Introduction
Osteoporosis is a disease characterized by changes
in bone microstructure, including thinning of trabeculae and an
increased susceptibility to brittle fractures (1). Osteoporosis primarily results from an
imbalance in bone metabolism, where bone formation is weakened
while bone resorption increases, leading to a loss of bone mass
(2). Factors such as aging,
inflammation and hormonal changes lead to a reduction in bone
formation (3); however, the
specific reasons have not yet been fully elucidated.
Bone marrow mesenchymal stem cells (BMSCs) are a
type of pluripotent stem cell with the ability to differentiate
into three lineages: Osteoblasts, adipocytes and chondrocytes
(4). It has previously been shown
that the differentiation strength of BMSCs is markedly associated
with bone changes in vivo (5). Research using tissue engineering
functional scaffolds has demonstrated that increasing the
osteogenic differentiation ability of BMSCs leads to a notable
increase in bone formation in vivo (6). Therefore, BMSCs are considered an
important target and functional cell for treating diseases
characterized by reduced bone formation (7,8).
However, the specific regulatory mechanism underlying the
osteogenic differentiation of BMSCs has not yet been fully
elucidated and requires further investigation.
Forkhead box (FOXJ3) possesses DNA-binding
transcriptional activation activity, RNA polymerase II specificity
and sequence-specific double-stranded DNA-binding activity.
Notably, FOXJ3 participates in the positive regulation of RNA
polymerase II transcription. Previous studies have shown that FOXJ3
is related to the progression of various diseases, such as
rheumatoid arthritis (9), and
spermatogenesis (10).
Furthermore, it has been reported to serve an important role in the
disease evolution process in cancer (11,12).
At the metabolic level, it has been reported that FOXJ3 can promote
the thermogenic effect of fat (13). In addition, FOXJ3 can promote the
formation of osteoclasts (14).
Fat metabolism and osteoclastogenesis in the bone marrow are
associated with bone formation and other processes. Given the
important role of BMSCs in bone formation, it is crucial to clarify
whether FOXJ3 affects the osteogenic differentiation function of
BMSCs and its role in bone metabolic diseases. However, to the best
of our knowledge, no relevant studies have yet been published.
The Wnt/β-catenin pathway is a crucial pathway that
serves important roles in various cell functions, including cell
proliferation (15) and
differentiation (16). It has
previously been shown that this pathway can promote the osteogenic
differentiation of BMSCs (17).
After activation of the Wnt/β-catenin protein and its entry into
the nucleus, it can activate the expression of osteogenic-related
molecules, promote the secretion of extracellular matrix proteins,
and the synthesis of alkaline phosphatase (ALP) and other
substances by BMSCs, thereby promoting mineralization (18). Therefore, the present study aims to
investigate whether FOXJ3 is involved in the osteogenic
differentiation of BMSCs and whether it exerts its regulatory
function through the Wnt/β-catenin pathway, thereby providing a new
therapeutic target for bone metabolic diseases.
Materials and methods
BMSC Treatment
Rat BMSCs were obtained from Wuhan Servicebio
Technology Co., Ltd. Since BMSCs from passages 3–6 exhibit a
homogeneous population, consistent morphology and robust osteogenic
differentiation functionality, all experiments were conducted using
cells within this passage range. Adherent BMSCs were cultured in
DMEM (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10%
fetal bovine serum (FBS; Zhejiang Tianhang Biotechnology Co., Ltd.)
in a humidified incubator maintained at 37°C with 5%
CO2.
Osteoblast differentiation
BMSCs were seeded at a density of 1×105
cells/well in 12-well plates. Following medium renewal on day 2,
the BMSCs were induced to differentiate by culturing them in
low-glucose DMEM supplemented with 10% FBS, 10−8 M
dexamethasone (Sigma-Aldrich; Merck KGaA; cat: D4902), 50 µg/ml
ascorbic acid 2-phosphate (Sigma-Aldrich, cat: 49752) and 10 mM
β-glycerophosphate (Sigma-Aldrich; Merck KGaA; cat. no. G9422). The
culture medium was refreshed every 3 days. Osteogenic
differentiation medium was supplemented with 5 µM SB216763
(19–21) or 10 µM XAV939 (22,23)
(both from Shanghai Aladdin Biochemical Technology Co., Ltd.) to
activate or inhibit the Wnt/β-catenin signaling pathway during BMSC
culture in 37°C, respectively. The DMSO group was supplemented with
the same volume of DMSO as the groups treated with SB216763 or
XAV939.
ALP activity assay
BMSCs were seeded at a density of 1×10 cells/well in
12-well plates. ALP activity was assessed following 10 days of
osteogenic differentiation, in accordance with the manufacturer's
protocol, using an ALP activity assay kit (Beyotime Biotechnology,
cat: C3206). Total protein concentrations in the lysates (Beyotime
Biotechnology, cat: P0013) were determined using the bicinchoninic
acid assay (Pierce; Thermo Fisher Scientific, Inc.). Results were
normalized to total protein content and expressed relative to the
control condition.
Alizarin Red S (ARS) staining
The degree of mineralization was determined by ARS
staining. BMSCs were seeded at a density of 1×105
cells/well in 12-well plates. After osteogenic differentiation, the
cells were fixed with 95% ethanol at 25°C for 30 min, followed by
incubation with 0.1% ARS solution (pH 4.2; Beijing Solarbio Science
& Technology Co., Ltd.) for 20 min at room temperature. To
quantify mineralization, the calcium-bound dye was solubilized
using 10% cetylpyridinium chloride (Sigma-Aldrich; Merck KGaA) for
1 h in 25°C, and the eluate was assayed spectrophotometrically at
562 nm. Staining intensity was captured in light microscope and
normalized to total protein content and reported relative to the
undifferentiated control.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from BMSCs using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), followed by cDNA synthesis via RT with random primers and an
M-MLV Reverse Transcriptase kit (Invitrogen; Thermo Fisher
Scientific, Inc.) according to manufacturer's protocol.
Subsequently, qPCR analyses were performed using a SYBR Green PCR
kit (Takara Biotechnology Co., Ltd.). Thermocycling conditions were
as follows: 94°C, 30 sec; 55°C, 30 sec. Step 3: 72°C, 1 min). GAPDH
expression was used for normalization. The ΔCq values were
calculated relative to GAPDH, and relative quantification of gene
expression was determined using the 2−ΔΔCq method
(24). Each sample was assessed in
triplicate. The primers used are shown in Table I.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Gene | Primer sequence,
5′-3′ |
|---|
| GAPDH | F:
AACCCTCAACAGGGATGCTT |
|
| R:
GTTCACACCGACCTTCACCA |
| FOXJ3 | F:
TTCTCTGGCATTGGGGCAAA |
|
| R:
CTGGCATAGCTGTACGGAGG |
| RUNX2 | F:
CAACCGAGTCAGTGAGTGCT |
|
| R:
CAAACCATACCCAGTCCCTGT |
| OCN | F:
CCGTTTAGGGCATGTGTTGC |
|
| R:
CCGTCCATACTTTCGAGGCA |
Western blotting
Cell protein was obtained using lysis buffer
(Beyotime Biotechnology, cat: P0013) and quantified by BCA method.
Protein lysates (30 µg/lane) underwent electrophoretic separation
on 10% SDS-polyacrylamide gels followed by wet transfer to PVDF
membranes (Sigma-Aldrich; Merck KGaA). Membranes were then blocked
for 1 h in 0.1% TBS-Tween (TBST) containing 5% non-fat dry milk at
room temperature. Primary antibody (FOXJ3: Solarbio, Cat: K008825P.
active β-catenin: Solarbio, Cat: K009589P. β-catenin: Solarbio,
Cat: K008788P. AKT: Solarbio, Cat: K109232P. p-AKT: Solarbio, Cat:
Cat:K000186M. ERK: Solarbio, Cat: K200062M. p-ERK: Solarbio, Cat:
K009730P. GAPDH: Solarbio, cat. no. K200057M) incubation was
performed overnight at 4°C (1:1,000). After washing, the blots were
incubated for 1 h at room temperature with a HRP-linked goat
anti-rabbit secondary antibody (1:1,000) (Solarbio, Cat. no. SE132
and SE134). Following three 5-min TBST washes, protein bands were
treated by ECL kit (Beyotime Biotechnology, cat: P0018S) and
detected by enhanced chemiluminescence after substrate application.
GAPDH blotting served as the normalization control. ImageJ software
(National Institutes of Health, V1.47) is used for protein
quantification.
Lentivirus production and
infection
A lentiviral vector encoding FOXJ3 (lentiviral
vector backbone: pCDH-EF1a-MCS-IRES-puro; OE-FOXJ3) was generated
by Guangzhou iGene Biotechnology Co., Ltd. In short, 293T cells
(Guangzhou iGene Biotechnology Co.) were co-transfected using a
third-generation lentiviral system, with plasmid ratios of 4 µg
(target plasmid): 3 µg (psPAX2): 1 µg (pMD2.G). Virus supernatants
were collected in batches at 48 and 72 h after transfection and
filtered through a 0.45 µm filter membrane. The virus particles
were concentrated by ultracentrifugation (~70,000-100,000 × g, 2 h)
in 4°C, and the precipitate was resuspended in a 500 µl of buffer.
Finally, the samples were aliquoted and stored at −80°C. When cells
reached 80–90% confluency, lentiviral transduction was performed
for 24 h at 37°C. The viral supernatant was diluted in complete
medium to achieve a multiplicity of infection of 10 and was applied
to cells supplemented with polybrene (8 µg/ml; Sigma-Aldrich; Merck
KGaA) in 24 h. The negative control was prepared by transducing the
cells with the lentiviral vector backbone lacking the target gene.
Transduction efficiency was assessed by RT-qPCR analysis of FOXJ3
mRNA levels. Subsequent experiments were performed in 24 h
later.
Small interfering (si)RNA
transfection
Gene silencing was performed using FOXJ3-targeting
siRNAs (Shanghai GenePharma Co., Ltd.), with a non-targeting
scrambled siRNA (Shanghai GenePharma Co., Ltd.) used as a negative
control. Transfection of 1×105 BMSCs was carried out
using 50 nM siRNA with Lipofectamine® RNAiMAX
(Invitrogen; Thermo Fisher Scientific, Inc.) according to standard
procedures in 37°C in 6 h. Subsequent experiments were performed in
6 h later. The siRNA sequences are shown in Table II.
 | Table II.siRNA sequences. |
Table II.
siRNA sequences.
| siRNA | siRNA sequence,
5′-3′ |
|---|
| siControl | Sense:
UUCUCCGAACGUGUCACGUTT |
|
| Antisense:
ACGUGACACGUUCGGAGA |
|
| ATT |
| siFOXJ3-1 | Sense:
CGGGCCUCAACUCCAUAUATT |
|
| Antisense:
UAUAUGGAGUUGAGGCCC |
|
| GTT |
| siFOXJ3-2 | Sense:
GGGAAGUGUACAUAGUUA |
|
| UTT |
|
| Antisense:
AUAACUAUGUACACUUCC |
|
| CTT |
| siFOXJ3-3 | Sense:
CUGGAGAGCAGCCUAACAUTT |
|
| Antisense:
AUGUUAGGCUGCUCUCCA |
|
| GTT |
Statistical analysis
All data were obtained from experiments repeated at
least three times. Results are presented as the mean ± standard
deviation. All statistical analyses were conducted using SPSS 18.0
(IBM Corp.). Paired Student's t-test was used for two-group
comparisons, whereas one-way ANOVA with Tukey's HSD post hoc
multiple comparisons test applied for multi-group analyses. The
Pearson correlation test was performed for correlation analyses.
P<0.05 was considered to indicate a statistically significant
difference.
Results
FOXJ3 expression is positively
associated with BMSC osteogenic differentiation
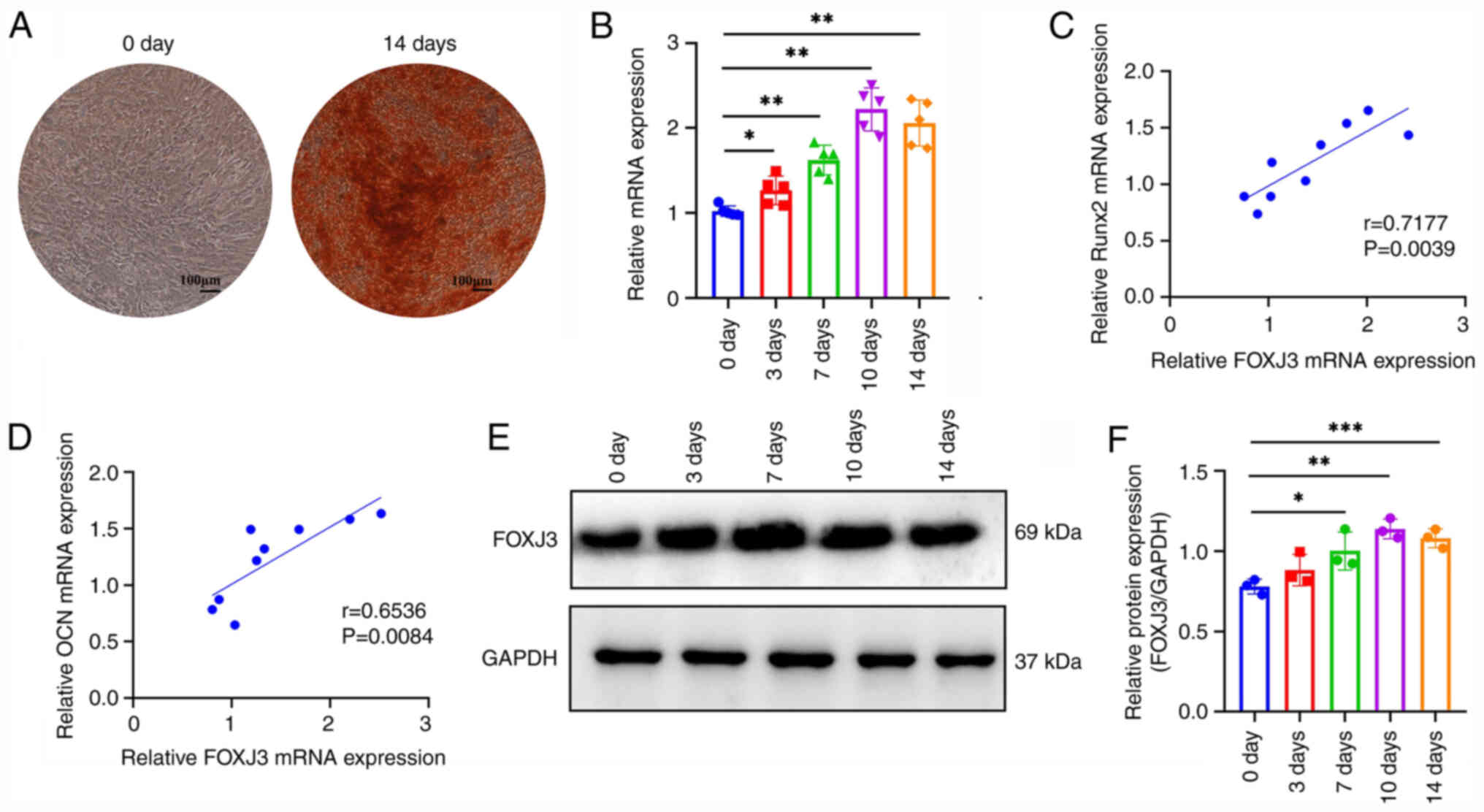
Firstly, osteogenic differentiation of BMSCs was
induced and dynamic changes in FOXJ3 expression were detected
during this differentiation process. The results of ARS staining
indicated that BMSCs were effectively differentiated into
osteoblasts after a 14-day culture in osteogenic induction medium
(Fig. 1A). RT-qPCR results
demonstrated that FOXJ3 expression progressively increased with
prolonged osteogenic induction time, reaching peak levels on day 10
of induction with a ~2-fold increase compared with that in the
non-induced group (Fig. 1B).
Furthermore, RT-qPCR analysis revealed a positive correlation
between FOXJ3 expression and the expression of osteogenesis-related
genes Runt-related transcription factor 2 (RUNX2) (Fig. 1C) and osteocalcin (OCN) (Fig. 1D). Additionally, western blotting
demonstrated a progressive elevation in FOXJ3 protein expression
with extended osteogenic induction, reaching a maximum on day 10 of
induction (Fig. 1E and F). These
findings collectively suggested that FOXJ3 may be positively
associated with osteogenic differentiation of BMSCs and could serve
a regulatory role in BMSCs osteogenic differentiation
processes.
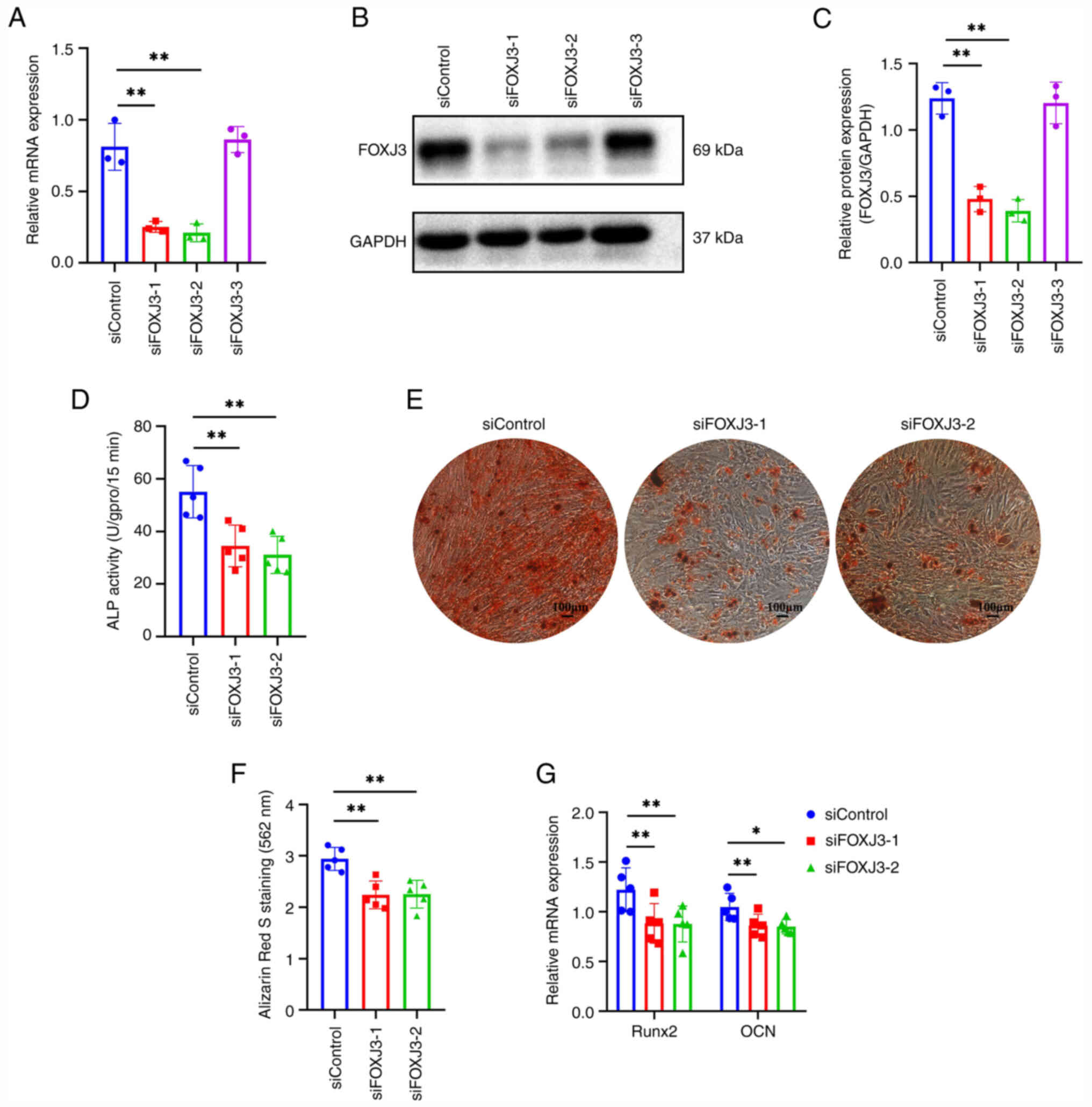
Loss of FOXJ3 in vitro inhibits the
osteogenic differentiation of BMSCs
To investigate the regulatory role of FOXJ3 in BMSCs
osteogenic differentiation, BMSCs were transfected with siRNA to
knock down FOXJ3 expression. The results of RT-qPCR demonstrated
that siFOXJ3-1 and siFOXJ3-2 exhibited significant knockdown
efficiencies, whereas siFOXJ3-3 showed no substantial effect
compared to the siControl group (Fig.
2A). Western blotting further confirmed that siFOXJ3-1 and
siFOXJ3-2 effectively reduced FOXJ3 protein expression in BMSCs
(Fig. 2B and C). Therefore,
siFOXJ3-1 and siFOXJ3-2 for the subsequent experiments. Following
FOXJ3 knockdown, osteogenic differentiation was induced in BMSCs.
Quantitative ALP analysis revealed decreased ALP activity in both
siFOXJ3-1 and siFOXJ3-2 groups compared with that in the control,
indicating that FOXJ3 knockdown suppressed ALP activity in BMSCs
(Fig. 2D). ARS staining showed
that the numbers of osteogenic nodules in the siFOXJ3-1 and
siFOXJ3-2 groups were markedly reduced compared with those in the
control group (Fig. 2E).
Quantitative analysis of ARS staining further confirmed that
knockdown of FOXJ3 expression inhibited osteogenic differentiation
of BMSCs (Fig. 2F). RT-qPCR
demonstrated that the expression of osteogenic
differentiation-related genes RUNX2 and OCN was suppressed
following FOXJ3 knockdown (Fig.
2G). These findings collectively demonstrated that FOXJ3
knockdown may impair the osteogenic differentiation potential in
BMSCs.
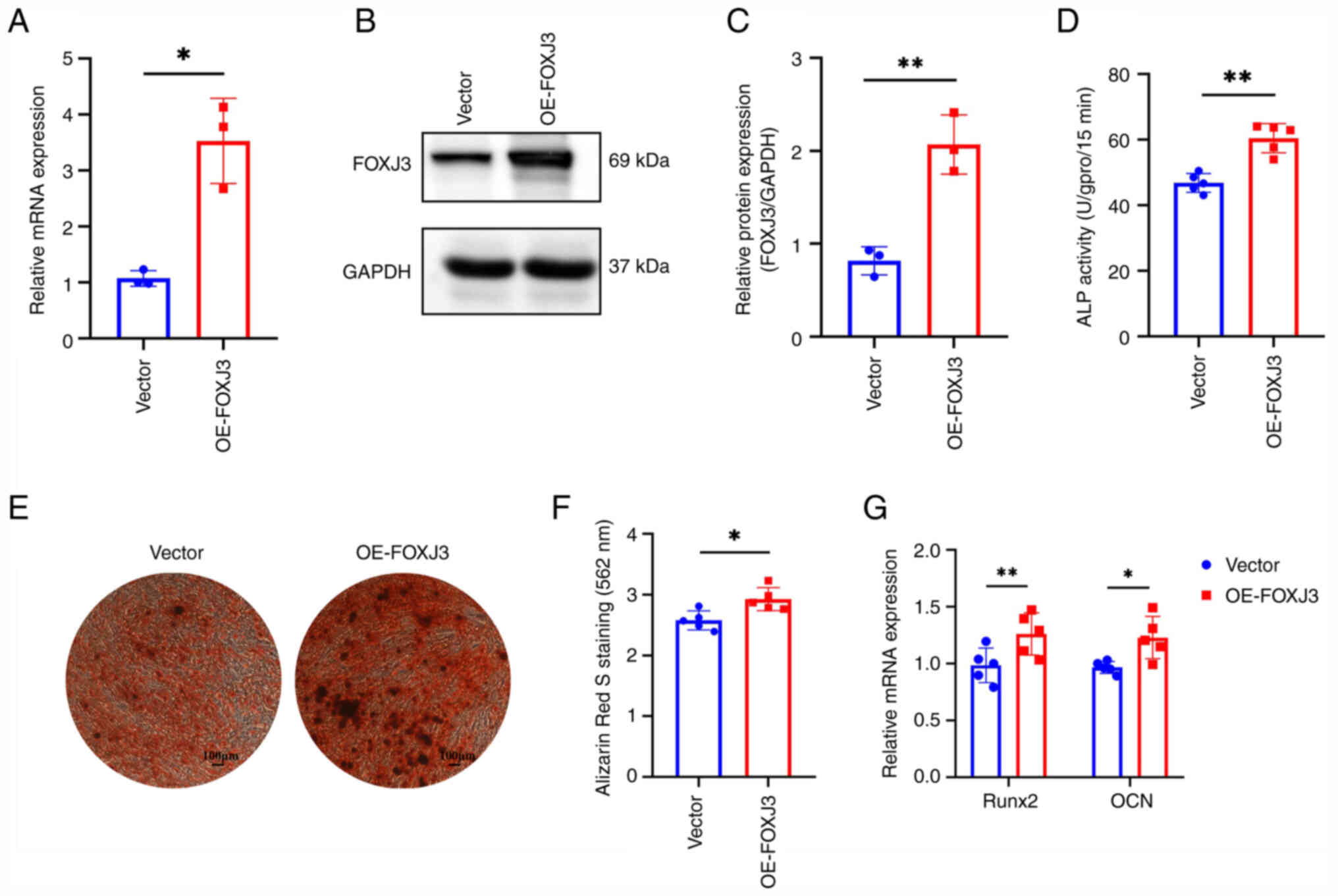
In vitro overexpression of FOXJ3
promotes BMSCs osteogenic differentiation
To further elucidate the regulatory role of FOXJ3 in
osteogenic differentiation, lentiviral infection was used to
overexpress FOXJ3 in BMSCs. The results of RT-qPCR showed that the
expression levels of FOXJ3 in BMSCs were significantly increased
after lentiviral infection (Fig.
3A). Western blotting also revealed that FOXJ3 protein
expression was elevated in the OE-FOXJ3 group compared with that in
the control group (Fig. 3B).
Protein semi-quantification demonstrated a ~2-fold increase in
protein expression in the OE-FOXJ3 group relative to the control
group (Fig. 2C). Following FOXJ3
OE, osteogenic differentiation was further induced in BMSCs.
Quantitative detection of ALP revealed that ALP activity in the
OE-FOXJ3 group was significantly increased compared with that in
the control group (Fig. 3D). ARS
staining results demonstrated a marked increase in osteogenic
nodules within the OE-FOXJ3 group relative to the control group
(Fig. 3E), and quantitative
analysis of ARS staining further confirmed that FOXJ3
overexpression enhanced osteogenic differentiation of BMSCs
(Fig. 3F). Additionally, RT-qPCR
revealed that the expression levels of the osteogenic
differentiation-related genes RUNX2 and OCN were upregulated
following FOXJ3 overexpression (Fig.
3G). These findings collectively demonstrated that FOXJ3
gain-of-function may promote osteogenic differentiation in
BMSCs.
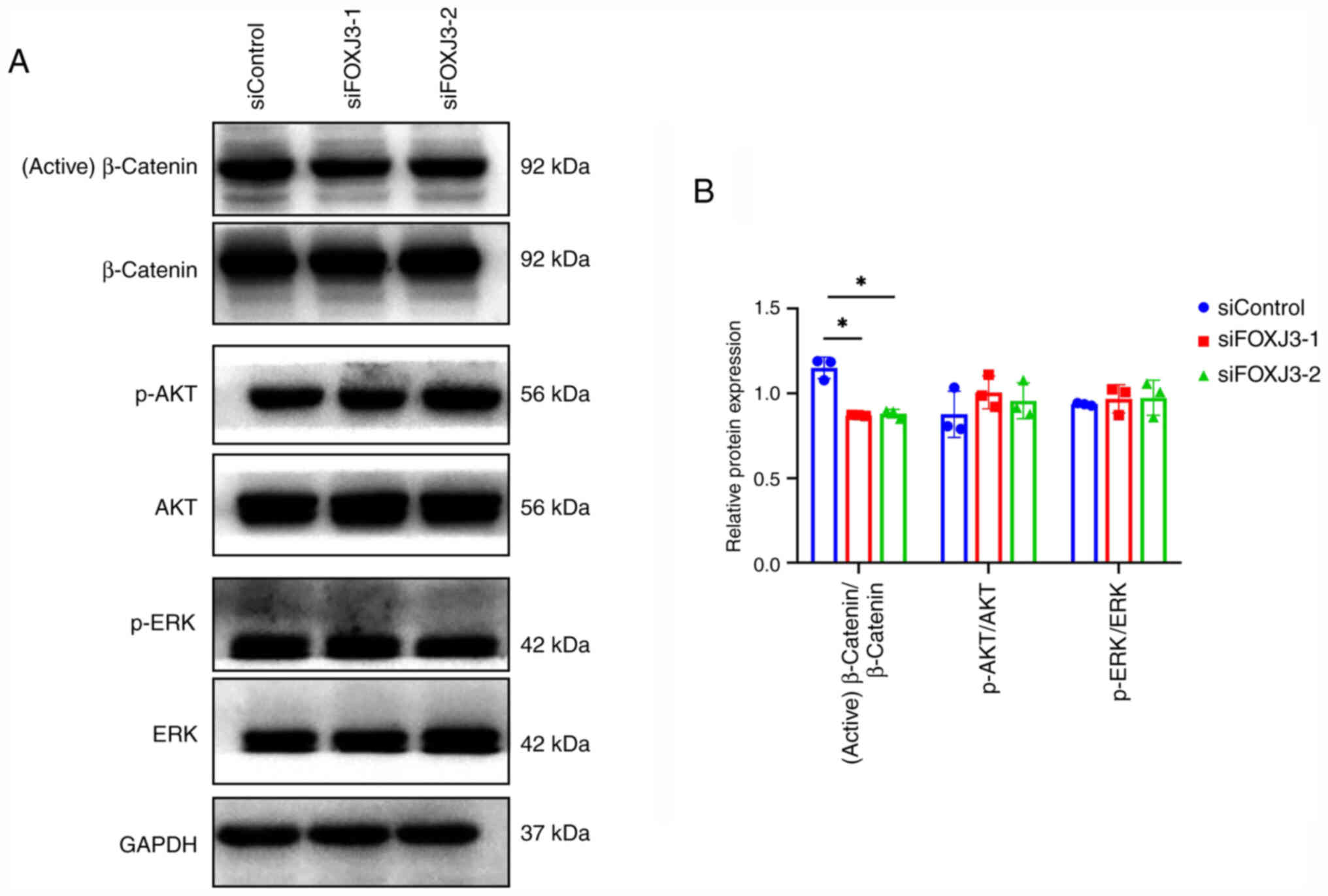
FOXJ3 regulates the Wnt/β-catenin
pathway
To investigate the mechanism by which FOXJ3
regulates BMSC osteogenic differentiation, osteogenic
differentiation was induced after knocking down FOXJ3 expression,
and the expression levels of proteins in common osteogenic
differentiation pathways, including the Wnt/β-catenin, PI3K/AKT and
MAPK/ERK pathways, were examined via western blotting. The results
revealed that the expression levels of active β-catenin were
decreased in the siFOXJ3-1 and siFOXJ3-2 groups compared with those
in the control group, whereas p-AKT and p-ERK expression showed no
significant differences (Fig. 4A and
B). These results indicated that FOXJ3 may primarily promote
BMSCs osteogenic differentiation by regulating the Wnt/β-catenin
pathway.
FOXJ3 modulates the osteogenic
differentiation of BMSCs through the Wnt/β-catenin pathway
To further elucidate the role of the Wnt/β-catenin
pathway in FOXJ3-mediated regulation of BMSCs osteogenic
differentiation, rescue experiments were performed using
pathway-specific inhibitors or agonists. Western blotting initially
confirmed alterations in the expression levels of proteins
associated with the Wnt/β-catenin pathway following combined FOXJ3
knockdown and treatment with SB216763, a Wnt/β-catenin pathway
agonist. The results showed that the expression of active β-catenin
in the siFOXJ3 group was decreased compared with that in the
siControl group; however, after the addition of the pathway agonist
SB216763, levels of active β-catenin were increased to levels
comparable with the control (Fig. S1A
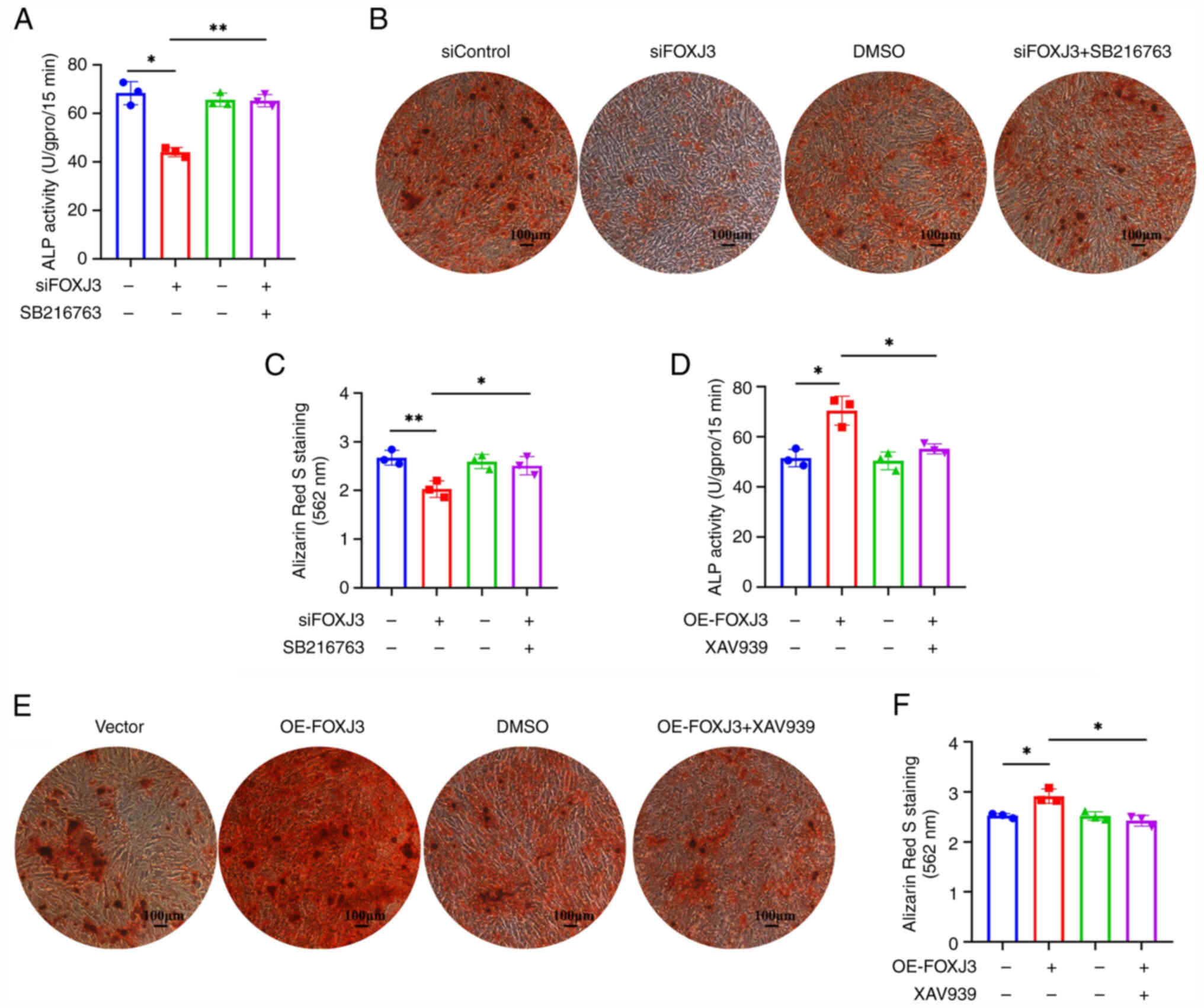
and B). Quantitative ALP analysis revealed that ALP activity
was reduced in the siFOXJ3 group compared with that in the
siControl group, whereas the addition of the pathway agonist
SB216763 significantly enhanced ALP activity (Fig. 5A). Furthermore, ARS staining and
quantification demonstrated fewer osteogenic nodules in the siFOXJ3
group compared with that in the siControl group, whereas the
addition of the pathway agonist SB216763 restored the osteogenic
differentiation capacity of BMSCs (Fig. 5B and C).
Furthermore, after overexpressing FOXJ3, the
findings were further validated using the Wnt/β-catenin pathway
inhibitor XAV939. Western blotting demonstrated that active
β-catenin expression was elevated in the OE-FOXJ3 group compared
with that in the vector group; however, this enhancement was
reversed following treatment with the pathway inhibitor XAV939,
restoring active β-catenin expression to control levels (Fig. S1C and D). ALP activity was
significantly enhanced in the OE-FOXJ3 group relative to the vector
group, whereas this effect was attenuated upon pathway inhibitor
treatment (Fig. 5D). ARS staining
and quantification showed increased osteogenic nodule formation in
the OE-FOXJ3 group compared with that in the vector group, whereas
this pro-osteogenic effect was abolished in the OE-FOXJ3 + XAV939
group, with nodule formation returning to baseline control levels
(Fig. 5E and F). Therefore, these
results indicated that FOXJ3 could regulate the osteogenic
differentiation of BMSCs in a Wnt/β-catenin pathway-dependent
manner.
Discussion
The present study provided compelling evidence
establishing the transcription factor FOXJ3 as a novel and
important positive regulator of osteogenic differentiation in BMSCs
and identified its crucial dependence on the Wnt/β-catenin
signaling pathway. The findings suggested the potential role of
FOXJ3 in in the development of osteoporosis, offering a promising
novel target for therapeutic intervention.
The pivotal role of BMSCs in maintaining bone
homeostasis and their dysfunction in osteoporosis is
well-established (25). As
multipotent progenitors residing in the bone marrow, BMSCs possess
the capacity to differentiate into osteoblasts, the bone-forming
cells essential for skeletal integrity and repair (26,27).
In osteoporosis, an age-related imbalance occurs where the
commitment of BMSCs shifts from osteogenesis towards adipogenesis,
coupled with a general decline in their osteogenic potential and
proliferative capacity (28).
Previous studies have indicated that numerous transcription factors
(including RUNX2 and Osterix/SP7) are master regulators of
osteogenesis (29,30). FOXJ3, a member of the FOX family of
transcription factors, which are characterized by a conserved
winged-helix DNA-binding domain, represents a hitherto unrecognized
regulator of cell differentiation (13). Although FOXJ3 has been implicated
in other biological processes, such as spermatogenesis and cellular
stress responses (12), its
specific functions in bone metabolism and BMSC biology have remain
unexplored. Notably, some other FOX members, such as FOXO1, have
been implicated in the oxidative stress response in bone, and FOXC2
has been shown to be involved in BMP2 signaling (31), thus indicating that FOXJ3 may also
be involved in osteogenesis.
The present study first revealed that FOXJ3 was
upregulated during in vitro osteogenic differentiation, and
further results indicated that a positive association existed
between FOXJ3 and osteogenic differentiation of BMSCs. Furthermore,
the siRNA-mediated knockdown of FOXJ3 resulted in a marked
suppression of the osteogenic potential of BMSCs. Conversely,
lentiviral overexpression of FOXJ3 robustly enhanced osteogenesis.
These findings are important in identifying FOXJ3 as a novel
modulator of BMSCs osteogenesis. While previous studies have
explored factors such as microRNAs (32), long non-coding RNAs (33) and epigenetic regulators (34) in BMSC osteogenesis, the
identification of the role of a transcription factor such as FOXJ3
may provide a novel mechanism and potential target. The present
results demonstrated that manipulating FOXJ3 levels alone was
sufficient to markedly alter the osteogenic differentiation
trajectory of BMSCs, highlighting its potency as a regulator.
FOXJ3, alongside other identified positive regulators of BMSCs
osteogenesis, such as specific isoforms of Dlk1, may expand the
known factors that potentially manipulate bone formation (35). Moreover, investigating FOXJ3
expression in well-characterized human osteoporosis cohorts,
particularly its association with bone mineral density, fracture
history or response to existing therapies, represents a critical
next step to validate its clinical relevance. Such studies could
further establish FOXJ3 as a potential diagnostic biomarker or
therapeutic target in osteoporosis.
The canonical Wnt pathway is a well-established and
powerful promoter of osteoblast differentiation and bone formation
(36). Here, FOXJ3 knockdown
specifically reduced the levels of active (non-phosphorylated)
β-catenin, while leaving the PI3K/AKT and MAPK/ERK pathways
unaffected. The selective impact of FOXJ3 on Wnt/β-catenin
signaling suggests a focused regulatory mechanism. This finding is
consistent with the results of previous studies emphasizing the
critical role of precise Wnt pathway modulation in bone anabolism
and its therapeutic exploitation (37,38).
For example, romosozumab, an anti-sclerostin antibody that enhances
Wnt signaling, has been shown to exert notable efficacy in treating
patients with osteoporosis (39).
The finding that FOXJ3 acts upstream of β-catenin activation adds a
novel layer to this complex regulatory network. Previous studies
have suggested that FOXJ3 can act as a recruited transcription
factor to promote osteoclast formation (40,41).
If both osteoclasts and osteoblasts exist in vivo, FOXJ3 may
have regulatory effects on both types of cells. Whether it promotes
or inhibits osteoporosis depends on whether its effect on bone
formation is greater than that on bone resorption. This not only
involves the quantity of osteoblasts and osteoclasts, but also the
activity of the cells and their proportion of their roles in bone
formation. The present study lacks animal experiments; therefore,
whether FOXJ3 will aggravate osteoporosis remains unknown. To
assess this, research using high-quality tools, such as gene
knockout mice, is needed.
The rescue experiments in the present study
demonstrated the pathway dependence and enhance the impact of the
study. The use of the specific Wnt/β-catenin agonist SB216763
effectively reversed the inhibitory effects of FOXJ3 knockdown on
β-catenin activation, ALP activity and mineralization. Conversely,
the pro-osteogenic effects of FOXJ3 overexpression were negated by
the Wnt pathway inhibitor XAV939. These experiments indicated that
the ability of FOXJ3 to promote BMSC osteogenic differentiation
requires a functional Wnt/β-catenin pathway, thus integrating FOXJ3
into a well-characterized and therapeutically relevant signaling
axis. However, the exact molecular mechanism by which FOXJ3
regulates β-catenin activation remains to be fully determined,
which is a promising direction for future research.
Placing the current findings within the broader
context of osteoporosis research underscores their potential
importance. Osteoporosis therapies have traditionally focused on
anti-resorptive agents (such as bisphosphonates and denosumab)
(42), however, while they are
effective, these treatments primarily prevent bone loss rather than
robustly rebuild bone. The development of true bone-forming
(anabolic) agents, such as teriparatide [a parathyroid hormone
(PTH) analogue], abaloparatide (a PTH-associated protein analogue)
and the aforementioned romosozumab, represents a major advance
(43). However, limitations
remain, including cost, administration routes and potential side
effects (44). Identifying novel
upstream regulators such as FOXJ3, which positively drives
osteogenesis through a fundamental anabolic pathway
(Wnt/β-catenin), provides novel options for therapeutic
development. Strategies may involve small molecules or biologics
designed to enhance FOXJ3 expression or activity directly within
BMSCs or osteoprogenitors, or gene therapy approaches. This
approach aligns with the growing interest in stem cell-based
therapies and targeting stem cell dysfunction in age-associated
diseases such as osteoporosis (45–47).
Enhancing the intrinsic osteogenic potential of endogenous BMSCs
via FOXJ3 modulation could offer a powerful strategy for bone
regeneration. Moreover, future studies should include in
vivo models, such as FOXJ3-knockout mice or local injection of
FOXJ3-modulating vectors in osteoporotic animal models, to further
validate its role.
In conclusion, the present study advances the
understanding of the molecular control of BMSC osteogenic
differentiation and the pathogenesis of osteoporosis. Robust
mechanistic evidence was provided demonstrating that FOXJ3 exerts
its pro-osteogenic effects primarily, if not exclusively, through
the potent Wnt/β-catenin signaling pathway. This dependency was
conclusively proven through targeted pathway rescue experiments.
The integration of functional cellular assays and mechanistic
pathway analysis provided a strong foundation for considering FOXJ3
as a promising new molecular target for the development of novel
anabolic therapies aimed at restoring bone formation in
osteoporosis and other bone-deficit conditions. While the present
study provided strong evidence for the role of FOXJ3 in
vitro and its clinical association, certain limitations warrant
mention and guide future research. First, the findings were based
on in vitro models, which may not fully recapitulate the
complex bone microenvironment. Second, the precise molecular
mechanism by which FOXJ3 regulates β-catenin remains unclear.
Third, clinical patient-derived data, to assess the association
between FOXJ3 expression and osteoporosis severity or treatment
outcomes, were not included. Thus, future research focusing on
in vivo validation and detailed mechanistic assessment will
be crucial to fully realize the therapeutic potential of targeting
the FOXJ3-Wnt/β-catenin axis, and to confirm the role of FOXJ3 in
osteoporosis and its translational potential.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Zhuhai Xiangshan Talent
Project (grant no. 2021XSYC-01) and the Supporting Project of
Natural Science Foundation of China (grant no. PT8217140653).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
HX performed the cell experiments and wrote the
initial manuscript and submitted the paper for publication. JL
contributed to some cell experiments. WH conducted the statistical
analysis of the data. YQ conceived the study, supervised the
research and revised the manuscript. HX and JL confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jin J: Screening for osteoporosis to
prevent fractures. JAMA. 333:5472025. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Reid IR and Billington EO: Drug therapy
for osteoporosis in older adults. Lancet. 399:1080–1092. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang B, He W, Pei Z, Guo Q, Wang J, Sun
M, Yang X, Ariben J, Li S, Feng W, et al: Plasma proteins,
circulating metabolites mediate causal inference studies on the
effect of gut bacteria on the risk of osteoporosis development.
Ageing Res Rev. 101:1024792024. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yin JQ, Zhu J and Ankrum JA: Manufacturing
of primed mesenchymal stromal cells for therapy. Nat Biomed Eng.
3:90–104. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dalle Carbonare L, Cominacini M, Trabetti
E, Bombieri C, Pessoa J, Romanelli MG and Valenti MT: The bone
microenvironment: New insights into the role of stem cells and cell
communication in bone regeneration. Stem Cell Res Ther. 16:1692025.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang L, Yuan X, Song R, Yuan Z, Zhao Y
and Zhang Y: Engineered 3D mesenchymal stem cell aggregates with
multifunctional prowess for bone regeneration: Current status and
future prospects. J Adv Res. Apr 11–2025.doi:
10.1016/j.jare.2025.04.008 (Epub ahead of print).
|
|
7
|
Wu KC, Chang YH, Ding DC and Lin SZ:
Mesenchymal stromal cells for aging cartilage regeneration: A
review. Int J Mol Sci. 25:129112024. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Artamonov MY and Sokov EL: Intraosseous
delivery of mesenchymal stem cells for the treatment of bone and
hematological diseases. Curr Issues Mol Biol. 46:12672–12693. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ban JY, Park HJ, Kim SK, Kim JW, Lee YA,
Choi IA, Chung JH and Hong SJ: Association of forkhead box J3
(FOXJ3) polymorphisms with rheumatoid arthritis. Mol Med Rep.
8:1235–1241. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ni L, Xie H and Tan L: Multiple roles of
FOXJ3 in spermatogenesis: A lesson from Foxj3 conditional knockout
mouse models. Mol Reprod Deve. 83:1060–1069. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jin J, Zhou S, Li C, Xu R, Zu L, You J and
Zhang B: MiR-517a-3p accelerates lung cancer cell proliferation and
invasion through inhibiting FOXJ3 expression. Life Sci. 108:48–53.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Challagundla KB, Pathania AS, Chava H,
Kantem NM, Dronadula VM, Coulter DW and Clarke M: FOXJ3, a novel
tumor suppressor in neuroblastoma. Mol Ther Oncol. 33:2009142025.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang J, Zhang Y, Zhou X, Song J, Feng Y,
Qiu T, Sheng S, Zhang M, Zhang X, Hao J, et al: Foxj3 Regulates
thermogenesis of brown and beige fat via induction of PGC-1α.
Diabetes. 73:178–196. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yuan L, Jiang N, Li Y, Wang X and Wang W:
RGS1 Enhancer RNA promotes gene transcription by recruiting
transcription factor FOXJ3 and facilitates osteoclastogenesis
through PLC-IP3R-dependent Ca2+ response in rheumatoid arthritis.
Inflammation. 48:447–463. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ding Y and Chen Q: Wnt/beta-catenin
signaling pathway: An attractive potential therapeutic target in
osteosarcoma. Front Oncol. 14:14569592024. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hosseini A, Dhall A, Ikonen N, Sikora N,
Nguyen S, Shen Y, Amaral MLJ, Jiao A, Wallner F, Sergeev P, et al:
Perturbing LSD1 and WNT rewires transcription to synergistically
induce AML differentiation. Nature. 642:508–518. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Arya PN, Saranya I and Selvamurugan N:
Crosstalk between Wnt and bone morphogenetic protein signaling
during osteogenic differentiation. World J Stem Cells. 16:102–113.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Abhishek Shah A, Chand D, Ahamad S, Porwal
K, Chourasia MK, Mohanan K, Srivastava KR and Chattopadhyay N:
Therapeutic targeting of Wnt antagonists by small molecules for
treatment of osteoporosis. Biochem Pharmacol. 230:1165872024.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gong W, Li M, Zhao L, Wang P, Wang X, Wang
B, Liu X and Tu X: Sustained release of a highly specific GSK3β
inhibitor SB216763 in the PCL scaffold creates an osteogenic niche
for osteogenesis, anti-adipogenesis, and potential angiogenesis.
Front Bioeng Biotechnol. 11:12152332023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tanthaisong P, Imsoonthornruksa S,
Ngernsoungnern A, Ngernsoungnern P, Ketudat-Cairns M and Parnpai R:
Enhanced chondrogenic differentiation of human umbilical cord
Wharton's jelly derived mesenchymal stem cells by GSK-3 inhibitors.
PLoS One. 12:e01680592017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yao J, Wu X, Qiao X, Zhang D, Zhang L, Ma
JA, Cai X, Boström KI and Yao Y: Shifting osteogenesis in vascular
calcification. JCI Insight. 6:e1430232021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rong X, Kou Y, Zhang Y, Yang P, Tang R,
Liu H and Li M: ED-71 Prevents Glucocorticoid-induced osteoporosis
by regulating osteoblast differentiation via notch and
Wnt/β-catenin pathways. Drug Des Devel Ther. 16:3929–3946. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang N, Zhang X, Li L, Xu T, Li M, Zhao Q,
Yu J, Wang J and Liu Z: Ginsenoside Rc promotes bone formation in
Ovariectomy-induced osteoporosis in vivo and osteogenic
differentiation in vitro. Int J Mol Sci. 23:61872022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Abdelbaset S, Mohamed Sob MA, Mutawa G,
El-Dein MA and Abou-El-Naga AM: Therapeutic potential of different
injection methods for bone marrow mesenchymal stem cell
transplantation in Buslfan-induced male rat infertility. J Stem
Cells Regen Med. 20:26–46. 2024.PubMed/NCBI
|
|
26
|
Pajarinen J, Lin T, Gibon E, Kohno Y,
Maruyama M, Nathan K, Lu L, Yao Z and Goodman SB: Mesenchymal stem
cell-macrophage crosstalk and bone healing. Biomaterials.
196:80–89. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sun Y, Wan B, Wang R, Zhang B, Luo P, Wang
D, Nie JJ, Chen D and Wu X: Mechanical stimulation on mesenchymal
stem cells and surrounding microenvironments in bone regeneration:
Regulations and applications. Front Cell Dev Biol. 10:8083032022.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu Y, Xie L, Wang M, Xiong Q, Guo Y, Liang
Y, Li J, Sheng R, Deng P, Wang Y, et al: Mettl3-mediated m6A RNA
methylation regulates the fate of bone marrow mesenchymal stem
cells and osteoporosis. Nat Commun. 9:47722018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chan WCW, Tan Z, To MKT and Chan D:
Regulation and role of transcription factors in osteogenesis. Int J
Mol Sci. 22:54452021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Komori T: Regulation of skeletal
development and maintenance by Runx2 and Sp7. Int J Mol Sci.
25:101022024. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang W, Zhang X, Li J, Zheng J, Hu X, Xu
M, Mao X and Ling J: Foxc2 and BMP2 Induce Osteogenic/odontogenic
differentiation and mineralization of human stem cells from apical
papilla. Stem Cells Int. 2018:23639172018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hong L, Sun H and Amendt BA: MicroRNA
function in craniofacial bone formation, regeneration and repair.
Bone. 144:1157892021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jin C, Jia L, Huang Y, Zheng Y, Du N, Liu
Y and Zhou Y: Inhibition of lncRNA MIR31HG promotes osteogenic
differentiation of human Adipose-derived stem cells. Stem Cells.
34:2707–2720. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu S, Liu D, Chen C, Hamamura K,
Moshaverinia A, Yang R, Liu Y, Jin Y and Shi S: MSC Transplantation
improves osteopenia via epigenetic regulation of notch signaling in
lupus. Cell Metab. 22:606–618. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Paradise CR, Galvan ML, Kubrova E, Bowden
S, Liu E, Carstens MF, Thaler R, Stein GS, van Wijnen AJ and
Dudakovic A: The epigenetic reader Brd4 is required for osteoblast
differentiation. J Cell Physiol. 235:5293–5304. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hu L, Chen W, Qian A and Li YP:
Wnt/β-catenin signaling components and mechanisms in bone
formation, homeostasis, and disease. Bone Res. 12:392024.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Huybrechts Y, Mortier G, Boudin E and Van
Hul W: WNT signaling and bone: Lessons from skeletal dysplasias and
disorders. Front Endocrinol (Lausanne). 11:1652020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Maeda K, Kobayashi Y, Koide M, Uehara S,
Okamoto M, Ishihara A, Kayama T, Saito M and Marumo K: The
regulation of bone metabolism and disorders by Wnt signaling. Int J
Mol Sci. 20:55252019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wu D, Li L, Wen Z and Wang G: Romosozumab
in osteoporosis: Yesterday, today and tomorrow. J Transl Med.
21:6682023. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Alexander MS, Shi X, Voelker KA, Grange
RW, Garcia JA, Hammer RE and Garry DJ: Foxj3 transcriptionally
activates Mef2c and regulates adult skeletal muscle fiber type
identity. Dev Biol. 337:396–404. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chen X, Wang Z, Duan N, Zhu G, Schwarz EM
and Xie C: Osteoblast-osteoclast interactions. Connect Tissue Res.
59:99–107. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Langdahl BL: Overview of treatment
approaches to osteoporosis. Br J Pharmacol. 178:1891–1906. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Khosla S and Hofbauer LC: Osteoporosis
treatment: Recent developments and ongoing challenges. Lancet
Diabetes Endocrinol. 5:898–907. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Shimizu R, Sukegawa S, Sukegawa Y,
Hasegawa K, Ono S, Nakamura T, Fujimura A, Fujisawa A, Nakano K,
Takabatake K, et al: Incidence and risk of Anti-Resorptive
Agent-related osteonecrosis of the jaw after tooth extraction: A
retrospective study. Healthcare (Basel). 10:13322022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tong Y, Tu Y, Wang J, Liu X, Su Q, Wang Y
and Wang W: Mechanisms and therapeutic strategies linking
mesenchymal stem cells senescence to osteoporosis. Front Endocrinol
(Lausanne). 16:16258062025. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Li H and Bai L: Advances in mesenchymal
stem cell and Exosome-based therapies for aging and age-related
diseases. Stem Cell Res Ther. 16:4012025. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Liang B, Burley G, Lin S and Shi YC:
Osteoporosis pathogenesis and treatment: Existing and emerging
avenues. Cell Mol Biol Lett. 27:722022. View Article : Google Scholar : PubMed/NCBI
|