Introduction
Osteoporosis (OP) is a systemic skeletal disorder
characterized by reduced bone mass and deterioration of bone
microarchitecture, resulting in increased bone fragility and a
higher risk of fractures. This condition markedly impairs the
quality of life of affected patients and may even lead to increased
mortality rates (1). As the global
population ages at an accelerating rate, the incidence of OP
continues to increase. In China alone, the number of individuals
aged ≥60 years is >210 million, posing considerable challenges
for the prevention and management of OP (2). Therefore, the development of
effective therapeutic strategies is of substantial clinical
importance.
Currently, clinical treatments for OP primarily
include pharmacological therapy, exercise interventions and
physical therapies (3,4). However, each of these approaches
presents notable limitations. For example, while bisphosphonates
effectively inhibit bone resorption, long-term use has been
associated with adverse effects such as osteonecrosis of the jaw
and gastrointestinal disturbances (5). Similarly, hormone replacement therapy
may increase the risk of cardiovascular events and breast cancer in
certain patient populations (6,7).
Therefore, the development of novel and safer therapeutic
strategies remains an important focus in current OP research.
In previous years, oxidative stress has emerged as a
notable contributor to the pathogenesis of OP. Factors such as
aging, iron overload and estrogen deficiency can disrupt the
balance between oxidative and antioxidant systems in the body,
leading to excessive accumulation of reactive oxygen species (ROS)
(8,9). These elevated ROS levels cause
oxidative damage to key macromolecules including DNA, lipids and
proteins, thereby accelerating cellular apoptosis (10). In the context of OP, oxidative
stress modulates signaling pathways, cytokine expression and
protein activity in bone marrow mesenchymal stem cells (BMSCs),
osteoblasts and osteoclasts (11).
Oxidative stress impairs the osteogenic differentiation potential
of BMSCs, inhibits osteoblast mineralization, and promotes the
activation, proliferation and maturation of osteoclasts (12,13).
This leads to a disrupted balance between bone formation and
resorption, impairs bone remodeling and ultimately accelerates the
progression of OP (14).
The research on natural drugs for the treatment of
OP has recently attracted widespread attention (15,16).
Hyperoside (Hyp), as a natural flavonoid compound existing in
various plants, such as Crataegus, Apocynum venetum and
Hypericum perforatum, exhibits multiple pharmacological
activities. Previous studies have demonstrated that Hyp has a
protective effect on the cardiovascular and nervous systems
(17,18). However, the therapeutic effect and
mechanism of Hyp on OP remain insufficiently characterized.
Therefore, the present study aimed to explore the therapeutic
effect and molecular mechanism of Hyp on OP, providing new ideas
and a theoretical basis for the treatment of OP through a
combination of in vivo and in vitro experiments.
Materials and methods
Reagents
Hyp (cat. no. HY-N0452) and LY294002 (cat. no.
HY-10108) were purchased from MedChemExpress with a purity of
99.5%. The RNA extraction kit (cat. no. AG21207) was selected from
Hunan Accurate Bio-Medical Technology Co., Ltd. Primer synthesis
was completed by Sangon Biotech Co., Ltd. The Cell Counting Kit-8
(CCK-8) (cat. no. C0037), alkaline phosphatase (ALP) staining kit
(cat. no. C3206) and Alizarin red S (ARS) staining kit (cat. no.
C0148S) were all purchased from Beyotime Biotechnology. The primary
antibodies runt-related transcription factor 2 (Runx2) (cat. no.
AF5186, rabbit), Osterix (cat. no. DF7731, rabbit), β-actin (cat.
no. AF7018, rabbit), phosphorylated (p)-PI3K (cat. no. AF3242,
rabbit), PI3K (cat. no. AF6241, rabbit), AKT (cat. no. AF0832,
rabbit), p-AKT (cat. no. AF0016, rabbit), as well as the
corresponding HRP-conjugated goat anti-rabbit IgG secondary
antibody (cat. no. S0001), were all purchased from Affinity
Biosciences. Isoflurane (cat. no. R510-22-10) was purchased from
Shenzhen Ruiwode Life Science Co., Ltd.
Animal experimentation
A total of 40 female C57BL/6J mice were purchased
from the Guangdong Provincial Medical Experimental Center [license
no. SCXK(YUE)2022-0002]. Female mice (age, 8 weeks; weight, 20–25
g) were randomly divided into control, model, low-concentration and
high-concentration groups (n=10 each) and housed in the specific
pathogen-free-grade Experimental Animal Center of The First
Affiliated Hospital of Guangzhou University of Chinese Medicine
(Guangzhou, China). The housing conditions were as follows:
22–25°C; 40–60% humidity; 12-h light/dark cycle; ad libitum
access to food/water. After 1 week of acclimation, except for the
control group, bilateral ovariectomy (OVX) was performed under
isoflurane inhalation anesthesia (5% for induction and 2% for
maintenance via precision vaporizer), with depth confirmed by loss
of pedal reflex. At 1-week post-surgery, the low- and
high-concentration groups were intragastrically administered 40 and
80 mg/kg Hyp, respectively, and the control and model groups
received an equal volume of vehicle (0.9% NaCl containing <0.1%
DMSO), once daily for 8 weeks. The selected doses were based on a
previous study reporting osteoprotective effects of Hyp in
ovariectomized mice (19). Humane
endpoints were defined as >20% weight loss, inability to access
food or water, severe distress or moribund state; health and
behavior were monitored daily. Euthanasia was performed by deep
anesthesia with 5% isoflurane followed by cervical dislocation,
with death confirmed by absence of heartbeat, respiration and
corneal reflex. The total experimental duration was 10 weeks. No
unexpected deaths occurred, and all procedures were approved by the
Animal Experiment Ethics Committee of The First Affiliated Hospital
of Guangzhou University of Chinese Medicine (approval no.
GZTCMF1-202403241).
Micro-CT scanning
The lower limbs of mice were positioned on the
scanning table of the MicroCT scanner (SkyScan 1276;
Bruker-Michrom, Inc.), with scanning parameters set to appropriate
values, including a voltage of 50 kV, a current of 200 µA and a
scanning resolution of 9 µm. The scan was subsequently performed.
After the scan was completed, specific reconstruction algorithms in
the scanning software were used to reconstruct the two-dimensional
projection images into three-dimensional images. Subsequently,
CT-analyser (CTAn) software (version 1.17.7.2; Bruker-Michrom,
Inc.) was used for data analysis. Initially, the scan data were
imported into the CTAn software, the appropriate region of interest
(ROI) was selected, typically starting with the distal femoral
growth plate, and 150 slices were analyzed upwards of this ROI
(Fig. S1). Using the standard
analysis function in CTAn, parameters such as trabecular thickness
(Tb.Th), trabecular separation (Tb.Sp), trabecular number (Tb.N)
and bone volume fraction (BV/TV) were calculated. Based on set
threshold values, the software automatically identified bone and
non-bone tissues and generated data for the aforementioned bone
structure parameters. Finally, the analysis results were exported
and the parameters compared between different groups to further
analyze trends and variations.
Hematoxylin and eosin (H&E) and
tartrate-resistant acid phosphatase (TRAP) staining
The mouse lower limb bone tissue samples were rinsed
with PBS (Gibco; Thermo Fisher Scientific, Inc.) and then fixed in
4% paraformaldehyde at 4°C for 24 h. Subsequently, they were
immersed in the decalcification solution for 2 weeks for
decalcification, followed by rinsing with distilled water. After
that, the samples were dehydrated with successive concentrations of
alcohol and treated with xylene for permeabilization. Subsequently,
the bone tissues were embedded in paraffin, trimmed and cut into
5-µm sections. The sections were placed in a 63°C constant
temperature oven for baking for 2 h. During the H&E staining
process, the sections were first dewaxed and hydrated, then stained
with hematoxylin solution for 5 min at room temperature (22–25°C).
After differentiation with hydrochloric acid alcohol and bluing
with tap water, the sections were stained with eosin solution for 2
min at room temperature. Finally, the sections were dehydrated with
a gradient alcohol series, permeabilized with xylene and sealed
with neutral balsam. Images were then captured using a light
microscope (CX43; Olympus Corporation).
For TRAP staining, the sections were similarly
dewaxed and rehydrated. TRAP staining was performed using a
commercial TRAP staining kit (cat. no. G1492; Beijing Solarbio
Science & Technology Co., Ltd.) according to the manufacturer's
instructions. Briefly, the sections were incubated in TRAP working
solution containing naphthol AS-BI phosphate and Fast Red Violet LB
salt in the presence of sodium tartrate at 37°C for 1 h in the
dark. After washing with distilled water, the sections were
counterstained lightly with hematoxylin, then dehydrated, cleared
in xylene and sealed with neutral balsam. TRAP-positive osteoclasts
appeared as red or purplish-red multinucleated cells located along
the trabecular bone surface. Representative images were obtained
under a light microscope, and osteoclast number was
semi-quantitatively evaluated by counting the number of
TRAP-positive cells per field under standardized magnification.
Acquisition of Hyp action targets
The structure of Hyp was obtained from the PubChem
database (https://pubchem.ncbi.nlm.nih.gov/) and then imported
into the SwissTargetPrediction (http://www.swisstargetprediction.ch/), PharmMapper
(http://www.lilab-ecust.cn/pharmmapper/), Super-Pred
(https://insilico-cyp.charite.de/SuperCYPsPred/) and
TargetNet (http://targetnet.scbdd.com/) databases to predict its
potential protein targets. After removing duplicates and
unannotated targets, all candidate targets were imported into the
UniProt database (https://www.uniprot.org/) using the parameters
‘reviewed’, ‘Homo sapiens’ and ‘gene name primary’ to
standardize the target names into official gene symbols. This
approach has been widely applied in previous network pharmacology
studies (20,21).
Prediction of OP targets and
differential gene analysis
The Gene Expression Omnibus database (https://www.ncbi.nlm.nih.gov/geo/) was searched
using ‘osteoporosis’ as the key word, and the species ‘Homo
Sapiens’ was selected to obtain the GSE35956 (22) gene chip dataset and the GSE80614
(23). gene chip dataset. In R
software (version 4.3.1; The R Foundation for Statistical
Computing), the parameters P<0.05 and |log (fold change) |>2
were set to screen for differential genes and draw a volcano plot.
Subsequently, searches and screenings were conducted in the
GeneCards (https://www.genecards.org/) and
Online Mendelian Inheritance in Man databases (https://www.omim.org/) using the aforementioned key
word. Finally, the datasets were integrated and duplicates were
removed to obtain the disease-target dataset.
Acquisition of potential action
targets of Hyp for treating OP
To conduct an in-depth study and elucidate the
interaction mechanisms of the active ingredients of Hyp in the
intervention and treatment of OP, a Venn analysis was performed
between the action targets of the active ingredients of Hyp and
OP-related targets. The overlapping region represents the potential
targets of the Hyp for treating OP. Subsequently, a Venn diagram
was generated to visualize these intersecting targets.
Construction of the protein-protein
interaction (PPI) network and screening of core targets
The intersecting targets between the OP and the Hyp
were imported into the Search Tool for the Retrieval of Interacting
Genes/Proteins (STRING) database (https://string-db.org/) for PPI analysis. Briefly, the
intersecting target proteins were imported into the STRING
database, the organism was set to Homo sapiens, and the
database query was performed to generate the PPI network. This
process was used to construct a PPI network model, thereby
facilitating the understanding of cellular functional interactions
between protein expression profiles and enabling a deeper
comprehension of protein functions at the cellular level. Following
retrieval, the PPI network diagram was exported and its
corresponding ‘.tsv’ format file was downloaded. The ‘.tsv’ file
was then imported into Cytoscape (version 3.9.1; http://cytoscape.org/) and the topological parameters
of each node in the network were analyzed using the CytoNCA plugin.
Target importance within the network was evaluated from three
dimensions: Node connectivity, betweenness centrality and closeness
centrality. Finally, a core target screening network was
constructed to identify the core targets.
Enrichment analysis of Gene Ontology
(GO) biological functions and Kyoto Encyclopedia of Genes and
Genomes (KEGG) information transmission pathways of core
targets
The Metascape database (https://metascape.org) enables enrichment of target
genes into their associated genomes and signaling pathways,
annotates gene functions, visualizes enrichment analysis results
and standardizes genes with different identification codes. To
further elucidate the gene functions of the screened targets and
their involvement in signaling pathways, these targets were
imported into the Metascape database for GO functional enrichment
analysis and KEGG pathway enrichment analysis. The Metascape
database was used to visualize the enrichment analysis results.
Based on ascending P-values, the top 10 results from each GO
category and the top 20 KEGG pathways were selected and visualized
using the platform.
Cell culture
Mouse primary BMSCs (cat. no. MIC-BIOSPECIES-s018)
were purchased from Guangzhou Xinyuan Biotechnology Co., Ltd. The
cells were cultured in α-Minimum Essential Medium (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin-streptomycin. Cultures were maintained in a humidified
incubator at 37°C with 5% CO2. The medium was refreshed
every 2 days. Once the cells reached 80–90% confluence, they were
detached using 0.25% trypsin-EDTA and passaged at a ratio of 1:3.
Cells at passages 3–6 were used for all subsequent experiments.
CCK-8 assay
The cells were seeded into a 96-well plate at a
density of 5×103 cells/well and cultured overnight to
allow adherence. For the Hyp cytotoxicity assay, the cells were
treated with different concentrations of Hyp (0, 10, 20, 40 and 80
µM) for 24 h at 37°C. For the oxidative stress model, the cells
were exposed to H2O2 at concentrations of 50,
100, 200 or 400 µM for 24 h at 37°C to evaluate the dose-dependent
cytotoxicity. To assess the protective effects of Hyp, the cells
were pretreated with different concentrations of Hyp (10, 20 and 40
µM) for 24 h, followed by exposure to 200 µM
H2O2 for 4 h at 37°C. After each treatment,
10 µl CCK-8 solution (Beyotime Biotechnology) was added to each
well and incubated for 1 h at 37°C. Finally, a microplate reader
was used to measure the absorbance at 450 nm.
5-Ethynyl-2′-deoxyuridine (EDU)
assay
An EDU incorporation assay (cat. no. C0075S;
Beyotime Biotechnology) was performed to evaluate the proliferation
of BMSCs. Briefly, BMSCs were pretreated with Hyp (20 or 40 µM) for
24 h at 37°C, followed by exposure to H2O2
(200 µM) for 4 h at 37°C. After treatment, the cells were washed
three times with PBS. Then, BMSCs were incubated with EDU for 2 h
at 37°C. The EDU working solution was prepared by diluting 10 mM
EDU stock with culture medium to a final concentration of 10 µM.
Following EDU incubation, the cells were fixed with 4%
paraformaldehyde for 15 min at room temperature. Cells were then
washed three times with PBS for 5 min each. After fixation, the
cells were permeabilized with 0.3% Triton X-100 in PBS for 15 min
and were subsequently washed again with wash buffer. EDU
incorporation was detected using an EDU detection kit, and the
proliferating cells were analyzed using a fluorescence
microscope.
ALP staining and ARS staining
BMSCs were seeded into 12-well plates. After cell
attachment to the surface, they were categorized into groups and
subjected to the respective interventions. Osteogenic induction was
initiated using an osteogenic induction medium containing 10 nM
dexamethasone, 50 µg/ml vitamin C and 10 mM β-sodium
glycerophosphate. The cells were divided into: i) A control group;
ii) a model group treated with 200 µM H2O2;
iii) a low-concentration group treated with 20 µM Hyp and 200 µM
H2O2; and iv) a high-concentration group
treated with 40 µM Hyp and 200 µM H2O2. The
culture medium was refreshed every 3 days. ALP staining was
performed on day 7. The cells were rinsed three times with PBS,
fixed with 4% paraformaldehyde for 30 min at room temperature, and
then stained using an ALP staining kit at 37°C for 30 min. ARS
staining was conducted on day 14. The cells were washed three times
with PBS, fixed with 4% paraformaldehyde for 30 min at room
temperature, and then stained with ARS at room temperature for 30
min. To quantitatively evaluate the degree of mineralization, the
ARS stain was eluted with 10% w/v cetylpyridinium chloride for 1 h,
and the OD value at 570 nm was measured using a microplate reader.
All images were captured under a light microscope (Olympus
Corporation), and quantitative analysis of mineralization intensity
was performed using ImageJ software (version 1.8.0; National
Institutes of Health).
Measurement of ROS level
The present study employed DCFH-DA to detect
intracellular ROS levels. After the aforementioned cell
intervention was completed, DCFH-DA (cat. no. S0033S; Beyotime
Biotechnology) was diluted to a final concentration of 10 µM in
serum-free medium and incubated at 37°C in the dark for 30 min. The
cells were then rinsed three times with PBS, and images were
captured under a fluorescence microscope. For flow cytometric
detection of ROS, after the incubation, the cells were gently
washed once with PBS to remove any excess fluorescent probe that
had not entered the cells. The cells were then digested with
trypsin and collected into centrifuge tubes. Following
centrifugation (300 × g, 5 min, 4°C), the supernatant was discarded
and 500 µl of PBS was added to resuspend the cells. The resulting
cell suspension was transferred into flow cytometry tubes and
analyzed on a BD FACSCalibur flow cytometer (BD Biosciences). Data
were processed using FlowJo software (version 10.8.1; BD
Biosciences).
Measurement of superoxide dismutase
(SOD) and malondialdehyde (MDA)
The BMSCs were seeded into 6-well plates. Once the
cells had adhered to the well surface, they were subjected to
intervention according to the aforementioned grouping for a
duration of 24 h. Upon completion of the intervention, cell samples
from each group were harvested. For the determination of
intracellular SOD level, the working solution was prepared
according to the protocol of the SOD activity detection kit (cat.
no. S0101; Beyotime Biotechnology). After incubation at 37°C for 30
min, the absorbance at 450 nm was measured using a microplate
reader. The SOD activity in the cell samples of each group was then
calculated based on the standard curve. For the detection of MDA
levels, the working solution was prepared according to the
instructions of the MDA detection kit (cat. no. S0131; Beyotime
Biotechnology). After thorough mixing with the samples, the mixture
was heated at 100°C for 15 min, cooled to room temperature in a
water bath and centrifuged at 1,000 × g for 10 min at room
temperature. A total of 200 µl supernatant was transferred into a
96-well plate, and the absorbance was measured at 532 nm using a
microplate reader. The MDA content in the cell samples of each
group was then calculated based on the standard curve.
Reverse transcription-quantitative PCR
(RT-qPCR)
The cell treatments were performed as
aforementioned, and total RNA was extracted from BMSCs using an RNA
extraction kit (cat. no. AG21207; Hunan Accurate Bio-Medical
Technology Co., Ltd.) according to the manufacturer's protocol.
After measuring the concentration, the RNA was reverse transcribed
into cDNA using the PrimeScript RT reagent kit (cat. no. RR047A;
Takara Bio, Inc.) with the following temperature protocol: 37°C for
15 min, 85°C for 5 sec, and hold at 4°C. qPCR was performed using a
SYBR® Green qPCR Mix (cat. no. AG11761; Hunan Accurate
Bio-Medical Technology Co., Ltd.). Using the cDNA of each group as
the template, and after adding the buffer and primers, PCR
amplification was performed according to the following parameters
described in the instruction manual: Initial denaturation at 95°C
for 30 sec, followed by 40 cycles of denaturation at 95°C for 5 sec
and annealing/extension at 60°C for 40 sec, in accordance with the
manufacturer's instructions. With 18S rRNA as the internal
control, the relative mRNA expression levels were calculated using
the 2−ΔΔCq method (24). The primer pair sequences for 18S
rRNA, Runx2, Alp, Osterix and type I collagen (Col1) are
listed in Table I.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Gene | Forward
(5′-3′) | Reverse
(5′-3′) |
|---|
| 18s |
TGGTTGCAAAGCTGAAACTTAAAG |
AGTCAAATTAAGCCGCAGGC |
| Runx2 |
GCCGGGAATGATGAGAACTA |
GGACCGTCCACTGTCACTTT |
| Alp |
CCAGAAAGACACCTTGACTGTGG |
TCTTGTCCGTGTCGCTCACCAT |
| Col1 |
CCTCAGGGTATTGCTGGACAAC |
CAGAAGGACCTTGTTTGCCAGG |
| Osterix |
GGCTTTTCTGCGGCAAGAGGTT |
CGCTGATGTTTGCTCAAGTGGTC |
Western blot analysis
The BMSCs were treated as aforementioned. Total
proteins were extracted from BMSCs using RIPA lysis buffer (cat.
no. P0013B; Beyotime Biotechnology) supplemented with a protease
and phosphatase inhibitor cocktail (cat. no. P1005; Beyotime
Biotechnology). Proteins were quantified by BCA assay and 30 µg
total protein was loaded per lane. Proteins were separated by
SDS-PAGE on 10% gels, then transferred to PVDF membranes (cat. no.
IPVH00010; MilliporeSigma). After blocking with 5% skim milk in TBS
containing 0.1% Tween-20 (TBST) for 2 h at room temperature, the
membranes were incubated overnight at 4°C with primary antibodies:
Runx2 (1:1,000), Osterix (1:1,000), PI3K (1:1,000), p-PI3K
(1:1,000), AKT (1:1,000), p-AKT (1:1,000) and β-actin (1:5,000).
Following three washes with TBST, membranes were incubated with
HRP-conjugated secondary antibodies (1:5,000) for 1.5 h at room
temperature. After further TBST washes, protein bands were
visualized using ECL Chemiluminescence Detection Kit (cat. no.
170-5061; Bio-Rad Laboratories, Inc.). Band densities were
semi-quantified using ImageJ software (version 1.8.0; National
Institutes of Health).
Immunohistochemistry
Paraffin-embedded mouse femur sections, prepared as
aforementioned for H&E and TRAP staining, were used for
immunohistochemical analysis. Briefly, the femurs were fixed in 4%
paraformaldehyde for 24 h at room temperature, decalcified in 10%
EDTA solution (pH 7.4) for 2 weeks, dehydrated, embedded in
paraffin and cut into 5-µm sections. After the sections had been
dewaxed, antigen retrieval was performed. The sections were then
treated with 3% H2O2 to block endogenous
peroxidase activity, followed by blocking with 5% normal goat serum
(cat. no. C0265; Beyotime Biotechnology) for 30 min at room
temperature. The sections were incubated overnight at 4°C with the
following primary antibodies: p-PI3K (cat. no. AF3242; 1:200) and
p-AKT (cat. no. AF0016; 1:200) (all from Affinity Biosciences). The
primary antibody was applied and incubated overnight at 4°C. On the
following day, after thorough washing with PBS, the corresponding
secondary antibody (1:200; cat. no. S0001; Affinity Biosciences)
was applied and incubated at room temperature for 2 h. The sections
were subsequently developed using a DAB chromogenic agent (cat. no.
P0203; Beyotime Biotechnology), counterstained with hematoxylin for
3 min at room temperature, dehydrated, cleared and mounted. The
staining results were observed and analyzed under a light
microscope (Olympus Corporation), and five random non-overlapping
fields per section were selected for semi-quantitative analysis
using ImageJ software (version 1.8.0; National Institutes of
Health).
Inhibitor treatment
To investigate the protective effects of Hyp and the
involvement of the PI3K/AKT signaling pathway, BMSCs were divided
into four groups: Control, model (H2O2), Hyp
and Hyp + LY294002. Cells in the control group were cultured under
standard conditions without any treatment. Cells in the model group
were exposed to 200 µM H2O2 for 4 h at 37°C
to induce oxidative stress. In the Hyp group, cells were pretreated
with Hyp (40 µM) for 24 h at 37°C, followed by 200 µM
H2O2 treatment for 4 h. To verify the role of
the PI3K/AKT signaling pathway, cells in the Hyp + LY294002 group
were pretreated with the PI3K inhibitor LY294002 (10 µM;
MedChemExpress) for 2 h at 37°C before Hyp intervention. After
pretreatment, the cells were exposed to Hyp (40 µM) for 24 h and
were subsequently treated with H2O2 (200 µM)
for 4 h at 37°C. LY294002 was dissolved in DMSO, and the final DMSO
concentration in the culture medium did not exceed 0.1% (v/v).
Statistical analysis
Statistical analyses were performed using SPSS
version 25.0 (IBM Corp.). Data are presented as the mean ± standard
deviation. Each experiment was independently repeated at least
three times. Differences among multiple groups were evaluated by
one-way analysis of variance (ANOVA). When significant differences
were detected by ANOVA, Tukey's honestly significant difference
post hoc test was applied for pairwise comparisons. P<0.05 was
considered to indicate a statistically significant difference.
Results
Hyp alleviates OP in mice induced by
OVX
The present study utilized micro-CT and H&E
staining to comprehensively assess structural alterations in the
lower limb bones of mice. Compared with in the control group, the
model group exhibited significant reductions in Tb.N, Tb.Th and
BV/TV, alongside a significant increase in Tb.Sp (Fig. 1A-E). These findings validated the
successful establishment of the OP model. Following Hyp
administration, these parameters significantly improved in a
dose-dependent manner, indicating that Hyp effectively promoted
trabecular bone formation, mitigated OVX-induced bone loss and
contributed to the restoration of bone microarchitecture.
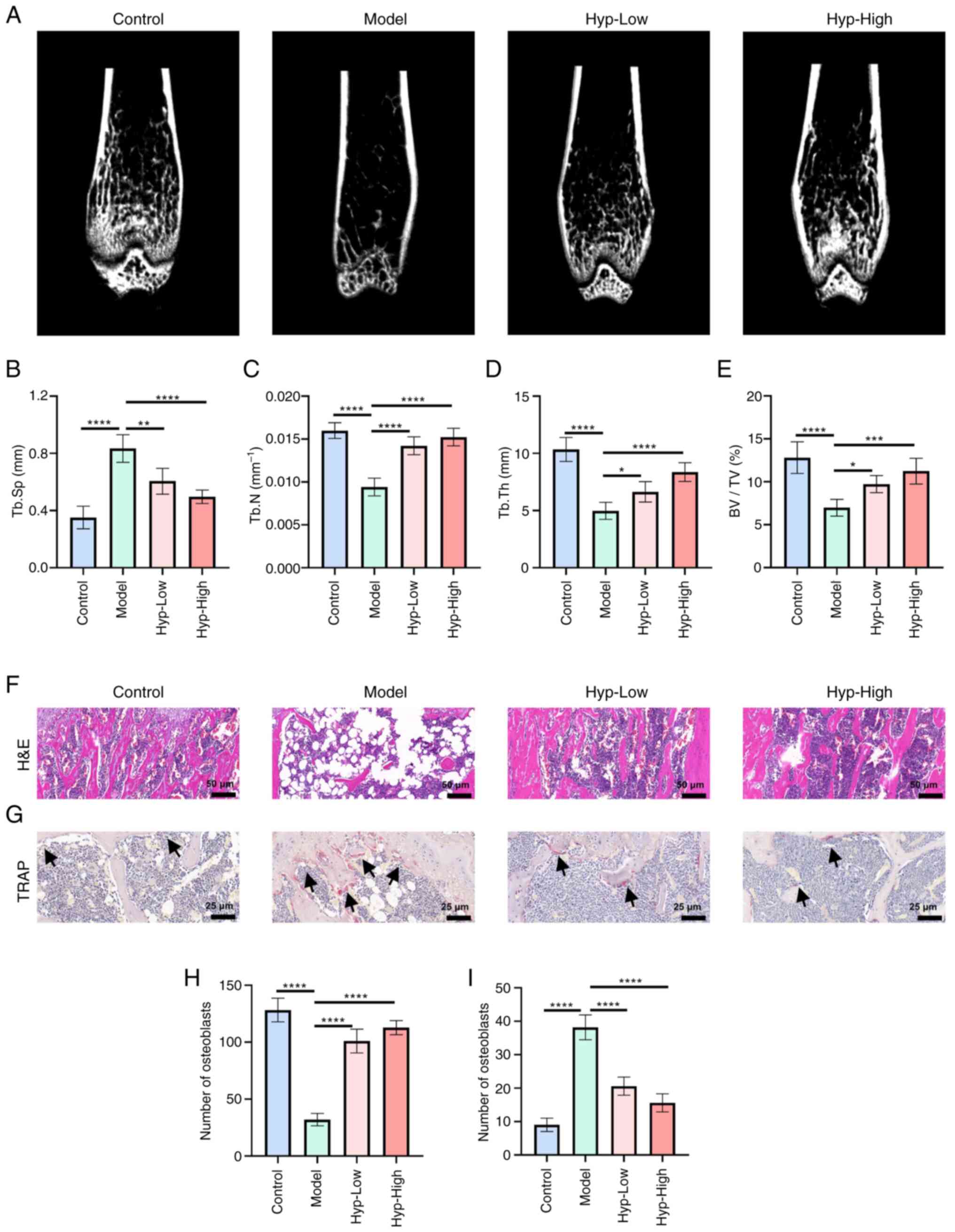 | Figure 1.Hyp alleviates ovariectomy-induced
osteoporosis in mice. (A) Representative micro-CT three-dimensional
reconstruction images of the mouse femur. Quantitative analyses of
(B) Tb.Sp, (C) Tb.N, (D) Tb.Th and (E) BV/TV (n=5). (F)
Representative H&E staining images of femoral sections (n=5;
×20 magnification). (G) Representative TRAP-stained sections
showing osteoclasts (indicated by black arrows) (n=5; ×40
magnification). (H) Quantification of osteoblast number per bone
surface (n=5). (I) Quantification of osteoclast number per bone
surface (n=5). Data are presented as the mean ± standard deviation.
*P<0.05, **P<0.01, ***P<0.001 and ****P<0.0001. Tb.Sp,
trabecular separation; Tb.N, trabecular number; Tb.Th, trabecular
thickness; BV/TV, bone volume fraction; TRAP, tartrate-resistant
acid phosphatase; Hyp, hyperoside. |
Histological evaluation by H&E staining further
corroborated these observations. In the OVX group, trabeculae were
sparse and attenuated, the marrow cavity was markedly expanded,
osteocyte density was reduced and cell morphology appeared
irregular (Fig. 1F). Additionally,
the number of empty lacunae was markedly elevated. By contrast,
bone sections from Hyp-treated mice exhibited more intact and
compact trabeculae, an increased number of osteocytes with
normalized morphology and a reduction in empty lacunae.
To further investigate cellular changes, the present
study quantitatively analyzed osteoblast and osteoclast
populations. The number of osteoblasts per bone surface was
significantly decreased in the OVX model mice but was restored in a
dose-dependent manner following Hyp treatment (Fig. 1H). Meanwhile, TRAP staining
revealed a substantial increase in TRAP-positive multinucleated
osteoclasts in the OVX group, indicative of enhanced bone
resorption (Fig. 1G and I).
Notably, Hyp significantly reduced osteoclast numbers per bone
surface compared with those in the OVX group. These results
underscored the dual modulatory effects of Hyp on bone regulation,
having simultaneously enhanced osteoblast activity and inhibited
osteoclastogenesis, thereby contributing to the maintenance of bone
homeostasis. Collectively, the present findings demonstrated that
Hyp not only ameliorated microarchitectural deterioration
associated with OP but also regulated key cellular mediators of
bone remodeling, supporting its therapeutic potential in the
management of OP.
Potential mechanism underlying the
effects of Hyp on the treatment of OP
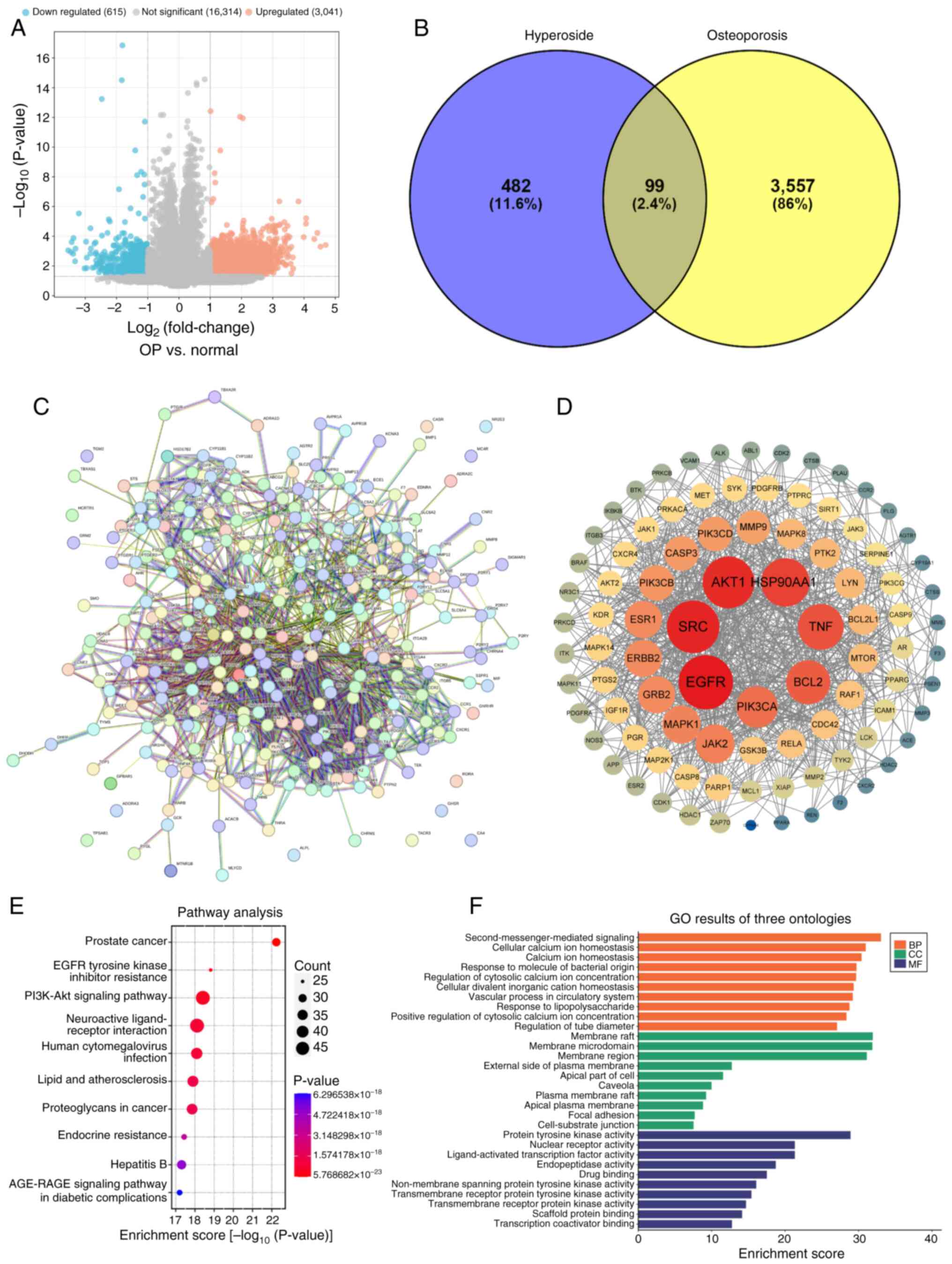
A volcano plot of the GSE35956 and GSE80614 gene
expression datasets are shown in Fig.
2A. A total of 581 drug targets and 3,565 disease targets
(Fig. 2B) were retrieved through
online databases. By conducting an intersection analysis with a
Venn diagram, 99 common target genes were determined and
subsequently designated as potential therapeutic targets for
further in-depth investigation (Fig.
2B). Subsequently, the STRING database was used to explore the
potential associations among these 99 common genes. Subsequently,
the analysis outcomes were imported into the Cytoscape software to
generate a PPI network for Hyp in OP (Fig. 2C and D). In the degree value
analysis of the PPI network, it was ascertained that the top 10
genes, namely Src, Hsp90aa1, Akt1, Egfr, Tnf, Pik3ca, Bcl2,
Grb2, Jak2 and Erbb2, were highly likely to assume a
central role in the therapeutic process of Hyp for OP. Further GO
molecular function analysis divulged that the top 5 functions of
Hyp encompassed ‘second-messenger-mediated signaling’, maintenance
of ‘cellular calcium ion homeostasis’, regulation of ‘calcium ion
homeostasis’, ‘response to molecule of bacterial origin’ and
‘regulation of cytosolic calcium ion concentration’ (Fig. 2F). This implied that Hyp may have
influenced intracellular signal transduction and ionic equilibrium
by regulating these molecular functions, thereby exerting a
beneficial effect on OP. Simultaneously, the KEGG enrichment
analysis results demonstrated that pathways such as ‘Prostate
cancer’, ‘EGFR tyrosine kinase inhibitor resistance’, ‘PI3K-Akt
signaling pathway’, ‘Neuroactive ligand-receptor interaction’ and
‘Human cytomegalovirus infection’ held significant implications in
the treatment of OP with Hyp (Fig.
2E).
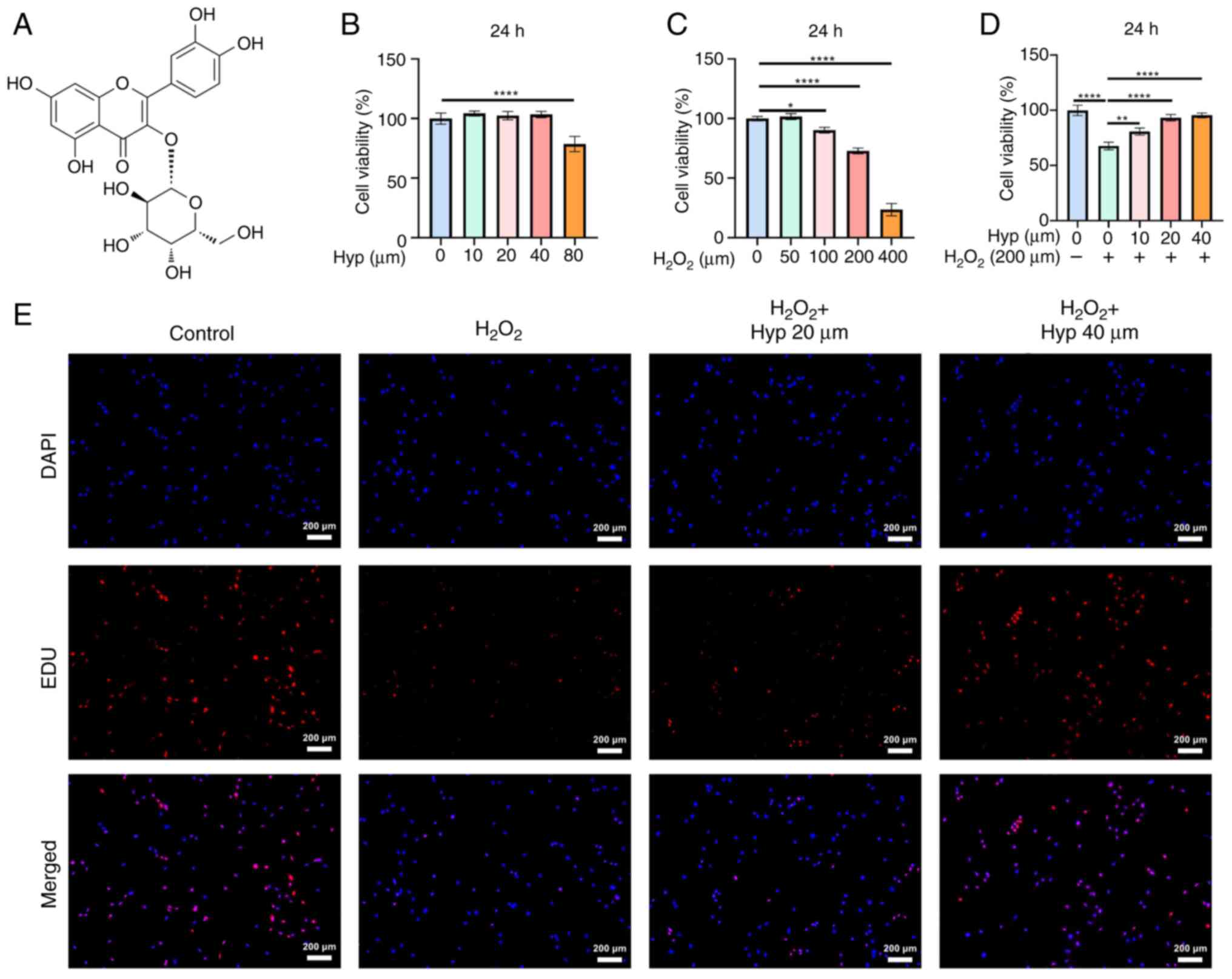
Effect of Hyp on the viability of
BMSCs
The chemical structure of Hyp is shown in Fig. 3A. The CCK-8 assay demonstrated that
Hyp concentrations ≤40 µM did not exhibit significant cytotoxicity
on BMSCs, with 20 and 40 µM selected for subsequent experiments
(Fig. 3B).
H2O2 at concentrations >100 µM
significantly inhibited BMSC proliferation, and 200 µM
H2O2 was chosen for inducing oxidative stress
in BMSCs (Fig. 3C). Notably, Hyp
effectively alleviated the inhibitory effect of
H2O2 on BMSC proliferation (Fig. 3D). Further analysis using EDU
labeling supported these findings. H2O2
treatment resulted in a reduced number of proliferating cells, as
indicated by lower EDU incorporation (Fig. 3E). By contrast, Hyp treatment
notably increased EDU-positive cells, suggesting its potential to
promote BMSC proliferation under oxidative stress.
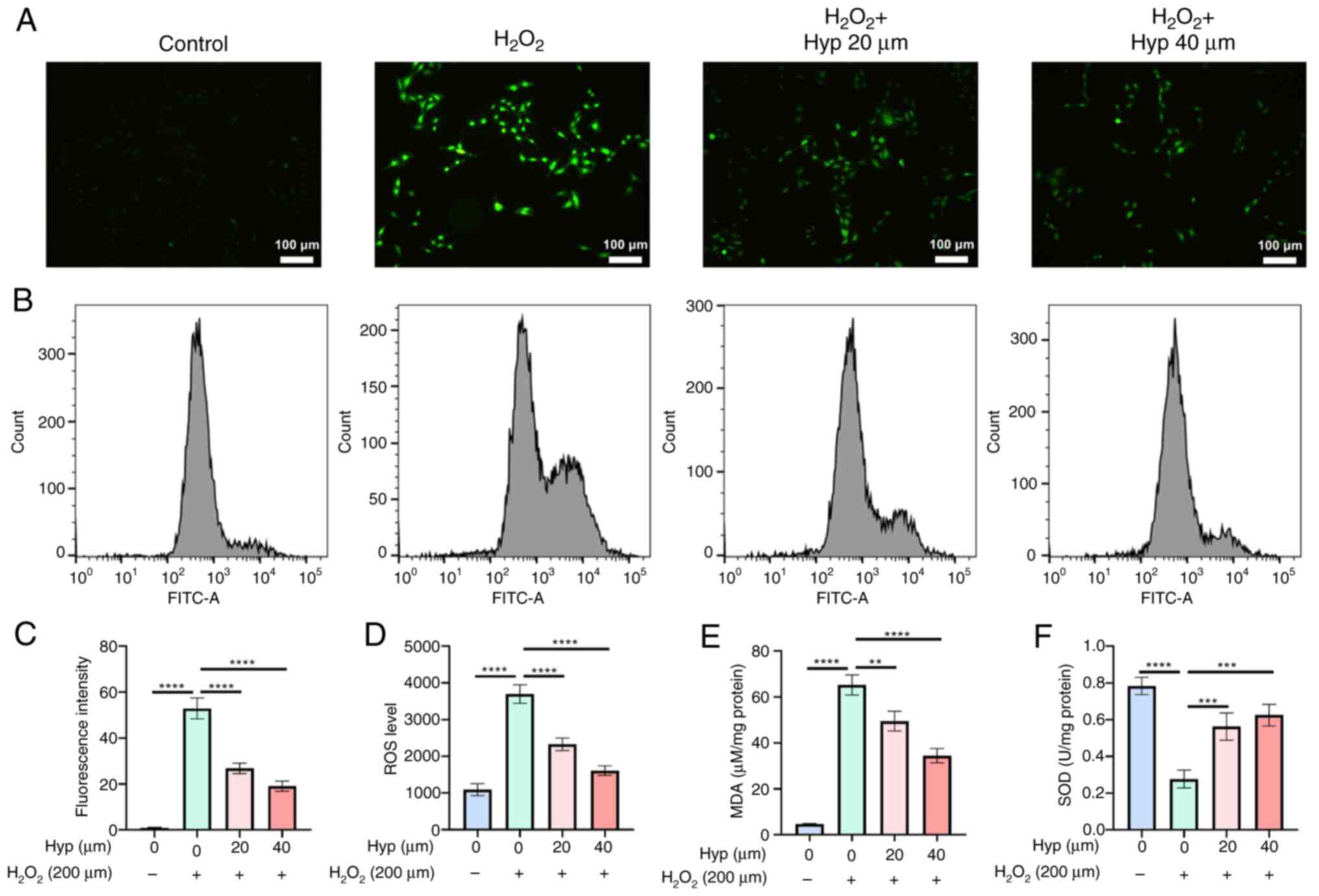
Hyp is capable of alleviating the
oxidative stress induced by H2O2
The DCFH-DA staining outcomes manifested that upon
the administration of H2O2, a conspicuous
augmentation in fluorescence intensity was observed, signifying a
pronounced elevation in the ROS levels within the cells (Fig. 4A and C). By contrast, when Hyp was
introduced, a reduction in the fluorescence intensity of BMSCs
ensued, implying a reduced ROS level. This was further corroborated
by flow cytometry, wherein the average ROS levels were
significantly elevated post-H2O2 treatment
and subsequently decreased after Hyp treatment (Fig. 4B and D). Furthermore, the results
of MDA detection assays indicated that H2O2
intervention led to a significant upsurge in MDA levels, whereas
Hyp treatment effectively mitigated these levels (Fig. 4E). Similarly, the SOD detection
assays revealed that H2O2 treatment induced a
significant decline in SOD levels, which were significantly
augmented following Hyp treatment (Fig. 4F). In summary, these comprehensive
findings demonstrated that Hyp possessed the capacity to alleviate
H2O2-induced oxidative stress levels in
BMSCs. This not only holds promise for safeguarding cells against
oxidative damage but also for maintaining cellular homeostasis,
potentially providing new options for therapeutic interventions
aimed at combating oxidative stress-related cellular
dysfunctions.
Hyp protects the osteogenic
differentiation ability of BMSCs
In the present study, the effect of Hyp on the
osteogenic differentiation ability of BMSCs was investigated under
oxidative stress conditions. The results of ALP and ARS staining
assays demonstrated that the osteogenic differentiation capacity of
BMSCs was significantly reduced after H2O2
intervention (Fig. 5A-D). However,
treatment with Hyp significantly enhanced the osteogenic
differentiation of BMSCs, as evidenced by the increased ALP
activity and the greater number of calcium nodules detected by ARS
staining. Furthermore, RT-qPCR analysis revealed that the
expression levels of Col1, Osterix, Runx2 and Alp
were significantly decreased upon H2O2
exposure (Fig. 5E-H). By contrast,
Hyp treatment led to a significant upregulation of these genes,
indicating its positive regulatory role in osteogenic gene
expression. Consistent with the RT-qPCR results, the results of
western blot analysis also showed that Hyp treatment significantly
increased the protein expression levels of Osterix and Runx2
(Fig. 5I-K). Collectively, these
results provided compelling evidence that Hyp effectively protected
the osteogenic differentiation ability of BMSCs under oxidative
stress conditions, suggesting its potential therapeutic application
in bone-related disorders associated with oxidative stress.
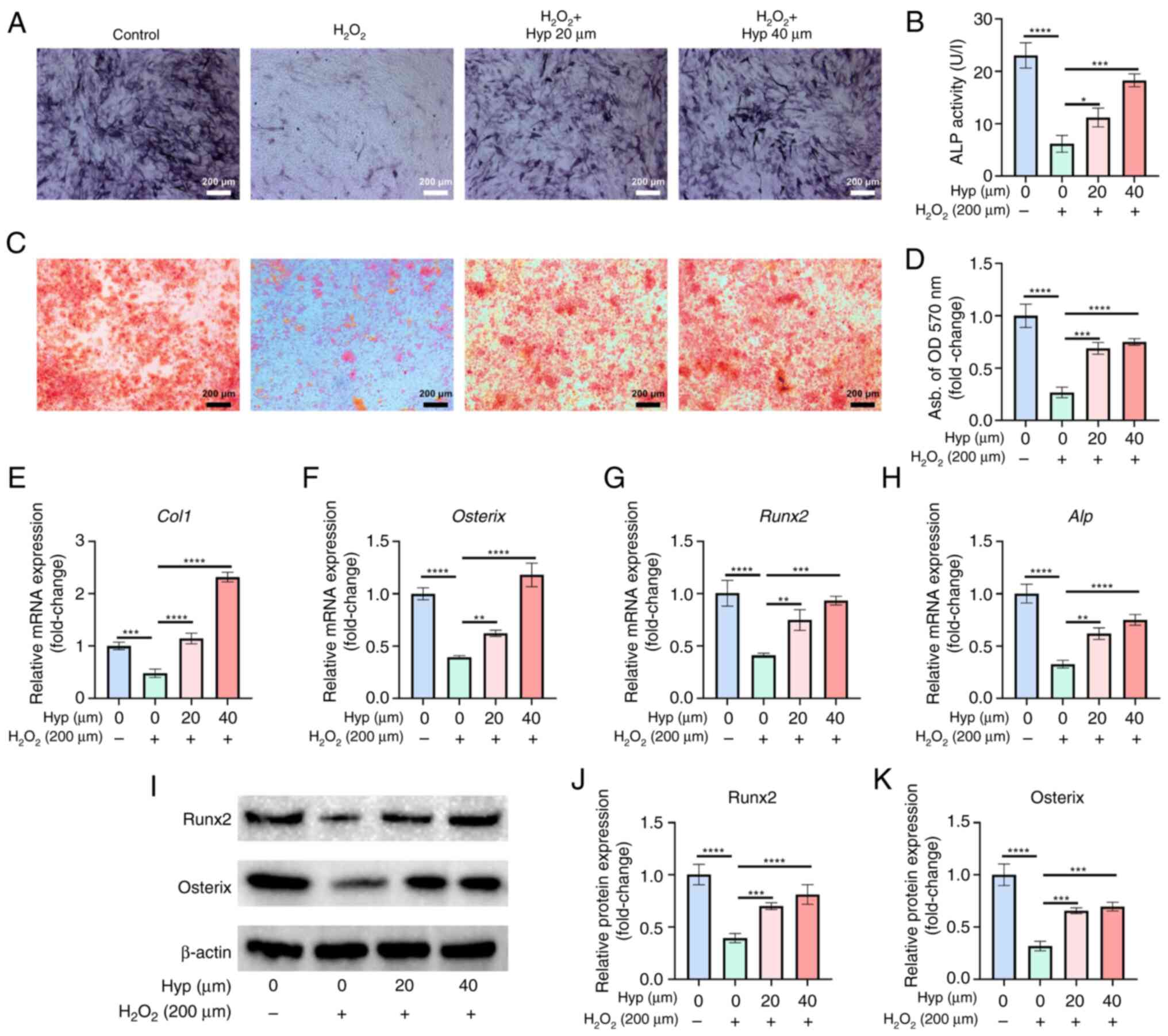 | Figure 5.Hyperoside rescues the osteogenic
differentiation function of bone marrow mesenchymal stem cells. (A)
Representative images of ALP staining and (B) quantification of ALP
activity (n=3; ×5 magnification). (C) Representative images of ARS
staining and (D) quantification of ARS staining (n=3; ×5
magnification). The mRNA expression levels of (E) Col1, (F)
Osterix, (G) Runx2 and (H) Alp (n=3). (I)
Representative western blot images, and semi-quantification of the
protein expression levels of (J) Runx2 and (K) Osterix (n=3). Data
are presented as the mean ± standard deviation. *P<0.05,
**P<0.01, ***P<0.001 and ****P<0.0001. OD, optical
density; Hyp, Hyperoside; Col1, type-I collagen; Runx2,
runt-related transcription factor 2; ARS, Alizarin red S;
ALP/Alp, alkaline phosphatase. |
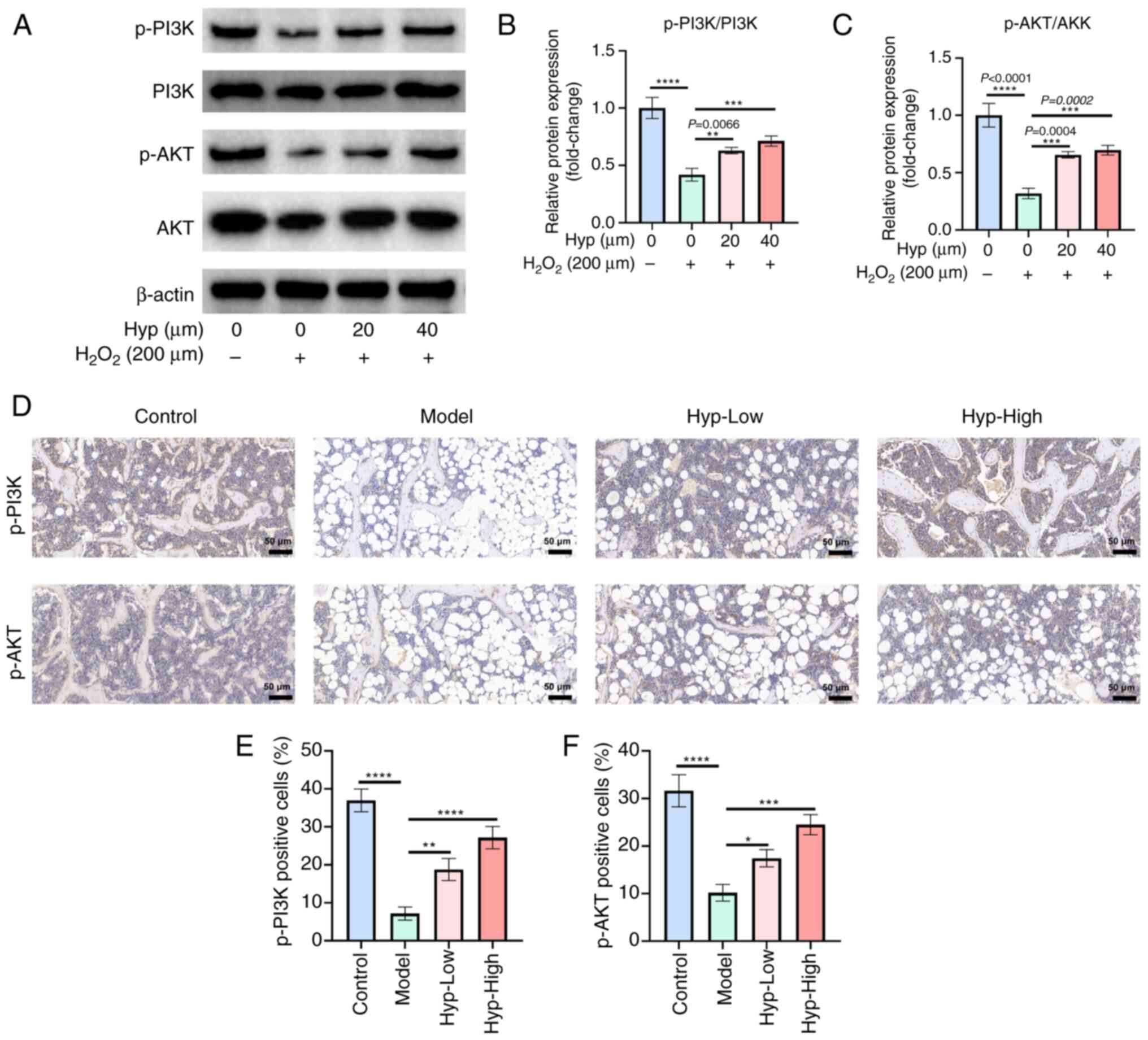
Hyp initiates activation of the
PI3K/AKT signaling pathway
Western blot analysis of BMSCs revealed that
H2O2 intervention led to a significant
decline in the phosphorylation levels of both PI3K and AKT. By
contrast, Hyp treatment effectively reversed this downward trend
and promoted the activation of PI3K and AKT phosphorylation
(Fig. 6A-C). This was further
supported by immunohistochemical analysis of mouse femoral tissues,
which indicated that the expression levels of p-PI3K and p-AKT were
significantly reduced in the model group compared with those in the
control group (Fig. 6D-F).
However, following Hyp intervention, a significant increase in the
expression of p-PI3K and p-AKT was observed.
Inhibition of the PI3K/AKT pathway
attenuates the antioxidative and osteogenic effects of Hyp
To investigate the role of the PI3K/AKT signaling
pathway in the antioxidative and osteogenic effects of Hyp, a
series of in vitro experiments were conducted using
LY294002, a specific inhibitor of the PI3K/AKT pathway. LY294002 is
a synthetic flavonoid derivative that acts as a reversible,
ATP-competitive inhibitor of PI3Ks, thereby blocking the
phosphorylation and subsequent activation of AKT and downstream
signaling molecules. DCFH-DA fluorescence staining revealed that
Hyp treatment significantly reduced
H2O2-induced intracellular ROS levels, as
evidenced by decreased green fluorescence intensity (Fig. 7A and B). However, co-treatment with
LY294002 partially yet significantly restored the fluorescence
signal and elevated ROS levels, suggesting that the antioxidative
capacity of Hyp was impaired when the PI3K/AKT pathway was
inhibited. Regarding osteogenic differentiation, ALP staining and
enzyme activity assays demonstrated that Hyp significantly
increased ALP activity, indicating the promotion of early
osteogenesis (Fig. 7C and D). By
contrast, the ALP activity and staining intensity were
significantly reduced in the Hyp + LY294002 group, approaching the
levels observed in the H2O2 group. These
findings suggested that the PI3K/AKT pathway was involved in
Hyp-induced early osteogenic differentiation. Furthermore, ARS
staining showed a substantial increase in mineralized nodule
formation in the Hyp-treated group, with quantitative analysis
supporting elevated calcium deposition (Fig. 7E and F). However, in the Hyp +
LY294002 group, both the ARS-stained area and calcium content were
markedly decreased, indicating compromised osteogenic
mineralization.
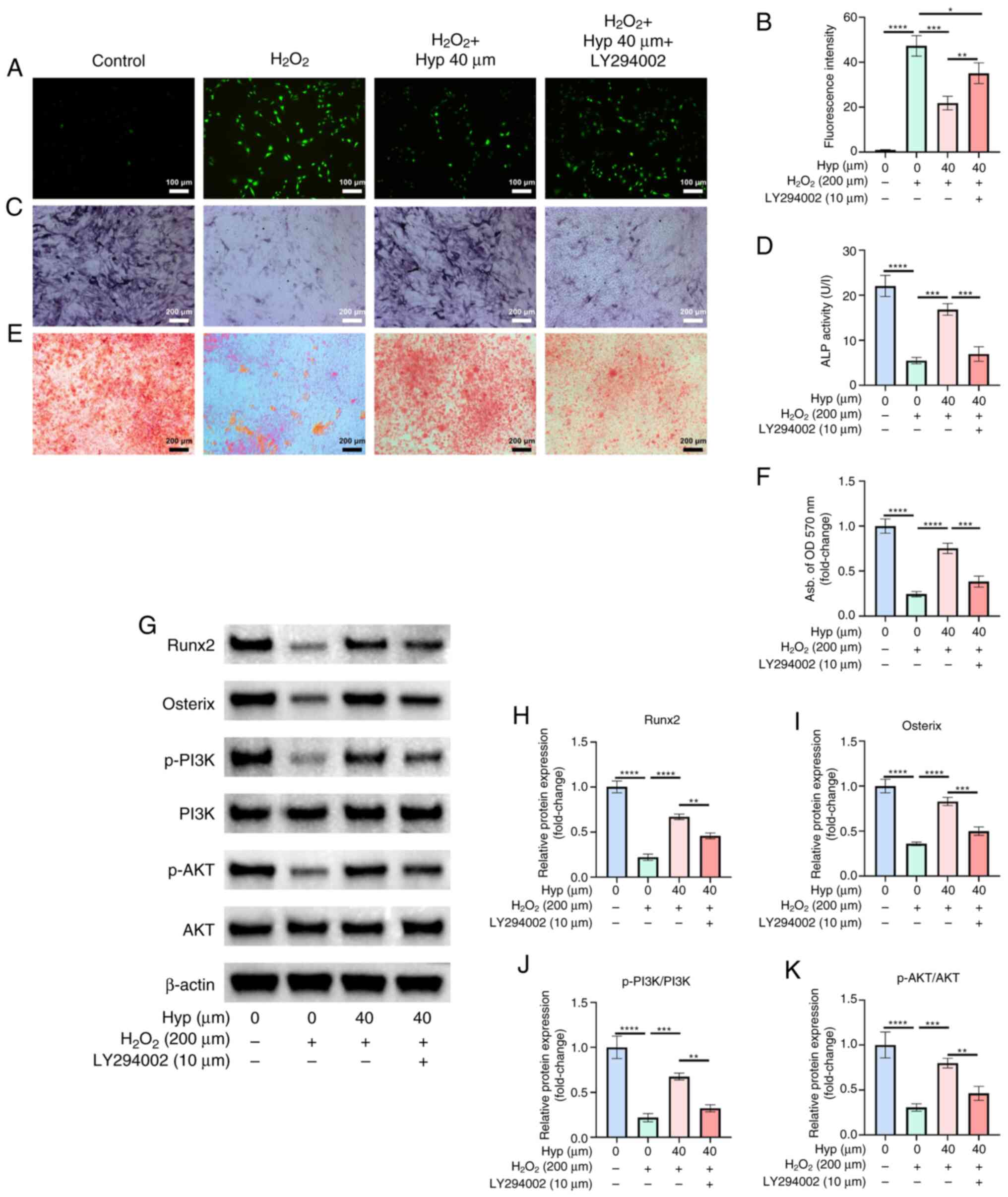 | Figure 7.Inhibition of the PI3K/AKT pathway
attenuates the antioxidative and osteogenic effects of Hyp. (A)
Representative fluorescence images of DCFH-DA staining and (B)
quantification (n=3; ×10 magnification). (C) Representative images
of ALP staining and (D) quantification of ALP activity (n=3; ×5
magnification). (E) Representative images of ARS staining and (F)
quantification of ARS absorbance levels (n=3; ×5 magnification).
(G) Representative western blotting images, and semi-quantification
of the protein expression levels of (H) Runx2, (I) Osterix, (J)
p-PI3K/PI3K and (K) p-AKT/AKT (n=3). Data are presented as the mean
± standard deviation. *P<0.05, **P<0.01, ***P<0.001 and
****P<0.0001. Hyp, Hyperoside; ALP, alkaline phosphatase; ARS,
Alizarin red S; Runx2, runt-related transcription factor 2; p-,
phosphorylated. |
Western blot analysis corroborated these findings.
Compared with in the model group, Hyp treatment significantly
upregulated the expression levels of p-PI3K and p-AKT, as well as
the key osteogenic transcription factors Runx2 and Osterix
(Fig. 7G-K). By contrast,
co-treatment with LY294002 resulted in a significant downregulation
of these proteins, demonstrating that LY294002 partially reversed
the effects of Hyp on PI3K/AKT signaling and its downstream
targets. Collectively, these results indicated that Hyp exerted its
antioxidative and pro-osteogenic effects by activating the PI3K/AKT
signaling pathway and that inhibition of this pathway significantly
attenuated its biological functions, highlighting the notable role
of PI3K/AKT signaling in mediating the effects of Hyp.
Discussion
Oxidative stress has been acknowledged to serve an
important role in the pathogenesis of OP, a view that has become
accepted in the current research field (11). With increases in age, alterations
in hormonal levels and the influence of other pathological factors,
the redox balance within the body is disrupted, leading to the
excessive generation of oxidative stress products such as ROS
(10,25) ROS can target intracellular
biomacromolecules, resulting in lipid peroxidation, protein
oxidative modification and DNA damage, thereby impairing the normal
function and viability of cells (26). In the skeletal system, oxidative
stress can markedly enhance the activity and number of osteoclasts,
thereby promoting the bone resorption process. Concurrently, it
inhibits the proliferation, differentiation and mineralization
capacity of osteoblasts, ultimately reducing bone formation. This
disruption in the balance of bone remodeling leads to bone mass
loss, and the occurrence and development of OP (27). A large number of in vivo and
in vitro studies have provided conclusive evidence for this,
prompting researchers to continuously explore effective antioxidant
strategies to combat OP (28,29).
Hyp is a natural flavanol glycoside compound that
has been shown to possess notable antioxidant and anti-inflammatory
activities (30,31). A study conducted by Chen et
al (19) demonstrated that Hyp
can prevent oxidative stress-induced liver injury in rats.
Additionally, Wu et al (32) revealed that Hyp can treat acute
kidney injury by regulating oxidative stress and cell apoptosis.
These findings collectively support the potent antioxidant
therapeutic effects of Hyp.
The present study found that Hyp exhibits a notable
ability to alleviate oxidative stress. The results of DCFH-DA
staining to measure the intracellular ROS levels showed that the
fluorescence intensity of the cells was significantly reduced after
Hyp treatment, indicating that Hyp effectively decreased
intracellular ROS generation. Meanwhile, the detection of MDA
content showed that Hyp reduced the MDA levels, and the detection
of SOD activity indicated that Hyp enhanced SOD activity. This
further supported the role of Hyp in alleviating oxidative stress
from the perspectives of lipid peroxidation levels and the
antioxidant enzyme system. This ability to reduce oxidative stress
is of notable importance for protecting cells from oxidative
damage, especially for cell types such as BMSCs that have a key
role in bone metabolism, thereby providing a favorable
microenvironment for maintaining their normal osteogenic
differentiation ability.
Oxidative stress refers to a state in which the
body, when exposed to various harmful stimuli, produces an
excessive amount of highly reactive molecules such as ROS and
reactive nitrogen species, leading to an imbalance between the
oxidative system and the antioxidant system, and ultimately causing
damage to cells and tissues (33,34).
Excessive oxidative stress can cause a notable accumulation of ROS
within cells, inflicting damage on cellular DNA. DNA damage may
trigger cell cycle arrest, thereby preventing osteoblast precursor
cells from undergoing normal mitosis and differentiation. Severe
DNA damage can also lead to cell apoptosis, reducing the number of
osteoblasts and thus inhibiting osteogenic differentiation
(35). Therefore, enhancing the
antioxidant capacity and osteogenic differentiation ability of
BMSCs is an important research direction for the treatment of
OP.
The results of the present study indicated that Hyp
not only reduced oxidative stress but also had a significant
protective effect on the osteogenic differentiation ability of
BMSCs. In the ALP and ARS staining experiments, the ALP activity of
the Hyp-treated group was significantly enhanced, and the ARS
staining showed a significant increase in the number of calcium
nodules, which are important markers of enhanced osteogenic
differentiation. At the molecular level, RT-qPCR and western blot
analysis revealed that Hyp upregulated the expression of
osteogenic-related genes and proteins, such as Col1, Osterix,
Runx2 and Alp. This series of results clearly
demonstrated the positive role of Hyp in protecting and promoting
the osteogenic differentiation of BMSCs. The mechanism of action of
Hyp may have involved reducing the interference of oxidative stress
on intracellular signal pathways, thus maintaining the normal
transduction of osteogenic differentiation-related signals,
enabling BMSCs to differentiate into osteoblasts in a relatively
stable environment and providing a strong cellular basis for bone
formation.
The PI3K/AKT signaling pathway, an important
intracellular signal transduction pathway, serves a central role in
the regulation of oxidative stress and OP, a concept that is widely
accepted in current research (29,36).
The activation of the PI3K/AKT signaling pathway can alleviate
oxidative stress through various mechanisms, including upregulating
the expression of antioxidant enzyme genes, enhancing the synthesis
of intracellular antioxidant substances and inhibiting the activity
of enzymes related to ROS production, thus maintaining the
intracellular redox balance (37,38).
In terms of bone metabolism, the activation of the PI3K/AKT
signaling pathway can promote the proliferation, differentiation
and survival of osteoblasts, while inhibiting osteoclast activity,
thus preserving the normal process of bone remodeling and
regulating OP (39,40). For instance, Wu et al
(41) demonstrated that
7,8-dihydroxyflavone can inhibit oxidative stress and promote bone
formation by activating the tyrosine kinase receptor B/PI3K/AKT
pathway. This pathway enhances the viability and osteogenic
differentiation ability of BMSCs inhibited by
H2O2-induced oxidative stress, thereby
improving OP (28). Several other
natural compounds, such as luteolin and curcumin, have also been
reported to regulate bone metabolism through the PI3K/AKT pathway
(42,43). While luteolin similarly promotes
osteoblast differentiation via the PI3K/AKT signaling pathway,
evidence regarding its role in redox regulation remains limited
(29). Curcumin has been shown to
promote osteogenic differentiation in association with PI3K/AKT and
nuclear factor erythroid 2-related factor 2 (Nrf2) activation,
suggesting a role in both bone formation and antioxidative
responses (43).
By contrast, the present study showed that Hyp
exerted both antioxidative and osteogenic effects in a
PI3K/AKT-dependent manner, highlighting its dual-function
specificity and potential novelty. In the present study, Hyp
significantly activated the PI3K/AKT signaling pathway. Western
blot analysis revealed that Hyp treatment partially reversed the
H2O2-induced reduction in p-PI3K and p-AKT
levels, and immunohistochemical staining further supported the
upregulation of p-PI3K and p-AKT expression following Hyp
administration. These results suggested that Hyp exerted both
antioxidative and osteogenic effects at least in part through
PI3K/AKT pathway activation.
To validate the specificity of this signaling axis,
the present study employed LY294002, a classical PI3K inhibitor,
and showed that co-treatment with LY294002 significantly attenuated
the beneficial effects of Hyp. Specifically, the reduction of ROS
levels by Hyp, as evidenced by DCFH-DA staining, was partially
reversed by LY294002, indicating a decline in the antioxidative
efficacy of Hyp. Similarly, ALP staining, ALP activity and ARS
staining demonstrated that LY294002 co-treatment inhibited
Hyp-induced early- and late-stage osteogenesis. Western blot
analysis further supported that LY294002 significantly suppressed
the Hyp-induced upregulation of p-PI3K, p-AKT and key osteogenic
transcription factors, such as Runx2 and Osterix. These findings
collectively suggested that the PI3K/AKT pathway was not only
activated by Hyp but was also important for its antioxidative and
osteogenic actions. The use of LY294002 as a pathway-specific
inhibitor provided robust mechanistic evidence supporting the
notable role of PI3K/AKT in mediating the therapeutic effects of
Hyp. These results align with prior studies highlighting the
importance of this pathway in OP treatment and offer a more
comprehensive mechanistic understanding of the pharmacological
profile of Hyp (44,45).
In summary, the results of the present study
indicated that Hyp holds notable promise in the treatment of OP and
suggested that its mechanism of action was multifaceted. The
results indicated that Hyp promoted bone homeostasis through
multiple mechanisms, including mitigating oxidative stress,
preserving osteogenic differentiation capacity and activating the
PI3K/AKT signaling pathway. These findings provide a solid
theoretical basis for further development of Hyp-based therapeutic
strategies for OP.
However, the present study also had certain
limitations. Firstly, the present study was mainly based on cell
and animal experimental models. Although these models can provide
important information regarding the mechanism of Hyp action, the
physiological environment and disease state of the human body are
notably complex. The efficacy, safety and pharmacokinetic
characteristics of Hyp in humans remain to be verified through
further clinical studies. Secondly, although the present study has
preliminarily revealed the association between Hyp and the PI3K/AKT
signaling pathway, the upstream and downstream molecular regulatory
networks of this pathway, as well as its potential crosstalk with
other signaling pathways, remain insufficiently elucidated.
Notably, it remains to be fully elucidated whether Hyp directly
regulates other redox-sensitive pathways such as the ROS/Kelch-like
ECH-associated protein 1/Nrf2 axis, which serves a central role in
maintaining intracellular oxidative balance in bone-related cells.
Therefore, further in-depth studies using targeted molecular
approaches, such as pathway-specific inhibitors, activators or
genetic knockdown models, are necessary to comprehensively uncover
the mechanistic landscape of the biological activity of Hyp.
Thirdly, the present study did not evaluate serum calcium and
estrogen levels. Direct biochemical measurements would provide
stronger support for the pathophysiological relevance and treatment
efficacy of Hyp. Future investigations should incorporate these
assessments to strengthen the comprehensiveness and translational
relevance of findings.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Science and Technology
Projects for Social Development in Dongguan City (grant no.
20221800900102).
Availability of data and materials
The datasets used or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LL and DX designed the study. LL and DX wrote the
manuscript. LL, HD and SZ performed animal experiments. LL and HD
carried out online data information collection. LL, HD, SZ and BW
performed the cell experiments. LL and DX performed the statistical
analysis. DX revised the manuscript. LL and DX confirm the
authenticity of all the raw data. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
All animal experimental procedures were approved by
the Animal Experiment Ethics Committee of The First Affiliated
Hospital of Guangzhou University of Chinese Medicine (approval no.
GZTCMF1-202403241) and were performed in accordance with
institutional and national guidelines for the care and use of
laboratory animals. According to the ethics committee, the
commercially purchased mouse primary BMSCs used in the present
study did not require additional ethics approval.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Walker MD and Shane E: Postmenopausal
osteoporosis. N Engl J Med. 389:1979–1991. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tang SS, Yin XJ, Yu W, Cui L, Li ZX, Cui
LJ, Wang LH and Xia W: Prevalence of osteoporosis and related
factors in postmenopausal women aged 40 and above in China.
Zhonghua Liu Xing Bing Xue Za Zhi. 43:509–516. 2022.(In Chinese).
PubMed/NCBI
|
|
3
|
LeBoff MS, Greenspan SL, Insogna KL,
Lewiecki EM, Saag KG, Singer AJ and Siris ES: The clinician's guide
to prevention and treatment of osteoporosis. Osteoporos Int.
33:2049–2102. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cosman F, Langdahl B and Leder BZ:
Treatment sequence for osteoporosis. Endocr Pract. 30:490–496.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Eid A and Atlas J: The role of
bisphosphonates in medical oncology and their association with jaw
bone necrosis. Oral Maxillofac Surg Clin North Am. 26:231–237.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chlebowski RT and Manson JE: Menopausal
hormone therapy and breast cancer. Cancer J. 28:169–175. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cho L, Kaunitz AM, Faubion SS, Hayes SN,
Lau ES, Pristera N, Scott N, Shifren JL, Shufelt CL, Stuenkel CA,
et al: Rethinking menopausal hormone therapy: For whom, what, when,
and how long? Circulation. 147:597–610. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Luo J, Mills K, le Cessie S, Noordam R and
van Heemst D: Ageing, age-related diseases and oxidative stress:
What to do next? Ageing Res Rev. 57:1009822020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Iantomasi T, Romagnoli C, Palmini G,
Donati S, Falsetti I, Miglietta F, Aurilia C, Marini F, Giusti F
and Brandi ML: Oxidative stress and inflammation in osteoporosis:
Molecular mechanisms involved and the relationship with microRNAs.
Int J Mol Sci. 24:37722023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Marcucci G, Domazetovic V, Nediani C,
Ruzzolini J, Favre C and Brandi ML: Oxidative stress and natural
antioxidants in osteoporosis: Novel preventive and therapeutic
approaches. Antioxidants (Basel). 12:3732023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kimball JS, Johnson JP and Carlson DA:
Oxidative stress and osteoporosis. J Bone Joint Surg Am.
103:1451–1461. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen Y, Cai Y, Chen C, Li M, Lu L, Yu Z,
Wang S, Fang L and Xu S: Aroclor 1254 induced inhibitory effects on
osteoblast differentiation in murine MC3T3-E1 cells through
oxidative stress. Front Endocrinol (Lausanne). 13:9406242022.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tanaka M, Inoue H, Takahashi N and Uehara
M: AMPK negatively regulates RANKL-induced osteoclast
differentiation by controlling oxidative stress. Free Radic Biol
Med. 205:107–115. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu C, Shen S, Zhang S, Huang M, Zhang L
and Chen X: Autophagy in bone remodeling: A regulator of oxidative
stress. Front Endocrinol (Lausanne). 13:8986342022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao Q, Feng J, Liu F, Liang Q, Xie M,
Dong J, Zou Y, Ye J, Liu G, Cao Y, et al: Rhizoma Drynariae-derived
nanovesicles reverse osteoporosis by potentiating osteogenic
differentiation of human bone marrow mesenchymal stem cells via
targeting ERα signaling. Acta Pharm Sin B. 14:2210–2227. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li B, Wang Y, Gong S, Yao W, Gao H, Liu M
and Wei M: Puerarin improves OVX-induced osteoporosis by regulating
phospholipid metabolism and biosynthesis of unsaturated fatty acids
based on serum metabolomics. Phytomedicine. 102:1541982022.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rao T, Tong H, Li J, Huang J, Yin Y and
Zhang J: Exploring the role and mechanism of hyperoside against
cardiomyocyte injury in mice with myocardial infarction based on
JAK2/STAT3 signaling pathway. Phytomedicine. 128:1553192024.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao K, Zhou F, Lu Y, Gao T, Wang R, Xie M
and Wang H: Hyperoside alleviates depressive-like behavior in
social defeat mice by mediating microglial polarization and
neuroinflammation via TRX1/NLRP1/Caspase-1 signal pathway. Int
Immunopharmacol. 145:1137312025. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen Y, Dai F, He Y, Chen Q, Xia Q, Cheng
G, Lu Y and Zhang Q: Beneficial effects of hyperoside on bone
metabolism in ovariectomized mice. Biomed Pharmacother.
107:1175–1182. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yan K, Zhang RK, Wang JX, Chen HF, Zhang
Y, Cheng F, Jiang Y, Wang M, Wu Z, Chen XG, et al: Using network
pharmacology and molecular docking technology, proteomics and
experiments were used to verify the effect of Yigu decoction (YGD)
on the expression of key genes in osteoporotic mice. Ann Med.
57:24492252025. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang PH, Wei YN, Xiao BJ, Li SY, Li XL,
Yang LJ, Pan HF and Chen GX: Curcumin for gastric cancer: Mechanism
prediction via network pharmacology, docking, and in vitro
experiments. World J Gastrointest Oncol. 16:3635–3650. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Benisch P, Schilling T, Klein-Hitpass L,
Frey SP, Seefried L, Raaijmakers N, Krug M, Regensburger M, Zeck S,
Schinke T, et al: The transcriptional profile of mesenchymal stem
cell populations in primary osteoporosis is distinct and shows
overexpression of osteogenic inhibitors. PLoS One. 7:e451422012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
van de Peppel J, Strini T, Tilburg J,
Westerhoff H, van Wijnen AJ and van Leeuwen JP: Identification of
three early phases of cell-fate determination during osteogenic and
adipogenic differentiation by transcription factor dynamics. Stem
Cell Reports. 8:947–960. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang C, Li H, Li J, Hu J, Yang K and Tao
L: Oxidative stress: A common pathological state in a high-risk
population for osteoporosis. Biomed Pharmacother. 163:1148342023.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Karthik V and Guntur AR: Energy metabolism
of osteocytes. Curr Osteoporos Rep. 19:444–451. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shinohara I, Morita M, Chow SK, Murayama
M, Sususki Y, Gao Q and Goodman SB: Pathophysiology of the effects
of oxidative stress on the skeletal system. J Orthop Res.
43:1059–1072. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li D, Zhao Z, Zhu L, Feng H, Song J, Fu J,
Li J, Chen Z and Fu H: 7,8-DHF inhibits BMSC oxidative stress via
the TRKB/PI3K/AKT/NRF2 pathway to improve symptoms of
postmenopausal osteoporosis. Free Radic Biol Med. 223:413–429.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chai S, Yang Y, Wei L, Cao Y, Ma J, Zheng
X, Teng J and Qin N: Luteolin rescues postmenopausal osteoporosis
elicited by OVX through alleviating osteoblast pyroptosis via
activating PI3K-AKT signaling. Phytomedicine. 128:1555162024.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jang E: Hyperoside as a potential natural
product targeting oxidative stress in liver diseases. Antioxidants
(Basel). 11:14372022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ku SK, Zhou W, Lee W, Han MS, Na M and Bae
JS: Anti-inflammatory effects of hyperoside in human endothelial
cells and in mice. Inflammation. 38:784–799. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wu L, Li Q, Liu S, An X, Huang Z, Zhang B,
Yuan Y and Xing C: Protective effect of hyperoside against renal
ischemia-reperfusion injury via modulating mitochondrial fission,
oxidative stress, and apoptosis. Free Radic Res. 53:727–736. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lennicke C and Cocheme HM: Redox
metabolism: ROS as specific molecular regulators of cell signaling
and function. Mol Cell. 81:3691–3707. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gebicki JM: Oxidative stress, free
radicals and protein peroxides. Arch Biochem Biophys. 595:33–39.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jomova K, Raptova R, Alomar SY, Alwasel
SH, Nepovimova E, Kuca K and Valko M: Reactive oxygen species,
toxicity, oxidative stress, and antioxidants: chronic diseases and
aging. Arch Toxicol. 97:2499–2574. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ren BC, Zhang YF, Liu SS, Cheng XJ, Yang
X, Cui XG, Zhao XR, Zhao H, Hao MF, Li MD, et al: Curcumin
alleviates oxidative stress and inhibits apoptosis in diabetic
cardiomyopathy via Sirt1-Foxo1 and PI3K-Akt signalling pathways. J
Cell Mol Med. 24:12355–12367. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Deng S, Dai G, Chen S, Nie Z, Zhou J, Fang
H and Peng H: Dexamethasone induces osteoblast apoptosis through
ROS-PI3K/AKT/GSK3β signaling pathway. Biomed Pharmacother.
110:602–608. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wei L, Chen W, Huang L, Wang H, Su Y,
Liang J, Lian H, Xu J, Zhao J and Liu Q: Alpinetin ameliorates bone
loss in LPS-induced inflammation osteolysis via ROS mediated
P38/PI3K signaling pathway. Pharmacol Res. 184:1064002022.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xin L, Feng HC and Zhang Q, Cen XL, Huang
RR, Tan GY and Zhang Q: Exploring the osteogenic effects of simiao
wan through activation of the PI3K/AKT pathway in osteoblasts. J
Ethnopharmacol. 338:1190232025. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang D, Liu Y, Tang D, Wei S, Sun J, Ruan
L, He L, Li R, Ren Q, Tian X and Chen Y: Induction of
PI3K/Akt-mediated apoptosis in osteoclasts is a key approach for
buxue tongluo pills to treat osteonecrosis of the femoral head.
Front Pharmacol. 12:7299092021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wu CH, Hung TH, Chen CC, Ke CH, Lee CY,
Wang PY and Chen SF: Post-injury treatment with
7,8-dihydroxyflavone, a TrkB receptor agonist, protects against
experimental traumatic brain injury via PI3K/Akt signaling. PLoS
One. 9:e1133972014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liang G, Zhao J, Dou Y, Yang Y, Zhao D,
Zhou Z, Zhang R, Yang W and Zeng L: Mechanism and experimental
verification of luteolin for the treatment of osteoporosis based on
network pharmacology. Front Endocrinol (Lausanne). 13:8666412022.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Xiong Y, Zhao B, Zhang W, Jia L, Zhang Y
and Xu X: Curcumin promotes osteogenic differentiation of
periodontal ligament stem cells through the PI3K/AKT/Nrf2 signaling
pathway. Iran J Basic Med Sci. 23:954–960. 2020.PubMed/NCBI
|
|
44
|
Lu J, Shi X, Fu Q, Han Y, Zhu L, Zhou Z,
Li Y and Lu N: New mechanistic understanding of osteoclast
differentiation and bone resorption mediated by P2X7 receptors and
PI3K-Akt-GSK3beta signaling. Cell Mol Biol Lett. 29:1002024.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Jiang Z, Deng L, Li M, Alonge E and Wang Y
and Wang Y: Ginsenoside Rg1 modulates PI3K/AKT pathway for enhanced
osteogenesis via GPER. Phytomedicine. 124:1552842024. View Article : Google Scholar : PubMed/NCBI
|