Introduction
Fluid and blood transfusion-based resuscitation
after hemorrhagic shock (HSR) can result in damage to multiple
organs (1,2). Acute lung injury (ALI) is common and
severe (3,4). However, the treatment options for
managing lung injury are currently limited and primarily involve
ventilation volume and positional changes (5,6).
Thus, effective pharmacological treatments are limited (6–8). Our
previous research demonstrated an increase in inflammatory
cytokines and exacerbated apoptosis in the HSR-ALI model (9,10).
In patients with HSR, tissue hypoxia induces an early inflammatory
response characterized by the release of pro-inflammatory cytokines
such as tumor necrosis factor-α (TNF-α). This promotes neutrophil
infiltration and vascular endothelial disruption. During
reperfusion, the reintroduction of oxygen leads to the generation
of reactive oxygen and nitrogen species (ROS/RNS), which further
exacerbate inflammation and apoptosis (11). These findings suggest that
targeting inflammation, apoptosis, and ROS/RNS might offer
potential therapeutic strategies for mitigating HSR-induced lung
injury.
After hemorrhagic shock (HS), patients are exposed
to a hyper-catecholaminergic state characterized by enhanced
sympathetic nervous system activity (12). In particular, β1 adrenergic
receptor activation leads to increased heart rate and myocardial
contractility, which consequently elevates metabolic demand and
causes an imbalance between oxygen supply and demand. This
imbalance may exacerbate ischemia-reperfusion injury and trigger
systemic inflammation (13).
β1-adrenergic receptor stimulation has been reported to enhance the
secretion of pro-inflammatory cytokines in human monocytes and
induce apoptosis when stimulated by β1-selective agonists (14,15).
Therefore, β1-blockade is considered a promising strategy for
suppressing these detrimental responses. Notably, the β2 receptor
pathway is preserved even with the use of β1 blockers. The β2
receptor pathway includes the 5′ adenosine monophosphate-activated
protein kinase (AMPK) pathway, which contributes to sustaining
homeostasis; reducing oxidative stress, mitigating mitochondrial
dysfunction, suppressing inflammatory responses, and activating
autophagy to maintain normal bodily functions (13,16,17).
Landiolol hydrochloride, a highly selective β1
blocker, has been employed to manage tachycardia and reduce the
risk of atrial fibrillation during the perioperative phase and
cardiac decompensation. The β1/β2 receptor selectivity ratio of
landiolol (255) is substantially higher than those of commonly used
β-blockers such as esmolol (33),
atenolol (4.3), metoprolol (2.3), and propranolol (0.68), as shown
in Table I (18,19).
It also exhibits approximately eightfold greater β1-selectivity
than does esmolol, with minimal activity on β2 receptors (18). Owing to this pharmacological
profile, landiolol was selected in this study to evaluate its
effects on lung injury following HSR. Although primarily used as an
anti-arrhythmic drug, recent studies have reported that β1 blockers
offer organ protection, including lung protection (20–25).
These findings have been primarily observed in sepsis and
ischemia-reperfusion models. In the lipopolysaccharide (LPS) model,
landiolol treatment has been shown to enhance serum levels of
TNF-α, interleukin (IL)-6, and high mobility group box 1 (HMGB1),
along with reducing TNF-α expression in the liver (20,24).
Conversely, some studies have reported no alterations in serum
levels of TNF-α, IL-1β, and IL-6, or in TNF-α and IL-6 expression
in the lungs, resulting in inconsistent findings (21,25).
 | Table I.β1/β2 receptor selectivity ratios of
commonly used β-blockers. |
Table I.
β1/β2 receptor selectivity ratios of
commonly used β-blockers.
| β-blocker | β1/β2 selectivity
ratio |
|---|
| Landiolol | 255 |
| Esmolol | 33 |
| Atenolol | 4.3 |
| Metoprolol | 2.3 |
| Propranolol | 0.68 |
In this study, landiolol was administered following
HSR. To our knowledge, no prior research has examined the effects
of landiolol on inflammatory cytokines and lung apoptosis in the
HSR-ALI model. The study aimed to explore the potential of
landiolol treatment after HSR for protecting lung tissue by
decreasing inflammatory responses and apoptosis.
Materials and methods
Animals
This research received approval from the Department
of Animal Resources at the Advanced Science Research Center,
Okayama University (OKU-2021247 on April 1, 2021 and OKU-2023436 on
April 24, 2023; Okayama, Japan) and adhered to the Guidelines for
the Care and Use of Laboratory Animals based on ARRIVE (26) and the 2020 AVMA euthanasia
guidelines (27). Male
Sprague-Dawley rats, weighing 350–430 g (Clea Japan, Inc., Tokyo,
Japan), were kept in temperature-regulated rooms (25°C) with a 12-h
light/dark cycle and had unrestricted access to water and food
prior to the experiments. A total of sixty-nine rats were used in
this study. Five rats were assigned to a control group without
undergoing any procedures. Since there was no statistically
significant difference between the control and sham groups, data of
the control group were excluded from statistical analysis.
Twenty-four rats were excluded from the analysis. Of these
twenty-four rats, nineteen were excluded due to technical issues
encountered during the HS procedure. These issues included failure
to monitor blood pressure due to thrombosis, inability to achieve
the target hypotensive state due to insufficient bleeding, or
catheter dislodgement that prevented continuation of the
experiment. All of these complications occurred during the HS
procedure, and animals that could not proceed with the study were
promptly euthanized. The other five rats died naturally from
worsening hypotension during the HS procedure, despite attempts at
resuscitation. The remaining forty rats successfully completed the
experimental protocol and their data were included in the final
analysis. These were divided equally into two groups: twenty in the
3-h model (n=5 each, four groups) and twenty in the 24-h model (n=5
each, four groups). All animals that successfully underwent the HSR
procedure survived. The sample size was decided based on previous
studies (28,29).
All rats were numbered sequentially upon arrival and
housed under identical conditions in uniformly sized cages placed
in the same location. All experimental procedures were consistently
performed under the same environmental conditions and followed a
predetermined order. Group assignment and drug preparation were
carried out by H. Shimizu. R. Sakamoto was responsible for
performing the HSR and sham procedures. Both R. Sakamoto and H.
Shimizu jointly conducted sampling and data analysis. Information
on group assignments was disclosed to R. Sakamoto only after sample
collection completion. The rats were euthanized for sampling under
2–3% isoflurane anesthesia by performing a laparotomy and
exsanguination via the abdominal aorta.
All rats included were confirmed to be healthy and
alive before starting the experiment. Isoflurane was used
throughout all procedures to provide adequate sedation and
analgesia. Humane endpoints were predefined based on criteria such
as ≥20% body weight loss or a marked decrease in physical activity.
However, none of the animals that successfully completed the HSR
procedure met these exclusion criteria during the study course. No
special housing conditions were required.
Drug preparation
Prior to administration, landiolol (Landiolol
Hydrochloride, Ono Pharmaceutical Co., Ltd., Osaka, Japan) was
dissolved in saline and tailored to the correct dosage (100
µg/kg/min per body weight in 2 ml of saline). The dosage of
landiolol used in this study was determined with reference to
previously published studies (20,21,24,25).
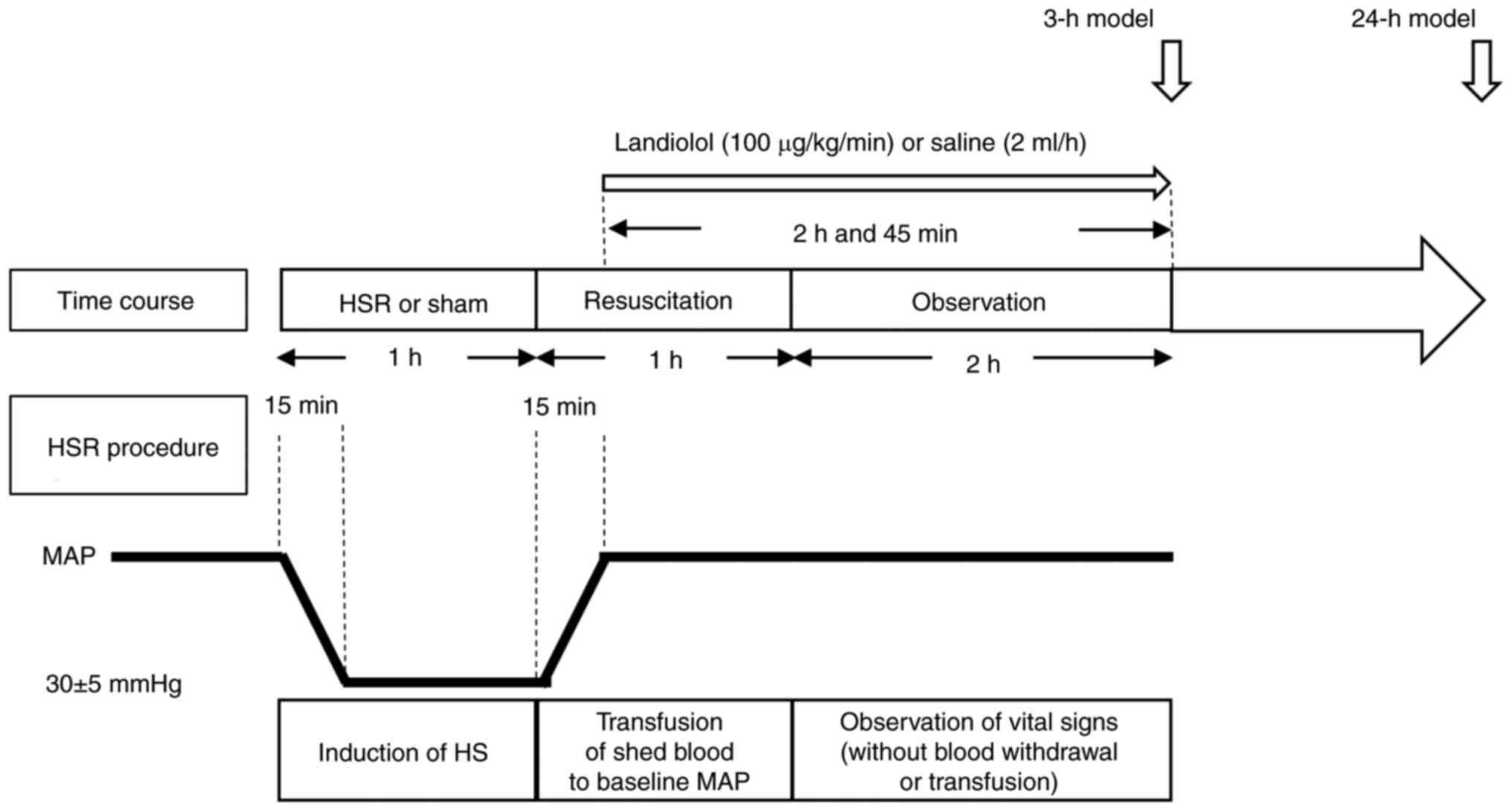
Hemorrhagic shock and resuscitation
(HSR) protocol
During the experiments, the rats were anesthetized
with isoflurane (0.8–2%) and underwent either sham or HSR surgery.
Inhalational anesthesia was performed in a chamber containing
isoflurane (4–5%) for induction. After confirming loss of both the
righting reflex and the tail pinch reflex, anesthesia was
maintained at a dosage of 0.8–2% via a face mask, in accordance
with the approved animal experiment protocol (OKU2023436), with the
most common concentrations being 5% for induction and 1.3 or 1% for
maintenance, during which anesthetic depth was assessed by the
absence of spontaneous movements. The vaporizer setting was reduced
to below 1% only when marked bradycardia (approximately half of the
normal heart rate) was observed. In the five rats that eventually
died, this bradycardia was followed within several minutes by
persistent hypotension, cardiac arrest and respiratory arrest.
During these events, the isoflurane concentration was initially
lowered and blood reinfusion was subsequently initiated in an
attempt to stabilize the hemodynamic condition. Although euthanasia
was also considered in these critical situations, it was difficult
to perform because the progression from bradycardia to cardiac and
respiratory arrest occurred within a very short period of time. The
concentration of isoflurane was verified using a vaporizer. The HSR
model was created as previously described (9,10,30,31).
The left femoral artery and vein were dissected using aseptic
procedures, and 22- and 24-gauge catheters were inserted,
respectively. The left femoral artery catheter was used to measure
blood pressure, whereas the left femoral vein catheter was used for
inducing HSR. After measuring the baseline blood pressure,
hemorrhage was initiated for over 15 min, aiming to maintain a mean
arterial blood pressure of 30 mmHg, which was achieved by bleeding
into a heparinized syringe (10 units/ml). The animals were
maintained at this blood pressure (30±5 mmHg) for 45 min through
additional blood withdrawal or shed blood infusion. Subsequently,
resuscitation was performed for 15 min by administering all the
shed blood until the pressure was restored to baseline levels. The
rats were kept under anesthesia for 2 h, during which only the
blood pressure and pulse rate were monitored (Fig. 1). The sham group underwent
identical procedures, except for bleeding. All rats maintained
spontaneous breathing throughout the experiment. All procedures
were performed on a heating pad, with continuous monitoring and
regulation to maintain the rectal temperature within the
physiological range.
Experimental design
To examine the effects of landiolol administration
on HSR-induced lung injury, the rats were randomly divided into the
following four groups: sham/saline (n=10), sham/landiolol (n=10),
HSR/saline (n=10), and HSR/landiolol (n=10). Landiolol (100
µg/kg/min) or vehicle (saline 2 ml/h) was injected into the tail
vein after HSR induction or sham surgery (Fig. 1). Continuous infusion was initiated
15 min after starting resuscitation and continued for 2 h and 45
min. The duration of administration was the same in both the 3 and
24-h models. At specific time points (3 h or 24 h) after
resuscitation, the animals were euthanized by phlebotomy under
isoflurane inhalation (2–3%). In our previous study, we found that
TNF-α mRNA expression peaked at 3 h after resuscitation in the rat
HSR-ALI model, supporting this time point for evaluating early
inflammatory gene expression (31). Meanwhile, previous studies have
shown that histological lung injury, lung wet/dry ratio, and
apoptosis markers such as cleaved caspase-3 are most prominent at
24 h post-insult in related ischemia-reperfusion injury models
(32,33). Therefore, the 24-h time point was
selected for assessing lung tissue damage and apoptosis. The left
lung was removed to determine the pulmonary wet-to-dry weight
ratio. The right upper lung was excised, quickly and gently rinsed
with saline, fixed in formalin, and stained for histological
analysis. Regarding the preparation of RNA and proteins, the right
middle and lower lungs were frozen immediately in liquid nitrogen
and stored at −80°C until further use.
Measurement of hemodynamic parameters
(blood pressure and heart rate)
To record the arterial pressure, an arterial
catheter was connected to a pressure transducer (Meritrans
DTSPlus® SCK-7874, Merit Medical Japan K.K, Shinjuku-ku,
Tokyo, Japan) attached to a bridge amplifier
(LifeScopeI® BSM-2303, Nihon Kohden Corporation,
Shinjuku-ku, Tokyo, Japan). The blood pressure and heart rate were
continuously recorded. The data were measured every 5 min and
saved.
Histopathological examination
After 24 h of resuscitation, the rats were
euthanized using the aforementioned method. Subsequently, the right
upper lobe of the lungs was excised and fixed in 10% neutral
buffered formalin, followed by paraffin embedding and sectioning at
a thickness of 5 µm for histological examinations. Following
deparaffinization and dehydration, the sections were stained with
hematoxylin and eosin (H&E) staining. The lung histological
changes were evaluated using blinded evaluation by five observers
using a light microscope in accordance with previously described
methods (34–36). In each rat, ten regions of lung
parenchyma were assessed on a scale from 0 (normal) to 3 (severe)
for four parameters: intravascular congestion, pulmonary edema,
inflammatory cell infiltration, and intra-alveolar hemorrhage. The
final results were reported as the median total score across these
parameters, with a maximum score of 30 for each parameter and 120
for the combined lung injury score.
Neutrophils in the lungs were stained using a
naphthol AS-D chloroacetate esterase staining kit (Sigma
Diagnostics, St. Louis, MO, USA) on sections adjacent to those used
for the histopathological analysis (30). An observer, blinded to the groups,
counted the positively stained cells in five nonconsecutive
sections per rat at ×400 magnification.
Terminal deoxynucleotidyl
transferase-mediated dUTP-fluorescein isothiocyanate nick-end
labeling staining
Transferase-mediated dUTP-fluorescein isothiocyanate
(FITC) nick-end labeling (TUNEL) staining was conducted using the
MEBSTAIN Apoptosis TUNEL Kit Direct (No. 8445; MBL, Nagano, Japan)
following the manufacturer's instructions. The sections were
briefly incubated with terminal deoxynucleotidyl transferase and
FITC-labeled dUTP, then counterstained with 0.5 µg/ml propidium
iodide. TUNEL-positive cells were counted in five non-consecutive
sections per rat at ×400 magnification by a blinded observer using
a Zeiss LSM510 confocal laser scanning microscope (Zeiss, Jena,
Germany).
Lung wet weight to dry weight
(wet/dry) ratio
The left lung tissue samples were collected 24 h
after resuscitation, weighed for their wet weight, and then dried
at 110°C for 24 h to obtain the dry weight. The wet-to-dry weight
ratio, calculated by dividing the wet weight by the dry weight, was
used as an indicator of pulmonary edema (9,31,37).
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
The total RNA was isolated from the lung tissues at
3 h after HSR using TRI REAGENT® (Molecular Research
Center, Inc., Cincinnati, OH, USA) following the manufacturer's
instructions. The total RNA was purified using the
RNeasy® Mini kit (Qiagen Sciences, Germantown, MD, USA).
After removing potentially contaminating DNA with DNase I
(RNase-Free DNase set; Qiagen GmbH, Hilden, Germany), reverse
transcription of the total RNA was performed using a
QuantiTect® Reverse Transcription Kit (Qiagen GmbH) to
generate first-strand cDNA. The PCR mixture was prepared using the
SYBR® Premix Ex Taq™ (Takara Bio Inc., Shiga, Japan).
PCR was performed using StepOnePlus (Thermo Fisher Scientific Inc.,
Waltham, MA, USA) according to the manufacturer's protocol. The
primer sequences for TNF-α, inducible nitric oxide synthase (iNOS),
and β-actin were as follows: 5′-GCCCTGGTATGAGCCCATGTA-3′ and
5′-CCTCACAGAGCAATGACTCCAAAG-3′ for TNF-α;
5′-CAAACTGTGTGCCTGGAGGTTC-3′ and 5′-AAGTAGGTGAGGGCTTGCCTGA-3′ for
iNOS; and 5′-AACCCTAAGGCCAACCGTGAA-3′ and
5′-CAGGGACAACACAGCCTGGA-3′ for β-actin, respectively. PCR
specificity was confirmed using melting curve analysis and DNA
sequencing. Quantification of gene expression was performed using a
standard curve method, as previously described (38,39).
The mRNA levels of TNF-α and iNOS were normalized to the mRNA level
of β-actin.
Protein extraction
The total proteins were extracted from a portion of
the right lung lobe collected 24 h after establishing the HSR
model, using Tissue Protein Extraction Reagent (T-PER) (Thermo
Fisher Scientific Inc.), according to the manufacturer's protocol.
Lung tissue was briefly homogenized in T-PER containing 5 mM
dithiothreitol, 5 mM ethylenediaminetetraacetic acid, a protease
inhibitor (cOmplete; Roche Diagnostics GmbH, Sigma-Aldrich, St.
Louis, MO, USA), and phosphatase inhibitors (PhosSTOP; Roche
Diagnostics GmbH, Sigma-Aldrich, St. Louis, MO, USA). The sample
was centrifuged at 10,000 × g for 30 min at 4°C, after which the
supernatants were collected and stored for later analysis. Protein
concentrations in the lung homogenates were measured using the
Pierce BCA™ Protein Assay Kit (Pierce, Rockford, IL, USA) following
the manufacturer's instructions, with readings taken on a Nivo 5
Multimode Microplate Reader (PerkinElmer, Shelton, CT, USA).
Western blot analysis
Samples with approximately 50 µg of protein were
loaded onto sodium dodecyl sulfate-polyacrylamide gels (10, 12, or
15% concentration) for electrophoresis, followed by the transfer of
proteins onto Amersham Hybond-PVDF membranes (GE Healthcare Life
Sciences, Chicago, IL, USA). The membranes were blocked at 25°C for
1 h using 4% (w/v) BlockAce (DS Pharma Biomedical Co., Ltd., Osaka,
Japan) or Blocking One-P (NACALAI TESQUE, Inc., Kyoto, Japan).
Subsequently, the membranes were incubated overnight at 4°C with
primary antibodies: cleaved caspase-3 [rabbit anti-cleaved
caspase-3 (Asp175), Cell Signaling, #9661, 1:500], Caspase-3
(rabbit anti-caspase-3, Cell Signaling, #9662, 1:1,000), GAPDH
[rabbit anti-GAPDH (FL 335), Santa Cruz, sc-25778, 1:5,000], pAMPKα
[rabbit anti-phosphorylated-AMPKα (Thr172)(40H9), Cell Signaling,
#2535, 1:1,000], and AMPKα (rabbit anti-AMPKα, Cell Signaling,
#2532 1:1,000). After washing in Tris-buffered saline with Tween
20, the membranes were incubated with secondary antibodies (goat
anti-rabbit IgG-HRP, Abcam, ab6721, 1:20,000 and anti-rabbit
IgG-HRP conjugate, Promega, W401B, 1:20,000) for 1 h at 25°C. The
membranes were then treated with Clarity Western ECL Substrate
(Bio-Rad, Hercules, CA, USA) following the manufacturer's
guidelines. Imaging was done with the ChemiDoc XRS Plus System
(Bio-Rad), with automatic exposure settings, and densitometry
analysis was conducted using Image Lab Version 5.0 software
(Bio-Rad).
Statistical analysis
The data are presented as the mean ± standard error
of the mean (SEM) or as the median (interquartile range), as
appropriate. Statistical analysis was performed using unpaired
Student's t-test, one-way analysis of variance (ANOVA) with
Tukey-Kramer multiple comparisons, or Kruskal-Wallis test followed
by Dunn's post hoc test for non-parametric data, as appropriate. A
two-sided P-value of <0.05 was considered statistically
significant. All analyses were performed using GraphPad Prism 10
(GraphPad Software Inc., San Diego, CA, USA).
Results
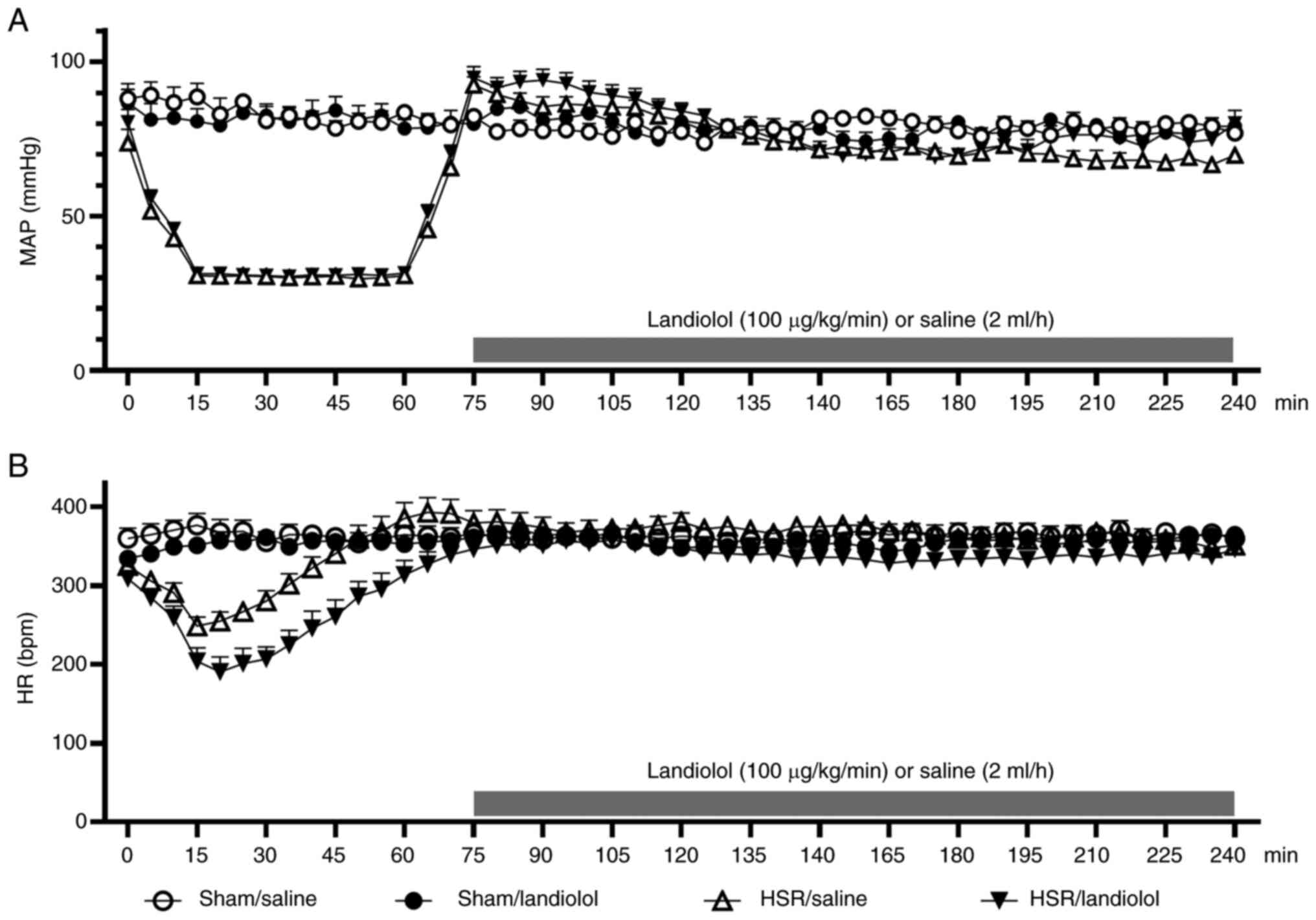
Landiolol administration does not
adversely affect the hemodynamic status after HSR
During the HS period, the mean arterial blood
pressure in the HSR groups was maintained within the target range
(30±5 mmHg) (Fig. 1). During the
subsequent resuscitation period, the heart rate and mean blood
pressure did not differ between the sham and HSR groups (Fig. 2A and B). However, during the
observation period after resuscitation, the HSR/saline group
exhibited a tendency toward decreased mean blood pressure compared
with that by the sham/saline group. In contrast, the HSR/landiolol
group showed no significant difference or tendency toward lower
blood pressure compared with that in the HSR/saline group. This
observation suggests that the HSR procedure could cause hypotension
after the procedure; however, landiolol administration did not
induce harmful blood pressure reduction. Regarding the heart rate,
no significant differences were observed among the four groups
after landiolol administration. No significant difference in blood
loss was observed between the HSR/saline and HSR/landiolol groups
(Fig. S1).
Effect of landiolol on HSR-Induced
lung histological damage
In this study, we examined the effect of landiolol
treatment on lung injury induced by HSR following HS.
Histopathological analysis showed that lung sections from the sham
group appeared approximately normal. In contrast, the HSR/saline
group displayed interstitial edema, marked by noticeable alveolar
septal thickening and inflammatory cell infiltration, 24 h after
HSR (Fig. 3A). Although there was
no statistically significant difference between the HSR/saline and
HSR/landiolol groups, the histopathological score in the
HSR/landiolol group was reduced to a level comparable with that of
the sham/saline group. Landiolol treatment after HSR tended to
attenuate these pathological changes, such as edema, inflammation,
and hemorrhage (Fig. 3A and B).
The impact of landiolol was further validated through
histopathological scoring by an independent, blinded researcher,
showing a decrease in the histopathological score (Fig. 3C).
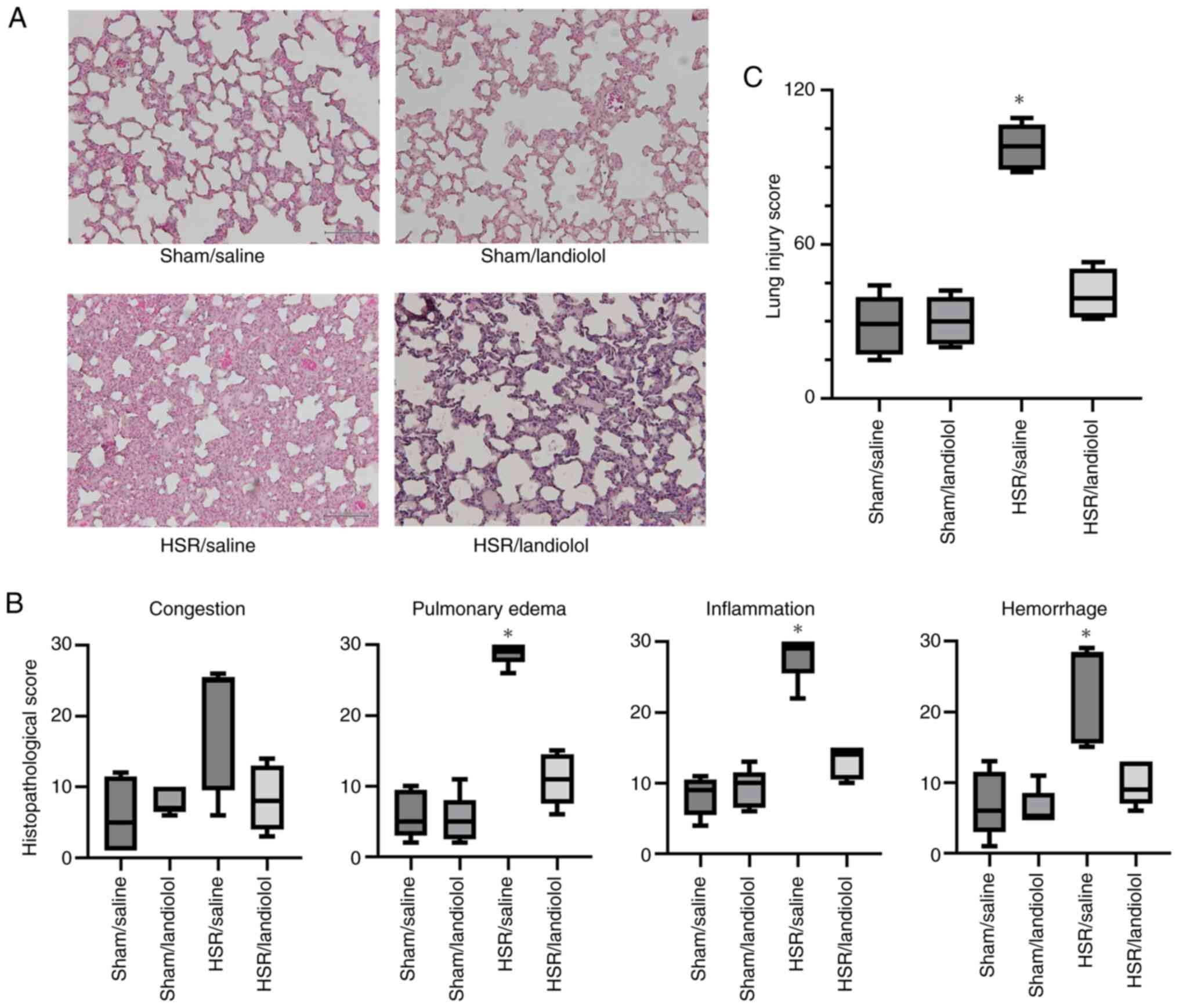 | Figure 3.Histological assessment of lung
injury following HSR. Lungs were collected from HSR model rats,
treated with or without landiolol, 24 h after resuscitation for
histological analysis. (A) Representative images from four
independent experiments (H&E staining; original magnification,
×200; scale bar, 100 µm). (B) The severity of histopathological
alterations in the lungs was scored for congestion, edema,
inflammation, and hemorrhage. Ten areas of lung parenchyma per rat
were rated on a scale from 0 (normal) to 3 (severe) for each
parameter, resulting in a maximum of 30 points per parameter.
Scoring was performed independently by five blinded observers, and
data are presented as median (interquartile range) and analyzed
using the Kruskal-Wallis test followed by Dunn's post hoc test. (C)
The total histopathological score was calculated by summing scores
across the four parameters, with a maximum possible score of 120.
Again, scoring was performed by five blinded observers, and the
median values (interquartile range) were analyzed using the
Kruskal-Wallis test followed by Dunn's post hoc test. The sum score
reflects the extent of lung tissue injury for each group.
*P<0.05 vs. sham/saline. Sham/saline and sham/landiolol groups
received sham surgery with saline or landiolol; HSR/saline and
HSR/landiolol groups underwent HSR with saline or landiolol
administration. HSR, hemorrhagic shock and resuscitation. |
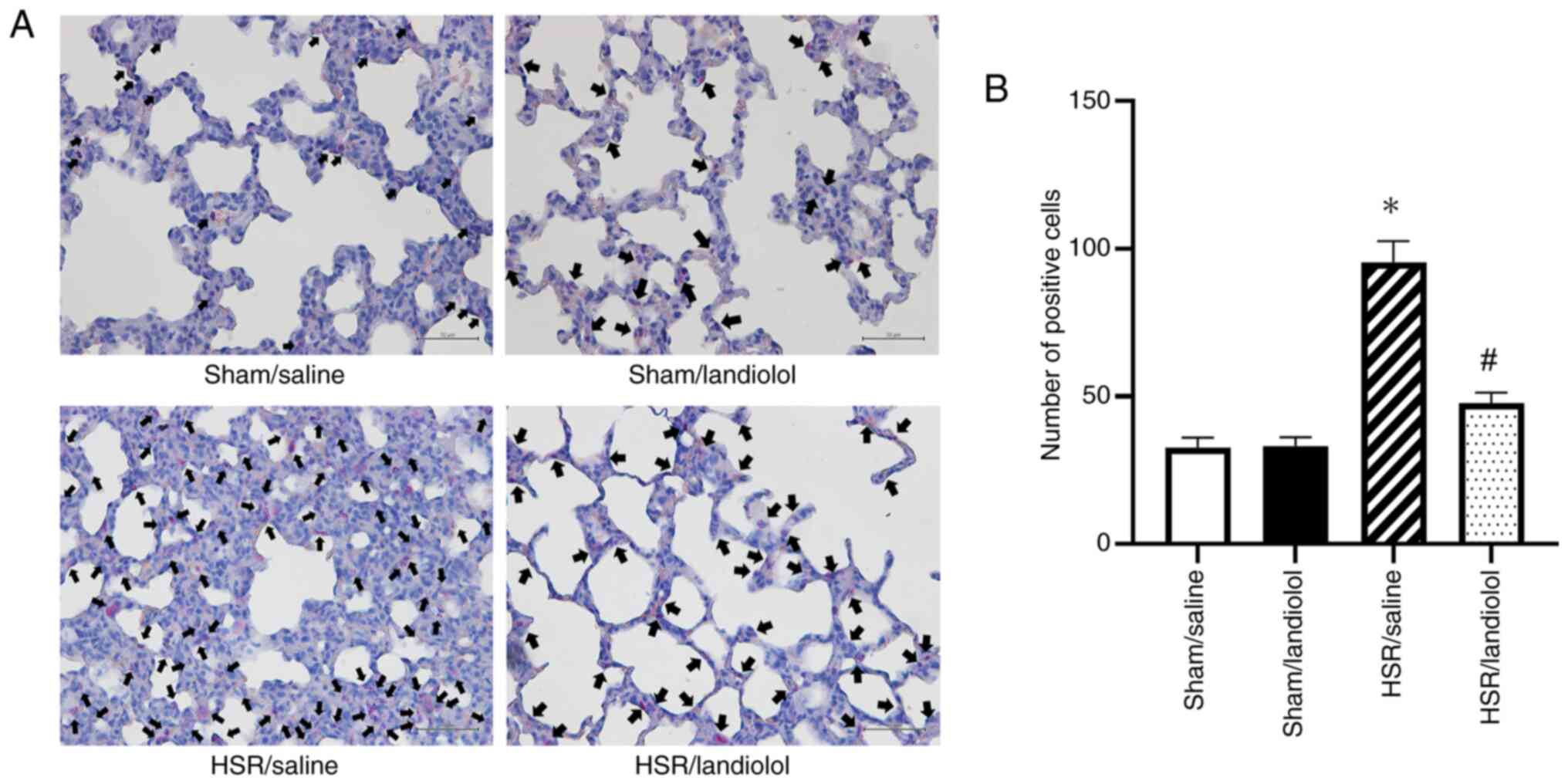
Effect of landiolol administration on
neutrophil accumulation in HSR-induced lung injury
To provide additional visual and quantitative
evidence of lung inflammation, neutrophils in the lung tissue were
stained and counted (Fig. 4A).
Neutrophil infiltration remained mild in the sham group, without
notable difference between the sham/saline and sham/landiolol
groups. However, in the HSR/saline group, the number of
infiltrating neutrophils was significantly higher 24 h after HSR
compared with the sham/saline and sham/landiolol groups. In
contrast, neutrophil infiltration was substantially lower in the
HSR/landiolol group than in the HSR/saline group (Fig. 4B). These neutrophil staining
results indicated that landiolol administration alleviated tissue
injury and decreased neutrophil infiltration.
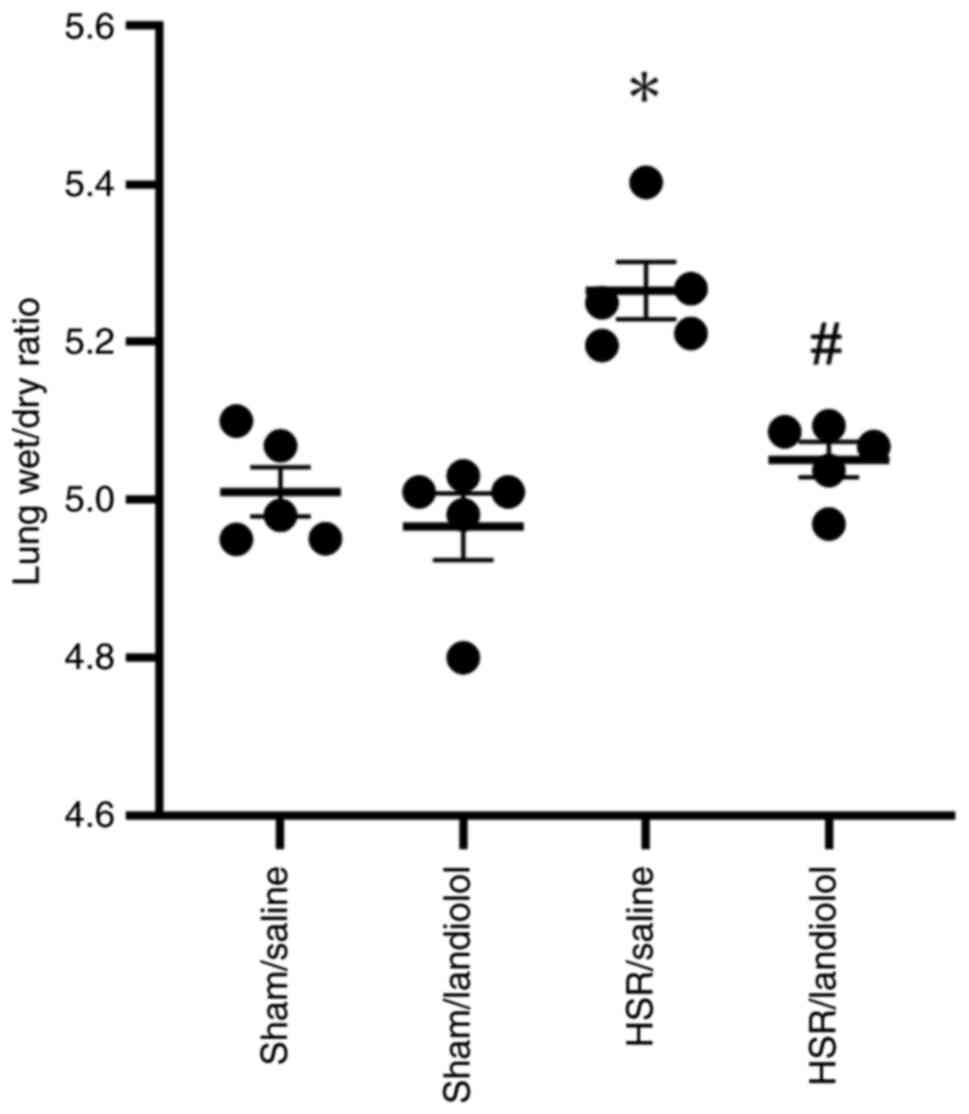
Effect of landiolol on lung wet/dry
weight ratio in HSR model rats
The lung wet/dry ratio, a parameter of lung edema,
was assessed in the 24 h models. Compared with those in the sham
groups, no differences were observed; the wet/dry ratio in the
sham/saline group was 5.01±0.03 and 4.97±0.04 in the sham/landiolol
group. The lung wet/dry ratio in the HSR/saline group significantly
increased compared with that in the sham groups (5.27±0.04).
However, landiolol administration significantly attenuated
HSR-induced lung edema, and the wet/dry ratio of HSR/landiolol was
statistically comparable to the sham groups (5.05±0.02) (Fig. 5). These results suggest that
landiolol administration could reduce lung edema in HSR model
rats.
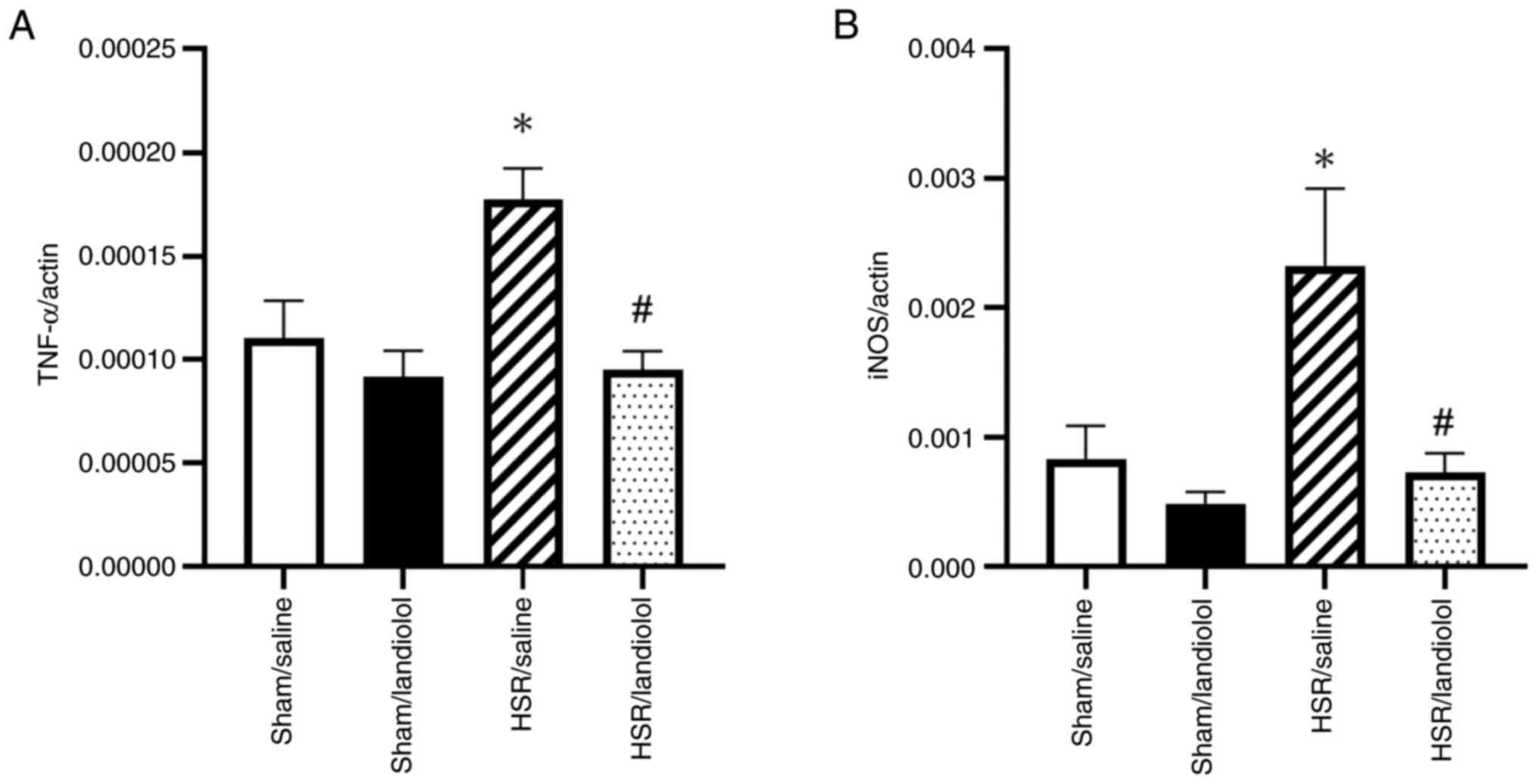
Effect of landiolol on gene
expressions of inflammatory mediators in HSR-induced lung
injury
To assess the anti-inflammatory effects of
landiolol, we analyzed the mRNA expression of TNF-α and iNOS in the
lungs 3 h after HSR using RT-qPCR. There were no statistically
significant differences in the levels of these inflammatory markers
between the sham groups. However, the HSR/saline group demonstrated
a significant increase in the TNF-α and iNOS expression compared
with that in the sham group. In contrast, landiolol administration
reduced the mRNA levels of TNF-α and iNOS by roughly 50 and 30%,
respectively, bringing them close to the levels observed in the
sham groups (Fig. 6A and B). These
findings indicate that landiolol administration lowered
inflammatory mRNA expression in the lungs of HSR model rats.
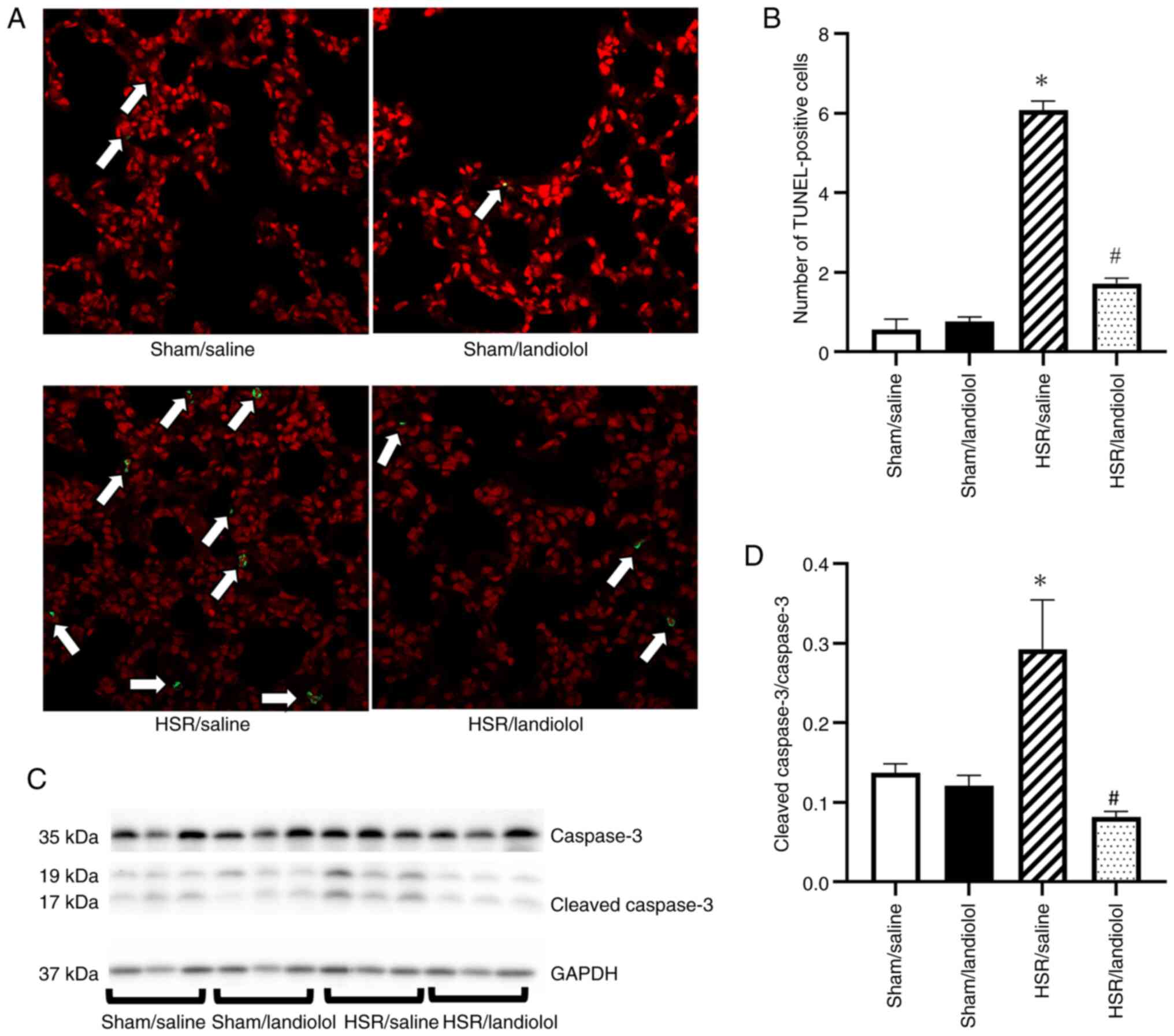
Effect of landiolol on apoptotic cell
death in HSR-induced lung injury
To assess apoptosis, we analyzed the TUNEL-positive
cells and cleaved caspase-3 expression in the lungs 24 h after HSR
using western blotting. The lung sections from the sham group had
very few TUNEL-positive cells, but their numbers increased
following the HSR procedure. In contrast, landiolol treatment
significantly reduced the number of TUNEL-positive cells compared
with the HSR/saline group (Fig. 7A and
B). Similarly, cleaved caspase-3 expression was minimally
detectable in the sham group but increased in the HSR group.
However, landiolol treatment effectively inhibited the increase in
cleaved caspase-3 (Fig. 7C and
D).
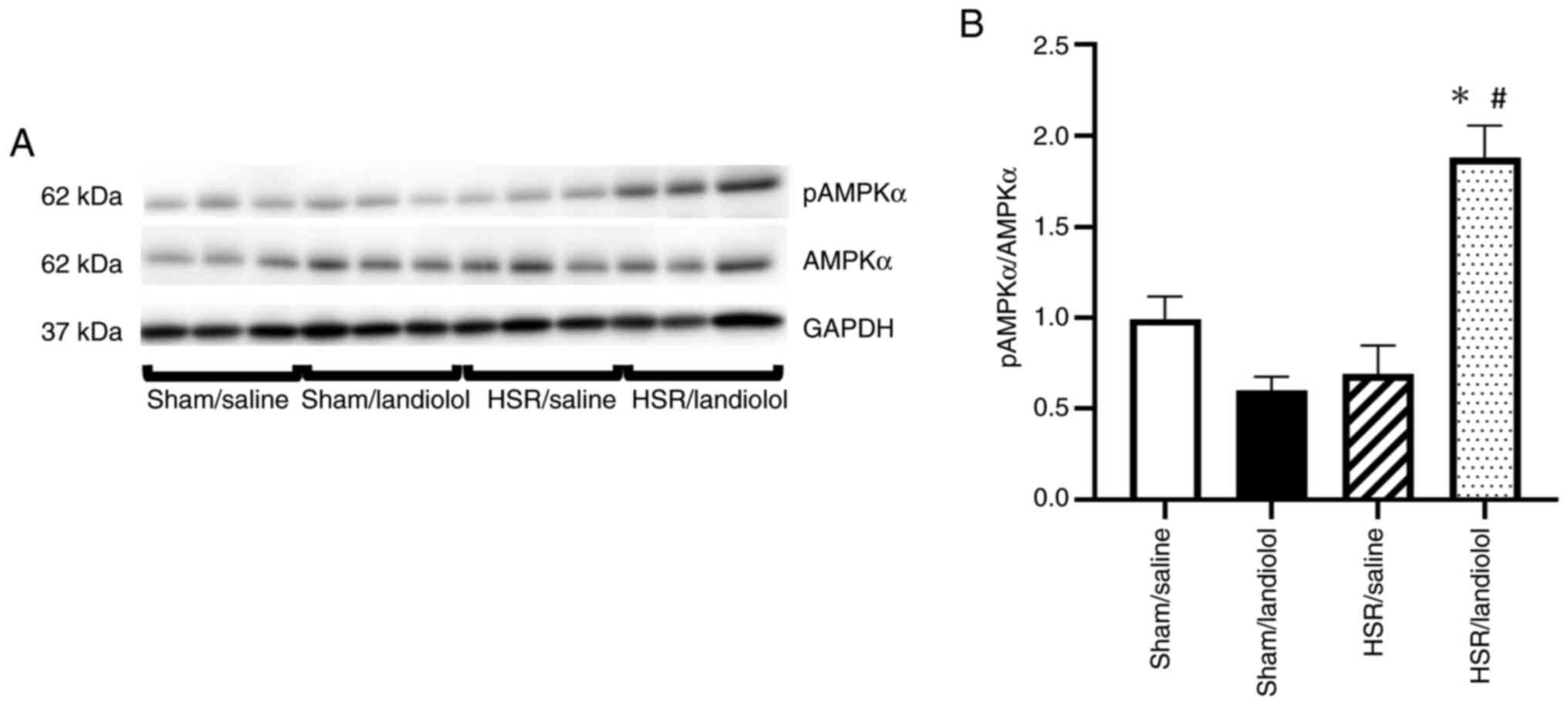
Effect of landiolol on the AMPK
pathway in the lungs following HSR
We examined pAMPKα via western blotting. pAMPKα is a
protein involved in mitochondrial biogenesis, autophagy, and
related processes. Regarding pAMPKα, we found no significant
differences between the sham/saline and sham/landiolol groups. The
HSR/saline group also showed no differences compared with the sham
group. However, landiolol treatment was found to increase pAMPKα
expression compared with the other three groups (Fig. 8A and B).
Discussion
This study showed that intravenous administration of
landiolol at a dose of 100 µg/kg/min following the HSR procedure
substantially alleviated lung injury induced by HSR. This finding
was supported by a trend toward reductions in histological
alterations, and was further substantiated by significant
reductions in neutrophil infiltration and lung edema. Furthermore,
landiolol administration markedly lowered the mRNA expression of
inflammatory mediators, including TNF-α and iNOS. Additionally,
landiolol administration reduced apoptotic cell death, as shown by
a decrease in the TUNEL-positive cells and cleaved caspase-3
expression. We investigated the pAMPKα expression as a mechanism
for the anti-inflammatory and anti-apoptotic effects of landiolol
and found by western blotting that pAMPKα expression was increased
when landiolol was administered after HSR procedures. In addition,
landiolol administration following the HSR procedure had no
discernible effect on the mean arterial blood pressure.
TNF-α promotes neutrophil infiltration and increases
vascular permeability, leading to endothelial injury. Neutrophils
act on vascular endothelial cells and produce superoxides and other
ROS, which cause cellular injury and edema (40). This endothelial disruption induces
the expression of iNOS, which generates reactive nitrogen species
such as nitric oxide, thereby exacerbating oxidative injury during
reperfusion (41). Furthermore,
endothelial damage results in vascular leakage, which exacerbates
tissue edema in the lungs (11).
Targeting upstream inflammatory mediators such as TNF-α,
iNOS-induced vascular injury, and neutrophil-mediated permeability
enhancement may help attenuate lung damage. For this reason, these
factors were selected as therapeutic targets in our study.
Landiolol is a drug used for treating arrhythmia;
however, previous studies have shown that landiolol treatment has
lung-protective effects. In a rat LPS model, Hagiwara et al
reported that landiolol administration reduced HMGB1 expression in
the lungs and serum and alleviated histological lung injury
(20). Matsuishi et al
reported that landiolol ameliorated histological findings and
PaO2 levels in a rat ALI model of early sepsis by
suppressing elevated levels of pulmonary endothelin-1 (25). Notably, our research is the first
to suggest that landiolol administration may play a protective role
against ALI in a non-septic HSR model. Considering our findings and
those of other studies (20,25),
landiolol appears to be highly effective in protecting the lungs
from ALI.
When investigating the mechanisms underlying the
protective effects of landiolol on ALI following HSR, we observed
that landiolol administration suppressed HSR-induced expression of
inflammatory genes. This finding aligns with those of previous
reports. Hagiwara et al showed that landiolol administration
reduces TNF-α and IL-6 levels in the serum of the LPS rat model
(20). Yoshino showed that
landiolol administration to the LPS rat model improves TNF-α
expression levels in the liver (24). Furthermore, Ackland et al
reported that the β1 adrenergic receptor blocker metoprolol
suppresses the expression of IFN-γ, IL-1β, IL-6, TNF-α, IL-18, and
MCP-1 in the liver when administered to the LPS rat model (42). The findings of our study also
indicated that landiolol administration decreased the expression of
inflammatory genes in the HSR-induced ALI model. Thus, landiolol
exhibits anti-inflammatory effects, representing a key mechanism in
its protective action against lung injury.
Our study demonstrated that landiolol inhibited
apoptosis in HSR-induced lung injury. Previous reports have also
shown that β1-blockers may have anti-apoptotic effects. Zaugg et
al reported that a β1 blocker atenolol suppressed
TUNEL-positive cells and increased the expression of the
anti-apoptotic protein Bcl2 in catecholamine-induced apoptosis
(15). Hashemi et al
reported that atenolol administration increases MEF2
transcriptional activity, which is involved in the anti-apoptotic
effects of catecholamine-induced apoptosis in rat cardiomyocytes
(43). Additionally, Taha et
al reported that the administration of atenolol suppressed the
gene expression of the pro-apoptosis protein caspase 1 and
increases the expression of Bcl2 in a rat intestinal
ischemia-reperfusion model (44).
These reports and our experimental results suggest that β1-blocker
has anti-apoptotic effects. Although the precise mechanism
underlying the anti-apoptotic effects of landiolol could not be
fully elucidated in this study, increased p-AMPKα expression
observed in the HSR/Landiolol group suggests a potential role of
AMPK pathway activation. AMPK is known to suppress oxidative
stress, promote autophagy, and preserve mitochondrial function, all
of which may contribute to the observed reduction in apoptosis
(16,17). In addition, the AMPK/Nrf2 signaling
pathway has been reported as a potential therapeutic target for
treating ALI. Activation of this pathway may upregulate downstream
effectors such as heme oxygenase-1 and modulate macrophage
activity, thereby attenuating inflammation (45). Further investigation into the
upstream and downstream signaling pathways of AMPK is warranted in
future studies. Additionally, in vitro studies have reported
that landiolol can scavenge multiple types of free radicals, which
may also contribute to its anti-apoptotic properties (46).
Our study had some limitations. First, our model
does not comply with the Berlin definition regarding the disuse of
positive end-expiratory pressure and image evaluations, oxygenation
index, pulmonary function indicator (47). However, conforming to this
definition for animal experiments is challenging. Therefore, we
performed the experiments according to the ALI definition for
animal experiments (48). The
severity of ALI for the animals was approximated using H&E
staining, neutrophil staining, and the wet/dry ratio. Second, this
HSR model is difficult to establish, with a mortality rate of
approximately 11% during the HS procedure, and several animals were
excluded due to complications. Third, we lacked survival rate data.
Although the mortality during the HS procedure was relatively high,
all rats that successfully underwent HSR treatment survived,
indicating that this represents a mild lung injury model. Forth,
the primary aim of this study was to assess the lung-protective
effects of landiolol administration; however, the detailed
mechanisms underlying these effects were not fully elucidated.
Previous studies have demonstrated the organ-protective role of
AMPK using Compound C, a specific AMPK inhibitor (49–51).
In the future, we plan to investigate whether the AMPK pathway
contributes to the protective effects observed in this study by
conducting validation experiments using Compound C. This study was
conducted using only male rats to ensure consistency with previous
experiments (9,10,28–31).
The potential influence of sex differences on the outcomes was not
assessed and remains a subject for future investigation.
In conclusion, landiolol administration after HSR
improved HSR-induced ALI through its anti-inflammatory and
anti-apoptotic effects, at least in part, mediated by the
activation of the AMPK pathway without severe hypotension. Further
research is required to elucidate the exact mechanisms and
pharmacological characteristics of landiolol. Thus, landiolol
administration may be a therapeutic approach for acute lung injury
following HSR.
Supplementary Material
Supporting Data
Acknowledgements
The StepOnePlus and Zeiss LSM510 devices used in
this study were obtained from the Central Research Laboratory,
Okayama University Medical School (Okayama, Japan). The authors
would also like to thank Mr. Kosuke Iguchi, Ms. Misako Yanagita and
Ms. Shukuko Wani (medical students at Okayama University, Okayama,
Japan) for their technical support.
Funding
This work was supported by a Japan Society for the Promotion of
Science Grant-in-Aid for Scientific Research (grant nos. JP19K09381
and JP23K08360).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
RS, HS and TT designed the study. RS wrote the first
draft of the manuscript. HS and HM critically revised the
manuscript for intellectual content. RS, HS, YLu and EO performed
the experiments. RS and HS confirm the authenticity of all the raw
data. RS, HS, YLi, RN and EO performed histological scoring, which
was conducted in a blinded manner. RS, HS, TT and HM analyzed and
interpreted the data. RS and HS performed the statistical analyses.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The animal experimental procedures in the present
study were approved by the Department of Animal Resources, Advanced
Science Research Center, Okayama University (approval no.
OKU-2021247 on April 1, 2021 and approval no. OKU-2023436 on April
24, 2023).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Use of artificial intelligence tools
During the preparation of this work, AI tools
(ChatGPT and Consensus) were used to improve the readability and
language of the manuscript, and subsequently, the authors revised
and edited the content produced by the AI tools as necessary,
taking full responsibility for the ultimate content of the present
manuscript.
Glossary
Abbreviations
Abbreviations:
|
ALI
|
acute lung injury
|
|
AMPK
|
5′ adenosine monophosphate-activated
protein kinase
|
|
ANOVA
|
analysis of variance
|
|
iNOS
|
inducible nitric oxide synthase
|
|
H&E
|
hematoxylin and eosin
|
|
HSR
|
hemorrhagic shock and
resuscitation
|
|
MAP
|
mean arterial blood pressure
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
SEM
|
standard error of the mean
|
|
TNF-α
|
tumor necrosis factor-α
|
|
IL
|
interleukin
|
|
TUNEL
|
terminal deoxynucleotidyl transferase
dUTP nick-end labeling
|
|
FITC
|
fluorescein isothiocyanate
|
|
HMGB1
|
high mobility group box 1
|
|
LPS
|
lipopolysaccharide
|
References
|
1
|
Dewar D, Moore FA, Moore EE and Balogh Z:
Postinjury multiple organ failure. Injury. 40:912–918. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ciesla DJ, Moore EE, Johnson JL, Cothren
CC, Banerjee A, Burch JM and Sauaia A: Decreased progression of
postinjury lung dysfunction to the acute respiratory distress
syndrome and multiple organ failure. Surgery. 140:640–648. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ware LB: Pathophysiology of acute lung
injury and the acute respiratory distress syndrome. Semin Respir
Crit Care Med. 27:337–349. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
ARDS Definition Task Force, . Ranieri VM,
Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E,
Camporota L and Slutsky AS: Acute respiratory distress syndrome:
The Berlin definition. JAMA. 307:2526–2533. 2012.PubMed/NCBI
|
|
5
|
Acute Respiratory Distress Syndrome
Network, . Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson
BT and Wheeler A: Ventilation with lower tidal volumes as compared
with traditional tidal volumes for acute lung injury and the acute
respiratory distress syndrome. N Engl J Med. 342:1301–1308. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tonelli AR, Zein J, Adams J and Ioannidis
JPA: Effects of interventions on survival in acute respiratory
distress syndrome: An umbrella review of 159 published randomized
trials and 29 meta-analyses. Intensive Care Med. 40:769–787. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Adhikari N, Burns KEA and Meade MO:
Pharmacologic therapies for adults with acute lung injury and acute
respiratory distress syndrome. Cochrane Database Syst Rev.
2004:CD0044772004.PubMed/NCBI
|
|
8
|
Peter JV, John P, Graham PL, Moran JL,
George IA and Bersten A: Corticosteroids in the prevention and
treatment of acute respiratory distress syndrome (ARDS) in adults:
Meta-analysis. BMJ. 336:1006–1009. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kanagawa F, Takahashi T, Inoue K, Shimizu
H, Omori E, Morimatsu H, Maeda S, Katayama H, Nakao A and Morita K:
Protective effect of carbon monoxide inhalation on lung injury
after hemorrhagic shock/resuscitation in rats. J Trauma.
69:185–194. 2010.PubMed/NCBI
|
|
10
|
Kumada Y, Takahashi T, Shimizu H, Nakamura
R, Omori E, Inoue K and Morimatsu H: Therapeutic effect of carbon
monoxide-releasing molecule-3 on acute lung injury after
hemorrhagic shock and resuscitation. Exp Ther Med. 17:3429–3440.
2019.PubMed/NCBI
|
|
11
|
Esiobu P and Childs EW: A rat model of
hemorrhagic shock for studying vascular hyperpermeability. Methods
Mol Biol. 1717:53–60. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Peitzman AB, Billiar TR, Harbrecht BG,
Kelly E, Udekwu AO and Simmons RL: Hemorrhagic shock. Curr Probl
Surg. 32:925–1002. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pérez-Schindler J, Philp A and
Hernandez-Cascales J: Pathophysiological relevance of the cardiac
β2-adrenergic receptor and its potential as a therapeutic target to
improve cardiac function. Eur J Pharmacol. 698:39–47. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Grisanti LA, Evanson J, Marchus E,
Jorissen H, Woster AP, DeKrey W, Sauter ER, Combs CK and Porter JE:
Pro-inflammatory responses in human monocytes are beta1-adrenergic
receptor subtype dependent. Mol Immunol. 47:1244–1254. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zaugg M, Xu W, Lucchinetti E, Shafiq SA,
Jamali NZ and Siddiqui MA: Beta-adrenergic receptor subtypes
differentially affect apoptosis in adult rat ventricular myocytes.
Circulation. 102:344–350. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Aslam M and Ladilov Y: Emerging role of
cAMP/AMPK signaling. Cells. 11:3082022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ding R, Wu W, Sun Z and Li Z:
AMP-activated protein kinase: An attractive therapeutic target for
ischemia-reperfusion injury. Eur J Pharmacol. 888:1734842020.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Plosker GL: Landiolol: A review of its use
in intraoperative and postoperative tachyarrhythmias. Drugs.
73:959–977. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bennett M, Chang CL, Tatley M, Savage R
and Hancox RJ: The safety of cardioselective β1-blockers
in asthma: Literature review and search of global pharmacovigilance
safety reports. ERJ Open Res. 7:00801–2020. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hagiwara S, Iwasaka H, Maeda H and Noguchi
T: Landiolol, an ultrashort-acting beta1-adrenoceptor antagonist,
has protective effects in an LPS-induced systemic inflammation
model. Shock. 31:515–520. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kiyonaga N, Moriyama T and Kanmura Y:
Effects of landiolol in lipopolysaccharide-induced acute kidney
injury in rats and in vitro. Shock. 52:e117–e123. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Goyagi T, Kimura T, Nishikawa T, Tobe Y
and Masaki Y: Beta-adrenoreceptor antagonists attenuate brain
injury after transient focal ischemia in rats. Anesth Analg.
103:658–663. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Iwata M, Inoue S, Kawaguchi M, Nakamura M,
Konishi N and Furuya H: Posttreatment but not pretreatment with
selective beta-adrenoreceptor 1 antagonists provides
neuroprotection in the hippocampus in rats subjected to transient
forebrain ischemia. Anesth Analg. 110:1126–1132. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yoshino Y, Jesmin S, Islam M, Shimojo N,
Sakuramoto H, Oki M, Khatun T, Suda M, Kawano S and Mizutani T:
Landiolol hydrochloride ameliorates liver injury in a rat sepsis
model by down regulating hepatic TNF-A. J Vasc Med Surg.
3:10001942015.
|
|
25
|
Matsuishi Y, Jesmin S, Kawano S, Hideaki
S, Shimojo N, Mowa CN, Akhtar S, Zaedi S, Khatun T, Tsunoda Y, et
al: Landiolol hydrochloride ameliorates acute lung injury in a rat
model of early sepsis through the suppression of elevated levels of
pulmonary endothelin-1. Life Sci. 166:27–33. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Percie du Sert N, Hurst V, Ahluwalia A,
Alam S, Avey MT, Baker M, Browne WJ, Clark A, Cuthill IC, Dirnagl
U, et al: The ARRIVE guidelines 2.0: Updated guidelines for
reporting animal research. J Cereb Blood Flow Metab. 40:1769–1777.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Leary S, Underwood W, Anthony R, Cartner
S, Grandin T, Greenacre C, Gwaltney-Brant S, McCrackin MA, Meyer R,
et al: AVMA Guidelines for the Euthanasia of Animals: 2020 Edition.
American Veterinary Medical Association; Schaumburg, IL, USA: pp.
1–121. 2020, https://www.avma.org/resources-tools/avma-policies/avma-guidelines-euthanasia-animalsMarch
1–2021
|
|
28
|
Kosaka J, Morimatsu H, Takahashi T,
Shimizu H, Kawanishi S, Omori E, Endo Y, Tamaki N, Morita M and
Morita K: Effects of biliverdin administration on acute lung injury
induced by hemorrhagic shock and resuscitation in rats. PLoS One.
8:e636062013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li Y, Shimizu H, Nakamura R, Lu Y,
Sakamoto R, Omori E, Takahashi T and Morimatsu H: The protective
effect of carbamazepine on acute lung injury induced by hemorrhagic
shock and resuscitation in rats. PLoS One. 19:e03096222024.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Umeda K, Takahashi T, Inoue K, Shimizu H,
Maeda S, Morimatsu H, Omori E, Akagi R, Katayama H and Morita K:
Prevention of hemorrhagic shock-induced intestinal tissue injury by
glutamine via heme oxygenase-1 induction. Shock. 31:40–49. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Maeshima K, Takahashi T, Uehara K, Shimizu
H, Omori E, Yokoyama M, Tani T, Akagi R and Morita K: Prevention of
hemorrhagic shock-induced lung injury by heme arginate treatment in
rats. Biochem Pharmacol. 69:1667–1680. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang Z, Zhang XR, Zhao Q, Wang SL, Xiong
LL, Zhang P, Yuan B, Zhang ZB, Fan SY, Wang TH and Zhang YH:
Knockdown of TNF-α alleviates acute lung injury in rats with
intestinal ischemia and reperfusion injury by upregulating IL-10
expression. Int J Mol Med. 42:926–934. 2018.PubMed/NCBI
|
|
33
|
Hassoun HT, Lie ML, Grigoryev DN, Liu M,
Tuder RM and Rabb H: Kidney ischemia-reperfusion injury induces
caspase-dependent pulmonary apoptosis. Am J Physiol Renal Physiol.
297:F125–F137. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Murakami K, McGuire R, Cox RA, Jodoin JM,
Bjertnaes LJ, Katahira J, Traber LD, Schmalstieg FC, Hawkins HK,
Herndon DN and Traber DL: Heparin nebulization attenuates acute
lung injury in sepsis following smoke inhalation in sheep. Shock.
18:236–241. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zegdi R, Fabre O, Cambillau M, Fornès P,
Tazi KA, Shen M, Hervé P, Carpentier A and Fabiani JN: Exhaled
nitric oxide and acute lung injury in a rat model of extracorporeal
circulation. Shock. 20:569–574. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jiang H, Meng F, Li W, Tong L, Qiao H and
Sun X: Splenectomy ameliorates acute multiple organ damage induced
by liver warm ischemia reperfusion in rats. Surgery. 141:32–40.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Stephens KE, Ishizaka A, Larrick JW and
Raffin TA: Tumor necrosis factor causes increased pulmonary
permeability and edema. Comparison to septic acute lung injury. Am
Rev Respir Dis. 137:1364–1370. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nakatsuka K, Matsuoka Y, Kurita M, Wang R,
Tsuboi C, Sue N, Kaku R and Morimatsu H: Intrathecal administration
of the α1 adrenergic antagonist phentolamine upregulates spinal
GLT-1 and improves mirror image pain in SNI model rats. Acta Med
Okayama. 76:255–263. 2022.PubMed/NCBI
|
|
40
|
McMillen MA, Huribal M and Sumpio B:
Common pathway of endothelial-leukocyte interaction in shock,
ischemia, and reperfusion. Am J Surg. 166:557–562. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cinelli MA, Do HT, Miley GP and Silverman
RB: Inducible nitric oxide synthase: Regulation, structure, and
inhibition. Med Res Rev. 40:158–189. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ackland GL, Yao ST, Rudiger A, Dyson A,
Stidwill R, Poputnikov D, Singer M and Gourine AV:
Cardioprotection, attenuated systemic inflammation, and survival
benefit of beta1-adrenoceptor blockade in severe sepsis in rats.
Crit Care Med. 38:388–394. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hashemi S, Salma J, Wales S and McDermott
JC: Pro-survival function of MEF2 in cardiomyocytes is enhanced by
β-blockers. Cell Death Discov. 1:150192015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Taha MO, Silva TDMAE, Ota KS, Vilela WJ,
Simões RS, Starzewski A and Fagundes DJ: The role of atenolol in
the modulation of the expression of genes encoding pro-(caspase-1)
and anti-(Bcl2L1) apoptotic proteins in endothelial cells exposed
to intestinal ischemia and reperfusion in rats. Acta Cir Bras.
33:1061–1066. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Huang Q, Ren YX, Yuan P, Huang M, Liu G,
Shi Y, Jia G and Chen M: Targeting the AMPK/Nrf2 pathway: A novel
therapeutic approach for acute lung injury. J Inflamm Res.
17:4683–4700. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Matsumoto S, Tokumaru O, Ogata K,
Kuribayashi Y, Oyama Y, Shingu C, Yokoi I and Kitano T:
Dose-dependent scavenging activity of the ultra-short-acting
β1-blocker landiolol against specific free radicals. J Clin Biochem
Nutr. 71:185–190. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ferguson ND, Fan E, Camporota L, Antonelli
M, Anzueto A, Beale R, Brochard L, Brower R, Esteban A, Gattinoni
L, et al: The Berlin definition of ARDS: An expanded rationale,
justification, and supplementary material. Intensive Care Med.
38:1573–1582. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Matute-Bello G, Downey G, Moore BB,
Groshong SD, Matthay MA, Slutsky AS and Kuebler WM; Acute Lung
Injury in Animals Study Group, : An official American Thoracic
Society workshop report: Features and measurements of experimental
acute lung injury in animals. Am J Respir Cell Mol Biol.
44:725–738. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Escobar DA, Botero-Quintero AM, Kautza BC,
Luciano J, Loughran P, Darwiche S, Rosengart MR, Zuckerbraun BS and
Gomez H: Adenosine monophosphate-activated protein kinase
activation protects against sepsis-induced organ injury and
inflammation. J Surg Res. 194:262–272. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Jiang WL, Zhao KC, Yuan W, Zhou F, Song
HY, Liu GL, Huang J, Zou JJ, Zhao B and Xie SP: MicroRNA-31-5p
exacerbates lipopolysaccharide-induced acute lung injury via
inactivating Cab39/AMPKα pathway. Oxid Med Cell Longev.
2020:88223612020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chen R, Cao C, Liu H, Jiang W, Pan R, He
H, Ding K and Meng Q: Macrophage Sprouty4 deficiency diminishes
sepsis-induced acute lung injury in mice. Redox Biol.
58:1025132022. View Article : Google Scholar : PubMed/NCBI
|