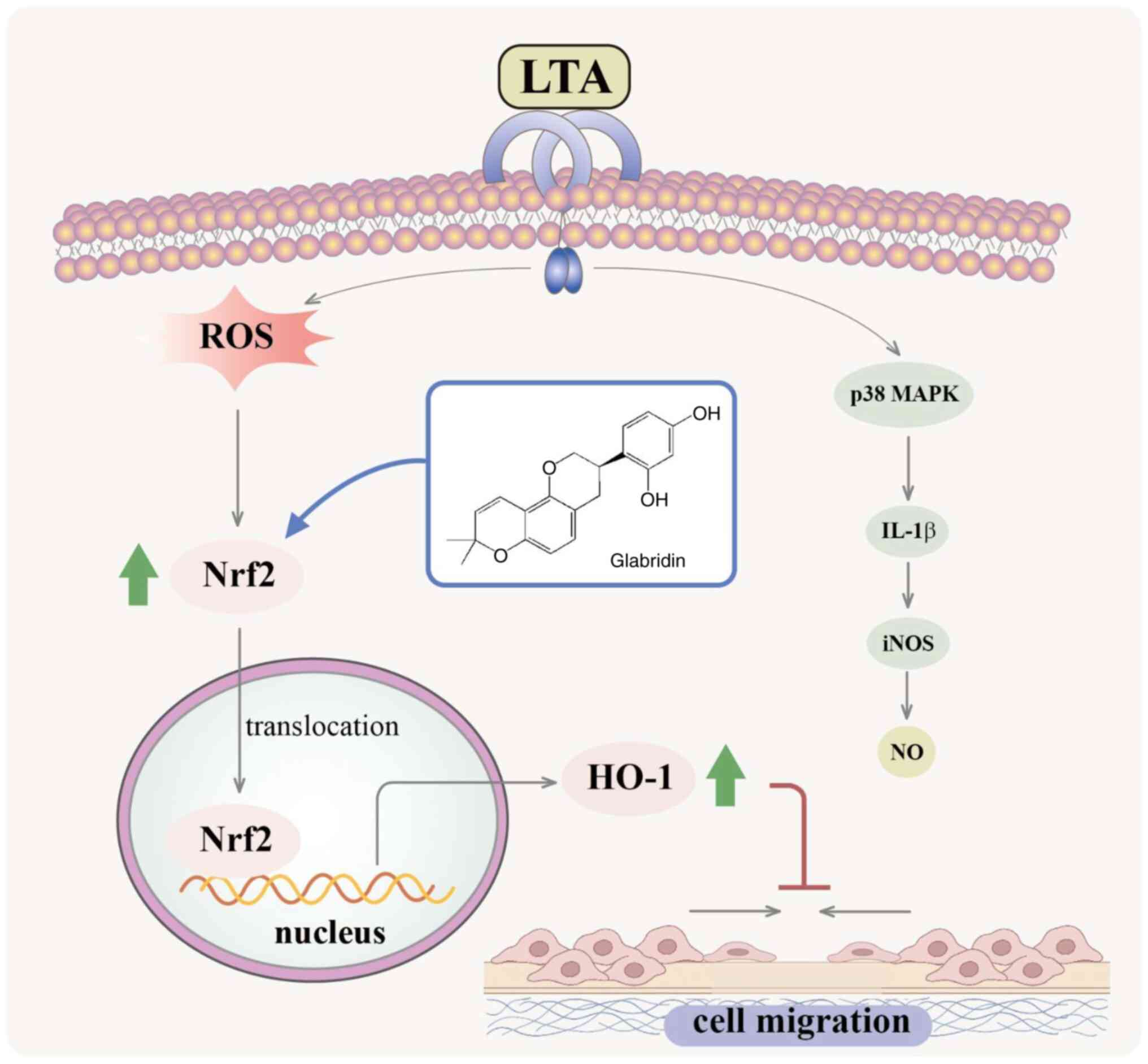
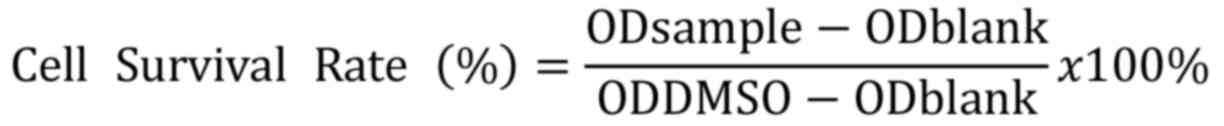Introduction
Gram-positive bacteria are a major class of
pathogens that trigger the host's innate immune response,
predominantly through their surface component lipoteichoic acid
(LTA), a potent immunostimulatory agent (1–3).
Alveolar macrophages are the resident immune cells in the alveolar
space, where they serve as the first line of defense against
inhaled pathogens and as key drivers of pulmonary immune responses
(4). LTA activates alveolar
macrophages through its interaction with Toll-like receptor 2
(TLR2), producing inflammatory cytokines and reactive oxygen
species (ROS). Although both mediators play a role in the host's
defensive response to infection, they can also contribute to lung
tissue injury (5,6). Given their central role in lung
immune surveillance, alveolar macrophages are uniquely vulnerable
to redox perturbations induced by microbial insults (7). Prolonged oxidative stress disrupts
redox balance and amplifies inflammatory signaling, causing
cellular dysfunction and damaging pulmonary tissue (8). Given that alveolar macrophages can be
both sources and targets of oxidative stress, careful regulation of
redox balance is required to sustain pulmonary homeostasis during
infection (7,9).
Cells combat oxidative injury through endogenous
antioxidant systems, which are predominantly mediated through the
nuclear factor erythroid 2-related factor 2 (Nrf2) pathway
(10–12). When Nrf2 is activated in response
to oxidative signals, it dissociates from its cytoplasmic
repressor, Keap1 and translocates to the nucleus, where it induces
transcription of antioxidant and cytoprotective genes such as heme
oxygenase-1 (HO-1) (12,13). Studies have demonstrated that the
Nrf2/HO-1 axis contributes to numerous oxidative injury models and
it may have protective effects against inflammatory lung diseases
(14,15). Although LTA induced macrophage
activation is well established, the extent to which Nrf2/HO-1
signaling contributes in alveolar macrophages remains unclear.
Additionally, whether pharmacological agents or naturally derived
products can induce protective effectors through this
redox-sensitive pathway under LTA-induced stress is unknown
(16).
Glabridin (GBD; Fig.
1A), a prenylated isoflavan derived from Glycyrrhiza
glabra (licorice root), has been reported to exhibit
antioxidant and anti-inflammatory properties in variouS cellular
models (17). These properties
take effect in part through the activation of Nrf2-dependent
pathways (18). However, whether
GBD can mitigate LTA-induced oxidative stress in alveolar
macrophages and restore redox balance through Nrf2/HO-1 signaling
remains to be elucidated. Moreover, the potential influence of this
pathway on macrophage migration under inflammatory conditions has
not been studied. The present study therefore investigated the
protective effects of GBD on LTA-induced oxidative stress and
macrophage migration, focusing on the potential involvement of
Nrf2/HO-1 signaling.
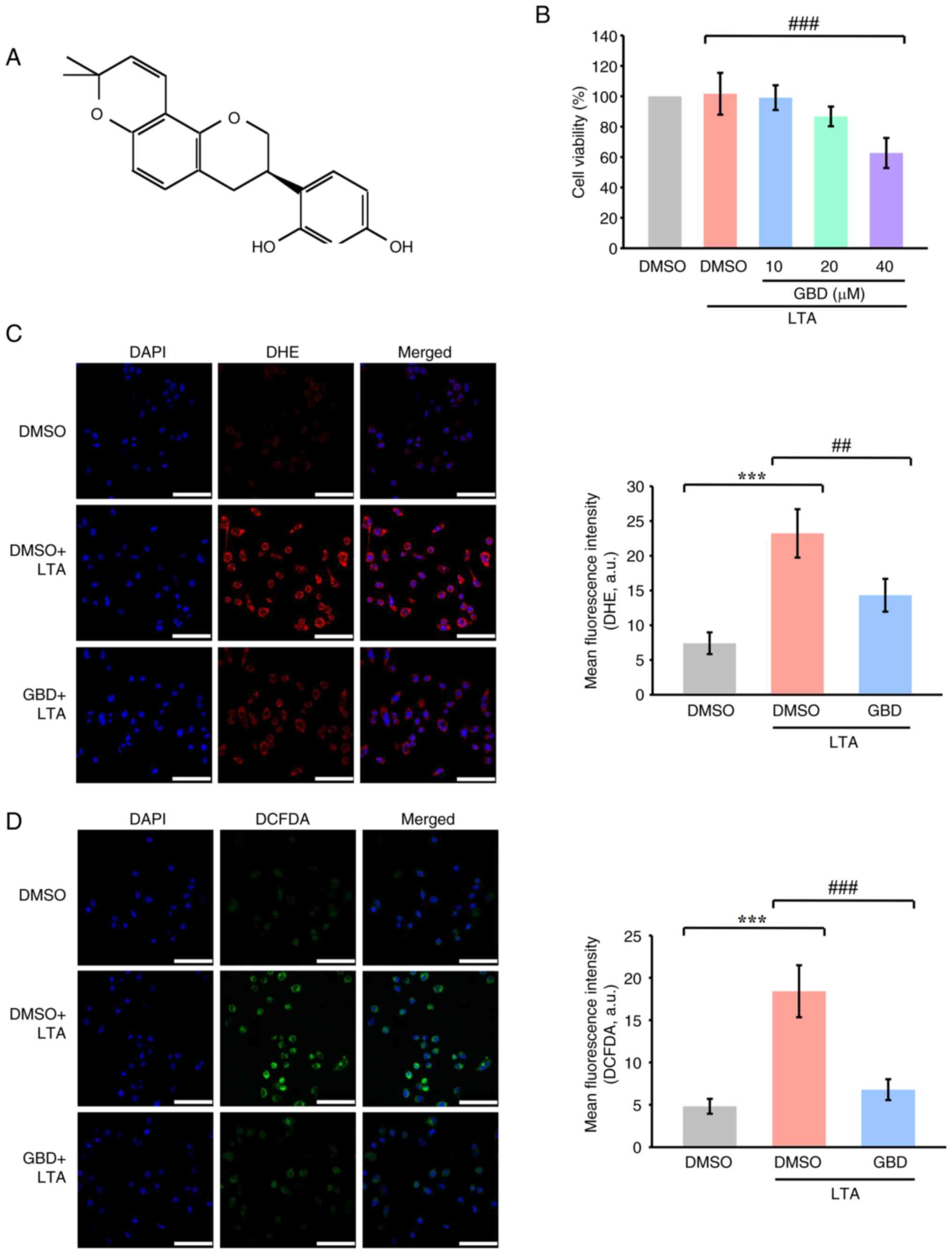 | Figure 1.GBD reduces LTA-induced ROS
production in MH-S cells. (A) Chemical structure of GBD. (B) MTT
assay of cell viability. Cells were pretreated with various
concentrations of GBD (10, 20 and 40 µM) or 0.1% DMSO for 30 min
and then stimulated with LTA (10 µg/ml) for 24 h. (C)
Representative fluorescence images and quantification of DHE
staining in MH-S cells. (D) Representative fluorescence images and
quantification of DCFDA staining. Cells were pretreated with GBD
(20 µM) for 1 h and then stimulated with LTA (10 µg/ml) for 30 min.
Experimental groups for C and D: CTRL, DMSO+LTA, and GBD+LTA. Scale
bar, 50 µm. Data are presented as means±SD (n=4). ***P<0.001 vs.
DMSO; ##P<0.01, and ###P<0.001 vs.
DMSO+LTA. GBD glabridin; LTA, lipoteichoic acid; ROS, reactive
oxygen species; DMSO, dimethyl sulfoxide; DHE, dihydroethidium
staining; DCFDA. 2′,7′-dichlorofluorescin diacetate. |
Materials and methods
Reagents and materials
GBD (≥98%; cat. no. 11843) and dihydroethidium (DHE;
cat. no. 12013) were purchased from Cayman Chemical Company. LTA
(cat. no. L2515), dimethyl sulfoxide (DMSO; cat. no. D8418), bovine
serum albumin (BSA; cat. no. A5611), 2′,7′-dichlorofluorescin
diacetate (DCFDA; cat. no. D6883), phenylmethylsulfonyl fluoride
(PMSF; cat. no. P7626), sodium orthovanadate (cat. no. S6508),
sodium pyrophosphate (cat. no. S6422), aprotinin (cat. no. A1153),
leupeptin (cat. no. L2884), sodium fluoride (NaF; cat. no. 201154),
and paraformaldehyde (PFA; cat. no. P6148) were purchased from
MilliporeSigma. Anti-Nrf2 (cat. no. GTX103322) polyclonal
antibodies (pAb) were purchased from GeneTex, Inc. Anti-HO-1 (cat.
no. 10701-1-AP) pAb and β-actin (cat. no. 60008-1-Ig) monoclonal
antibodies were purchased from Proteintech Group, Inc. Hybond-P
polyvinylidene difluoride (PVDF) membranes (cat. no. GE10600023)
and enhanced chemiluminescence Western blotting detection reagent
(cat. no. GERPN2232) were obtained from Cytiva. Horseradish
peroxidase (HRP)-conjugated donkey anti-rabbit immunoglobulin G
(IgG; cat. no. AP182P), and sheep anti-mouse IgG (cat. no.
SAB3701093) were obtained from MilliporeSigma. GBD was dissolved in
DMSO, stored at 4°C, and diluted to the working concentration in
cell culture medium before use. DMSO (0.1%) served as the vehicle
control for all experiments.
Cell culture
MH-S cells, a murine alveolar macrophage cell line,
were obtained from ATCC (cat. no. CRL-2019) and cultured in
RPMI-1640 medium (cat. no. A1049101; Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% fetal bovine serum (FBS;
cat. no. 26140079; Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin-streptomycin. Cells were maintained at 37°C in a 5%
CO2 humidified atmosphere. Cells were used for
experiments between passages 4 and 8. The cell line was confirmed
to be free of mycoplasma contamination using a Mycoplasma Detection
Kit (cat. no. rep-mys-10; InvivoGen).
Cell viability assay
The MH-S cells were seeded in 24-well culture plates
at a density of 1×105 cells per well and cultured in
RPMI-1640 medium containing 10% FBS for 24 h. Once the cells
reached the required confluence, they were treated with varying
concentrations of GBD (10–40 µM) or 0.1% DMSO for 30 min and then
subjected to LTA stimulation (10 µg/ml) for 24 h at 37°C. An MTT
assay (cat. no. AM0815-0005; Bionovas Biotechnology Co., Ltd.) was
performed to assesS cell viability, with results expressed as a
percentage calculated using the following formula:
Measurement of intracellular ROS
Intracellular ROS production was assessed with DCFDA
and DHE. Cells were cultured on coverslips in 6-well plates at a
density of 5×104 cells/well and treated with 0.1% DMSO
or 20 µM GBD. The cells then underwent LTA stimulation for 30 min
followed by incubation with DCFDA (10 µM) or DHE (5 µM) for 30 min
at 37°C in the dark. The cells were washed twice with
phosphate-buffered saline (PBS), and the coverslips were mounted
onto glass slides with Fluoroshield medium containing DAPI (cat.
no. ab104139; Abcam). A DM6 CS confocal microscope (Leica
Microsystems GmbH) with a ×63 oil immersion objective was used for
immediate fluorescence signal visualization. Fluorescence intensity
was quantified using ImageJ software, version 1.54g (NIH).
Immunofluorescence staining assay
Cells were cultured on coverslips at a density of
5×104 per well and pretreated with 0.1% DMSO, 20 µM GBD,
or 5 µM ML385 (cat. no. 21114; Cayman Chemical Company) for 30 min.
The cells were subsequently stimulated with LTA for 3 or 6 h and
fixed after treatment in 4% PFA for 10 min at room temperature. The
fixed cells were permeabilized with 0.1% Triton X-100, followed by
blocking in 5% BSA for 30 min. The prepared cells were incubated
with target-specific primary antibodies (1:100) overnight at 4°C.
After thorough PBS washing, the specimens were exposed to Alexa
Fluor 488-conjugated goat anti-rabbit IgG secondary antibody
(1:2,000; cat. no. ab150077; Abcam) for 1 h at room temperature.
Following three additional PBS washes, the coverslips were mounted
onto glass slides with Fluoroshield medium containing DAPI.
Cell imaging was performed with the aforementioned
confocal microscope system. ImageJ software was used to analyze
fluorescence intensity. For Nrf2 nuclear translocation, nuclear
regions of interest (ROIs) were identified through DAPI staining.
For HO-1 analysis, ROIs were selected around individual cells to
calculate the mean fluorescence intensity (MFI) of the whole cell
was calculated after background subtraction.
Cell transfection
Cells were seeded in 6-well plates at a density of
2×105 cells/well and cultured until 80% confluence.
Cells were then transfected with either a small interfering (si)RNA
targeting Nrf2 [Nfe2l2 siRNA: 5′-AGCAUUUUAACAUGUUAACAG-3′
(sense) and 5′-GUUAACAUGUUAAAAUGCUAU-3′ (antisense)] or a negative
control siRNA [si-Ctrl: 5′-UUCUCCGAACGUGUCACGUTT-3′ (sense) and
reverse 5′-ACGUGACACGUUCGGAGAATT-3′ (antisense); cat. no.
HY-RS09246; MedChemExpress], using a transfection reagent (cat. no.
HY-K2017, MedChemExpress) and 50 nM siRNA diluted in serum-free
medium, according to the manufacturer's instructions. Following 6 h
incubation at 37°C, the transfection mixture was replaced with
complete medium, and cells were further cultured for 24 h before
subsequent treatments. Knockdown efficiency was verified by reverse
transcription-quantitative (RT-q) PCR.
RT-qPCR
Total RNA was extracted from MH-S cells
(5×106 cells) with a NucleoSpin RNA Kit (cat. no.
740955.50; Macherey-Nagel) according to the manufacturer's
instructions. RNA purity and concentration were assessed with a
NanoDrop spectrophotometer (Thermo Fisher Scientific, Inc.).
Complementary DNA was synthesized from 2 µg total RNA with the
SuperScript IV First-Strand Synthesis System (cat. no. 18091050;
Thermo Fisher Scientific, Inc.). RT-qPCR was performed with Fast
SYBR Green Master Mix (cat. no. 4385612; Applied Biosystems; Thermo
Fisher Scientific, Inc.). Amplification was performed with a
StepOnePlus Real-Time PCR System (Applied Biosystems; Thermo Fisher
Scientific, Inc.) with an initial hot-start activation at 95°C for
1 min, followed by 45 cycles of denaturation at 95°C for 5 sec and
annealing/extension at 60°C for 1 min. The following primers were
used: Nrf2, forward 5′-AGCAGGACATGGAGCAAGTT-3′ and reverse
5′-TTCTTTTTCCAGCGAGGAGA-3′; HO-1, forward
5′-GCACTATGTAAAGCGTCTCC-3′ and reverse 5′-GACTCTGGTCTTTGTGTTCC-3′;
and GAPDH, forward 5′-GAACATCATCCCTGCATCCA-3′ and reverse
5′-GCCAGTGAGCTTCCCGTTCA-3′. GAPDH was used as the internal control.
The primer sequences used for IL-1β, TNF-α, IL-6 and SOD1 are
listed in Table SI. Relative gene
expression levels were calculated according to the
2−ΔΔCq method (19).
All experiments were independently repeated ≥4 times.
Determination of nitric oxide and malondialdehyde
levels. The detailed procedures for the determination of nitric
oxide (NO) and malondialdehyde (MDA) levels are described in the
Supplementary Methods.
Western blot analysis
MH-S cells (8×105 per ml) were cultured
and preincubated with DMSO, GBD, or ML385 for 30 min at 37°C. Cells
underwent LTA stimulation or were left untreated for 3 or 6 h. The
cells were lysed in lysis buffer containing aprotinin (10 µg/ml),
PMSF (1 mM), leupeptin (2 µg/ml), NaF (10 mM), sodium orthovanadate
(1 mM), and sodium pyrophosphate (5 mM) for 1 h at 4°C.
Subsequently, the samples were centrifuged at 13,500 × g for 30
min, and protein concentration was determined through a Bradford
protein assay (cat. no. 5000006; Bio-Rad Laboratories, Inc.). Equal
amounts of total protein (50 µg) were separated through 10%
SDS-PAGE and then transferred onto polyvinylidene difluoride
membranes (MilliporeSigma). Membranes were blocked in 5% BSA in
Tris-buffered saline with 0.1% Tween-20 (TBST) at room temperature
for 1 h and incubated with primary antibodies (anti-Nrf2, cat. no.
GTX103322, GeneTex; anti-HO-1, cat. no. 10701-1-AP and
anti-β-actin, cat. no. 60008-1-Ig, Proteintech) diluted 1:1,000 in
TBST overnight at 4°C. The membranes were then washed and incubated
with HRP-conjugated secondary antibodies (donkey anti-rabbit IgG,
cat. no. AP182P; and sheep anti-mouse IgG, cat. no. SAB3701093,
MilliporeSigma) diluted 1:5,000 in TBST for 1 h at room
temperature. Protein bands were quantified with a video
densitometer and BioProfil BioLight software (v2000.01; Vilber
Lourmat).
Wound healing assay
Cell migration was assessed through a wound healing
assay performed in 24-well plates. A sterile 20-µl pipette
tip was used to create a scratch in the confluent cell monolayer.
Cells were incubated in serum-free medium with or without
treatment. Images were captured at 0, 6, and 24 h after the scratch
was created. Wound width was analyzed with ImageJ software, version
1.54g (NIH). The wound closure percentage was calculated according
to the following formula:
Transwell migration assay
Cell migration was assessed using Transwell inserts
with 8 µm pore size (SPL Life Sciences, FL, USA) in 24-well
plates. MH-S cells (1×105) were resuspended in medium
containing 2% FBS and seeded into the upper chamber in 200
µl medium. The lower chambers were also filled with 600
µl of medium containing 2% FBS, and the plates were
incubated at 37°C for 30–60 min to allow cell
attachment. Cells in the upper chamber were then treated with DMSO,
GBD, or ML385 for 30 min in medium containing 2% FBS at
37°C. After treatment, the medium in the lower chambers was
replaced with fresh medium containing 2% FBS, with or without LTA
(10 µg/ml), to serve as the chemoattractant.
After 6 or 24 h of incubation at 37°C
in a humidified 5% CO2 incubator, non-migrated cells on
the upper surface of the membrane were gently removed with cotton
swabs. Cells that migrated to the lower surface were fixed with 4%
PFA for 15 min at room temperature, washed twice with PBS,
and stained with 0.2% crystal violet for 3 min at room
temperature. Excess stain was removed by washing twice with PBS.
Migrated cells were imaged and counted under an inverted microscope
at ×100 magnification. Relative migration was calculated as the
ratio of the number of migrated cells in each treatment group to
that in the DMSO group, which was defined as 1.
Statistical analysis
At least four independent replicates were performed
for all experiments. Data are expressed as means±standard deviation
(SD). Significance was determined through one-way analysis of
variance followed by a Student-Newman-Keuls post hoc test. For
datasets containing >3 groups, Tukey's post hoc test was used.
P<0.05 was considered to indicate a statistically significant
difference.
Results
GBD attenuates LTA-induced oxidative
stress in MH-S cells
An MTT assay was performed to assess GBD
cytotoxicity in MH-S cells. MH-S cells were treated with 10, 20 and
40 µM of GBD in the presence of LTA (10 µg/ml) for 24 h. Whereas 40
µM of GBD reduced cell viability below 80%, 10 and 20 µM had no
significant cytotoxic effects (Fig.
1B). To determine the effective concentration, cells were
treated with 0, 5, 10, 20, and 30 µM GBD for 24 h. Increasing
concentrations of GBD reduced proliferation, with an
IC50 of ~18 µM (Fig.
S1). Based on both of these findings, 20 µM was selected as the
working concentration for subsequent experiments.
LTA stimulation markedly increased intracellular ROS
production, as indicated by elevated fluorescence in both DCFDA and
DHE staining (Fig. 1C and D),
which suggests the oxidative environment in the MH-S cells was
enhanced. Pretreatment with 20 µM GBD markedly suppressed
LTA-induced ROS accumulation. Quantitative analysis revealed
markedly reduced fluorescence intensity in GBD-treated cells
compared with the LTA group, as measured by DHE and DCFDA staining,
indicating that GBD effectively mitigated LTA-induced oxidative
stress.
GBD activates Nrf2 signaling in
LTA-stimulated MH-S cells
The present study investigated whether Nrf2
signaling contributed to the antioxidative effects of GBD. Confocal
microscopy revealed that LTA stimulation alone resulted in minimal
Nrf2 nuclear localization, whereas GBD pretreatment markedly
increased Nrf2 nuclear accumulation (Fig. 2A).
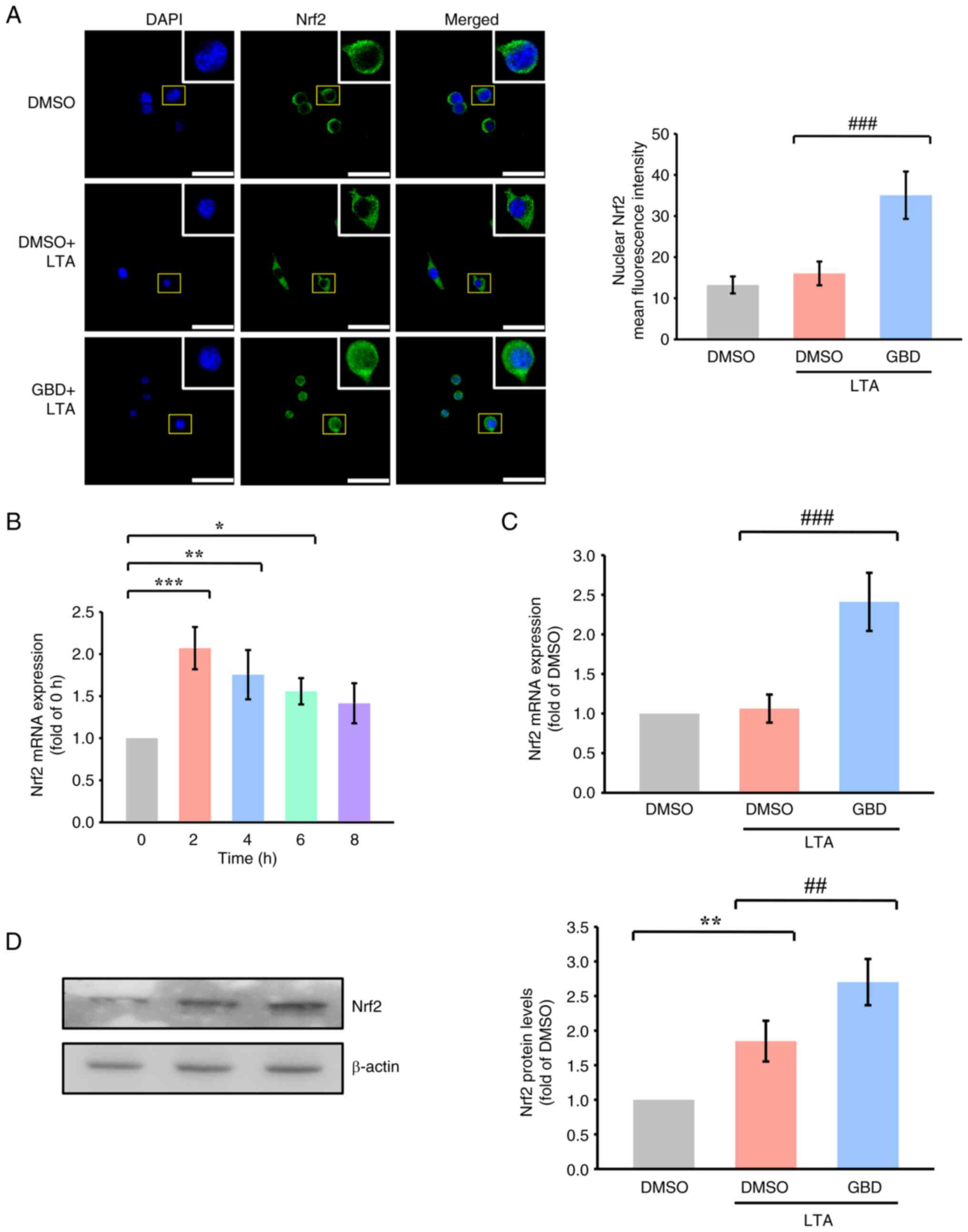 | Figure 2.GBD activates Nrf2 signaling in
response to LTA. MH-S cells were pretreated with GBD for 30 min and
then stimulated with LTA. (A) Confocal images indicating Nrf2
nuclear translocation 3 h poststimulation. Scale bar, 50 µm. (B)
Time-course of Nrf2 mRNA expression after LTA stimulation (0, 2, 4,
6, and 8 h). (C) Nrf2 mRNA expression was determined through
reverse transcription-quantitative PCR 2 h poststimulation, as
described in the materials and methods section. (D) Western blot
analysis of Nrf2 protein levels 3 h after LTA stimulation. Data are
presented as means±SD. *P<0.05, **P<0.01 and ***P<0.001
vs. 0 h or DMSO; ##P<0.01 and
###P<0.001 vs. DMSO+LTA. GBD glabridin; Nrf2, nuclear
factor erythroid 2-related factor 2; LTA, lipoteichoic acid; DMSO,
dimethyl sulfoxide. |
Time-course analysis indicated that Nrf2 mRNA peaked
2 h after LTA stimulation (Fig.
2B); thus, 2 h was selected as the representative time point
for mRNA analysis. Nrf2 mRNA levels were markedly elevated in the
GBD+LTA group compared with in the DMSO+LTA group (Fig. 2C). Western blot analysis performed
at 3 h post-LTA stimulation showed that Nrf2 protein levels were
increased following GBD treatment (Fig. 2D).
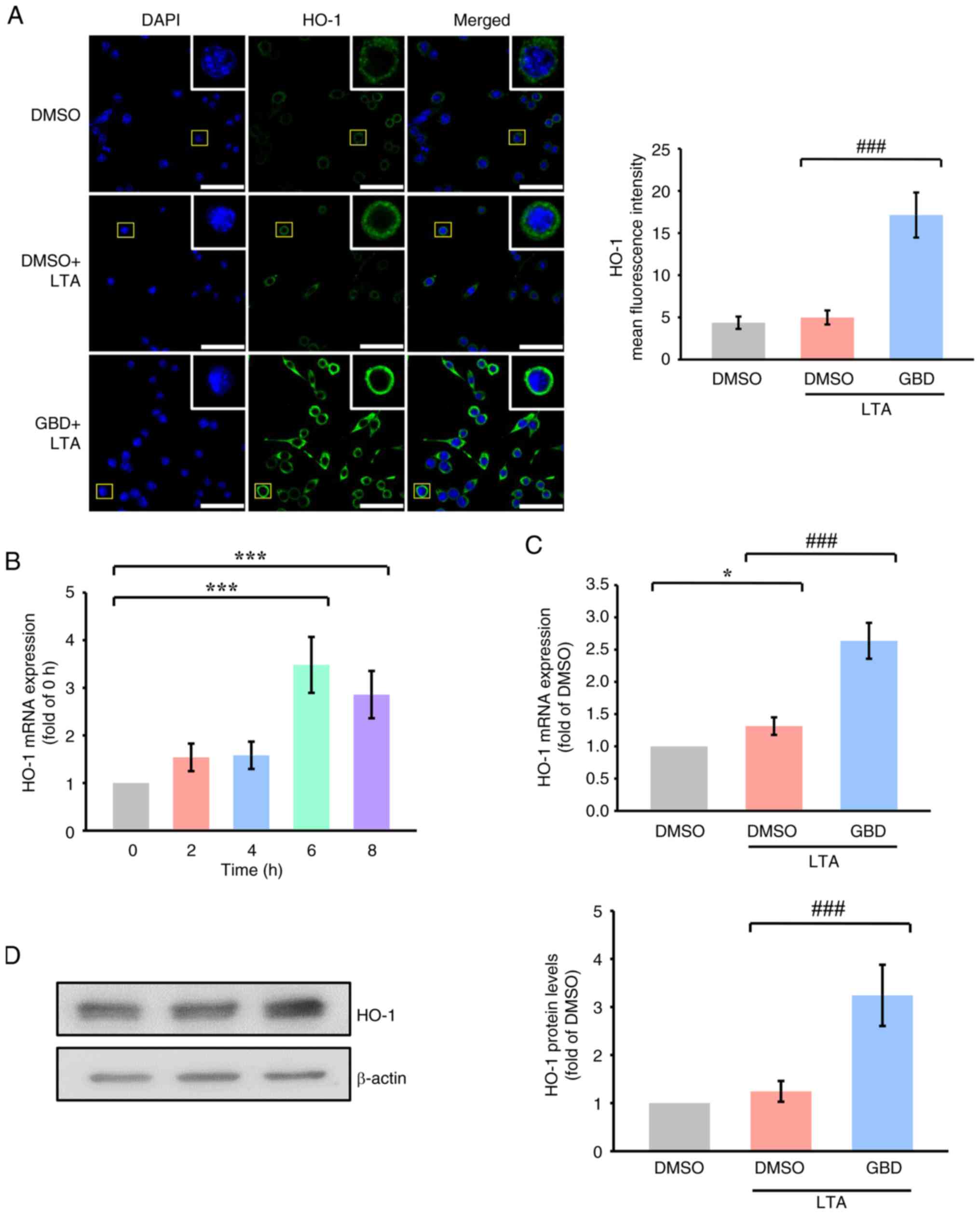
GBD upregulates HO-1 expression
following Nrf2 activation
HO-1 expression was further examined as a downstream
target of Nrf2. Confocal imaging revealed that GBD increased HO-1
fluorescence intensity 6 h after LTA stimulation relative to LTA
treatment alone (Fig. 3A).
Time-course analysis revealed that HO-1 mRNA expression peaked 6 h
following LTA stimulation (Fig.
3B); thus, 6 h was selected as the representative time point
for subsequent analysis. GBD also markedly upregulated HO-1 mRNA
and protein levels 6 h after treatment (Fig. 3C and D), confirming its downstream
activation of Nrf2. HO-1 is a cytoprotective enzyme with
antioxidant properties. Its upregulation by GBD may represent one
mechanism through Nrf2 activation contributes to redox homeostasis.
These results align with the observed decrease in ROS accumulation
and support the role of HO-1 as a key effector in GBD-mediated
regulation of oxidative stress.
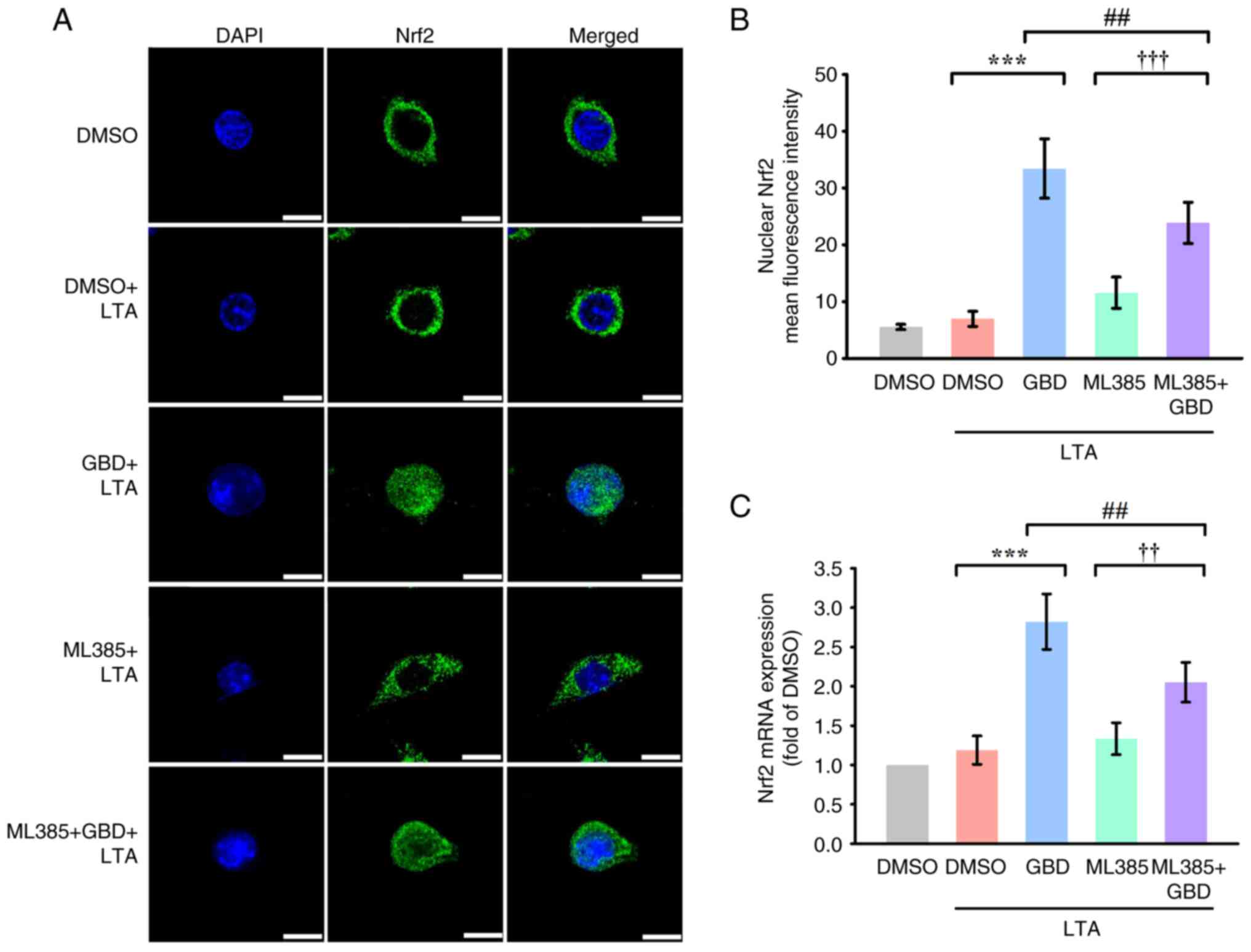
ML385 attenuates GBD-induced
activation of Nrf2 and HO-1
The selective inhibitor ML385 (5 µM) was used to
investigate the Nrf2-dependence of GBD-induced activation of Nrf2
and HO-1. Confocal microscopy indicated that ML385 markedly reduced
the nuclear accumulation of Nrf2 in GBD-treated cells at 3 h
(Fig. 4A and B). Additionally,
RT-qPCR analysis revealed that ML385 suppressed GBD-induced
expression of Nrf2 mRNA (Fig. 4C),
indicating transcriptional inhibition.
ML385 attenuated HO-1 fluorescence intensity at 6 h,
as confirmed through confocal imaging (Fig. 5A and B), and markedly decreased
HO-1 mRNA levels in GBD-treated cells (Fig. 5C). These findings suggested that
GBD-mediated upregulation of the Nrf2/HO-1 pathway is substantially
dependent on Nrf2 activity, given that pharmacological inhibition
of Nrf2 impaired both nuclear localization and downstream gene
expression.
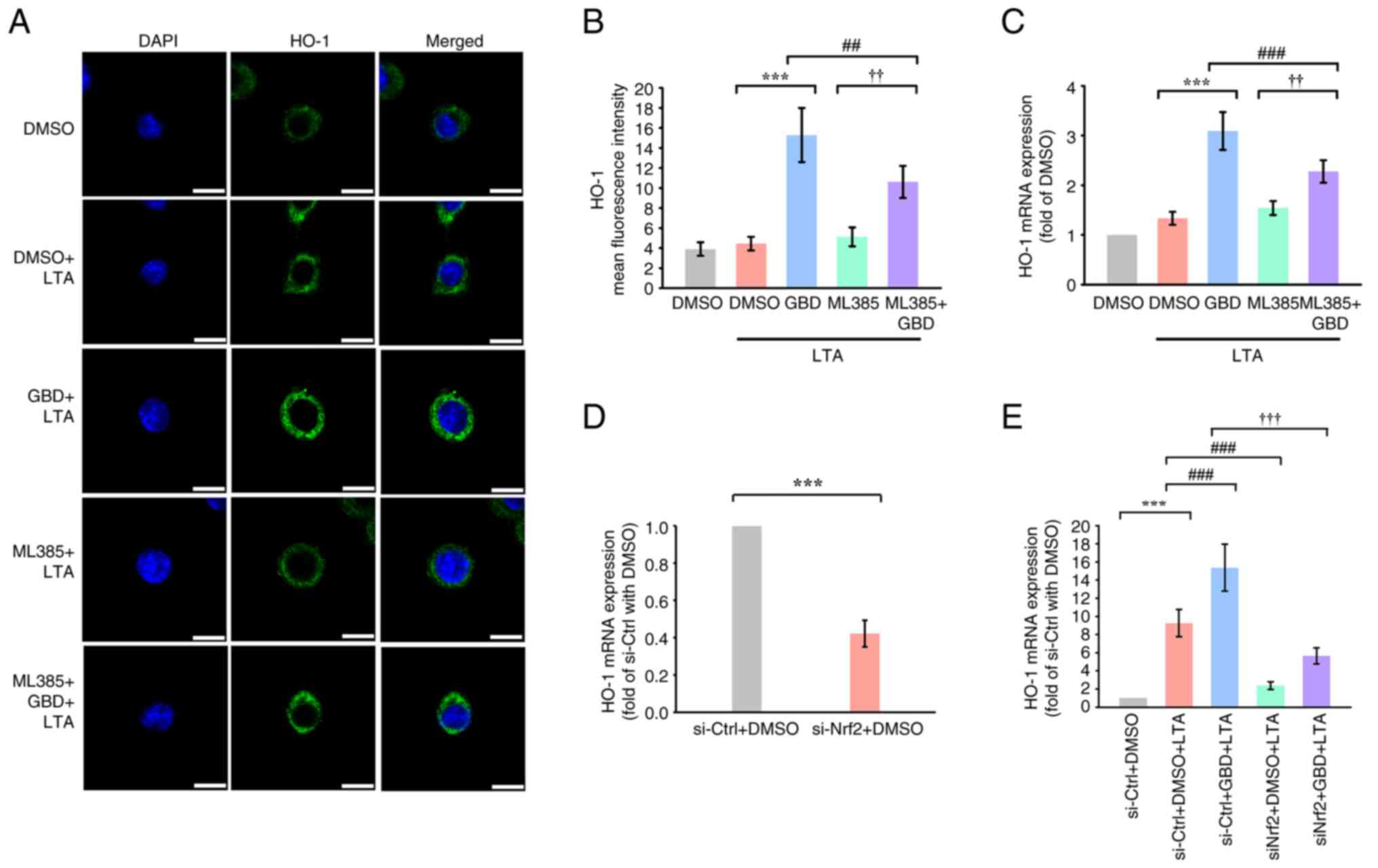 | Figure 5.Pharmacological and genetic
inhibition of Nrf2 attenuates GBD-induced HO-1 expression. MH-S
cells were pretreated with the indicated compounds for 30 min and
then stimulated with LTA. (A) Immunofluorescence analysis of HO-1
expression 6 h poststimulation. Scale bar, 10 µm. (B)
Immunofluorescence image evaluation of HO-1 mean fluorescence
intensity. (C) HO-1 mRNA expression 6 h poststimulation, determined
through RT-qPCR. (D and E) Effect of Nrf2 knockdown on GBD-induced
HO-1 expression. MH-S cells were transfected with control siRNA
(siNC) or Nrf2 siRNA (siNrf2) for 6 h, followed by the indicated
treatments. HO-1 mRNA levels were measured by RT-qPCR. Data are
presented as means ± SD. ***P<0.001 vs. DMSO+LTA (B-C) or
si-Ctrl+DMSO (D and E); ##P<0.01 and
###P<0.001 vs. GBD+LTA (B-C) or si-Ctrl+DMSO+LTA (E);
††P<0.01 and †††P<0.001 vs. ML385+LTA
(B-C) or si-Ctrl+GBD+LTA (E). Nrf2, nuclear factor erythroid
2-related factor 2; GBD, glabridin; LTA, lipoteichoic acid; HO-1,
heme oxygenase-1; RT-qPCR, reverse transcription-quantitative PCR;
si, short interfering; DMSO, dimethyl sulfoxide. |
Genetic inhibition of Nrf2 reduces
GBD-induced HO-1 expression
To further validate the role of Nrf2 in mediating
GBD's effects, the present study performed siRNA knockdown
experiments. Transfection with siRNA targeting Nrf2 (siNrf2)
markedly reduced Nrf2 mRNA expression under basal conditions
compared with a negative control siRNA (si-Ctrl; Fig. S2). Furthermore, Nrf2 knockdown
markedly attenuated the GBD-induced upregulation of HO-1 mRNA
(Fig. 5D and E). These results,
together with the pharmacological inhibition by ML385, supported
the involvement of Nrf2 in GBD-mediated HO-1 induction.
In addition to the Nrf2/HO-1 pathway, GBD also
influenced inflammatory and oxidative stress-related factors in
LTA-stimulated MH-S cells. GBD significantly decreased the mRNA
expression of IL-1β, TNF-α and IL-6, while the expression of SOD1
remained unchanged at 6 h (Fig.
S3). Furthermore, GBD reduced MDA and NO levels at 24 h. These
findings provide additional evidence supporting the antioxidative
and anti-inflammatory potential of GBD.
Nrf2 contributes to the inhibitory
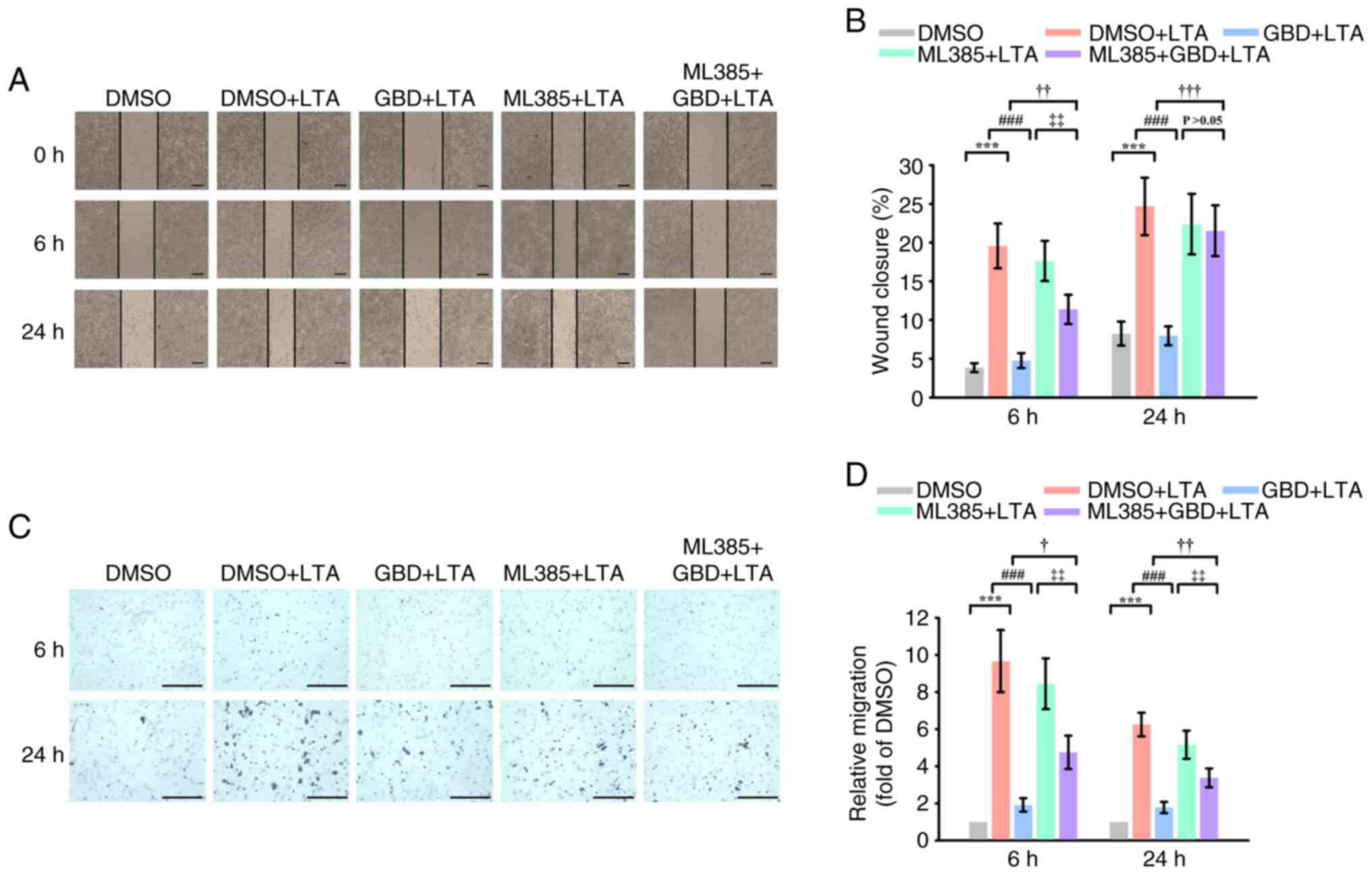
effect of GBD on LTA-induced macrophage migration
Wound healing assays revealed that LTA markedly
promoted MH-S cell migration at 6 and 24 h. GBD pretreatment
suppressed this effect at both time points (Fig. 6A and B). Notably, cotreatment with
Nrf2 inhibitor ML385 attenuated the antimigratory effects of GBD,
as evidenced by markedly higher closure in the ML385+GBD+LTA group
than in the GBD+LTA group at 6 and 24 h. In addition, migration in
ML385+GBD+LTA group remained lower than in ML385+LTA group at 6 h,
although this difference was not sustained at 24 h. These findings
suggested that activation of the Nrf2/HO-1 axis at least partially
mediates the inhibitory effects of GBD.
To further validate the antimigratory effects of
GBD, Transwell assays were performed to assess directional cell
migration. LTA stimulation markedly enhanced macrophage migration
at both 6 and 24 h compared with the DMSO group (Fig. 6D). GBD pretreatment markedly
attenuated this increase. Consistent with Nrf2 involvement, ML385
treatment in combination with LTA promoted migration, and
importantly, cotreatment with ML385 partially reversed the
inhibitory effect of GBD. These results corroborated the
wound-healing findings and indicated that the antimigratory effects
of GBD were mediated, at least in part, by Nrf2.
Discussion
Regulation of macrophage migration has gained
increasing attention in inflammatory contexts, given that the
aberrant migratory behavior of activated macrophages contributes to
tissue damage and disease progression (20). Control over immune cell movement
could limit excessive inflammatory responses and promote resolution
(21). Although several studies
have demonstrated the involvement of oxidative and inflammatory
signaling in macrophage motility (22,23),
the specific modulatory role of natural compounds remains
uncertain. The findings of the present study suggested that GBD
suppresses macrophage migration following LTA stimulation and that
this effect was associated with the activation of the Nrf2/HO-1
pathway (Fig. 7). Thus, GBD may be
a novel avenue for therapies targeting macrophage dynamics in
inflammation-related conditions.
Notably, lipopolysaccharide (LPS), which is derived
from Gram-negative bacteria, primarily activates TLR4 signaling.
Although both LTA and LPS initiate innate immune responses, their
receptor engagement, downstream signaling profiles and temporal
dynamics differ. Clinically, LPS-related endotoxemia is commonly
associated with sepsis caused by Gram-negative infections, such as
Escherichia coli or Pseudomonas aeruginosa. By
contrast, LTA is implicated in Gram-positive bacterial infections,
including Staphylococcus aureus, Streptococcus pneumoniae,
and Enterococcus species, which contribute to pneumonia,
endocarditis, and skin and soft tissue infections (24). In research on pulmonary diseases,
both LPS and LTA have been employed as experimental stimuli to
mimic bacterial infection and elicit innate immune responses in
lung models. In animal studies, LPS is widely used to induce acute
lung injury or acute respiratory distress syndrome (ARDS) because
of its potent ability to activate neutrophils, disrupt endothelial
barriers, and promote cytokine storms through TLR4-dependent
signaling (25,26). By contrast, LTA activates alveolar
macrophages, modulates surfactant expression, and contributes to
the development of Gram-positive pneumonia (27). Although LTA-induced responses are
generally less systemically severe than LPS-induced responses are,
evidence increasingly suggests that chronic or repeated exposure to
LTA can provoke sustained lung inflammation, epithelial damage, and
macrophage-driven remodeling (27,28).
These differential effects underscore a need to distinguish between
Gram-negative and Gram-positive bacterial stimuli in evaluations of
pulmonary immune responses and corresponding therapeutic
strategies. In the present study, LTA stimulation induced a marked
increase in alveolar macrophage migration, elevated ROS levels and
activated the Nrf2/HO-1 pathway. These findings indicated that
Gram-positive bacterial components influence macrophage behavior
through stress-responsive mechanisms, providing novel insights into
cell dynamics during bacterial exposure.
Alveolar macrophages are specialized tissue-resident
immune cells located within the alveolar lumen, where they play a
critical role in maintaining pulmonary homeostasis and coordinating
immune surveillance (29). Unlike
circulating monocytes or recruited inflammatory macrophages,
alveolar macrophages originate from fetal monocytes and their
population is self-renewing (30,31).
Under steady-state conditions, they exhibit a relatively quiescent
phenotype, clearing inhaled particles, apoptotic cells and
pathogens without eliciting excessive inflammation (32). Due to their position at the
interface between the airway epithelium and the external
environment, their activity must be tightly regulated to avoid
compromising alveolar barrier integrity (33). In the present study, MH-S cells
were employed as an in vitro model because of their
established relevance in studies of pulmonary innate immunity
(34). This model provided a
suitable framework to investigate the regulatory effects of GBD on
alveolar macrophage migration.
The Nrf2/HO-1 pathway is a central regulator of
cellular homeostasis under stress (35–37).
Upon activation, the transcription factor Nrf2 translocates to the
nucleus to induce cytoprotective genes (38). HO-1 was chosen as the canonical
marker because it is not only a robustly induced Nrf2 target in
macrophages (39), but also
because its enzymatic products actively regulate inflammation and
macrophage migration (40,41), positioning it at the mechanistic
nexus of GBD's effects. While this pathway is known to modulate
immune cell behavior, including migration (42,43),
its role in response to specific bacterial components such as LTA
was unclear.
To further refine the mechanistic profile, the
present study also examined additional inflammatory and oxidative
stress markers. GBD displayed a selective pattern of activity. GBD
markedly suppressed LTA-induced IL-1β mRNA expression, yet had no
effect on TNF-α or IL-6 levels. Similarly, while the steady-state
antioxidant enzyme SOD1 remained unchanged, GBD markedly reduced
later-stage oxidative markers, including 24-h lipid peroxidation
(MDA) and nitric oxide (NO) production. These supplementary
findings suggested that HO-1 is likely to be a prominent downstream
effector of GBD in this setting, consistent with reports that the
Nrf2/HO-1 axis can provide real-time protection against oxidative
insults (37,38). and that HO-1 products can modulate
inflammatory cell migration (44,45).
Dysregulated macrophage migration has been
implicated in inflammatory lung diseases, contributing to both
excessive inflammation and impaired pathogen clearance (46). While alveolar macrophages are
generally sessile under homeostatic conditions (4), they can adopt a migratory phenotype
upon microbial stimulation to support immune surveillance (33,47).
To assess GBD's effect on this process, the present study employed
two complementary approaches: The wound-healing assay a
well-established method for assessing collective motility and the
Transwell assay for directional chemotaxis. In both systems, LTA
stimulation increased macrophage migration, whereas GBD treatment
reduced this response. The inhibitory effect was attenuated by the
Nrf2 inhibitor ML385, suggesting that Nrf2 signaling contributes to
this regulation. Consistent outcomes from both assays supported the
reliability of these findings and provided a rationale for further
validation of the underlying mechanism.
To rigorously validate the essential role of Nrf2,
the present study employed both pharmacological inhibition and
genetic knockdown approaches. The Nrf2 inhibitor ML385 partly
reversed the anti-migratory effects of GBD, while silencing Nrf2
with siRNA markedly attenuated the GBD-induced upregulation of HO-1
mRNA. This convergence of evidence from two distinct methodologies
supported the involvement of Nrf2/HO-1 signaling in mediating the
effect of GBD. Furthermore, the specific downstream effectors
mediating this anti-migratory response remain to be elucidated.
Future studies should therefore examine additional Nrf2 targets,
including other cytoprotective enzymes beyond HO-1, such as NQO1,
as well as proteins directly involved in cell motility,
particularly matrix metalloproteinases (such as MMP-2 and MMP-9),
which are functionally linked to macrophage migration (48).
The present study offered novel mechanistic insights
regarding how GBD affects macrophage behavior under LTA
stimulation. LTA exposure was sufficient to induce pronounced
migratory activity in alveolar macrophages, highlighting its
capacity to modulate innate immune cell motility. GBD pretreatment
markedly reduced macrophage migration, possibly because of the
increased nuclear accumulation of Nrf2 and elevated HO-1
expression. Pharmacological inhibition with ML385 and genetic
knockdown with siRNA both attenuated the antimigratory effects of
GBD, supporting the involvement of Nrf2 activation in mediating
this response. To the best of our knowledge, this is the first
study to demonstrate that GBD modulated LTA-induced alveolar
macrophage migration through mechanisms involving the Nrf2/HO-1
pathway. These findings revealed a novel functional aspect of GBD
in controlling macrophage dynamics, suggesting it holds potential
relevance in preventing dysregulated cell migration associated with
pulmonary inflammation.
The clinical implications of modulating macrophage
migration are extensive. Aberrant cell motility is a recognized
pathological feature in a wide range of diseases; for instance,
excessive macrophage infiltration drives pulmonary conditions such
as COPD and ARDS, while insufficient migration can impair pathogen
clearance and tissue repair (46,49–52).
Beyond the lung, macrophage trafficking is also pivotal in the
progression of cancer, atherosclerosis and autoimmune diseases
(53). The present study thus
positioned GBD as a potential modulator in these contexts. Notably,
the temporal dynamics of GBD's antimigratory effects have important
therapeutic implications. The rapid onset of action suggested
potential utility in acute inflammatory conditions where early
intervention is critical. However, the attenuated long-term effects
indicated that sustained therapeutic benefits may require optimized
dosing regimens or combination approaches. This nuanced profile
supported the strategy of using pharmacological agents to modulate,
rather than abolish, macrophage migration to restore immune
balance. Therefore, GBD may hold potential as a modulator for
diseases involving immune cell dysregulation.
Macrophage migration and polarization are
functionally interlinked, particularly under inflammatory
conditions where M1-polarized cells exhibit enhanced motility
(54–56). This relationship is often
bidirectional, as migration into a pro-inflammatory
microenvironment can further reinforce M1 polarization (57). In this context, the LTA-induced
migration observed may reflect a shift toward an activated,
pro-inflammatory M1-like state. Measurement of nitric oxide (NO)
production, a marker often associated with M1 activation, supported
this notion. However, a more comprehensive assessment using
canonical polarization markers (such as iNOS, CD86 and Arg1) will
be valuable in future studies to further establish this link.
While the present study established a mechanistic
link between GBD, Nrf2/HO-1 activation and suppressed macrophage
migration, several limitations should be acknowledged. One
limitation is that the investigation was confined to in
vitro models. Future in vivo studies are necessary to
validate the physiological relevance of the findings and to assess
downstream pathophysiological implications, such as the effect of
GBD on alveolar-capillary barrier integrity. Another limitation is
that the mechanistic focus was restricted to the Nrf2/HO-1 axis.
The role of other critical components of cell motility,
particularly adhesion molecules (such as integrins, selectins), was
not assessed and represents an important avenue for future
research. A further limitation is that the analysis of oxidative
stress was not exhaustive. Future work should therefore include a
more comprehensive profiling of oxidative stress parameters; for
instance, by measuring protein carbonyl formation and assessing the
cellular glutathione antioxidant system. Finally, for GBD to
advance as a pharmacologically viable compound, its pharmacokinetic
profile, particularly bioavailability and metabolic stability, will
require comprehensive evaluation (58). The observed time-dependent
attenuation of GBD's effects in the present study also highlighted
the need to investigate optimal dosing regimens for sustained
therapeutic efficacy.
The present study demonstrated that GBD attenuated
LTA-induced alveolar macrophage migration by activating the
Nrf2/HO-1 signaling pathway. It integrated molecular, cellular and
functional analyses to reveal a regulatory mechanism by which GBD
affected macrophage motility in response to bacterial stimulation.
These findings broadened the current understanding of the
biological effects of GBD and suggested its potential as a
modulatory agent of immune cell behavior in disease contexts.
Future in vivo investigations and pharmacological profiling
are required to further validate the therapeutic utility and
translational applicability of GBD to inform the development of
novel therapeutic strategies for inflammatory lung diseases.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Science and
Technology Council, Taiwan, R.O.C. (grant nos. NSTC
112-2320-B-038-037-MY3 and NSTC 113-2320-B341-002-MY3), Shin Kong
Wu Ho-Su Memorial Hospital (grant no. 2025SKHAND011) and Chi Mei
Medical Center-Taipei Medical University (grant no.
111CM-TMU-07).
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
JRS designed the study. CHH and CMY wrote the
manuscript. CHH, CMY, CCC, TLY, AGD and CCH performed the
experiments. CCC, TLY and AGD analyzed the data. CMY and JRS
confirm the authenticity of all the raw data. All authors have read
and approved the final manuscript.
Ethical approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Takeuchi O, Hoshino K, Kawai T, Sanjo H,
Takada H, Ogawa T, Takeda K and Akira S: Differential roles of TLR2
and TLR4 in recognition of gram-negative and gram-positive
bacterial cell wall components. Immunity. 11:443–451. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pai AB, Patel H, Prokopienko AJ, Alsaffar
H, Gertzberg N, Neumann P, Punjabi A and Johnson A: Lipoteichoic
acid from staphylococcus aureus induces lung endothelial cell
barrier dysfunction: Role of reactive oxygen and nitrogen species.
PLoS One. 7:e492092012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Percy MG and Gründling A: Lipoteichoic
acid synthesis and function in gram-positive bacteria. Annu Rev
Microbiol. 68:81–100. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hussell T and Bell TJ: Alveolar
macrophages: Plasticity in a tissue-specific context. Nat Rev
Immunol. 14:81–93. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Boyd AR, Shivshankar P, Jiang S, Berton MT
and Orihuela CJ: Age-related defects in TLR2 signaling diminish the
cytokine response by alveolar macrophages during murine
pneumococcal pneumonia. Exp Gerontol. 47:507–518. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu CF, Drocourt D, Puzo G, Wang JY and
Riviere M: Innate immune response of alveolar macrophage to house
dust mite allergen is mediated through TLR2/-4 co-activation. PLoS
One. 8:e759832013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Aggarwal S, Dimitropoulou C, Lu Q, Black
SM and Sharma S: Glutathione supplementation attenuates
lipopolysaccharide-induced mitochondrial dysfunction and apoptosis
in a mouse model of acute lung injury. Front Physiol. 3:1612012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Birben E, Sahiner UM, Sackesen C, Erzurum
S and Kalayci O: Oxidative stress and antioxidant defense. World
Allergy Organ J. 5:9–19. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Malainou C, Abdin SM, Lachmann N, Matt U
and Herold S: Alveolar macrophages in tissue homeostasis,
inflammation, and infection: Evolving concepts of therapeutic
targeting. J Clin Invest. 133:e1705012023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Harvey CJ, Thimmulappa RK, Sethi S, Kong
X, Yarmus L, Brown RH, Feller-Kopman D, Wise R and Biswal S:
Targeting Nrf2 signaling improves bacterial clearance by alveolar
macrophages in patients with COPD and in a mouse model. Sci Transl
Med. 3:78ra322011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Itoh K, Chiba T, Takahashi S, Ishii T,
Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, et
al: An Nrf2/small Maf heterodimer mediates the induction of phase
II detoxifying enzyme genes through antioxidant response elements.
Biochem Biophys Res Commun. 236:313–322. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ma Q: Role of nrf2 in oxidative stress and
toxicity. Annu Rev Pharmacol Toxicol. 53:401–426. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Itoh K, Wakabayashi N, Katoh Y, Ishii T,
Igarashi K, Engel JD and Yamamoto M: Keap1 represses nuclear
activation of antioxidant responsive elements by Nrf2 through
binding to the amino-terminal Neh2 domain. Genes Dev. 13:76–86.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ryter SW, Alam J and Choi AM: Heme
oxygenase-1/carbon monoxide: From basic science to therapeutic
applications. Physiol Rev. 86:583–650. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Meng X, Hu L and Li W: Baicalin
ameliorates lipopolysaccharide-induced acute lung injury in mice by
suppressing oxidative stress and inflammation via the activation of
the Nrf2-mediated HO-1 signaling pathway. Naunyn Schmiedebergs Arch
Pharmacol. 392:1421–1433. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Luan R, Ding D and Yang J: The protective
effect of natural medicines against excessive inflammation and
oxidative stress in acute lung injury by regulating the Nrf2
signaling pathway. Front Pharmacol. 13:10390222022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shin J, Choi LS, Jeon HJ, Lee HM, Kim SH,
Kim KW, Ko W, Oh H and Park HS: Synthetic glabridin derivatives
inhibit LPS-induced inflammation via MAPKs and NF-κB Pathways in
RAW264.7 macrophages. Molecules. 28:21352023. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shu L, Zhang Z, Wang N, Yin Q, Chao Y and
Ge X: Glabridin ameliorates hemorrhagic shock induced acute kidney
injury by activating Nrf2/HO-1 pathway. Biochim Biophys Acta Mol
Basis Dis. 1871:1678102025. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Murrey MW, Ng IT and Pixley FJ: The role
of macrophage migratory behavior in development, homeostasis and
tumor invasion. Front Immunol. 15:14800842024. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rodriguez-Morales P and Franklin RA:
Macrophage phenotypes and functions: Resolving inflammation and
restoring homeostasis. Trends Immunol. 44:986–998. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tran N and Mills EL: Redox regulation of
macrophages. Redox Biol. 72:1031232024. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Breßer M, Siemens KD, Schneider L,
Lunnebach JE, Leven P, Glowka TR, Oberländer K, De Domenico E,
Schultze JL, Schmidt J, et al: Macrophage-induced enteric
neurodegeneration leads to motility impairment during gut
inflammation. EMBO Mol Med. 17:301–335. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gatica S, Fuentes B, Rivera-Asin E,
Ramírez-Céspedes P, Sepúlveda-Alfaro J, Catalán EA, Bueno SM,
Kalergis AM, Simon F, Riedel CA and Melo-Gonzalez F: Novel evidence
on sepsis-inducing pathogens: From laboratory to bedside. Front
Microbiol. 14:11982002023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
He J, Zhao Y, Fu Z, Chen L, Hu K, Lin X,
Wang N, Huang W, Xu Q, He S, et al: A novel tree shrew model of
lipopolysaccharide-induced acute respiratory distress syndrome. J
Adv Res. 56:157–165. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang Y, Han Z, Jiang A, Wu D, Li S, Liu
Z, Wei Z, Yang Z and Guo C: Protective effects of pterostilbene on
lipopolysaccharide-induced acute lung injury in mice by inhibiting
NF-κB and activating Nrf2/HO-1 signaling pathways. Front Pharmacol.
11:5918362021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Knapp S, von Aulock S, Leendertse M,
Haslinger I, Draing C, Golenbock DT and van der Poll T:
Lipoteichoic acid-induced lung inflammation depends on TLR2 and the
concerted action of TLR4 and the platelet-activating factor
receptor. J Immunol. 180:3478–3484. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hoogerwerf JJ, de Vos AF, Bresser P, van
der Zee JS, Pater JM, de Boer A, Tanck M, Lundell DL, Her-Jenh C,
Draing C, et al: Lung inflammation induced by lipoteichoic acid or
lipopolysaccharide in humans. Am J Respir Crit Care Med. 178:34–41.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hou F, Xiao K, Tang L and Xie L: Diversity
of macrophages in lung homeostasis and diseases. Front Immunol.
12:7539402021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Guilliams M, De Kleer I, Henri S, Post S,
Vanhoutte L, De Prijck S, Deswarte K, Malissen B, Hammad H and
Lambrecht BN: Alveolar macrophages develop from fetal monocytes
that differentiate into long-lived cells in the first week of life
via GM-CSF. J Exp Med. 210:1977–1992. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Röszer T: Understanding the biology of
self-renewing macrophages. Cells. 7:1032018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Guan F, Wang R, Yi Z, Luo P, Liu W, Xie Y,
Liu Z, Xia Z, Zhang H and Cheng Q: Tissue macrophages: Origin,
heterogenity, biological functions, diseases and therapeutic
targets. Signal Transduct Target Ther. 10:932025. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ahmad S, Nasser W and Ahmad A: Epigenetic
mechanisms of alveolar macrophage activation in chemical-induced
acute lung injury. Front Immunol. 15:14889132024. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mbawuike IN and Herscowitz HB: MH-S, a
murine alveolar macrophage cell line: Morphological, cytochemical,
and functional characteristics. J Leukoc Biol. 46:119–127. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Loboda A, Damulewicz M, Pyza E, Jozkowicz
A and Dulak J: Role of Nrf2/HO-1 system in development, oxidative
stress response and diseases: An evolutionarily conserved
mechanism. Cell Mol Life Sci. 73:3221–3247. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li N, Hao L, Li S, Deng J, Yu F, Zhang J,
Nie A and Hu X: The NRF-2/HO-1 signaling pathway: A promising
therapeutic target for metabolic dysfunction-associated steatotic
liver disease. J Inflamm Res. 17:8061–8083. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Su H, Wang Z, Zhou L, Liu D and Zhang N:
Regulation of the Nrf2/HO-1 axis by mesenchymal stem cells-derived
extracellular vesicles: Implications for disease treatment. Front
Cell Dev Biol. 12:13979542024. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
O'Rourke SA, Shanley LC and Dunne A: The
Nrf2-HO-1 system and inflammaging. Front Immunol. 15:14570102024.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Alam J and Cook JL: How many transcription
factors does it take to turn on the heme oxygenase-1 gene? Am J
Respir Cell Mol Biol. 36:166–174. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Vijayan V, Wagener FADTG and Immenschuh S:
The macrophage heme-heme oxygenase-1 system and its role in
inflammation. Biochem Pharmacol. 153:159–167. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang L and He C: Nrf2-mediated
anti-inflammatory polarization of macrophages as therapeutic
targets for osteoarthritis. Front Immunol. 13:9671932022.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Feng R, Morine Y, Ikemoto T, Imura S,
Iwahashi S, Saito Y and Shimada M: Nrf2 activation drive
macrophages polarization and cancer cell epithelial-mesenchymal
transition during interaction. Cell Commun Signal. 16:542018.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Han H, Gao Y, Chen B, Xu H, Shi C, Wang X,
Liang Y, Wu Z, Wang Z, Bai Y and Wu C: Nrf2 inhibits M1 macrophage
polarization to ameliorate renal ischemia-reperfusion injury
through antagonizing NF-κB signaling. Int Immunopharmacol.
143:1133102024. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jagadeesh ASV, Fang X, Kim SH,
Guillen-Quispe YN, Zheng J, Surh YJ and Kim SJ: Non-canonical vs.
canonical functions of heme oxygenase-1 in cancer. J Cancer Prev.
27:7–15. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Freitas A, Alves-Filho JC, Secco DD, Neto
AF, Ferreira SH, Barja-Fidalgo C and Cunha FQ: Heme
oxygenase/carbon monoxide-biliverdin pathway down regulates
neutrophil rolling, adhesion and migration in acute inflammation.
Br J Pharmacol. 149:345–354. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Cheng P, Li S and Chen H: Macrophages in
lung injury, repair, and fibrosis. Cells. 10:4362021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chen S, Saeed AFUH, Liu Q, Jiang Q, Xu H,
Xiao GG, Rao L and Duo Y: Macrophages in immunoregulation and
therapeutics. Signal Transduct Target Ther. 8:2072023. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ngo V and Duennwald ML: Nrf2 and oxidative
stress: A general overview of mechanisms and implications in human
disease. Antioxidants (Basel). 11:23452022. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Matthay MA and Zemans RL: The acute
respiratory distress syndrome: Pathogenesis and treatment. Annu Rev
Pathol. 6:147–163. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Barnes PJ: Inflammatory mechanisms in
patients with chronic obstructive pulmonary disease. J Allergy Clin
Immunol. 138:16–27. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Gordon S and Plüddemann A: Macrophage
clearance of apoptotic cells: A critical assessment. Front Immunol.
9:1272018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wynn TA and Vannella KM: Macrophages in
tissue repair, regeneration, and fibrosis. Immunity. 44:450–462.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Gordon S, Plüddemann A and Martinez
Estrada F: Macrophage heterogeneity in tissues: Phenotypic
diversity and functions. Immunol Rev. 262:36–55. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Mantovani A, Biswas SK, Galdiero MR, Sica
A and Locati M: Macrophage plasticity and polarization in tissue
repair and remodelling. J Pathol. 229:176–185. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Murray PJ, Allen JE, Biswas SK, Fisher EA,
Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence
T, et al: Macrophage activation and polarization: Nomenclature and
experimental guidelines. Immunity. 41:14–20. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Auffray C, Fogg D, Garfa M, Elain G,
Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G and
Geissmann F: Monitoring of blood vessels and tissues by a
population of monocytes with patrolling behavior. Science.
317:666–670. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Lawrence T and Natoli G: Transcriptional
regulation of macrophage polarization: Enabling diversity with
identity. Nat Rev Immunol. 11:750–761. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Xie L, Diao Z, Xia J, Zhang J, Xu Y, Wu Y,
Liu Z, Jiang C, Peng Y, Song Z, et al: Comprehensive evaluation of
metabolism and the contribution of the hepatic first-pass effect in
the bioavailability of glabridin in rats. J Agric Food Chem.
71:1944–1956. 2023. View Article : Google Scholar : PubMed/NCBI
|