Introduction
Diabetes mellitus (DM) is a prevalent metabolic and
endocrine disorder characterized by the persistent elevation of
blood sugar levels, termed hyperglycemia, occurring as a result of
impaired insulin secretion and/or function (1). It is estimated that >10.5% of the
global adult population now have this condition (2). DM is a major public health burden
associated with high health care and societal costs, early death
and serious morbidity. Sustained hyperglycemia gradually induces
and aggravates damage to the nervous system, cardiovascular,
kidneys, eyes, and other systemic tissues and organs, resulting in
complications such as cardiovascular and cerebrovascular disease,
diabetic kidney disease (DKD), diabetic retinopathy (DR) and
diabetic neuropathy (DN) (1,3).
Diabetic eye complications mainly include DR,
diabetic cataract and diabetic keratopathy (DK), the latter of
which has historically been neglected. DK is classified as either
primary and secondary, with primary DK developing due to chronic
hyperglycemia, and secondary DK occurring due to diabetic trauma
and following surgery (4). Chronic
hyperglycemia leads to progressive damage to multiple organ
systems, including corneal tissues (5). DK has been reported to occur in
47–64% of diabetic patients (6,7). The
clinical manifestations of DK include dry eye, persistent corneal
epithelial erosion, superficial punctate keratopathy, delayed
epithelial regeneration and decreased corneal sensitivity (8,9). In
more advanced stages, corneal ulceration and scarring may occur,
leading to corneal opacity, and ultimately impaired vision or
permanent vision loss (10).
Nuclear proteins (NPs) are synthesized in the
cytoplasm and specifically targeted to the nucleus of a cell
(11). They include histones and
non-histone proteins, such as structural proteins of the nuclear
matrix and lamina, RNA and DNA polymerases, and gene regulatory
proteins (12). The transport of
NPs from the cytoplasm into the nucleus is mediated by nuclear
localization signals (NLSs), which induce the NPs to pass through
the nuclear pore complex (NPC) (13). NPs play important roles in the
transmission of genetic information, such as in DNA replication,
RNA transcription and processing, and DNA damage repair (14). Certain NPs have been implicated as
mediators of pathological conditions affecting the eye, including
DR, DK and optic neuropathy; examples include peroxisome
proliferator-activated receptors (PPARs) (15,16),
high-mobility group box 1 (HMGB1) (17) and enhancer of zeste homolog 2
(EZH2) (18). Therefore, the roles
of NPs in DK are of considerable research interest. In the present
review, the pathogenesis of DK is described and the roles of
various NPs in the pathophysiological processes of DK are
discussed.
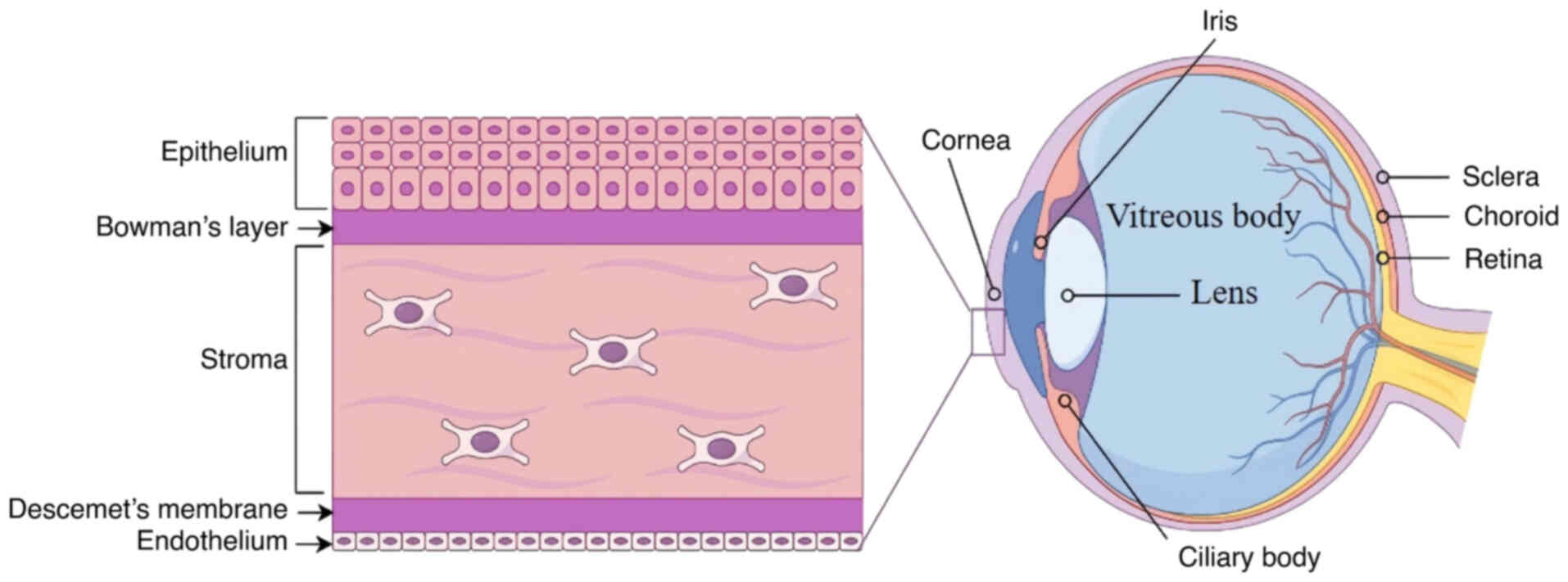
Structure and function of the cornea
The eye is a sensory organ responsible for visual
perception, transmitting light-derived information to the brain to
generate visual images. The eyeball consists of three distinct
anatomical layers: The outer layer including the cornea and sclera;
the middle layer consisting of the iris, ciliary body and choroid;
and the inner neural layer known as the retina. The intraocular
cavity contains the anterior chamber, posterior chamber and
vitreous cavity, and the major intraocular contents include the
aqueous humor, lens and vitreous body (15,19).
The cornea, forming the anterior portion of the eye,
is an avascular, transparent tissue that allows light to enter the
visual system. It not only protects the inner eye but also provides
approximately two-thirds of the total refractive power of the eye
(20–22). The cornea is a convex structure
composed of five distinct layers, which are, in order from the
anterior to the posterior surface: Epithelium, Bowman's layer,
stroma, Descemet's membrane and endothelium (23). Its anterior surface is covered by
the tear film, which provides lubrication, protection through
soluble immune factors, and a smooth optical surface (24,25).
The epithelium is ~53 µm thick and consists of 5–7
cellular layers composed of three distinct cell types, namely
squamous cells, wing cells and basal epithelial cells that adhere
to the underlying basement membrane (26). The basal epithelial cells have
proliferative capacity, which is important for cell renewal and
repair (27). Bowman's layer,
located posterior to the epithelial basement membrane, is 8–12 µm
thick, acellular and lacks the ability to regenerate (28). It is composed of randomly arranged
type I, III, V and XII collagen fibrils together with proteoglycans
(29).
Beneath Bowman's layer lies the corneal stroma,
constituting ~90% of the corneal thickness (30). The stroma primarily consists of
extracellular matrix (ECM) and a small number of keratocytes. The
keratocytes produce type I and type V collagen, along with
proteoglycans, to assemble collagen fibrils and maintain the ECM
(31). The stroma is highly
organized, to ensure both mechanical strength and light
transparency. The subepithelial nerve plexus, located between
Bowman's layer and the anterior stroma, comprises stromal nerve
branches that perforate Bowman's layer and form the sub-basal
epithelial nerve plexus that supplies the corneal epithelium
(32).
Underlying the stroma is Descemet's membrane, a
3-µm, acellular fibrous layer secreted by the underlying
endothelial cells (33).
Descemet's membrane not only provides an ‘adhesive scaffold’ and
barrier protection for corneal endothelial cells, but also
maintains the shape and hydration of the cornea (33). The corneal endothelium, located on
the posterior surface of the cornea, consists of a single layer of
flat, hexagonal cells that are connected in a mosaic honeycomb
pattern (15). These cells
preserve corneal clarity and visual acuity by maintaining the
relatively dehydrated status of the stroma through high-density
ionic pumps in their basolateral membranes (27). Since the corneal endothelium has
very limited regenerative capacity, damage to the endothelial cells
results in permanent loss, leading to corneal edema and blindness
(Fig. 1).
DK
Diabetes induces morphological and functional
alterations in the cornea. Continuous hyperglycemia can affect all
corneal layers, including the epithelium, nerves, stroma and
endothelium, with the nerves and epithelial cells being
particularly vulnerable (10). DK
exhibits several clinical manifestations, including persistent
corneal epithelial erosion, superficial punctate keratopathy,
delayed epithelial regeneration, corneal edema and decreased
corneal sensitivity (8,34). Persistent corneal epithelial
defects can progress to corneal scarring and ulceration, ultimately
leading to corneal opacity and the risk of decreased visual acuity
or permanent vision loss (35,36).
Diabetic corneal epitheliopathy
Corneal epitheliopathy is one of the most common and
long-term complications of DM (37). The rapid repair ability of the
epithelium is essential for the maintenance of corneal transparency
and homeostasis, particularly in the event of injury or infection
(8). However, DM can increase
susceptibility to spontaneous corneal trauma, and epithelial
lesions take longer to heal in patients with DM. Disrupted
epithelial cell junctions, cellular edema and reduced microvilli
have been observed in the corneas of diabetic rats by scanning
electron microscopy (38).
Hyperglycemia can disrupt the tight-junction
complexes of corneal epithelial cells, leading to epithelial
dysfunction and stromal edema (39,40).
The density of corneal basal epithelial cells has been found to be
significantly reduced in patients with both type 1 DM (T1DM) and
type 2 DM (T2DM), which impairs the ability of the endothelium to
regulate the corneal fluid balance, leading to corneal edema
(41).
Hyperglycemia has been demonstrated to impair
epidermal growth factor receptor signaling in corneal epithelial
cells, ultimately contributing to delayed wound healing (42). The activity of proteolytic enzymes
such as matrix metalloproteinases (MMPs) is increased during the
wound-healing process in corneal epithelial cells exposed to high
glucose (HG) and in the corneal epithelium of diabetic rats,
thereby interfering with wound healing and tissue remodeling
(43). Moreover, Di et al
(44) reported that an excessive
inflammatory response resulted in increased levels of
proinflammatory cytokines and delayed corneal epithelial wound
healing in diabetic model mice.
Diabetic corneal neuropathy (DCN)
The cornea is the most richly innervated structure
in the human body. Corneal nerves provide protective and trophic
functions, including the maintenance of corneal sensitivity and the
regulation of corneal wound healing through the release of
neuropeptides, neurotrophins and growth factors (8). DCN results from trigeminal nerve
impairment caused by chronic hyperglycemia, primarily affecting the
small Aδ and C nerve fibers. This condition leads to structural
abnormalities and reduced corneal innervation, which causes corneal
epithelial breakdown, delayed wound healing, and progression to
corneal ulceration, melting and perforation (5,45).
Corneal confocal microscopy has become the standard
method of assessing the cornea at a cellular level in vivo
(46). Studies using this
technique have shown that corneal nerve parameters, including
subbasal nerve fiber density, fiber length and number of fibers,
are reduced in patients with T1DM or T2DM, and exhibit an
association with diabetic peripheral neuropathy (47–51).
Furthermore, corneal sensitivity in patients with diabetes is
significantly lower compared with that in non-diabetic controls
(52). Notably, these changes are
inversely associated with the duration of DM (53).
Diabetic corneal stroma lesions
DM can cause both structural and functional changes
in the corneal stroma, leading to a loss of corneal transparency
and threatening vision (54). The
accumulation of advanced glycosylation end products (AGEs) promotes
non-enzymatic crosslinking between collagen molecules and
proteoglycans, resulting in corneal sclerosis and thickening. The
levels of collagen I and III in the corneal stroma of patients with
DM have been observed to be significantly increased compared with
those in healthy controls, which is consistent with fibrosis and
disruption of the matrix structure, leading to thickening and
scarring (55). In addition,
Ossabaw mini pigs with T2DM exhibit disorganized stromal collagen
fibrils, with decreased levels of collagen IV and upregulated
expression levels of MMP-9 (56).
In addition, Kalteniece et al (57) found that anterior, mid and
posterior stromal keratocyte density was significantly reduced in
diabetic patients with and without diabetic peripheral neuropathy
compared with those in non-diabetic controls, suggesting an
association with corneal sub-basal plexus nerve damage in patients
with DM. However, Gad et al (58) observed downregulations in corneal
nerve fiber density and anterior and mid-stromal keratocyte
densities in children with T1DM, but no correlation between nerve
and keratocyte loss.
Diabetic corneal endotheliopathy
The corneal endothelium plays a critical role in the
maintenance of appropriate stromal hydration via tight junctions
and Na+/K+-ATPase pump activity (59). In diabetes, structural and
functional impairments in the corneal endothelium reduce
endothelial pump efficiency, leading to stromal edema, which can be
detected clinically as increased central corneal thickness (CCT).
These impairments are exacerbated with disease progression, and can
manifest as corneal haze and decreased vision (60,61).
Numerous studies on the effects of DM on the corneal endothelium
have reported that patients with DM exhibit a higher CCT, reduced
corneal endothelial cell density (ECD), decreased percentages of
hexagonal cells, and increased cell area variation coefficients
compared with those in controls (62–67).
Furthermore, a multivariate analysis of patients with DM
demonstrated that a low ECD is significant associated with elevated
glycated hemoglobin levels, longer DM duration and more advanced DR
(68).
DK results from prolonged hyperglycemia, which
induces severe morphological and functional alterations in the
cornea. Chronic hyperglycemia activates multiple pathological
mechanisms, such as the accumulation of AGEs, oxidative stress,
activation of the polyol pathway and protein kinase C pathways,
chronic inflammation, immune cell activation, and reduced
neurotrophic innervation (9).
These mechanisms complement each other and jointly promote the
development of DK.
NPs
NPs are proteins that localize to the cell nucleus,
and include structural proteins, transcription factors and other
functional proteins. They are synthesized in the cytoplasm and are
transported into the nucleus through NPCs (69). This transport relies on NLSs and
nuclear export signals within the primary structure of the NP. The
NPC, composed of ~30 different protein components called
nucleoporins, regulates the movement of molecules across the
nuclear envelope (70). Small
molecules such as ions, metabolites and <40-kDa proteins can
diffuse freely though the NPC, whereas larger molecules require
specific carrier proteins for transport into and out of the nucleus
(69).
There are numerous regulatory NPs that interact with
DNA, RNA, nucleosomes and other proteins to form biomolecular
condensates, thereby functionally impacting gene expression. These
NPs include DNA- and RNA-binding proteins, as well as transcription
factors (14).
Deoxyribonucleoproteins are involved in the regulation of DNA
replication and transcription (14), whereas ribonucleoproteins
participate in post-transcriptional regulatory processes (71). Some NPs also mediate
post-translational modifications, such as acetylation and
methylation, which regulate the physiological activities of cells
and influence cell transformation and progression (72). For example, EZH2, a histone
methyltransferase of the polycomb repressive complex 2 (PRC2)
complex, promotes the trimethylation of histone H3 at lysine 27
(H3K27me3), which alters the chromatin structure and represses gene
transcription (73,74). PEST-containing nuclear protein
(PCNP) is a small NP of only 178 amino acids that contains two PEST
sequences, rich in proline, glutamic acid, serine and threonine.
PCNP is involved in vital cellular processes such as cell
proliferation and has been implicated in tumorigenesis (75).
Role of NPs in DK
In the cell nuclei of the cornea, NPs regulate gene
expression and modulate cellular responses. DK is associated with
the dysregulation of NPs, leading to molecular and cellular
alterations. Certain NPs have been well studied, including PPAR,
HMGB1, EZH2, phosphatase and tensin homolog (PTEN) and sirtuin 1
(SIRT1); however, these proteins have not been systematically
analyzed in relation to DK in previous reviews.
PPARs
PPARs are a group of ligand-activated transcription
factors belonging to the nuclear hormone receptor family (76). The PPAR family comprises three
isotypes: PPARα, PPARγ and PPARδ (also known as PPARβ or PPARβ/δ),
with genes located on human chromosomes 22, 3 and 6, respectively
(77,78). PPARs play crucial roles in glucose
and lipid metabolism, and are involved in cell proliferation and
differentiation, inflammation, angiogenesis and insulin sensitivity
(79). PPARs have been
investigated as therapeutic targets, and PPAR agonists represent a
promising treatment approach for metabolic diseases such as
diabetes, diabetic complications and neurodegeneration (80–82).
Within the eye, PPARα and PPARγ are expressed in the
cornea, conjunctiva, retina, meibomian glands and lacrimal glands,
whereas PPARδ is located in the cornea, retina and lacrimal glands
(15). Matlock et al
(83) demonstrated that in both
human diabetic corneas and the corneas of diabetic rats, the
expression of PPARα was significantly downregulated. In addition,
experiments using PPARα−/− mice revealed a marked
decline in corneal nerve densities, accompanied by an increased
incidence of epithelial lesions in the central cornea. These
findings suggest that PPARα plays a protective role against
diabetes-induced corneal nerve degeneration, potentially by
maintaining neuronal integrity and modulating pathological
processes associated with diabetes (83). The PPARα agonist fenofibrate has
been shown to alleviate mitochondrial dysfunction and promote
corneal wound healing in diabetic mice (84). Furthermore, a study of 30 patients
with T2DM revealed that oral treatment with fenofibrate for 30 days
significantly improved corneal nerve regeneration, reduced nerve
edema, and improved the density and width of corneal nerve fibers
(85). In addition, both topical
and oral fenofibrate are able to ameliorate DCN in diabetic mice,
with topical fenofibrate significantly reducing neuroinflammation
(86). These findings highlight
the therapeutic potential of PPARα agonists in the treatment of
DK.
Selective agonists to PPARγ, including
thiazolidinedione class drugs such as troglitazone, can decrease
insulin resistance and inhibit inflammation, fibrosis and corneal
scarring (87). PPARγ
downregulates the expression of TGF-β1-induced connective tissue
growth factor, thereby exerting anti-fibrogenic effects that
inhibit the development of corneal scarring (88). Rosiglitazone, a PPARγ agonist, was
shown to alleviate ocular surface damage, enhance corneal
sensitivity and increase tear production in a mouse model of
diabetes-related dry eye. These effects were accompanied by the
upregulation of antioxidant enzyme expression, indicating that
oxidative stress was reduced in the lacrimal glands of the diabetic
mice (89). Furthermore, the
PPARβ/δ agonist GW501516 was reported to suppress inflammatory
cytokine release, promote neovascularization by promoting the
expression of VEGF-α, and increase the infiltration of M2
macrophages and vascular endothelial cells into the corneal wound
area (90).
HMGB1
A ubiquitously expressed and evolutionarily
conserved chromosomal protein, HMGB1 plays diverse roles in the
mediation of inflammation. It is the most abundant and well-studied
member of the HMG superfamily. HMGB1 was first identified in bovine
thymus in 1973 by Goodwin and Johns (91), and its name reflects its high
migration ability in polyacrylamide gel electrophoresis. It is
mainly located in the nucleus, wherein it binds to DNA and
regulates chromatin remodeling, gene transcription and DNA damage
repair (92). The HMGB1 protein
comprises a single polypeptide chain of 215 amino acids, which
contains binding sites for DNA as well as for Toll-like receptor 4
(TLR4) and receptor for AGEs (RAGE), which promote inflammation
(93). HMGB1 can be passively
released by necrotic cells or actively secreted by activated immune
cells into the extracellular milieu (94). By interacting with its receptors,
RAGE and TLR, HMGB1 ultimately activates NF-κB and stimulates the
production of proinflammatory cytokines, including IL-6, IL-1β and
TNF-α, while also inducing its own expression and that of its
receptors, creating a positive feedback loop of inflammation
(95). HMGB1 has been implicated
in numerous diseases, including cancer, arthritis (96), diabetes and autoimmune diseases
(97,98).
It has been reported that HMGB1 levels are
associated with diabetic complications, with increased levels of
HMGB1 being observed in both diabetic patients and animal models of
diabetes (99,100). HMGB1 promotes insulin resistance
and inflammation by upregulating the expression of RAGE, activating
the TLR4/JNK/NF-κB pathway and reducing activation of the insulin
receptor substrate-1 signaling pathway (101). Studies have also demonstrated the
involvement of HMGB1 in the occurrence and development of DR
(102,103). Furthermore, HMGB1 exacerbates
neuronal apoptosis and autophagy defects in DN by binding to TLR4,
with increased HMGB1 and TLR4 levels promoting apoptosis and
impairing autophagy (104). Hou
et al (17) reported that
the corneas of diabetic mice with DK exhibit significantly
upregulated protein expression levels of HMGB1 and its receptors,
indicating that HMGB1 and its receptors play a key role in the
development of DK. In another study, a dipotassium
glycyrrhizinate-based micelle formulation encapsulating genistein
blocked HMGB1 signaling, which promoted diabetic corneal and nerve
wound healing (105). These
studies suggest that targeting HMGB1 may be a promising therapeutic
strategy to enhance corneal and nerve repair in patients with
diabetes.
EZH2
EZH2 is a protein that contributes to the regulation
of gene activity in cells. The EZH2 gene is located on chromosome
7q35 and comprises 20 exons, encoding a protein comprising 746
amino acids (106). Acting as a
catalytic subunit of PRC2, EZH2 promotes transcriptional silencing
through H3K27me3 (73,74). EZH2 also forms complexes with
transcriptions factors or directly binds to the promoters of target
genes to regulate gene transcription. As a key regulator in DNA
damage repair, the cell cycle, cell differentiation, autophagy,
apoptosis and immunological modulation, EZH2 has been implicated in
a variety of diseases, including cancer and ocular disorders
(18). Notably, the upregulation
of EZH2 expression has been reported in ocular tumors (107), corneal injury (108), cataracts (109) and DR (110).
Studies suggest that EZH2 is actively involved in
the pathogenesis of DK. Myofibroblast transdifferentiation induced
by TGF-β plays an important role in corneal wound healing but can
cause cornea scarring fibrosis when excessively activated (111). In a mouse model of corneal
injury, EZH2 expression was upregulated, and the EZH2 inhibitor
EPZ-6438 suppressed corneal myofibroblast activation and ECM
protein synthesis (112). Corneal
neovascularization is another key process in wound healing. In a
mouse model of alkali burn-induced corneal neovascularization, the
EZH2 inhibitor 3-deazaneplanocin A reduced EZH2 expression and the
release of proangiogenic factors, thereby alleviating oxidative
stress and abnormal neovascularization in the cornea (113). These findings suggest that EZH2
promotes corneal injury and may serve as a novel and valuable
therapeutic target. In addition, in human corneal endothelial cells
cultured in vitro, EZH2 promoted apoptosis by mediating the
H3K27me3-dependent suppression of heme oxygenase-1 gene
transcription, further supporting the potential of EZH2 as a target
for the treatment of corneal apoptosis (108).
EZH2 has also been implicated in DR. Upregulated
expression of EZH2 has been detected in HG-induced human retinal
endothelial cells and in diabetic animal models, and the inhibition
of EZH2 activity has been shown to reduce MMP-9 and VEGF levels
both in vitro and in vivo (110,114).
PTEN
PTEN is a tumor suppressor gene located at 10q23. It
encodes a 403-amino acid protein containing five main functional
domains: An N-terminal phosphatidyl-inositol-4,5-diphosphate
(PIP2)-binding domain, a phosphatase domain, a
membrane-targeting C2 domain, a C-terminal tail, and a PDZ binding
motif (115). PTEN is normally
present in the nucleus and cytoplasm of cells in normal tissue, but
absent from neoplastic tissues (115). Nuclear PTEN contributes to genome
stability by regulating damage repair, cell-cycle progression, DNA
replication and chromatin organization (116).
PTEN was the first tumor suppressor gene with
phosphatase function identified in humans, and plays a role in
tumor inhibition by regulating cell-cycle progression, apoptosis
and cell migration (117). The
PTEN protein exhibits both lipid- and protein-phosphatase activity.
Its lipid-phosphatase function dephosphorylates
phosphatidyl-inositol-3,4,5-triphosphate (PIP3) to form
PIP2, which antagonizes PI3K signaling and blocks the
activation of AKT to inhibit tumorigenesis, while its
protein-phosphatase activity dephosphorylates various protein
substrates, influencing various signaling pathways (118,119). It has been identified that PTEN
has three alternative translational isoforms: PTENα, PTENβ and
PTENε, which each have tumor-suppressive functions (118). Consequently, the dysfunction of
PTEN due to genetic mutation, epigenetic silencing or
post-translational modifications contributes to the development and
progression of numerous cancers.
The PI3K/Akt signaling pathway is a major network
activated in response to insulin or insulin-like growth factor 1
(120). As a negative regulator
of this pathway, PTEN also serves as an inhibitor of insulin
signaling. It dephosphorylates PIP3 to PIP2,
which suppresses insulin-induced PI3K signaling, ultimately leading
to decreased insulin sensitivity and insulin resistance, a key
pathogenic process in T2DM (117). The tissue-specific deletion of
PTEN improves insulin sensitivity and protects against systemic
insulin resistance, suggesting a potential strategy for therapeutic
intervention in both cancer and DM (117).
During the development of DR, hyperglycemia induces
the dysregulation of mitophagy, which causes the accumulation of
dysfunctional mitochondria and activation of the inflammasome,
processes that are mainly regulated by the PTEN-induced putative
kinase protein 1/parkin pathway (121).
The role of PTEN in keratopathy has been
investigated in a number of studies. One study demonstrated that in
hyperglycemia-induced diabetic mice, neuropeptide FF (NPFF) levels
were significantly reduced, while the application of NPFF promoted
corneal nerve injury recovery and epithelial wound healing, and
downregulated PTEN expression (122). Another study found that PTEN mRNA
and protein expression levels were upregulated in diabetic cornea,
which hindered corneal epithelial regeneration and Akt activation,
while the inhibition of PTEN by the topical administration of
dipotassium bisperoxo(picolinato)oxovanadate (V) dihydrate
[bpV(pic)] or potassium bisperoxo (1,10-phenanthroline) oxovanadate
(V) trihydrate [bpV(phen)] facilitated corneal epithelial
regeneration by reactivating Akt signaling. These treatments also
improved the recovery of corneal nerve fiber density and
sensitivity (123). Similarly,
Zhang et al (124)
demonstrated that the intracameral injection of bpV(pic) promoted
the proliferation and migration of corneal endothelial cells, and
enhanced corneal endothelial wound healing in a rat model.
Furthermore, human umbilical cord mesenchymal stem cell-derived
small extracellular vesicles overexpressing miR-21 were shown to
enhance the recovery of corneal epithelial wounds by suppressing
PTEN expression (125).
SIRTs
SIRTs are a family of NAD+-dependent
lysine deacetylases that act on histones and other proteins,
thereby regulating diverse cellular processes such as
proliferation, apoptosis and metabolism (126). Seven subtypes of SIRTs, namely
SIRT1-7, have been identified in mammals (127). They are involved in numerous
physiological and pathological processes, including energy
metabolism, oxidative stress responses, inflammation and
carcinogenesis (126).
Accordingly, the SIRT family has emerged as a potential therapeutic
target for various types of pathologies, including cancer,
cardiovascular disease and respiratory disease (128). SIRT1 and SIRT2 exist in the cell
nucleus and cytoplasm, while SIRT3-5 are predominantly
mitochondrial, and SIRT6 and SIRT7 are primarily located in the
cell nucleus (129). SIRT1 is the
most well studied member of the SIRT family.
Numerous studies have reported that SIRTs,
particularly SIRT1-6, are associated with biological processes
involved in the development and progression of DM (130). SIRTs participate in the onset and
development of DM by regulating glucose metabolism and maintaining
insulin homeostasis. Most evidence suggests that SIRT1-4 and SIRT6
have protective effects against the development of DM, while SIRT5
promotes DM progression. However, downregulation of SIRT1 and SIRT2
has been reported to improve DM in some contexts, likely due to
cell- and tissue-specific effects (128).
In terms of DM complications, SIRT1 and SIRT3 have
been shown to exert protective effects against DKD (131), DR (132), DN (133) and diabetic cardiomyopathy
(134). Mechanistically, studies
indicate that SIRT1 mediates its protective effects by inhibiting
endoplasmic reticulum stress (ERS) and suppressing the NF-κB
inflammatory signaling pathway (135). Furthermore, SIRT1 activators have
been demonstrated to exert a beneficial impact by reversing
T2DM-related complications and promoting diabetic wound healing,
underscoring their therapeutic potential in T2DM (136,137).
In DR, SIRT1, SIRT3, SIRT5 and SIRT6 play important
roles by regulating insulin sensitivity, inflammatory responses and
glucose metabolism (138). More
specifically, in DK, Wei et al (139) reported that SIRT1 alleviates
disease progression by regulating ERS and decreasing the apoptosis
of corneal epithelial cells in diabetic rat corneal tissues.
Furthermore, another study found that SIRT1 expression was
downregulated in the trigeminal sensory neurons of diabetic mice.
However, the overexpression of SIRT1 led to the upregulation of its
downstream effector miR-182, which mitigated the harmful effects of
hyperglycemia by stimulating diabetic corneal nerve regeneration.
These findings suggest that targeting SIRT1 may be a potential
therapeutic approach for diabetic sensory nerve regeneration and DK
(140). Similarly, Hu et
al (141) demonstrated that
HG (25 mM D-glucose) reduced SIRT3 expression and impaired
mitophagy in both TKE2 cells and corneal tissues from
Ins2Akita/+mice. Conversely, the overexpression of SIRT3
has been shown to promote wound healing by upregulating mitophagy
under HG conditions (142). These
results indicate that SIRT3 may positively impact corneal repair in
DK (Table I).
 | Table I.Nuclear proteins involved in diabetic
keratopathy. |
Table I.
Nuclear proteins involved in diabetic
keratopathy.
| Nuclear
proteins | Protein class | Isotypes | Genes | Biological
effects | Agonists and
inhibitors |
|---|
| PPARs | Nuclear receptors,
transcription factors (77) | PPARα, PPARγ, PPARδ
(77) | PPARA (22q13.31),
PPARG (3p25.2), PPARD (6p21.31) (78) | Protects the
corneal nerve (83);
anti-fibrogenic (87) |
Fenofibratea (84–86),
thiazolidinedione class drugsa (87,89),
GW501516a (90) |
| HMGB1 | Chromosomal protein
(92,93) | - | HMGB1 (93) | Promotes the
development of diabetic keratopathy (17) and diabetic neuropathy | Dipotassium
glycyrrhi zinate-based micelle formulation encapsulating active
agentsb (105) |
| EZH2 | Catalytic subunit
of polycomb repressive complex 2 (73,74) | - | EZH2 (7q35)
(106) | Promotes corneal
scarring and fibrosis (111,112), corneal oxidative stress and
neovascularization (113) and
human corneal endothelial cell apoptosis (108) |
EPZ-6438b (112), 3-deazaneplanocin Ab (113) |
| PTEN | Phosphatase and
tumor suppressor gene (115,117) | PTENα, PTENβ, PTENε
(118) | PTEN (10q23)
(115) | Decreases insulin
sensitivity and causes insulin resistance (117); inhibits corneal epithelium and
nerve regeneration (122,123) |
BpV(pic)b and BpV(Phen)b (123); BpV(Pic)b (124); HUMSC-sEVsb (125) |
| SIRTs |
NAD+-dependent lysine
deacetylases (126) | SIRT1, SIRT2,
SIRT3, SIRT4, SIRT5, SIRT6, SIRT7 (128) | SIRT1 (10q21.3),
SIRT2 (19q13.3), SIRT3 (11p15.5), SIRT4 (12q24.31), SIRT5 (6p23),
SIRT6 (19p13.3), SIRT7 (17q25.3) (127) | Regulates
endoplasmic reticulum stress and decreases corneal epithelial cell
apoptosis (139); stimulates
diabetic corneal nerve regeneration (140); promotes wound healing (141,142) | - |
Conclusions and prospects
DM is a global public health problem, and its
complications seriously impact quality of life. Although DK has a
high prevalence it has long been overlooked (2). Clinically, it is characterized by dry
eye, corneal epithelial erosions, superficial punctate keratopathy
and delayed epithelial regeneration, which in severe cases may
result in impaired vision or permanent vision loss.
NPs regulate gene expression and cellular
physiological activities in the cell nucleus, and their
dysregulation is closely associated with the development of
multiple diseases. In DK, the aberrant expression or dysfunction of
NPs such as PPARs, HMGB1, EZH2, PTEN and SIRT1 has been implicated
in the pathological process of keratopathy. PPAR agonists have
shown therapeutic potential in DK by ameliorating corneal
neuropathy and enhancing wound healing, while the blockade of HMGB1
suppresses inflammatory responses and promotes corneal healing.
EZH2 contributes to corneal scar formation, and its inhibition may
prevent corneal fibrosis. PTEN negative regulates the PI3K/Akt
pathway, and its inhibition helps to promote corneal epithelial
regeneration and nerve repair. Conversely, SIRT1 exerts protective
effects by attenuating ERS and inflammation. Collectively, these
findings suggest novel targets and strategies for the treatment of
DK.
While other reviews have broadly summarized the
corneal complications of DM, the present review is the first to
specifically examine the functional interplay between specific NPs,
namely PPARs, EZH2 and SIRTs, in all corneal layers in DK. However,
research on NPs in DK is at an early stage, and numerous questions
remain to be addressed. For example, the specific molecular
mechanisms underlying the functions of NPs in DK have not yet been
fully elucidated, and their interactions with other cell signaling
pathways merit further exploration. In addition, the clinical
application of NPs as potential biomarkers or therapeutic targets
requires validation in additional clinical studies to establish
their safety and efficacy.
In conclusion, NPs play complex and diverse roles in
DK. Investigating these mechanisms contributes to an in-depth
understanding of the pathophysiological process of DK, and also
provides an important theoretical basis for the development of
novel diagnostic and therapeutic strategies. Continued research in
this field may be expected to provide more effective solutions for
DK and improve the quality of life of diabetic patients.
Acknowledgements
Not applicable.
Funding
This study was supported by grants from the Henan Provincial
Science and Technology Development Program of China (grant no.
232102310104), and Cultivation Project for Innovation Team in
Teachers' Teaching Proficiency by Zhengzhou Health College (grant
no. 2024jxcxtd01).
Availability of data and materials
Not applicable.
Authors' contributions
HX and ZJ were responsible for conceptualization,
methodology, investigation, formal analysis and writing the
original draft of the manuscript. YW collected and collated
clinical literature on DK phenotypes and classification, assisted
in analyzing clinical evidence of corneal confocal microscopy for
DK, and verifying key clinical data such as DK prevalence to ensure
the review's clinical conclusions were evidence-based. XH and WD
made contributed to data analysis and interpretation by designing
key figures, conducting statistical analysis of visualized data,
drafting the “Structure and Function of the Cornea” section and
figure legends, and critically revising the manuscript. YC and QZ
contributed to data acquisition by curating and verifying
experimental resources and to data interpretation by optimizing
figure layouts and cross-validating visualized data, while also
revising figure descriptions and approving the final version. XJ,
SJ and YD contributed to conceptualization, resources, supervision,
funding acquisition, and the review and editing of the manuscript.
Data authentication is not applicable. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
DK
|
diabetic keratopathy
|
|
DM
|
diabetes mellitus
|
|
NPs
|
nuclear proteins
|
|
PPARs
|
peroxisome proliferator-activated
receptors
|
|
HMGB1
|
high-mobility group box 1
|
|
EZH2
|
enhancer of zeste homolog 2
|
|
PTEN
|
phosphatase and tensin homolog
|
|
SIRT
|
sirtuin
|
|
DR
|
diabetic retinopathy
|
|
NLSs
|
nuclear localization signals
|
|
NPC
|
nuclear pore complex
|
|
CCT
|
central corneal thickness
|
|
ECD
|
endothelial cell density
|
|
AGEs
|
advanced glycosylation end
products
|
|
PCNP
|
PEST-containing nuclear protein
|
|
DCN
|
diabetic corneal neuropathy
|
|
PRC2
|
polycomb repressive complex 2
|
References
|
1
|
Bodke H, Wagh V and Kakar G: Diabetes
mellitus and prevalence of other comorbid conditions: A systematic
review. Cureus. 15:e493742023.PubMed/NCBI
|
|
2
|
Sun H, Saeedi P, Karuranga S, Pinkepank M,
Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JCN, Mbanya JC, et
al: IDF diabetes atlas: Global, regional and country-level diabetes
prevalence estimates for 2021 and projections for 2045. Diabetes
Res Clin Pract. 183:1091192022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cole JB and Florez JC: Genetics of
diabetes mellitus and diabetes complications. Nat Rev Nephrol.
16:377–390. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhu L, Titone R and Robertson DM: The
impact of hyperglycemia on the corneal epithelium: Molecular
mechanisms and insight. Ocul Surf. 17:644–654. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhao H, He Y, Ren YR and Chen BH: Corneal
alteration and pathogenesis in diabetes mellitus. Int J Ophthalmol.
12:1939–1950. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lyu Y, Zeng X, Li F and Zhao S: The effect
of the duration of diabetes on dry eye and corneal nerves. Cont
Lens Anterior Eye. 42:380–385. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Teo ZL, Tham YC, Yu M, Chee ML, Rim TH,
Cheung N, Bikbov MM, Wang YX, Tang Y, Lu Y, et al: Global
prevalence of diabetic retinopathy and projection of burden through
2045: Systematic review and meta-analysis. Ophthalmology.
128:1580–1591. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Priyadarsini S, Whelchel A, Nicholas S,
Sharif R, Riaz K and Karamichos D: Diabetic keratopathy: Insights
and challenges. Surv Ophthalmol. 65:513–529. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Han SB, Yang HK and Hyon JY: Influence of
diabetes mellitus on anterior segment of the eye. Clin Interv
Aging. 14:53–63. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Buonfiglio F, Wasielica-Poslednik J,
Pfeiffer N and Gericke A: Diabetic keratopathy: Redox signaling
pathways and therapeutic prospects. Antioxidants (Basel).
13:1202024. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pelley JW: 17-Protein synthesis and
degradation. Elsevier's Integrated Biochemistry. Pelley JW: Mosby,
Philadelphia: pp. 147–158. 2007, View Article : Google Scholar
|
|
12
|
Goodman SR: Chapter 5-regulation of gene
expression. Medical Cell Biology. (Third Edition). Goodman SR:
Academic Press; San Diego, CA: pp. 149–190. 2008
|
|
13
|
Bernhofer M, Goldberg T, Wolf S, Ahmed M,
Zaugg J, Boden M and Rost B: NLSdb-major update for database of
nuclear localization signals and nuclear export signals. Nucleic
Acids Res. 46:D503–D508. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li W and Jiang H: Nuclear protein
condensates and their properties in regulation of gene expression.
J Mol Biol. 434:1671512022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Escandon P, Vasini B, Whelchel AE,
Nicholas SE, Matlock HG, Ma JX and Karamichos D: The role of
peroxisome proliferator-activated receptors in healthy and diseased
eyes. Exp Eye Res. 208:1086172021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Khatol P, Saraf S and Jain A: Peroxisome
proliferated activated receptors (PPARs): Opportunities and
challenges for ocular therapy. Crit Rev Ther Drug Carrier Syst.
35:65–97. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hou Y, Lan J, Zhang F and Wu X: Expression
profiles and potential corneal epithelial wound healing regulation
targets of high-mobility group box 1 in diabetic mice. Exp Eye Res.
202:1083642021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Peng Y, Bui CH, Zhang XJ, Chen JS, Tham
CC, Chu WK, Chen LJ, Pang CP and Yam JC: The role of EZH2 in ocular
diseases: A narrative review. Epigenomics. 15:557–570. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rocher N: Anatomy and physiology of the
human eye. Soins. 30–31. 2010.(In French). PubMed/NCBI
|
|
20
|
Mobaraki M, Abbasi R, Omidian Vandchali S,
Ghaffari M, Moztarzadeh F and Mozafari M: Corneal repair and
regeneration: Current concepts and future directions. Front Bioeng
Biotechnol. 7:1352019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Meek KM and Knupp C: Corneal structure and
transparency. Prog Retin Eye Res. 49:1–16. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lavker RM, Kaplan N, Wang J and Peng H:
Corneal epithelial biology: Lessons stemming from old to new. Exp
Eye Res. 198:1080942020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Doughty MJ and Jonuscheit S: Corneal
structure, transparency, thickness and optical density
(densitometry), especially as relevant to contact lens wear-a
review. Cont Lens Anterior Eye. 42:238–245. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Luke RA, Braun RJ, Driscoll TA, Begley CG
and Awisi-Gyau D: Parameter estimation for evaporation-driven tear
film thinning. Bull Math Biol. 82:712020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Braun RJ, King-Smith PE, Begley CG, Li L
and Gewecke NR: Dynamics and function of the tear film in relation
to the blink cycle. Prog Retin Eye Res. 45:132–164. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wilson SL, El Haj AJ and Yang Y: Control
of scar tissue formation in the cornea: Strategies in clinical and
corneal tissue engineering. J Funct Biomater. 3:642–687. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Melnyk S and Bollag WB: Aquaporins in the
cornea. Int J Mol Sci. 25:37482024. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sridhar MS: Anatomy of cornea and ocular
surface. Indian J Ophthalmol. 66:190–194. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wilson SE: Bowman's layer in the
cornea-structure and function and regeneration. Exp Eye Res.
195:1080332020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
DelMonte DW and Kim T: Anatomy and
physiology of the cornea. J Cataract Refract Surg. 37:588–598.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hassell JR and Birk DE: The molecular
basis of corneal transparency. Exp Eye Res. 91:326–335. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Marfurt CF, Cox J, Deek S and Dvorscak L:
Anatomy of the human corneal innervation. Exp Eye Res. 90:478–492.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
de Oliveira RC and Wilson SE: Descemet's
membrane development, structure, function and regeneration. Exp Eye
Res. 197:1080902020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ljubimov AV: Diabetic complications in the
cornea. Vision Res. 139:138–152. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mukhija R, Gupta N, Vashist P, Tandon R
and Gupta SK: Population-based assessment of visual impairment and
pattern of corneal disease: Results from the CORE (corneal opacity
rural epidemiological) study. Br J Ophthalmol. 104:994–998. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chang YS, Tai MC, Ho CH, Chu CC, Wang JJ,
Tseng SH and Jan RL: Risk of corneal ulcer in patients with
diabetes mellitus: A retrospective large-scale cohort study. Sci
Rep. 10:73882020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yeung A and Dwarakanathan S: Diabetic
keratopathy. Dis Mon. 67:1011352021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Huang C, Liao R, Wang F and Tang S:
Characteristics of reconstituted tight junctions after corneal
epithelial wounds and ultrastructure alterations of corneas in type
2 diabetic rats. Curr Eye Res. 41:783–790. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shih KC, Lam KS and Tong L: A systematic
review on the impact of diabetes mellitus on the ocular surface.
Nutr Diabetes. 7:e2512017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Alfuraih S, Barbarino A, Ross C, Shamloo
K, Jhanji V, Zhang M and Sharma A: Effect of high glucose on ocular
surface epithelial cell barrier and tight junction proteins. Invest
Ophthalmol Vis Sci. 61:32020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liu T, Sun DP, Li DF, Bi WJ and Xie LX:
Observation and quantification of diabetic keratopathy in type 2
diabetes patients using in vivo laser confocal microscopy. Zhonghua
Yan Ke Za Zhi. 56:754–760. 2020.(In Chinese). PubMed/NCBI
|
|
42
|
Xu KP, Li Y, Ljubimov AV and Yu FS: High
glucose suppresses epidermal growth factor
receptor/phosphatidylinositol 3-kinase/Akt signaling pathway and
attenuates corneal epithelial wound healing. Diabetes.
58:1077–1085. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lan X, Zhang W, Zhu J, Huang H, Mo K, Guo
H, Zhu L, Liu J, Li M, Wang L, et al: dsRNA induced IFNβ-MMP13 axis
drives corneal wound healing. Invest Ophthalmol Vis Sci. 63:142022.
View Article : Google Scholar
|
|
44
|
Di G, Du X, Qi X, Zhao X, Duan H, Li S,
Xie L and Zhou Q: Mesenchymal stem cells promote diabetic corneal
epithelial wound healing through TSG-6-dependent stem cell
activation and macrophage switch. Invest Ophthalmol Vis Sci.
58:4344–4354. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bikbova G, Oshitari T, Baba T, Bikbov M
and Yamamoto S: Diabetic corneal neuropathy: Clinical perspectives.
Clin Ophthalmol. 12:981–987. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Liu YC, Lin MT and Mehta JS: Analysis of
corneal nerve plexus in corneal confocal microscopy images. Neural
Regen Res. 16:690–691. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kaplan H, Yüzbaşıoğlu S, Vural G and
Gümüşyayla Ş: Investigation of small fiber neuropathy in patients
with diabetes mellitus by corneal confocal microscopy. Neurophysiol
Clin. 54:1029552024. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Mokhtar SBA, van der Heide FCT, Oyaert
KAM, van der Kallen CJH, Berendschot TTJM, Scarpa F, Colonna A, de
Galan BE, van Greevenbroek MMJ, Dagnelie PC, et al: (Pre)diabetes
and a higher level of glycaemic measures are continuously
associated with corneal neurodegeneration assessed by corneal
confocal microscopy: The maastricht study. Diabetologia.
66:2030–2041. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Carmichael J, Fadavi H, Ishibashi F,
Howard S, Boulton AJM, Shore AC and Tavakoli M: Implementation of
corneal confocal microscopy for screening and early detection of
diabetic neuropathy in primary care alongside retinopathy
screening: Results from a feasibility study. Front Endocrinol
(Lausanne). 13:e8915752022. View Article : Google Scholar
|
|
50
|
Banerjee M, Mukhopadhyay P and Ghosh S,
Basu M, Pandit A, Malik R and Ghosh S: Corneal confocal microscopy
abnormalities in children and adolescents with type 1 diabetes.
Endocr Pract. 29:692–698. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
De Clerck EEB, Schouten JSAG, Berendschot
TTJM, Koolschijn RS, Nuijts RMMA, Schram MT, Schaper NC, Henry RMA,
Dagnelie PC, Ruggeri A, et al: Reduced corneal nerve fibre length
in prediabetes and type 2 diabetes: The maastricht study. Acta
Ophthalmol. 98:485–491. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Mvilongo C, Akono ME, Nkoudou D, Nanfack
C, Nomo A, Dim R and Eballé AO: Clinical profile of corneal
sensitivity in diabetic patients: A case-control study. J Fr
Ophtalmol. 47:1042122024. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Schiano Lomoriello D, Abicca I, Parravano
M, Giannini D, Russo B, Frontoni S and Picconi F: Early alterations
of corneal subbasal plexus in uncomplicated type 1 diabetes
patients. J Ophthalmol. 2019:98182172019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Torricelli AA and Wilson SE: Cellular and
extracellular matrix modulation of corneal stromal opacity. Exp Eye
Res. 129:151–160. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Priyadarsini S, McKay TB, Sarker-Nag A,
Allegood J, Chalfant C, Ma JX and Karamichos D: Complete metabolome
and lipidome analysis reveals novel biomarkers in the human
diabetic corneal stroma. Exp Eye Res. 153:90–100. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Sinha NR, Balne PK, Bunyak F, Hofmann AC,
Lim RR, Mohan RR and Chaurasia SS: Collagen matrix perturbations in
corneal stroma of Ossabaw mini pigs with type 2 diabetes. Mol Vis.
27:666–678. 2021.PubMed/NCBI
|
|
57
|
Kalteniece A, Ferdousi M, Azmi S, Marshall
A, Soran H and Malik RA: Keratocyte density is reduced and related
to corneal nerve damage in diabetic neuropathy. Invest Ophthalmol
Vis Sci. 59:3584–3590. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Gad H, Al-Jarrah B, Saraswathi S, Mohamed
S, Kalteniece A, Petropoulos IN, Khan A, Ponirakis G, Singh P,
Khodor SA, et al: Corneal confocal microscopy identifies a
reduction in corneal keratocyte density and sub-basal nerves in
children with type 1 diabetes mellitus. Br J Ophthalmol.
106:1368–1372. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Eghrari AO, Riazuddin SA and Gottsch JD:
Overview of the cornea: Structure, function, and development. Prog
Mol Biol Transl Sci. 134:7–23. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
El-Agamy A and Alsubaie S: Corneal
endothelium and central corneal thickness changes in type 2
diabetes mellitus. Clin Ophthalmol. 11:481–486. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Goldstein AS, Janson BJ, Skeie JM, Ling JJ
and Greiner MA: The effects of diabetes mellitus on the corneal
endothelium: A review. Surv Ophthalmol. 65:438–450. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Yalcın SO, Kaplan AT and Sobu E: Corneal
endothelial cell morphology and optical coherence tomography
findings in children with type 1 diabetes mellitus. Eur J
Ophthalmol. 33:1331–1339. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Amador-Muñoz DP, Conforti V, Matheus LM,
Molano-Gonzalez N and Payán-Gómez C: Diabetes mellitus type 1 has a
higher impact on corneal endothelial cell density and pachymetry
than diabetes mellitus type 2, independent of age: A
meta-regression model. Cornea. 41:965–973. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Chowdhury B, Bhadra S, Mittal P and Shyam
K: Corneal endothelial morphology and central corneal thickness in
type 2 diabetes mellitus patients. Indian J Ophthalmol.
69:1718–1724. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Kim YJ and Kim TG: The effects of type 2
diabetes mellitus on the corneal endothelium and central corneal
thickness. Sci Rep. 11:83242021. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Taşlı NG, Icel E, Karakurt Y, Ucak T,
Ugurlu A, Yilmaz H and Akbas EM: The findings of corneal specular
microscopy in patients with type-2 diabetes mellitus. BMC
Ophthalmol. 20:2142020. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Zhang K, Zhao L, Zhu C, Nan W, Ding X,
Dong Y and Zhao M: The effect of diabetes on corneal endothelium: A
meta-analysis. BMC Ophthalmol. 21:782021. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Papadakou P, Chatziralli I, Papathanassiou
M, Lambadiari V, Siganos CS, Theodossiadis P and Kozobolis V: The
effect of diabetes mellitus on corneal endothelial cells and
central corneal thickness: A case-control study. Ophthalmic Res.
63:550–554. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Sekimoto T and Yoneda Y: Intrinsic and
extrinsic negative regulators of nuclear protein transport
processes. Genes Cells. 17:525–535. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Cronshaw JM, Krutchinsky AN, Zhang W,
Chait BT and Matunis MJ: Proteomic analysis of the mammalian
nuclear pore complex. J Cell Biol. 158:915–927. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Jeon P, Ham HJ, Park S and Lee JA:
Regulation of cellular ribonucleoprotein granules: From assembly to
degradation via post-translational modification. Cells.
11:20632022. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Shen F, Kirmani KZ, Xiao Z, Thirlby BH,
Hickey RJ and Malkas LH: Nuclear protein isoforms: Implications for
cancer diagnosis and therapy. J Cell Biochem. 112:756–760. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Simon JA and Lange CA: Roles of the EZH2
histone methyltransferase in cancer epigenetics. Mutat Res.
647:21–29. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Glancy E, Ciferri C and Bracken AP:
Structural basis for PRC2 engagement with chromatin. Curr Opin
Struct Biol. 67:135–144. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Khan NH, Chen HJ, Fan Y, Surfaraz M,
Ahammad MF, Qin YZ, Shahid M, Virk R, Jiang E, Wu DD and Ji XY:
Biology of PEST-containing nuclear protein: A potential molecular
target for cancer research. Front Oncol. 12:7845972022. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Brown JD and Plutzky J: Peroxisome
proliferator-activated receptors as transcriptional nodal points
and therapeutic targets. Circulation. 115:518–533. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Chow BJ, Lee IXY, Liu C and Liu YC:
Potential therapeutic effects of peroxisome proliferator-activated
receptors on corneal diseases. Exp Biol Med (Maywood).
249:101422024. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Kim IS, Silwal P and Jo EK: Peroxisome
proliferator-activated receptor-targeted therapies: Challenges upon
infectious diseases. Cells. 12:6502023. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Mirza AZ, Althagafi II and Shamshad H:
Role of PPAR receptor in different diseases and their ligands:
Physiological importance and clinical implications. Eur J Med Chem.
166:502–513. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Jain N, Bhansali S, Kurpad AV, Hawkins M,
Sharma A, Kaur S, Rastogi A and Bhansali A: Effect of a dual PPAR
α/γ agonist on insulin sensitivity in patients of type 2 diabetes
with hypertriglyceridemia-randomized double-blind
placebo-controlled trial. Sci Rep. 9:190172019. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Lin Y, Wang Y and Li PF: PPARα: An
emerging target of metabolic syndrome, neurodegenerative and
cardiovascular diseases. Front Endocrinol (Lausanne).
13:10749112022. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Hu P, Li K, Peng X, Kan Y, Li H, Zhu Y,
Wang Z, Li Z, Liu HY and Cai D: Nuclear receptor PPARα as a
therapeutic target in diseases associated with lipid metabolism
disorders. Nutrients. 15:47722023. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Matlock HG, Qiu F, Malechka V, Zhou K,
Cheng R, Benyajati S, Whelchel A, Karamichos D and Ma JX:
Pathogenic role of PPARα downregulation in corneal nerve
degeneration and impaired corneal sensitivity in diabetes.
Diabetes. 69:1279–1291. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Liang W, Huang L, Whelchel A, Yuan T, Ma
X, Cheng R, Takahashi Y, Karamichos D and Ma JX: Peroxisome
proliferator-activated receptor-α (PPARα) regulates wound healing
and mitochondrial metabolism in the cornea. Proc Natl Acad Sci USA.
120:e22175761202023. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Teo CHY, Lin MT, Lee IXY, Koh SK, Zhou L,
Goh DS, Choi H, Koh HWL, Lam AYR, Lim PS, et al: Oral peroxisome
proliferator-activated receptor-α agonist enhances corneal nerve
regeneration in patients with type 2 diabetes. Diabetes.
72:932–946. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Mansoor H, Lee IXY, Lin MT, Ang HP, Xue
YC, Krishaa L, Patil M, Koh SK, Tan HC, Zhou L and Liu YC: Topical
and oral peroxisome proliferator-activated receptor-α agonist
ameliorates diabetic corneal neuropathy. Sci Rep. 14:134352024.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Jeon KI, Kulkarni A, Woeller CF, Phipps
RP, Sime PJ, Hindman HB and Huxlin KR: Inhibitory effects of PPARγ
ligands on TGF-β1-induced corneal myofibroblast transformation. Am
J Pathol. 184:1429–4145. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Jeon KI, Phipps RP, Sime PJ and Huxlin KR:
Inhibitory effects of PPARγ ligands on TGF-β1-induced CTGF
expression in cat corneal fibroblasts. Exp Eye Res. 138:52–58.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Wang J, Chen S, Zhao X, Guo Q, Yang R,
Zhang C, Huang Y, Ma L and Zhao S: Effect of PPARγ on oxidative
stress in diabetes-related dry eye. Exp Eye Res. 231:1094982023.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Tobita Y, Arima T, Nakano Y, Uchiyama M,
Shimizu A and Takahashi H: Peroxisome proliferator-activated
receptor beta/delta agonist suppresses inflammation and promotes
neovascularization. Int J Mol Sci. 21:52962020. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Goodwin GH and Johns EW: Isolation and
characterisation of two calf-thymus chromatin non-histone proteins
with high contents of acidic and basic amino acids. Eur J Biochem.
40:215–219. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Thomas JO and Stott K: H1 and HMGB1:
Modulators of chromatin structure. Biochem Soc Trans. 40:341–346.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Kang R, Chen R, Zhang Q, Hou W, Wu S, Cao
L, Huang J, Yu Y, Fan XG, Yan Z, et al: HMGB1 in health and
disease. Mol Aspects Med. 40:1–116. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Bell CW, Jiang W, Reich CF III and
Pisetsky DS: The extracellular release of HMGB1 during apoptotic
cell death. Am J Physiol Cell Physiol. 291:C1318–C1325. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
van Beijnum JR, Buurman WA and Griffioen
AW: Convergence and amplification of toll-like receptor (TLR) and
receptor for advanced glycation end products (RAGE) signaling
pathways via high mobility group B1 (HMGB1). Angiogenesis.
11:91–99. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Zhang S, Zhong J, Yang P, Gong F and Wang
CY: HMGB1, an innate alarmin, in the pathogenesis of type 1
diabetes. Int J Clin Exp Pathol. 3:24–38. 2009.PubMed/NCBI
|
|
97
|
Lotze MT and Tracey KJ: High-mobility
group box 1 protein (HMGB1): Nuclear weapon in the immune arsenal.
Nat Rev Immunol. 5:331–342. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Taniguchi N, Kawahara K, Yone K,
Hashiguchi T, Yamakuchi M, Goto M, Inoue K, Yamada S, Ijiri K,
Matsunaga S, et al: High mobility group box chromosomal protein 1
plays a role in the pathogenesis of rheumatoid arthritis as a novel
cytokine. Arthritis Rheum. 48:971–981. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Dasu MR, Devaraj S, Park S and Jialal I:
Increased toll-like receptor (TLR) activation and TLR ligands in
recently diagnosed type 2 diabetic subjects. Diabetes Care.
33:861–868. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Skrha J Jr, Kalousová M, Svarcová J,
Muravská A, Kvasnička J, Landová L, Zima T and Skrha J:
Relationship of soluble RAGE and RAGE ligands HMGB1 and EN-RAGE to
endothelial dysfunction in type 1 and type 2 diabetes mellitus. Exp
Clin Endocrinol Diabetes. 120:277–281. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Wu H, Chen Z, Xie J, Kang LN, Wang L and
Xu B: High mobility group box-1: A missing link between diabetes
and its complications. Mediators Inflamm. 2016:38961472016.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Steinle JJ: Role of HMGB1 signaling in the
inflammatory process in diabetic retinopathy. Cell Signal.
73:1096872020. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Feng L, Liang L, Zhang S, Yang J, Yue Y
and Zhang X: HMGB1 downregulation in retinal pigment epithelial
cells protects against diabetic retinopathy through the
autophagy-lysosome pathway. Autophagy. 18:320–339. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Guo X, Shi Y, Du P, Wang J, Han Y, Sun B
and Feng J: HMGB1/TLR4 promotes apoptosis and reduces autophagy of
hippocampal neurons in diabetes combined with OSA. Life Sci.
239:1170202019. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Hou Y, Xin M, Li Q and Wu X: Glycyrrhizin
micelle as a genistein nanocarrier: Synergistically promoting
corneal epithelial wound healing through blockage of the HMGB1
signaling pathway in diabetic mice. Exp Eye Res. 204:1084542021.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Liu Y and Yang Q: The roles of EZH2 in
cancer and its inhibitors. Med Oncol. 40:1672023. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Cao J, Pontes KC, Heijkants RC, Brouwer
NJ, Groenewoud A, Jordanova ES, Marinkovic M, van Duinen S,
Teunisse AF, Verdijk RM, et al: Overexpression of EZH2 in
conjunctival melanoma offers a new therapeutic target. J Pathol.
245:433–444. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Lin Y, Su H, Zou B and Huang M: EZH2
promotes corneal endothelial cell apoptosis by mediating H3K27me3
and inhibiting HO-1 transcription. Curr Eye Res. 48:1122–1132.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Zhang L, Wang L, Hu XB, Hou M, Xiao Y,
Xiang JW, Xie J, Chen ZG, Yang TH, Nie Q, et al: MYPT1/PP1-mediated
EZH2 dephosphorylation at S21 promotes epithelial-mesenchymal
transition in fibrosis through control of multiple families of
genes. Adv Sci (Weinh). 9:e21055392022. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Thomas AA, Feng B and Chakrabarti S:
ANRIL: A regulator of VEGF in diabetic retinopathy. Invest
Ophthalmol Vis Sci. 58:470–480. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Wilson SE: Corneal myofibroblasts and
fibrosis. Exp Eye Res. 201:1082722020. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Liao K, Cui Z, Zeng Y, Liu J, Wang Y, Wang
Z, Tang S and Chen J: Inhibition of enhancer of zeste homolog 2
prevents corneal myofibroblast transformation in vitro. Exp Eye
Res. 208:1086112021. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Wan SS, Pan YM, Yang WJ, Rao ZQ and Yang
YN: Inhibition of EZH2 alleviates angiogenesis in a model of
corneal neovascularization by blocking FoxO3a-mediated oxidative
stress. FASEB J. 34:10168–10181. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Duraisamy AJ, Mishra M and Kowluru RA:
Crosstalk between histone and DNA methylation in regulation of
retinal matrix metalloproteinase-9 in diabetes. Invest Ophthalmol
Vis Sci. 58:6440–6448. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Liu T, Wang Y, Wang Y and Chan AM:
Multifaceted regulation of PTEN subcellular distributions and
biological functions. Cancers (Basel). 11:12472019. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Ho J, Cruise ES, Dowling RJO and Stambolic
V: PTEN nuclear functions. Cold Spring Harb Perspect Med.
10:a0360792020. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Li A, Qiu M, Zhou H, Wang T and Guo W:
PTEN, insulin resistance and cancer. Curr Pharm Des. 23:3667–3676.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Liu A, Zhu Y, Chen W, Merlino G and Yu Y:
PTEN dual lipid- and protein-phosphatase function in tumor
progression. Cancers (Basel). 14:36662022. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Li X, Yang P, Hou X and Ji S:
Post-translational modification of PTEN protein: Quantity and
activity. Oncol Rev. 18:14302372024. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Li YZ, Di Cristofano A and Woo M:
Metabolic role of PTEN in insulin signaling and resistance. Cold
Spring Harb Perspect Med. 10:a0361372020. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
D'Amico AG, Maugeri G, Magrì B, Bucolo C
and D'Agata V: Targeting the PINK1/Parkin pathway: A new
perspective in the prevention and therapy of diabetic retinopathy.
Exp Eye Res. 247:1100242024. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Dai Y, Zhao X, Chen P, Yu Y, Wang Y and
Xie L: Neuropeptide FF promotes recovery of corneal nerve injury
associated with hyperglycemia. Invest Ophthalmol Vis Sci.
56:7754–7765. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Li J, Qi X, Wang X, Li W, Li Y and Zhou Q:
PTEN inhibition facilitates diabetic corneal epithelial
regeneration by reactivating Akt signaling pathway. Transl Vis Sci
Technol. 9:52020. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Zhang W, Yu F, Yan C, Shao C, Gu P, Fu Y,
Sun H and Fan X: PTEN inhibition accelerates corneal endothelial
wound healing through increased endothelial cell division and
migration. Invest Ophthalmol Vis Sci. 61:192020. View Article : Google Scholar
|
|
125
|
Liu X, Li X, Wu G, Qi P, Zhang Y, Liu Z,
Li X, Yu Y, Ye X, Li Y, et al: Umbilical cord mesenchymal stem
cell-derived small extracellular vesicles deliver miR-21 to promote
corneal epithelial wound healing through PTEN/PI3K/Akt pathway.
Stem Cells Int. 2022:12525572022. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Penteado AB, Hassanie H, Gomes RA, Silva
Emery FD and Goulart Trossini GH: Human sirtuin 2 inhibitors, their
mechanisms and binding modes. Future Med Chem. 15:291–311. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Vassilopoulos A, Fritz KS, Petersen DR and
Gius D: The human sirtuin family: Evolutionary divergences and
functions. Hum Genomics. 5:485–496. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Wu QJ, Zhang TN, Chen HH, Yu XF, Lv JL,
Liu YY, Liu YS, Zheng G, Zhao JQ, Wei YF, et al: The sirtuin family
in health and disease. Signal Transduct Target Ther. 7:4022022.
View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Tao Z, Jin Z, Wu J, Cai G and Yu X:
Sirtuin family in autoimmune diseases. Front Immunol.
14:11862312023. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Guarente L: Franklin H: Epstein lecture:
Sirtuins, aging, and medicine. N Engl J Med. 364:2235–2244. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Hong Q, Zhang L, Das B, Li Z, Liu B, Cai
G, Chen X, Chuang PY, He JC and Lee K: Increased podocyte Sirtuin-1
function attenuates diabetic kidney injury. Kidney Int.
93:1330–1343. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Hammer SS, Vieira CP, McFarland D, Sandler
M, Levitsky Y, Dorweiler TF, Lydic TA, Asare-Bediako B,
Adu-Agyeiwaah Y, Sielski MS, et al: Fasting and fasting-mimicking
treatment activate SIRT1/LXRα and alleviate diabetes-induced
systemic and microvascular dysfunction. Diabetologia. 64:1674–1689.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Chandrasekaran K, Salimian M, Konduru SR,
Choi J, Kumar P, Long A, Klimova N, Ho CY, Kristian T and Russell
JW: Overexpression of Sirtuin 1 protein in neurons prevents and
reverses experimental diabetic neuropathy. Brain. 142:3737–3752.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Li L, Zeng H, He X and Chen JX: Sirtuin 3
alleviates diabetic cardiomyopathy by regulating TIGAR and
cardiomyocyte metabolism. J Am Heart Assoc. 10:e0189132021.
View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Zhao K, Zhang H and Yang D: SIRT1 exerts
protective effects by inhibiting endoplasmic reticulum stress and
NF-κB signaling pathways. Front Cell Dev Biol. 12:14055462024.
View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Mihanfar A, Akbarzadeh M, Ghazizadeh
Darband S, Sadighparvar S and Majidinia M: SIRT1: A promising
therapeutic target in type 2 diabetes mellitus. Arch Physiol
Biochem. 130:13–28. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Prabhakar PK, Singh K, Kabra D and Gupta
J: Natural SIRT1 modifiers as promising therapeutic agents for
improving diabetic wound healing. Phytomedicine. 76:1532522020.
View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Nebbioso M, Lambiase A, Armentano M,
Tucciarone G, Sacchetti M, Greco A and Alisi L: Diabetic
retinopathy, oxidative stress, and sirtuins: An in depth look in
enzymatic patterns and new therapeutic horizons. Surv Ophthalmol.
67:168–183. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Wei S, Fan J, Zhang X, Jiang Y, Zeng S,
Pan X, Sheng M and Chen Y: Sirt1 attenuates diabetic keratopathy by
regulating the endoplasmic reticulum stress pathway. Life Sci.
265:1187892021. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Wang Y, Zhao X, Wu X, Dai Y, Chen P and
Xie L: microRNA-182 mediates Sirt1-induced diabetic corneal nerve
regeneration. Diabetes. 65:2020–2031. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Hu J, Kan T and Hu X: Sirt3 regulates
mitophagy level to promote diabetic corneal epithelial wound
healing. Exp Eye Res. 181:223–231. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Zhang B, Cui S, Bai X, Zhuo L, Sun X, Hong
Q, Fu B, Wang J, Chen X and Cai G: SIRT3 overexpression antagonizes
high glucose accelerated cellular senescence in human diploid
fibroblasts via the SIRT3-FOXO1 signaling pathway. Age (Dordr).
35:2237–2253. 2013. View Article : Google Scholar : PubMed/NCBI
|