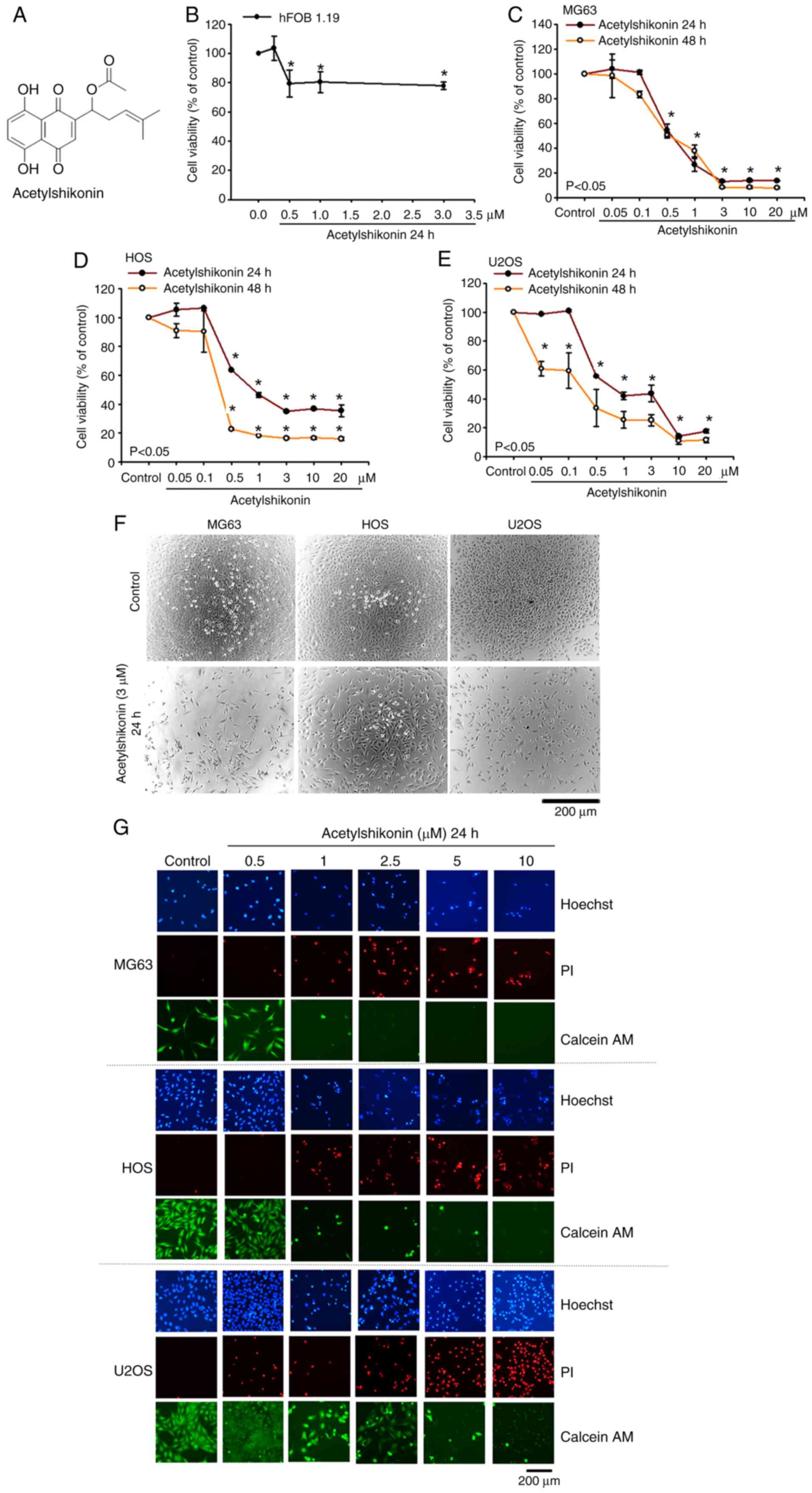
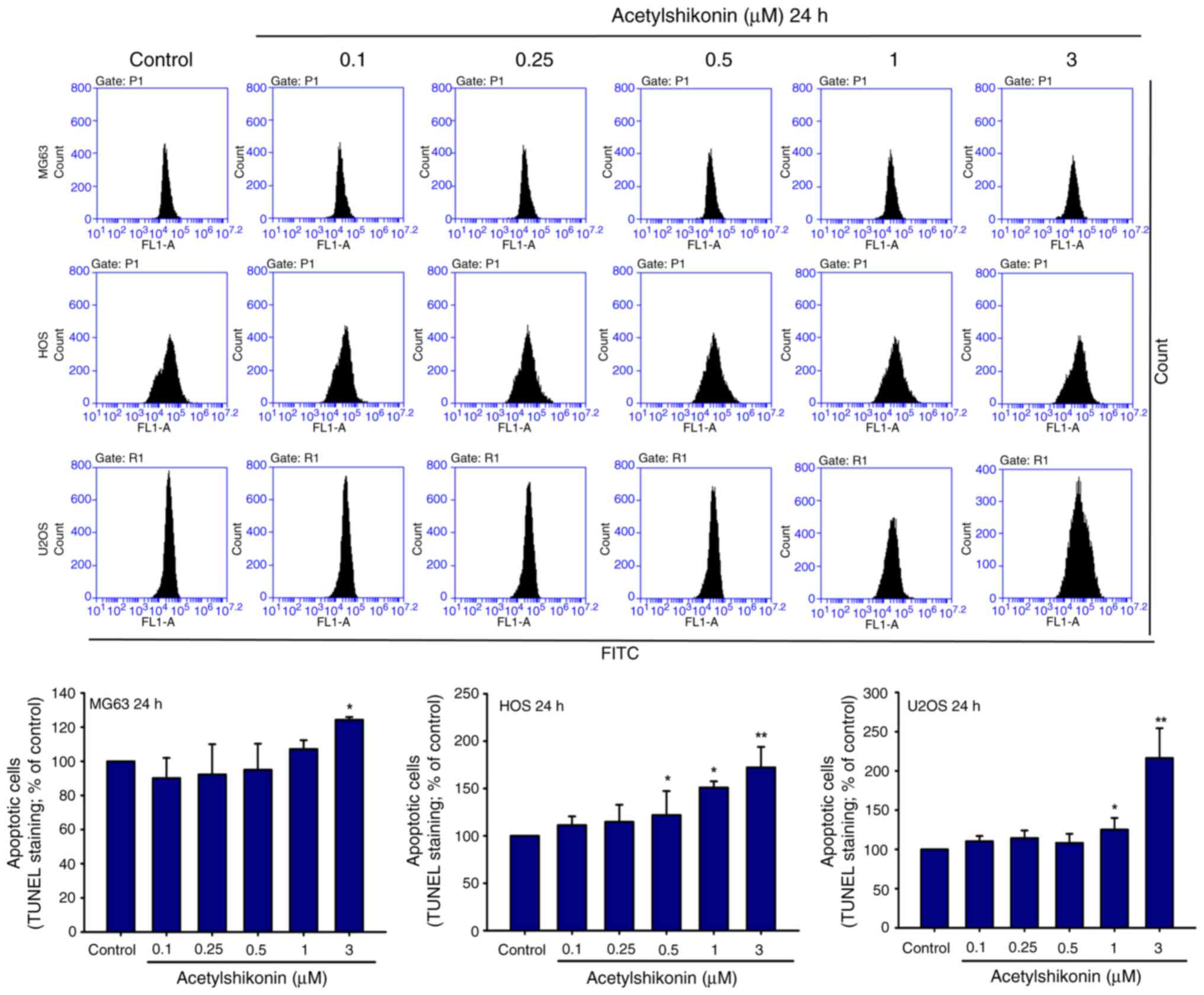
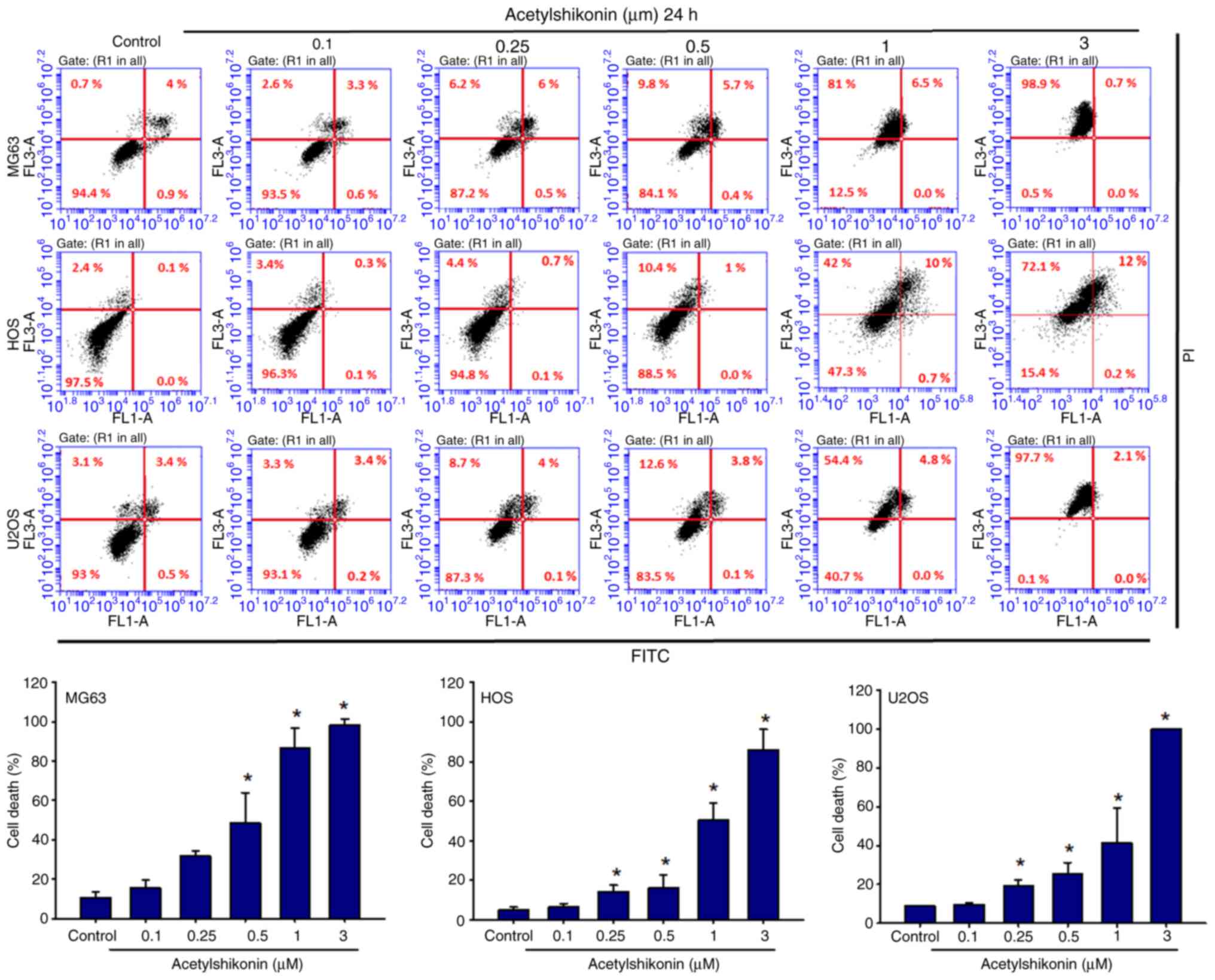
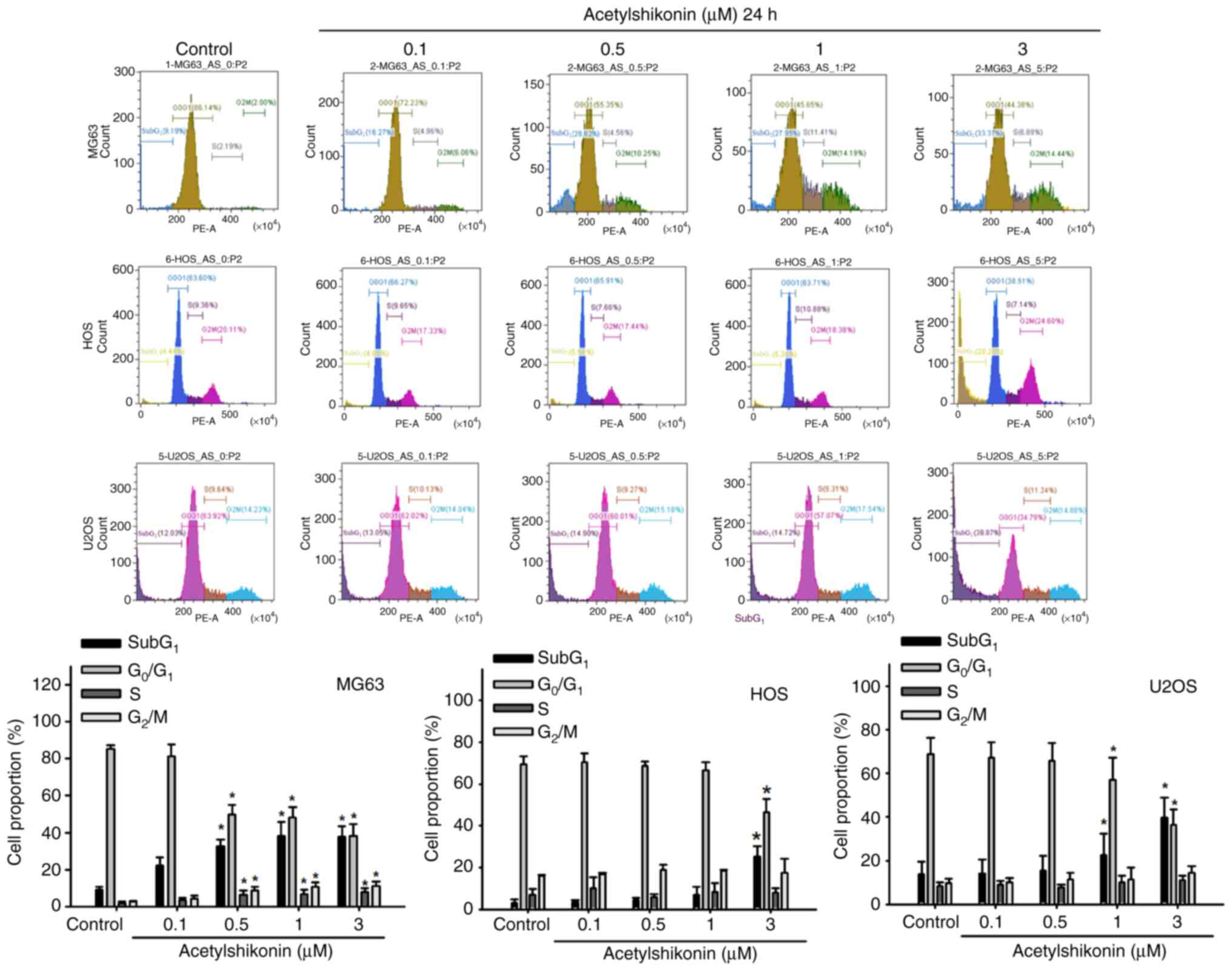
|
1
|
Ottaviani G and Jaffe N: The epidemiology
of osteosarcoma. Cancer Treat Res. 152:3–13. 2009. View Article : Google Scholar
|
|
2
|
Meltzer PS and Helman LJ: New Horizons in
the treatment of osteosarcoma. N Engl J Med. 385:2066–2076. 2021.
View Article : Google Scholar
|
|
3
|
Belayneh R, Fourman MS, Bhogal S and Weiss
KR: Update on osteosarcoma. Curr Oncol Rep. 23:712021. View Article : Google Scholar
|
|
4
|
Lilienthal I and Herold N: Targeting
molecular mechanisms underlying treatment efficacy and resistance
in osteosarcoma: A review of current and future strategies. Int J
Mol Sci. 21:68852020. View Article : Google Scholar
|
|
5
|
Haider T, Pandey V, Banjare N, Gupta PN
and Soni V: Drug resistance in cancer: Mechanisms and tackling
strategies. Pharmacol Rep. 72:1125–1151. 2020. View Article : Google Scholar
|
|
6
|
Mohammad RM, Muqbil I, Lowe L, Yedjou C,
Hsu HY, Lin LT, Siegelin MD, Fimognari C, Kumar NB, Dou QP, et al:
Broad targeting of resistance to apoptosis in cancer. Semin Cancer
Biol. 35 (Suppl 1):S78–S103. 2015. View Article : Google Scholar
|
|
7
|
Wang J, Li M, Guo P and He D: Correction:
Survival benefits and challenges of adjuvant chemotherapy for
high-grade osteosarcoma: A population-based study. J Orthop Surg
Res. 18:8342023. View Article : Google Scholar
|
|
8
|
Hiraga H and Ozaki T: Adjuvant and
neoadjuvant chemotherapy for osteosarcoma: JCOG bone and soft
tissue tumor study group. Jpn J Clin Oncol. 51:1493–1497. 2021.
View Article : Google Scholar
|
|
9
|
Zhang C, Liu X, Jin S, Chen Y and Guo R:
Ferroptosis in cancer therapy: A novel approach to reversing drug
resistance. Mol Cancer. 21:472022. View Article : Google Scholar
|
|
10
|
Zhou N and Bao J: FerrDb: A manually
curated resource for regulators and markers of ferroptosis and
ferroptosis-disease associations. Database (Oxford).
2020:baaa0212020. View Article : Google Scholar
|
|
11
|
Zhou Q, Meng Y, Li D, Yao L, Le J, Liu Y,
Sun Y, Zeng F, Chen X and Deng G: Ferroptosis in cancer: From
molecular mechanisms to therapeutic strategies. Signal Transduct
Target Ther. 9:552024. View Article : Google Scholar
|
|
12
|
Nie Q, Hu Y, Yu X, Li X and Fang X:
Induction and application of ferroptosis in cancer therapy. Cancer
Cell Int. 22:122022. View Article : Google Scholar
|
|
13
|
Wu Y, Yu C, Luo M, Cen C, Qiu J, Zhang S
and Hu K: Ferroptosis in cancer treatment: Another way to rome.
Front Oncol. 10:5711272020. View Article : Google Scholar
|
|
14
|
Hayashi M: Pharmacological studies of
Shikon and Tooki. (2) Pharmacological effects of the pigment
components, Shikonin and acetylshikonin. Nihon Yakurigaku Zasshi.
73:193–203. 1977.(In Japanese). View Article : Google Scholar
|
|
15
|
Wang Q, Wang J, Wang J, Ju X and Zhang H:
Molecular mechanism of shikonin inhibiting tumor growth and
potential application in cancer treatment. Toxicol Res (Camb).
10:1077–1084. 2021. View Article : Google Scholar
|
|
16
|
Hu S, Li Y, Zhou J, Xu K, Pang Y,
Weiskirchen R, Ocker M and Ouyang F: Identification of
acetylshikonin as a novel tubulin polymerization inhibitor with
antitumor activity in human hepatocellular carcinoma cells. J
Gastrointest Oncol. 14:2574–2586. 2023. View Article : Google Scholar
|
|
17
|
Cha HS, Lee HK, Park SH and Nam MJ:
Acetylshikonin induces apoptosis of human osteosarcoma U2OS cells
by triggering ROS-dependent multiple signal pathways. Toxicol In
Vitro. 86:1055212023. View Article : Google Scholar
|
|
18
|
Majtnerova P and Rousar T: An overview of
apoptosis assays detecting DNA fragmentation. Mol Biol Rep.
45:1469–1478. 2018. View Article : Google Scholar
|
|
19
|
Loo DT: TUNEL assay. An overview of
techniques. Methods Mol Biol. 203:21–30. 2002.
|
|
20
|
Wang B, Wang Y, Zhang J, Hu C, Jiang J, Li
Y and Peng Z: ROS-induced lipid peroxidation modulates cell death
outcome: Mechanisms behind apoptosis, autophagy, and ferroptosis.
Arch Toxicol. 97:1439–1451. 2023. View Article : Google Scholar
|
|
21
|
Rizwan H, Pal S, Sabnam S and Pal A: High
glucose augments ROS generation regulates mitochondrial dysfunction
and apoptosis via stress signalling cascades in keratinocytes. Life
Sci. 241:1171482020. View Article : Google Scholar
|
|
22
|
Battaglia AM, Chirillo R, Aversa I, Sacco
A, Costanzo F and Biamonte F: Ferroptosis and cancer: Mitochondria
meet the ‘Iron Maiden’ cell death. Cells. 9:15052020. View Article : Google Scholar
|
|
23
|
Fu B, Lou Y, Wu P, Lu X and Xu C: Emerging
role of necroptosis, pyroptosis, and ferroptosis in breast cancer:
New dawn for overcoming therapy resistance. Neoplasia.
55:1010172024. View Article : Google Scholar
|
|
24
|
Sajeev A, Sailo B, Unnikrishnan J,
Talukdar A, Alqahtani MS, Abbas M, Alqahtani A, Sethi G and
Kunnumakkara AB: Unlocking the potential of Berberine: Advancing
cancer therapy through chemosensitization and combination
treatments. Cancer Lett. 597:2170192024. View Article : Google Scholar
|
|
25
|
Chen X, Comish PB, Tang D and Kang R:
Characteristics and biomarkers of ferroptosis. Front Cell Dev Biol.
9:6371622021. View Article : Google Scholar
|
|
26
|
Chen F, Kang R, Tang D and Liu J:
Ferroptosis: Principles and significance in health and disease. J
Hematol Oncol. 17:412024. View Article : Google Scholar
|
|
27
|
Yadav S, Sharma A, Nayik GA, Cooper R,
Bhardwaj G, Sohal HS, Mutreja V, Kaur R, Areche FO, AlOudat M, et
al: Review of shikonin and derivatives: Isolation, chemistry,
biosynthesis, pharmacology and toxicology. Front Pharmacol.
13:9057552022. View Article : Google Scholar
|
|
28
|
Iranzadeh S, Dalil D, Kohansal S and
Isakhani M: Shikonin in breast cancer treatment: A comprehensive
review of molecular pathways and innovative strategies. J Pharm
Pharmacol. 76:967–982. 2024. View Article : Google Scholar
|
|
29
|
Qi K, Li J, Hu Y, Qiao Y and Mu Y:
Research progress in mechanism of anticancer action of shikonin
targeting reactive oxygen species. Front Pharmacol. 15:14167812024.
View Article : Google Scholar
|
|
30
|
Lin SS, Chang TM, Wei AI, Lee CW, Lin ZC,
Chiang YC, Chi MC and Liu JF: Acetylshikonin induces necroptosis
via the RIPK1/RIPK3-dependent pathway in lung cancer. Aging (Albany
NY). 15:14900–14914. 2023. View Article : Google Scholar
|
|
31
|
Zhang Z, Bai J, Zeng Y, Cai M, Yao Y, Wu
H, You L, Dong X and Ni J: Pharmacology, toxicity and
pharmacokinetics of acetylshikonin: A review. Pharm Biol.
58:950–958. 2020. View Article : Google Scholar
|
|
32
|
Sun DX, Tian HF, Meng ZY, Du A, Yuan D, Gu
RL, Wu ZN and Dou GF: Quantitative determination of acetylshikonin
in macaque monkey blood by LC-ESI-MS/MS after precolumn
derivatization with 2-mercaptoethanol and its application in
pharmacokinetic study. Acta Pharmacol Sin. 29:1499–1506. 2008.
View Article : Google Scholar
|
|
33
|
Cho MH, Paik YS and Hahn TR: Physical
stability of shikonin derivatives from the roots of Lithospermum
erythrorhizon cultivated in Korea. J Agric Food Chem. 47:4117–4120.
1999. View Article : Google Scholar
|
|
34
|
Zhang Q, Hao S, Wei G, Liu X and Miao Y:
The p53-mediated cell cycle regulation is a potential mechanism for
emodin-suppressing osteosarcoma cells. Heliyon. 10:e268502024.
View Article : Google Scholar
|
|
35
|
Engert F, Kovac M, Baumhoer D, Nathrath M
and Fulda S: Osteosarcoma cells with genetic signatures of BRCAness
are susceptible to the PARP inhibitor talazoparib alone or in
combination with chemotherapeutics. Oncotarget. 8:48794–48806.
2017. View Article : Google Scholar
|
|
36
|
Wei J, Liu Z, He J, Liu Q, Lu Y, He S,
Yuan B, Zhang J and Ding Y: Traditional Chinese medicine reverses
cancer multidrug resistance and its mechanism. Clin Transl Oncol.
24:471–482. 2022. View Article : Google Scholar
|
|
37
|
Miao K, Liu W, Xu J, Qian Z and Zhang Q:
Harnessing the power of traditional Chinese medicine monomers and
compound prescriptions to boost cancer immunotherapy. Front
Immunol. 14:12772432023. View Article : Google Scholar
|
|
38
|
Yuan J, Liu Y, Zhang T, Zheng C, Ding X,
Zhu C, Shi J and Jing Y: Traditional Chinese medicine for breast
cancer treatment: A bibliometric and visualization analysis. Pharm
Biol. 62:499–512. 2024. View Article : Google Scholar
|
|
39
|
Argenziano M, Tortora C, Pota E, Di Paola
A, Di Martino M, Di Leva C, Di Pinto D and Rossi F: Osteosarcoma in
children: Not only chemotherapy. Pharmaceuticals (Basel).
14:9232021. View Article : Google Scholar
|
|
40
|
Giliberti G, Marrapodi MM, Di Feo G, Pota
E, Di Martino M, Di Pinto D, Rossi F and Di Paola A: Curcumin and
methotrexate: A promising combination for osteosarcoma treatment
via hedgehog pathway inhibition. Int J Mol Sci. 25:113002024.
View Article : Google Scholar
|
|
41
|
Hsu PC, Tsai CC, Lin YH and Kuo CY:
Therapeutic targeting of apoptosis, autophagic cell death,
necroptosis, pyroptosis, and ferroptosis pathways in oral squamous
cell carcinoma: Molecular mechanisms and potential strategies.
Biomedicines. 13:17452025. View Article : Google Scholar
|
|
42
|
Ma X, Zhao J and Feng H: Targeting iron
metabolism in osteosarcoma. Discov Oncol. 14:312023. View Article : Google Scholar
|
|
43
|
Zhao J, Zhao Y, Ma X, Zhang B and Feng H:
Targeting ferroptosis in osteosarcoma. J Bone Oncol. 30:1003802021.
View Article : Google Scholar
|