Introduction
Lung cancer remains one of the leading causes of
tumor-related deaths worldwide, accounting for ~25% of global
cancer fatalities (1,2). It is primarily classified into two
histological types: Small cell lung cancer (SCLC) and non-SCLC
(NSCLC) (3,4). NSCLC, which includes adenocarcinoma,
squamous cell carcinoma and large cell lung cancer, represents ~85%
of all lung cancer cases (5).
Currently, chemotherapy is the first-line treatment for SCLC and
stage IV NSCLC (6); however,
chemotherapy often involves administering the maximum tolerated
intravenous dose of the relevant drug, which can display result in
marked toxicity to healthy tissues. Furthermore, despite the
initial effectiveness of chemotherapy, drug resistance and toxicity
notably limit its long-term clinical success (7). Despite progress in early detection
and treatment strategies, the overall 5-year survival rate for lung
cancer remains at <20% (8).
Therefore, developing new therapeutic agents with improved efficacy
and safety profiles for lung cancer treatment is of substantial
importance.
Ferroptosis is an iron-dependent form of regulated
cell death driven by the accumulation of lipid peroxidation
products and reactive oxygen species (ROS) (9). Unlike apoptosis, a process that is
characterized by notable morphological changes such as cell
membrane rupture and nuclear fragmentation (10), ferroptosis is marked by
iron-dependent lipid peroxidation that causes cellular damage and
death. This process involves excessive ROS accumulation and the
disruption of antioxidant defense systems, distinguishing it from
traditional apoptotic pathways. Notably, induction of ferroptosis
has emerged as a promising therapeutic strategy, particularly for
malignancies resistant to conventional treatments, positioning
ferroptosis as a novel approach to induce cancer cell death
(11–13).
System Xc−, composed of the subunits
solute carrier family 7 member 11 (SLC7A11/xCT) and solute carrier
family 3 member 2, functions as a cystine/glutamate antiporter that
imports cystine into cells while exporting glutamate (14,15).
Inhibition of SLC7A11 by compounds such as erastin blocks cystine
uptake, leading to depletion of intracellular L-cysteine and
reduced synthesis of glutathione (GSH) (16). GSH is an important cofactor for GSH
peroxidase 4 (GPX4), which detoxifies lipid peroxides by converting
them into non-toxic lipid alcohols (17,18).
When L-cysteine is depleted, GPX4 activity is inhibited, resulting
in excessive lipid peroxide accumulation and triggering ferroptosis
(16). Studies have detected the
upregulation of xCT in patients with NSCLC, and targeting xCT has
been shown to suppress tumor growth both in vitro and in
vivo (19,20).
Panax notoginseng, a member of the Araliaceae
family, has been widely used as a traditional Chinese medicine for
thousands of years. Notoginsenoside R1 (NG-R1), one of the primary
bioactive compounds extracted from the root of Panax
notoginseng, exhibits diverse pharmacological activities,
including cardiovascular protection (21), neuroprotection (22) and anticancer effects (23). NG-R1 has been shown to reduce the
incidence of lung cancer in a urethane-induced mouse model by
improving lung barrier permeability and ameliorating
histopathological changes (24).
Additionally, the ethanol extract of Panax notoginseng
inhibits migration, invasion, adhesion and metastasis of colorectal
cancer cells by modulating the expression of key regulatory
molecules (25). Despite numerous
reports on the antitumor efficacy of NGs, to the best of our
knowledge, no studies have investigated NG-induced ferroptosis in
tumor cells. The present study aimed to explore the effects of
NG-R1 on NSCLC using the human NSCLC cell line A549.
Materials and methods
Cell line, reagents and
antibodies
Human NSCLC A549 cells were obtained from Shanghai
Fuheng Biotechnology Co., Ltd. (cat. no. FH0045). NG-R1 was
purchased from Shanghai Macklin Biochemical Co., Ltd. (cat. no.
N814983). The ROS detection kit (cat. no. S0033), YF 594 Click-iT
EdU Kit (cat. no. C0078S), BCA protein concentration assay kit
(cat. no. P0012S), 2′,7′-dichlorofluorescein diacetate (DCFH-DA;
cat. no. S1105S), RIPA lysis buffer (cat. no. P0013B) and
protease-phosphatase inhibitor cocktail (cat. no. P1045) were all
sourced from Beyotime Biotechnology. Primary antibodies against
GPX4 (cat. no. ab125066), ferritin heavy chain 1 (FTH1) (cat. no.
ab183781), transferrin receptor 1 (TfR1) (cat. no. ab214039) and
SLC7A11 (cat. no. ab175186) were purchased from Abcam. GAPDH
monoclonal antibody (cat. no. AF7021), secondary antibody (goat
anti-rabbit IgG, HRP-conjugated; cat. no. S0001) and ECL
luminescent substrate (cat. no. K002) were obtained from Affinity
Biosciences. Cell culture plates including 96-well (cat. no. 3599),
24-well (cat. no. 3527) and 6-well (cat. no. 3516), as well as
Matrigel® and Transwell inserts (cat. no. 3422), were
purchased from Corning, Inc. Crystal Violet Staining Solution
(0.1%; cat. no. G1063) was supplied by Beijing Solarbio Science
& Technology Co., Ltd. Fetal bovine serum (FBS; cat. no.
A5256701) and Dulbecco's modified Eagle's medium (DMEM; cat. no.
11965092) were purchased from Gibco; Thermo Fisher Scientific, Inc.
Equipment used included a CO2 incubator (model XD-101;
SANYO), precision balance (model AUW-220D; Shimadzu Corporation),
refrigerated centrifuge (model MicroCL 21R; Thermo Fisher
Scientific, Inc.), inverted biological light microscope (model
IX73; Olympus Corporation), microplate reader (model ×800; Tecan
Group, Ltd.), electrophoresis and protein transfer system (model
041BR121667; Bio-Rad Laboratories, Inc.), gel imaging system (model
Tanon-4600, Tanon Science and Technology Co., Ltd.) and flow
cytometer (CytoFLEX V2-B2-R2; Beckman Coulter, Inc.).
Cell culture
Human NSCLC A549 cells were cultured in high-glucose
DMEM supplemented with 10% FBS and 100 U/ml penicillin-streptomycin
at 37°C in a humidified atmosphere containing 5% CO2.
Cells were passaged at a 1:3 ratio every 3 days upon reaching ~90%
confluency. Regular mycoplasma contamination tests were performed
and yielded negative results.
Cell viability test
A549 cells in the logarithmic growth phase were
seeded into 96-well plates at a density of 5×103
cells/well and allowed to adhere for 24 h at 37°C. Subsequently,
the cells were treated with various concentrations of NG-R1 (0,
0.1, 0.2, 0.4, 0.8, 1.6 and 2.0 mg/ml) for 72 h at 37°C. Following
treatment, 20 µl MTT solution (0.5 mg/ml) was added to each well
and incubated for 4 h at 37°C. The supernatant was then carefully
removed, and 150 µl DMSO was added to each well to dissolve the
formazan crystals. Plates were gently shaken for 10 min at room
temperature, and absorbance (A) was measured at 570 nm using a
microplate reader; cell viability was calculated using the formula:
Cell viability (%)=(Adrug group/Ablank control
group) ×100. The half-maximal inhibitory concentration
(IC50) was determined using the IC50
calculator, an online tool provided by AAT Bioquest (https://www.aatbio.com/tools/ic50-calculator).
Cell proliferation detection
A549 cells in the logarithmic growth phase were
seeded into 24-well plates at a density of 2.5×104
cells/well and allowed to adhere for 24 h at 37°C. Subsequently,
the cells were treated with varying concentrations of NG-R1 (0,
0.4, 0.8 and 1.6 mg/ml) for 72 h at 37°C. Following treatment, the
supernatant was discarded and 10 µM EdU solution was added to each
well for a 2-h incubation at 37°C. EdU staining was performed using
the YF 594 Click-iT EdU Kit, followed by nuclear counterstaining
with Hoechst 33342, according to the manufacturer's protocol,
including three PBS washes before microscopic imaging. Images were
captured using an inverted fluorescence microscope and subsequently
analyzed with ImageJ software (version 1.53k; National Institutes
of Health).
Wound healing assay
A549 cells in the logarithmic growth phase were
seeded into 6-well plates at a density of 1.0×106
cells/well and cultured in medium containing 10% FBS for 24 h at
37°C to allow adhesion. Subsequently, a 10-µl pipette tip was used
to create three straight scratches per well. Floating cells were
removed by washing with PBS and images of the scratch area at 0 h
(W0 h) were captured using an inverted biological light
microscope. Subsequently, cells were treated with different
concentrations of NG-R1 (0, 0.4, 0.8 and 1.6 mg/ml) in medium
containing 2% FBS and incubated for 72 h at 37°C before images were
captured again. Images were analyzed using ImageJ software and the
cell migration rate was calculated using the formula: Cell
migration rate (%)=[(W0 h-W72
h)/W0 h] ×100.
Cell invasion detection
Serum-free high-glucose DMEM was used to dilute
Matrigel basement membrane matrix at a ratio of 1:8. Then, 50 µl
diluted Matrigel was added to the upper chamber of each Transwell
insert and incubated at 37°C for 1 h to allow gelation, after which
any residual liquid was carefully aspirated. A549 cells were
suspended in serum-free high-glucose DMEM at a density of
1×105 cells/ml. A volume of 200 µl of this cell
suspension was added per well to the upper chamber coated with
Matrigel. The lower chamber was filled with 600 µl high-glucose
DMEM containing 10% FBS and different concentrations of NG-R1 (0,
0.4, 0.8 and 1.6 mg/ml) and the setup was incubated for 72 h at
37°C. After incubation, non-invasive cells on the upper surface of
the membrane were gently removed with a cotton swab. The invasive
cells on the lower membrane surface were fixed with 4%
paraformaldehyde for 15 min at room temperature, washed twice with
PBS, stained with 0.1% crystal violet for 10 min at room
temperature and washed twice with PBS. Once dried, images of the
invaded cells were captured using an inverted biological light
microscope and analyzed using ImageJ software.
Detection of intracellular ROS
A549 cells in the logarithmic growth phase were
seeded into 6-well plates at a density of 5.0×105
cells/well and allowed to adhere for 24 h at 37°C. Subsequently,
the cells were treated with varying concentrations of NG-R1 (0,
0.4, 0.8 and 1.6 mg/ml) for 72 h at 37°C. The supernatant was then
discarded, and 1 ml DCFH-DA probe (diluted 1:1,000 in serum-free
medium) was added to each well. Cells were incubated at 37°C for 20
min, washed twice with PBS and subsequently digested with 0.05%
trypsin. The cells were collected, resuspended in PBS and analyzed
by flow cytometry recording 10,000 events per sample. Subsequent
data analysis was performed using FlowJo software (version 10.0; BD
Biosciences) to measure DCFH-DA fluorescence intensity, which
reflects intracellular ROS levels.
Detection of expression levels of
ferroptosis-related proteins
A549 cells, after being treated with varying
concentrations of NG-R1 (0, 0.4, 0.8 and 1.6 mg/ml) for 72 h at
37°C, were lysed using RIPA buffer supplemented with 1% protease
and phosphatase inhibitor cocktail. Protein concentrations were
determined using a BCA assay kit. Subsequently, 20 µg total protein
was mixed with loading buffer and denatured at 100°C for 10 min.
Proteins were then separated by SDS-PAGE on 12% gels and
transferred onto PVDF membranes using the wet transfer method.
Thereafter, the membranes were blocked with 5% skim milk at room
temperature for 1 h and the blocked membranes were incubated with
specific primary antibodies at 4°C overnight. The primary
antibodies and their dilutions were as follows: Anti-GPX4
(1:1,000), anti-FTH1 (1:1,000), anti-TfR1 (1:1,000), anti-SLC7A11
(1:1,000), and anti-GAPDH (1:5,000). Following primary antibody
incubation, the membranes were incubated with an HRP-conjugated
secondary antibody (1:5,000) at room temperature for 30 min.
Protein bands were visualized using the aforementioned ECL kit
according to the manufacturer's instructions and images were
captured with the Tanon gel imaging system. Band intensities were
semi-quantified with ImageJ software.
Statistical analysis
Experimental results from three independent
experiments are presented as the mean ± standard deviation. All
data were analyzed using GraphPad Prism 7.0 software (Dotmatics) by
one-way ANOVA followed by Dunnett's multiple comparisons test,
which compared all treatment groups against a single control group.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effect of NG-R1 on A549 cell
viability
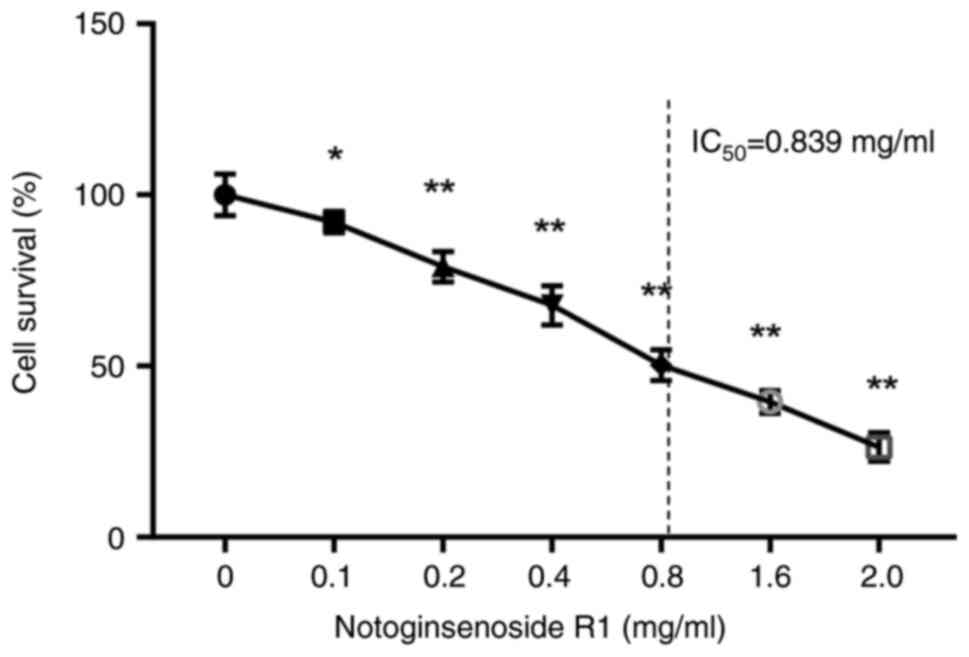
As shown in Fig. 1,
NG-R1 exhibited significant dose-dependent cytotoxicity against
A549 cells. The IC50 value for the inhibitory effect of
NG-R1 was calculated to be 0.839 mg/ml. Therefore, the
concentrations of 0.4, 0.8 and 1.6 mg/ml were selected for
subsequent experiments.
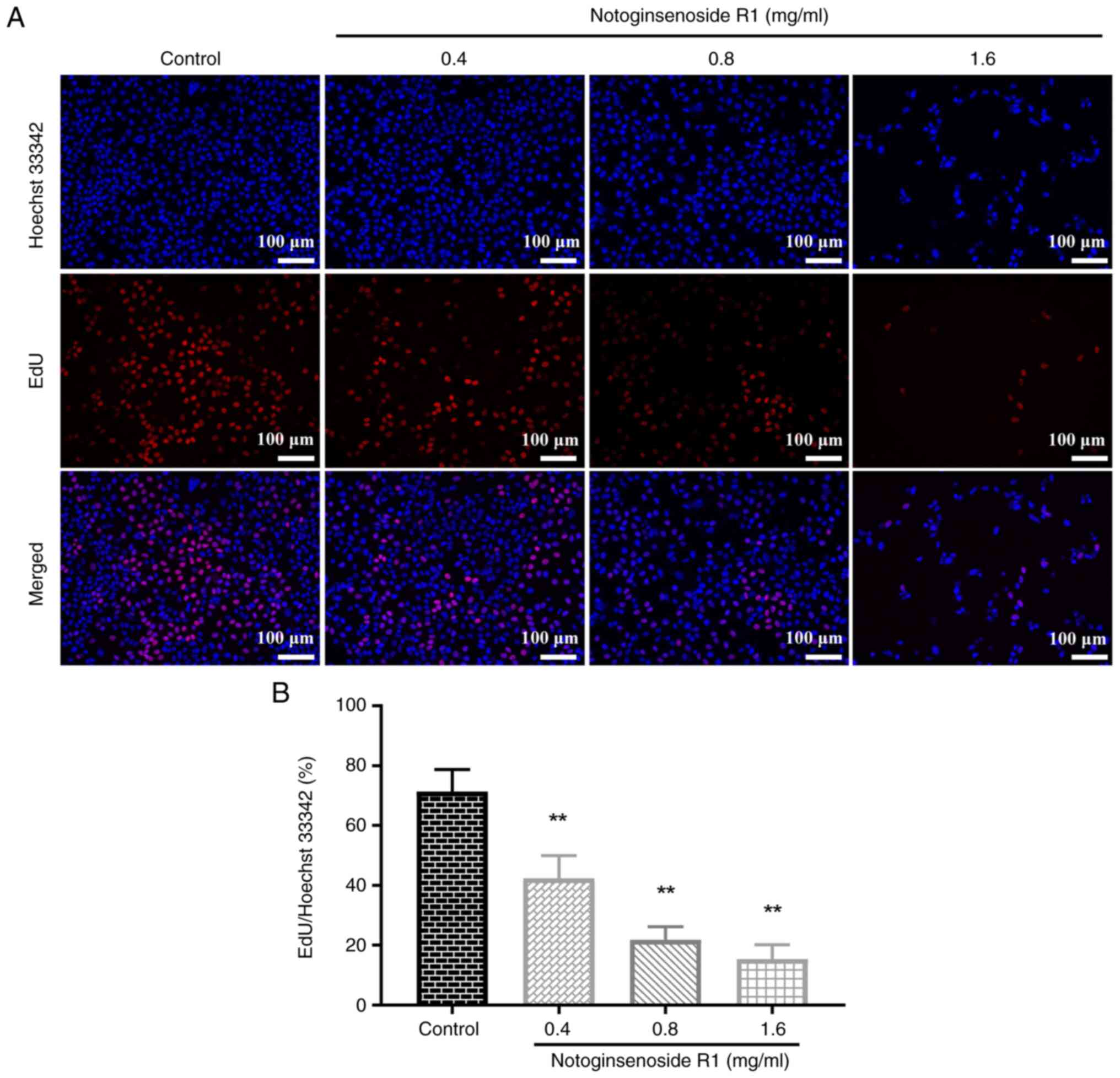
Effect of NG-R1 on A549 cell
proliferation
The BeyoClick EdU-594 cell proliferation detection
kit was used to assess the effect of NG-R1 on A549 cell
proliferation. As shown in Fig. 2,
treatment with 0.4 mg/ml NG-R1 significantly reduced the
proliferation rate of A549 cells compared with that in the control
group (P<0.01). Furthermore, the proliferation rate of A549
cells decreased progressively with increasing concentrations of
NG-R1, indicating that NG-R1 significantly inhibited A549 cell
proliferation in a dose-dependent manner (all P<0.01).
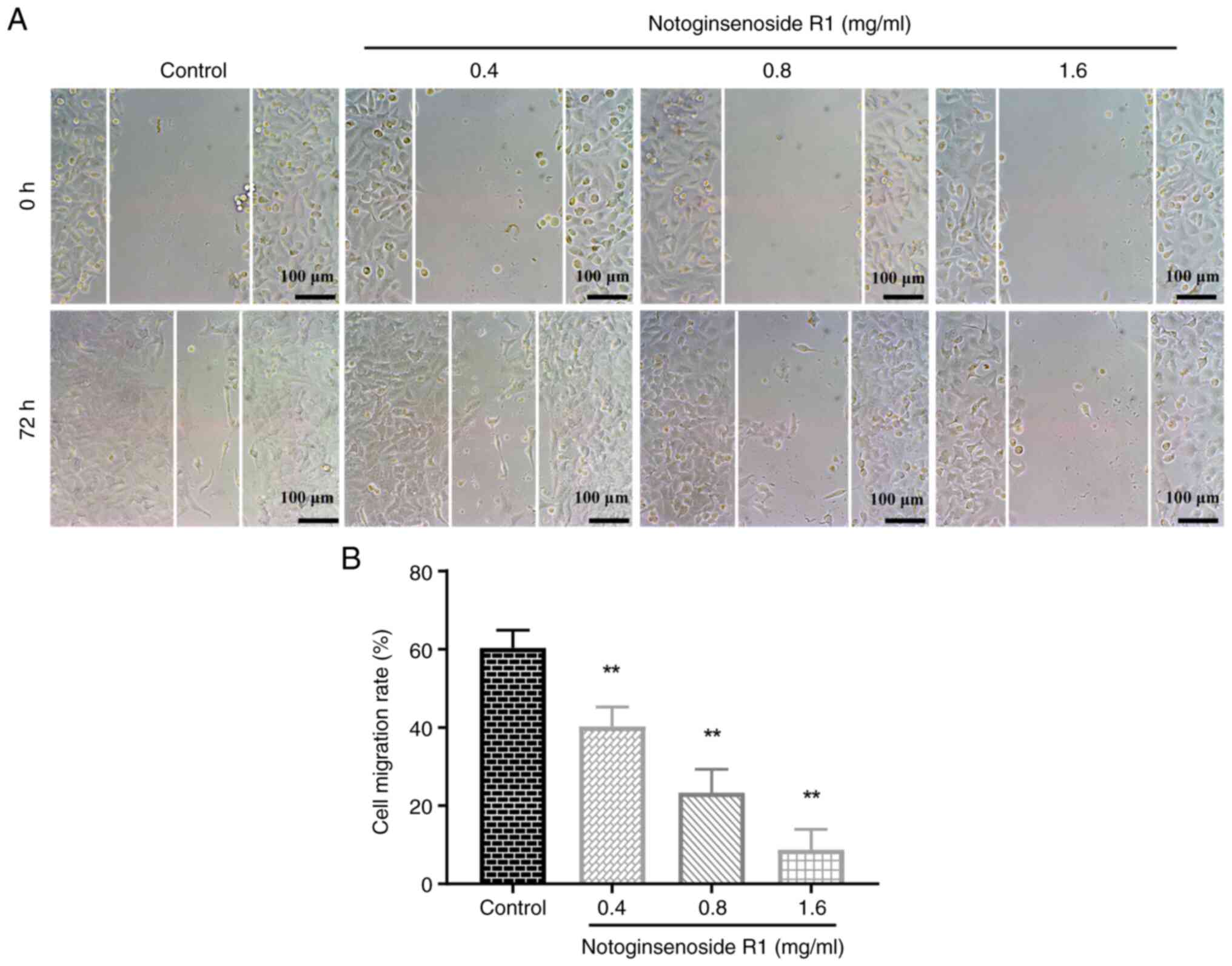
Effect of NG-R1 on A549 cell
migration
The wound healing assay results demonstrated that
the migration rates in the 0.4, 0.8 and 1.6 mg/ml NG-R1 treatment
groups were significantly lower than those in the control group
(all P<0.01; Fig. 3),
indicating that NG-R1 effectively inhibited the migration of A549
cells.
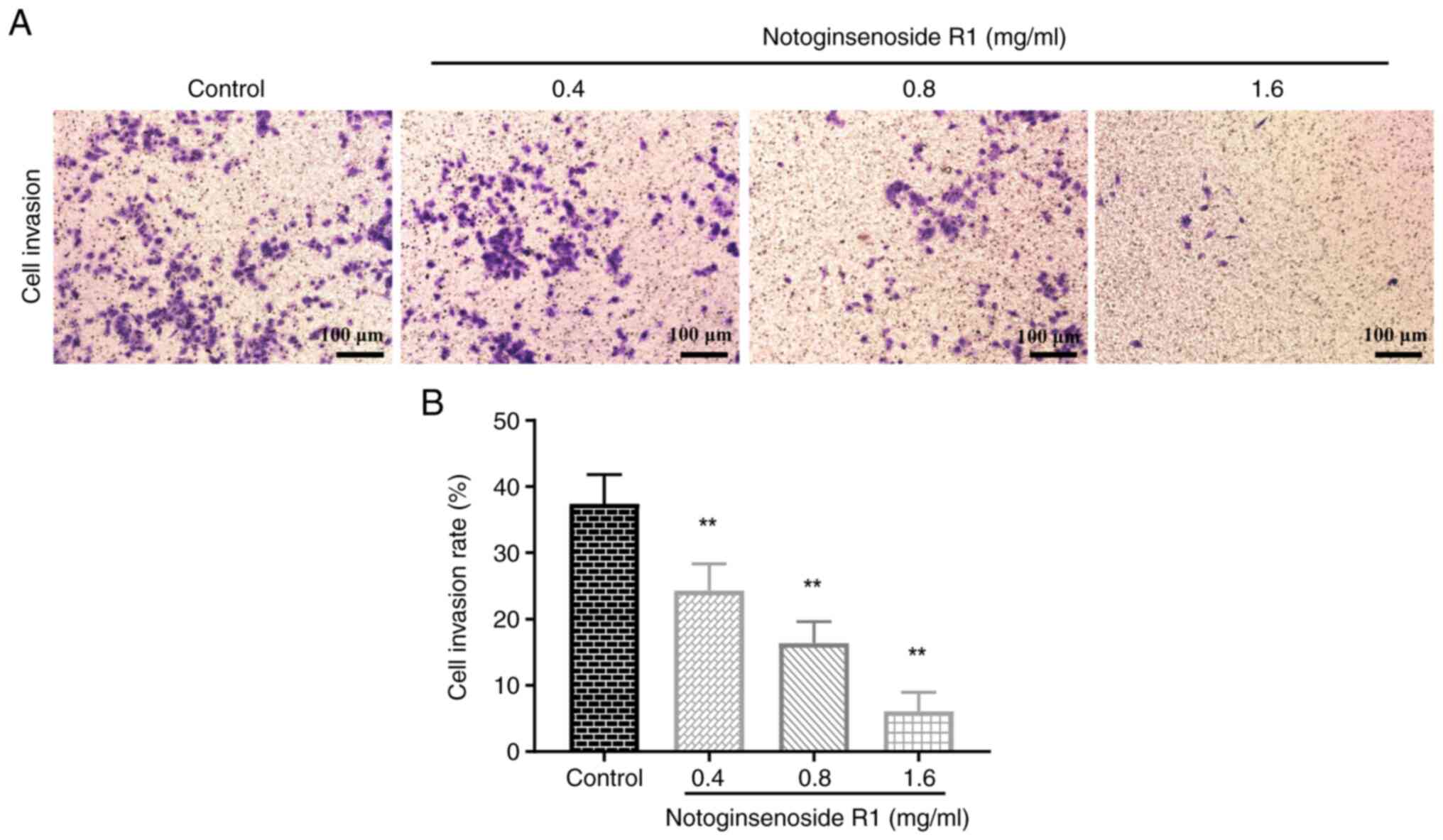
Effect of NG-R1 on A549 cell
invasion
Compared with that in the control group, the
invasion rate of A549 cells was significantly reduced in a
dose-dependent manner following 72 h of treatment with 0.4, 0.8 and
1.6 mg/ml NG-R1 (all P<0.01; Fig.
4), indicating that NG-R1 inhibited the invasive ability of
A549 cells.
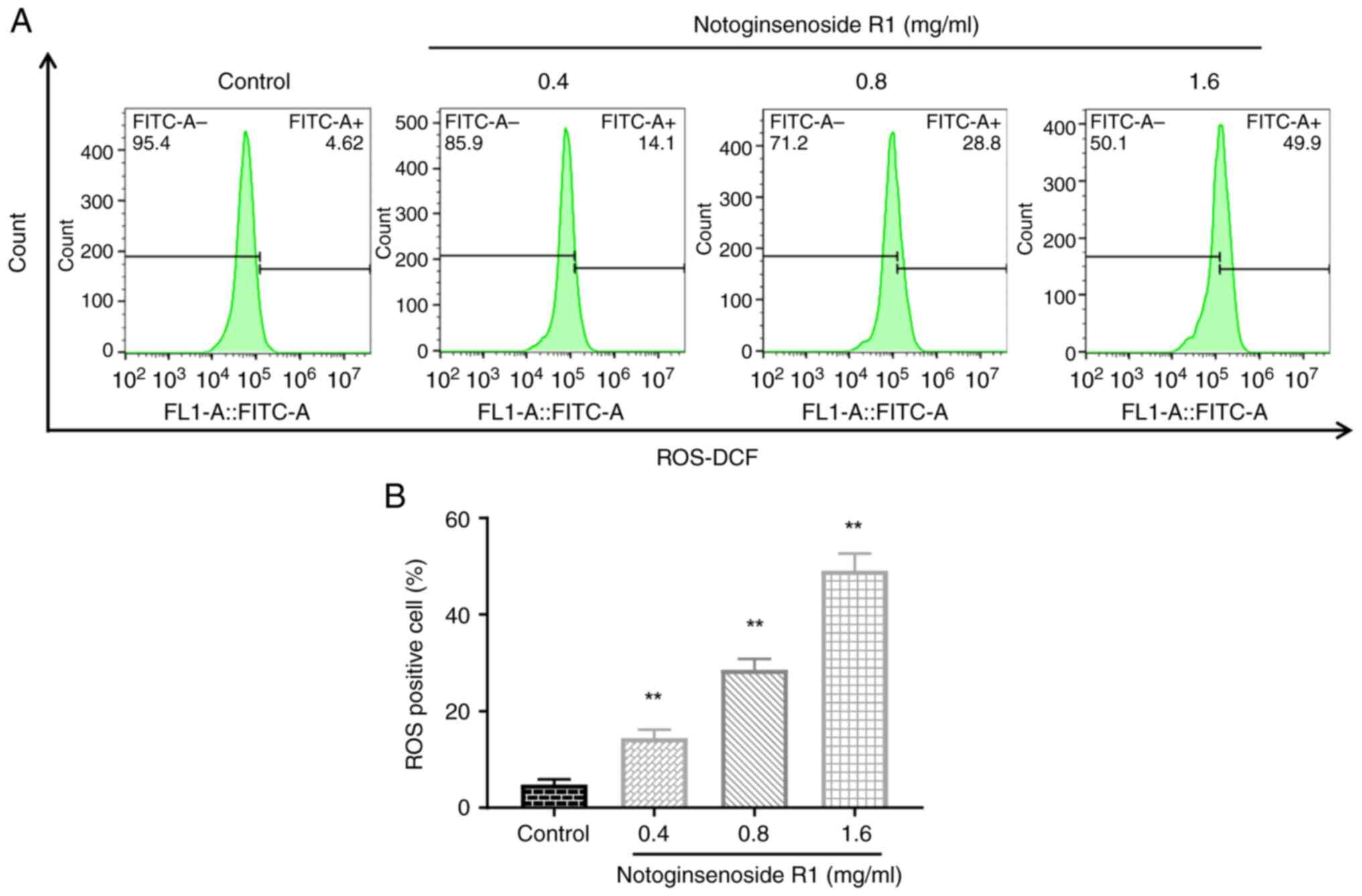
Effect of NG-R1 on the ROS levels of
A549 cells
The fluorescent probe DCFH-DA was used to measure
ROS levels in A549 cells following 72 h of treatment with various
concentrations of NG-R1. As shown in Fig. 5, treatment with 0.4, 0.8 or 1.6
mg/ml NG-R1 significantly increased ROS levels in A549 cells (all
P<0.01), which may indicate the induction of ferroptosis.
Effect of NG-R1 on the expression
levels of ferroptosis-related proteins in A549 cells
As shown in Fig. 6,
treatment with 0.4 mg/ml NG-R1 did not significantly alter TfR1
protein levels, whereas 0.8 and 1.6 mg/ml concentrations
significantly increased TfR1 expression in A549 cells (all
P<0.01). Additionally, NG-R1 treatment at concentrations of 0.4,
0.8 and 1.6 mg/ml significantly downregulated the protein
expression levels of SLC7A11 (P<0.05, P<0.01), FTH1
(P<0.05 and P<0.01) and GPX4 (P<0.05 and P<0.01). These
results suggested that NG-R1 may modulate ferroptosis-related
proteins and induce ferroptosis in A549 cells.
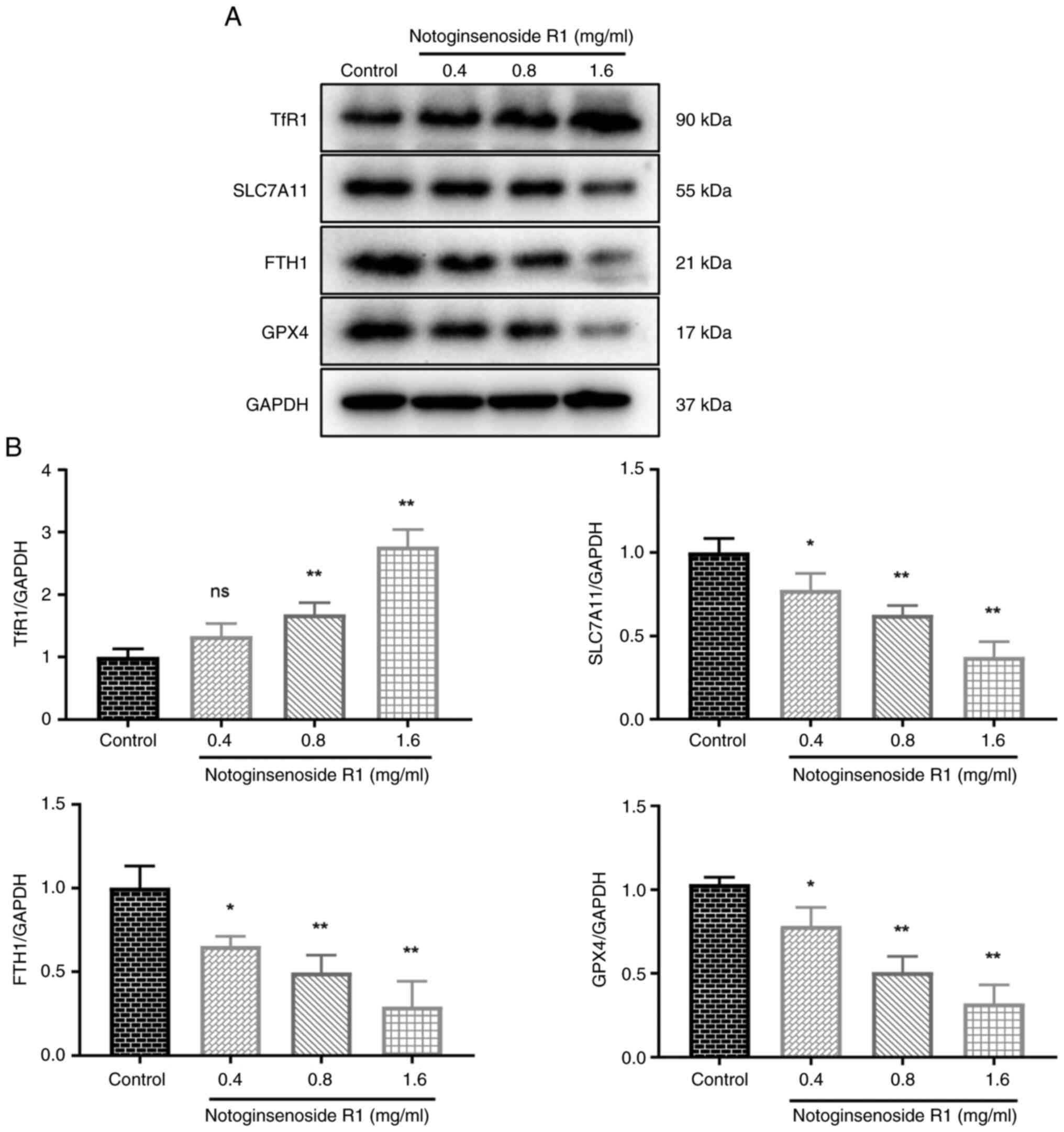 | Figure 6.Effect of Notoginsenoside R1 on the
expression levels of ferroptosis-related proteins (n=3). (A)
Representative western blot images of the ferroptosis-related
proteins. (B) Protein expression levels of TfR1, SLC7A11, FTH1 and
GPX4 were examined by western blotting. Data are presented as the
mean ± SD. The one-way ANOVA results were as follows: TfR1:
F (3, 8), 41.41; SLC7A11: F (3, 8), 28.67; FTH1:
F (3, 8), 19.68; GPX4: F (3, 8), 32.63. *P<0.05
and **P<0.01 vs. control group. FTH1, ferritin heavy chain 1;
GPX4, glutathione peroxidase 4; ns, non-significant; SLC7A11,
solute carrier family 7 member 11; TfR1, transferrin receptor
1. |
Discussion
The present study is, to the best of our knowledge,
the first to demonstrate that NG-R1 induces ferroptosis in human
NSCLC A549 cells and to explore its underlying mechanisms. The
results of the present study showed that NG-R1 significantly
inhibited the viability, proliferation, migration and invasion of
A549 cells in a dose-dependent manner. Notably, western blot
analysis revealed that NG-R1 upregulated TfR1 while downregulating
SLC7A11, GPX4 and FTH1, suggesting that its anticancer effects may
have been mediated through ferroptosis in A549 cells (16).
The findings of the present study are consistent
with those of previous studies demonstrating the anticancer effects
of NG-R1 and its analogs, particularly against NSCLC (23,26).
In a similar manner to established ferroptosis inducers such as
erastin, NG-R1 promotes ferroptosis by elevating intracellular ROS
levels (27). The pronounced
inhibition of A549 cell proliferation, migration and invasion
observed in the present study further supports the potential of
NG-R1 as an effective anticancer agent, providing new scientific
evidence for its application in NSCLC treatment (23,26).
To further elucidate the molecular mechanisms
underlying NG-R1-induced ferroptosis, the present study examined
changes in the expression of key ferroptosis-related proteins. The
results of the present study suggested that NG-R1 may regulate iron
metabolism through multiple pathways, thereby activating
ferroptosis, based on the findings from western blot analysis of
related proteins. Notably, NG-R1 significantly downregulated
SLC7A11, an important protein responsible for maintaining
intracellular GSH levels and suppressing lipid peroxidation
(27). SLC7A11 is commonly
upregulated in NSCLC cells, contributing to tumor growth (19), and its downregulation by NG-R1 may
reduce GSH synthesis, leading to increased lipid peroxidation and
ferroptosis induction. The precise regulatory mechanism of NG-R1 on
SLC7A11 warrants further investigation, potentially involving
signaling pathways such as those mediated by nuclear factor
erythroid 2-related factor 2 (Nrf2), p53 or other transcription
factors (28,29). Additionally, NG-R1 significantly
decreased the expression of GPX4, an important enzyme responsible
for repairing lipid peroxides (21,27).
This reduction may be secondary to GSH depletion following SLC7A11
downregulation, resulting in impaired GPX4 activity and the
accumulation of toxic lipid peroxides, a hallmark of ferroptosis.
Furthermore, NG-R1 could have modulated iron metabolism by
significantly upregulating TfR1 and downregulating FTH1, thereby
increasing the labile iron pool and promoting ferroptosis via
enhanced Fenton reaction-mediated lipid peroxidation (13,27,30).
Collectively, these findings suggest that NG-R1 induced ferroptosis
in NSCLC cells by orchestrating the suppression of antioxidant
defenses and dysregulation of iron homeostasis.
Although the present study provides evidence that
NG-R1 induced ferroptosis in A549 cells, several important
limitations remain. First, the lack of rescue experiments using
ferroptosis-specific inhibitors, such as ferrostatin-1 or
liproxstatin-1, and iron chelators such as deferoxamine, limits the
suggestion that the observed cytotoxicity is primarily due to
ferroptosis. Such experiments are important to exclude other forms
of cell death, including apoptosis and necroptosis (27). Second, while alterations in key
ferroptosis-related proteins were observed, the upstream regulatory
mechanisms by which NG-R1 modulates proteins such as SLC7A11 and
TfR1 remain ambiguous. Future investigations should focus on
elucidating how NG-R1 influences these targets at the
transcriptional and post-transcriptional levels, including the
potential involvement of signaling pathways such as Nrf2, p53 and
activating transcription factor 4 (28,29).
In conclusion, in the present study, NG-R1 was shown
to inhibit the proliferation and migration of A549 cells, while
significantly increasing intracellular ROS levels. Western blot
analysis revealed that treatment with NG-R1 significantly
upregulated TfR1 expression, and downregulated SLC7A11, GPX4 and
FTH1 levels. These findings suggest that NG-R1 may induce
ferroptosis in A549 cells and thus holds potential as an anti-lung
cancer agent.
Acknowledgements
Not applicable.
Funding
The present work was supported by a grant from the Science and
the Technology Plan Project of Yunnan Provincial Department of
Science and Technology (grant no. 202101BA070001-267).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YZ was responsible for conceptualization, data
curation, formal analysis, funding acquisition, investigation,
methodology, project administration, resources, and writing,
reviewing and editing the manuscript. ZM was responsible for data
curation [data collection, cleaning (which involved data inspection
and error correction: examining the collected raw data for
recording errors, anomalies, or clearly illogical inputs)and
validation], formal analysis, investigation, and writing, reviewing
and editing the manuscript. YD was responsible for formal analysis,
investigation and project administration. YZ and ZM confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dubey AK, Gupta U and Jain S: Epidemiology
of lung cancer, approaches for its prediction: A systematic review
and analysis. Chin J Cancer. 35:712016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Colombet M, Soerjomataram I,
Parkin DM, Piñeros M, Znaor A and Bray F: Cancer statistics for the
year 2020: An overview. Int J Cancer. 149:778–789. 2021. View Article : Google Scholar
|
|
3
|
Jurisic V, Obradovic J, Nikolic N, Javorac
J, Perin B and Milasin J: Analyses of P16INK4a gene
promoter methylation relative to molecular, demographic, clinical
parameters characteristics in non-small cell lung cancer patients:
A pilot study. Mol Biol Rep. 50:971–979. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Petrović M, Bukumirić Z, Zdravković V,
Mitrović S, Atkinson HD and Jurišić V: The prognostic significance
of the circulating neuroendocrine markers chromogranin A,
pro-gastrin-releasing peptide, and neuron-specific enolase in
patients with small-cell lung cancer. Med Oncol. 31:8232014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Socinski MA and Pennell NA: Best practices
in treatment selection for patients with advanced NSCLC. Cancer
Control. 23 (4 Suppl):S2–S4. 2016. View Article : Google Scholar
|
|
6
|
Zappa C and Mousa SA: Non-small cell lung
cancer: Current treatment and future advances. Transl Lung Cancer
Res. 5:288–300. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pilkington G, Boland A, Brown T, Oyee J,
Bagust A and Dickson R: A systematic review of the clinical
effectiveness of first-line chemotherapy for adult patients with
locally advanced or metastatic non-small cell lung cancer. Thorax.
70:359–367. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Luo YH, Wang C, Xu WT, Zhang Y, Zhang T,
Xue H, Li YN, Fu ZR, Wang Y and Jin CH: 18β-Glycyrrhetinic acid Has
Anti-cancer effects via inducing apoptosis and G2/M cell cycle
arrest, and inhibiting migration of A549 lung cancer cells. Onco
Targets Ther. 14:5131–5144. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Proneth B and Conrad M: Ferroptosis and
necroinflammation, a yet poorly explored link. Cell Death Differ.
26:14–24. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Galluzzi L, Vitale I, Aaronson SA, Abrams
JM, Adam D, Agostinis P, Alnemri ES, Altucci L, Amelio I, Andrews
DW, et al: Molecular mechanisms of cell death: Recommendations of
the Nomenclature Committee on Cell Death 2018. Cell Death Differ.
25:486–541. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hassannia B, Vandenabeele P and Berghe TV:
Targeting ferroptosis to iron out cancer. Cancer Cell. 35:830–849.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liang C, Zhang X, Yang M and Dong X:
Recent progress in ferroptosis inducers for cancer therapy. Adv
Mater. 31:19041972019. View Article : Google Scholar
|
|
13
|
Bebber CM, Müller F, Clemente LP, Weber J
and Karstedt SV: Ferroptosis in cancer cell biology. Cancers.
12:1642020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lewerenz J, Hewett SJ, Huang Y, Lambros M,
Gout PW, Kalivas PW, Massie A, Smolders I, Methner A, Pergande M,
et al: The Cystine/glutamate Antiporter system x(c)(−) in health,
disease: From molecular mechanisms to novel therapeutic
opportunities. Antioxid Redox Signal. 18:522–555. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bridges RJ, Natale NR and Patel SA: System
xc− Cystine/glutamate antiporter: An update on molecular
pharmacology and roles within the CNS. Br J Pharmacol. 165:20–34.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta
R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS,
et al: Ferroptosis: An Iron-dependent form of nonapoptotic cell
death. Cell. 149:1060–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang WS, SriRamaratnam R, Welsch ME,
Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji
AF, Clish CB, et al: Regulation of ferroptotic cancer cell death by
GPX4. Cell. 156:317–331. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ursini F, Maiorino M, Valente M, Ferri L
and Gregolin C: Purification from pig liver of a protein which
protects liposomes and biomembranes from peroxidative degradation
and exhibits glutathione peroxidase activity on phosphatidylcholine
hydroperoxides. Biochim Biophys Acta. 710:197–211. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ji X, Qian J, Rahman SMJ, Siska PJ, Zou Y,
Harris BK, Hoeksema MD, Trenary IA, Heidi C, Eisenberg R, et al:
xCT (SLC7A11)-mediated metabolic reprogramming promotes non-small
cell lung cancer progression. Oncogene. 37:5007–5019. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Koppula P, Zhang Y, Zhuang L and Gan B:
Amino acid transporter SLC7A11/xCT at the crossroads of regulating
redox homeostasis and nutrient dependency of cancer. Cancer Commun
(Lond). 38:122018.PubMed/NCBI
|
|
21
|
Sun B, Xiao J, Sun XB and Wu Y:
Notoginsenoside R1 attenuates cardiac dysfunction in endotoxemic
mice: An insight into oestrogen receptor activation and PI3K/Akt
signalling. British J Pharmacology. 168:1758–1770. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Guo Q, Li P, Wang Z, Cheng Y, Wu H, Yang
B, Du S and Lu Y: Brain distribution pharmacokinetics and
integrated pharmacokinetics of Panax Notoginsenoside R1,
Ginsenosides Rg1, Rb1, Re and Rd in rats after intranasal
administration of Panax Notoginseng Saponins assessed by
UPLC/MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci.
969:264–271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee CY, Hsieh SL, Hsieh S, Tsai CC, Hsieh
LC, Kuo YH and Wu CC: Inhibition of human colorectal cancer
metastasis by notoginsenoside R1, an important compound from
Panax notoginseng. Oncol Rep. 37:399–407. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang W, Shu H, Fang L, Tang N, Li Y, Guo
B and Meng F: Cancer inhibition mechanism of lung cancer mouse
model based on dye trace method. Saudi J Biol Sci. 27:1155–1162.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hsieh SL, Hsieh S, Kuo YH, Wang JJ, Wang
JC and Wu CC: Effects of Panax notoginseng on the metastasis
of human colorectal cancer cells. Am J Chin Med. 44:851–870. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou Y, Zhang X, Wang X, Li Y, Liu Z,
Zhang J, Chen L, Xu Y, Yang Y and Wang Z: Ferroptosis in cancer:
From molecular mechanisms to therapeutic applications. Nat Rev
Cancer. 24:1–15. 2024.PubMed/NCBI
|
|
27
|
Xie Y, Hou W, Song X, Yu Y, Huang J, Sun
X, Kang R and Tang D: Ferroptosis: Process and function. Cell Death
Differ. 23:369–79. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Muckenthaler MU, Galy B and Hentze MW:
Systemic iron homeostasis and the iron-responsive
element/iron-regulatory protein (IRE/IRP) regulatory network. Annu
Rev Nutr. 28:197–213. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang WS and Stockwell BR: Synthetic lethal
screening identifies compounds activating iron-dependent,
nonapoptotic cell death in oncogenic-RAS-harboring cancer cells.
Chem Biol. 15:234–245. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ganz T and Nemeth E: Hepcidin and iron
homeostasis. Biochim Biophys Acta. 1823:1434–1443. 2012. View Article : Google Scholar : PubMed/NCBI
|