Rheumatoid arthritis (RA) is a chronic autoimmune
inflammatory disorder primarily characterized by symmetrical,
multi-joint pain and swelling, often accompanied by local joint
destruction. The underlying pathogenesis remains incompletely
understood, but it involves a complex interplay of genetic,
environmental, and immune factors, ultimately leading to the
breakdown of immune tolerance and systemic immune dysregulation
(1). RA markedly impairs the
quality of life of patients. Over the past three decades, its
global prevalence has steadily increased, with the 2020 RA Global
Burden of Disease Study reporting an estimated prevalence of ~0.2%,
placing a substantial burden on the global economy (2).
Despite significant advances in the treatment of RA,
outcomes remain unsatisfactory. Due to its complex pathogenesis, no
drug or therapy can fully halt RA progression, and most treatments
are associated with notable side effects (3). Epidemiological and translational
studies have increasingly emphasized the critical role of mucosal
interactions with symbiotic bacteria in RA development (4–6).
Research on high-risk RA populations has revealed lung mucosal
inflammation, as well as the production of local anti-citrullinated
protein antibodies (ACPAs) and rheumatoid factor, supporting the
‘mucosal origin hypothesis’ (7,8).
Further investigations have identified distinct mucosal mechanisms,
including those of the intestinal mucosa, that drive RA progression
(9). The intestine, home to the
largest concentration of innate and adaptive immune cells, forms a
vital interface between the internal environment and external
factors, linking environmental influences to systemic immunity
(10,11). Alterations in the gut microbiota,
which may respond to external environmental and changes or be
driven by systemic inflammation can occur before the onset of
arthritis. Emerging research increasingly highlights the critical
role of the ‘gut-joint axis’ in the transition from preclinical to
clinical RA.
The relationship between intestinal microecological
imbalance and RA involves complex physiological and pathological
mechanisms. Studies from RA animal models and preclinical RA
(PreRA) populations indicate that gut alterations may precede the
onset of the disease and potentially serve as latent triggers of
systemic inflammation (12–16).
Conversely, a reverse ‘gut-joint axis’ mechanism may also exist,
although further research is required to substantiate this
hypothesis. For example, increasing levels of inflammatory factors
in the joints may spread peripherally, contributing to or
exacerbating damage to the intestinal mucosal barrier and
microbiota dysbiosis (17,18). Additionally, pain may alter the
metabolic products of gut microbiota and intestinal hormones
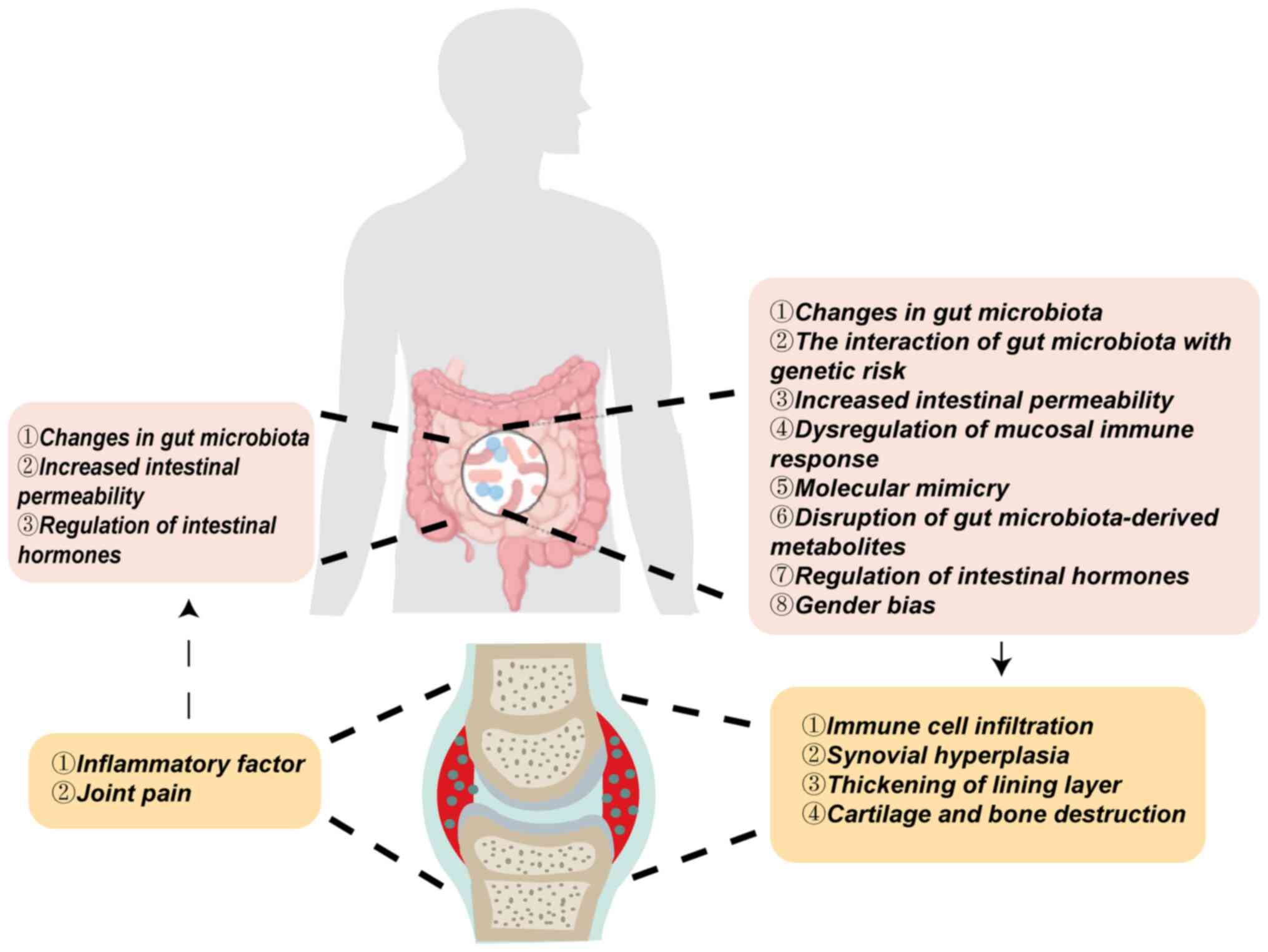
(19,20). The present review summarized the
‘gut-joint axis’ mechanism in RA, highlighting changes in gut
microbiota, interactions between microbiota and genetic risks,
alterations in intestinal permeability, dysregulated mucosal immune
responses, molecular mimicry, disruption of microbial-derived
metabolites, gut hormone regulation and sex bias. These factors,
alone or in combination, contribute to the onset and progression of
RA (Fig. 1).
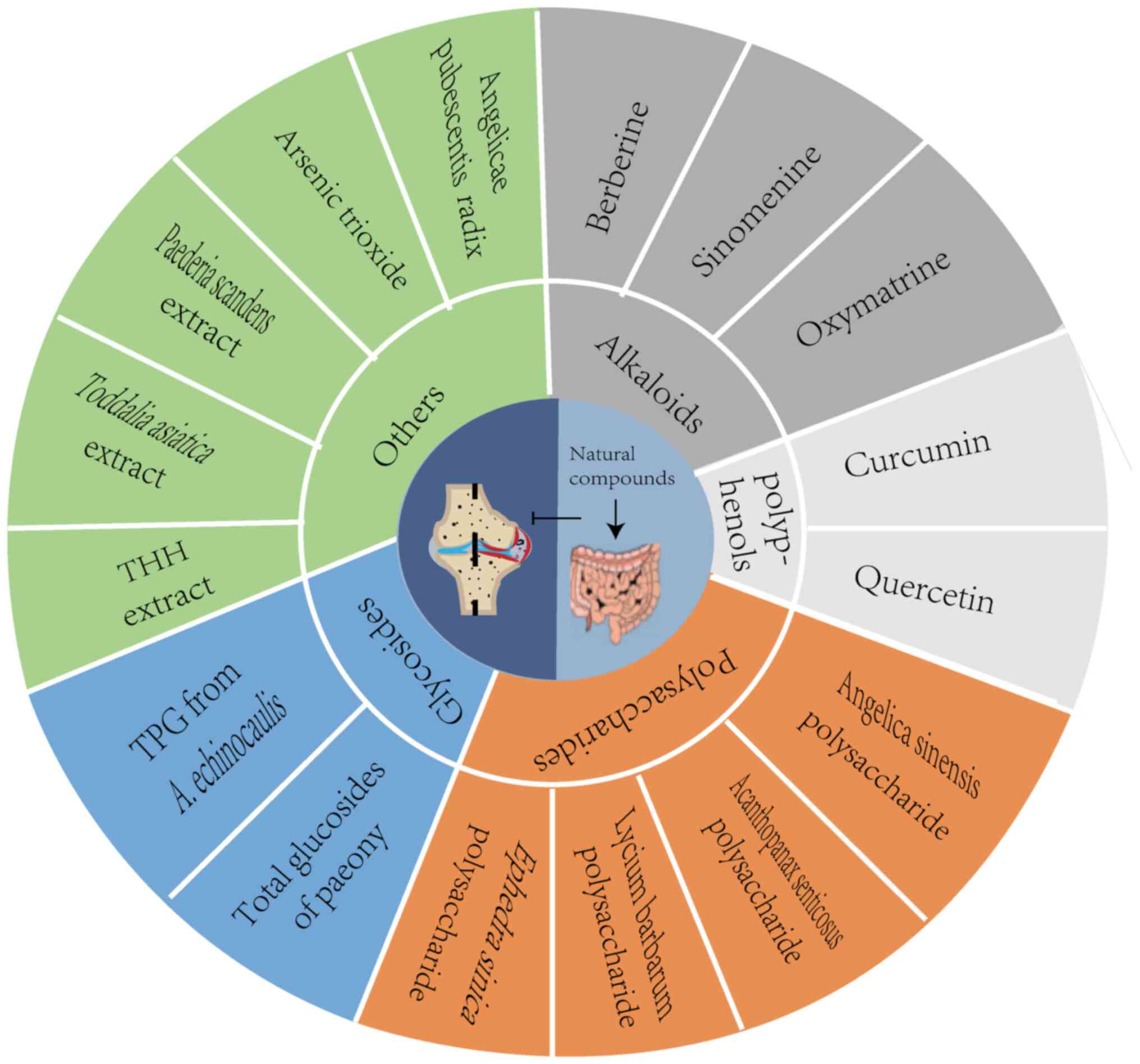
Natural compounds, biologically active substances
derived from natural sources, have garnered significant attention
due to their diverse biological effects, including antioxidant,
anti-inflammatory, anti-fibrotic, anti-tumor and anti-angiogenesis
properties. Given the limitations of current RA treatments, the
present review explored several natural compounds that exert
anti-RA effects through the ‘gut-joint axis’ mechanism, offering
new insights and potential strategies for innovative RA therapies
(Fig. 2).
The gut microbiota plays a pivotal role in
regulating host immunity and influencing the progression of RA
through various mechanisms (21,22).
Several recurring patterns have been identified in the intestinal
microbiota of patients with RA, including an increase in the
abundance of Prevotella and Lactobacillus and a
decrease in Faecalibacterium and Bacteroidetes
(14,23–27).
Among these, the pathogenic role of Prevotella in RA has
been extensively studied. Prevotella is closely associated
with the RA risk gene human leukocyte antigen
(HLA)-DRB1 and is involved in the disease's adaptive
immune response (12). Notably,
antibodies against Prevotella copri (P. copri) are specific
to patients with RA, with antibody levels to other symbiotic
bacteria in patients with RA are similar or lower compared with
those in other patients with arthritis or healthy controls
(28,29). Moreover, when P. copri
enters the systemic circulation, it can selectively accumulate in
the joints, potentially inducing synovial hyperplasia and
deformation by promoting synovial tissue growth (30). P. copri is one of several
intestinal microbiota species with pathogenic effects on RA and
their mechanisms have been comprehensively reviewed (31,32).
Genome-wide association studies have identified
several RA susceptibility alleles, including HLA-DRB1,
PTPN22, and PADI4 genes (33,34).
Alleles within the class II loci of HLA, particularly those
in DRB1, show a strong association with RA risk in
individuals positive for autoantibodies, with >70% of
ACPA-positive patients with RA carrying the HLA-DRB1 allele
(35). Environmental factors may
serve as significant triggers for individuals with a genetic
predisposition to develop RA. Notable microbiome changes have been
observed in the intestines of healthy individuals carrying the RA
risk allele HLA-DRB1, including an increase in
Lachnospiraceae, Clostridiaceae and Bifidobacterium
longum species (36). Wells
et al (12) classified
Prevotella species based on predicted types and assigned
numerical names according to the SILVA database. The authors cohort
study found that, even in the absence of clinically detectable
disease, the genetic risk of RA was associated with specific gut
microbiota. The Prevotella_7 strain (whose predicted type
remains uncertain) was particularly strongly linked to the RA
genotype.
The intestine plays a critical role as a conduit
between the body's internal environment and external factors. When
the intestinal mucosal barrier is compromised, non-self antigens
can penetrate and invade the body, triggering and exacerbating
systemic inflammatory responses. This can lead to the breakdown of
self-immune tolerance and the disruption of immune homeostasis
(37). Additionally, increased
intestinal permeability allows immune cells to migrate beyond the
intestine, potentially causing extraintestinal inflammation
(38,39). Clinical studies have shown that
intestinal permeability is elevated in both preclinical and
established patients with RA (24,39–42).
Individuals with high zonulin levels (>10 ng/ml) during the
early stages of RA are at greater risk of developing the disease
within a year (39). Furthermore,
persistent arthritis may further compromise intestinal
permeability, facilitating the invasion of joint synovial fluid by
intestinal microorganisms in the IV stage of RA (17). In vitro studies have also
indicated that intestinal bacteria from PreRA subjects directly
modulate the expression of zonula occludens-1 in Caco-2 cells at
both the transcriptional and protein levels (5). Collinsella, a genus within the
Actinobacteria phylum, is markedly enriched in the gut microbiota
of patients with RA. Its overgrowth disrupts intestinal barrier
function and is independently associated with disease-related
inflammatory activity (14,43).
In collagen-induced arthritis (CIA) mice, serum zonulin levels and
intestinal permeability are elevated prior to arthritis onset, with
this barrier dysfunction being microbiota-driven. Transfer of
CIA-derived gut microbiota to germ-free (GF) recipients results in
increased gut permeability. Moreover, pre-arthritic administration
of a zonulin antagonist selectively restores epithelial integrity
and reduces subsequent joint inflammation (39). Hypoxia-inducible factor (HIF) plays
a pivotal role in regulating tight junction proteins such as
claudin-1, thereby orchestrating adaptive barrier responses in the
mucosa (44,45). HIF-2α, specifically expressed in
the mucosal epithelium, is essential for maintaining gut barrier
integrity and regulating intestinal iron absorption. Certain
bacterial metabolites, such as 1,3-diaminopropane, reuterin, and
short-chain fatty acids (SCFAs), suppress HIF-2α, influencing
intestinal barrier function (46).
Wen et al (42)
demonstrated that inhibiting intestinal HIF-2α preserves epithelial
barrier integrity by selectively downregulating claudin-15, thereby
reducing arthritis severity. Consequently, restoring intestinal
tight junctions may be a key intervention in the prevention and
treatment of RA.
Studies suggest that the initiation of autoimmune
responses in RA begins in gut-associated lymphoid tissue (GALT),
distant from the joints, involving both innate and adaptive immune
responses (5,39,47,48).
Increased intestinal permeability facilitates the transport of
intestinal immune cells to the joints. Takahashi et al
(49) performed cell transport
analysis using KikGR mice and observed that both follicular helper
T (Tfh) cells and B cells migrated from the colonic patch to the
draining lymph nodes following immunization. In the CIA model, it
has been confirmed the distal GALT is a primary site for initiating
the autoimmune response. The intestinal lamina propria is
recognized as the main source of T helper 17 (Th17) cells, and
during the immune initiation phase in CIA mice, an increase in Th17
cell content in intestinal lamina propria correlates strongly with
arthritis severity (47,50,51).
In transgenic K/BxN arthritis mice, a population of α4β7-expressing
Th17 cells was found in the spleen, where they assist in the
generation of autoantibodies against glucose-6-phosphate isomerase
(52). Another potential source of
α4β7+ Th17 cells in the spleen is the migration of
intestinal CD103+ dendritic cells (DCs) to the spleen.
Marietta et al (38)
demonstrated that CD103+ DCs, located in the intestinal
lamina propria, migrate to the spleen in CIA mice, suggesting that
these DCs may have originated from the intestine.
Molecular mimicry, a process in which microbial
antigens share structural similarities with autoantigens, can lead
to the cross-activation of autoreactive T or B cells, thereby
triggering autoimmunity. Certain gut microbiota have been
implicated in causing autoimmune responses and systemic
inflammation through molecular mimicry, resulting in joint tissue
damage (53). For instance,
Escherichia coli heat-shock protein (DnaJ) shares a
molecular mimicry mechanism with the HLA-DRB1*0401 molecule,
as both exhibit the amino acid sequence QKRAA (54). Preclinical RA studies have
identified potential epitopes in Citrobacter, Clostridium,
Bacteroides and Eggerthella that resemble collagen XI
and HLA-DRB1*0401 epitopes (12,13,55,56).
Additionally, Bacteroides fragilis 3_1_12, a strain from the
Bacteroidaceae family, may mimic the collagen II peptide
(57–59). Similarly, Prevotella
epitopes show high sequence homology with T-cell epitopes of
N-acetylglucosamine-6-sulfonamide and serpin A, presented by
HLA-DR (60–62). These microorganisms may trigger
autoimmune responses and antibody production via molecular
mimicry, thereby accelerating RA progression. Thus, molecular
mimicry may represent a key mechanism by which the gut microbiota
influences the pathogenesis and development of RA.
The disruption of metabolites derived from the
intestinal microbiota plays a pivotal role in the ‘gut-joint axis’
of RA. Preclinical high-risk populations have already shown
intestinal microbiota dysbiosis and alterations in metabolomics
(5). Among these metabolites,
SCFAs have been studied most extensively studied and are considered
key immunomodulatory compounds closely associated with RA (63). SCFAs primarily exert their effects
on the immune system by modulating histone acetyltransferases and
deacetylases (64). In patients
with RA, fecal levels of acetate, propionate, butyrate, and
valerate are markedly reduced (49,63,65),
with a characteristic deficiency of butyrate producers and an
enrichment of butyrate consumers in the intestinal microbiota of
these individuals (66,67). The protective role of butyrate in
RA has been widely investigated, particularly its effects on
balancing Th17 and T regulatory (Treg) cells in the intestine
system, as well as its regulation of B cells and autoantibody
production (63,67,68).
Additionally, Kyoto Encyclopedia of Genes and Genomes (KEGG)
pathway enrichment analysis has revealed alterations in amino acid
and lipid metabolism in PreRA and established patients with RA
(5,69). A retrospective clinical cohort
study in Sweden found that patients with RA and positive ACPA
exhibited elevated levels of lysophospholipid and tryptophan
metabolism several years before diagnosis, compared with
undiagnosed individuals with RA (70). Following this, Seymour et al
(71) demonstrated in animal
studies that indole, a tryptophan metabolite produced by bacteria
such as P. copri, Collinsella and Ruminococcus, plays
a pivotal role in the development of CIA by enhancing Th17 immunity
both locally in the gut and systemically. Bile acids (BAs) are
vital for immune regulation, particularly in balancing Th17 and
Treg cell populations (72–75),
and contribute to the maintenance of fat metabolism. However, high
concentrations of BAs can have cytotoxic effects on colonic
epithelial cells (76). While
studies on BA levels in RA animal models remain inconclusive
(77–79), accumulating evidence suggests that
BAs play a protective and preventative role in RA (18,80).
The intestine harbors a significant number of
endocrine cells that secrete various intestinal hormones. Hormones
such as vasoactive intestinal peptide (VIP), somatostatin (SOM),
substance P (SP) and gastrin-releasing peptide are implicated in
the progression of RA (81–83).
VIP can promote the Th1/Th2 balance and enhance the differentiation
of Treg cells and follicular regulatory T (Tfr) cells, thereby
reducing joint inflammation in RA animal models (84–86).
SP has been reported to stimulate the proliferation of synovial
cells (87,88) and serum SP levels are considered
indicative of disease activity and subclinical inflammation in
patients with RA (89). However,
other studies suggest that SP treatment can reduce inflammatory
cytokines, such as tumor necrosis factor-α (TNF-α) and
interleukin-17 (IL-17), while increasing the expression of the
anti-inflammatory cytokine IL-10, leading to significant
improvements in RA symptoms (90,91).
Consequently, the exact mechanism by which SP influences RA remains
to be further elucidated.
Significant sex differences exist in RA, with a
prevalence rate in females that is notably higher than in males,
often at a ratio of 4:1 or more (92,93).
The influence of sex hormones on RA has been extensively studied. A
sudden drop in estrogen levels and a decrease in androgen can both
increase RA risk (94). Sex
hormone deficiency enhances intestinal permeability, expands the
Th17 cell pool and upregulates osteoclastogenic factors, such as
TNF-α, RANKL and IL-17, in the bone marrow, with the gut microbiota
playing a central role in this process (95). However, butyrate supplementation
has been shown to prevent estrogen-deficiency-induced bone loss
(96) and alleviate arthritis by
activating the estrogen-related receptor α on Th17 cells,
modulating IL-17 and IL-10 production (68). Sex-specific differences in the gut
microbiota have been well established (97). Compared to women, men have markedly
lower levels of Prevotella (36) and P. copri antibodies, which
are considered specific to RA, exhibit a sex disparity, with a
male-to-female ratio of 1:9. This difference may markedly
contribute to the female predominance observed in RA (28). Additionally, Eggerthella lenta
(E. lenta), a potential pathogenic bacterium in RA, is found in
higher levels in female patients with RA (98). Animal studies have shown that
female mice treated with E. lenta display increased
intestinal permeability, elevated IgG-RF levels, an expansion of
IL-17- and IFN-γ-producing B cells, and higher concentrations of
BAs and succinyl carnitine, indicating a sex-specific immune
response to E. lenta (98).
Despite some evidence, the role of sex-dependent differences in the
human microbiota in RA remains poorly understood. Preclinical and
clinical data are both still limited, and more comprehensive
evidence is needed to support or challenge these findings.
Natural compounds exert a positive effect on human
health by regulating various mechanisms, offering potential
therapeutic benefits for a range of diseases. Several natural
compounds, including sinomenine and Tripterygium wilfordii
are widely used in the clinical treatment of RA in China. Clinical
studies exploring the use of natural compounds for RA treatment
have entered the initial exploration stage (Table I). However, these studies face
challenges such as low quality, weak evidence and small sample
size, necessitating high-quality clinical trials to improved
support the therapeutic effects of these compounds on RA. Basic
research has consistently confirmed the protective effects of
natural compounds on RA, elucidating the underlying mechanisms. For
most high-molecular-weight, low-bioavailability natural compounds,
which accumulate in large quantities in the gastrointestinal tract
after oral administration, the intestine appears to be a key target
for their action in treating RA. This section focuses on natural
compounds that treat or enhance RA therapy through the ‘gut-joint
axis’ (Table II).
Alkaloids, primarily found in plants, are key
components of numerous Chinese herbal medicines. Higher plants from
the Liliaceae, Berberidaceae, Ranunculaceae, Leguminosae,
Amaryllidaceae, Papaveraceae and Solanaceae families are rich in
alkaloids. These compounds are characterized by nitrogen atoms in
their chemical structure, which is a defining feature and their
biological efficacy is attributed to the specific arrangement of
atoms in their molecular structure (99). Alkaloids exhibit significant
biological activities, including antibacterial, antiviral,
anti-inflammatory, and anti-tumor effects, suggesting their
potential medicinal value in the RA treatment (100–102).
Berberine, an isoquinoline alkaloid extracted from
various medicinal plants such as Coptis, Phellodendron and
Berberis, possesses a broad range of pharmacological effects,
including anti-tumor, anti-oxidative, anti-inflammatory,
hypoglycemic and lipid-lowering properties. Clinical studies have
reported its therapeutic efficacy in treating conditions such as
cancer, digestive system disorders, and metabolic diseases
(103–107). Due to its ability to inhibit
toxins and bacteria and protect the intestinal mucosal barrier,
berberine has shown significant advantages in the treatment of
digestive disorders (108). A
large portion of orally administered berberine remains in the
intestine, with an extremely low bioavailability of only 0.1%
(109). Oral administration of
200 mg/kg berberine markedly improved CIA in rats, although the
peak drug concentration in the blood was <0.06 µM (110), which is much lower than the in
vitro minimum effective concentration of 1–50 µM (109,111). Additionally, intravenous
berberine did not improve arthritis in rats, suggesting that its
anti-arthritis effects are intestinally dependent (110). Further mechanistic studies
revealed that oral berberine selectively increased cortistatin
levels, a neuropeptide derived from the intestine, both in the
intestines and serum of arthritic rats. It also promoted
cortistatin expression in intestinal nerve and endocrine cells. The
upregulated cortistatin then entered systemic circulation, where it
suppressed the immune response of Th17 cells, alleviating arthritis
symptoms (110). Moreover,
berberine markedly increased the levels of intestinal SCFAs,
particularly butyrate, enhanced the abundance of butyrate-producing
bacteria and stimulated the expression and activity of butyryl-CoA:
Acetate CoA transferase (BUT) (112). When BUT inhibitors or
broad-spectrum antibiotics were applied, the regulatory effect of
berberine on the intestinal internal environment and its
anti-arthritis effects were markedly reduced, indicating that
berberine promotes butyrate production through the intestinal
microbiota, positioning it as a potential therapeutic agent for RA
(112). This effect is highly
dependent on the intestinal microbiota (112).
Matrine, a natural bioactive compound extracted from
the roots of the leguminous plant Sophora flavescens, is one
of its main pharmacologically active components. It possesses a
broad range of pharmacological and biological effects, including
antiviral, anti-tumor, antioxidant and antibacterial properties,
with the added advantage of low toxicity and minimal side effects
(125). A number of studies have
demonstrated that matrine can effectively alleviate arthritis
symptoms in RA model animal by regulating immune responses,
inhibiting synovial vessel formation, and suppressing
fibroblast-such as synovial cell proliferation, among other
mechanisms (126–128). Oxymatrine, a derivative of
matrine with a distinct oxygen structure, exhibits similar
pharmacological effects. Animal studies have shown that oxymatrine
also exerts anti-RA effects (129–131). In CIA mice, oxymatrine treatment
markedly improved arthritis symptoms, reduced the abundance of
Firmicutes in the gut and increased the abundance of
Bacteroidota, Patescibacteria and Campylobacterota,
thereby reshaping the gut microbiota towards more normal levels
(131). Moreover, oxymatrine
treatment corrected the imbalance in CD4+ T cell subsets
in CIA mice, restoring intestinal immune balance by regulating the
Th1/Th2, Treg/Th17 and Tfr/Tfh cell ratios (131). Limited clinical studies also
suggest that adding oxymatrine to routine RA treatment can more
effectively reduce inflammatory markers and balance the immune
response in patients (132,133).
Polyphenols, commonly found in plants such as
vegetables, fruits and soybeans, consist primarily of aromatic
rings and hydroxyl groups. These compounds are renowned for their
potent anti-inflammatory and antioxidant properties, which offer
significant benefits for human health and the prevention of chronic
diseases. Due to these properties, polyphenols are emerging as
promising candidate drugs for the management of RA.
Curcumin, the main active compound in turmeric,
directly targets the intestines and exhibits potent
anti-inflammatory, immune-regulatory and intestinal
microbiota-modulating effects (134,135). Extensive basic and clinical
studies have confirmed curcumin's significant benefits in improving
RA (136). In clinical settings,
curcumin not only alleviates symptoms and reduces serum
inflammatory markers but also demonstrates a high safety profile
(137–140). However, curcumin is characterized
by poor gastrointestinal absorption and low oral bioavailability,
with an absolute bioavailability of only 1%. In rats, oral
administration of 100 mg/kg curcumin resulted in a plasma peak of
only 0.02 µM, far below the minimum effective concentration of 10
µM needed to inhibit synovial cell and lymphocyte activation in
vitro (141–144). Consequently, curcumin's
therapeutic effects on RA may be primarily mediated through its
extensive pharmacological effect on the intestine. Yang et
al (145) indicated that the
anti-arthritis effects of oral curcumin depend on the intestine.
Curcumin activates the cAMP/PKA and Ca2+/CaMKII
signaling pathways, increasing the number of SOM-positive cells in
the small intestine and raising SOM levels in both the intestine
and serum. This process alleviates inflammation and immune
dysfunction, thereby inhibiting the progression of adjuvant-induced
arthritis (AIA) in rats. However, when a SOM inhibitor is used or
curcumin is administered intraperitoneally, this anti-arthritis
effect is markedly diminished.
Quercetin, a flavonoid commonly found in fruits and
vegetables, possesses multiple biological activities, including
anti-inflammatory, antioxidant, anti-cancer and liver-protective
effects. Clinical studies on quercetin for treating RA are limited.
While quercetin appears to positively affect arthritis symptoms,
its ability to enhance antioxidant capacity and modulate
inflammation in patients with RA remains controversial (146,147). Animal studies, however, have
shown that quercetin's potent antioxidant properties not only
improve RA but also alleviate side effects caused by traditional RA
treatments (148,149). Moreover, research has
demonstrated that RA is associated with enteric neurodegenerative
changes that impair intestinal functions such as absorption,
secretion, and immunity (150,151). Oral administration of quercetin
can increase the expression of glial cell-derived neurotrophic
factor, glial fibrillary acidic protein and VIP in the intestine.
This effect helps reverse the decreased density of intestinal
neurons and glial cells in RA mice, restores the morphological
changes in the myenteric and submucosal plexuses, and alleviates
both intestinal and joint inflammation (152,153).
Natural polysaccharides are macromolecular
substances primarily extracted from plants, algae, animals, fungi
and bacteria. These polysaccharides exhibit a wide range of
important biological activities, including anti-hyperlipidemic,
antioxidant, anti-tumor, anti-hepatotoxicity, and immunomodulatory
effects (154). Several Chinese
herbal medicines, such as Angelica sinensis, wolfberry,
Acanthopanax senticosus and Ephedra, contain natural
polysaccharides that have been shown to alleviate RA by protecting
the intestines and modulating the intestinal microbiota.
LBP, a key active component of wolfberry, is not
directly digested or absorbed by the human body. Instead, it
reaches the large intestine where it is metabolized by the gut
microbiota. LBP is highly valued for its medicinal properties,
including enhancing barrier function, anti-inflammatory,
antioxidant, anti-tumor effects and its ability to regulate the
intestinal microbiota (163–167). LBP exerts an anti-CIA effect
regulates chondrocyte proliferation, reduces the expression of
inflammatory cytokines such as IL-1α, IL-1β, IL-12 and IL-17 and
restores anti-inflammatory cytokine IL-10 levels (168–170). Additionally, LBP may alter the
gut microbiota composition, increasing the abundance of microbial
taxa that produce S-adenosylmethionine, which induces DNA
hypermethylation of RA-related genes (such as Dpep3, Gstm6,
Slc27a2, Col11a2, Sycp2, SNORA22) in intestinal epithelial
cells. This suppresses gene expression, reduces inflammatory
cytokine levels, and mitigates inflammation (170). The elimination of LBP's anti-CIA
effect by antibiotics further suggests that its therapeutic action
relies on the intestinal microbiota (171).
Paeony, a traditional Chinese herbal medicine, is
highly regarded for its medicinal properties. TGP, extracted from
the roots of paeony, serves as an immunomodulator and is widely
used in China for RA treatment. Although clinical studies have not
yet conclusively demonstrated that combining TGP with conventional
drugs offers superior efficacy in treating RA, they have shown that
TGP can mitigate liver function damage caused by drug treatments
(175–178). The primary chemical components of
TGP include paeoniflorin, albiflorin, hydroxy-paeoniflorin, paeonin
and benzoylpaeoniflorin, all of which are monoterpene glycosides
with low bioavailability and poor absorption (179,180). These compounds tend to accumulate
in the intestine and can be metabolized by the intestinal
microbiota (181–183). TGP treatment reduces arthritis
severity in CIA rats, restructures the gut microbiota by correcting
78% of differential taxonomic profiles and enriches beneficial
symbionts, thereby restoring intestinal ecological balance.
Additionally, TGP markedly downregulates intestinal IFN-γ and
secretory IgA production, modulates mucosal immunity, induces
autoimmune tolerance and suppressed inflammatory responses
(180). TGP also inhibits the
expression of VEGF in CIA rats, regulating synovial
neovascularization and abnormal synovial cell proliferation
(184). These findings suggest
that the therapeutic effects of TGP on RA may be mediated through
intestinal regulatory mechanisms.
THH is a commonly used drug in China, known for its
immunosuppressive, anti-tumor and anti-inflammatory properties
(187). Numerous studies have
demonstrated that THH extract improves joint indices, reduces
swelling and mitigates joint damage in RA animal models (188,189). Further investigations have
revealed that THH extract markedly elevates the colonic mRNA levels
of tight-junction proteins and mucins in RA mice. It also reduces
the abundance of pathogenic bacteria, such as Marvinbryantia,
Desulfovibrio, Parabobacterides, Bacteroides and
Butyricimonas, while enriching beneficial taxa such as
ifidobacterium, Akkermansia, Lactobacillus and
Roseburia. These alterations help restore microbial balance,
reprogram metabolic pathways, enhance SCFA production, and modulate
BA metabolism (190,191). Additionally, THH treatment
reduces the expression of pro-inflammatory cytokines (IL-1β, IL-8,
IL-17) and increases anti-inflammatory cytokine IL-10 in the colon
of RA model mice. It also inhibits NLRP3 inflammasome activation
and blocks the TLR4/MyD88/MAPK signaling pathways in muscles and
plasma, reducing joint inflammation and protecting the joints
(190,191). However, these therapeutic effects
were not observed in RA mice with gut microbiota deficiency,
confirming that a healthy gut microbiota is essential for the
therapeutic efficacy of THH extract (190,191).
RTA, a liana primarily found in China and Southeast
Asia, has anti-inflammatory and analgesic properties, making it
widely used for treating chronic pain and gastrointestinal
disorders (192–194). RTA extract reduces serum
inflammatory markers in RA model animals, increases chondrocyte
survival in inflammatory environments, and improves arthritis
symptoms (194–196). Research by Qin et al
(197) suggests that the
therapeutic the mechanism of RTA extract in RA may involve
modulating intestinal immune responses and restoring microbial
balance. Following RTA treatment, Th17 (IL-17A, RORC, IL-1β and
IL-6) and Treg (IL-10 and FOXP3) cell-related protein and mRNA
levels in the colon tissues of AIA rats were downregulated and
upregulated, respectively, promoting the restoration of the
Th17//Treg balance. Additionally, RTA extract reshaped the
intestinal microbiota of AIA rats, increasing beneficial bacteria
and reducing RA-associated strains, such as Liilactobacillus
and Streptococcus.
ATO, a compound with a long history of use in
treating various diseases, was approved by the U.S. Food and Drug
Administration in 2000 for treating acute promyelocytic leukemia,
eventually emerging as a cutting-edge treatment for lymphoma, solid
tumors, and other conditions (200). Studies suggest that ATO holds
potential in RA treatment by balancing immune cells and inhibiting
angiogenesis (201–203). Additionally, regulating
intestinal microecology and abnormal metabolites may be part of
ATO's therapeutic action in RA (204). Specifically, ATO treatment
increased the richness and diversity of the intestinal microbiota
in CIA mice, restoring the imbalance between the two major phyla,
Firmicutes and Bacteroidetes (204). On a metabolic level, ATO
regulates several metabolites, including (−)-β-pinene, a substrate
involved in lipid metabolism related to RA. ATO may alleviate RA
symptoms by regulating lipid metabolism through the modulation of
(−)-β-pinene levels (204).
The ‘gut-joint axis’ is not exclusive to RA but
also occurs in other immune-mediated arthritis diseases, with
distinct mechanisms at play. Studies have shown that ~50% of
patients with spondyloarthritis (SpA) develop subclinical
intestinal inflammation, which can progress to inflammatory bowel
disease (IBD), most commonly Crohn's disease (210,211). By contrast, subclinical
intestinal inflammation in RA predominantly involves the small
intestine and is characterized by lymphocyte and macrophage
infiltration (39,210,211). Furthermore, the composition of
the intestinal microbiota differs between RA and SpA:
Prevotella is markedly and specifically enriched in RA,
whereas no distinct microbial signature has yet been identified for
SpA. However, dysbiosis-driven activation of the IL-23/IL-17 axis
plays a pivotal role in the pathogenesis of both SpA and IBD
(212). Additionally, whereas
CD4+ T cells that dominate the ‘gut-joint axis’ in RA,
CD8+ T cells bearing identical T-cell receptor
clonotypes are the predominant immune population in both the gut
and joints of SpA patients (210,213).
Overall, alterations in the microbiota are a key
factor in the ‘gut-joint axis’ of RA. These changes not only
influence mucosal and systemic autoimmune responses via
metabolites, molecular mimicry and other mechanisms but are also
closely linked to the efficacy and side effects of RA treatments,
particularly methotrexate (24,214,215). Research focusing on specific
microbiota and metabolomics may offer a more targeted approach to
understanding the ‘gut-joint axis’ in RA. Since the intestinal
microbiota is influenced by numerous factors, broadly studying all
microbiota changes to pinpoint the pathogenesis of diseases may
lead to incorrect conclusions.
Natural compounds possess diverse biological
activities and can improve RA by regulating the intestinal
microecology. Importantly, the anti-RA effects of certain compounds
are dependent on the intestine. The intestinal microbiota
represents a potential target for the treatment of RA, particularly
with high-molecular-weight and low-bioavailability natural
compounds. Although existing reviews have catalogued the potential
mechanisms by which natural compounds ameliorate RA and
occasionally mention the gut-joint axis, the present review
presented a more comprehensive and systematic summary of the
anti-RA effects of natural compounds based on this mechanism
(216–219). The present review highlighted the
protective effects of natural compounds such as alkaloids,
polyphenols, polysaccharides and plant extracts on the intestinal
mucosa and the regulation of intestinal flora homeostasis in RA
model animals, ultimately improving RA symptoms. It explored the
anti-RA potential and mechanisms of these compounds, providing a
scientific foundation and new insights for the development of novel
therapeutic drugs. Several natural compounds have already been
widely used in clinical practice, while others, with solid
scientific support for drug development, should be prioritized. For
example, berberine, a well-studied natural compound, has shown
significant therapeutic effects across various diseases, suggesting
its broad potential in RA drug development. Lycium barbarum
polysaccharides, one of the key active components of L.
barbarum, also exhibit notable efficacy in treating RA.
Moreover, L. barbarum, being a readily available and
low-cost source, could be a valuable candidate for drug
development.
Despite their therapeutic potential, most natural
compounds suffer from low oral bioavailability and tend to
accumulate in the gastrointestinal tract after oral administration.
While this accumulation may be advantageous for RA treatment
through the ‘gut-joint axis’ mechanism, improving the
bioavailability of these compounds could further enhance their
efficacy in disease recovery. Additionally, challenges in
translating natural compounds into clinical drugs include
structural instability, poor solubility and unclear toxic or side
effects. Hence, novel drug discovery remains a protracted,
multi-parameter endeavor that demands rigorous, head-to-toe
optimization and integrated assessment of all pharmacological,
pharmacokinetic and safety attributes. However, as pharmacological
mechanisms of natural compounds are being dissected at ever-greater
depth, our knowledge of these compounds has broadened and their
multifaceted profiles are now improved understood.
At present, new drug delivery methods such as
nanoparticles are developing rapidly. It is expected that in the
future, there will be more basic research and clinical trials on
the treatment of RA using natural compounds through the ‘gut-joint
axis’ pathway. This is precisely what is needed at present.
Not applicable.
Funding: No funding was received.
Not applicable.
WH was responsible for conceptualization, writing
the original draft, writing, reviewing and editing. RL was
responsible for writing, reviewing and editing and providing
illustrations and tables. ZZ was responsible for writing, reviewing
and editing. Data authentication is not applicable. All authors
read and approved the final manuscript.
Not applicable.
Not applicable.
|
1
|
Di Matteo A, Bathon JM and Emery P:
Rheumatoid arthritis. Lancet. 402:2019–2033. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
GBD 2021 Rheumatoid Arthritis
Collaborators, . Global, regional, and national burden of
rheumatoid arthritis, 1990–2020, and projections to 2050: A
systematic analysis of the Global Burden of Disease Study 2021.
Lancet Rheumatol. 5:e594–e610. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Aletaha D and Smolen JS: Diagnosis and
management of rheumatoid arthritis: A Review. JAMA. 320:1360–1372.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brewer RC, Lanz TV, Hale CR, Sepich-Poore
GD, Martino C, Swafford AD, Carroll TS, Kongpachith S, Blum LK,
Elliott SE, et al: Oral mucosal breaks trigger anti-citrullinated
bacterial and human protein antibody responses in rheumatoid
arthritis. Sci Transl Med. 15:eabq84762023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Luo Y, Tong Y, Wu L, Niu H, Li Y, Su LC,
Wu Y, Bozec A, Zaiss MM, Qing P, et al: Alteration of gut
microbiota in individuals at High-Risk for rheumatoid arthritis
associated with disturbed metabolome and the initiation of
arthritis through the triggering of mucosal immunity imbalance.
Arthritis Rheumatol. 75:1736–1748. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
de Jesus VC, Singh M, Schroth RJ,
Chelikani P and Hitchon CA: Association of bitter taste receptor
T2R38 polymorphisms, oral microbiota, and rheumatoid arthritis.
Curr Issues Mol Biol. 43:1460–1472. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
van Delft MAM and Huizinga TWJ: An
overview of autoantibodies in rheumatoid arthritis. J Autoimmun.
110:1023922020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kronzer VL and Sparks JA: Occupational
inhalants, genetics and the respiratory mucosal paradigm for
ACPA-positive rheumatoid arthritis. Ann Rheum Dis. 82:303–305.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Holers VM, Demoruelle KM, Buckner JH,
James EA, Firestein GS, Robinson WH, Steere AC, Zhang F, Norris JM,
Kuhn KA and Deane KD: Distinct mucosal endotypes as initiators and
drivers of rheumatoid arthritis. Nat Rev Rheumatol. 20:601–613.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao T, Wei Y, Zhu Y, Xie Z, Hai Q, Li Z
and Qin D: Gut microbiota and rheumatoid arthritis: From
pathogenesis to novel therapeutic opportunities. Front Immunol.
13:10071652022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jiao Y, Wu L, Huntington ND and Zhang X:
Crosstalk between gut microbiota and innate immunity and its
implication in autoimmune diseases. Front Immunol. 11:2822020.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wells PM, Adebayo AS, Bowyer RCE, Freidin
MB, Finckh A, Strowig T, Lesker TR, Alpizar-Rodriguez D, Gilbert B,
Kirkham B, et al: Associations between gut microbiota and genetic
risk for rheumatoid arthritis in the absence of disease: A
cross-sectional study. Lancet Rheumatol. 2:e418–e427. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang X, Zhang D, Jia H, Feng Q, Wang D,
Liang D, Wu X, Li J, Tang L, Li Y, et al: The oral and gut
microbiomes are perturbed in rheumatoid arthritis and partly
normalized after treatment. Nat Med. 21:895–905. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen J, Wright K, Davis JM, Jeraldo P,
Marietta EV, Murray J, Nelson H, Matteson EL and Taneja V: An
expansion of rare lineage intestinal microbes characterizes
rheumatoid arthritis. Genome Med. 8:432016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Inamo J: Non-causal association of gut
microbiome on the risk of rheumatoid arthritis: A Mendelian
randomisation study. Ann Rheum Dis. 80:e1032021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jeong Y, Kim JW, You HJ, Park SJ, Lee J,
Ju JH, Park MS, Jin H, Cho ML, Kwon B, et al: Gut microbial
composition and function are altered in patients with early
rheumatoid arthritis. J Clin Med. 8:6932019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cheng M, Zhao Y, Cui Y, Zhong C, Zha Y, Li
S, Cao G, Li M, Zhang L, Ning K and Han J: Stage-specific roles of
microbial dysbiosis and metabolic disorders in rheumatoid
arthritis. Ann Rheum Dis. 81:1669–1677. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sun H, Guo Y, Wang H, Yin A, Hu J, Yuan T,
Zhou S, Xu W, Wei P, Yin S, et al: Gut commensal Parabacteroides
distasonis alleviates inflammatory arthritis. Gut. 72:1664–1677.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu X, Zhan G, Chen R, Wang D, Guan S and
Xu H: Gut microbiota and its role in stress-induced hyperalgesia:
Gender-specific responses linked to different changes in serum
metabolites. Pharmacol Res. 177:1061292022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Barton JR, Londregan AK, Alexander TD,
Entezari AA, Covarrubias M and Waldman SA: Enteroendocrine cell
regulation of the gut-brain axis. Front Neurosci. 17:12729552023.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fan L, Xia Y, Wang Y, Han D, Liu Y, Li J,
Fu J, Wang L, Gan Z, Liu B, et al: Gut microbiota bridges dietary
nutrients and host immunity. Sci China Life Sci. 66:2466–2514.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen S, Dan L, Xiang L, He Q, Hu D and Gao
Y: The role of gut flora-driven Th cell responses in preclinical
rheumatoid arthritis. J Autoimmun. 154:1034262025. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Alpizar-Rodriguez D, Lesker TR, Gronow A,
Gilbert B, Raemy E, Lamacchia C, Gabay C, Finckh A and Strowig T:
Prevotella copri in individuals at risk for rheumatoid
arthritis. Ann Rheum Dis. 78:590–593. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Scher JU, Sczesnak A, Longman RS, Segata
N, Ubeda C, Bielski C, Rostron T, Cerundolo V, Pamer EG, Abramson
SB, et al: Expansion of intestinal Prevotella copri
correlates with enhanced susceptibility to arthritis. Elife.
2:e012022013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Picchianti-Diamanti A, Panebianco C,
Salemi S, Sorgi ML, Di Rosa R, Tropea A, Sgrulletti M, Salerno G,
Terracciano F, D'Amelio R, et al: Analysis of gut microbiota in
rheumatoid arthritis patients: Disease-related dysbiosis and
modifications induced by etanercept. Int J Mol Sci. 19:29382018.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu X, Zeng B, Zhang J, Li W, Mou F, Wang
H, Zou Q, Zhong B, Wu L, Wei H and Fang Y: Role of the gut
microbiome in modulating arthritis progression in mice. Sci Rep.
6:305942016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hu Q, Wu C, Yu J, Luo J and Peng X:
Angelica sinensis polysaccharide improves rheumatoid
arthritis by modifying the expression of intestinal Cldn5, Slit3
and Rgs18 through gut microbiota. Int J Biol Macromol. 209:153–161.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pianta A, Arvikar S, Strle K, Drouin EE,
Wang Q, Costello CE and Steere AC: Evidence of the immune relevance
of Prevotella copri, a gut microbe, in patients with
rheumatoid arthritis. Arthritis Rheumatol. 69:964–975. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pianta A, Chiumento G, Ramsden K, Wang Q,
Strle K, Arvikar S, Costello CE and Steere AC: Identification of
novel, immunogenic HLA-DR-Presented Prevotella copri
peptides in patients with rheumatoid arthritis. Arthritis
Rheumatol. 73:2200–2205. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao Y, Chen B, Li S, Yang L, Zhu D, Wang
Y, Wang H, Wang T, Shi B, Gai Z, et al: Detection and
characterization of bacterial nucleic acids in culture-negative
synovial tissue and fluid samples from rheumatoid arthritis or
osteoarthritis patients. Sci Rep. 8:143052018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Peng Y, Huang Y, Li H, Li C, Wu Y, Wang X,
Wang Q, He J and Miao C: Associations between rheumatoid arthritis
and intestinal flora, with special emphasis on RA pathologic
mechanisms to treatment strategies. Microb Pathog. 188:1065632024.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu Z, Wu Y, Luo Y, Wei S, Lu C, Zhou Y,
Wang J, Miao T, Lin H, Zhao Y, et al: Self-Balance of intestinal
flora in spouses of patients with rheumatoid arthritis. Front Med
(Lausanne). 7:5382020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Padyukov L: Genetics of rheumatoid
arthritis. Semin Immunopathol. 44:47–62. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Saevarsdottir S, Stefansdottir L, Sulem P,
Thorleifsson G, Ferkingstad E, Rutsdottir G, Glintborg B,
Westerlind H, Grondal G, Loft IC, et al: Multiomics analysis of
rheumatoid arthritis yields sequence variants that have large
effects on risk of the seropositive subset. Ann Rheum Dis.
81:1085–1095. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Larid G, Pancarte M, Offer G, Clavel C,
Martin M, Pradel V, Auger I, Lafforgue P, Roudier J, Serre G and
Balandraud N: In rheumatoid arthritis patients, HLA-DRB1*04:01 and
rheumatoid nodules are associated with ACPA to a particular fibrin
epitope. Front Immunol. 12:6920412021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Asquith M, Sternes PR, Costello ME,
Karstens L, Diamond S, Martin TM, Li Z, Marshall MS, Spector TD, le
Cao KA, et al: HLA alleles associated with risk of ankylosing
spondylitis and rheumatoid arthritis influence the gut microbiome.
Arthritis Rheumatol. 71:1642–1650. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Paray BA, Albeshr MF, Jan AT and Rather
IA: Leaky gut and autoimmunity: An Intricate balance in individuals
health and the diseased State. Int J Mol Sci. 21:97702020.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Marietta EV, Murray JA, Luckey DH, Jeraldo
PR, Lamba A, Patel R, Luthra HS, Mangalam A and Taneja V:
Suppression of inflammatory arthritis by human Gut-derived
prevotella histicola in humanized mice. Arthritis Rheumatol.
68:2878–2888. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tajik N, Frech M, Schulz O, Schälter F,
Lucas S, Azizov V, Dürholz K, Steffen F, Omata Y, Rings A, et al:
Targeting zonulin and intestinal epithelial barrier function to
prevent onset of arthritis. Nat Commun. 11:19952020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Audo R, Sanchez P, Riviere B, Mielle J,
Tan J, Lukas C, Macia L, Morel J and Immediato Daien C: Rheumatoid
arthritis is associated with increased gut permeability and
bacterial translocation which are reversed by inflammation control.
Rheumatology (Oxford). Aug 10–2022.(Epub ahead of print). doi:
10.1093/rheumatology/keac454.
|
|
41
|
Heidt C, Kammerer U, Fobker M, Ruffer A,
Marquardt T and Reuss-Borst M: Assessment of intestinal
permeability and inflammation Bio-markers in patients with
rheumatoid arthritis. Nutrients. 15:23862023. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wen J, Lyu P, Stolzer I, Xu J, Gießl A,
Lin Z, Andreev D, Kachler K, Song R, Meng X, et al: Epithelial
HIF2α expression induces intestinal barrier dysfunction and
exacerbation of arthritis. Ann Rheum Dis. 81:1119–1130. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ruiz-Limón P, Mena-Vázquez N,
Moreno-Indias I, Manrique-Arija S, Lisbona-Montañez JM, Cano-García
L, Tinahones FJ and Fernández-Nebro A: Collinsella is associated
with cumulative inflammatory burden in an established rheumatoid
arthritis cohort. Biomed Pharmacother. 153:1135182022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Schwartz AJ, Das NK, Ramakrishnan SK, Jain
C, Jurkovic MT, Wu J, Nemeth E, Lakhal-Littleton S, Colacino JA and
Shah YM: Hepatic hepcidin/intestinal HIF-2α axis maintains iron
absorption during iron deficiency and overload. J Clin Invest.
129:336–348. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Saeedi BJ, Kao DJ, Kitzenberg DA,
Dobrinskikh E, Schwisow KD, Masterson JC, Kendrick AA, Kelly CJ,
Bayless AJ, Kominsky DJ, et al: HIF-dependent regulation of
claudin-1 is central to intestinal epithelial tight junction
integrity. Mol Biol Cell. 26:2252–2262. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Das NK, Schwartz AJ, Barthel G, Inohara N,
Liu Q, Sankar A, Hill DR, Ma X, Lamberg O, Schnizlein MK, et al:
Microbial metabolite signaling is required for systemic Iron
homeostasis. Cell Metab. 31:115–130.e6. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Rogier R, Evans-Marin H, Manasson J, van
der Kraan PM, Walgreen B, Helsen MM, van den Bersselaar LA, van de
Loo FA, van Lent PL, Abramson SB, et al: Alteration of the
intestinal microbiome characterizes preclinical inflammatory
arthritis in mice and its modulation attenuates established
arthritis. Sci Rep. 7:156132017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chriswell ME, Lefferts AR, Clay MR, Hsu
AR, Seifert J, Feser ML, Rims C, Bloom MS, Bemis EA, Liu S, et al:
Clonal IgA and IgG autoantibodies from individuals at risk for
rheumatoid arthritis identify an arthritogenic strain of
Subdoligranulum. Sci Transl Med. 14:eabn51662022. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Takahashi D, Hoshina N, Kabumoto Y, Maeda
Y, Suzuki A, Tanabe H, Isobe J, Yamada T, Muroi K, Yanagisawa Y, et
al: Microbiota-derived butyrate limits the autoimmune response by
promoting the differentiation of follicular regulatory T cells.
EBioMedicine. 58:1029132020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ivanov II, Atarashi K, Manel N, Brodie EL,
Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, et al:
Induction of intestinal Th17 cells by segmented filamentous
bacteria. Cell. 139:485–498. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Tan TG, Sefik E, Geva-Zatorsky N, Kua L,
Naskar D, Teng F, Pasman L, Ortiz-Lopez A, Jupp R, Wu HJ, et al:
Identifying species of symbiont bacteria from the human gut that,
alone, can induce intestinal Th17 cells in mice. Proc Natl Acad Sci
USA. 113:E8141–E8150. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wu HJ, Ivanov II, Darce J, Hattori K,
Shima T, Umesaki Y, Littman DR, Benoist C and Mathis D:
Gut-residing segmented filamentous bacteria drive autoimmune
arthritis via T helper 17 cells. Immunity. 32:815–827. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Rojas M, Restrepo-Jimenez P, Monsalve DM,
Pacheco Y, Acosta-Ampudia Y, Ramírez-Santana C, Leung PSC, Ansari
AA, Gershwin ME and Anaya JM: Molecular mimicry and autoimmunity. J
Autoimmun. 95:100–123. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Albani S, Keystone EC, Nelson JL, Ollier
WE, La Cava A, Montemayor AC, Weber DA, Montecucco C, Martini A and
Carson DA: Positive selection in autoimmunity: Abnormal immune
responses to a bacterial dnaJ antigenic determinant in patients
with early rheumatoid arthritis. Nat Med. 1:448–452. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Alcaide-Ruggiero L, Molina-Hernandez V,
Granados MM and Dominguez JM: Main and minor types of collagens in
the articular cartilage: The role of collagens in repair tissue
evaluation in chondral defects. Int J Mol Sci. 22:133292021.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Gomez A, Luckey D, Yeoman CJ, Marietta EV,
Berg Miller ME, Murray JA, White BA and Taneja V: Loss of sex and
age driven differences in the gut microbiome characterize
arthritis-susceptible 0401 mice but not arthritis-resistant 0402
mice. PLoS One. 7:e360952012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhou C, Zhao H, Xiao XY, Chen BD, Guo RJ,
Wang Q, Chen H, Zhao LD, Zhang CC, Jiao YH, et al: Metagenomic
profiling of the pro-inflammatory gut microbiota in ankylosing
spondylitis. J Autoimmun. 107:1023602020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Miyoshi M and Liu S: Collagen-induced
arthritis models. Methods Mol Biol. 2766:3–7. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Liang B, Ge C, Lönnblom E, Lin X, Feng H,
Xiao L, Bai J, Ayoglu B, Nilsson P, Nandakumar KS, et al: The
autoantibody response to cyclic citrullinated collagen type II
peptides in rheumatoid arthritis. Rheumatology (Oxford).
58:1623–1633. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Pianta A, Arvikar SL, Strle K, Drouin EE,
Wang Q, Costello CE and Steere AC: Two rheumatoid
arthritis-specific autoantigens correlate microbial immunity with
autoimmune responses in joints. J Clin Invest. 127:2946–2956. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Wang JY and Roehrl MH: Glycosaminoglycans
are a potential cause of rheumatoid arthritis. Proc Natl Acad Sci
USA. 99:14362–14367. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Hua R, Ni Q, Eliason TD, Han Y, Gu S,
Nicolella DP, Wang X and Jiang JX: Biglycan and chondroitin sulfate
play pivotal roles in bone toughness via retaining bound water in
bone mineral matrix. Matrix Biol. 94:95–109. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Rosser EC, Piper CJM, Matei DE, Blair PA,
Rendeiro AF, Orford M, Alber DG, Krausgruber T, Catalan D, Klein N,
et al: Microbiota-derived metabolites suppress arthritis by
amplifying Aryl-hydrocarbon receptor activation in regulatory B
cells. Cell Metab. 31:837–851.e10. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Correa-Oliveira R, Fachi JL, Vieira A,
Sato FT and Vinolo MA: Regulation of immune cell function by
short-chain fatty acids. Clin Transl Immunol. 5:e732016. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Yao Y, Cai X, Zheng Y, Zhang M, Fei W, Sun
D, Zhao M, Ye Y and Zheng C: Short-chain fatty acids regulate B
cells differentiation via the FFA2 receptor to alleviate rheumatoid
arthritis. Br J Pharmacol. 179:4315–4329. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Wang Y, Wei J, Zhang W, Doherty M, Zhang
Y, Xie H, Li W, Wang N, Lei G and Zeng C: Gut dysbiosis in
rheumatic diseases: A systematic review and meta-analysis of 92
observational studies. EBioMedicine. 80:1040552022. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
He J, Chu Y, Li J, Meng Q, Liu Y, Jin J,
Wang Y, Wang J, Huang B, Shi L, et al: Intestinal
butyrate-metabolizing species contribute to autoantibody production
and bone erosion in rheumatoid arthritis. Sci Adv. 8:eabm15112022.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kim DS, Kwon JE, Lee SH, Kim EK, Ryu JG,
Jung KA, Choi JW, Park MJ, Moon YM, Park SH, et al: Attenuation of
rheumatoid inflammation by sodium butyrate through reciprocal
targeting of HDAC2 in osteoclasts and HDAC8 in T cells. Front
Immunol. 9:15252018. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Wang M, Huang J, Fan H, He D, Zhao S, Shu
Y, Li H, Liu L, Lu S, Xiao C and Liu Y: Treatment of rheumatoid
arthritis using combination of methotrexate and tripterygium
glycosides Tablets-A quantitative plasma pharmacochemical and
pseudotargeted metabolomic approach. Front Pharmacol. 9:10512018.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Surowiec I, Arlestig L, Rantapaa-Dahlqvist
S and Trygg J: Metabolite and lipid profiling of biobank plasma
samples collected prior to onset of rheumatoid arthritis. PLoS One.
11:e01641962016. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Seymour BJ, Trent B, Allen BE, Berlinberg
AJ, Tangchittsumran J, Jubair WK, Chriswell ME, Liu S, Ornelas A,
Stahly A, et al: Microbiota-dependent indole production stimulates
the development of collagen-induced arthritis in mice. J Clin
Invest. 134:e1676712023. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Cao S, Meng X, Li Y, Sun L, Jiang L, Xuan
H and Chen X: Bile acids elevated in chronic periaortitis could
activate Farnesoid-X-receptor to suppress IL-6 production by
macrophages. Front Immunol. 12:6328642021. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Fiorucci S, Biagioli M, Zampella A and
Distrutti E: Bile acids activated receptors regulate innate
immunity. Front Immunol. 9:18532018. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Hu J, Wang C, Huang X, Yi S, Pan S, Zhang
Y, Yuan G, Cao Q, Ye X and Li H: Gut microbiota-mediated secondary
bile acids regulate dendritic cells to attenuate autoimmune uveitis
through TGR5 signaling. Cell Rep. 36:1097262021. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Zou F, Qiu Y, Huang Y, Zou H, Cheng X, Niu
Q, Luo A and Sun J: Effects of short-chain fatty acids in
inhibiting HDAC and activating p38 MAPK are critical for promoting
B10 cell generation and function. Cell Death Dis. 12:5822021.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Ignacio Barrasa J, Olmo N, Perez-Ramos P,
Santiago-Gómez A, Lecona E, Turnay J and Antonia Lizarbe M:
Deoxycholic and chenodeoxycholic bile acids induce apoptosis via
oxidative stress in human colon adenocarcinoma cells. Apoptosis.
16:1054–1067. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Cheng X, Pi Z, Zheng Z, Liu S, Song F and
Liu Z: Combined 16S rRNA gene sequencing and metabolomics to
investigate the protective effects of Wu-tou decoction on
rheumatoid arthritis in rats. J Chromatogr B Analyt Technol Biomed
Life Sci. 1199:1232492022. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Liu M, Li S, Cao N, Wang Q, Liu Y, Xu Q,
Zhang L, Sun C, Xiao X and Yao J: Intestinal flora, intestinal
metabolism, and intestinal immunity changes in complete Freud's
adjuvant-rheumatoid arthritis C57BL/6 mice. Int Immunopharmacol.
125:1110902023. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Wang Z, Yu Y, Liao J, Hu W, Bian X, Wu J
and Zhu YZ: S-Propargyl-Cysteine remodels the gut microbiota to
alleviate rheumatoid arthritis by regulating bile acid metabolism.
Front Cell Infect Microbiol. 11:6705932021. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Liu J, Peng F, Cheng H, Zhang D, Zhang Y,
Wang L, Tang F, Wang J, Wan Y, Wu J, et al: Chronic cold
environment regulates rheumatoid arthritis through modulation of
gut microbiota-derived bile acids. Sci Total Environ.
903:1668372023. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Gonzalez-Rey E, Delgado-Maroto V, Souza
Moreira L and Delgado M: Neuropeptides as therapeutic approach to
autoimmune diseases. Curr Pharm Des. 16:3158–3172. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Delgado M, Pozo D and Ganea D: The
significance of vasoactive intestinal peptide in immunomodulation.
Pharmacol Rev. 56:249–290. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Imhof AK, Gluck L, Gajda M, Lupp A, Bräuer
R, Schaible HG and Schulz S: Differential antiinflammatory and
antinociceptive effects of the somatostatin analogs octreotide and
pasireotide in a mouse model of immune-mediated arthritis.
Arthritis Rheum. 63:2352–2362. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Jimeno R, Leceta J, Martínez C,
Gutiérrez-Cañas I, Pérez-García S, Carrión M, Gomariz RP and
Juarranz Y: Effect of VIP on the balance between cytokines and
master regulators of activated helper T cells. Immunol Cell Biol.
90:178–186. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Jimeno R, Gomariz RP, Gutierrez-Canas I,
Martinez C, Juarranz Y and Leceta J: New insights into the role of
VIP on the ratio of T-cell subsets during the development of
autoimmune diabetes. Immunol Cell Biol. 88:734–745. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Leceta J, Garin MI and Conde C: Mechanism
of immunoregulatory properties of vasoactive intestinal peptide in
the K/BxN mice model of autoimmune arthritis. Front Immunol.
12:7018622021. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
O'Connor TM, O'Connell J, O'Brien DI,
Goode T, Bredin CP and Shanahan F: The role of substance P in
inflammatory disease. J Cell Physiol. 201:167–180. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Raap T, Justen HP, Miller LE, Cutolo M,
Scholmerich J and Straub RH: Neurotransmitter modulation of
interleukin 6 (IL-6) and IL-8 secretion of synovial fibroblasts in
patients with rheumatoid arthritis compared to osteoarthritis. J
Rheumatol. 27:2558–2565. 2000.PubMed/NCBI
|
|
89
|
Barbosa-Cobos RE, Lugo-Zamudio G,
Flores-Estrada J, Becerril-Mendoza LT, Rodríguez-Henríquez P,
Torres-González R, Moreno-Eutimio MA, Ramirez-Bello J and Moreno J:
Serum substance P: An indicator of disease activity and subclinical
inflammation in rheumatoid arthritis. Clin Rheumatol. 37:901–908.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Jiang MH, Chung E, Chi GF, Ahn W, Lim JE,
Hong HS, Kim DW, Choi H, Kim J and Son Y: Substance P induces
M2-type macrophages after spinal cord injury. Neuroreport.
23:786–792. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Hong HS and Son Y: Substance P ameliorates
collagen II-induced arthritis in mice via suppression of the
inflammatory response. Biochem Biophys Res Commun. 453:179–184.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Galarza-Delgado DA, Azpiri-Lopez JR,
Colunga-Pedraza IJ, Cárdenas-de la Garza JA, Vera-Pineda R,
Wah-Suárez M, Arvizu-Rivera RI, Martínez-Moreno A, Ramos-Cázares
RE, Torres-Quintanilla FJ, et al: Prevalence of comorbidities in
Mexican mestizo patients with rheumatoid arthritis. Rheumatol Int.
37:1507–1511. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Castillo-Canon JC, Trujillo-Caceres SJ,
Bautista-Molano W, Valbuena-Garcia AM, Fernandez-Avila DG and
Acuna-Merchan L: Rheumatoid arthritis in Colombia: A clinical
profile and prevalence from a national registry. Clin Rheumatol.
40:3565–3573. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Niu Q, Hao J, Li Z and Zhang H: Helper T
cells: A potential target for sex hormones to ameliorate rheumatoid
arthritis? (Review). Mol Med Rep. 30:2152024. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Li JY, Chassaing B, Tyagi AM, Vaccaro C,
Luo T, Adams J, Darby TM, Weitzmann MN, Mulle JG, Gewirtz AT, et
al: Sex steroid deficiency-associated bone loss is microbiota
dependent and prevented by probiotics. J Clin Invest.
126:2049–2063. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Porwal K, Pal S, Kulkarni C, Singh P,
Sharma S, Singh P, Prajapati G, Gayen JR, Ampapathi RS, Mullick A
and Chattopadhyay N: A prebiotic, short-chain
fructo-oligosaccharides promotes peak bone mass and maintains bone
mass in ovariectomized rats by an osteogenic mechanism. Biomed
Pharmacother. 129:1104482020. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Dominianni C, Sinha R, Goedert JJ, Pei Z,
Yang L, Hayes RB and Ahn J: Sex, body mass index, and dietary fiber
intake influence the human gut microbiome. PLoS One.
10:e01245992015. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Balakrishnan B, Luckey D, Wright K, Davis
JM, Chen J and Taneja V: Eggerthella lenta augments
preclinical autoantibody production and metabolic shift mimicking
senescence in arthritis. Sci Adv. 9:eadg11292023. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Bhambhani S, Kondhare KR and Giri AP:
Diversity in chemical structures and biological properties of plant
alkaloids. Molecules. 26:33742021. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Ettefagh KA, Burns JT, Junio HA, Kaatz GW
and Cech NB: Goldenseal (Hydrastis canadensis L.) extracts
synergistically enhance the antibacterial activity of berberine via
efflux pump inhibition. Planta Med. 77:835–840. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Zhu M, Wang H, Chen J and Zhu H:
Sinomenine improve diabetic nephropathy by inhibiting fibrosis and
regulating the JAK2/STAT3/SOCS1 pathway in streptozotocin-induced
diabetic rats. Life Sci. 265:1188552021. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Samra YA, Said HS, Elsherbiny NM, Liou GI,
El-Shishtawy MM and Eissa LA: Cepharanthine and Piperine ameliorate
diabetic nephropathy in rats: Role of NF-kappaB and NLRP3
inflammasome. Life Sci. 157:187–199. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Naz I, Masoud MS, Chauhdary Z, Shah MA and
Panichayupakaranant P: Anti-inflammatory potential of
berberine-rich extract via modulation of inflammation biomarkers. J
Food Biochem. 46:e143892022. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Alorabi M, Cavalu S, Al-Kuraishy HM,
Al-Gareeb AI, Mostafa-Hedeab G, Negm WA, Youssef A, El-Kadem AH,
Saad HM and Batiha GE: Pentoxifylline and berberine mitigate
diclofenac-induced acute nephrotoxicity in male rats via modulation
of inflammation and oxidative stress. Biomed Pharmacother.
152:1132252022. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Luo R, Liao Z, Song Y, Yin H, Zhan S, Li
G, Ma L, Lu S, Wang K, Li S, et al: Berberine ameliorates oxidative
stress-induced apoptosis by modulating ER stress and autophagy in
human nucleus pulposus cells. Life Sci. 228:85–97. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Samadi P, Sarvarian P, Gholipour E,
Asenjan KS, Aghebati-Maleki L, Motavalli R, Hojjat-Farsangi M and
Yousefi M: Berberine: A novel therapeutic strategy for cancer.
IUBMB Life. 72:2065–2079. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Pirillo A and Catapano AL: Berberine, a
plant alkaloid with lipid- and glucose-lowering properties: From in
vitro evidence to clinical studies. Atherosclerosis. 243:449–461.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Song D, Hao J and Fan D: Biological
properties and clinical applications of berberine. Front Med.
14:564–582. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Liu YT, Hao HP, Xie HG, Lai L, Wang Q, Liu
CX and Wang GJ: Extensive intestinal first-pass elimination and
predominant hepatic distribution of berberine explain its low
plasma levels in rats. Drug Metab Dispos. 38:1779–1784. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Yue M, Xia Y, Shi C, Guan C, Li Y, Liu R,
Wei Z and Dai Y: Berberine ameliorates collagen-induced arthritis
in rats by suppressing Th17 cell responses via inducing cortistatin
in the gut. FEBS J. 284:2786–2801. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Tan XS, Ma JY, Feng R, Ma C, Chen WJ, Sun
YP, Fu J, Huang M, He CY, Shou JW, et al: Tissue distribution of
berberine and its metabolites after oral administration in rats.
PLoS One. 8:e779692013. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Yue M, Tao Y, Fang Y, Lian X, Zhang Q, Xia
Y, Wei Z and Dai Y: The gut microbiota modulator berberine
ameliorates collagen-induced arthritis in rats by facilitating the
generation of butyrate and adjusting the intestinal hypoxia and
nitrate supply. FASEB J. 33:12311–12323. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Sun Y, Yao Y and Ding CZ: A combination of
sinomenine and methotrexate reduces joint damage of collagen
induced arthritis in rats by modulating osteoclast-related
cytokines. Int Immunopharmacol. 18:135–141. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Liu W, Zhang Y, Zhu W, Ma C, Ruan J, Long
H and Wang Y: Sinomenine inhibits the progression of rheumatoid
arthritis by regulating the secretion of inflammatory cytokines and
Monocyte/macrophage subsets. Front Immunol. 9:22282018. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Huang RY, Pan HD, Wu JQ, Zhou H, Li ZG,
Qiu P, Zhou YY, Chen XM, Xie ZX, Xiao Y, et al: Comparison of
combination therapy with methotrexate and sinomenine or leflunomide
for active rheumatoid arthritis: A randomized controlled clinical
trial. Phytomedicine. 57:403–410. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Chen JJ, Sun Y and Huang CB: Clinical
observation of sinomenine hydrochloride in the treat-to-target
therapy of rheumatoid arthritis and patient-reported outcomes. J
Chin Med Materials. 228–231. 2025.
|
|
117
|
Xiang G, Gao M, Qin H, Shen X, Huang H,
Hou X and Feng Z: Benefit-risk assessment of traditional Chinese
medicine preparations of sinomenine using multicriteria decision
analysis (MCDA) for patients with rheumatoid arthritis. BMC
Complement Med Ther. 23:372023. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Li X, He L, Hu Y, Duan H, Li X, Tan S, Zou
M, Gu C, Zeng X, Yu L, et al: Sinomenine suppresses osteoclast
formation and Mycobacterium tuberculosis H37Ra-induced bone loss by
modulating RANKL signaling pathways. PLoS One. 8:e742742013.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Chen DP, Wong CK, Leung PC, Fung KP, Lau
CB, Lau CP, Li EK, Tam LS and Lam CW: Anti-inflammatory activities
of Chinese herbal medicine sinomenine and Liang Miao San on tumor
necrosis factor-α-activated human fibroblast-like synoviocytes in
rheumatoid arthritis. J Ethnopharmacol. 137:457–468. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Tong B, Yu J, Wang T, Dou Y, Wu X, Kong L,
Dai Y and Xia Y: Sinomenine suppresses collagen-induced arthritis
by reciprocal modulation of regulatory T cells and Th17 cells in
gut-associated lymphoid tissues. Mol Immunol. 65:94–103. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Wang H, Liao H, Ochani M, Justiniani M,
Lin X, Yang L, Al-Abed Y, Wang H, Metz C, Miller EJ, et al:
Cholinergic agonists inhibit HMGB1 release and improve survival in
experimental sepsis. Nat Med. 10:1216–1221. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Yi L, Luo JF, Xie BB, Liu JX, Wang JY, Liu
L, Wang PX, Zhou H and Dong Y: α7 nicotinic acetylcholine receptor
is a novel mediator of sinomenine Anti-Inflammation effect in
macrophages stimulated by lipopolysaccharide. Shock. 44:188–195.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Hayashi S, Hamada T, Zaidi SF, Oshiro M,
Lee J, Yamamoto T, Ishii Y, Sasahara M and Kadowaki M: Nicotine
suppresses acute colitis and colonic tumorigenesis associated with
chronic colitis in mice. Am J Physiol Gastrointest Liver Physiol.
307:G968–G978. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Yue M, Zhang X, Dou Y, Wei Z, Tao Y, Xia Y
and Dai Y: Gut-sourced vasoactive intestinal polypeptide induced by
the activation of α7 nicotinic acetylcholine receptor substantially
contributes to the anti-inflammatory effect of sinomenine in
collagen-induced arthritis. Front Pharmacol. 9:6752018. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Zhang H, Chen L, Sun X, Yang Q, Wan L and
Guo C: Matrine: A promising natural product with various
pharmacological activities. Front Pharmacol. 11:5882020. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Ao L, Gao H, Jia L, Liu S, Guo J, Liu B
and Dong Q: Matrine inhibits synovial angiogenesis in
collagen-induced arthritis rats by regulating HIF-VEGF-Ang and
inhibiting the PI3K/Akt signaling pathway. Mol Immunol. 141:13–20.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Niu Y, Dong Q and Li R: Matrine regulates
Th1/Th2 cytokine responses in rheumatoid arthritis by attenuating
the NF-κB signaling. Cell Biol Int. 41:611–621. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Zou Y, Li Q, Liu D, Li J, Cai Q, Li C,
Zhao Q and Xu W: Therapeutic effects of matrine derivate MASM in
mice with collagen-induced arthritis and on fibroblast-like
synoviocytes. Sci Rep. 7:24542017. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Cao G, Mao Z, Niu T, Wang P, Yue X, Wang
X, Ma A and Zhang Y: Oxymatrine ameliorates rheumatoid arthritis by
regulation of Tfr/Tfh cell balance via the TLR9-MyD88-STAT3
signaling pathway. J Sci Food Agric. 103:6017–6024. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Zhang G, Liu B, Zeng Z, Chen Q, Feng Y and
Ning X: Oxymatrine hydrazone (OMTH) synthesis and its protective
effect for rheumatoid arthritis through downregulation of MEK/NF-κB
pathway. Environ Toxicol. 36:2448–2453. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Cao G, Li J, Mao Z and Zhang Y: Oxymatrine
alleviates Collagen-induced arthritis in mice by regulating the
immune balance of T cells. Molecules. 28:58792023. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Shi F, Li S and Shi H: Oxymatrine
modulates immune balance in rheumatoid arthritis and its effect on
patients' inflammatory profile. Hainan Med J. 29:2997–3000.
2018.
|
|
133
|
Li W and Zhang SN: Effect of oxymatrine on
immune balance and its influence on the inflammatory status in
patients with rheumatoid arthritis. China Modern Med. 26:18–21.
2019.
|
|
134
|
Kacena C: Effects of the curcuminoid and
Non-curcuminoid compounds of turmeric on the gut microbiome and
inflammation: Potential use in the treatment and prevention of
disease. Nutr Rev. 83:1771–1783. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Hasanzadeh S, Read MI, Bland AR, Majeed M,
Jamialahmadi T and Sahebkar A: Curcumin: An inflammasome silencer.
Pharmacol Res. 159:1049212020. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Kou H, Huang L, Jin M, He Q, Zhang R and
Ma J: Effect of curcumin on rheumatoid arthritis: A systematic
review and meta-analysis. Front Immunol. 14:11216552023. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Chandran B and Goel A: A randomized, pilot
study to assess the efficacy and safety of curcumin in patients
with active rheumatoid arthritis. Phytother Res. 26:1719–1725.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Pourhabibi-Zarandi F, Rafraf M, Zayeni H,
Asghari-Jafarabadi M and Ebrahimi AA: The efficacy of curcumin
supplementation on serum total antioxidant capacity,
malondialdehyde, and disease activity in women with rheumatoid
arthritis: A randomized, double-blind, placebo-controlled clinical
trial. Phytother Res. 38:3552–3563. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Pourhabibi-Zarandi F, Rafraf M, Zayeni H,
Asghari-Jafarabadi M and Ebrahimi AA: Effects of curcumin
supplementation on metabolic parameters, inflammatory factors and
obesity values in women with rheumatoid arthritis: A randomized,
double-blind, placebo-controlled clinical trial. Phytother Res.
36:1797–1806. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Amalraj A, Varma K, Jacob J, Divya C,
Kunnumakkara AB, Stohs SJ and Gopi S: A novel highly bioavailable
curcumin formulation improves symptoms and diagnostic indicators in
rheumatoid arthritis patients: A randomized, double-blind,
placebo-controlled, two-dose, three-arm, and parallel-group study.
J Med Food. 20:1022–1030. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Yang KY, Lin LC, Tseng TY, Wang SC and
Tsai TH: Oral bioavailability of curcumin in rat and the herbal
analysis from Curcuma longa by LC-MS/MS. J Chromatogr B Analyt
Technol Biomed Life Sci. 853:183–189. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Jang DJ, Kim ST, Oh E and Lee K: Enhanced
oral bioavailability and antiasthmatic efficacy of curcumin using
redispersible dry emulsion. Biomed Mater Eng. 24:917–930.
2014.PubMed/NCBI
|
|
143
|
Kloesch B, Becker T, Dietersdorfer E,
Kiener H and Steiner G: Anti-inflammatory and apoptotic effects of
the polyphenol curcumin on human fibroblast-like synoviocytes. Int
Immunopharmacol. 15:400–405. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Huang G, Xu Z, Huang Y, Duan X, Gong W,
Zhang Y, Fan J and He F: Curcumin protects against collagen-induced
arthritis via suppression of BAFF production. J Clin Immunol.
33:550–557. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Yang Y, Wu X, Wei Z, Dou Y, Zhao D, Wang
T, Bian D, Tong B, Xia Y, Xia Y and Dai Y: Oral curcumin has
anti-arthritic efficacy through somatostatin generation via
cAMP/PKA and Ca(2+)/CaMKII signaling pathways in the small
intestine. Pharmacol Res. 95-96:71–81. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Javadi F, Ahmadzadeh A, Eghtesadi S,
Aryaeian N, Zabihiyeganeh M, Rahimi Foroushani A and Jazayeri S:
The effect of quercetin on inflammatory factors and clinical
symptoms in women with rheumatoid arthritis: A Double-blind,
randomized controlled trial. J Am Coll Nutr. 36:9–15. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Javadi F, Eghtesadi S, Ahmadzadeh A,
Aryaeian N, Zabihiyeganeh M, Foroushani AR and Jazayeri S: The
effect of quercetin on plasma oxidative status, C-reactive protein
and blood pressure in women with rheumatoid arthritis. Int J Prev
Med. 5:293–301. 2014.PubMed/NCBI
|
|
148
|
Tang M, Zeng Y, Peng W, Xie X, Yang Y, Ji
B and Li F: Pharmacological aspects of natural quercetin in
rheumatoid arthritis. Drug Des Devel Ther. 16:2043–2053. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Liu X, Tao T, Yao H, Zheng H, Wang F and
Gao Y: Mechanism of action of quercetin in rheumatoid arthritis
models: Meta-analysis and systematic review of animal studies.
Inflammopharmacology. 31:1629–1645. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Cojocaru M, Cojocaru IM, Silosi I and
Vrabie CD: Gastrointestinal manifestations in systemic autoimmune
diseases. Maedica (Bucur). 6:45–51. 2011.PubMed/NCBI
|
|
151
|
Souza ID, Ribeiro JS, Bersani-Amado CA and
Zanoni JN: Analysis of myosin-V immunoreactive myenteric neurons
from arthritic rats. Arq Gastroenterol. 48:205–210. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Piovezana Bossolani GD, Silva BT, Colombo
Martins Perles JV, Lima MM, Vieira Frez FC, Garcia de Souza SR,
Sehaber-Sierakowski CC, Bersani-Amado CA and Zanoni JN: Rheumatoid
arthritis induces enteric neurodegeneration and jejunal
inflammation, and quercetin promotes neuroprotective and
anti-inflammatory actions. Life Sci. 238:1169562019. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Silva BTD, Martins-Perles JVC, Bossolani
GDP, Lima MM, Sehaber-Sierakowski CC, Gremaschi LB, Cunha JPSE,
Bersani-Amado CA and Zanoni JN: Quercetin and ibuprofen combination
displayed anti-inflammatory effects and also extenuates the enteric
neurons damage of arthritic rats. An Acad Bras Cienc. 96 (Suppl
1):e202302442024. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Zhao Y, Yan B, Wang Z, Li M and Zhao W:
Natural polysaccharides with immunomodulatory activities. Mini Rev
Med Chem. 20:96–106. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Ho Do M, Seo YS and Park HY:
Polysaccharides: Bowel health and gut microbiota. Crit Rev Food Sci
Nutr. 61:1212–1224. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Luo J, Yang Q, Jiang W, Liu Y, Hu Q and
Peng X: The interaction between Angelica sinensis
polysaccharide ASP-2pb and specific gut bacteria alleviates
rheumatoid arthritis in rats. Int J Biol Macromol. 301:1404732025.
View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Lim H, Min DS, Yun HE, Kim KT, Sun YN, Dat
LD, Kim YH and Kim HP: Impressic acid from Acanthopanax koreanum,
possesses matrix metalloproteinase-13 down-regulating capacity and
protects cartilage destruction. J Ethnopharmacol. 209:73–81. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Takahashi Y, Tanaka M, Murai R,
Kuribayashi K, Kobayashi D, Yanagihara N and Watanabe N:
Prophylactic and therapeutic effects of Acanthopanax
senticosus Harms extract on murine collagen-induced arthritis.
Phytother Res. 28:1513–1519. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Kang MC, Kim SY, Kim EA, Lee JH, Kim YS,
Yu SK, Chae JB, Choe IH, Cho JH and Jeon YJ: Antioxidant activity
of polysaccharide purified from Acanthopanax koreanum Nakai stems
in vitro and in vivo zebrafish model. Carbohydr Polym. 127:38–46.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Fu J, Fu J, Yuan J, Zhang N, Gao B, Fu G,
Tu Y and Zhang Y: Anti-diabetic activities of Acanthopanax
senticosus polysaccharide (ASP) in combination with metformin.
Int J Biol Macromol. 50:619–623. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Lee JB, Tanikawa T, Hayashi K, Asagi M,
Kasahara Y and Hayashi T: Characterization and biological effects
of two polysaccharides isolated from Acanthopanax sciadophylloides.
Carbohydr Polym. 116:159–166. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Liu A, Zhang M, Wu Y, Zhang C, Zhang Q, Su
X, Zhu X, Shi W, Liu J, Zhang Y, et al: ASPS exhibits
Anti-rheumatic effects by reprogramming gut microbiota and
increasing serum γ-Glutamylcysteine Level. Adv Sci (Weinh).
10:e22056452023. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Liu L, Sha XY, Wu YN, Chen MT and Zhong
JX: Lycium barbarum polysaccharides protects retinal
ganglion cells against oxidative stress injury. Neural Regen Res.
15:1526–1531. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Ran L, Chen F, Zhang J, Mi J, Lu L, Yan Y
and Cao Y: Antitumor effects of pollen polysaccharides from Chinese
wolfberry on DU145 cells via the PI3K/AKT pathway in vitro and in
vivo. Int J Biol Macromol. 152:1164–1173. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Li W, Gao M and Han T: Lycium
barbarum polysaccharides ameliorate intestinal barrier
dysfunction and inflammation through the MLCK-MLC signaling pathway
in Caco-2 cells. Food Funct. 11:3741–3748. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Fu YW, Peng YF, Huang XD, Yang Y, Huang L,
Xi Y, Hu ZF, Lin S, So KF and Ren CR: Lycium barbarum
polysaccharide-glycoprotein preventative treatment ameliorates
aversive. Neural Regen Res. 16:543–549. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Kalinkovich A and Livshits G: A cross talk
between dysbiosis and Gut-associated immune system governs the
development of inflammatory arthropathies. Semin Arthritis Rheum.
49:474–484. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Liu Y, Lv J, Yang B, Liu F, Tian Z, Cai Y,
Yang D, Ouyang J, Sun F, Shi Y and Xia P: Lycium barbarum
polysaccharide attenuates type II collagen-induced arthritis in
mice. Int J Biol Macromol. 78:318–323. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Cai S, Sun J and Wei X: Lycium
barbarum polysaccharide inhibits NF-κB pathway to reduce the
level of inflammatory cytokines in osteoarthritis chondrocytes. Xi
Bao Yu Fen Zi Mian Yi Xue Za Zhi. 34:989–993. 2018.(In Chinese).
PubMed/NCBI
|
|
170
|
Lai W, Wang C, Lai R, Peng X and Luo J:
Lycium barbarum polysaccharide modulates gut microbiota to
alleviate rheumatoid arthritis in a rat model. NPJ Sci Food.
6:342022. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Liu Y, Liu L, Luo J and Peng X:
Metabolites from specific intestinal bacteria in vivo fermenting
Lycium barbarum polysaccharide improve collagenous arthritis
in rats. Int J Biol Macromol. 226:1455–1467. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Cao W, Liu J, Dai Y, Zhou Y, Li R and Yu
P: Bibliometric analysis of marine traditional chinese medicine in
pharmacopoeia of the People's Republic of China: Development,
differences, and trends directions. Evid Based Complement Alternat
Med. 2022:39719672022. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Zheng Q, Mu X, Pan S, Luan R and Zhao P:
Ephedrae herba: A comprehensive review of its traditional uses,
phytochemistry, pharmacology, and toxicology. J Ethnopharmacol.
307:1161532023. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Ma Y, Wei X, Peng J, Wei F, Wen Y, Liu M,
Song B, Wang Y, Zhang Y and Peng T: Ephedra sinica polysaccharide
regulate the anti-inflammatory immunity of intestinal microecology
and bacterial metabolites in rheumatoid arthritis. Front Pharmacol.
15:14146752024. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Chen Z, Li XP, Li ZJ, Xu L and Li XM:
Reduced hepatotoxicity by total glucosides of paeony in combination
treatment with leflunomide and methotrexate for patients with
active rheumatoid arthritis. Int Immunopharmacol. 15:474–477. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Xiang N, Li XM, Zhang MJ, Zhao DB, Zhu P,
Zuo XX, Yang M, Su Y, Li ZG, Chen Z and Li XP: Total glucosides of
paeony can reduce the hepatotoxicity caused by Methotrexate and
Leflunomide combination treatment of active rheumatoid arthritis.
Int Immunopharmacol. 28:802–807. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Wang Y and Xing HY: Clinical observation
on effect of total glucosides of paeony combined with methotrexate
on rheumatoid arthritis. Zhongguo Zhong Xi Yi Jie He Za Zhi.
27:839–840. 2007.(In Chinese). PubMed/NCBI
|
|
178
|
Luo J, Jin DE, Yang GY, Zhang YZ, Wang JM,
Kong WP and Tao QW: Total glucosides of paeony for rheumatoid
arthritis: A systematic review of randomized controlled trials.
Complement Ther Med. 34:46–56. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Fei F, Yang H, Peng Y, Wang P, Wang S,
Zhao Y, Huang J, Yu X, Feng S, Sun R, et al: Sensitive analysis and
pharmacokinetic study of the isomers paeoniflorin and albiflorin
after oral administration of total glucosides of white paeony
capsule in rats. J Chromatogr B Analyt Technol Biomed Life Sci.
1022:30–37. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Xia SM, Shen R, Sun XY, Shen LL, Yang YM,
Ke Y, Wang Y, Chen DY and Han XM: Development and validation of a
sensitive liquid chromatography-tandem mass spectrometry method for
the determination of paeoniflorin in rat brain and its application
to pharmacokinetic study. J Chromatogr B Analyt Technol Biomed Life
Sci. 857:32–39. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Zhang W and Dai SM: Mechanisms involved in
the therapeutic effects of Paeonia lactiflora Pallas in rheumatoid
arthritis. Int Immunopharmacol. 14:27–31. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Sun B, Dai G, Bai Y, Zhang W, Zhu L, Chu
J, Pan R and Ju W: Determination of paeoniflorin in rat plasma by
Ultra-high performance liquid Chromatography-tandem mass
spectrometry and its application to a pharmacokinetic study. J
Chromatogr Sci. 55:1006–1012. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Tong L, Wan M, Zhou D, Gao J, Zhu Y and Bi
K: LC-MS/MS determination and pharmacokinetic study of albiflorin
and paeoniflorin in rat plasma after oral administration of Radix
Paeoniae Alba extract and Tang-Min-Ling-Wan. Biomed Chromatogr.
24:1324–1331. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Peng J, Lu X, Xie K, Xu Y, He R, Guo L,
Han Y, Wu S, Dong X, Lu Y, et al: Dynamic alterations in the gut
microbiota of Collagen-induced arthritis rats following the
prolonged administration of total glucosides of paeony. Front Cell
Infect Microbiol. 9:2042019. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Bao BL and Wang ZZ: Resource and
pharmacognostical study on Aralia echinocaulis. Zhongguo
Zhong Yao Za Zhi. 29:508–510. 2004.(In Chinese). PubMed/NCBI
|
|
186
|
Li Y, Dai M, Wang L and Wang G:
Polysaccharides and glycosides from Aralia echinocaulis
protect rats from arthritis by modulating the gut microbiota
composition. J Ethnopharmacol. 269:1137492021. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Zhao J, Zhang F, Xiao X, Wu Z, Hu Q, Jiang
Y, Zhang W, Wei S, Ma X and Zhang X: Tripterygium hypoglaucum
(Levl.) hutch and its main bioactive components: recent advances in
pharmacological activity, pharmacokinetics and potential toxicity.
Front Pharmacol. 12:7153592021. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Zhou X, Liu Q, Zhou X, Zhang J, Liu W,
Zhao X and Luo N: THH Relieves CIA inflammation by reducing
inflammatory-related cytokines. Cell Biochem Biophys. 78:367–374.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Long C, Yang Y, Wang Y, Zhang X, Zhang L,
Huang S, Yang D, Qiao X, Yang Y and Guo Y: Role of
Glutamine-glutamate/GABA cycle and potential target GLUD2 in
alleviation of rheumatoid arthritis by Tripterygium hypoglaucum
(levl.) Hutch based on metabolomics and molecular pharmacology. J
Ethnopharmacol. 281:1145612021. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Hu J, Ni J, Zheng J, Guo Y, Yang Y, Ye C,
Sun X, Xia H, Liu Y and Liu H: Tripterygium hypoglaucum extract
ameliorates adjuvant-induced arthritis in mice through the gut
microbiota. Chin J Nat Med. 21:730–744. 2023.PubMed/NCBI
|
|
191
|
Zheng J, Hu J, Yang Y, Xiong L, Yang H,
Zhang Z, Jiang N and Liu H: Suppressive effect of Tripterygium
hypoglaucum (Levl.) Hutch extract on rheumatoid arthritis in mice
by modulating inflammasome and bile acid metabolism. Biomed
Pharmacother. 167:1154942023. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Orwa JA, Jondiko IJ, Minja RJ and Bekunda
M: The use of Toddalia asiatica (L) Lam. (Rutaceae) in
traditional medicine practice in East Africa. J Ethnopharmacol.
115:257–262. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Sukieum S, Sang-Aroon W and Yenjai C:
Coumarins and alkaloids from the roots of Toddalia asiatica. Nat
Prod Res. 32:944–952. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Yang K, Tong L, Chen C, Zhang P, Pi H,
Ruan H and Wu J: Therapeutic effects of extracts from Radix
Toddaliae Asiaticae on collagen-induced arthritis in Balb/c mice. J
Ethnopharmacol. 146:355–362. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Tong L, Chen T, Chen Z, Zhang P, Pi H,
Ruan H and Wu J: Anti-inflammatory activity of omphalocarpin
isolated from radix toddaliae asiaticae. J Ethnopharmacol.
155:1553–1560. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Yan C and Wu J: Effect of Toddalia
asiatica extract combined with miR-483 on proliferation, apoptosis
and inflammatory factors expression of osteoarthritis chondrocyte.
Pak J Pharm Sci. 33:1333–1340. 2020.PubMed/NCBI
|
|
197
|
Qin H, Fu Y, Zhou K, Song H, Fang G, Chen
Q and Pang Y: Toddalia asiatica extract attenuates adjuvant-induced
arthritis by modulating colon Th17/Treg balance and colony
homeostasis. J Ethnopharmacol. 313:1165422023. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Chen BC, He HY, Niu K, Rui K, Huang JG,
Xie YQ and Xiao M: Network pharmacology-based approach uncovers the
JAK/STAT signaling mechanism underlying Paederia scandens
extract treatment of rheumatoid arthritis. Am J Transl Res.
14:5295–5307. 2022.PubMed/NCBI
|
|
199
|
Xiao M, Fu X, Ni Y, Chen J, Jian S, Wang
L, Li L and Du G: Protective effects of Paederia scandens
extract on rheumatoid arthritis mouse model by modulating gut
microbiota. J Ethnopharmacol. 226:97–104. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Swindell EP, Hankins PL, Chen H,
Miodragovic DU and O'Halloran TV: Anticancer activity of
small-molecule and nanoparticulate arsenic(III) complexes. Inorg
Chem. 52:12292–12304. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Zhang J, Li C, Zheng Y, Lin Z, Zhang Y and
Zhang Z: Inhibition of angiogenesis by arsenic trioxide via
TSP-1-TGF-β1-CTGF-VEGF functional module in rheumatoid arthritis.
Oncotarget. 8:73529–73546. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Zhang J, Ma Y, Zhang Y, Niu S, Chu M and
Zhang Z: Angiogenesis is inhibited by arsenic trioxide through
downregulation of the CircHIPK3/miR-149-5p/FOXO1/VEGF functional
module in rheumatoid arthritis. Front Pharmacol. 12:7516672021.
View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Li C, Zhang J, Wang W, Wang H, Zhang Y and
Zhang Z: Arsenic trioxide improves Treg and Th17 balance by
modulating STAT3 in treatment-naive rheumatoid arthritis patients.
Int Immunopharmacol. 73:539–551. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Niu S, Zhu X, Zhang J, Ma Y, Lang X, Luo
L, Li W, Zhao Y and Zhang Z: Arsenic trioxide modulates the
composition and metabolic function of the gut microbiota in a mouse
model of rheumatoid arthritis. Int Immunopharmacol. 111:1091592022.
View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Kang JI, Hong JY, Choi JS and Lee SK:
Columbianadin inhibits cell proliferation by inducing apoptosis and
necroptosis in HCT116 colon cancer cells. Biomol Ther (Seoul).
24:320–327. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Lim HJ, Lee JH, Choi JS, Lee SK, Kim YS
and Kim HP: Inhibition of airway inflammation by the roots of
Angelica decursiva and its constituent, columbianadin. J
Ethnopharmacol. 155:1353–1361. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Li R, Zhao C, Yao M, Song Y, Wu Y and Wen
A: Analgesic effect of coumarins from Radix angelicae pubescentis
is mediated by inflammatory factors and TRPV1 in a spared nerve
injury model of neuropathic pain. J Ethnopharmacol. 195:81–88.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Su X, Wu B, Zhang W, Ji YH, Wang Q and Tan
ZY: Inhibitory effects of columbianadin on nociceptive behaviors in
a neuropathic pain model, and on Voltage-gated calcium currents in
dorsal root ganglion neurons in mice. Front Pharmacol. 10:15222019.
View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Chen S, Wang Y, Zhang L, Han Y, Liang C,
Wang S, Qi L, Pang X, Li J and Chang Y: Therapeutic effects of
columbianadin from Angelicae Pubescentis radix on the progression
of collagen-induced rheumatoid arthritis by regulating inflammation
and oxidative stress. J Ethnopharmacol. 316:1167272023. View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Gracey E, Vereecke L, McGovern D, Fröhling
M, Schett G, Danese S, De Vos M, Van den Bosch F and Elewaut D:
Revisiting the Gut-joint axis: Links between gut inflammation and
spondyloarthritis. Nat Rev Rheumatol. 16:415–433. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
211
|
de Winter JJ, van Mens LJ, van der Heijde
D, Landewe R and Baeten DL: Prevalence of peripheral and
extra-articular disease in ankylosing spondylitis versus
non-radiographic axial spondyloarthritis: A meta-analysis.
Arthritis Res Ther. 18:1962016. View Article : Google Scholar : PubMed/NCBI
|
|
212
|
Guggino G, Mauro D, Rizzo A, Alessandro R,
Raimondo S, Bergot AS, Rahman MA, Ellis JJ, Milling S, Lories R, et
al: Inflammasome activation in ankylosing spondylitis is associated
with gut dysbiosis. Arthritis Rheumatol. 73:1189–1199. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
213
|
Mauro D, Nakamura A, Haroon N and Ciccia
F: The gut-enthesis axis and the pathogenesis of Spondyloarthritis.
Semin Immunol. 58:1016072021. View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Fan J, Jiang T and He D: Advances in the
implications of the gut microbiota on the treatment efficacy of
disease-modifying anti-rheumatic drugs in rheumatoid arthritis.
Front Immunol. 14:11890362023. View Article : Google Scholar : PubMed/NCBI
|
|
215
|
Qiao J, Zhang SX, Chang MJ, Cheng T, Zhang
JQ, Zhao R, Song S, Liu GY, Chang JS and Li XF: Specific enterotype
of gut microbiota predicted clinical effect of methotrexate in
patients with rheumatoid arthritis. Rheumatology (Oxford).
62:1087–1096. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
216
|
Liu X, Wang Z, Qian H, Tao W, Zhang Y, Hu
C, Mao W and Guo Q: Natural medicines of targeted rheumatoid
arthritis and its action mechanism. Front Immunol. 13:9451292022.
View Article : Google Scholar : PubMed/NCBI
|
|
217
|
Han Y, Chen S, Liu C, Sun H, Jia Z, Shi J,
Li J and Chang Y: A comprehensive review of natural products in
rheumatoid arthritis: Therapeutic potential and mechanisms. Front
Immunol. 16:15010192025. View Article : Google Scholar : PubMed/NCBI
|
|
218
|
Guo W, Lv G, Yang D, Zhang W, Li N, Hu J,
Wu Y, Pi Z and Lin Z: Advances in the effect of gut-joint axis
dysfunction on rheumatoid arthritis and the intervention of natural
products. Chin J Analytical Chemistry. 52:1003542024. View Article : Google Scholar
|
|
219
|
Liang Y, Liu M, Cheng Y, Wang X and Wang
W: Prevention and treatment of rheumatoid arthritis through
traditional Chinese medicine: Role of the gut microbiota. Front
Immunol. 14:12339942023. View Article : Google Scholar : PubMed/NCBI
|