Introduction
Diabetes mellitus has emerged as a major global
health concern, with its prevalence steadily rising. According to
the International Diabetes Federation, the global prevalence of
diabetes was estimated at 10.5% (536.6 million people) in 2024, and
projections indicate that it will increase to 12.2% (783.2 million
people) by 2045 (1). Diabetic
osteoporosis (DOP), a notable complication of diabetes, is becoming
increasingly prevalent (2).
Epidemiological data reveals the gravity of the situation. A
meta-analysis of 21 studies involving 11,603 patients with type 2
diabetes mellitus (T2DM) found a high osteoporosis prevalence of
27.67% (95% confidence interval, 21.37-33.98%) (3). Another study reported that >35% of
patients with diabetes experience bone loss, with ~20% meeting the
diagnostic criteria for osteoporosis (4). The incidence of fractures, a severe
consequence of DOP, is also notably high. In patients with type 1
diabetes mellitus, the relative risk for hip fracture was reported
as 4.93 (95% confidence interval, 3.06-7.95), while in patients
with T2DM the risk was 1.33 (95% confidence interval, 1.19-1.49)
(5).
The pathogenesis of DOP is primarily attributed to
either absolute or relative insulin deficiency, which leads to the
formation and accumulation of advanced glycation end products
(AGEs) (6,7). These AGEs disrupt the cross-linking
of collagen in the bone matrix, resulting in alterations to bone
microstructure, decreasing bone density per unit volume, decreasing
bone strength and increasing bone fragility, thereby compromising
bone quality in diabetic patients and elevating the risk of
fractures (8,9). In current medical practice, the
traditional medical treatment of DOP has certain limitations
(10). For instance, drugs such as
thiazolidinediones may affect bone metabolism and increase the risk
of fractures. Although insulin has the effect of promoting bone
synthesis, observational studies have shown that the fracture risk
also increases in insulin users (11). The therapeutic effects of some
drugs are not satisfactory, and the efficacy of these drugs in
treating related complications and the applicable population remain
unclear. Further research is needed to clarify these aspects
(12). Over the years, numerous
animal models have been established to study the pathophysiology of
T2DM-associated osteoporosis. For instance, the combination of a
high-fat diet and streptozotocin (STZ) in rodents has been widely
used to induce T2DM-like conditions, which are then associated with
subsequent development of osteoporosis-like bone changes (13,14).
These models have shown characteristic features such as reduced
bone mineral density (BMD), deteriorated bone microstructure and
imbalanced bone remodeling, closely mimicking the human DOP
phenotype (15,16).
Zinc carnosine (ZnC), also known as polaprezinc, is
an orally available biochelate composed of L-carnosine and zinc
ions (17). ZnC is broken down
during intestinal absorption into two compounds: L-carnosine and
zinc. Carnosine, a naturally active dipeptide, is abundant in
mammalian skeletal muscle and brain, and is synthesized in the body
from β-alanine and L-histidine. Zinc exhibits a range of beneficial
properties, including anti-inflammatory, antioxidant, anti-aging,
antitumor and immune regulatory effects, the scavenging of oxygen
free radicals, prevention of AGEs, chelation of bivalent metal ions
(such as zinc2+ and copper2+), and
anti-protein carboxylation and anti-glycosylation effects (18–20).
Studies have confirmed that carnosine can effectively reduce
reactive oxygen species (ROS) levels in chondrocytes injured by
hydrogen peroxide (H2O2), thereby inhibiting
chondrocyte degeneration and bone loss (21,22).
Zinc is an important trace element necessary for the growth,
development and maintenance of bone health, and it is considered a
key factor in bone metabolism (23). Additionally, zinc ions exhibit
anti-glycosylation and anti-oxidation effects (24,25).
Research has shown that zinc ions can promote the differentiation
of human bone marrow mesenchymal stem cells and mouse bone marrow
monocytes into osteoblasts and osteoclasts (26). By mediating the receptor activator
of nuclear factor-κB ligand (RANKL)/RANK/osteoprotegerin (OPG) and
Wnt signaling pathways, zinc ions positively influence bone
metabolism by upregulating the expression of key genes involved in
bone formation (20,27,28).
In the latest review by Yudhani et al(29), zinc was summarized to play a
notable role in alleviating obesity, particularly in lipid
metabolism, appetite regulation, insulin signaling and inflammatory
responses.
The present study aims to fill this research gap by
focusing on ZnC. The originality of the present study lies in
comprehensively evaluating the potential of ZnC in ameliorating
DOP. To the best of our knowledge, previous studies have not
examined the effects of ZnC in its chelated form on DOP, and did
not conduct a thorough investigation into the complex
pathophysiological processes of DOP. The present study explores the
effects of ZnC on bone loss and bone quality in a T2DM mouse model
through a multi-parameter assessment, including imaging,
biomechanics, histology and molecular biology analysis. By doing
so, the present study aims to provide novel insights into the
treatment of DOP and potentially identify ZnC as a new therapeutic
option.
In conclusion, AGEs induced by T2DM trigger
oxidative stress, stimulate the production of inflammatory
cytokines and ROS, and provoke an inflammatory response in
osteoblasts and osteoclasts. This cascade of events induced by AGEs
enhances the bone resorption activity of osteoclasts, thereby
contributing to the development of DOP (30,31).
Both L-carnosine and zinc ions exhibit notable antioxidation and
anti-glycosylation effects and are closely associated with bone
metabolism. The chelation of carnosine with trace element zinc ions
forms ZnC, which may inhibit the accumulation of AGEs in the bone
microenvironment by clearing ROS. The removal of ROS could reduce
the persistent progression of chronic low-level inflammation in a
high-glucose environment, lower the expression of inflammatory
factors that induce insulin resistance and decrease osteoblast
apoptosis, thereby ameliorating osteoporosis both directly and
indirectly (32,33). The present study employed a T2DM
mouse model to investigate the impact and mechanism of oral ZnC
supplementation on DOP. The present investigation was based on
evidence of bone loss and deteriorating bone quality in diabetic
mice and assessed various parameters, including imaging,
biomechanics, histology and molecular biology analysis results.
Materials and methods
Experimental animals
All experimental protocols were conducted in
compliance with the requirements of the Animal Ethics Committee and
have been approved by the Experimental Animal Ethics Committee of
North China University of Science and Technology (Tangshan, China;
approval no. 2023-SY-230). A total of 24 specific pathogen-free
(SPF) C57BL/6J 6-week-old male mice were purchased from Beijing
Huafukang Biotechnology Co., Ltd. The mice were housed under
standard conditions at a temperature of 22±2°C and a humidity of
50±5%, with a 12-h light/12-h dark cycle, ensuring access to
adequate water. The mean body weight of the mice was ~18±3 g and
they were fed with a standard diet for 1 week to allow for
adaptation.
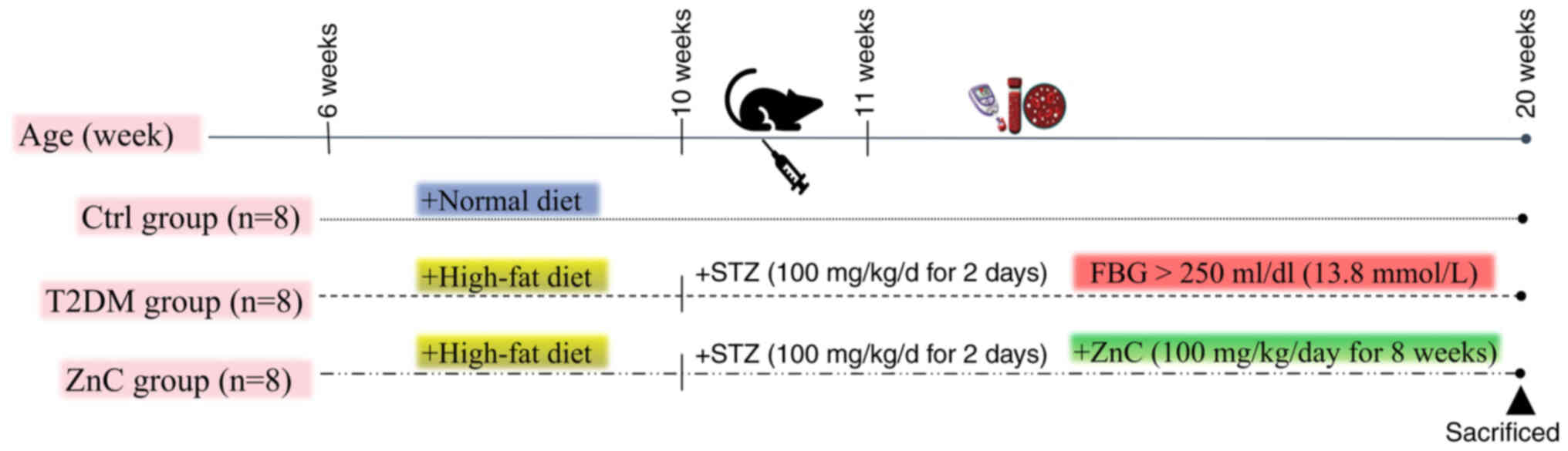
Animal modeling and grouping
A total of 24 6-week-old SPF male C57BL/6J mice were
used in the experiment. The mice were randomly divided into 3
groups, with 8 mice in each group. During the experiment, general
observations were conducted on the animals every day, including
appearance, behavior and activity status. A detailed examination
was performed once a week, including measuring body weight,
observing eating and drinking habits, and checking for any disease
symptoms. If abnormal behaviors were observed in the animals, such
as lethargy, reduced activity or diarrhea, the observation
frequency was increased. In the present experiment, anesthetic
agents were not administered during the observation and handling
procedures, as these did not involve invasive interventions. All
mice survived until the end of the experiment and no mice died.
The experiment lasted for 20 weeks, starting from
week 1 and ending at week 20. In the first 4 weeks, 16 mice were
fed a high-sugar and high-fat diet to induce metabolic disorders of
sugar, fat and insulin resistance. Subsequently, starting from week
5, 12 h of fasting was followed by intraperitoneal injections of
STZ at a dose of 100 mg/(kg/day) for 2 consecutive days. STZ was
dissolved in 0.1 mol/l citrate-sodium citrate buffer (pH 4.5). This
treatment was designed to damage pancreatic β cells and establish a
model of T2DM. The control group received standard food and was
intraperitoneally injected with citrate-sodium citrate buffer (0.1
mol/l; pH 4.5) as a pH-matched control vehicle at week 5 (34–36).
This ensured that any observed differences could be attributed to
the effects of STZ or ZnC (33,37,38).
After completion of the STZ injections, the fasting blood glucose
(FBG) levels of the mice were monitored on designated days. Mice
with FBG levels >250 mg/dl (13.8 mmol/l) on 3 consecutive days
were confirmed as T2DM models. After the successful construction of
the model, the ZnC group and the T2DM group underwent corresponding
treatments for 8 weeks. The ZnC group was orally administered 100
mg/(kg/day) ZnC, while the T2DM group was orally administered 1
ml/(kg/day) 0.9% sodium chloride solution once daily. At the end of
week 20 in the experiment, all mice were euthanized via cervical
dislocation. After performing the cervical dislocation, signs such
as cessation of breathing, disappearance of heartbeat and dilation
of pupils were observed to confirm the death of the animals. At the
same time, all experimental mice underwent autopsy to further
confirm the death status, ensuring the accuracy of the experimental
data and the reliability of the experimental results, and for the
collection of tissue samples for subsequent experiments (Fig. 1).
Micro-computed tomography (micro-CT)
analysis
The right tibia of each mouse was scanned using
high-resolution micro-CT (SkyScan1176; Bruker Corporation) to
assess BMD and microstructural parameters. The scanned images were
reconstructed and analyzed using Avatar software [version 1.7.4.0;
Pingseng Medical Technology (Kunshan) Co., Ltd.]. For trabecular
bone, the region of interest (ROI) was defined as starting at 0.2
mm distal to the growth plate and extending 5% of the tibial length
distally, thereby excluding the growth plate and primary spongy
body. The following parameters were obtained: BMD
(g/cm3) bone volume/tissue volume (BV/TV; %), number of
bone trabeculae (Tb.N; 1/mm), bone trabecular thickness (Tb.Th;
µm), bone trabecular separation (Tb.Sp; mm) and structural model
index (SMI). For cortical bone, the ROI for selected scans
commenced at 40% of the tibia length distal to the proximal growth
plate and extended 10% of the tibia length distally. Within this
ROI, thickness of cortical bone (Ct.Th; µm), area of cortical bone
(Ct.Ar, %) and cortical porosity (Ct.Po, %) were calculated. Ct.Ar
refers to the absolute cortical bone area within the ROI, while
total bone area was not measured in this study.
Determination of biomechanical
properties
The right femurs were utilized for biomechanical
testing using a universal electronic testing machine (cat. no.
MMT-250NV-10; Shimadzu Corporation). The load-measuring accuracy of
the testing instrument was 0.01 N, with a span length of 6 mm and a
loading speed of 2 mm/min. Based on the load-displacement curve,
the maximum load (Max-Load; N) and the maximum bending stress
(Max-Stress; N/mm2) were obtained.
Hematoxylin and eosin (H&E)
staining and tartrate-resistant acid phosphatase (TRAP)
staining
Left tibial tissues were processed for histological
examination using H&E staining and TRAP staining. For bone
tissue decalcification, the left tibia was initially fixed in 4%
paraformaldehyde at room temperature (25°C) for 24 h, followed by
decalcification in a 17% EDTA solution at room temperature (25°C)
12 weeks, with the solution being changed weekly. Section
preparation involved a series of steps: Dehydration, clearing,
paraffin embedding and embedding utilizing an automatic embedding
machine. Paraffin blocks were sectioned at a thickness of 5 µm
using a rotary microtome (Leica RM2235, Leica Biosystems). The
trimmed wax blocks were subjected to continuous sectioning. First,
the sections were pre-cooled on an ice stage to prevent tissue
fragmentation, followed by fine trimming. Subsequently, the
sections were spread in warm water at 45°C to obtain flat and
wrinkle-free sections. Next, the sections were picked up and placed
in a 60°C oven for overnight drying to ensure tight adhesion.
Finally, the dried sections were stored at room temperature for
subsequent staining and immunohistochemical experiments.
The tissue sections were placed in an oven at 60°C
and baked for 2 h to remove the wax. Successive immersions were
performed in 100% xylene I, 100% xylene II, and 100, 95, 80, 70 and
60% ethanol, with each solution applied for 10 min, to complete the
dewaxing and rehydration process. Staining and immunohistochemical
experiments were then performed.
H&E staining was performed, which included
hematoxylin (cat. no. BL702B; Biosharp Life Sciences) staining at
room temperature for 3–5 min, differentiation using a 1%
hydrochloric acid solution in ethanol for 20 sec, bluing with 1%
ammonia for 5 min, eosin staining (cat. no. BA4098; Zhuhai Beisuo
Biotechnology Co., Ltd.) for 30 sec and sealing with neutral gum
following dehydration and clearing.
For TRAP staining, a staining solution composed of
0.1 M acetic acid buffer, 1 mg/ml hexazo para-fuchsin and naphthol
AS-BI phosphate (all from Sigma-Aldrich, USA) was prepared.
Sections were dewaxed, hydrated, stained and incubated for 3 h,
after which they were washed, counterstained, differentiated,
dehydrated, cleared and sealed to ensure optimal staining quality
for subsequent observation and analysis. Histological sections were
examined using a BX53 optical microscope (Olympus Corporation) at
×40 and ×100 magnification. The osteoclast number was obtained by
counting the number of TRAP-positive cells (39).
Immunohistochemical staining
After sections that had been dewaxed to water were
washed with phosphate buffered saline [PBS; cat. no. AC08L033;
Life-iLab; Heyuan Liji (Shanghai) Biotechnology Co., Ltd.] for 10
min, antigen repair was performed by incubation with 0.05% trypsin
[cat. no. AC15L821; Life-iLab; Heyuan Liji (Shanghai) Biotechnology
Co., Ltd.] at 37°C for 30 min. To block endogenous peroxidase
activity, 3% H2O2 was incubated at room
temperature for 10 min. The sections were then incubated with the
corresponding primary antibody at 4°C overnight. The primary
antibodies used and their dilution ratios are as follows: Type I
collagen (COL-I; 1:200; cat. no. AF7001; Affinity Biosciences),
osteocalcin (OCN, 1:200; cat. no. 16157-1-AP; Proteintech Group,
Inc.) and OPG (1:200; cat. no 31766-1-AP; Proteintech Group, Inc.).
The next day, after the sections were rinsed with PBS, they were
incubated with secondary antibody (cat. no. 2414D1020; Beijing
Zhongshan Jinqiao Biotechnology Co., Ltd.), and diaminobenzidine
(cat. no. ZLI-9018; Beijing Zhongshan Jinqiao Biotechnology Co.,
Ltd.) was used for color development, which was terminated by
washing with PBS. After counterstaining with hematoxylin for 1 min,
differentiation was performed with 1% hydrochloric acid ethanol for
3 sec, followed by rinsing with tap water for 15 min to return to
blue. Subsequently, dehydration was carried out with gradient
ethanol at 60, 70, 80, 90 and 100% for 5 min each in sequence,
followed by 100% xylene clearing for 5 min and finally mounting
with resin. All sections were imaged under an optical microscope
(Olympus BX53; Olympus Corporation) at a magnification of ×200. The
region under the growth plate was selected as the ROI, and Image J
software (National Institutes of Health) was used to analyze the
average optical density (AOD) within the ROI of each section.
Reverse transcription-quantitative PCR
(RT-qPCR)
For RT-qPCR, femurs were ground and lysed using
Trizol (cat. no. DP424; Tiangen Biotech Co., Ltd.) reagent, at room
temperature (25°C) for 5 min. Chloroform (MilliporeSigma) was
added, and the mixture was vortexed for 15 sec and incubated at
room temperature for 3 min, followed by centrifugation at 12,000 ×
g for 15 min at 4°C to separate the aqueous phase containing RNA.
RNA was precipitated by adding isopropyl alcohol, incubated at room
temperature for 10 min, and pelleted by centrifugation at 12,000 ×
g for 10 min at 4°C. The RNA pellet was washed with 70% ethanol,
centrifuged at 7,500 × g for 5 min at 4°C and air-dried before
resuspension in RNase-free water. The purified RNA was dissolved in
nuclease-free water, and its concentration and purity were assessed
using a UV spectrophotometer (cat. no. SLAN-96S; Shanghai Hongshi
Medical Technology Co., Ltd.). A 20-µl reaction mixture was
prepared, and first-strand cDNA was synthesized using a reverse
transcription kit (cat. no. ZR103; Beijing Zhuangmeng International
Biogene Technology Co., Ltd.) according to the manufacturer's
protocol. The cDNA was subsequently subjected to qPCR in a 20-µl
reaction volume using the SYBR® Premix Ex Taq™ II kit
(cat. no. ZR103-1; Beijing Zoman Biotechnology Co., Ltd.) with SYBR
Green as the fluorophore. With β-actin as the internal reference
gene, the 2−∆∆Cq method (40) was used to calculate the relative
mRNA expression levels of the target genes relative to the control
group. The genes analyzed included OPG, OCN and RANKL, which were
selected since they are key regulators of bone metabolism and
remodeling, serving as important markers of osteogenic
differentiation and bone turnover. The primer synthesis was
completed by Sangon Biotech Co., Ltd. The primer sequences used are
shown in Table I. The PCR
conditions were as follows: 94°C for 5 min, followed by 35 cycles
of 94°C for 30 sec, 52–58°C for 30 sec and 72°C for 30 sec.
 | Table I.Primer sequences of selected
genes. |
Table I.
Primer sequences of selected
genes.
| Gene | Primer sequence
(5′-3′) |
|---|
| OCN |
|
|
Forward |
GAGGGCAATAAGGTAGTGAA |
|
Reverse |
CATAGATGCGTTTGTAGGC |
| OPG |
|
|
Forward |
CAGAGCGAAACACAGTTTG |
|
Reverse |
CACACAGGGTGACATCTATTC |
| RANKL |
|
|
Forward |
TGTACTTTCGAGCGCAGATG |
|
Reverse |
CCACAATGTGTTGCAGTTCC |
| β-actin |
|
|
Forward |
GATCAGCAAGCAGGAGTACGA |
|
Reverse |
GGTGTAAAACGCAGCTCAGTAAC |
Statistical analysis
All data are presented in the form of mean ±
standard deviation. The present study used SPSS 22.0 software (IBM
Corp.) for all statistical analyses. For the differences between
groups that follow a normal distribution, a one-way analysis of
variance (ANOVA) was first used to confirm the overall group
differences. If the ANOVA results showed a statistically
significant difference (P<0.05), then the LSD (least significant
difference) post-hoc test was further used to conduct pairwise
comparisons between each group to clearly identify which groups had
differences. Each group had a sample size of 8 mice (n=8), and
comparisons were made between groups. The significance level α was
set at 0.05. During the analysis, the actual P-value of each test
was clearly indicated. P<0.05 was considered to indicate a
statistically significant difference; if P≥0.05, it indicated that
there was no statistically significant difference between the
groups.
Results
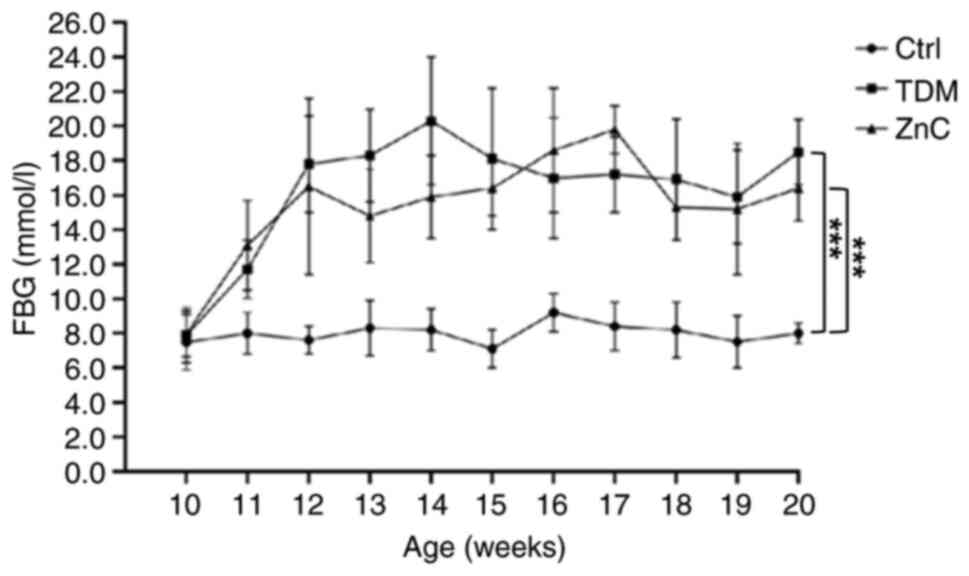
Changes in FBG in each group of
mice
Notable differences in blood glucose concentrations
were observed in the T2DM model group and the ZnC intervention
group when compared with the control group. However, there was no
notable difference in blood glucose concentration between the ZnC
group and the T2DM group, indicating a lack of a substantial
hypoglycemic effect following the ZnC intervention (Fig. 2).
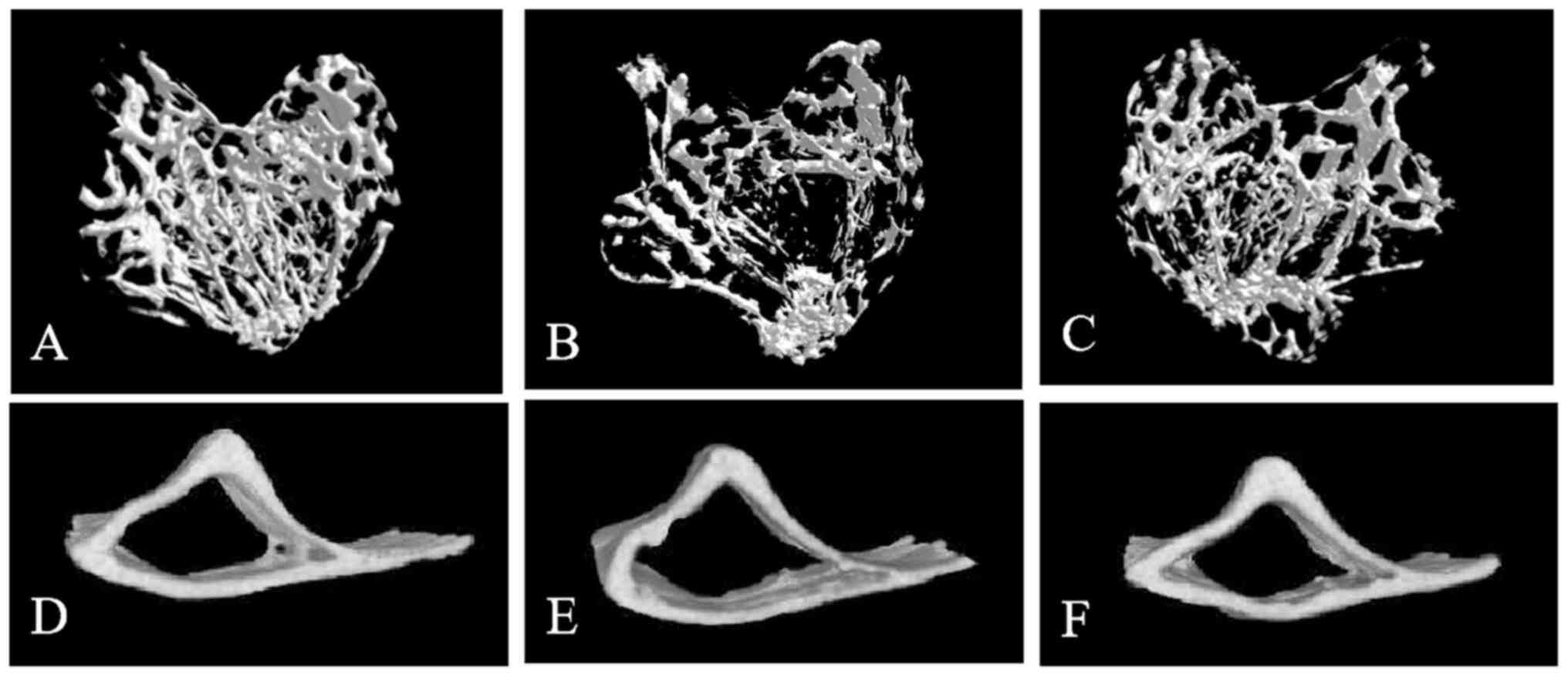
Micro-CT analysis of cancellous and
cortical bone parameters
The tibial bone tissue of mice was examined using
micro-CT, and the three-dimensional reconstruction images revealed
the microstructure of both cancellous and cortical bone. The bone
microstructure in the control group appeared generally normal. By
contrast, the cancellous bone microstructure in the T2DM model
group exhibited significant alterations compared with that of the
control group, including thinning of the proximal growth plate, a
reduction in the number of trabeculae beneath the growth plate,
decreased trabecular thickness, increased trabecular spacing, and
enhanced separation and thinning of the cortical bone. Quantitative
analysis of tibial bone parameters further validated osteoporosis
in the T2DM model. Notably, the microstructural changes observed in
the tibia of the T2DM model group were significantly mitigated in
the ZnC intervention group.
Further analysis of tibial BMD and bone
microstructure parameters revealed that compared with the Control
group, the BMD, BV/TV, Tb.N, Ct.Th and Ct.Ar in the T2DM model
group were significantly decreased (P<0.05). Notably, there were
no significant differences in SMI, Tb.Th and Ct.Po between the ZnC
group and the T2DM model group (P>0.05). However, compared with
the T2DM model group, the BMD, BV/TV, Tb.N, Ct.Th and Ct.Ar in the
ZnC group were significantly increased, and Tb.Sp was significantly
decreased (P<0.05) (Figs. 3 and
4).
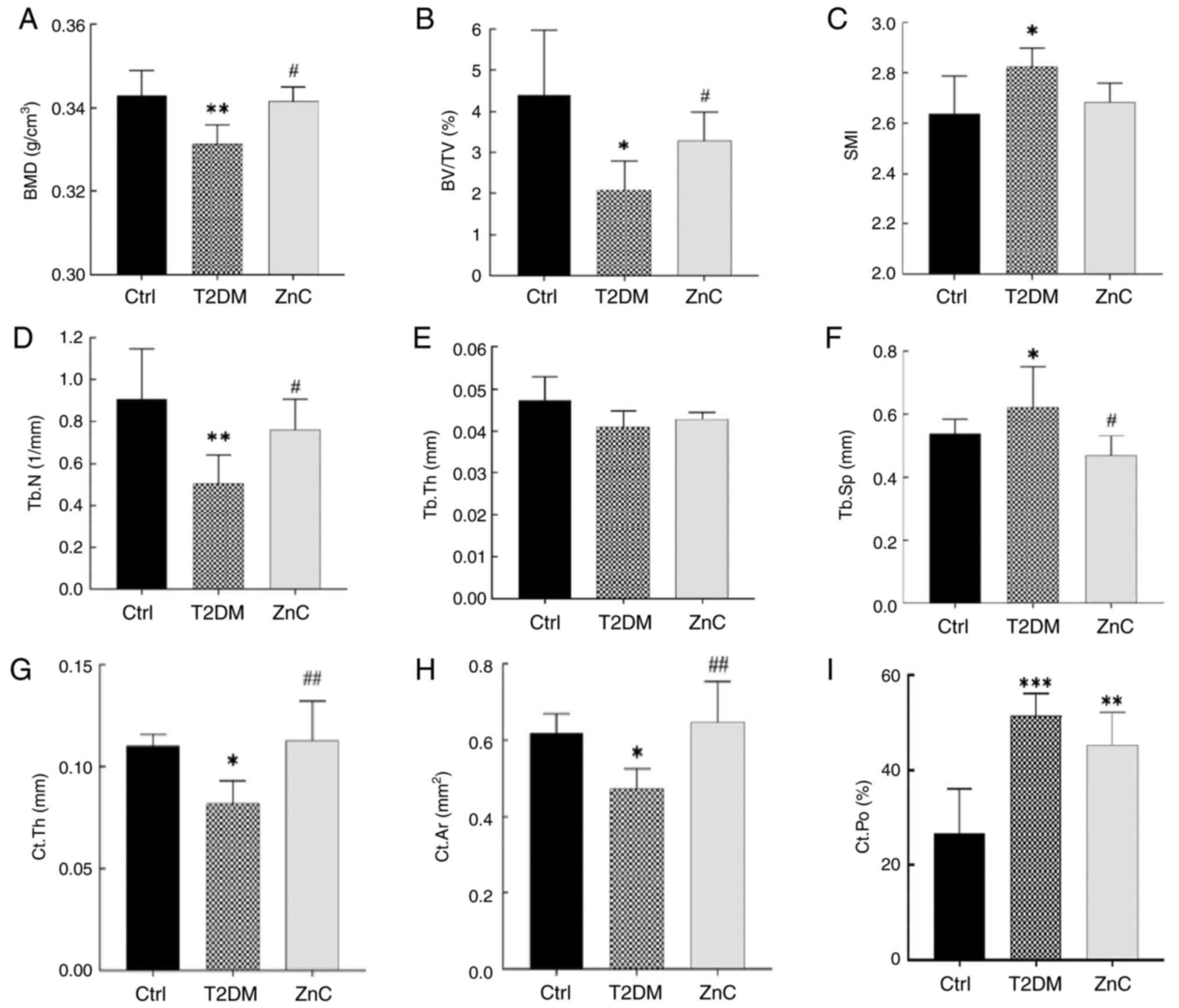 | Figure 4.Analysis of tibial BMD and bone
microstructure parameters in mice. *P<0.05, **P<0.01 and
***P<0.001 vs. Ctrl group; #P<0.05 and
##P<0.01 vs. T2DM model group. (A) BMD, bone mineral
density; (B) BV/TV, bone volume/tissue volume; (C) SMI, structural
model index; (D) Tb.N, number of bone trabeculae; (E) Tb.Th, bone
trabecular thickness; (F) Tb.Sp, bone trabecular separation; (G)
Ct.Th, thickness of cortical bone; (H) Ct.Ar, area of cortical
bone; (I) Ct.Po, porosity of cortical bone. Ctrl, control; T2DM,
type 2 diabetes mellitus; ZnC, zinc carnosine. |
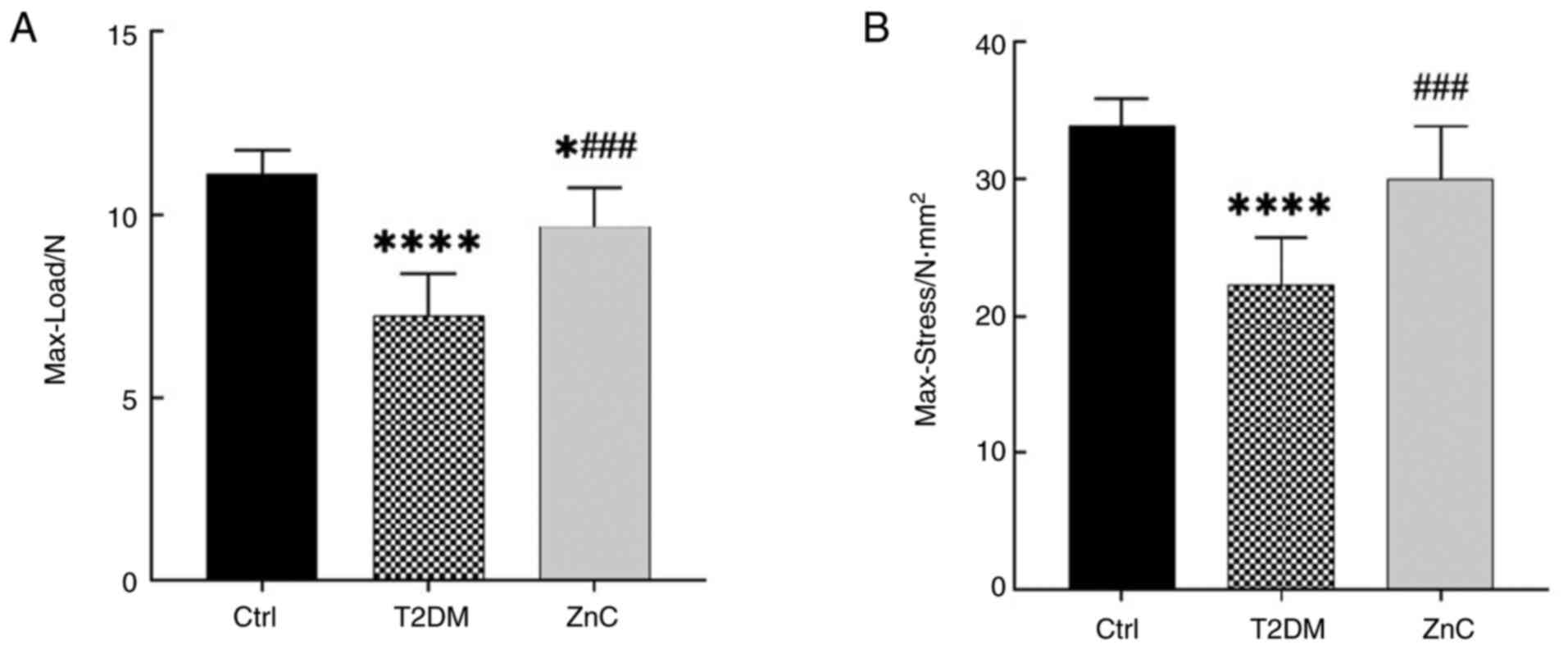
Three-point bending test
The biomechanical properties of the femur were
assessed using a three-point bending test (Fig. 5). When compared with the control
group, the Max-Load in both the T2DM model group and the ZnC
intervention group was significantly reduced (P<0.05). However,
compared with the T2DM model group, the Max-Load and Max-Stress in
the ZnC intervention group were significantly increased
(P<0.05).
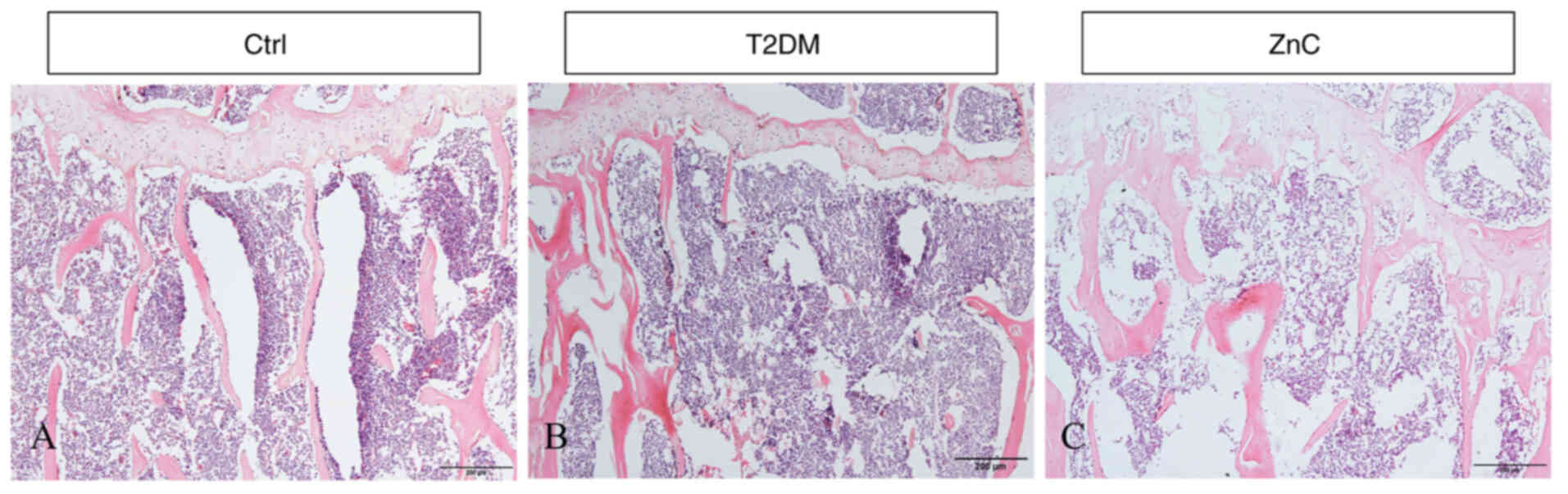
Histological staining results
Compared with that in the control group, the growth
plate in the T2DM model group exhibited a notable reduction in
thickness, with considerable damage to the lamellar trabecular
structure in the lower bone marrow cavity. The distance between
trabeculae increased and the presence of lipid vacuoles was noted.
Additionally, the marginal cortical bone was thinner, resulting in
a more pronounced loss of total bone mass. By contrast, the ZnC
intervention group showed an increase in growth plate thickness, a
reduction in the extent of partial destruction of both trabecular
and cortical bone and a decrease in total bone mass loss when
compared with the T2DM model group (Fig. 6). This is consistent with the
results shown in Fig. 4: that is,
compared with the control group, BMD, BV/TV, Tb.N, Ct.Th and Ct.Ar
in the T2DM model group were all significantly decreased
(P<0.05); while ZnC intervention could significantly reverse
these indicators (P<0.05).
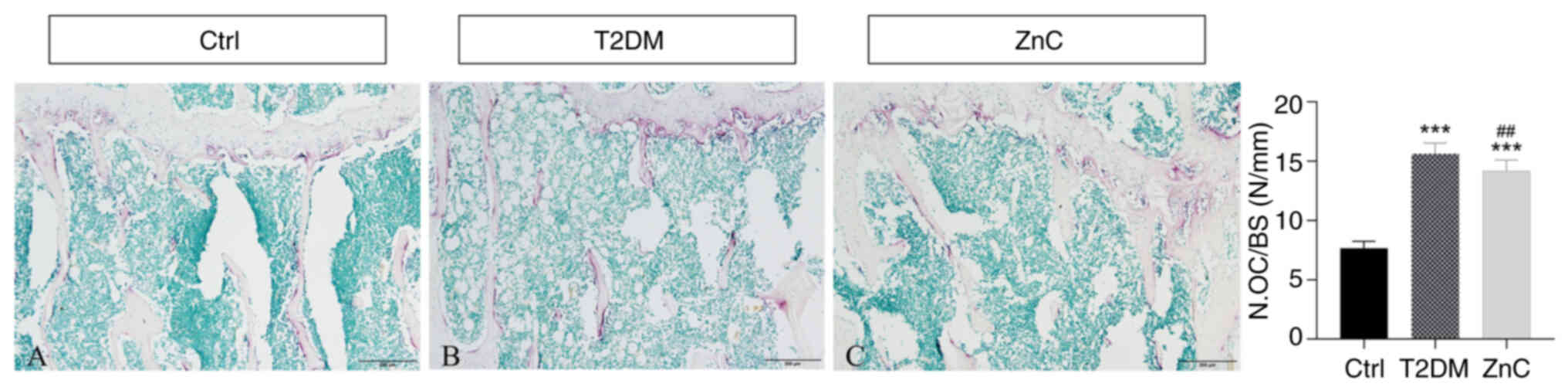
TRAP staining
Compared with the control group, the T2DM model
group exhibited a significantly higher density of TRAP-positive
multinucleated cells (≥3 nuclei/cell) (P<0.01) localized
predominantly in osteoclast-active regions; specifically, beneath
the growth plate and adjacent to the medullary cavity along
trabecular surfaces (P<0.05). This pattern indicated enhanced
osteoclast-mediated bone resorption in diabetic conditions.
Conversely, ZnC intervention significantly suppressed
osteoclastogenesis, evidenced by a marked reduction in
TRAP-positive cell density relative to the T2DM model group
(P<0.05), as quantified by histomorphometric parameter of
measuring osteoclast number/bone surface (41,42)
(Fig. 7).
Immunohistochemical detection of
protein expression of bone metabolism-related factors in each
group
Protein expression of bone metabolism-related
factors was evaluated in each group via immunohistochemistry.
Quantitative analysis of histochemical staining images revealed no
significant differences in the expression levels of bone formation
markers type I collagen (COL-I), osteocalcin (OCN) and OPG between
the T2DM group and the control group (all P>0.05). By contrast,
the AOD of RANKL, a key osteoclast-activating factor, was
significantly elevated in the T2DM group compared with that in the
control group (P<0.05).
Following ZnC intervention, the AOD of osteogenic
genes COL-I and OCN increased significantly compared with that in
both the control and T2DM groups (all P<0.05), indicating
enhanced bone formation. Meanwhile, OPG expression remained
unchanged (P>0.05). Notably, ZnC treatment significantly
suppressed RANKL expression compared with that in the T2DM model
group (P<0.05), suggesting inhibition of osteoclast-mediated
bone resorption (Fig. 8).
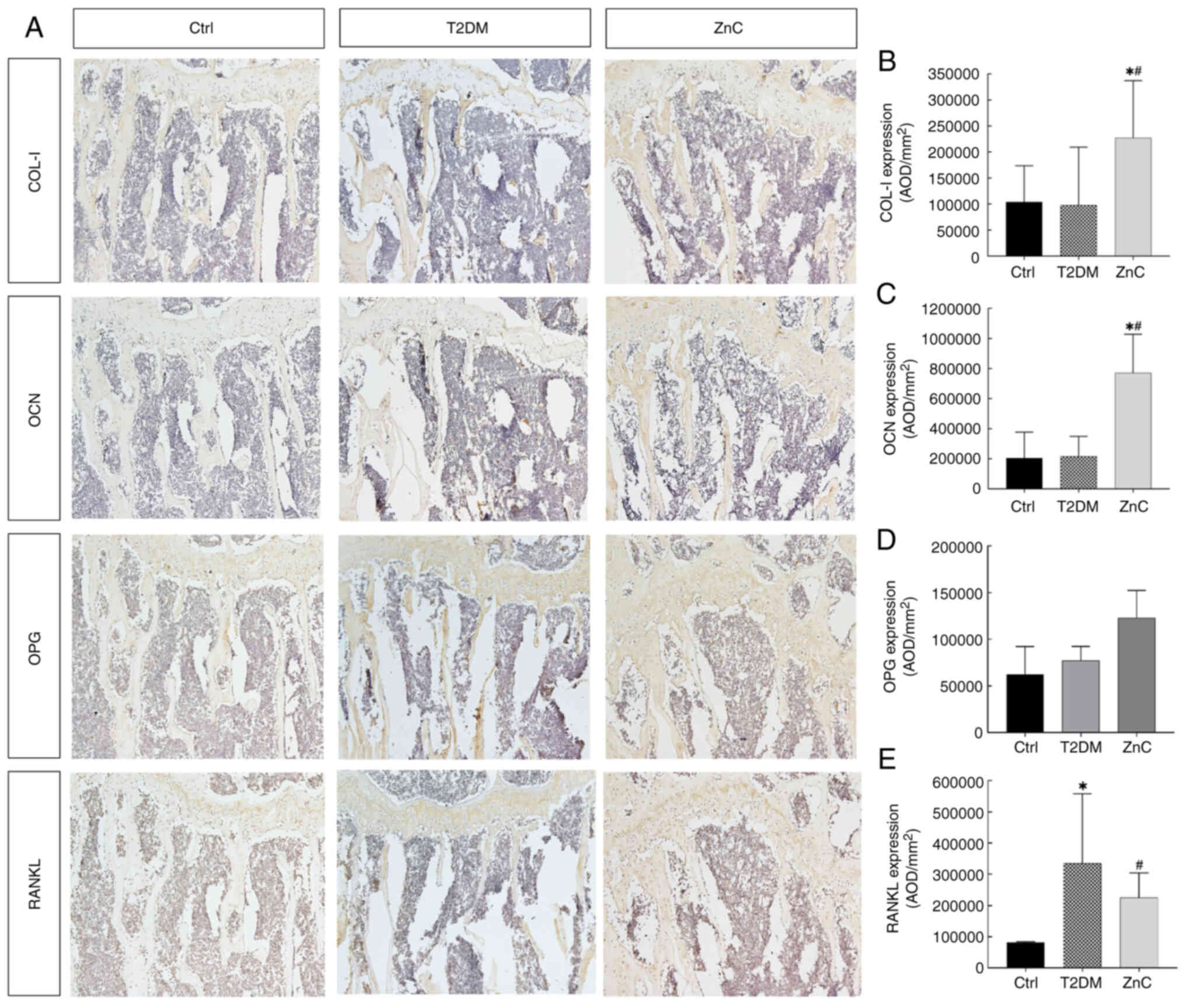 | Figure 8.COL-I, OCN, OPG and RANKL protein
expression levels in each group after 8 weeks of intervention. (A)
COL-I, OCN, OPG and RANKL protein expression level of each
treatment group. Average optical density of (B) COL-I, (C) OCN, (D)
OPG and (E) RANKL in each group. *P<0.05 vs. Ctrl group;
#P<0.05 vs. T2DM model group. COL-I, type I collagen;
OCN, osteocalcin; OPG, osteoprotegerin; RANKL, receptor activator
of nuclear factor-κB ligand; Ctrl, control; T2DM, type 2 diabetes
mellitus; ZnC, zinc carnosine; AOD, average optical density
value. |
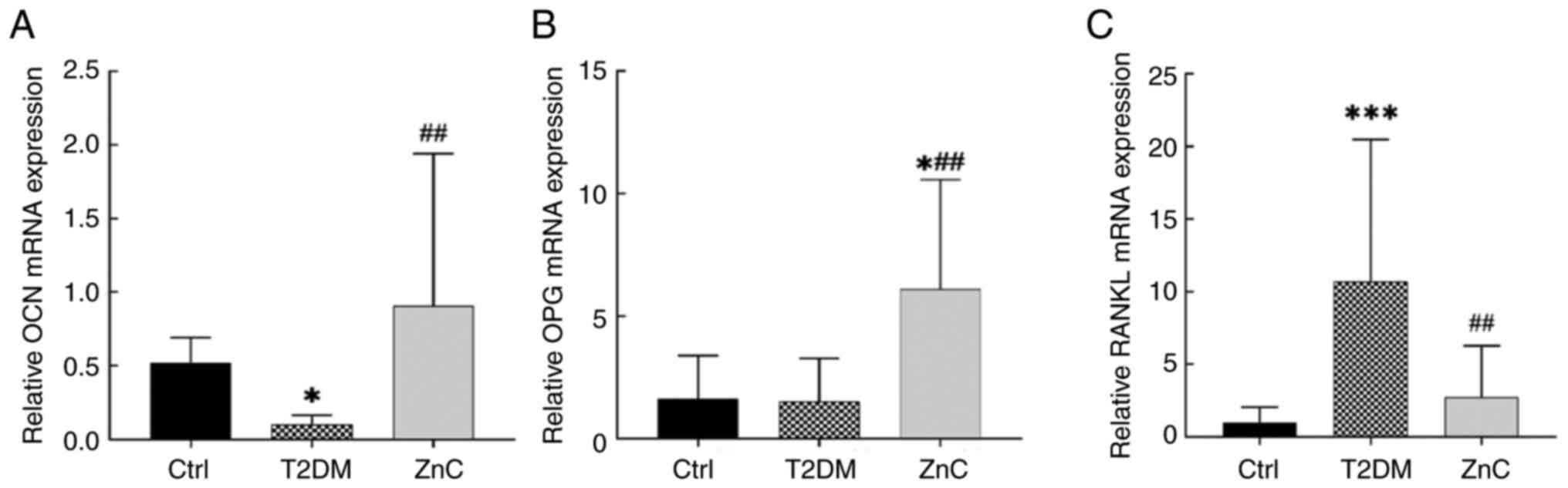
mRNA expression levels of bone
metabolism-related factors
Compared with the control group, the mRNA expression
of OCN in mice of the T2DM model group was significantly decreased
(P<0.05), while the difference in the expression level of OPG
was not statistically significant (P>0.05); however, the
expression level of RANKL was significantly increased (P<0.05).
When compared with the T2DM group, the ZnC group exhibited
significant upregulation of OCN and OPG mRNA expression
(P<0.05), and downregulation of RANKL mRNA expression
(P<0.05) (Fig. 9).
Discussion
ZnC is a zinc peptide that is widely used in the
treatment of peptic ulcers, gastrointestinal tumors, Alzheimer's
disease, diabetes complications and other diseases, and its safety
and lack of toxicity have been demonstrated (43,44).
In the present study, a mouse model of DOP was
established by administering a high-fat diet in conjunction with
intraperitoneal injections of a moderate dose of STZ (45,46).
Continuous monitoring of FBG levels and body mass changes confirmed
the onset of insulin resistance after 4 weeks of high-fat diet
feeding. Following two consecutive injections of moderate doses of
100 mg/(kg/day) STZ, blood glucose levels gradually increased over
the course of 1 week, successfully establishing the T2DM model. In
the present study, it was observed that the FBG levels of mice
receiving ZnC intervention for 8 weeks were lower than those of the
diabetes model group without ZnC intervention; however, this
difference was not statistically significant, suggesting that ZnC
does not have a notable hypoglycemic effect. Previous meta-analyses
have indicated that ZnC may possess therapeutic potential in
diabetes, as it can reduce glycated hemoglobin and FBG levels
(47,48). Further studies are necessary to
conclusively determine whether ZnC has a hypoglycemic effect.
ZnC has been demonstrated to markedly alleviate bone
mass loss associated with postmenopausal osteoporosis, senile
osteoporosis, apraxia osteoporosis, autoimmune osteoporosis and
secondary osteoporosis resulting from vitamin D or calcium
deficiency (49–52). In the present study, micro-CT
analysis revealed that BMD, BV/TV, Tb.N, Ct.Th and Ct.Ar were
significantly reduced in the diabetes model group, whilst Tb.Sp,
SMI and the Ct.Po were significantly increased compared with the
control group. After ZnC intervention, these parameters were
partially restored toward control group levels, showing significant
improvement compared with the T2DM group. The data of the
three-point bending mechanics test indicated that the Max-Load and
Max-Stress of the diabetic model group were significantly reduced
compared with those of the control group. No significant
differences were observed between the ZnC intervention group and
the T2DM model group, indicating that ZnC treatment did not
significantly improve femoral biomechanical properties. The
aforementioned results showed that STZ injection combined with a
high-fat diet for 8 weeks could induce a significant DOP phenotype
in mice and that the associated osteoporosis was significantly
improved after ZnC intervention. ZnC intervention partially
mitigated diabetic bone mass loss, as evidenced by improvements in
bone microstructure and histological parameters, although
biomechanical properties were not significantly restored.
ZnC, a bidirectional modulator, may help to treat
osteoporosis and other bone diseases. By increasing the expression
levels of protein kinase and protein phosphatase in osteoblasts,
ZnC stimulates newly synthesized bone proteins in osteoblasts,
enhances the activity of zinc-activated aminoacyl-tRNA synthetase
in translation, and stimulates bone formation and calcification
(51,53–55).
ZnC can also upregulate the OPG/RANKL ratio and block the
RANKL/RANK interaction between osteoclast precursors and
osteoclasts, thereby effectively inhibiting the formation,
differentiation and apoptosis of osteoclasts, and mitigating
DOP-associated bone loss (56–60).
In the present study, immunohistochemical and histological analyses
demonstrated that oral supplementation of 100 mg/(kg/day) ZnC
alleviated diabetes-induced bone loss, improved high sugar-induced
abnormalities in bone microstructure, and regulated bone
metabolism-related factors by increasing osteogenic proteins
(COL-I, OCN and OPG) and decreasing the osteoclast-related factor
RANKL, accompanied by a reduction in TRAP-positive cells, which may
reduce the stimulating effect of diabetes on osteoclast
differentiation by promoting osteoblast differentiation, and
inhibiting osteoclast differentiation and bone resorption function,
and exhibits a protective role in cortical bone and cancellous bone
during abnormal bone loss.
In conclusion, ZnC effectively alleviated the DOP
phenotype in diabetic mice, improved bone homeostasis and mitigated
the abnormal osteoclast activity under the high glucose-metabolic
state. ZnC was shown to inhibit bone resorption and promote bone
formation by suppressing osteoclast activity and enhancing
osteoblast differentiation, demonstrating its potential as a
therapeutic drug for bone remodeling-related diseases (including
osteoporosis). However, the present study also had certain
limitations, as follows: i) The present study was conducted only in
mouse models, and the animal-based experimental results exhibit a
level of uncertainty when extrapolated to humans; and ii) although
the present study explored the effects of ZnC on bone
metabolism-related factors and initially revealed its mechanism of
action, the specific molecular signaling pathways have not been
fully clarified. Future research should consider conducting
clinical studies to verify the efficacy and safety of ZnC in
humans; at the same time, in-depth explorations of its mechanism of
action at the cellular and molecular levels can provide a
theoretical basis for developing more effective osteoporosis
treatment strategies.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Central Government-Guided
Local Science and Technology Development Foundation of Hebei
Province (grant nos. 226Z7709G and 246Z7744G), the Natural Science
Foundation of Hebei Province (grant no. H2022209054) and the Basic
Scientific Research Foundation of Universities in Hebei Province
(grant no. JYG2021005).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
FT and JG conceived and designed the study,
supervised the project, interpreted the data, critically revised
the manuscript for important intellectual content and approved the
final manuscript. FT and JG are responsible for the overall
integrity of the work. HY made substantial contributions to the
conception of the work, performed all experiments, analyzed and
interpreted the data, drafted the manuscript and approved the final
version. JG and HY confirm the authenticity of all raw data. QL and
YH made substantial contributions to data acquisition, performed
the statistical analysis, interpreted the results and approved the
final manuscript. ZY contributed to data collection, validation of
the experimental results and approval of the final manuscript. LX,
YX and XH performed the H&E and TRAP staining experiments,
contributed to the analysis and interpretation of the histological
data, and approved the final manuscript. DH secured funding for the
study, provided methodological guidance, contributed to the
critical review of the manuscript and approved the final version.
All authors have read and approved the final manuscript, and agree
to be accountable for all aspects of the work.
Ethics approval and consent to
participate
All experiments were approved by the Institutional
Animal Care and Use Committee of North China University of Science
and Technology (Tangshan, China; approval no. 2023-SY-230).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ZnC
|
zinc carnosine
|
|
DOP
|
diabetic osteoporosis
|
|
T2DM
|
type 2 diabetes mellitus
|
|
STZ
|
streptozotocin
|
|
micro-CT
|
micro-computed tomography
|
|
BMD
|
bone mineral density
|
|
BV/TV
|
bone volume/tissue volume
|
|
Tb.N
|
number of bone trabeculae
|
|
Ct.Th
|
thickness of cortical bone
|
|
Ct.Ar
|
area of cortical bone
|
|
Tb.Sp
|
bone trabecular separation
|
|
Ct.Po
|
porosity of cortical bone
|
|
ROS
|
reactive oxygen species
|
|
ROI
|
region of interest
|
|
Tb.Th
|
bone trabecular thickness
|
|
SMI
|
structural model index
|
|
Max-Load
|
maximum load
|
|
COL-I
|
type I collagen
|
|
OCN
|
osteocalcin
|
|
AOD
|
average optical density value
|
|
FBG
|
fasting blood glucose
|
References
|
1
|
Gao HX, Regier EE and Close KL:
International diabetes federation world diabetes Congress 2015. J
Diabetes. 8:300–302. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Carballido-Gamio J: Imaging techniques to
study diabetic bone disease. Curr Opin Endocrinol Diabetes Obes.
29:350–360. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu X, Chen F, Liu L and Zhang Q:
Prevalence of osteoporosis in patients with diabetes mellitus: A
systematic review and meta-analysis of observational studies. BMC
Endocr Disord. 23:12023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schacter GI and Leslie WD: Diabetes and
osteoporosis: Part I, epidemiology and pathophysiology. Endocrinol
Metab Clin North Am. 50:275–228. 20215. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vilaca T, Schini M, Harnan S, Sutton A,
Poku E, Allen IE, Cummings SR and Eastell R: The risk of hip and
non-vertebral fractures in type 1 and type 2 diabetes: A systematic
review and meta-analysis update. Bone. 137:1154572020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Asadipooya K and UY EM: Advanced glycation
end products (AGEs), receptor for AGEs, diabetes, and bone: Review
of the literature. J Endocr Soc. 3:1799–1818. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yamagishi S: Role of advanced glycation
end products (AGEs) in osteoporosis in diabetes. Curr Drug Targets.
12:2096–2102. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee HS and Hwang JS: Impact of type 2
diabetes mellitus and antidiabetic medications on bone metabolism.
Curr Diab Rep. 20:782020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Napoli N, Chandran M, Pierroz DD,
Abrahamsen B, Schwartz AV and Ferrari SL; IOF Bone and Diabetes
Working Group, : Mechanisms of diabetes mellitus-induced bone
fragility. Nat Rev Endocrinol. 13:208–219. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jankovic J: Complications and limitations
of drug therapy for Parkinson's disease. Neurology. 55 (12 Suppl
6):S2–S6. 2000.PubMed/NCBI
|
|
11
|
Pietschmann P, Patsch JM and Schernthaner
G: Diabetes and bone. Horm Metab Res. 42:763–768. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Holstege A: Long-term drug treatments to
improve prognosis of patients with liver cirrhosis and to prevent
complications due to portal hypertension. Z Gastroenterol.
57:983–996. 2019.(In German). PubMed/NCBI
|
|
13
|
Huang KC, Chuang PY, Yang TY, Tsai YH, Li
YY and Chang SF: Diabetic rats induced using a high-fat diet and
low-dose streptozotocin treatment exhibit gut microbiota dysbiosis
and osteoporotic bone pathologies. Nutrients. 16:12202024.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Boyar H, Turan B and Severcan F: FTIR
spectroscopic investigation of mineral structure of streptozotocin
induced diabetic rat femur and tibia. Spectroscopy. 17:627–633.
2003. View Article : Google Scholar
|
|
15
|
Lu R, Zheng Z, Yin Y and Jiang Z:
Genistein prevents bone loss in type 2 diabetic rats induced by
streptozotocin. Food Nutr Res. 64:10.29219/fnr.v64.3666. 2020.
View Article : Google Scholar
|
|
16
|
Wu X, Gong H, Hu X, Shi P, Cen H and Li C:
Effect of verapamil on bone mass, microstructure and mechanical
properties in type 2 diabetes mellitus rats. BMC Musculoskelet
Disord. 23:3632022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ghodsi R and Kheirouri S: Carnosine and
advanced glycation end products: A systematic review. Amino Acids.
50:1177–1186. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Corona C, Frazzini V, Silvestri E,
Lattanzio R, La Sorda R, Piantelli M, Canzoniero LM, Ciavardelli D,
Rizzarelli E and Sensi SL: Effects of dietary supplementation of
carnosine on mitochondrial dysfunction, amyloid pathology, and
cognitive deficits in 3×Tg-AD mice. PLoS One. 6:e179712011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jukić I, Kolobarić N, Stupin A, Matić A,
Kozina N, Mihaljević Z, Mihalj M, Šušnjara P, Stupin M, Ćurić ŽB,
et al: Carnosine, small but mighty-prospect of use as functional
ingredient for functional food formulation. Antioxidants (Basel).
10:10372021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ko EA, Park YJ, Yoon DS, Lee KM, Kim J,
Jung S, Lee JW and Park KH: Drug repositioning of polaprezinc for
bone fracture healing. Commun Biol. 5:4622022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Busa P, Lee SO, Huang N, Kuthati Y and
Wong CS: Carnosine alleviates knee osteoarthritis and promotes
synoviocyte protection via activating the Nrf2/HO-1 signaling
pathway: An in-vivo and in-vitro study. Antioxidants (Basel).
11:12092022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang T, Yan G and Guan M: Zinc
homeostasis in bone: Zinc transporters and bone diseases. Int J Mol
Sci. 21:12362020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kheirouri S, Alizadeh M and Maleki V: Zinc
against advanced glycation end products. Clin Exp Pharmacol
Physiol. 45:491–498. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Marreiro DD, Cruz KJ, Morais JB, Beserra
JB, Severo JS and de Oliveira AR: Zinc and oxidative stress:
Current mechanisms. Antioxidants (Basel). 6:242017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Brzóska MM and Rogalska J: Protective
effect of zinc supplementation against cadmium-induced oxidative
stress and the RANK/RANKL/OPG system imbalance in the bone tissue
of rats. Toxicol Appl Pharmacol. 272:208–220. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bartmański M, Pawłowski Ł, Knabe A, Mania
S, Banach-Kopeć A, Sakowicz-Burkiewicz M and Ronowska A: The effect
of marginal Zn(2+) excess released from titanium coating on
differentiation of human osteoblastic cells. ACS Appl Mater
Interfaces. 16:48412–48427. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shiota J, Tagawa H, Izumi N, Higashikawa S
and Kasahara H: Effect of zinc supplementation on bone formation in
hemodialysis patients with normal or low turnover bone. Ren Fail.
37:57–60. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Saito M and Marumo K: Collagen cross-links
as a determinant of bone quality: A possible explanation for bone
fragility in aging, osteoporosis, and diabetes mellitus. Osteoporos
Int. 21:195–214. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yudhani RD, Pakha DN, Wiyono N and Wasita
B: Molecular mechanisms of zinc in alleviating obesity: Recent
updates (Review). World Acad Sci J. 6:702024. View Article : Google Scholar
|
|
30
|
Gao X, Al-Baadani MA, Wu M, Tong N, Shen
X, Ding X and Liu J: Study on the local anti-osteoporosis effect of
polaprezinc-loaded antioxidant electrospun membrane. Int J
Nanomedicine. 17:17–29. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
An Y, Zhang H, Wang C, Jiao F, Xu H, Wang
X, Luan W, Ma F, Ni L, Tang X, et al: Activation of
ROS/MAPKs/NF-κB/NLRP3 and inhibition of efferocytosis in
osteoclast-mediated diabetic osteoporosis. FASEB J.
33:12515–201927. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li M, Sun Z, Zhang H and Liu Z: Recent
advances on polaprezinc for medical use (Review). Exp Ther Med.
22:14452021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Furman BL: Streptozotocin-induced diabetic
models in mice and rats. Curr Protoc Pharmacol. 70:5.47.1–5.20.
2015.PubMed/NCBI
|
|
34
|
Furman BL: Streptozotocin-induced diabetic
models in mice and rats. Curr Protoc. 1:e782021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Islam MS and Loots DT: Experimental rodent
models of type 2 diabetes: A review. Methods Find Exp Clin
Pharmacol. 31:249–261. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Amri J, Alaee M, Babaei R, Salemi Z,
Meshkani R, Ghazavi A, Akbari A and Salehi M: Biochanin-A has
antidiabetic, antihyperlipidemic, antioxidant, and protective
effects on diabetic nephropathy via suppression of TGF-β1 and PAR-2
genes expression in kidney tissues of STZ-induced diabetic rats.
Biotechnol Appl Biochem. 69:2112–2121. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Muhammad HJ, Shimada T, Fujita A and Sai
Y: Sodium citrate buffer improves pazopanib solubility and
absorption in gastric acid-suppressed rat model. Drug Metab
Pharmacokinet. 55:1009952024. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Krebs H: Citric acid cycle: A chemical
reaction for life. Nurs Mirror. 149:30–32. 1979.PubMed/NCBI
|
|
39
|
Sun Q, Tian FM, Liu F, Fang JK, Hu YP,
Lian QQ, Zhou Z and Zhang L: Denosumab alleviates intervertebral
disc degeneration adjacent to lumbar fusion by inhibiting endplate
osteochondral remodeling and vertebral osteoporosis in
ovariectomized rats. Arthritis Res Ther. 23:1522021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Livák F and Petrie HT: Somatic generation
of antigen-receptor diversity: A reprise. Trends Immunol.
22:608–612. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tsuji-Naito K: Aldehydic components of
cinnamon bark extract suppresses RANKL-induced osteoclastogenesis
through NFATc1 downregulation. Bioorg Med Chem. 16:9176–9183. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Matsui S, Hiraishi C, Sato R, Kojima T,
Matoba K, Fujimoto K and Yoshida H: Association of metformin
administration with the serum levels of zinc and homocysteine in
patients with type 2 diabetes: A cross-sectional study. Diabetol
Int. 16:394–402. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Goyal SN, Reddy NM, Patil KR, Nakhate KT,
Ojha S, Patil CR and Agrawal YO: Challenges and issues with
streptozotocin-induced diabetes-A clinically relevant animal model
to understand the diabetes pathogenesis and evaluate therapeutics.
Chem Biol Interact. 244:49–63. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
El Dib R, Gameiro OL, Ogata MS, Módolo NS,
Braz LG, Jorge EC, do Nascimento P Jr and Beletate V: Zinc
supplementation for the prevention of type 2 diabetes mellitus in
adults with insulin resistance. Cochrane Database Syst Rev.
2015:CD0055252015.PubMed/NCBI
|
|
45
|
Sureshkumar K, Durairaj M, Srinivasan K,
Goh2 KW, Undela K, Mahalingam VT, Ardianto C, Ming LC and Ganesan
RM: Effect of L-carnosine in patients with age-related diseases: A
systematic review and meta-analysis. Front Biosci (Landmark Ed).
28:182023. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Cararo JH, Streck EL, Schuck PF and
Ferreira Gda C: Carnosine and related peptides: Therapeutic
potential in age-related disorders. Aging Dis. 6:369–379. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yamaguchi M and Ehara Y: Zinc decrease and
bone metabolism in the femoral-metaphyseal tissues of rats with
skeletal unloading. Calcif Tissue Int. 57:218–223. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sugiyama T, Tanaka H and Kawai S:
Improvement of periarticular osteoporosis in postmenopausal women
with rheumatoid arthritis by beta-alanyl-L-histidinato zinc: A
pilot study. J Bone Miner Metab. 18:335–338. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kato H, Ochiai-Shino H, Onoder S, Saito A,
Shibahara T and Azuma T: Promoting effect of 1,25(OH)2 vitamin D3
in osteogenic differentiation from induced pluripotent stem cells
to osteocyte-like cells. Open Biol. 5:1402012015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Seo HJ, Cho YE, Kim T, Shin H and Kwun IS:
Zinc may increase bone formation through stimulating cell
proliferation, alkaline phosphatase activity and collagen synthesis
in osteoblastic MC3T3-E1 cells. Nutr Res Pract. 4:356–361. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yamaguchi M, Goto M, Uchiyama S and
Nakagawa T: Effect of zinc on gene expression in osteoblastic
MC3T3-E1 cells: Enhancement of Runx2, OPG, and regucalcin mRNA
expressions. Mol Cell Biochem. 312:157–166. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yang Y, Wang Y, Kong Y, Zhang X, Zhang H,
Gang Y and Bai L: Carnosine prevents type 2 diabetes-induced
osteoarthritis through the ROS/NF-κB pathway. Front Pharmacol.
9:5982018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yamaguchi M and Uchiyama S: Receptor
activator of NF-kappaB ligand-stimulated osteoclastogenesis in
mouse marrow culture is suppressed by zinc in vitro. Int J Mol Med.
14:81–85. 2004.PubMed/NCBI
|
|
54
|
Thomas S and Jaganathan BG: Signaling
network regulating osteogenesis in mesenchymal stem cells. J Cell
Commun Signal. 16:47–61. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Amarasekara DS, Kim S and Rho J:
Regulation of osteoblast differentiation by cytokine networks. Int
J Mol Sci. 22:28512021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Lin W, Hu S, Li K, Shi Y, Pan C, Xu Z, Li
D, Wang H, Li B and Chen H: Breaking osteoclast-acid vicious cycle
to rescue osteoporosis via an acid responsive organic
framework-based neutralizing and gene editing platform. Small.
20:e23075952024. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Amin N, Clark CCT, Taghizadeh M and
Djafarnejad S: Zinc supplements and bone health: The role of the
RANKL-RANK axis as a therapeutic target. J Trace Elem Med Biol.
57:1264172020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Molenda M and Kolmas J: The role of zinc
in bone tissue health and regeneration-a review. Biol Trace Elem
Res. 201:5640–5651. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Ono T, Hayashi M, Sasaki F and Nakashima
T: RANKL biology: Bone metabolism, the immune system, and beyond.
Inflamm Regen. 40:22020. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Boyce BF and Xing L: Functions of
RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem
Biophys. 473:139–146. 2008. View Article : Google Scholar : PubMed/NCBI
|