Rep. 6:236652016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shin M, Kang HS, Park JH, Bae JH, Song DK
and Im SS: Recent insights into insulin-like growth factor binding
protein 2 transcriptional regulation. Endocrinol Metab (Seoul).
32:11–17. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Spitschak M and Hoeflich A: Potential
functions of IGFBP-2 for ovarian folliculogenesis and
steroidogenesis. Front Endocrinol (Lausanne). 9:1192018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Haouzi D, Assou S, Monzo C, Vincens C,
Dechaud H and Hamamah S: Altered gene expression profile in cumulus
cells of mature MII oocytes from patients with polycystic ovary
syndrome. Hum Reprod. 27:3523–3530. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kamangar BB, Gabillard JC and Bobe J:
Insulin-like growth factor-binding protein (IGFBP)-1, −2, −3, −4,
−5, and −6 and IGFBP-related protein 1 during rainbow trout
postvitellogenesis and oocyte maturation: molecular
characterization, expression profiles, and hormonal regulation.
Endocrinology. 147:2399–2410. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Somigliana E, Viganò P, Rossi G, Carinelli
S, Vignali M and Panina-Bordignon P: Endometrial ability to implant
in ectopic sites can be prevented by interleukin-12 in a murine
model of endometriosis. Hum Reprod. 14:2944–2950. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ren XU, Wang Y, Xu G and Dai L: Effect of
rapamycin on endometriosis in mice. Exp Ther Med. 12:101–106. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lu Y, Nie J, Liu X, Zheng Y and Guo SW:
Trichostatin A, a histone deacetylase inhibitor, reduces lesion
growth and hyperalgesia in experimentally induced endometriosis in
mice. Hum Reprod. 25:1014–1025. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tilly JL: Ovarian follicle counts-not as
simple as 1, 2, 3. Reprod Biol Endocrinol. 1:112003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Luo LL, Huang J, Fu YC, Xu JJ and Qian YS:
Effects of tea polyphenols on ovarian development in rats. J
Endocrinol Invest. 31:1110–1118. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tian Y, Shen W, Lai Z, Shi L, Yang S, Ding
T, Wang S and Luo A: Isolation and identification of ovarian
theca-interstitial cells and granulose cells of immature female
mice. Cell Biol Int. 39:584–590. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hernandez-Segura A, Nehme J and Demaria M:
Hallmarks of cellular senescence. Trends Cell Biol. 28:436–453.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fragouli E, Lalioti MD and Wells D: The
transcriptome of follicular cells: biological insights and clinical
implications for the treatment of infertility. Hum Reprod Update.
20:1–11. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Laschke MW, Elitzsch A, Scheuer C,
Holstein JH, Vollmar B and Menger MD: Rapamycin induces regression
of endometriotic lesions by inhibiting neovascularization and cell
proliferation. Br J Pharmacol. 149:137–144. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Augoulea A, Mastorakos G, Lambrinoudaki I,
Christodoulakos G and Creatsas G: The role of the oxidative-stress
in the endometriosis-related infertility. Gynecol Endocrinol.
25:75–81. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mansour G, Sharma RK, Agarwal A and
Falcone T: Endometriosis-induced alterations in mouse metaphase II
oocyte microtubules and chromosomal alignment: A possible cause of
infertility. Fertil Steril. 94:1894–1899. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ding GL, Chen XJ, Luo Q, Dong MY, Wang N
and Huang HF: Attenuated oocyte fertilization and embryo
development associated with altered growth factor/signal
transduction induced by endometriotic peritoneal fluid. Fertil
Steril. 93:2538–2544. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Papaconstantinou J: The role of signaling
pathways of inflammation and oxidative stress in development of
senescence and aging phenotypes in cardiovascular disease. Cells.
8:13832019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kishi H, Kitahara Y, Imai F, Nakao K and
Suwa H: Expression of the gonadotropin receptors during follicular
development. Reprod Med Biol. 17:11–19. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Harrison DE, Strong R, Sharp ZD, Nelson
JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter
CS, et al: Rapamycin fed late in life extends lifespan in
genetically heterogeneous mice. Nature. 460:392–395. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Weichhart T: mTOR as regulator of
lifespan, aging, and cellular senescence: A mini-review.
Gerontology. 64:127–134. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shen B, Wang Y, Cheng J, Peng Y, Zhang Q,
Li Z, Zhao L, Deng X and Feng H: Pterostilbene alleviated NAFLD via
AMPK/mTOR signaling pathways and autophagy by promoting Nrf2.
Phytomedicine. 109:1545612023. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chung KW, Lee EK, Lee MK, Oh GT, Yu BP and
Chung HY: Impairment of PPARα and the fatty Acid oxidation pathway
aggravates renal fibrosis during aging. J Am Soc Nephrol.
29:1223–1237. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chin C, Ravichandran R, Sanborn K, Fleming
T, Wheatcroft SB, Kearney MT, Tokman S, Walia R, Smith MA, Flint
DJ, et al: Loss of IGFBP2 mediates alveolar type 2 cell senescence
and promotes lung fibrosis. Cell Rep Med. 4:1009452023. View Article : Google Scholar : PubMed/NCBI
|