Inflammatory bowel disease (IBD) is a chronic and
recurrent inflammatory disorder of the gastrointestinal tract,
which mainly includes Crohn's disease (CD) and ulcerative colitis
(UC) (1–4). UC is an idiopathic chronic
inflammatory condition affecting the colonic mucosa, characterized
by alternating periods of flare-ups and remission. The disease
typically originates in the rectum and extends continuously
throughout the colon (5). Lesions
are predominantly confined to the colonic mucosa, with ulcers
manifesting as the primary symptom, alongside diarrhea, abdominal
pain and weight loss, among which bloody diarrhea is a hallmark of
UC (6). CD is a progressive
chronic inflammatory disease that can affect any part of the
gastrointestinal tract. The most commonly affected parts are the
terminal ileum and colon. In addition to symptoms such as diarrhea
and abdominal pain, complications such as stenosis, fistula or
abscesses may occur over time (7,8). The
incidence of IBD is higher in developed countries. According to
recent epidemiological evidence, ~3 million individuals in the USA
are affected by IBD (9). The
majority of patients experience symptom onset aged 20–39 years,
with a lower incidence rate observed in individuals aged >50
years (3). The pathogenesis of IBD
involves multifactorial interactions, including environmental
triggers, genetic susceptibility, immune dysregulation and gut
microbiota alterations. Available evidence suggests that abnormal
immune responses, including both innate and adaptive immune
response, are important causes of chronic intestinal inflammation
in patients with IBD (10,11). Chronic, persistent inflammation is
a hallmark of IBD and is associated with an increased risk of
intestinal malignancies (12,13).
Notably, liver X receptor (LXR), as a cholesterol sensor, has
recently been shown to mitigate inflammation-driven carcinogenesis
by restoring immune-metabolic balance (14). Long-term inflammation can induce
damage and dysregulated proliferation of colonic mucosal cells,
ultimately leading to the development of cancer, such as colorectal
cancer (CRC) and colitis-associated cancer (15,16).
Therefore, a major challenge in controlling IBD and colon cancer is
managing the occurrence and development of intestinal inflammation,
which may be an effective method to treat digestive system diseases
linked to intestinal inflammation.
LXR is a ligand-activated transcription factor
belonging to the nuclear hormone receptor family, comprising two
subtypes: LXRα and LXRβ (17).
LXRα is highly expressed in metabolically active tissues, including
the liver, small intestine, kidney, adipose tissue and macrophages,
while LXRβ is ubiquitously expressed throughout the body (17,18).
LXR has been identified to function as a heterodimer with retinoid
X receptor (RXR). The LXR/RXR heterodimer can be activated by
ligands or agonists of LXR or RXR individually, or by both
simultaneously, resulting in a synergistic effect (19,20).
The activation of LXR has been reported to regulate the expression
of a series of genes associated with cholesterol transport, glucose
metabolism and the modulation of inflammatory responses (21,22).
LXR regulates cholesterol metabolism by inducing the expression of
a series of target genes, including adenosine triphosphate
(ATP)-binding cassette transporter family members such as
ATP-binding cassette transporter A1 (ABCA1), ATP-binding cassette
subfamily G member (ABCG)1, ABCG5 and ABCG8, which promote the
absorption, transport and excretion of cholesterol in the intestine
(23,24). The LXR/RXR heterodimer has been
identified as a key regulator of ABCA1 expression in vivo
(25). In addition, the genes
involved in the LXR-induced regulation of the cholesterol
metabolism pathway include sterol regulatory element-binding
protein (SREBP)2, which can activate cholesterol biosynthesis and
uptake pathways at low cholesterol levels. These mechanisms work
together to maintain the balance of cholesterol in the body
(26–28). Although the most widely studied
function of LXR is to regulate cholesterol metabolism, previous
studies have shown that LXR is also involved in the regulation of
inflammatory responses and tumor progression, particularly in
enteritis and intestinal cancer (29,30).
Existing studies have shown that LXR can reduce inflammatory
response and intestinal mucosal injury by reducing inflammatory
cell infiltration, inhibiting the secretion of pro-inflammatory
cytokines and enhancing the integrity of the intestinal mucosal
barrier (14,31,32).
For example, LXR activation can reduce the release of inflammatory
mediators by promoting macrophage polarization to anti-inflammatory
phenotypes and can enhance the phagocytosis of macrophages toward
tumor-related inflammatory cells (33–36).
In addition, the activation of LXR can directly inhibit the
proliferation or promote the apoptosis of intestinal tumor cells by
regulating cholesterol metabolism or related genes, thus inhibiting
tumor growth (37–39). The present review summarizes the
pleiotropic effects of LXR on IBD and CRC pathogenesis, focusing on
three interconnected axes: i) Intestinal barrier preservation; ii)
immune cell reprogramming; and iii) metabolic regulation.
Therapeutic opportunities and challenges in targeting LXR signaling
are further discussed.
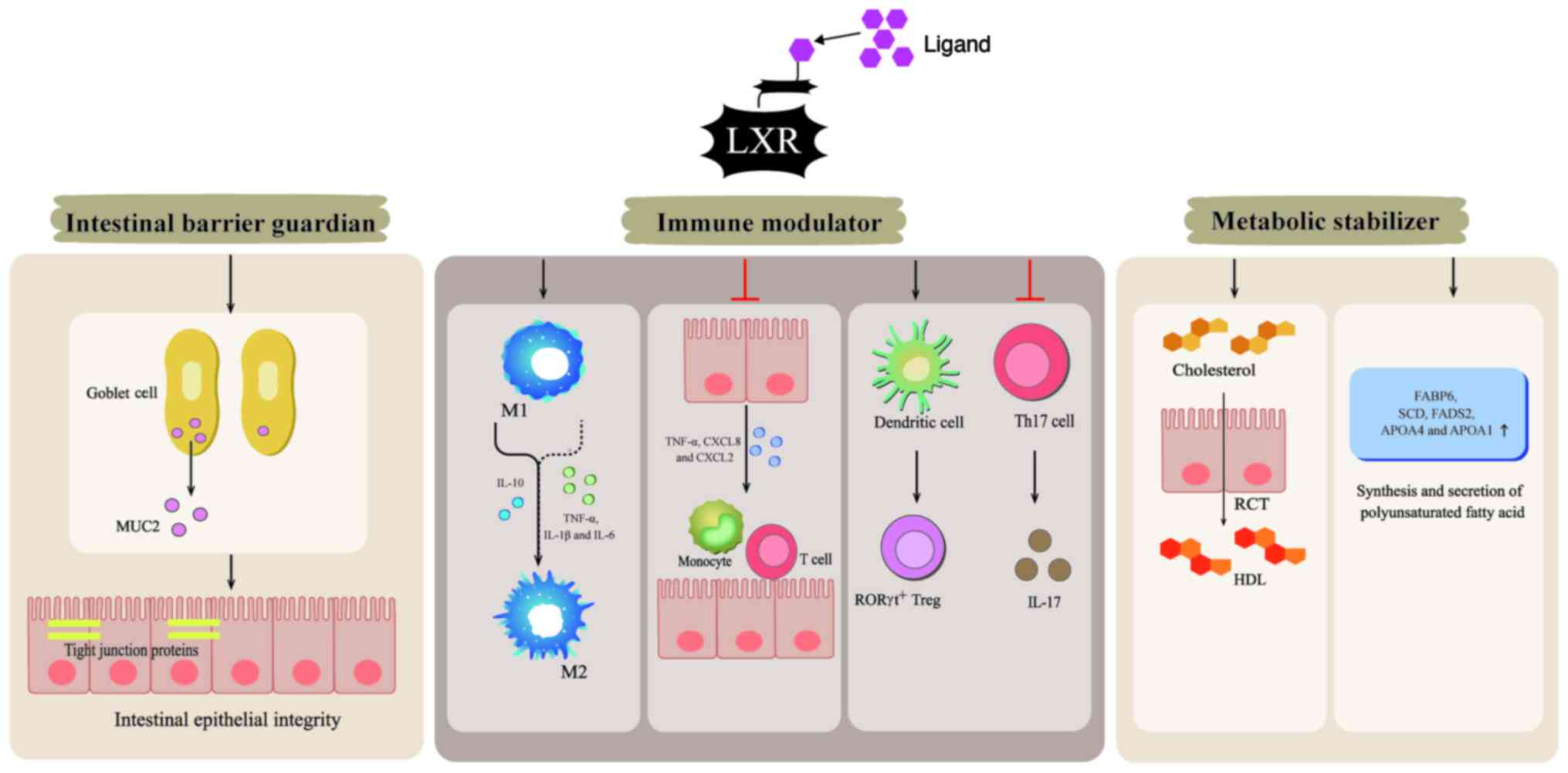
As a nuclear receptor superfamily member, LXR has
emerged as a central regulator of intestinal homeostasis through
its dual roles in metabolic regulation and immune modulation
(29,40). LXR maintains mucosal integrity
through dual mechanisms: The intestinal barrier serves as the first
line of defense in maintaining intestinal homeostasis and it is
mainly composed of the mucus layer on the surface of intestinal
epithelial cells, which is formed by the synthesis and secretion of
large amounts of mucin 2 (MUC2) by goblet cells. MUC2 serves a key
role in protecting the intestinal tract from harmful substances and
numerous microorganisms. By contrast, defects in the synthesis and
secretion of MUC2, as well as alterations in its glycosylation
structure, may lead to intestinal diseases (41–43).
Furthermore, tight junction proteins in intestinal epithelial
cells, such as tight junction protein 1 and claudins, are an
important part of the intestinal barrier (44,45).
Under physiological conditions, LXR, with its function primarily
mediated by LXRβ, maintains MUC2 secretion from goblet cells and
sustains the expression of tight junction proteins (46).
The activation of LXR has emerged as a potent
protective mechanism in intestinal inflammatory diseases. It is
worth noting that the distinct LXR subtypes have different roles:
LXRα primarily governs immune responses in myeloid immune cells,
such as macrophages and dendritic cells (DCs), whereas LXRβ
orchestrates immune responses within the intestinal epithelium
(47). Among them, LXRα serves a
notable role in modulating macrophage polarization, shifting their
phenotype from pro-inflammatory M1 to anti-inflammatory M2
subtypes, which is important for resolving intestinal inflammation
and maintaining immune homeostasis (29,48).
LXRβ effectively mediates the initiation and progression of
inflammatory responses by suppressing the transcription of
inflammatory factors and chemokines in intestinal epithelial cells,
thereby reducing immune cell infiltration (47). Furthermore, an imbalance between T
helper 17 (Th17) and regulatory T (Treg) cells is an important
factor in the pathogenesis of autoimmune diseases, such as
rheumatoid arthritis, systemic lupus erythematosus and IBD
(49,50). The activation of LXRα and LXRβ
alleviates the occurrence and progression of intestinal
inflammatory diseases by regulating the balance of Th17/Treg cells
in the intestine through distinct mechanisms. The excessive
activation of Th17 cells or excessive immunosuppressive activity
induced by Treg cells may contribute to the development of cancer,
such as colorectal, breast and ovarian cancer. Therefore, the
activation of LXR is important for regulating the balance of
Th17/Treg cells, and inhibiting tumor immune escape and growth
(51).
LXR, which acts as a cholesterol sensor, has been
reported to regulate cholesterol transport to the liver and its
excretion as bile acids, so as to maintain cholesterol homeostasis.
In particular, LXR is involved in reverse cholesterol transport
(RCT), which refers to the transport of cholesterol from peripheral
tissues to the liver, where it is excreted in the form of bile acid
(52–54). In the intestine, LXRα is
predominantly expressed in fully differentiated cells of the
colonic epithelium and ileal villi, mediating the reverse excretion
of cholesterol, while LXRβ is typically expressed in the intestinal
mucosal epithelium, promoting cholesterol absorption. The relative
distribution and interaction of these two subtypes jointly regulate
the absorption and excretion of intestinal cholesterol (55).
Furthermore, the activation of LXR, particularly
LXRα, promotes fatty acid desaturation and storage by upregulating
fatty acid-binding protein 6, stearoyl-CoA desaturase (SCD) and
fatty acid desaturase 2, thereby facilitating dynamic lipid
buffering within intracellular lipid droplets. Complementing these
processes, LXR stimulates the synthesis and secretion of
apolipoprotein (APO)A4 and APOA1, which are important for
chylomicron assembly and basolateral trafficking of dietary lipids
(56–58). These synergistic mechanisms
maintain the homeostatic equilibrium of intestinal lipid
absorption, metabolism and excretion. Therefore, the activation of
LXR can regulate intestinal cholesterol homeostasis, thus
preventing the occurrence of metabolic diseases. Notably, the
intestinal vs. hepatic tissue-specific effects of LXR agonists
warrant further investigation, as systemic activation of LXR may
lead to hepatic steatosis, a key challenge for therapeutic
development (30).
Briefly, the activation of LXR inhibits intestinal
inflammation and tumorigenesis by enhancing intestinal barrier
function and immune regulation, as well as regulating cholesterol
metabolism (Fig. 1).
In the pathological process of IBD, as a nuclear
receptor and transcriptional regulator, LXR ameliorates colonic
pathology and intestinal inflammation by maintaining the intestinal
barrier function, inhibiting the release of inflammatory mediators
and modulating immune cell function. In terms of CRC, LXR
activation in tumor cells is responsible for apoptosis or
pyroptosis by blocking the cell cycle and regulating cholesterol
metabolism, thus inhibiting tumor cell proliferation. In addition,
the activation of LXR within the tumor microenvironment (TME) also
inhibits tumor progression by fostering a robust antitumor immune
response. The role of LXR in IBD and CRC pathogenesis is summarized
as follows.
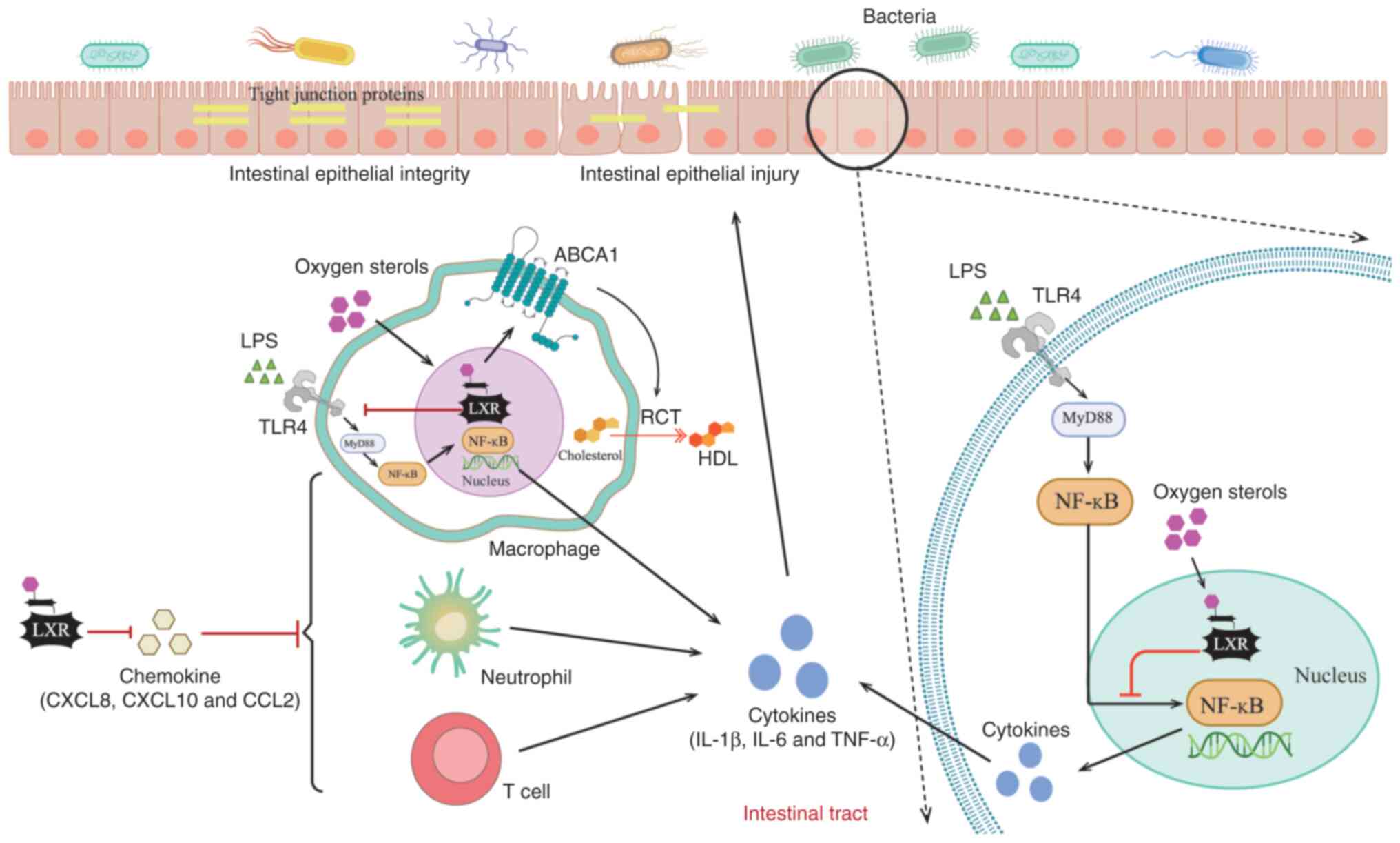
Various immune factors contribute to the development
of IBD. These include immune cells, such as macrophages,
neutrophils and lymphocytes, as well as the inflammatory cytokines
and chemokines they produce (47,59–61).
Furthermore, epithelial cells can secrete a variety of cytokines
and chemokines, which promote the migration and infiltration of
other immune cells, thus aggravating the inflammatory response
(47,59,62).
Current evidence has indicated that the activation of LXR can
inhibit the inflammatory response by regulating the differentiation
and infiltration of lymphocytes. LXRβ activation suppresses
pro-inflammatory cytokine release and CD4+ T-cell
infiltration, thereby preventing aberrant Th17 cell
differentiation. In parallel, LXRα activation mitigates
inflammation by driving DC-dependent Treg cell differentiation.
Together, these mechanisms reduce the production of inflammatory
mediators, such as TNF-α (46,47,63–66).
Previous studies in mice with colitis have revealed that impaired
oxysterol-LXR signaling disrupts the Th17/Treg cell balance through
increased Th17 polarization and diminished Treg cell populations.
This imbalance is associated with elevated levels of
pro-inflammatory cytokines, such as IL-17 and IL-23 (44,64,67).
However, specific drug treatments, such as Si-Ni-San, can target
oxysterol/LXR signaling, which helps to restore the balance of
Th17/Treg cells, thus protecting the body from intestinal diseases
caused by inflammatory response (44).
In addition, experimental models have shown that LXR
deficiency exacerbates colitis by increasing the infiltration of
immune cells and the release of inflammatory mediators into the
colon (46,47). The activation of LXR, primarily of
the isoform LXRα, reduces the accumulation of immune cells at
inflammatory sites and inhibits the release of pro-inflammatory
mediators, including IL-17, TNF-α, IL-1β, C-X-C motif chemokine
(CXCL)8, CXCL10 and C-C motif chemokine (CCL)2, thus alleviating
intestinal inflammation and injury (68–70).
Additionally, the activation of LXR attenuates the recruitment of
MyD88, an innate immune signal transduction adaptor, and TNF
receptor associated factor 6 via ABCA1-dependent changes in
membrane lipid tissue; furthermore, LXR activation inhibits the
Toll-like receptor (TLR)2, TLR4 and TLR9 signaling pathways,
particularly TLR4 signaling and the NF-κB axis, and reduces the
release of pro-inflammatory factors, such as TNF-α and IL-1β
(32,71). These anti-inflammatory effects
following LXR activation are observed not only in immune cells,
such as macrophages and DCs, but also in intestinal epithelial
cells (71–74).
The anti-inflammatory effect of epithelial LXR
activation is mediated through two primary mechanisms. Primarily,
in response to high intracellular cholesterol, LXRα activation in
intestinal epithelial cells orchestrates cholesterol homeostasis by
upregulating efflux transporters, such as ABCA1 and ABCG1, and
inhibiting the uptake mediator NPC1-like intracellular cholesterol
transporter 1 (75). This promotes
RCT and high-density lipoprotein synthesis, thereby preventing
excessive accumulation of cholesterol in enterocytes and exerting
an anti-inflammatory effect (56,76–79).
It has been shown that the activation of LXR can regulate lipid
metabolism by increasing the expression of ABCA1 and promoting the
outflow of cholesterol (75,80),
thus inhibiting the NF-κB signaling pathway and the release of
pro-inflammatory factors (72,76,81,82).
Similarly, LXRβ expression in colonic epithelial cells exerts
anti-inflammatory effects. The activation of LXRβ markedly
suppresses the expression of inflammatory and chemotactic factors,
such as TNF-α, CXCL8 and CXCL2, thereby reducing the recruitment of
immune cells, including macrophages and T cells, and ultimately
curbing the propagation of inflammation (47).
Intestinal barrier homeostasis is important for
preventing IBD. Compromise of the mucosal barrier can initiate IBD
and even the development of CRC. Both LXRα and LXRβ are expressed
in colonic epithelial cells and work cooperatively to maintain
intestinal barrier integrity (46,72).
A study has shown that LXRβ deletion leads to the development of
higher severity colitis than LXRα deletion (47). Further mechanistic analysis has
suggested that LXRβ may directly regulate goblet cell
differentiation and sustain MUC2 expression, whereas loss of LXRβ
can lead to reduced goblet cell numbers and disruption of the mucus
layer, thereby impairing the intestinal mucosal barrier (46). Notably, LXRα/β double knockout mice
have been shown to exhibit a loss of estrogen receptor β (ERβ),
which leads to the dysfunction of goblet cell secretion and the
notable downregulation of the hemidesmosomal protein plectin,
resulting in mucosal damage and the deterioration of epithelial
cell connections, suggesting that LXRα indirectly regulates
epithelial structure integrity through ERβ expression (46,47).
These findings in LXR-deficient mice suggest that LXR contributes
to intestinal barrier integrity not only in its activated state but
also through basal expression in the absence of ligand binding.
Nonetheless, the specific regulatory mechanisms of LXR isoforms in
intestinal barrier function remain to be fully elucidated. Further
investigations using subtype-selective genetic knockout models are
required to elucidate their distinct roles in cellular
differentiation.
LXR also serves as an important regulator of
intestinal stem cell proliferation, differentiation dynamics and
epithelial repair processes (30).
In dextran sulfate sodium-induced colitis models, treatment with
the LXR agonist GW3965 hydrochloride was shown to promote the
proliferation of crypt cells, rather than directly mitigating
damage. Upon intestinal injury, LXR activation enhances epithelial
regeneration and tissue repair through modulation of cholesterol
homeostasis and induction of amphiregulin expression (30,83,84).
Although these findings highlight the regenerative role of LXR, the
precise molecular mechanisms governing LXR-mediated intestinal
regeneration require further elucidation. Additionally, one study
demonstrated that LXR agonists such as T0901317 promote fecal
cholesterol excretion by promoting RCT and intestinal cholesterol
excretion in diabetic and obese mice, thus preventing excessive
accumulation of cholesterol in intestinal epithelial cells
(24).
Briefly, LXR serves an important role in regulating
intestinal inflammation by maintaining the integrity of the
intestinal barrier, regulating the release of chemokines and
mediating the function of immune cells. Notably, chronic
inflammation in IBD establishes a tumor-promoting microenvironment
through sustained cytokine signaling and oxidative stress (85,86).
Given the anti-inflammatory function of LXR, it is plausible that
LXR activation may attenuate chronic inflammation-driven
tumorigenesis during IBD progression. Therefore, LXR is not only a
key molecule in the development of human intestinal inflammation
and experimental colitis, but also a potential target for the
treatment of intestinal cancer (Fig.
2).
CRC is a prevalent malignancy of the
gastrointestinal tract, which mainly occurs in the colon (87,88).
At present, CRC is one of the most common types of cancer worldwide
and the second leading cause of cancer-related mortality (89,90).
The incidence and mortality of CRC vary according to ethnicity and
its incidence is usually closely associated with diet, such as a
high consumption of red meat and processed meat, and lifestyle,
including a lack of physical activity, smoking, obesity and heavy
drinking (91–93). In previous years, the overall
incidence of CRC, particularly rectal cancer and distal colon
cancer, has decreased in individuals aged >50 years old, but
increased in those aged <50 years old (94). Notably, surgery, radiotherapy,
chemotherapy and other cancer treatment methods cannot cure
advanced or metastatic CRC, thus identifying a target for the
treatment of colon cancer is important (90,95).
Recent research has demonstrated that LXR activation within tumor
cells and the TME controls CRC progression, indicating that
elucidating the mechanisms of LXR activity could offer promising
new targets for clinical therapy (96,97).
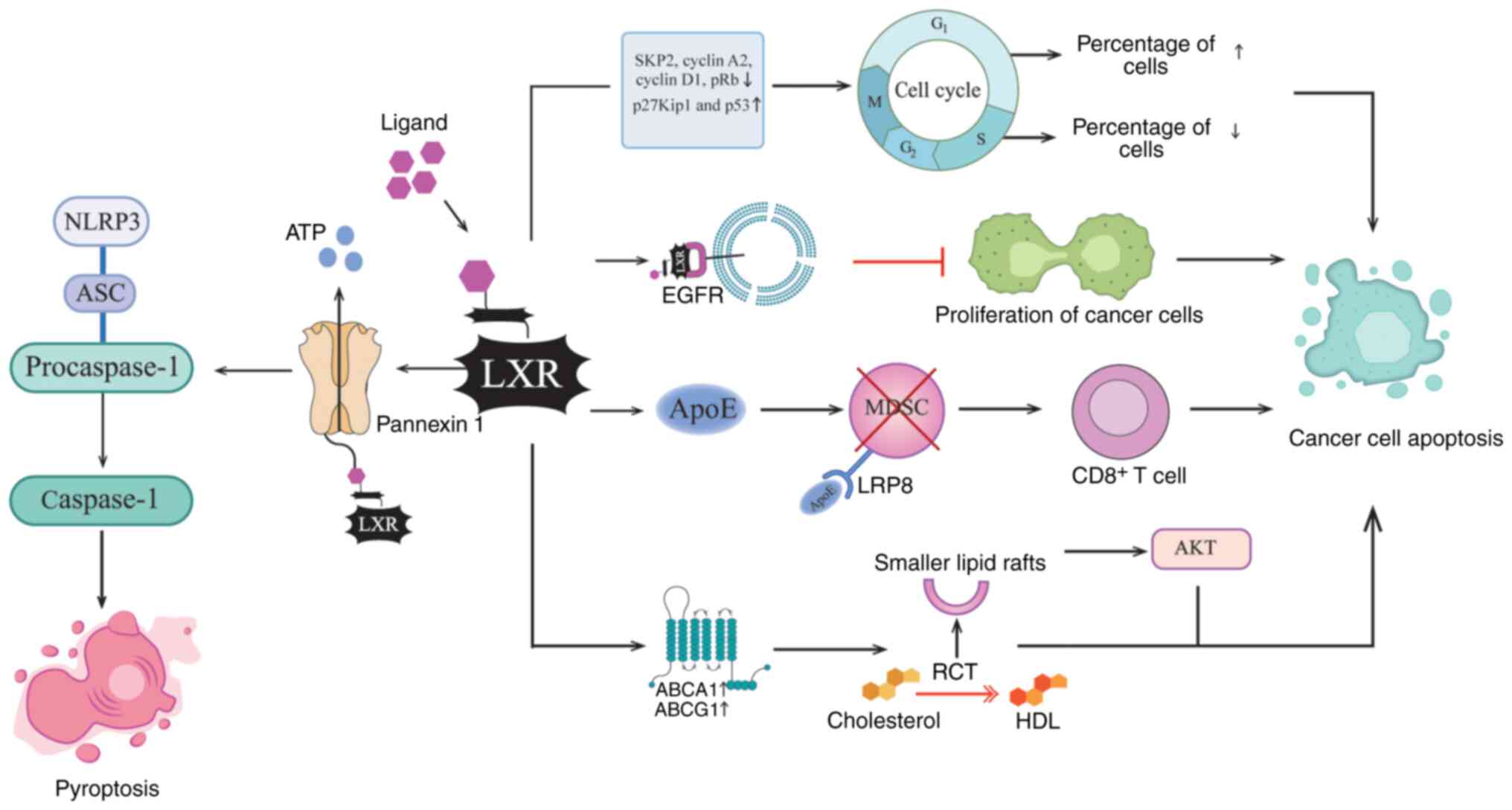
Studies have shown that the occurrence of intestinal
tumors is associated with the loss of control of cell proliferation
and with cholesterol metabolism disorders (98–100). During cell proliferation, the
expression of LXRα and LXRβ is upregulated. Activation of LXR
downregulates key cell cycle promoters including S-phase
kinase-related protein 2, cyclin A2 and cyclin D1, while
upregulating cell cycle inhibitors p27Kip1 and p53. These changes
lead to G1 phase cell cycle arrest and inhibit cell
cycle progression in cancer cells (81,97,101). The antitumor effect of LXR is
also dependent on inducing apoptosis by activating the caspase-3
pathway (102). A study has shown
that ergosterol, as an agonist of both LXR subtypes, can induce the
apoptosis of CRC cells by upregulating pro-apoptotic proteins Bax
and cleaved caspase-3, downregulating the anti-apoptotic protein
Bcl-2, and synergistically inhibiting pathways such as the PI3K/AKT
and Ras/MAPK signaling pathways (97). In addition, the homeostatic
regulation of cholesterol mediated by LXR demonstrates potent
anti-neoplastic activity by inhibiting the growth of colon cancer
and other tumors, as research has found that the activation of LXR
can inhibit the survival of cancer cells by regulating
intracellular cholesterol levels (103–107). Specifically, the activation of
LXR, predominantly LXRα, mediates the upregulation of the ABCA1 and
ABCG1 genes, which stimulates the reverse transport and efflux of
cholesterol and damages the structure of lipid rafts, making them
smaller and thinner. The decrease of the plasma membrane
cholesterol steady-state level leads to the downregulation of AKT
phosphorylation in the lipid layer and promotes cancer cell
apoptosis (104,108).
Notably, LXR activation induces not only apoptosis
but also pyroptosis in cancer cells. Specifically, in colon cancer,
LXR promotes caspase-1 activation to trigger pyroptosis (107). The primary mechanism involves
LXRβ activation, which facilitates the opening of the pannexin 1
channel on the cell membrane, leading to ATP release. Extracellular
ATP activates P2X purinoceptor 7, thereby inducing the assembly of
the NOD-like-receptor pyrin domain containing 3 (NLRP3)
inflammasome and subsequent caspase-1 activation. This process
ultimately results in pyroptosis of colon cancer cells (109,110). This mechanism establishes NLRP3
inflammasome activation as a novel LXR-dependent antitumor
modality, which has important clinical relevance for targeting LXR
in colon cancer therapies.
Furthermore, studies have revealed that LXR
activation can influence tumor progression by modulating antitumor
immunity within the TME. During the development of malignant
tumors, immunosuppressive cells such as myeloid-derived suppressor
cells (MDSCs) expand and inhibit the antitumor immune response,
thereby promoting tumor cell proliferation and metastasis (111–114). Studies have revealed that the
LXRβ-selective agonist abequolixron (RGX-104) inhibits tumor growth
by reducing the survival of immunosuppressive cells within the TME.
Specifically, LXRβ activation upregulates the transcription of
ApoE, which binds to LDL receptor-related protein 8 (LRP8) on
MDSCs. This interaction impairs MDSC survival, enhances
CD8+ T-cell activation and potentiates antitumor
immunity in vivo (18,111,115–117). Furthermore, in zearalenone
(Zen)-induced Sprague-Dawley rats, intestinal injury,
immunosuppression and dysregulated lipid metabolism have been
observed. Zen can markedly reduce the level of the LXR endogenous
agonist 27-hydroxycholesterol, downregulate LXRα and LXRβ, and
inhibit the expression of ApoE (118). ApoE deficiency promotes the
proliferation of intestinal MDSCs and inhibits T-cell function,
thus increasing the risk of immunosuppressive TME formation
(18,118,119). Notably, administration of either
LXR or ApoE agonists can reverse T-cell suppression, restoring the
core activity of this pathway in preventing tumorigenesis (118).
LXRα, as a transcription factor, can regulate the
expression of several cancer-related genes (120,121). Epidermal growth factor receptor
(EGFR) is highly expressed in several types of cancer, and is
closely associated with the proliferation, invasion and metastasis
of tumors (122–124). Previous studies have shown that
LXRα can directly bind to the promoter region of EGFR and inhibit
its transcription, thus blocking the downstream signaling pathway
mediated by EGFR and inhibiting the proliferation of CRC cells
(122,125). This transcriptional regulation
mechanism unveils an epigenetic dimension of LXR-mediated tumor
suppression, providing a possible novel target for the treatment of
CRC. In addition, emerging research has revealed a
context-dependent duality of LXR signaling in colorectal
carcinogenesis. A recent study demonstrated that ATPase
H+ transporting V0 subunit A1 (ATP6VOA1), an intrinsic
regulator in tumor cells, promotes the synthesis of cholesterol
metabolite 24-hydroxycholesterol (24-OHC) by enhancing both
exogenous cholesterol uptake and intracellular cholesterol
accumulation in CRC cells (96).
As a natural agonist of LXR, 24-OHC simultaneously activates both
LXRα and LXRβ subtypes. In contrast to the conventional view of
LXR-mediated tumor suppression, this activation of the LXR pathway
promotes CRC cell secretion of elevated levels of TGF-β1 into the
TME in a paracrine manner. The released TGF-β1 subsequently
activates SMAD family member 3 signaling in CD8+ T
cells, ultimately suppressing their antitumor cytotoxicity.
Notably, the pharmacological inhibition of the ATP6VOA1/24-OHC
pathway blocks LXR activation and prevents immunosuppressive
reprogramming, thereby attenuating CRC tumor growth (96,126,127). These findings demonstrate that
context-specific LXR inhibition can exert antitumor effects,
expanding the paradigm of LXR function in oncogenesis and
highlighting the context-dependent duality of LXR activity in tumor
regulation. These insights establish a novel therapeutic strategy
for CRC through the precise modulation of LXR signaling pathways.
Emerging evidence has suggested that the circadian regulation of
LXR activity may influence therapeutic efficacy. Previous studies
have demonstrated diurnal oscillation of LXRα expression in colonic
epithelium, with peak activity coinciding with lipid absorption
phases. This chronobiological dimension introduces new
considerations for optimizing dosing schedules of LXR-targeted
therapies (128–130).
In summary, LXR activation exerts multifaceted
antitumor effects by inducing cell cycle arrest, promoting
apoptosis and pyroptosis via NLRP3 inflammasome activation, and
suppressing cholesterol metabolism in cancer cells. LXR also
enhances antitumor immunity by inhibiting MDSCs and dampening
EGFR-mediated proliferation. It is worth noting that all
aforementioned pleiotropic effects are contingent upon LXR
activation status and cellular context. However, emerging evidence
has suggested a context-dependent dual role for LXR: The activation
of LXR may also promote the secretion of immunosuppressive factors
by tumor cells, thereby undermining antitumor immunity. These
pleiotropic actions highlight the therapeutic potential of
targeting LXR in CRC, although its precise mechanisms require
further elucidation. Continued research is important to fully
exploit LXR as a novel therapeutic target or agent (Fig. 3).
Given the notable role of the LXR pathway in
combating IBD and CRC, the discovery and development of LXR
agonists holds considerable therapeutic potential for intestinal
diseases. As a nuclear transcription factor, LXR is activated by
its natural ligand oxysterol, a cholesterol derivative that
regulates the transcription of target genes (131). Commonly used synthetic agonists,
such as T0901317 and GW3965 hydrochloride, activate the downstream
pathway of LXR by simulating the activity of oxysterols, the
natural ligands for LXR, subsequently regulating immune function
and lipid homeostasis (31,72).
For example, the synthetic ligand GW3965 hydrochloride promotes
RCT, inhibits the NF-κB signal cascade by activating the LXR/ABCA1
pathway and inhibits the production of pro-inflammatory factors,
such as IL-8 and CCL28 (72).
T0901317 inhibits the proliferation of colon cancer stem cells by
activating LXR signaling, and upregulates ABCA1, ABCG5 and ABCG8,
disrupting membrane integrity in CRC cells and inducing apoptosis
(58). Subtype-selective agonists
offer improved safety profiles compared with general LXR agonists.
The LXRβ-specific agonist RGX-104 depletes MDSCs through the
LXRβ/ApoE/LRP8 axis, enhances CD8+ T-cell activity and
suppresses tumor growth in CRC models, and has advanced to phase
Ib/II clinical trials (trial no. NCT02922764), suggesting superior
tumor suppression compared with non-selective agonists (18). However, to date, no agonists
specifically targeting LXRα for alleviating IBD or CRC have been
identified. Sterol-based LXR agonists, such as DMHCA and MePipHCA,
have been shown to alleviate intestinal inflammation in DSS-induced
IBD mouse model of IBD by activating ABCA1 and ABCG1, and
inhibiting pro-inflammatory factors, such as IL-1β and CCL2,
without inducing SREBP1c-mediated lipogenic pathways, thereby
avoiding hepatotoxicity (31).
Although LXR synthetic agonists have shown potential
in treating IBD and CRC in preclinical studies, LXR targeted
therapy still faces notable obstacles. Synthetic agonists may
promote hepatic steatosis via lipogenesis mediated by LXRα/SREBP1c
(132), and cholestasis via ABCG5
and ABCG8 upregulation, leading to hepatotoxicity (133). LXRα predominantly regulates lipid
metabolism, while LXRβ mainly regulates immune cells; both LXRα and
LXRβ are widely co-expressed in the body, so the design of
LXR-targeting subtype-selective drugs is complex (120). Furthermore, LXR activation in CRC
may paradoxically promote immunosuppression via the
ATP6V0A1/LXR/TGF-β1 axis, thereby inhibiting CD8+ T-cell
function and undermining antitumor immunity, which complicates the
development of LXR-targeted therapies (96).
Combination strategies represent promising
approaches to enhance LXR-directed therapy. RGX-104 combined with
photosensitizer Ce6-mediated photodynamic therapy depletes MDSCs
and activates the caspase-3/gasdermin-E-dependent cell apoptosis
pathway (119). Further synergy
with anti-programmed cell death-1 antibody treatment could promote
CD8+ T-cell infiltration and activation, amplifying
systemic antitumor immunity, although this strategy remains
unexplored in IBD and CRC contexts (119,134). In addition, the LXR inverse
agonist SR9243 specifically targets CRC stem cells (CSCs) and
inhibits the expression of lipid synthesis genes SCD1 and fatty
acid synthase by specifically binding to the allosteric site of
LXR, resulting in the obstruction of lipid production and
metabolism. However, this inhibitory effect does not affect the
LXR-mediated RCT pathway. This inhibition of lipid metabolism leads
to the accumulation of reactive oxygen species in CSCs, which
eventually induces apoptosis (39,58).
This discovery provides a novel concept for targeted therapy based
on LXR allosteric inhibition.
LXR serves a notable role at the intersection of
lipid metabolism and immune regulation, positioning it as a
promising therapeutic target linking IBD and CRC. Current
understanding of LXR function predominantly depends on animal model
research and no LXR agonist has progressed to phase III clinical
trials of IBD or CRC. Given that LXR is widely expressed in the
body, existing agonists are often accompanied by the risk of
hepatotoxicity. Furthermore, there is insufficient targeted
research on LXR subtypes, which makes it difficult to develop
intestine-specific treatments. Nevertheless, the latest progress in
subtype selection and combination therapy provides a feasible way
forward. Developing intestine-specific agonists and combined
regimens may be key strategies in unlocking the potential of
metabolism and immunotherapy in treating IBD and CRC. However, the
ways in which LXR activation and inhibition affect the progress of
enteritis through interactions with intestinal microflora remain to
be fully elucidated. Clarifying the role of LXR in IBD and CRC may
provide new targets for the treatment of these diseases and broaden
the application prospects of LXR.
Not applicable.
The present study was supported by the National Natural Science
Foundation of China (grant nos. 82000525 and 81873883), the Science
and Technology Support Plan for Youth Innovation of Colleges and
Universities of Shandong Province of China (grant no. 2021KJ106)
and the Shandong Provincial Natural Science Foundation (grant no.
ZR2023MH359). In addition, the present study was funded by the
Youth Innovation Team Project for Talent Introduction and
Cultivation in Universities of Shandong Province.
Not applicable.
YL, ML and SL conceived the study and edited the
manuscript. MB, JZ, JQ, HZ and XF contributed to the search and
analysis of literature and the review of the manuscript. Data
authentication is not applicable. All authors read and approved the
final version of the manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Guan Q: A comprehensive review and update
on the pathogenesis of inflammatory bowel disease. J Immunol Res.
2019:72472382019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Singh N and Bernstein CN: Environmental
risk factors for inflammatory bowel disease. United European
Gastroenterol J. 10:1047–1053. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bruner LP, White AM and Proksell S:
Inflammatory bowel disease. Prim Care. 50:411–427. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gilliland A, Chan JJ, De Wolfe TJ, Yang H
and Vallance BA: Pathobionts in inflammatory bowel disease:
Origins, underlying mechanisms, and implications for clinical care.
Gastroenterology. 166:44–58. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sun Y, Zhang Z, Zheng CQ and Sang LX:
Mucosal lesions of the upper gastrointestinal tract in patients
with ulcerative colitis: A review. World J Gastroenterol.
27:2963–2978. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Voelker R: What is ulcerative colitis?
JAMA. 331:7162024. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dolinger M, Torres J and Vermeire S:
Crohn's disease. Lancet. 403:1177–1191. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cockburn E, Kamal S, Chan A, Rao V, Liu T,
Huang JY and Segal JP: Crohn's disease: An update. Clin Med (Lond).
23:549–557. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lewis JD, Parlett LE, Funk MLJ, Brensinger
C, Pate V, Wu Q, Dawwas GK, Weiss A, Constant BD, et al: Incidence,
Prevalence, and Racial and Ethnic Distribution of Inflammatory
Bowel Disease in the United States. Gastroenterology.
165:1197–1205. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Saez A, Herrero-Fernandez B, Gomez-Bris R,
Sánchez-Martinez H and Gonzalez-Granado JM: Pathophysiology of
inflammatory bowel disease: Innate immune system. Int J Mol Sci.
24:15262023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yue N, Hu P, Tian C, Kong C, Zhao H, Zhang
Y, Yao J, Wei Y, Li D and Wang L: Dissecting innate and adaptive
immunity in inflammatory bowel disease: Immune
compartmentalization, microbiota crosstalk, and emerging therapies.
J Inflamm Res. 17:9987–10014. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hisamatsu T, Miyoshi J, Oguri N, Morikubo
H, Saito D, Hayashi A, Omori T and Matsuura M:
Inflammation-associated carcinogenesis in inflammatory bowel
disease: Clinical features and molecular mechanisms. Cells.
14:5672025. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fathima A and Jamma T: UDCA ameliorates
inflammation driven EMT by inducing TGR5 dependent SOCS1 expression
in mouse macrophages. Sci Rep. 14:242852024. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Endo-Umeda K, Kim E, Thomas DG, Liu W, Dou
H, Yalcinkaya M, Abramowicz S, Xiao T, Antonson P, Gustafsson JÅ,
et al: Myeloid LXR (Liver X Receptor) Deficiency induces
inflammatory gene expression in foamy macrophages and accelerates
atherosclerosis. Arterioscler Thromb Vasc Biol. 42:719–731. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shah SC and Itzkowitz SH: Colorectal
cancer in inflammatory bowel disease: Mechanisms and management.
Gastroenterology. 162:715–730.e3. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou RW, Harpaz N, Itzkowitz SH and
Parsons RE: Molecular mechanisms in colitis-associated colorectal
cancer. Oncogenesis. 12:482023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Griffett K, Hayes M, Bedia-Diaz G,
Appourchaux K, Sanders R, Boeckman MP, Koelblen T, Zhang J,
Schulman IG, Elgendy B and Burris TP: Antihyperlipidemic activity
of gut-restricted LXR inverse agonists. ACS Chem Biol.
17:1143–1154. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tavazoie MF, Pollack I, Tanqueco R,
Ostendorf BN, Reis BS, Gonsalves FC, Kurth I, Andreu-Agullo C,
Derbyshire ML, Posada J, et al: LXR/ApoE activation restricts
innate immune suppression in cancer. Cell. 172:825–840. e182018.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nishimaki-Mogami T, Tamehiro N, Sato Y,
Okuhira K, Sai K, Kagechika H, Shudo K, Abe-Dohmae S, Yokoyama S,
Ohno Y, et al: The RXR agonists PA024 and HX630 have different
abilities to activate LXR/RXR and to induce ABCA1 expression in
macrophage cell lines. Biochem Pharmacol. 76:1006–1013. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Brahma M, Ghosal S, Maruthi M and Kalangi
SK: Endocytosis of LXRs: Signaling in liver and disease. Prog Mol
Biol Transl Sci. 194:347–375. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mukwaya A, Lennikov A, Xeroudaki M,
Mirabelli P, Lachota M, Jensen L, Peebo B and Lagali N:
Time-dependent LXR/RXR pathway modulation characterizes capillary
remodeling in inflammatory corneal neovascularization.
Angiogenesis. 21:395–413. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hiebl V, Ladurner A, Latkolik S and Dirsch
VM: Natural products as modulators of the nuclear receptors and
metabolic sensors LXR, FXR and RXR. Biotechnol Adv. 36:1657–1698.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gu Y, Bi X, Liu X, Qian Q, Wen Y, Hua S,
Fu Q, Zheng Y and Sun S: Roles of ABCA1 in chronic obstructive
pulmonary disease. COPD. 22:24937012025. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Srivastava RAK, Cefalu AB, Srivastava NS
and Averna M: NPC1L1 and ABCG5/8 induction explain synergistic
fecal cholesterol excretion in ob/ob mice co-treated with PPAR-α
and LXR agonists. Mol Cell Biochem. 473:247–262. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Matsuo M: ABCA1 and ABCG1 as potential
therapeutic targets for the prevention of atherosclerosis. J
Pharmacol Sci. 148:197–203. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang B and Tontonoz P: Liver X receptors
in lipid signalling and membrane homeostasis. Nat Rev Endocrinol.
14:452–463. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Aljabban J, Rohr M, Borkowski VJ, Nemer M,
Cohen E, Hashi N, Aljabban H, Boateng E, Syed S, Mohammed M, et al:
Probing predilection to Crohn's disease and Crohn's disease flares:
A crowd-sourced bioinformatics approach. J Pathol Inform.
13:1000942022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Saito H, Tachiura W, Nishimura M, Shimizu
M, Sato R and Yamauchi Y: Hydroxylation site-specific and
production-dependent effects of endogenous oxysterols on
cholesterol homeostasis: Implications for SREBP-2 and LXR. J Biol
Chem. 299:1027332023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
de Freitas FA, Levy D, Reichert CO,
Cunha-Neto E, Kalil J and Bydlowski SP: Effects of oxysterols on
immune cells and related diseases. Cells. 11:12512022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Das S, Parigi SM, Luo X, Fransson J, Kern
BC, Okhovat A, Diaz OE, Sorini C, Czarnewski P, Webb AT, et al:
Liver X receptor unlinks intestinal regeneration and tumorigenesis.
Nature. 637:1198–1206. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yu S, Li S, Henke A, Muse ED, Cheng B,
Welzel G, Chatterjee AK, Wang D, Roland J, Glass CK and Tremblay M:
Dissociated sterol-based liver X receptor agonists as therapeutics
for chronic inflammatory diseases. FASEB J. 30:2570–2579. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
George M, Lang M, Gali CC, Babalola JA,
Tam-Amersdorfer C, Stracke A, Strobl H, Zimmermann R, Panzenboeck U
and Wadsack C: Liver X receptor activation attenuates
oxysterol-induced inflammatory responses in fetoplacental
endothelial cells. Cells. 12:11862023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yan C, Zheng L, Jiang S, Yang H, Guo J,
Jiang LY, Li T, Zhang H, Bai Y, Lou Y, et al: Exhaustion-associated
cholesterol deficiency dampens the cytotoxic arm of antitumor
immunity. Cancer Cell. 41:1276–1293.e11. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lin CY and Gustafsson JÅ: Targeting liver
X receptors in cancer therapeutics. Nat Rev Cancer. 15:216–224.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
de la Aleja AG, Herrero C,
Torres-Torresano M, Schiaffino MT, Del Castillo A, Alonso B, Vega
MA, Puig-Kröger A, Castrillo A and Corbí ÁL: Inhibition of LXR
controls the polarization of human inflammatory macrophages through
upregulation of MAFB. Cell Mol Life Sci. 80:962023. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Snodgrass RG, Benatzy Y, Schmid T,
Namgaladze D, Mainka M, Schebb NH, Lütjohann D and Brüne B:
Efferocytosis potentiates the expression of arachidonate
15-lipoxygenase (ALOX15) in alternatively activated human
macrophages through LXR activation. Cell Death Differ.
28:1301–1316. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sharma B, Gupta V, Dahiya D, Kumar H,
Vaiphei K and Agnihotri N: Clinical relevance of cholesterol
homeostasis genes in colorectal cancer. Biochim Biophys Acta Mol
Cell Biol Lipids. 1864:1314–1327. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang Q, Ren M, Feng F, Chen K and Ju X:
Treatment of colon cancer with liver X receptor agonists induces
immunogenic cell death. Mol Carcinog. 57:903–910. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dianat-Moghadam H, Abbasspour-Ravasjani S,
Hamishehkar H, Rahbarghazi R and Nouri M: LXR inhibitor
SR9243-loaded immunoliposomes modulate lipid metabolism and
stemness in colorectal cancer cells. Med Oncol. 40:1562023.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bhunia AK and Al-Sadi R: Editorial:
Intestinal epithelial barrier disruption by enteric pathogens.
Front Cell Infect Microbiol. 13:11347532023. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liu Y, Yu X, Zhao J, Zhang H, Zhai Q and
Chen W: The role of MUC2 mucin in intestinal homeostasis and the
impact of dietary components on MUC2 expression. Int J Biol
Macromol. 164:884–891. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yao D, Dai W, Dong M, Dai C and Wu S: MUC2
and related bacterial factors: Therapeutic targets for ulcerative
colitis. EBioMedicine. 74:1037512021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang H, Wang X, Zhao L, Zhang K, Cui J
and Xu G: Biochanin a ameliorates DSS-induced ulcerative colitis by
improving colonic barrier function and protects against the
development of spontaneous colitis in the Muc2 deficient mice. Chem
Biol Interact. 395:1110142024. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang A, Yang X, Lin J, Wang Y, Yang J,
Zhang Y, Tian Y, Dong H, Zhang Z and Song R: Si-Ni-San alleviates
intestinal and liver damage in ulcerative colitis mice by
regulating cholesterol metabolism. J Ethnopharmacol.
336:1187152025. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Klepsch V, Moschen AR, Tilg H, Baier G and
Hermann-Kleiter N: Nuclear Receptors Regulate Intestinal
Inflammation in the Context of IBD. Front Immunol. 10:10702019.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Song X, Wu W, Dai Y, Warner M, Nalvarte I,
Antonson P, Varshney M and Gustafsson JÅ: Loss of ERβ in aging
LXRαβ knockout mice leads to colitis. Int J Mol Sci. 24:124612023.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Jakobsson T, Vedin LL, Hassan T, Venteclef
N, Greco D, D'Amato M, Treuter E, Gustafsson JÅ and Steffensen KR:
The oxysterol receptor LXRβ protects against DSS- and TNBS-induced
colitis in mice. Mucosal Immunol. 7:1416–1428. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Vassiliou E and Farias-Pereira R: Impact
of lipid metabolism on macrophage polarization: Implications for
inflammation and tumor immunity. Int J Mol Sci. 24:120322023.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yan JB, Luo MM, Chen ZY and He BH: The
function and role of the Th17/Treg cell balance in inflammatory
bowel disease. J Immunol Res. 2020:88135582020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Astier AL and Kofler DM: Editorial:
Dysregulation of Th17 and Treg cells in autoimmune diseases. Front
Immunol. 14:11518362023. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Knochelmann HM, Dwyer CJ, Bailey SR, Amaya
SM, Elston DM, Mazza-McCrann JM and Paulos CM: When worlds collide:
Th17 and Treg cells in cancer and autoimmunity. Cell Mol Immunol.
15:458–469. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
King RJ, Singh PK and Mehla K: The
cholesterol pathway: Impact on immunity and cancer. Trends Immunol.
43:78–92. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Korach-André M and Gustafsson JÅ: Liver X
receptors as regulators of metabolism. Biomol Concepts. 6:177–190.
2015. View Article : Google Scholar
|
|
54
|
Xu H, Xin Y, Wang J, Liu Z, Cao Y, Li W,
Zhou Y, Wang Y and Liu P: The TICE pathway: Mechanisms and
potential clinical applications. Curr Atheroscler Rep. 25:653–662.
2023. View Article : Google Scholar
|
|
55
|
Modica S, Gofflot F, Murzilli S, D'Orazio
A, Salvatore L, Pellegrini F, Nicolucci A, Tognoni G, Copetti M,
Valanzano R, et al: The intestinal nuclear receptor signature with
epithelial localization patterns and expression modulation in
tumors. Gastroenterology. 138:636–648. 648.e1–12. 2010. View Article : Google Scholar
|
|
56
|
Willemsen S, Yengej FAY, Puschhof J,
Rookmaaker MB, Verhaar MC, van Es J, Beumer J and Clevers H: A
comprehensive transcriptome characterization of individual nuclear
receptor pathways in the human small intestine. Proc Natl Acad Sci
USA. 121:e24111891212024. View Article : Google Scholar
|
|
57
|
Kikuchi T, Orihara K, Oikawa F, Han SI,
Kuba M, Okuda K, Satoh A, Osaki Y, Takeuchi Y, Aita Y, et al:
Intestinal CREBH overexpression prevents high-cholesterol
diet-induced hypercholesterolemia by reducing Npc1l1 expression.
Mol Metab. 5:1092–1102. 2016. View Article : Google Scholar
|
|
58
|
Dianat-Moghadam H, Khalili M, Keshavarz M,
Azizi M, Hamishehkar H, Rahbarghazi R and Nouri M: Modulation of
LXR signaling altered the dynamic activity of human colon
adenocarcinoma cancer stem cells in vitro. Cancer Cell Int.
21:1002021. View Article : Google Scholar
|
|
59
|
Lazennec G and Richmond A: Chemokines and
chemokine receptors: New insights into cancer-related inflammation.
Trends Mol Med. 16:133–144. 2010. View Article : Google Scholar
|
|
60
|
Kobayashi T, Siegmund B, Le Berre C, Wei
SC, Ferrante M, Shen B, Bernstein CN, Danese S, Peyrin-Biroulet L
and Hibi T: Ulcerative colitis. Nat Rev Dis Primers. 6:742020.
View Article : Google Scholar
|
|
61
|
Saez A, Gomez-Bris R, Herrero-Fernandez B,
Mingorance C, Rius C and Gonzalez-Granado JM: Innate lymphoid cells
in intestinal homeostasis and inflammatory bowel disease. Int J Mol
Sci. 22:76182021. View Article : Google Scholar
|
|
62
|
dos Santos Ramos A, Viana GCS, de Macedo
Brigido M and Almeida JF: Neutrophil extracellular traps in
inflammatory bowel diseases: Implications in pathogenesis and
therapeutic targets. Pharmacol Res. 171:1057792021. View Article : Google Scholar
|
|
63
|
Kiss M, Czimmerer Z and Nagy L: The role
of lipid-activated nuclear receptors in shaping macrophage and
dendritic cell function: From physiology to pathology. J Allergy
Clin Immunol. 132:264–286. 2013. View Article : Google Scholar
|
|
64
|
Parigi SM, Das S, Frede A, Cardoso RF,
Tripathi KP, Doñas C, Hu YOO, Antonson P, Engstrand L, Gustafsson
JÅ and Villablanca EJ: Liver X receptor regulates Th17 and RORγt+
Treg cells by distinct mechanisms. Mucosal Immunol. 14:411–419.
2021. View Article : Google Scholar
|
|
65
|
Qin T, Yang J, Huang D, Zhang Z, Huang Y,
Chen H and Xu G: DOCK4 stimulates MUC2 production through its
effect on goblet cell differentiation. J Cell Physiol.
236:6507–6519. 2021. View Article : Google Scholar
|
|
66
|
Herold M, Breuer J, Hucke S, Knolle P,
Schwab N, Wiendl H and Klotz L: Liver X receptor activation
promotes differentiation of regulatory T cells. PLoS One.
12:e01849852017. View Article : Google Scholar
|
|
67
|
Li H, Fan C, Lu H, Feng C, He P, Yang X,
Xiang C, Zuo J and Tang W: Protective role of berberine on
ulcerative colitis through modulating enteric glial
cells-intestinal epithelial cells-immune cells interactions. Acta
Pharm Sin B. 10:447–461. 2020. View Article : Google Scholar
|
|
68
|
Lee JW, Wang P, Kattah MG, Youssef S,
Steinman L, DeFea K and Straus DS: Differential regulation of
chemokines by IL-17 in colonic epithelial cells. J Immunol.
181:6536–6545. 2008. View Article : Google Scholar
|
|
69
|
Jakobsson T, Treuter E, Gustafsson JÅ and
Steffensen KR: Liver X receptor biology and pharmacology: new
pathways, challenges and opportunities. Trends Pharmacol Sci.
33:394–404. 2012. View Article : Google Scholar
|
|
70
|
Delfini M, Stakenborg N, Viola MF and
Boeckxstaens G: Macrophages in the gut: Masters in multitasking.
Immunity. 55:1530–1548. 2022. View Article : Google Scholar
|
|
71
|
Xiong T, Zheng X, Zhang K, Wu H, Dong Y,
Zhou F, Cheng B, Li L, Xu W, Su J, et al: Ganluyin ameliorates
DSS-induced ulcerative colitis by inhibiting the enteric-origin
LPS/TLR4/NF-κB pathway. J Ethnopharmacol. 289:1150012022.
View Article : Google Scholar
|
|
72
|
Miranda-Bautista J, Rodríguez-Feo JA,
Puerto M, López-Cauce B, Lara JM, González-Novo R, Martín-Hernández
D, Ferreiro-Iglesias R, Bañares R and Menchén L: Liver X receptor
exerts anti-inflammatory effects in colonic epithelial cells via
ABCA1 and its expression is decreased in human and experimental
inflammatory bowel disease. Inflamm Bowel Dis. 27:1661–1673. 2021.
View Article : Google Scholar
|
|
73
|
Qu Y, Li X, Xu F, Zhao S, Wu X, Wang Y and
Xie J: Kaempferol alleviates murine experimental colitis by
restoring gut microbiota and inhibiting the LPS-TLR4-NF-κB axis.
Front Immunol. 12:6798972021. View Article : Google Scholar
|
|
74
|
Li B, Jiang XF, Dong YJ, Zhang YP, He XL,
Zhou CL, Ding YY, Wang N, Wang YB, Cheng WQ, et al: The effects of
Atractylodes macrocephala extract BZEP self-microemulsion based on
gut-liver axis HDL/LPS signaling pathway to ameliorate metabolic
dysfunction-associated fatty liver disease in rats. Biomed
Pharmacother. 175:1165192024. View Article : Google Scholar
|
|
75
|
Pang J, Xu H, Wang X, Chen X, Li Q, Liu Q,
You Y, Zhang H, Xu Z, Zhao Y, et al: Resveratrol enhances
trans-intestinal cholesterol excretion through selective activation
of intestinal liver X receptor alpha. Biochem Pharmacol.
186:1144812021. View Article : Google Scholar
|
|
76
|
Ito A, Hong C, Rong X, Zhu X, Tarling EJ,
Hedde PN, Gratton E, Parks J and Tontonoz P: LXRs link metabolism
to inflammation through Abca1-dependent regulation of membrane
composition and TLR signaling. Elife. 4:e080092015. View Article : Google Scholar
|
|
77
|
Režen T, Rozman D, Kovács T, Kovács P,
Sipos A, Bai P and Mikó E: The role of bile acids in
carcinogenesis. Cell Mol Life Sci. 79:2432022. View Article : Google Scholar
|
|
78
|
Breevoort SR, Angdisen J and Schulman IG:
Macrophage-independent regulation of reverse cholesterol transport
by liver X receptors. Arterioscler Thromb Vasc Biol. 34:1650–1660.
2014. View Article : Google Scholar
|
|
79
|
Lifsey HC, Kaur R, Thompson BH, Bennett L,
Temel RE and Graf GA: Stigmasterol stimulates transintestinal
cholesterol excretion independent of liver X receptor activation in
the small intestine. J Nutr Biochem. 76:1082632020. View Article : Google Scholar
|
|
80
|
Liang L, Xie Q, Sun C, Wu Y, Zhang W and
Li W: Phospholipase A2 group IIA correlates with circulating
high-density lipoprotein cholesterol and modulates cholesterol
efflux possibly through regulation of PPAR-γ/LXR-α/ABCA1 in
macrophages. J Transl Med. 19:4842021. View Article : Google Scholar
|
|
81
|
Lo Sasso G, Bovenga F, Murzilli S,
Salvatore L, Di Tullio G, Martelli N, D'Orazio A, Rainaldi S, Vacca
M, Mangia A, et al: Liver X receptors inhibit proliferation of
human colorectal cancer cells and growth of intestinal tumors in
mice. Gastroenterology. 144:1497–1507. 1507e1–13. 2013. View Article : Google Scholar
|
|
82
|
Zhu X, Lee JY, Timmins JM, Brown JM,
Boudyguina E, Mulya A, Gebre AK, Willingham MC, Hiltbold EM, Mishra
N, et al: Increased cellular free cholesterol in
macrophage-specific Abca1 knock-out mice enhances pro-inflammatory
response of macrophages. J Biol Chem. 283:22930–22941. 2008.
View Article : Google Scholar
|
|
83
|
Pentinmikko N, Iqbal S, Mana M, Andersson
S, Cognetta AB III, Suciu RM, Roper J, Luopajärvi K, Markelin E,
Gopalakrishnan S, et al: Notum produced by Paneth cells attenuates
regeneration of aged intestinal epithelium. Nature. 571:398–402.
2019. View Article : Google Scholar
|
|
84
|
Liebergall SR, Angdisen J, Chan SH, Chang
Y, Osborne TF, Koeppel AF, Turner SD and Schulman IG: Inflammation
triggers liver X receptor dependent lipogenesis. Mol Cell Biol.
40:e00364–19. 2020. View Article : Google Scholar
|
|
85
|
Fantini MC, Favale A, Onali S and
Facciotti F: Tumor infiltrating regulatory T cells in sporadic and
colitis-associated colorectal cancer: The red little riding hood
and the wolf. Int J Mol Sci. 21:67442020. View Article : Google Scholar
|
|
86
|
Zhang H, Shi Y, Lin C, He C, Wang S, Li Q,
Sun Y and Li M: Overcoming cancer risk in inflammatory bowel
disease: New insights into preventive strategies and pathogenesis
mechanisms including interactions of immune cells, cancer signaling
pathways, and gut microbiota. Front Immunol. 14:13389182024.
View Article : Google Scholar
|
|
87
|
Tao XY, Li QQ and Zeng Y: Clinical
application of liquid biopsy in colorectal cancer: Detection,
prediction, and treatment monitoring. Mol Cancer. 23:1452024.
View Article : Google Scholar
|
|
88
|
Dougherty MW and Jobin C: Intestinal
bacteria and colorectal cancer: Etiology and treatment. Gut
Microbes. 15:21850282023. View Article : Google Scholar
|
|
89
|
Benson AB, Venook AP, Al-Hawary MM, Arain
MA, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Farkas L, et
al: Colon cancer, version 2.2021, NCCN clinical practice guidelines
in oncology. J Natl Compr Canc Netw. 19:329–359. 2021. View Article : Google Scholar
|
|
90
|
Miller KD, Nogueira L, Devasia T, Mariotto
AB, Yabroff KR, Jemal A, Kramer J and Siegel RL: Cancer treatment
and survivorship statistics, 2022. CA Cancer J Clin. 72:409–436.
2022.
|
|
91
|
Carethers JM: Racial and ethnic
disparities in colorectal cancer incidence and mortality. Adv
Cancer Res. 151:197–229. 2021. View Article : Google Scholar
|
|
92
|
Rawla P, Sunkara T and Barsouk A:
Epidemiology of colorectal cancer: Incidence, mortality, survival,
and risk factors. Prz Gastroenterol. 14:89–103. 2019.
|
|
93
|
Li N, Lu B, Luo C, Cai J, Lu M, Zhang Y,
Chen H and Dai M: Incidence, mortality, survival, risk factor and
screening of colorectal cancer: A comparison among China, Europe,
and Northern America. Cancer Lett. 522:255–268. 2021. View Article : Google Scholar
|
|
94
|
Li J, Ma X, Chakravarti D, Shalapour S and
DePinho RA: Genetic and biological hallmarks of colorectal cancer.
Genes Dev. 35:787–820. 2021. View Article : Google Scholar
|
|
95
|
Wen J, Min X, Shen M, Hua Q, Han Y, Zhao
L, Liu L, Huang G, Liu J and Zhao X: ACLY facilitates colon cancer
cell metastasis by CTNNB1. J Exp Clin Cancer Res. 38:4012019.
View Article : Google Scholar
|
|
96
|
Huang TX, Huang HS, Dong SW, Chen JY,
Zhang B, Li HH, Zhang TT, Xie Q, Long QY, Yang Y, et al:
ATP6V0A1-dependent cholesterol absorption in colorectal cancer
cells triggers immunosuppressive signaling to inactivate memory
CD8+ T cells. Nat Commun. 15:56802024. View Article : Google Scholar
|
|
97
|
Taank Y and Agnihotri N: Exploring the
anticancer potential of ergosterol and ergosta-5,22,25-triene-3-ol
against colorectal cancer: Insights from experimental models and
molecular mechanisms. Biochem Pharmacol. 240:1170612025. View Article : Google Scholar
|
|
98
|
Huang B, Song BL and Xu C: Cholesterol
metabolism in cancer: Mechanisms and therapeutic opportunities. Nat
Metab. 2:132–141. 2020. View Article : Google Scholar
|
|
99
|
Matthews HK, Bertoli C and de Bruin RAM:
Cell cycle control in cancer. Nat Rev Mol Cell Biol. 23:74–88.
2022. View Article : Google Scholar
|
|
100
|
Ren YM, Zhuang ZY, Xie YH, Yang PJ, Xia
TX, Xie YL, Liu ZH, Kang ZR, Leng XX, Lu SY, et al: BCAA-producing
Clostridium symbiosum promotes colorectal tumorigenesis through the
modulation of host cholesterol metabolism. Cell Host Microbe.
32:1519–1535. e72024. View Article : Google Scholar
|
|
101
|
Warns J, Marwarha G, Freking N and Ghribi
O: 27-hydroxycholesterol decreases cell proliferation in colon
cancer cell lines. Biochimie. 153:171–180. 2018. View Article : Google Scholar
|
|
102
|
Zhang W, Jiang H, Zhang J, Zhang Y, Liu A,
Zhao Y, Zhu X, Lin Z and Yuan X: Liver X receptor activation
induces apoptosis of melanoma cell through caspase pathway. Cancer
Cell Int. 14:162014. View Article : Google Scholar
|
|
103
|
Dufour J, Viennois E, De Boussac H, Baron
S and Lobaccaro JM: Oxysterol receptors, AKT and prostate cancer.
Curr Opin Pharmacol. 12:724–728. 2012. View Article : Google Scholar
|
|
104
|
Pommier AJC, Alves G, Viennois E, Bernard
S, Communal Y, Sion B, Marceau G, Damon C, Mouzat K, Caira F, et
al: Liver X Receptor activation downregulates AKT survival
signaling in lipid rafts and induces apoptosis of prostate cancer
cells. Oncogene. 29:2712–2723. 2010. View Article : Google Scholar
|
|
105
|
Wan W, Hou Y, Wang K, Cheng Y, Pu X and Ye
X: The LXR-623-induced long non-coding RNA LINC01125 suppresses the
proliferation of breast cancer cells via PTEN/AKT/p53 signaling
pathway. Cell Death Dis. 10:2482019. View Article : Google Scholar
|
|
106
|
Ding X, Zhang W, Li S and Yang H: The role
of cholesterol metabolism in cancer. Am J Cancer Res. 9:219–227.
2019.
|
|
107
|
Piccinin E, Cariello M and Moschetta A:
Lipid metabolism in colon cancer: Role of liver X receptor (LXR)
and Stearoyl-CoA Desaturase 1 (SCD1). Mol Aspects Med.
78:1009332021. View Article : Google Scholar
|
|
108
|
Kashiwagi K, Sato-Yazawa H, Ishii J, Kohno
K, Tatsuta I, Miyazawa T, Takagi M, Chiba H and Yazawa T: LXRβ
activation inhibits the proliferation of small-cell lung cancer
cells by depleting cellular cholesterol. Anticancer Res.
42:2923–2930. 2022. View Article : Google Scholar
|
|
109
|
Derangère V, Chevriaux A, Courtaut F,
Bruchard M, Berger H, Chalmin F, Causse SZ, Limagne E, Végran F,
Ladoire S, et al: Liver X receptor β activation induces pyroptosis
of human and murine colon cancer cells. Cell Death Differ.
21:1914–1924. 2014. View Article : Google Scholar
|
|
110
|
Rébé C, Derangère V and Ghiringhelli F:
Induction of pyroptosis in colon cancer cells by LXRβ. Mol Cell
Oncol. 2:e9700942015. View Article : Google Scholar
|
|
111
|
No authors listed. LXR agonism depletes
MDSCs to promote antitumor immunity. Cancer Discov. 8:2632018.
View Article : Google Scholar
|
|
112
|
Wang L, Lynch C, Pitroda SP, Piffkó A,
Yang K, Huser AK, Liang HL and Weichselbaum RR: Radiotherapy and
immunology. J Exp Med. 221:e202321012024. View Article : Google Scholar
|
|
113
|
Wang H, Zhou F, Qin W, Yang Y, Li X and
Liu R: Metabolic regulation of myeloid-derived suppressor cells in
tumor immune microenvironment: Targets and therapeutic strategies.
Theranostics. 15:2159–2184. 2025. View Article : Google Scholar
|
|
114
|
Zhao H, Teng D, Yang L, Xu X, Chen J,
Jiang T, Feng AY, Zhang Y, Frederick DT, Gu L, et al:
Myeloid-derived itaconate suppresses cytotoxic CD8+ T cells and
promotes tumour growth. Nat Metab. 4:1660–1673. 2022. View Article : Google Scholar
|
|
115
|
Killock D: Immunotherapy: Targeting MDSCs
with LXR agonists. Nat Rev Clin Oncol. 15:200–201. 2018. View Article : Google Scholar
|
|
116
|
Liang H and Shen X: LXR activation
radiosensitizes non-small cell lung cancer by restricting
myeloid-derived suppressor cells. Biochem Biophys Res Commun.
528:330–335. 2020. View Article : Google Scholar
|
|
117
|
Hinshaw DC and Shevde LA: The tumor
microenvironment innately modulates cancer progression. Cancer Res.
79:4557–4566. 2019. View Article : Google Scholar
|
|
118
|
Ruan H, Zhang J, Wang Y, Huang Y, Wu J, He
C, Ke T, Luo J and Yang M: 27-Hydroxycholesterol/liver X
receptor/apolipoprotein E mediates zearalenone-induced intestinal
immunosuppression: A key target potentially linking zearalenone and
cancer. J Pharm Anal. 14:371–388. 2024. View Article : Google Scholar
|
|
119
|
Qiu W, Su W, Xu J, Liang M, Ma X, Xue P,
Kang Y, Sun ZJ and Xu Z: Immunomodulatory-photodynamic
nanostimulators for invoking pyroptosis to augment tumor
immunotherapy. Adv Healthc Mater. 11:e22012332022. View Article : Google Scholar
|
|
120
|
Pontini L and Marinozzi M: Shedding light
on the roles of liver X receptors in cancer by using chemical
probes. Br J Pharmacol. 178:3261–3276. 2021. View Article : Google Scholar
|
|
121
|
Wang M, Ma LJ, Yang Y, Xiao Z and Wan JB:
n-3 Polyunsaturated fatty acids for the management of alcoholic
liver disease: A critical review. Crit Rev Food Sci Nutr. 59
(sup1):S116–S129. 2018. View Article : Google Scholar
|
|
122
|
Gabitova L, Restifo D, Gorin A, Manocha K,
Handorf E, Yang DH, Cai KQ, Klein-Szanto AJ, Cunningham D, Kratz
LE, et al: Endogenous sterol metabolites regulate growth of
EGFR/KRAS-dependent tumors via LXR. Cell Rep. 12:1927–1938. 2015.
View Article : Google Scholar
|
|
123
|
Chen T, Xu J and Fu W: EGFR/FOXO3A/LXR-α
axis promotes prostate cancer proliferation and metastasis and
dual-targeting LXR-α/EGFR shows synthetic lethality. Front Oncol.
10:16882020. View Article : Google Scholar
|
|
124
|
Wu Y, Yu DD, Hu Y, Cao HX, Yu SR, Liu SW
and Feng JF: LXR ligands sensitize EGFR-TKI-resistant human lung
cancer cells in vitro by inhibiting Akt activation. Biochem Biophys
Res Commun. 467:900–905. 2015. View Article : Google Scholar
|
|
125
|
Liang X, Cao Y, Xiang S and Xiang Z:
LXRα-mediated downregulation of EGFR suppress colorectal cancer
cell proliferation. J Cell Biochem. 120:17391–17404. 2019.
View Article : Google Scholar
|
|
126
|
Trebska-McGowan K, Chaib M, Alvarez MA,
Kansal R, Pingili AK, Shibata D, Makowski L and Glazer ES: TGF-β
alters the proportion of infiltrating immune cells in a pancreatic
ductal adenocarcinoma. J Gastrointest Surg. 26:113–121. 2022.
View Article : Google Scholar
|
|
127
|
Park BV, Freeman ZT, Ghasemzadeh A,
Chattergoon MA, Rutebemberwa A, Steigner J, Winter ME, Huynh TV,
Sebald SM, Lee SJ, et al: TGFβ1-mediated SMAD3 enhances PD-1
expression on antigen-specific T cells in cancer. Cancer Discov.
6:1366–1381. 2016. View Article : Google Scholar
|
|
128
|
Kovač U, Skubic C, Bohinc L, Rozman D and
Režen T: Oxysterols and gastrointestinal cancers around the clock.
Front Endocrinol (Lausanne). 10:4832019. View Article : Google Scholar
|
|
129
|
Chen R, Zuo Z, Li Q, Wang H, Li N, Zhang
H, Yu X and Liu Z: DHA substitution overcomes high-fat diet-induced
disturbance in the circadian rhythm of lipid metabolism. Food
Funct. 11:3621–3631. 2020. View Article : Google Scholar
|
|
130
|
Le Martelot G, Claudel T, Gatfield D,
Schaad O, Kornmann B, Lo Sasso G, Moschetta A and Schibler U:
REV-ERBalpha participates in circadian SREBP signaling and bile
acid homeostasis. PLoS Biol. 7:e10001812009. View Article : Google Scholar
|
|
131
|
Wang Q, Wang J, Wang J and Zhang H:
Molecular mechanism of liver X receptors in cancer therapeutics.
Life Sci. 273:1192872021. View Article : Google Scholar
|
|
132
|
Nguyen LH, Cho YE, Kim S, Kim Y, Kwak J,
Suh JS, Lee J, Son K, Kim M, Jang ES, et al: Discovery of
N-Aryl-N'-[4-(aryloxy)cyclohexyl]squaramide-based inhibitors of
LXR/SREBP-1c signaling pathway ameliorating steatotic liver
disease: Navigating the role of SIRT6 activation. J Med Chem.
67:17608–17628. 2024. View Article : Google Scholar
|
|
133
|
Ghosh S, Devereaux MW, Anderson AL, Gehrke
S, Reisz JA, D'Alessandro A, Orlicky DJ, Lovell M, El Kasmi KC,
Shearn CT and Sokol RJ: NF-κB regulation of LRH-1 and ABCG5/8
potentiates phytosterol role in the pathogenesis of parenteral
nutrition-associated cholestasis. Hepatology. 74:3284–3300. 2021.
View Article : Google Scholar
|
|
134
|
Kato Y, Tabata K, Yachie-Kinoshita A,
Ozawa Y, Yamada K, Ito J, Tachino S, Hori Y, Matsuki M, Matsuoka Y,
et al: Lenvatinib plus anti-PD-1 antibody combination treatment
activates CD8+ T cells through reduction of tumor-associated
macrophage and activation of the interferon pathway. PLoS One.
14:e02125132019. View Article : Google Scholar
|