Introduction
Osteoarthritis (OA) is a prevalent chronic joint
disorder that poses a notable global health burden. With the aging
population, the prevalence of OA is expected to rise, making it a
critical public health issue (1).
According to recent statistics, OA affects >300 million people
worldwide, with a higher prevalence in individuals aged ≥60 years
(1). The economic cost of OA is
substantial, with direct medical expenses and indirect costs due to
lost productivity estimated at billions of dollars annually; this
underscores the urgent need for effective therapeutic strategies to
manage and potentially reverse OA progression (2).
The pathophysiological hallmarks of OA include
articular cartilage degeneration, abnormal bone remodeling and
synovial inflammation (3).
Cartilage degeneration is characterized by the loss of chondrocytes
and the breakdown of the extracellular matrix, leading to reduced
joint function and increased pain (4). Abnormal bone remodeling manifests as
subchondral bone sclerosis and the formation of osteophytes, which
further contribute to joint dysfunction (5). Synovial inflammation is characterized
by the infiltration of inflammatory cells and the production of
proinflammatory cytokines such as IL-1β) and tumor necrosis
factor-α (TNF-α), exacerbating cartilage and bone damage. These
pathological changes are interconnected, creating a vicious cycle
that accelerates OA progression (5).
Despite the notable burden of OA, traditional
treatments have limitations (6).
Current therapeutic approaches primarily focus on symptom
management, such as pain relief and improved joint function, rather
than addressing the underlying disease mechanisms (7). Most research has concentrated on
local factors within the joint microenvironment, such as
chondrocyte apoptosis and inflammatory mediators (8,9).
However, these approaches have shown limited efficacy in halting or
reversing OA progression; therefore, there is a growing recognition
of the need to explore systemic factors that may influence OA
development and progression.
One such systemic factor is the gut microbiome,
which has emerged as a potential regulator of OA through the
gut-bone-cartilage axis (10,11).
The gut microbiome comprises trillions of microorganisms that serve
crucial roles in host metabolism, immune function and inflammation
(12). Previous studies have shown
that the gut microbiota and its metabolites can influence bone and
cartilage homeostasis through various mechanisms, including immune
modulation and signaling pathways such as Wnt/β-catenin (10,13–15).
For example, animal studies have demonstrated that alterations in
the gut microbiota composition can lead to changes in bone density
and cartilage integrity, suggesting a potential link between gut
dysbiosis and OA development (11,16).
Additionally, the Wnt/β-catenin signaling pathway has been
implicated in OA pathogenesis, with a study showing that its
dysregulation can contribute to cartilage degeneration and bone
remodeling (17). These findings
highlight the potential of targeting the gut microbiome and
Wnt/β-catenin signaling as novel therapeutic strategies for OA.
The present study aims to provide a comprehensive
review of the emerging evidence supporting the gut-bone-cartilage
axis in OA and the role of microbial regulation of Wnt/β-catenin
signaling in joint remodeling. The current understanding of the
influence of the gut microbiome on OA pathogenesis is summarized,
discussing the mechanisms underlying the gut-bone-cartilage axis
and exploring the therapeutic potential of targeting this axis. By
integrating insights from various studies, the present review aims
to provide information on novel options for OA treatment and
management.
Gut microbiota-derived metabolites:
Regulators of Wnt/β-catenin signaling
The gut microbiome, comprising a diverse array of
microorganisms, serves a crucial role in host metabolism and immune
function (18). These
microorganisms, which include bacteria, fungi and viruses, reside
primarily in the gastrointestinal tract and contribute to the
breakdown of dietary fibers, the production of vitamins and the
regulation of immune responses (19). Among the various metabolites
produced by the gut microbiome, short-chain fatty acids (SCFAs),
bile acids and tryptophan derivatives have garnered marked
attention due to their systemic effects on bone and cartilage
metabolism (20–22) (Table
I).
 | Table I.Gut microbial metabolites and their
regulation of the Wnt/β-catenin pathway in OA. |
Table I.
Gut microbial metabolites and their
regulation of the Wnt/β-catenin pathway in OA.
| First author,
year | Metabolite
category | Specific
metabolite | Source of
metabolite | Mechanism of action
on Wnt/β-catenin pathway | Model/study
type | Key findings | (Refs.) |
|---|
|
Montalvany-Antonucci et al,
2019 | SCFAs | Butyrate | Produced by gut
microbiota through fermentation of dietary fibers | Inhibits HDACs,
enhancing β-catenin stability; this leads to increased expression
of Wnt target genes in osteoblasts, promoting bone formation | Animal model | Butyrate
supplementation reduces cartilage degradation and inflammation in
animal models of OA by modulating the Wnt/β-catenin pathway | (20) |
| Hou et al,
2023 | Bile acids | Secondary bile
acids | Metabolized by gut
microbiota from primary bile acids Wnt-induced inflammation | FXR activation
inhibits the Wnt/β-catenin pathway in osteoblasts, reducing bone
resorption; in chondrocytes, it suppresses | Mechanistic
study | Altered bile acid
metabolism is associated with changes in bone density and cartilage
integrity in patients with OA | (21) |
| Lucas et al,
2018 | Tryptophan
derivatives | Indole-3-propionic
acid | Produced by gut
microbiota from tryptophan | Acts as an
antioxidant and modulates Wnt/β-catenin signaling by interacting
with aryl hydrocarbon receptors, reducing inflammatory responses in
chondrocytes | Cell culture | Indole-3-propionic
acid protects chondrocytes from oxidative stress and inhibits
Wnt/β-catenin pathway overactivation | (22) |
| Lee et al,
2019 | SCFAs | Propionate | Produced by gut
microbiota through fermentation of dietary fibers | It upregulates Wnt
inhibitors like DKK1, reducing Wnt activation in osteoblasts and
chondrocytes | Animal model | Propionate
supplementation has a protective effect on cartilage in animal
models of OA | (25) |
| Rios et al,
2019 | Bile acids | Deoxycholic
acid | Metabolized by gut
microbiota from primary bile acids | Activates TLR4 and
NF-κB pathways, leading to increased production of pro-inflammatory
cytokines | Animal model | Deoxycholic acid
administration is associated with exacerbated joint inflammation
and cartilage damage in animal models of OA | (26) |
| Guan et al,
2020 | Vitamin
metabolites | Vitamin K2 | Influenced by gut
microbiota metabolism | Vitamin K2
deficiency decreases osteocalcin activation, impairing Wnt-mediated
bone formation | Animal model | Dysbiosis affects
vitamin K2 availability, impacting bone metabolism and potentially
contributing to OA | (27) |
| Collins et
al, 2021 | LPS | Gram-negative
bacterial cell wall component | Released by gut
microbiota, particularly in dysbiosis | Activates TLR4
pathway, leading to pro-inflammatory cytokine production | Animal model | Elevated LPS levels
are associated with increased joint inflammation and cartilage
damage in animal models of OA | (28) |
| Pedersini et
al, 2020 | Vitamin
metabolites | Vitamin D | Influenced by gut
microbiota, metabolism | Regulates
Wnt/β-catenin signaling by enhancing the expression of vitamin D
receptor and influencing the activity of related proteins | Animal model | Vitamin D
deficiency is associated with exacerbated OA progression while
supplementation improves joint health | (29) |
| Xie et al,
2020 | LPS | Lipid A | Released by gut
microbiota, particularly in dysbiosis | Activates TLR4 and
NF-κB pathways, leading to increased production of pro-inflammatory
cytokines | Animal model | Lipid A
administration is associated with increased joint inflammation and
cartilage damage in animal models of OA | (33) |
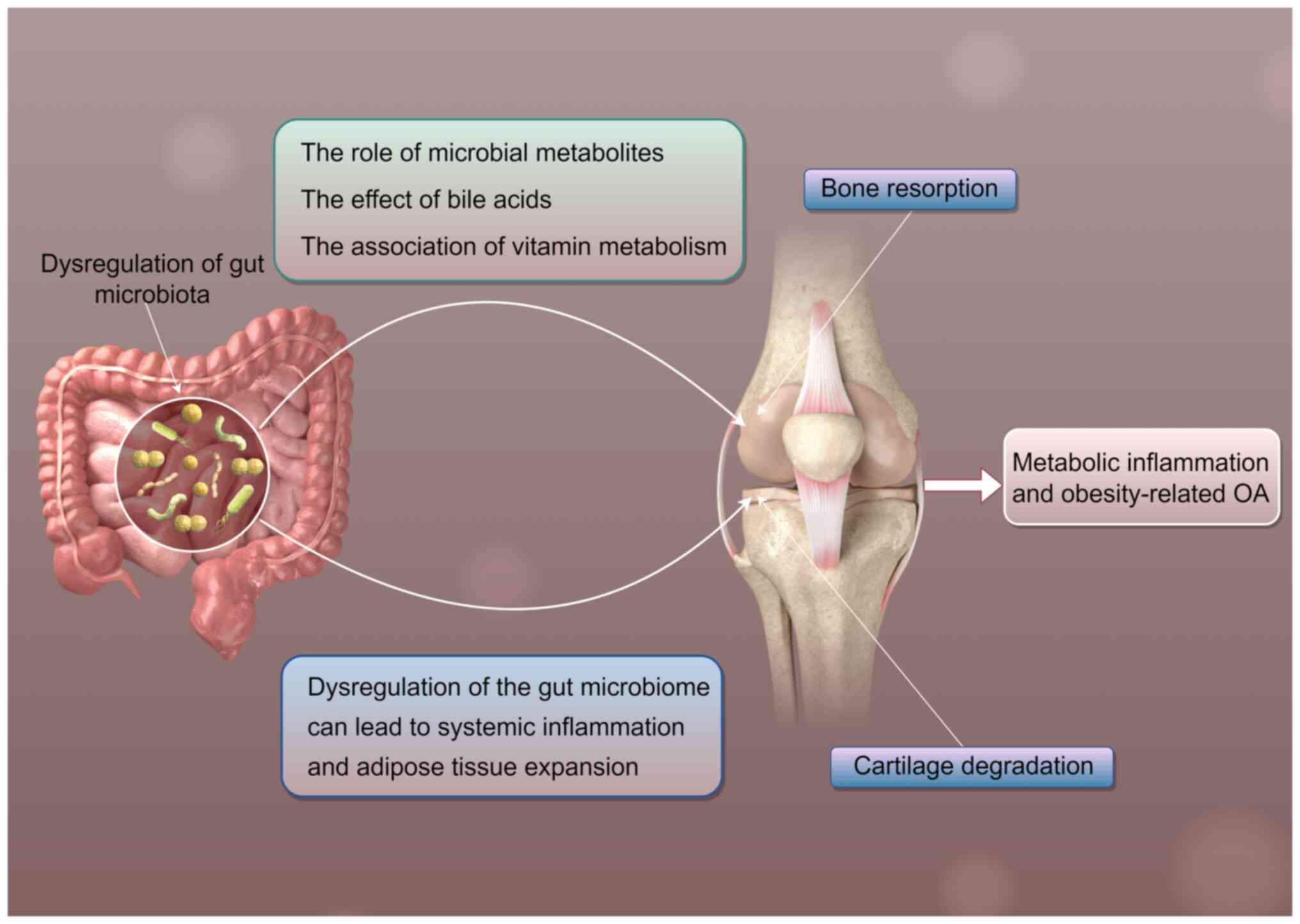
Key metabolites linking the microbiota
to the Wnt pathway [SCFAs, bile acids and lipopolysaccharide
(LPS)]
The gut microbiome is characterized by its high
diversity, with thousands of different species coexisting in a
complex ecosystem (19). Bacteria
from the Firmicutes, Bacteroidetes, Actinobacteria and
Proteobacteria phyla are the most abundant. These microorganisms
perform a variety of functions, including the fermentation of
indigestible carbohydrates to produce SCFAs, such as acetate,
propionate and butyrate (20).
These SCFAs not only serve as an energy source for colonic
epithelial cells but also have systemic effects on metabolism and
inflammation (18,22) (Fig.
1).
The metabolites produced by the gut microbiome
notably impact bone metabolism, influencing both bone formation and
resorption (23,24). SCFAs, particularly butyrate, have
been shown to regulate bone density through multiple mechanisms.
Butyrate inhibits osteoclast differentiation, thereby reducing bone
resorption and protecting bone density (25). This effect is mediated by the
inhibition of histone deacetylases (HDACs), which serve a crucial
role in osteoclastogenesis. On the other hand, LPS, a component of
the outer membrane of gram-negative bacteria, can promote bone
resorption by activating the Toll-like receptor 4 (TLR4) pathway.
This leads to the production of pro-inflammatory cytokines such as
IL-1β and TNF-α, which stimulate osteoclast activity and bone
resorption (26) (Fig. 1).
Vitamin metabolism is another area where the gut
microbiome exerts its influence on bone health. Vitamin K2,
essential for the activation of bone matrix proteins such as
osteocalcin, is influenced by the gut microbiome. Guan et al
(27) showed that alterations in
the gut microbiome can affect the availability of vitamin K2,
thereby impacting bone metabolism (Fig. 1).
Metabolite-driven Wnt
activation/inhibition mechanisms
The gut microbiome also indirectly influences
cartilage metabolism through the induction of systemic
inflammation. Dysbiosis, or an imbalance in the gut microbiome, has
been associated with increased levels of pro-inflammatory cytokines
such as IL-1β and TNF-α (28).
These cytokines can promote the degradation of cartilage matrix
components, such as collagen and proteoglycans, by stimulating the
production of matrix metalloproteinases (MMPs) and aggrecanases in
chondrocytes (29). This
inflammatory cascade accelerates cartilage degeneration and
contributes to the progression of OA.
Moreover, the gut microbiome is implicated in the
development of metabolic inflammation and obesity-related OA
(30). Obesity is an important
risk factor for OA, and studies have shown that the gut microbiome
can influence energy metabolism and fat storage (31,32).
Dysbiosis can lead to increased energy harvest from the diet and
promote adipose tissue expansion, contributing to obesity (33). The resulting mechanical stress on
joints, combined with systemic inflammation, exacerbates cartilage
degeneration and joint damage in OA.
Butyrate (from Firmicutes) inhibits HDACs, enhancing
β-catenin nuclear translocation and activating Wnt signaling in
osteoblasts (20). Conversely, LPS
(from gram-negative bacteria) binds TLR4, suppressing Wnt via
NF-κB-induced sclerostin expression (34), promoting osteoclastogenesis and
cartilage breakdown.
Notably, the influence of the gut microbiota on
Wnt/β-catenin signaling is not limited to direct metabolic
products. The gut microbiota also modulates the immune response of
the host, which in turn affects Wnt/β-catenin signaling. For
example, probiotics such as Lactobacillus have been shown to
modulate the immune system and reduce inflammation by regulating
the balance between T helper 17 (Th17) and regulatory T (Treg)
cells, thereby inhibiting the overactivation of the Wnt/β-catenin
pathway (35). This
immune-mediated regulation provides another layer of complexity in
the role of the gut microbiota in OA pathogenesis.
Additionally, a recent study has highlighted the
role of bile acids in regulating Wnt/β-catenin signaling (21). Bile acids, upon metabolism by gut
microbiota, can activate farnesoid X receptor (FXR) and other
signaling pathways that intersect with Wnt/β-catenin (21). This crosstalk is particularly
relevant in the context of bone and cartilage homeostasis, as
evidenced by altered bile acid profiles in patients with OA,
characterized by a significant decrease in the levels of secondary
bile acids (such as deoxycholic acid and lithocholic acid) and a
relative increase in primary bile acids (such as cholic acid and
chenodeoxycholic acid) compared to healthy individuals (21).
This crosstalk is particularly relevant in the
context of bone and cartilage homeostasis, as evidenced by studies
showing altered bile acid profiles in patients with OA,
characterized by a significant decrease in the levels of secondary
bile acids (such as deoxycholic acid and lithocholic acid) and a
relative increase in primary bile acids (such as cholic acid and
chenodeoxycholic acid) compared to healthy individuals (21).
In summary, gut microbiota-derived metabolites exert
notable effects on Wnt/β-catenin signaling through multiple
mechanisms, involving direct metabolic actions, immune modulation
and interactions with other signaling pathways. These findings
underscore the multifaceted role of the gut microbiome in OA
pathogenesis and highlight potential therapeutic targets for
modulating joint remodeling in OA.
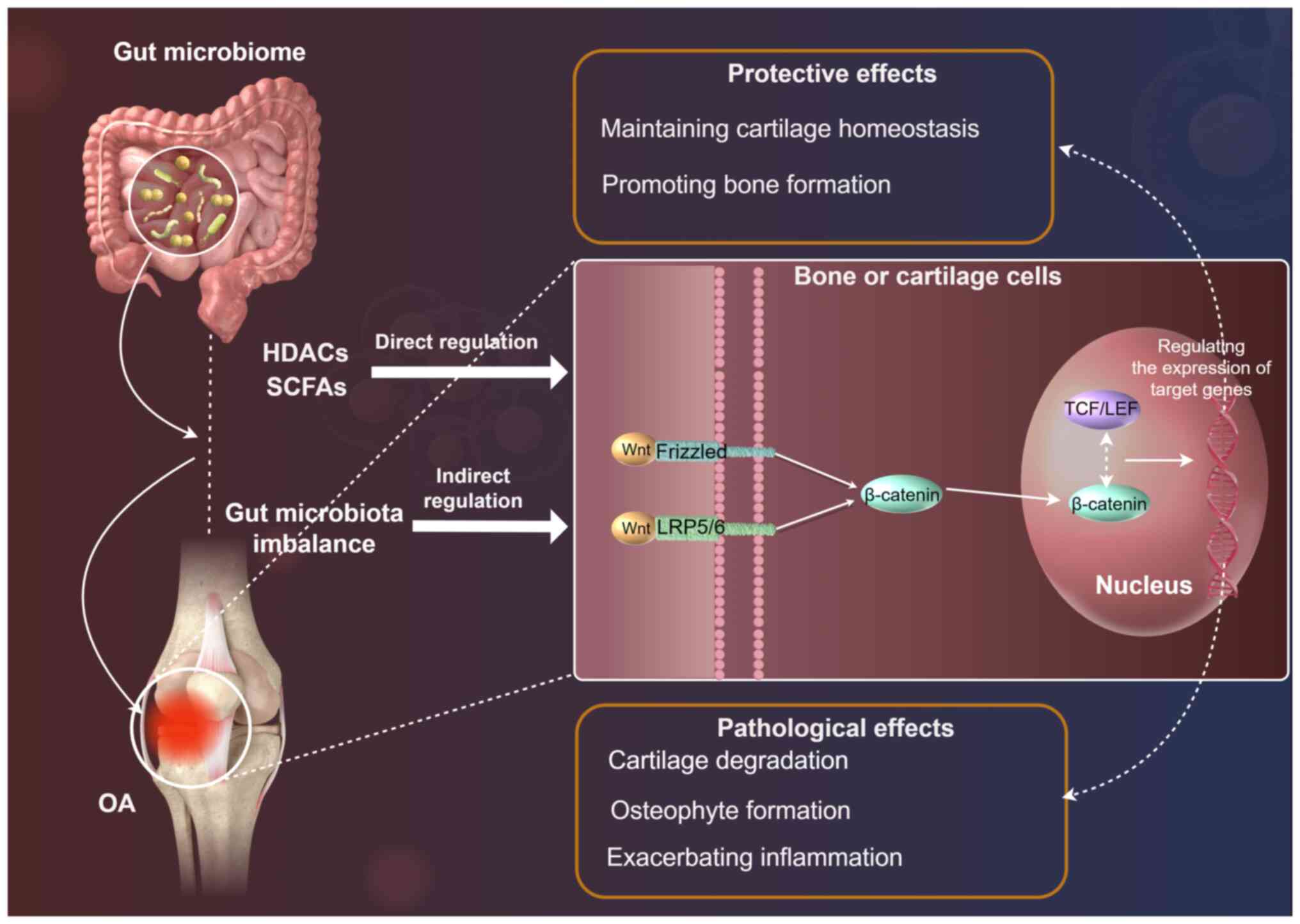
Wnt/β-catenin dysregulation: Direct driver
of OA structural pathology
The Wnt/β-catenin signaling pathway serves a crucial
role in bone formation, cartilage homeostasis and joint development
(36). This pathway is involved in
the regulation of cell proliferation, differentiation and survival,
and it is thus essential in maintaining the structural integrity of
the musculoskeletal system. In OA, the Wnt/β-catenin pathway
exhibits a dual role, with both protective and pathological effects
(36). Understanding this dual
role is essential for developing targeted therapeutic strategies to
manage OA progression (Fig.
2).
Wnt hyperactivation
The Wnt/β-catenin signaling pathway is a highly
conserved pathway that regulates various cellular processes,
including bone formation and cartilage homeostasis (36). In the canonical Wnt pathway, Wnt
ligands bind to Frizzled (FZD) receptors and LRP5/6 co-receptors,
leading to the accumulation of β-catenin in the cytoplasm (37,38).
This accumulated β-catenin translocates to the nucleus, where it
interacts with TCF/LEF transcription factors to regulate the
expression of target genes involved in OA pathogenesis, such as
MMP13, ADAMTS5 (a disintegrin and metalloproteinase with
thrombospondin motifs 5), and cyclin D1 (37). These genes are involved in cell
proliferation, differentiation and survival, making the
Wnt/β-catenin pathway a critical regulator of bone and cartilage
metabolism (38). β-catenin
accumulation in chondrocytes, driven by canonical Wnt signaling
activation, functions as a transcriptional co-activator that
directly binds to T-cell factor/lymphoid enhancer factor (TCF/LEF)
sites in the promoters of genes encoding catabolic enzymes. This
leads to the upregulation of MMP13 and ADAMTS5 (39–41).
MMP13 specifically cleaves type II collagen, the primary structural
component of articular cartilage, while ADAMTS5 is the major
aggrecanase responsible for aggrecan core protein degradation
(39,40). This direct degradation of collagen
II and aggrecan is a central mechanism driving cartilage matrix
loss in OA. Elevated fecal LPS levels, indicative of gut dysbiosis
and increased gram-negative bacterial load/intestinal permeability,
promote systemic inflammation and can activate TLR4 signaling
within joint tissues. TLR4 signaling has been shown to potentiate
Wnt/β-catenin signaling and/or induce the expression of
inflammatory cytokines (such as IL-1β and TNF-α) that further
stimulate chondrocyte catabolism, including MMP13 and ADAMTS5
production (28,34). Thus, the clinical association
between elevated fecal LPS levels and accelerated cartilage loss
can be mechanistically explained, at least in part, by LPS-mediated
enhancement of the Wnt/β-catenin pathway and downstream
inflammatory cascades, culminating in the upregulation of these key
matrix-degrading enzymes and subsequent cartilage destruction
(28,34).
Wnt dysregulation
In OA, the Wnt/β-catenin pathway is often
dysregulated, leading to both protective and pathological effects
(39). At the chondrocyte level,
excessive activation of the Wnt/β-catenin pathway can result in the
hypertrophy of chondrocytes and the upregulation of MMPs, which
contribute to cartilage degradation (40,41).
This process is characterized by the loss of cartilage matrix
components, such as collagen and proteoglycans, leading to reduced
joint function and increased pain.
On the other hand, the Wnt/β-catenin pathway also
serves a role in bone remodeling (42). β-catenin promotes the
differentiation of osteoblasts, which are responsible for bone
formation (43,44). However, abnormal activation of this
pathway can lead to the formation of osteophytes, bony outgrowths
that contribute to joint deformity and reduced mobility (45,46).
This dual role of the Wnt/β-catenin pathway in OA highlights the
complexity of its regulation and the need for targeted therapeutic
interventions.
Regulation of the Wnt pathway by the
gut microbiome
The gut microbiome has emerged as a key regulator of
the Wnt/β-catenin pathway, influencing both bone and cartilage
metabolism (47). Furthermore, the
gut-brain axis may be involved in the indirect regulation of the
Wnt/β-catenin pathway through neural communication, although
further research is needed to elucidate its role in bone and
cartilage metabolism (48).
Overall, these diverse mechanisms highlight the complexity of how
gut microbial metabolites modulate the Wnt/β-catenin signaling
pathway in distant bones and cartilages, and underscore the
potential of targeting this axis for OA treatment.
At the cellular level, the Wnt/β-catenin pathway
exhibits distinct effects on different cell types. In osteoblasts,
β-catenin promotes differentiation and bone formation by
upregulating osteogenic genes such as Runx2 and Osterix (49). Conversely, in chondrocytes,
excessive activation of the Wnt/β-catenin pathway can lead to
hypertrophy and the upregulation of MMPs, which contribute to
cartilage degradation (50). This
cell-specific regulation underscores the complexity of the
Wnt/β-catenin pathway in OA pathogenesis.
The gut microbiome can indirectly regulate the
Wnt/β-catenin pathway through the immune-metabolic network.
Dysbiosis, or an imbalance in the gut microbiome, can lead to
increased levels of pro-inflammatory cytokines, such as IL-1β and
TNF-α. These cytokines can influence the expression of Wnt
pathway-related genes, such as sclerostin, an inhibitor of Wnt
signaling (51). Furthermore, the
Wnt/β-catenin pathway involves intricate feedback mechanisms. For
example, the upregulation of Dickkopf-1 and secreted FZD-related
protein acts as a negative feedback loop to modulate Wnt signaling
intensity (52,53). These feedback mechanisms are
essential for maintaining the balance of Wnt/β-catenin signaling in
bone and cartilage homeostasis.
Gut-joint connection: Evidence, mechanisms
and debates
The gut-bone-cartilage triad has emerged as a
promising area of research in understanding the pathogenesis of OA
(10). However, despite
advancements, there are still controversies and gaps in the
knowledge that need to be addressed. The present review aims to
integrate the existing evidence and highlight the controversies in
current research (Table II).
 | Table II.Gut microbiota and OA-related
studies. |
Table II.
Gut microbiota and OA-related
studies.
| First author,
year | Gut microbiota
category/intervention | Model/study
type | Key findings | (Refs.) |
|---|
| Ulici et al,
2018 | Germ-free mice | Animal model | OA induced by
destabilization of the medial meniscus is reduced in germ-free
mice | (54) |
| Chen et al,
2021 | FMT | Animal model | FMT from patients
with OA to germ-free mice results in joint inflammation and
cartilage damage | (55) |
| Fortuna et
al, 2021 | Comparative
analysis of fecal microbiota | Clinical study | Patients with OA
show decreased gut microbiota diversity and specific bacterial
species associated with disease severity | (56) |
| Hahn et al,
2021 | Probiotic
intervention | Clinical trial | Probiotic
supplementation improves OA symptoms in some patients but not in
others | (57) |
| Ilesanmi-Oyelere
et al, 2021 | Gut microbiota and
Wnt/β-catenin pathway | Mechanistic
study | Microbial
metabolites can directly modulate Wnt/β-catenin signaling in
chondrocytes and osteoblasts | (58) |
| Lan et al,
2021 | Time-dependent
effect of gut microbiota | Longitudinal
study | Early-life exposure
to specific gut microbiota may influence joint health later in
life | (59) |
| Luna et al,
2021 | Probiotic
intervention | Animal model |
Lactobacillus can regulate the
balance between Th17 and Treg cells, inhibiting the overactivation
of the Wnt/β-catenin pathway | (35) |
| Pedersini et
al, 2021 | Prebiotic
intervention | Animal model | A high-fiber diet
increases the production of butyrate, reducing inflammation and
cartilage degradation | (60) |
| Mi et al,
2023 | High-fiber diet and
gut microbiota | Intervention
study | A high-fiber diet
promotes the growth of beneficial gut bacteria and increases
butyrate production, reducing inflammation and cartilage
degradation | (61) |
| Piva et al,
2023 | FMT | Animal model | FMT alleviates
joint inflammation and cartilage degeneration by restoring a
healthy gut microbiome | (62) |
| Wang et al,
2023 | Small molecule
inhibitors | Animal model | ICG-001 inhibits
the Wnt/β-catenin pathway and decreases cartilage degeneration | (63) |
| Zhao et al,
2024 | Gene editing
(CRISPR/Cas9) | Animal model | CRISPR/Cas9 editing
of genes involved in the Wnt/β-catenin pathway, such as sclerostin,
enhances bone formation and reduces cartilage degeneration | (65) |
Evidence for the
microbiota-metabolite-Wnt-structural axis
Rodent models have provided robust pre-clinical
evidence linking the gut microbiome to OA pathogenesis. In
germ-free C57BL/6 mice subjected to destabilization of the medial
meniscus (DMM), Ulici et al (54) observed more severe cartilage
degeneration and subchondral bone sclerosis than in
specific-pathogen-free (SPF) controls, indicating that the absence
of commensal microbes exacerbates OA-related joint damage.
Complementing these findings, Chen et al (55) performed fecal microbiota
transplantation (FMT) from human knee-OA patients into 8-week-old
germ-free Swiss Webster mice and documented increased synovial
inflammation and accelerated cartilage loss within eight weeks.
Collins et al (28)
employed high-fat diet (HFD)-fed C57BL/6J mice to model metabolic
OA. The authors demonstrated that HFD-induced dysbiosis,
characterized by reduced Lactobacillus and increased
Desulfovibrionaceae, was associated with elevated systemic
lipopolysaccharide (LPS) levels and greater cartilage erosion
independent of body weight. In a rat model, Rios et al
(26) demonstrated that prebiotic
supplementation (oligofructose) in male Wistar rats mitigated
HFD-driven joint inflammation by restoring gut-barrier integrity
and reducing TLR4/NF-κB activation. Collectively, these murine and
rat studies establish a causal role for the microbiome-joint axis
in OA development and provide multiple translational platforms for
mechanistic dissection.
Resolving controversies through a
unified framework
While animal studies have provided evidence for the
role of the gut microbiome in OA, clinical research has yielded
contradictory results. Fortuna et al (56) reported a decrease in gut microbiota
diversity in patients with OA, with specific bacterial species,
such as Prevotella, showing a positive association with
disease severity. However, other studies have failed to replicate
these findings, suggesting that the relationship between gut
microbiota and OA may be more complex (55,57).
For instance, Chen et al reported no significant difference
in α-diversity between older women with OA and healthy controls,
and found no consistent association between specific bacterial taxa
and OA status (55). Similarly,
Hahn et al demonstrated that neither global microbiome
diversity nor the relative abundance of Prevotella correlated with
OA severity or pain scores in a mouse model (57), suggesting that the relationship
between gut microbiota and OA may be more complex than initially
thought.
Probiotic interventions have also shown inconsistent
results in clinical trials. Hahn et al (57) reported improvements in OA symptoms
following probiotic supplementation, whereas Fortuna et al
(56) observed no significant
changes in knee joint function or systemic inflammation after
12-week prebiotic/probiotic intervention in obese adults with knee
OA, and Ilesanmi-Oyelere et al (58) found no notable effects of synbiotic
supplementation on OA-related biomarkers in a randomized controlled
trial. These discrepancies may be attributed to differences in
study design, patient populations and the specific probiotic
strains used.
Despite the growing body of evidence linking the gut
microbiome to OA, gaps remain in the understanding of the
underlying mechanisms. One key area of uncertainty is the
cell-specific interaction between gut microbiota and the
Wnt/β-catenin pathway. Ilesanmi-Oyelere et al (58) suggested that microbial metabolites
can directly modulate Wnt/β-catenin signaling in chondrocytes and
osteoblasts, but the exact cell types and molecular mechanisms
involved remain unclear.
Another area of controversy is the time-dependent
effect of gut microbiota on OA development. Lan et al
(59) suggested that early-life
exposure to specific gut microbiota may influence joint health
later in lif. However, subsequent germ-free and antibiotic
perturbation studies by Ulici et al (54) and Chen et al (55) failed to detect any significant
association between neonatal microbial composition and OA
susceptibility in adulthood, indicating that the role of host
developmental stages in modulating the impact of gut microbiota on
OA progression remains to be elucidated.
Therapeutic strategies and translational
medicine prospects
The gut-bone-cartilage triad and the role of
microbial regulation of Wnt/β-catenin signaling in OA joint
remodeling offer promising options for therapeutic interventions.
The present review aims to explore various strategies targeting the
gut microbiome and the Wnt/β-catenin pathway, as well as the
potential of gene editing and carrier technologies in OA treatment
(Table II). Therapies targeting
the microbiota-metabolite-Wnt axis (such as probiotics increasing
butyrate/Wnt inhibition and FMT restoring anti-inflammatory taxa)
show promise in normalizing joint remodeling (35,60,61).
Targeting the gut microbiome
Probiotics and prebiotics
Probiotics, such as Lactobacillus, have shown
potential in modulating the immune system and reducing
inflammation. Luna et al (35) demonstrated that
Lactobacillus can regulate the balance between Th17 and Treg
cells, thereby inhibiting the overactivation of the Wnt/β-catenin
pathway. Prebiotics, which are non-digestible food components that
promote the growth of beneficial gut bacteria, can also serve a
role in OA management. For example, a high-fiber diet has been
shown to increase the production of butyrate, a SCFA that inhibits
β-catenin signaling, thereby reducing inflammation and cartilage
degradation (60).
Diet control
Dietary interventions are a promising approach to
modulating the gut microbiome and its effects on OA. A high-fiber
diet has been shown to promote the growth of beneficial gut
bacteria, leading to increased production of butyrate, which can
inhibit β-catenin signaling and reduce inflammation (61). Additionally, a balanced diet rich
in antioxidants and anti-inflammatory nutrients can mitigate the
effects of OA by reducing systemic inflammation and promoting joint
health (61).
FMT
FMT, which involves transferring fecal microbiota
from a healthy donor to a recipient, has shown promising results in
OA animal models. Piva et al (62) demonstrated that FMT can alleviate
joint inflammation and cartilage degeneration by restoring a
healthy gut microbiome. This approach holds potential for clinical
applications, although further research is needed to optimize the
procedure and ensure its safety and efficacy.
Targeting the Wnt pathway with small
molecule drugs
The Wnt/β-catenin pathway has emerged as a promising
target for OA therapy. Small molecule inhibitors, such as ICG-001,
have been shown to inhibit the Wnt/β-catenin pathway and reduce
cartilage degeneration (63).
However, the use of inhibitors must be balanced against the
potential for adverse effects, as the Wnt/β-catenin pathway serves
a crucial role in bone formation and homeostasis (36). On the other hand, agonists, such as
lithium salts, can promote bone formation by activating the
Wnt/β-catenin pathway (64). The
therapeutic potential of these agents must be carefully evaluated
in clinical trials to determine their safety and efficacy.
Gene editing technologies, such as CRISPR/Cas9,
offer a powerful tool for modulating the Wnt/β-catenin pathway in
OA (65). Zhao et al
(65) demonstrated that
CRISPR/Cas9 can be used to edit genes involved in the Wnt/β-catenin
pathway, such as SCLEROSTIN, which is an inhibitor of Wnt
signaling. Lentiviral vectors have been used to deliver CRISPR/Cas9
systems to target cells, enabling precise gene editing and
modulation of the Wnt/β-catenin pathway. This approach holds
potential for developing novel therapies for OA, although further
research is needed to address technical challenges, and ensure the
safety and efficacy of these technologies.
Critical analysis of intervention
strategies
When evaluating the proposed intervention strategies
for OA targeting the gut-bone-cartilage triad and Wnt/β-catenin
signaling, a comprehensive analysis of feasibility, specificity,
safety and ethical considerations is essential.
Gene editing technologies
Gene editing tools such as CRISPR/Cas9 offer precise
modulation of the Wnt/β-catenin pathway. While preclinical studies
show promise in enhancing bone formation and reducing cartilage
degradation, several concerns must be addressed. The specificity of
CRISPR/Cas9 remains a challenge due to potential off-target
effects, which could lead to unintended genetic modifications in
various cell types (66). Safety
concerns include the risk of immune responses to the Cas9 protein
and the long-term consequences of altering gene expression. Ethical
considerations revolve around the permanent nature of germline
editing, even though somatic cell editing is primarily used for OA.
The irreversible changes to the genome necessitate the careful
balancing of risks and benefits (67).
Targeting the gut microbiome
Interventions targeting the gut microbiome, such as
probiotics, prebiotics, FMT and dietary modifications, show
potential for regulating the Wnt/β-catenin pathway. These
approaches are relatively feasible and have fewer ethical concerns
compared with gene editing. However, challenges remain in achieving
specificity, as the complex composition of the gut microbiome makes
it difficult to target specific bacterial strains without affecting
the overall microbial balance (68). Safety concerns include the risk of
introducing pathogenic bacteria through FMT or altering the
microbiome in ways that could lead to other health issues (69). Long-term studies are needed to
assess the safety and effectiveness of these interventions.
Small molecule drugs
Small molecule inhibitors and agonists of the
Wnt/β-catenin pathway provide another option for OA treatment.
These drugs are relatively easy to administer and have
well-established pharmacokinetic profiles, enhancing their
feasibility (37). However,
ensuring their specificity to the Wnt/β-catenin pathway is
challenging, as they may interact with other signaling pathways.
Safety concerns involve potential toxicities and side effects,
particularly with long-term use (37,70).
Rigorous clinical trials are necessary to evaluate their safety and
efficacy in patients with OA.
Future research directions and
challenges
The gut-bone-cartilage triad and the microbial
regulation of Wnt/β-catenin signaling in OA joint remodeling
present opportunities for future research and therapeutic
development (71,72). However, several challenges need to
be addressed to fully realize the potential of these approaches.
The present review explores future research directions and
challenges in this field.
One of the most promising future directions in OA
research is the integration of multiomics approaches, including
metagenomics, metabolomics and single-cell sequencing (73,74).
These techniques can provide a comprehensive understanding of the
complex interactions between the gut microbiome and the host,
particularly in the context of OA (74). Metagenomics can identify specific
microbial species and their functional capabilities, whereas
metabolomics can reveal the metabolic products of these
microorganisms and their effects on host metabolism (75). Single-cell sequencing can provide
insights into the heterogeneity of cell populations within the
joint, including chondrocytes, osteoblasts and immune cells, and
how they respond to microbial signals (76). For example, SCFAs produced by the
gut microbiota can influence bone metabolism by enhancing the bone
morphogenetic protein, Wnt and osteoprotegerin signaling pathways,
and inhibiting the RANKL signaling pathway (20,77,78).
By integrating these multiomics approaches, researchers can
identify key microbial species and their metabolites that serve a
role in OA progression, and develop targeted therapies to modulate
these interactions.
Personalized medicine, which is based on the host
genotype and gut microbiome characteristics of the host, holds
potential for OA treatment (79).
The gut microbiome varies among individuals and this variability
can influence the efficacy of therapeutic interventions. By
understanding the specific microbial composition and functional
capabilities of the gut microbiome of individuals, clinicians can
tailor treatments to target the specific pathways involved in OA
progression (14,16,17).
For example, studies have shown that certain bacterial species,
such as Lactobacillus rhamnosus GG, can increase the
production of the osteogenic Wnt ligand Wnt10b, promoting bone
formation (80). By identifying
individuals with specific microbial profiles, clinicians can
recommend personalized interventions, such as probiotics or
prebiotics, to modulate the gut microbiome and improve joint
health.
The development of new therapeutic strategies for OA
requires the integration of cross-disciplinary technologies,
including gene editing and carrier technologies (81,82).
Carrier technologies, such as lentiviral vectors, can be used to
deliver CRISPR/Cas9 systems to target cells, enabling precise gene
editing and modulation of the Wnt/β-catenin pathway (83). These technologies can be further
optimized to enhance their safety and efficacy, making them viable
options for clinical application.
Conclusion
The gut-bone-cartilage triad and the microbial
regulation of Wnt/β-catenin signaling in OA joint remodeling offer
promising opportunities for future research and therapeutic
development. By integrating multiomics approaches, personalized
medicine and cross-disciplinary technologies, researchers may gain
a deeper understanding of the complex interactions between the gut
microbiome and the host, and develop targeted therapies to modulate
these interactions. Future research should focus on addressing the
challenges in these areas to fully realize the potential of these
approaches in OA treatment.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
RZ, LZ, YW, BT, XK and JZ conceived the study. RZ
and JZ performed the literature review. RZ, LZ, YW, BT and XK wrote
the manuscript. Data authentication is not applicable. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wang Z, Xiao Z, Sun C, Xu G and He J:
Global, regional and national burden of osteoarthritis in
1990–2021: A systematic analysis of the global burden of disease
study 2021. BMC Musculoskelet Disord. 25:10212024. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Laires PA, Canhão H, Rodrigues AM, Eusébio
M, Gouveia M and Branco JC: The impact of osteoarthritis on early
exit from work: Results from a population-based study. BMC Public
Health. 18:4722018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Peng R, Shang J, Jiang N, Chi-Jen H, Gu Y,
Xing B, Hu R, Wu B, Wang D, Xu X and Lu H: Klf10 is involved in
extracellular matrix calcification of chondrocytes alleviating
chondrocyte senescence. J Transl Med. 22:522024. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Akkiraju H and Nohe A: Role of
chondrocytes in cartilage formation, progression of osteoarthritis
and cartilage regeneration. J Dev Biol. 3:177–192. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cho Y, Jeong S, Kim H, Kang D, Lee J, Kang
SB and Kim JH: Disease-modifying therapeutic strategies in
osteoarthritis: Current status and future directions. Exp Mol Med.
53:1689–1696. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fleischmann R: Have we found a true
disease-modifying osteoarthritis drug (DMOAD) or is there still
much work to be done? Rheumatology (Oxford). 64:2345–2346. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yao Q, Wu X, Tao C, Gong W, Chen M, Qu M,
Zhong Y, He T, Chen S and Xiao G: Osteoarthritis: Pathogenic
signaling pathways and therapeutic targets. Signal Transduct Target
Ther. 8:562023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guan M, Yu Q, Zhou G, Wang Y, Yu J, Yang W
and Li Z: Mechanisms of chondrocyte cell death in osteoarthritis:
Implications for disease progression and treatment. J Orthop Surg
Res. 19:5502024. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Deng H, Xue P, Zhou X, Wang Y and Liu W:
CCL4/CCR5 regulates chondrocyte biology and OA progression.
Cytokine. 183:1567462024. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hao X, Shang X, Liu J, Chi R, Zhang J and
Xu T: The gut microbiota in osteoarthritis: Where do we stand and
what can we do? Arthritis Res Ther. 23:422021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chisari E, Wouthuyzen-Bakker M, Friedrich
AW and Parvizi J: The relation between the gut microbiome and
osteoarthritis: A systematic review of literature. PLoS One.
16:e02613532021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nigam M, Devi K, Coutinho HDM and Mishra
AP: Exploration of gut microbiome and inflammation: A review on key
signalling pathways. Cell Signal. 118:1111402024. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li J, Ho WTP, Liu C, Chow SK, Ip M, Yu J,
Wong HS, Cheung WH, Sung JJY and Wong RMY: The role of gut
microbiota in bone homeostasis. Bone Joint Res. 10:51–59. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lyu Z, Hu Y, Guo Y and Liu D: Modulation
of bone remodeling by the gut microbiota: A new therapy for
osteoporosis. Bone Res. 11:312023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Marchese L, Contartese D, Giavaresi G, Di
Sarno L and Salamanna F: The complex interplay between the gut
microbiome and osteoarthritis: A systematic review on potential
correlations and therapeutic approaches. Int J Mol Sci. 25:1432023.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu F, Zhu C and Wu W: Senile
osteoarthritis regulated by the gut microbiota: From mechanisms to
treatments. Int J Mol Sci. 26:15052025. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Y, Fan X, Xing L and Tian F: Wnt
signaling: A promising target for osteoarthritis therapy. Cell
Commun Signal. 17:972019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang W and Cong Y: Gut microbiota-derived
metabolites in the regulation of host immune responses and
immune-related inflammatory diseases. Cell Mol Immunol. 18:866–877.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Grant ET, Parrish A, Boudaud M, Hunewald
O, Hirayama A, Ollert M, Fukuda S and Desai MS: Dietary fibers
boost gut microbiota-produced B vitamin pool and alter host immune
landscape. Microbiome. 12:1792024. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Montalvany-Antonucci CC, Duffles LF, de
Arruda JAA, Zicker MC, de Oliveira S, Macari S, Garlet GP, Madeira
MFM, Fukada SY, Andrade I Jr, et al: Short-chain fatty acids and
FFAR2 as suppressors of bone resorption. Bone. 125:112–121. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hou Y, Li J and Ying S: Tryptophan
metabolism and gut microbiota: A novel regulatory axis integrating
the microbiome, immunity, and cancer. Metabolites. 13:11662023.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lucas S, Omata Y, Hofmann J, Böttcher M,
Iljazovic A, Sarter K, Albrecht O, Schulz O, Krishnacoumar B,
Krönke G, et al: Short-chain fatty acids regulate systemic bone
mass and protect from pathological bone loss. Nat Commun. 9:552018.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yan J, Herzog JW, Tsang K, Brennan CA,
Bower MA, Garrett WS, Sartor BR, Aliprantis AO and Charles JF: Gut
microbiota induce IGF-1 and promote bone formation and growth. Proc
Natl Acad Sci USA. 113:E7554–E7563. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li JY, Chassaing B, Tyagi AM, Vaccaro C,
Luo T, Adams J, Darby TM, Weitzmann MN, Mulle JG, Gewirtz AT, et
al: Sex steroid deficiency-associated bone loss is microbiota
dependent and prevented by probiotics. J Clin Invest.
126:2049–2063. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee JY, Mannaa M, Kim Y, Kim J, Kim GT and
Seo YS: Comparative analysis of fecal microbiota composition
between rheumatoid arthritis and osteoarthritis patients. Genes
(Basel). 10:7482019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rios JL, Bomhof MR, Reimer RA, Hart DA,
Collins KH and Herzog W: Protective effect of prebiotic and
exercise intervention on knee health in a rat model of diet-induced
obesity. Sci Rep. 9:38932019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guan Z, Jia J, Zhang C, Sun T, Zhang W,
Yuan W, Leng H and Song C: Gut microbiome dysbiosis alleviates the
progression of osteoarthritis in mice. Clin Sci (Lond).
134:3159–3174. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Collins KH, Schwartz DJ, Lenz KL, Harris
CA and Guilak F: Taxonomic changes in the gut microbiota are
associated with cartilage damage independent of adiposity, high fat
diet, and joint injury. Sci Rep. 11:145602021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pedersini P, Turroni S and Villafañe JH:
Gut microbiota and physical activity: Is there an evidence-based
link? Sci Total Environ. 727:1386482020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cani PD and Van Hul M: Gut microbiota in
overweight and obesity: Crosstalk with adipose tissue. Nat Rev
Gastroenterol Hepatol. 21:164–183. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cani PD, Possemiers S, Van de Wiele T,
Guiot Y, Everard A, Rottier O, Geurts L, Naslain D, Neyrinck A,
Lambert DM, et al: Changes in gut microbiota control inflammation
in obese mice through a mechanism involving GLP-2-driven
improvement of gut permeability. Gut. 58:1091–1103. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Collins KH, Paul HA, Reimer RA, Seerattan
RA, Hart DA and Herzog W: Relationship between inflammation, the
gut microbiota, and metabolic osteoarthritis development: Studies
in a rat model. Osteoarthritis Cartilage. 23:1989–1998. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xie LL, Zhao YL, Yang J, Cheng H, Zhong
ZD, Liu YR and Pang XL: Electroacupuncture prevents osteoarthritis
of high-fat diet-induced obese rats. Biomed Res Int.
2020:93809652020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zeng XZ, Zhang YY, Yang Q, Wang S, Zou BH,
Tan YH, Zou M, Liu SW and Li XJ: Artesunate attenuates LPS-induced
osteoclastogenesis by suppressing TLR4/TRAF6 and
PLCγ1-Ca2+-NFATc1 signaling pathway. Acta Pharmacol Sin.
41:229–236. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Luna M, Guss JD, Vasquez-Bolanos LS,
Alepuz AJ, Dornevil S, Strong J, Alabi D, Shi Q, Pannellini T,
Otero M, et al: Obesity and load-induced posttraumatic
osteoarthritis in the absence of fracture or surgical trauma. J
Orthop Res. 39:1007–1016. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hu L, Chen W, Qian A and Li YP:
Wnt/β-catenin signaling components and mechanisms in bone
formation, homeostasis, and disease. Bone Res. 12:392024.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Maurice MM and Angers S: Mechanistic
insights into Wnt-β-catenin pathway activation and signal
transduction. Nat Rev Mol Cell Biol. 26:371–388. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ren DN, Chen J, Li Z, Yan H, Yin Y, Wo D,
Zhang J, Ao L, Chen B, Ito TK, et al: LRP5/6 directly bind to
Frizzled and prevent Frizzled-regulated tumour metastasis. Nat
Commun. 6:69062015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ma B, van Blitterswijk CA and Karperien M:
A Wnt/β-catenin negative feedback loop inhibits
interleukin-1-induced matrix metalloproteinase expression in human
articular chondrocytes. Arthritis Rheum. 64:2589–2600. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Landman EB, Miclea RL, van Blitterswijk CA
and Karperien M: Small molecule inhibitors of WNT/β-catenin
signaling block IL-1β- and TNFα-induced cartilage degradation.
Arthritis Res Ther. 15:R932013. View
Article : Google Scholar : PubMed/NCBI
|
|
41
|
Takamatsu A, Ohkawara B, Ito M, Masuda A,
Sakai T, Ishiguro N and Ohno K: Verapamil protects against
cartilage degradation in osteoarthritis by inhibiting Wnt/β-catenin
signaling. PLoS One. 9:e926992014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bao Q, Chen S, Qin H, Feng J, Liu H, Liu
D, Li A, Shen Y, Zhao Y, Li J and Zong Z: An appropriate
Wnt/β-catenin expression level during the remodeling phase is
required for improved bone fracture healing in mice. Sci Rep.
7:26952017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang X, Qu Z, Zhao S, Luo L and Yan L:
Wnt/β-catenin signaling pathway: Proteins' roles in osteoporosis
and cancer diseases and the regulatory effects of natural compounds
on osteoporosis. Mol Med. 30:1932024. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bao Q, Chen S, Qin H, Feng J, Liu H, Liu
D, Li A, Shen Y, Zhong X, Li J and Zong Z: Constitutive β-catenin
activation in osteoblasts impairs terminal osteoblast
differentiation and bone quality. Exp Cell Res. 350:123–131. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Salazar VS, Mbalaviele G and Civitelli R:
The pro-osteogenic action of beta-catenin requires interaction with
BMP signaling, but not Tcf/Lef transcriptional activity. J Cell
Biochem. 104:942–952. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Feng SY, Cao MN, Gao CC, Li YX, Lei J and
Fu KY: Akt2 inhibition alleviates temporomandibular joint
osteoarthritis by preventing subchondral bone loss. Arthritis Res
Ther. 27:432025. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hao J, Liu C, Gu Z, Yang X, Lan X and Guo
X: Dysregulation of Wnt/β-catenin signaling contributes to
intestinal inflammation through regulation of group 3 innate
lymphoid cells. Nat Commun. 15:28202024. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ohara TE and Hsiao EY:
Microbiota-neuroepithelial signalling across the gut-brain axis.
Nat Rev Microbiol. 23:371–384. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhou X, Zhang Z, Feng JQ, Dusevich VM,
Sinha K, Zhang H, Darnay BG and de Crombrugghe B: Multiple
functions of Osterix are required for bone growth and homeostasis
in postnatal mice. Proc Natl Acad Sci USA. 107:12919–12924. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Usami Y, Gunawardena AT, Iwamoto M and
Enomoto-Iwamoto M: Wnt signaling in cartilage development and
diseases: Lessons from animal studies. Lab Invest. 96:186–196.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Sharvari S, Meganathan P and Vedagiri H:
Gut microbial dysbiosis induced exacerbations influence the
progression of colorectal cancer. Biol Bull Rev. 14:724–739. 2024.
View Article : Google Scholar
|
|
52
|
Mehta A, Motavaf M, Raza D, McLure AJ,
Osei-Opare KD, Bordone LA and Gru AA: Revolutionary approaches to
hair regrowth: Follicle neogenesis, Wnt/ß-catenin signaling, and
emerging therapies. Cells. 14:7792025. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kwack MH, Sung YK, Chung EJ, Im SU, Ahn
JS, Kim MK and Kim JC: Dihydrotestosterone-inducible dickkopf 1
from balding dermal papilla cells causes apoptosis in follicular
keratinocytes. J Invest Dermatol. 128:262–269. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ulici V, Kelley KL, Azcarate-Peril MA,
Cleveland RJ, Sartor RB, Schwartz TA and Loeser RF: Osteoarthritis
induced by destabilization of the medial meniscus is reduced in
germ-free mice. Osteoarthritis Cartilage. 26:1098–1109. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Chen J, Wang A and Wang Q: Dysbiosis of
the gut microbiome is a risk factor for osteoarthritis in older
female adults: A case control study. BMC Bioinformatics.
22:2992021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Fortuna R, Hart DA, Sharkey KA, Schachar
RA, Johnston K and Reimer RA: Effect of a prebiotic supplement on
knee joint function, gut microbiota, and inflammation in adults
with co-morbid obesity and knee osteoarthritis: Study protocol for
a randomized controlled trial. Trials. 22:2552021. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Hahn AK, Wallace CW, Welhaven HD, Brooks
E, McAlpine M, Christiansen BA, Walk ST and June RK: The microbiome
mediates epiphyseal bone loss and metabolomic changes after acute
joint trauma in mice. Osteoarthritis Cartilage. 29:882–893. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ilesanmi-Oyelere BL, Roy NC and Kruger MC:
Modulation of bone and joint biomarkers, gut microbiota, and
inflammation status by synbiotic supplementation and weight-bearing
exercise: Human study protocol for a randomized controlled trial.
JMIR Res Protoc. 10:e301312021. View
Article : Google Scholar : PubMed/NCBI
|
|
59
|
Lan H, Hong W, Qian D, Peng F, Li H, Liang
C, Du M, Gu J, Mai J, Bai B and Peng G: Quercetin modulates the gut
microbiota as well as the metabolome in a rat model of
osteoarthritis. Bioengineered. 12:6240–6250. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Pedersini P, Savoldi M, Berjano P and
Villafañe JH: A probiotic intervention on pain hypersensitivity and
microbiota composition in patients with osteoarthritis pain: Study
protocol for a randomized controlled trial. Arch Rheumatol.
36:296–301. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Mi Y, Yi N, Xu X, Zeng F, Li N, Tan X,
Gong Z, Yan K, Kuang G and Lu M: Prebiotics alleviate cartilage
degradation and inflammation in post-traumatic osteoarthritic mice
by modulating the gut barrier and fecal metabolomics. Food Funct.
14:4065–4077. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Piva F, Gervois P, Karrout Y, Sané F and
Romond MB: Gut-joint axis: Impact of bifidobacterial cell wall
lipoproteins on arthritis development. Nutrients. 15:48612023.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Wang X, Wu Y, Liu Y, Chen F, Chen S, Zhang
F, Li S, Wang C, Gong Y, Huang R, et al: Altered gut microbiome
profile in patients with knee osteoarthritis. Front Microbiol.
14:11534242023. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Meffre D, Grenier J, Bernard S, Courtin F,
Dudev T, Shackleford G, Jafarian-Tehrani M and Massaad C: Wnt and
lithium: A common destiny in the therapy of nervous system
pathologies? Cell Mol Life Sci. 71:1123–1148. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Zhao L, Lai Y, Jiao H, Li J, Lu K and
Huang J: CRISPR-mediated Sox9 activation and RelA inhibition
enhance cell therapy for osteoarthritis. Mol Ther. 32:2549–2562.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Naeem M, Majeed S, Hoque MZ and Ahmad I:
Latest developed strategies to minimize the off-target effects in
CRISPR-cas-mediated genome editing. Cells. 9:16082020. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Klermund J, Rhiel M, Kocher T, Chmielewski
KO, Bischof J, Andrieux G, El Gaz M, Hainzl S, Boerries M, Cornu
TI, et al: On- and off-target effects of paired CRISPR-Cas nickase
in primary human cells. Mol Ther. 32:1298–1310. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Hitch TCA, Hall LJ, Walsh SK, Leventhal
GE, Slack E, de Wouters T, Walter J and Clavel T: Microbiome-based
interventions to modulate gut ecology and the immune system.
Mucosal Immunol. 15:1095–1113. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Porcari S, Fusco W, Spivak I, Fiorani M,
Gasbarrini A, Elinav E, Cammarota G and Ianiro G: Fine-tuning the
gut ecosystem: The current landscape and outlook of artificial
microbiome therapeutics. Lancet Gastroenterol Hepatol. 9:460–475.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Yu M, Yang Y, Sykes M and Wang S:
Small-molecule inhibitors of tankyrases as prospective therapeutics
for cancer. J Med Chem. 65:5244–5273. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Zhong X, Zhang F, Yin X, Cao H, Wang X,
Liu D, Chen J and Chen X: Bone homeostasis and gut
microbial-dependent signaling pathways. J Microbiol Biotechnol.
31:765–774. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Ticinesi A, Siniscalchi C, Meschi T and
Nouvenne A: Gut microbiome and bone health: Update on mechanisms,
clinical correlations, and possible treatment strategies.
Osteoporos Int. 36:167–191. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Liu Y, Da W, Xu MJ, Xiao CX, Deng T, Zhou
SL, Chen XT, Zhou YJ, Tang L, Nie Y, et al: Single-cell
transcriptomics reveals novel chondrocyte and osteoblast subtypes
and their role in knee osteoarthritis pathogenesis. Signal
Transduct Target Ther. 10:402025. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Li J, Yang X, Chu Q, Xie L, Ding Y, Xu X,
Timko MP and Fan L: Multi-omics molecular biomarkers and database
of osteoarthritis. Database (Oxford). 2022:baac0522022. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Wang Y, Zeng T, Tang D, Cui H, Wan Y and
Tang H: Integrated multi-omics analyses reveal lipid metabolic
signature in osteoarthritis. J Mol Biol. 437:1688882025. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Ji Q, Zheng Y, Zhang G, Hu Y, Fan X, Hou
Y, Wen L, Li L, Xu Y, Wang Y and Tang F: Single-cell RNA-seq
analysis reveals the progression of human osteoarthritis. Ann Rheum
Dis. 78:100–110. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Feng B, Lu J, Han Y, Han Y, Qiu X and Zeng
Z: The role of short-chain fatty acids in the regulation of
osteoporosis: new perspectives from gut microbiota to bone health:
A review. Medicine (Baltimore). 103:e394712024. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Kumar SS, Fathima A, Srihari P and Jamma
T: Host-gut microbiota derived secondary metabolite mediated
regulation of Wnt/β-catenin pathway: A potential therapeutic axis
in IBD and CRC. Front Oncol. 14:13925652024. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Choi YR, Collins KH, Lee JW, Kang HJ and
Guilak F: Genome engineering for osteoarthritis: From designer
cells to disease-modifying drugs. Tissue Eng Regen Med. 16:335–343.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Tyagi AM, Yu M, Darby TM, Vaccaro C, Li
JY, Owens JA, Hsu E, Adams J, Weitzmann MN, Jones RM and Pacifici
R: The microbial metabolite butyrate stimulates bone formation via
T regulatory cell-mediated regulation of WNT10B expression.
Immunity. 49:1116–1131.e7. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Chaudhry N, Muhammad H, Seidl C, Downes D,
Young DA, Hao Y, Zhu L and Vincent TL: Highly efficient
CRISPR-Cas9-mediated editing identifies novel mechanosensitive
microRNA-140 targets in primary human articular chondrocytes.
Osteoarthritis Cartilage. 30:596–604. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Seidl CI, Fulga TA and Murphy CL:
CRISPR-Cas9 targeting of MMP13 in human chondrocytes leads to
significantly reduced levels of the metalloproteinase and enhanced
type II collagen accumulation. Osteoarthritis Cartilage.
27:140–147. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Ortinski PI, O'Donovan B, Dong X and
Kantor B: Integrase-deficient lentiviral vector as an all-in-one
platform for highly efficient CRISPR/Cas9-mediated gene editing.
Mol Ther Methods Clin Dev. 5:153–164. 2017. View Article : Google Scholar : PubMed/NCBI
|