Introduction
Diabetes mellitus is a metabolic disease
characterized by persistent hyperglycemia. Type 2 diabetes mellitus
(T2DM) accounts for ~90% of all cases of diabetes. In the disease
process, due to impaired insulin sensitivity, the body's uptake and
utilization of glucose are blocked, which leads to the
clinicopathological diagnosis characterized by glucose intolerance
(1). At present, research on the
pathogenesis of diabetes continues to advance, and insulin
resistance has been found to be the main feature and pathogenic
factor of T2DM (2). The insulin
receptor (IR) substrate (IRS)/PI3K/Akt pathway is the classical
insulin metabolic pathway (3).
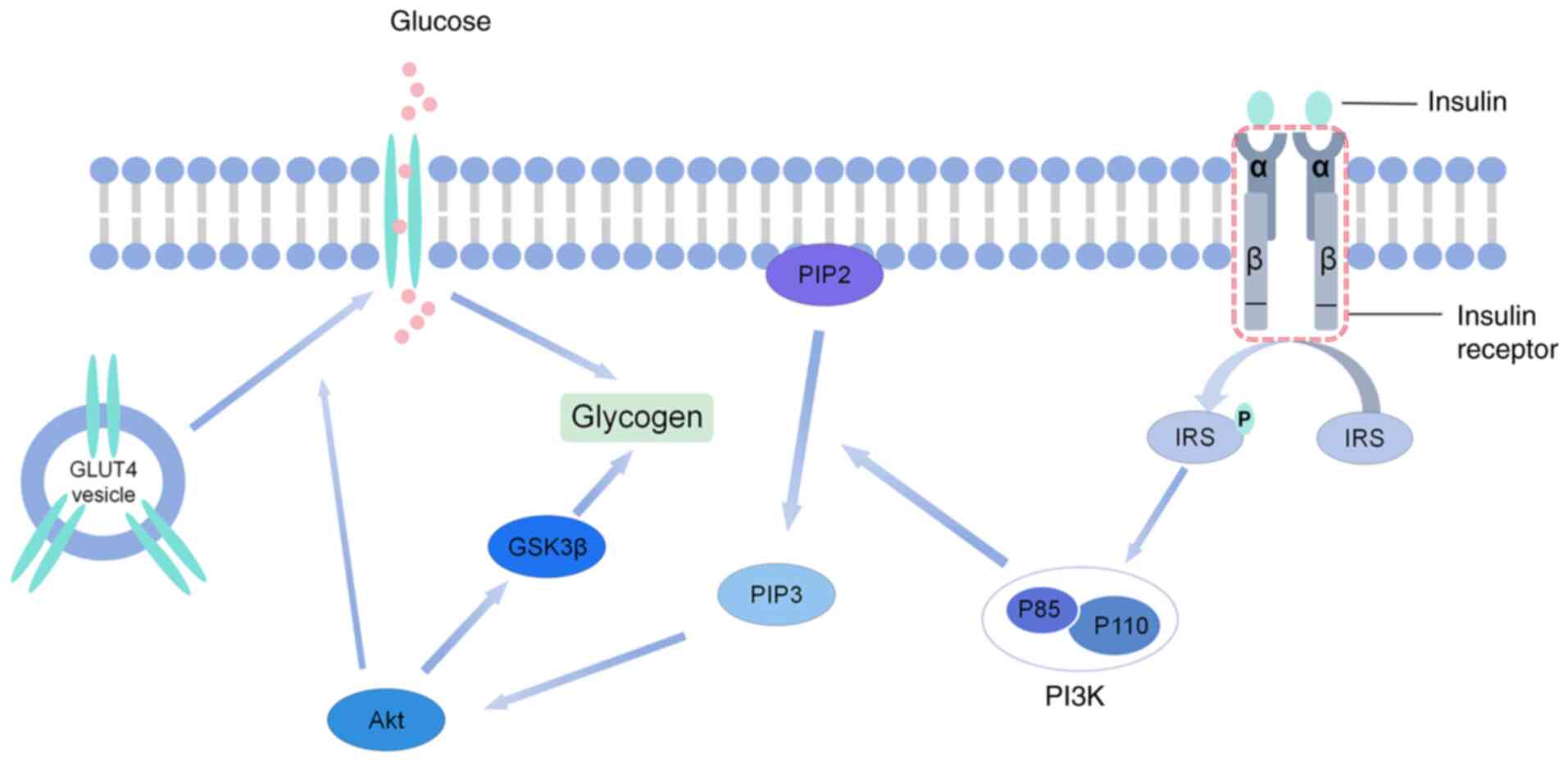
Glucose transporter type 2 (GLUT2) is constitutively expressed in
the liver and pancreatic β cells, and its transport activity is
predominantly driven by a glucose concentration gradient. In its
physiological state, hepatic GLUT2 maintains glucose homeostasis
through bidirectional transport (4). In the early stage of insulin
resistance, where the IRS-1/PI3K/Akt pathway is not completely
inactivated, Akt may inhibit glycogen synthesis by phosphorylating
glycogen synthase kinase 3β (GSK3β) and indirectly enhance
GLUT2-mediated gluconeogenesis (5). GLUT2 activity is predominantly driven
by concentration gradients for glucose transport in tissues such as
the liver, independent of insulin stimulation. However, membrane
translocation of GLUT4 in muscle and adipose tissue is strictly
dependent on insulin signaling. After PI3K is activated by tyrosine
phosphorylation of IRS-1, phosphatidylinositol 3,4,5-triphosphate
(PIP3) is generated to recruit 3-phosphoinositide-dependent protein
kinase 1 and activate Akt (6).
Activated Akt promotes the fusion of GLUT4 vesicles to the cell
membrane by phosphorylating TBC1 domain family member 4 (AS160)
(7). Serine phosphorylation of
IRS-1, such as at Ser307, inhibits tyrosine phosphorylation in
insulin resistance, leading to the blockade of the PI3K/PIP3/Akt
pathway and reduced GLUT4 translocation (8).
Insulin regulates glucose uptake and metabolism in
insulin-dependent tissues, such as muscle and fat, by activating
specific signaling pathways and inducing GLUT4 transport, thereby
maintaining the blood glucose balance (9). By regulating the IRS/PI3K/Akt
signaling pathway, gluconeogenesis can be reduced, liver glycogen
synthesis can be promoted, insulin resistance can be reduced and
blood glucose levels in the body can be restored to normal
(10).
IRS/PI3K/Akt signaling
The insulin-related signaling pathway is the
molecular basis for the regulation of insulin metabolism, and the
IRS/PI3K/Akt signaling pathway serves a key role in improving
glucose metabolism and conversion in vivo (11). Its mechanism of action is that
islet β cells secrete insulin, which binds to corresponding
receptors on target cells and serves a role in lowering blood
glucose in vivo. IR, a member of the tyrosine kinase family,
is a tetrameric protein structure composed of two α subunits and
two β subunits. When insulin is not bound to its receptor, the
tyrosine kinase in the cell dissociates, forms a λ type and the
kinase activity is autoinhibited (12). When insulin binds to the α subunit
of IR on the target cell, the protein conformation changes, the β
subunit is activated and some tyrosine residues within the cell are
autophosphorylated, and these are subsequently recognized and
recruited by the phosphotyrosine binding domain of the adaptor
protein. Blocking of insulin signaling leads to insulin resistance
and metabolic system disorders (13).
As blood glucose levels continue to rise in the
body, insulin binds to the IR on the cell surface and
autophosphorylates downstream IRS proteins (14). IRS-1 was the first identified IR
substrate, and is widely expressed in a variety of tissues, such as
muscle and adipose tissue, and cell types. IRS-1 is one of the key
molecules mediating insulin and insulin-like growth factor-1
signaling (15). Following
insulin-IR binding, IRS-1 is subsequently recruited and
phosphorylated, and specific tyrosine residues on phosphorylated
IRS-1 provide binding sites for the p85 regulatory subunit of PI3K,
through which PI3K is activated (16,17).
IRS-2 is abundantly expressed in the liver, islet β cells, adipose
tissue and other tissues. IRS-2 serves an important role in
regulating glucose metabolism in the liver, and the survival and
function of islet β cells. Similar to IRS-1, insulin signaling
activates the IR and phosphorylates IRS-2 to bind to the p85
subunit of PI3K. In addition, IRS-2 activates PI3K and its
downstream signaling pathways (18–20).
IRS-3 is predominantly expressed in adipose tissue and the liver,
while IRS-4 is expressed in endocrine glands, such as the
pituitary, thyroid and adrenal glands, and some tumor cells.
Following its activation by the corresponding receptors, IRS-4 can
recruit the p85 subunit of PI3K by phosphorylation to activate
PI3K, and participate in the regulation of cell growth,
proliferation and metabolism (21). Tyrosyl-phosphorylated IRS proteins
bind to the Src homology 2 domain signaling molecule of the PI3K
regulatory subunit p85 and recruit the catalytic subunit p110 of
PI3K to activate PI3K. Tyrosyl-phosphorylated IRS binds to
downstream PI3K in activated cells and promotes PI3K activation
(22).
PI3K is a downstream effector of IRS. Upon binding
to IRS proteins phosphorylated by tyrosine, PI3K activates
phosphatidylinositol phosphorylation to generate PIP3, which in
turn activates the serine/threonine protein kinase Akt (23). Akt activates a variety of
substrates and mediates a variety of insulin-acting organisms,
thereby promoting glucose transporters in the cell membrane to
accelerate glucose uptake and utilization, a process important for
maintaining a normal glycemic range (24). A key role of insulin in maintaining
blood glucose is to induce the translocation of GLUT4 from
intracellular storage sites to the plasma membrane, which promotes
glucose uptake by cells and reduces insulin resistance (25) (Fig.
1).
Effects of the IRS/PI3K/Akt pathway on
insulin resistance
Insulin resistance is an important factor in the
development of diabetes and is characterized by a reduced ability
of insulin to promote tissue glucose uptake and utilization
(26). Impaired IRS signaling
induces and exacerbates insulin resistance. Previous studies have
shown that glucose metabolism is predominantly manifested in target
tissues through the IRS-1/PI3K/Akt pathway, which is an important
signaling pathways that regulates the blood glucose balance through
insulin (27,28). With the development of diabetes
research, the importance of the IRS/PI3K/Akt signaling pathway in
insulin resistance has been gradually realized. Abnormal activation
of this pathway leads to the weakened response of liver, muscle and
other tissues to insulin, so that blood glucose levels cannot be
effectively controlled (29). The
regulation of this pathway can effectively alleviate insulin
resistance and provide novel ideas for the treatment of
diabetes.
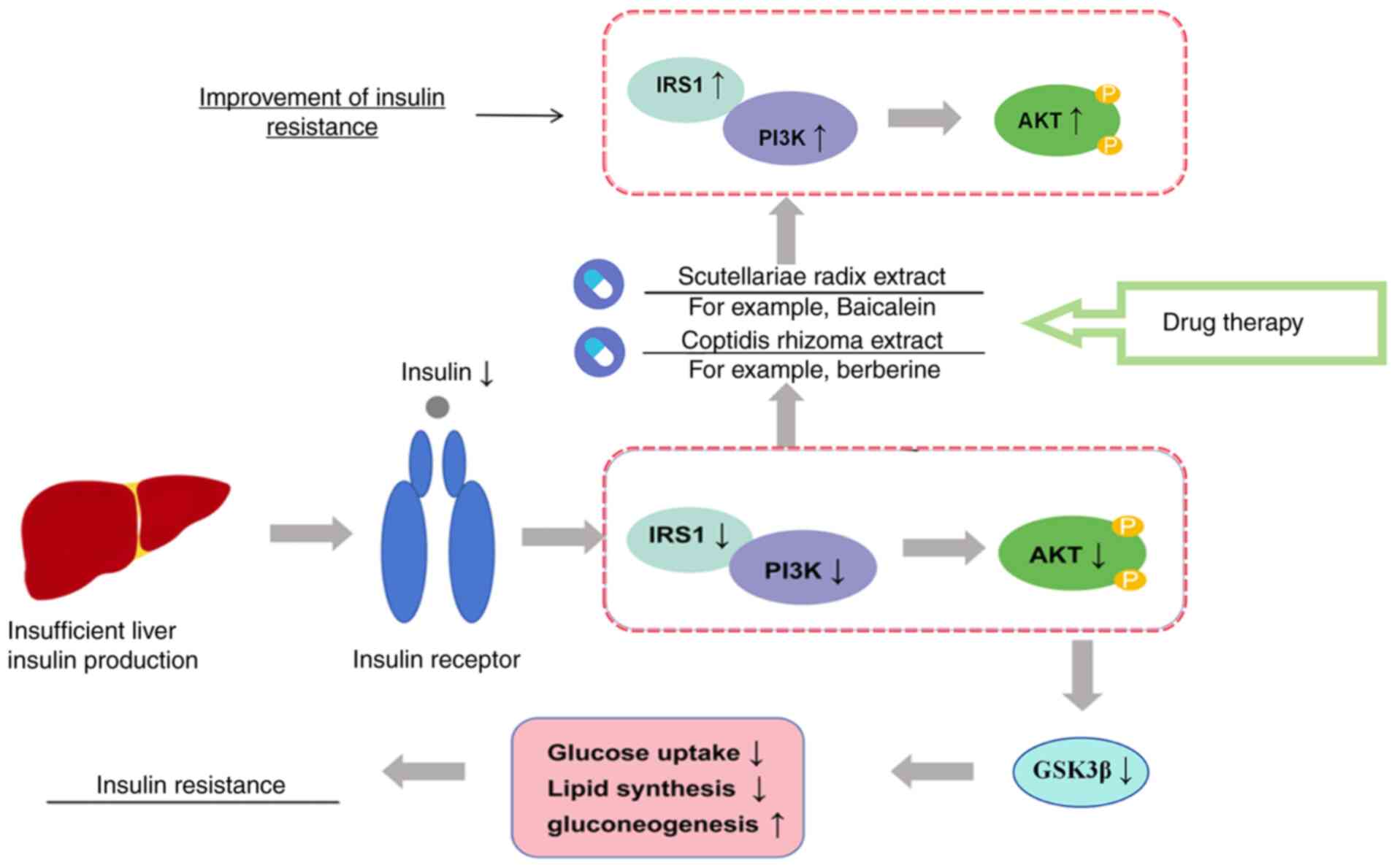
The liver serves an important role in glucose
metabolism; when the body exhibits insufficient insulin secretion,
the liver produces excessive glucose, which disrupts the balance of
peripheral glucose consumption and leads to continuous blood
glucose elevation (30). Hepatic
insulin resistance is an important pathological feature of T2DM
(31). Under physiological
conditions, the initial step for insulin to exert its biological
effects is its specific binding to the IR on the surface of target
cells. This binding triggers conformational changes and
autophosphorylation activation, thereby initiating a downstream
signal transduction cascade. IRS-1, as a key downstream adaptor
molecule, can bind to the intracellular domain phosphorylated by
the activated IR through its specific domain, thereby completing
insulin signaling (32). By
activating PI3K and its downstream target Akt, glucose metabolism
disorders in the liver can be directly improved, and thus, it also
plays a role in the intervention of insulin resistance (33). The stimulation of the PI3K/Akt
signaling pathway promotes glucose uptake and activates GSK3β,
thereby promoting glycogen synthesis (34). Previous studies have shown that
some natural drug extracts or fractions improve insulin resistance
and reduce blood glucose levels by activating the IRS/PI3K/Akt
pathway (35–37). Studies have found that natural
medicinal plants and tea can also activate the PI3K/Akt signaling
pathway and improve the sensitivity of skeletal muscle to insulin,
thereby effectively reducing insulin resistance in diabetic mice
(38–40). This opens up novel possibilities
for the treatment of diabetes. In summary, the IRS/PI3K/Akt
signaling pathway is one of the key signaling pathways in the
pathogenesis of diabetes. By inhibiting the IRS/PI3K/Akt pathway
and down-regulating GSK3β, it reduces glucose uptake and lipid
synthesis, and increases gluconeogenesis, thereby causing insulin
resistance. Drugs (such as Baicalein and berberine), by enhancing
the activity of IRS-1/PI3K and promoting Akt phosphorylation,
alleviate insulin resistance and play a crucial role in the
pathogenesis of diabetes (41–43)
(Fig. 2).
Interrelationship between the IRS/PI3K/Akt
and AMP-activated protein kinase (AMPK) signaling pathways
In insulin resistance, there is a complex
relationship between the IRS/PI3K/Akt and AMPK signaling pathways.
These pathways affect and regulate each other and participate in
the occurrence and development of insulin resistance (44). AMPK can regulate the IRS/PI3K/Akt
pathway by phosphorylating IRS. When exercise or energy stress
occurs, AMPK is activated, and this can phosphorylate certain
serine residues on IRS-1. This phosphorylation affects the binding
of IRS-1 to IR and its interaction with PI3K, which in turn
regulates downstream signaling (45). In general, moderate AMPK activation
causes the phosphorylation of AS160 at Thr642, which promotes the
fusion of GLUT4 vesicles to the cell membrane. In addition, AMPK
activates tuberin, inhibits mTOR complex 1, reduces the
phosphorylation of ribosomal protein S6 kinase β-1 (S6K1) on IRS-1,
decreases the negative feedback phosphorylation of S6K1 on IRS-1,
and protects the integrity and signal transduction efficiency of
the IRS/PI3K/Akt pathway. Activated Akt inhibits AMPK through
phosphorylation, and this negative feedback regulation can prevent
the excessive activation of AMPK and maintain the intracellular
metabolic balance. However, when energy metabolism is abnormal,
AMPK is overactivated to inhibit β cell stress and cell death,
thereby promoting the occurrence and development of diabetes
(46,47).
Insulin can also increase intracellular ATP levels
by increasing intracellular glucose uptake and metabolism, thereby
activating AMPK. The IRS/PI3K/Akt signaling pathway increases
glucose uptake and utilization by cells by promoting GLUT4
translocation to the cell membrane, while promoting glycogen
synthesis and inhibiting gluconeogenesis (48). AMPK also promotes glucose uptake
through the activation of downstream
6-phosphofructose-2-kinase/fructose-2,6-biphosphatase 2, increasing
glycolysis. AMPK also inhibits the expression of
gluconeogenesis-related genes and reduces gluconeogenesis (49). The IRS/PI3K/Akt and AMPK signaling
pathways work together to maintain the balance of glucose
metabolism, and during insulin resistance, this synergy is
dysregulated, leading to increased blood glucose levels.
Research progress regarding the IRS/PI3K/Akt
pathway in the treatment of diabetes
The IRS/PI3K/Akt pathway is the key pathway of
insulin signal transduction and serves a central role in the
occurrence and development of insulin resistance. This article
elaborates on how drugs can improve insulin resistance by
regulating the IRS/PI3K/Akt pathway. The existing research has been
summarized in the present review and can be used to help alleviate
insulin resistance caused by diabetes by regulating the
IRS/PI3K/Akt pathway.
Natural drug extracts
Some monomeric active ingredients in natural drug
extracts have been found to improve glucose metabolism and insulin
resistance by regulating the IRS/PI3K/Akt signaling pathway
(Table I). It has been found that
the abundance and diversity of gut microbiota are associated with
the occurrence and development of diabetes. Diosmetin regulates
Corynebacterium glutamicum through the IRS/PI3K/Akt
signaling pathway, reduces the firmicute/bacteroidete ratio,
notably increases the abundance of Corynebacterium
glutamicum and alters the intestinal flora (50). In KK-Ay type diabetic mice,
compared with that in the control group, the glucose metabolism of
the mice treated with Diosmetin improved significantly (51). Astragaloside IV (AS-IV) could
markedly reduce blood lipid and glucose levels, as well as insulin
resistance and oxidative stress in T2DM model mice. AS-IV was also
shown to protect the liver and pancreatic cell structure.
High-throughput 16S ribosomal RNA gene sequencing was used to
determine the composition of gut microbiota in the model mice. As a
result, AS-IV was found to increase the levels of butyrate, and
improve the abundance and diversity of intestinal flora in model
mice. The mechanism by which AS-IV alleviates insulin resistance
was linked to the regulation of the AMPK/sirtuin 1 (SIRT1) and
PI3K/Akt signaling pathways (52).
 | Table I.Research on the regulation of the
insulin receptor substrate/PI3K/Akt signaling pathway by natural
drug extracts and its role in improving insulin resistance. |
Table I.
Research on the regulation of the
insulin receptor substrate/PI3K/Akt signaling pathway by natural
drug extracts and its role in improving insulin resistance.
| First author/s,
year | Medicine | In
vitro/in vivo | Experimental
subject | Dosages | Remarks | (Refs.) |
|---|
| Gong et al,
2021 | Diosmetin | In vivo | KK-Ay mice | 20 and 60
mg/kg | Reshaped the
imbalanced intestinal flora and improve glucose metabolism. | (51) |
| Gong et al,
2023 | Astragaloside
IV | In vivo | T2DM mice | 25, 50 and 100
mg/kg | Improved the
abnormal levels of blood lipids, blood glucose, insulin resistance
and oxidative stress in T2DM mice. | (52) |
| Gong et al,
2023 | Astragaloside
IV | In
vitro | HepG2 cells | 12.5, 25 and 50
µM | By regulating the
AMPK/SIRT1 and PI3K/AKT signaling pathways, oxidative stress and
insulin resistance can be improved. | (52) |
| Tan et al,
2022 |
3,3′,4,5′-tetramethoxy-trans-stilbene | In
vitro | HepG2 cells | 2.5 µM | Increased glucose
consumption and glycogen synthesis and upregulated the antioxidant
activity of nuclear factor erythroid 2-related factor 2. | (53) |
| Feng et al,
2021 | Anemarrhena
saponins | In vivo | Insulin-resistant
Sprague-Dawley rats | 100, 200 and 400
mg/kg | Promoted insulin
signal transduction and alleviated liver damage caused by insulin
resistance. | (54) |
| Yang et al,
2020 | Guava leaf
extract | In vivo | KK-Ay mice | 1,638 mg/kg | Improved insulin
resistance in KK-Ay diabetic mice and exerted an anti-diabetic
effect. | (55) |
| Wang et al,
2022 | Potentilla
bifurca flavonoids | In
vitro | 3T3-L1
adipocytes | - | Improved the
disorder of glycolipid metabolism in 3T3-L1 adipocytes and improved
insulin resistance. | (59) |
3,3′,4,5′-tetramethoxy-trans-stilbene (2.5 µM) can
upregulate the phosphorylation of GSK3βSer9, inhibit the
phosphorylation of IRS-1Ser307, increase the levels of
IRS-1 and IRS-2, activate the PI3K/Akt pathway and promote glycogen
synthesis. It has been shown to reduce the oxidative stress level
of HepG2 cells by upregulating nuclear factor erythroid 2-related
factor 2, as well as to regulate insulin sensitivity and
homeostasis, thus improving insulin resistance (53). Anemarrhena saponins have
been shown to have lipid-lowering and glucose-lowering effects. In
an experiment investigating the effect of treatment with
Anemarrhena saponins in insulin-resistant rats, the
phosphorylation levels of IRS-1, PI3K and Akt were increased in
model rats compared with controls. The mRNA expression levels of
glucose-6-phosphatase (G6pase), phosphoenolpyruvate carboxykinase
(PEPCK) and GSK3β were notably decreased (54).
A study showed that guava leaves have anti-diabetic
effects. After 8 weeks of treatment with guava leaf extract in
KK-Ay diabetic mice, the body weight, fasting blood glucose,
fasting insulin and insulin resistance index of diabetic model mice
were markedly decreased, while the insulin sensitivity index was
increased. The protein and gene expression levels of IRS-1, PI3K
and Akt in the liver were also upregulated, suggesting that guava
leaf extract could improve insulin resistance in KK-Ay diabetic
mice by regulating the IRS/PI3K/Akt signaling pathway, and may
serve a role in the treatment of diabetes (55).
Abnormal glucose and lipid metabolism are associated
with insulin resistance and the development of T2DM (56). Impaired insulin function results in
notable reductions in glucose uptake, glucose consumption and
glycogen storage, as well as marked increases in plasma glucose
levels (57). Oxidative stress can
disrupt normal insulin signaling, and increase the risk of insulin
resistance, glucose and lipid metabolism disorders, and diabetes
(58). Flavonoids from
Potentilla bifurca can effectively improve insulin
resistance. Experiments have shown that this flavonoid component
enhanced glucose uptake in adipocytes from the 3T3-L1 cell line.
Insulin resistance can be improved by regulating the content of
p-Akt/Akt, IKKβ and p-NF-κBp65/NF-κBp65 in the IRS/PI3K/Akt
signaling pathway (59).
Traditional medicine
T2DM is a metabolic disease characterized by
hyperglycemia, with insulin resistance representing the leading
cause (60). 1-deoxynojirimycin,
an inhibitor of intestinal α-glucosidase, can effectively inhibit
the conversion of glucose in the human body and is superior to the
α-glucosidase inhibitor acarbose in terms of absorption (61). The glucose and insulin tolerance of
db/db mice were improved following a 4-week course of intravenous
injections with 1-deoxynojirimycin. This inhibitor also enhanced
GLUT4 translocation, and phosphorylation of Ser473-Akt, p85-PI3K,
Tyr1361-IR-β and Tyr612-IRS-1 in the skeletal muscle of db/db mice,
suggesting that 1-deoxynojirimycin enhances insulin sensitivity
through the IRS-1/PI3K/Akt pathway and increases the translocation
of GLUT4 to the membrane, leading to an increase in muscle glycogen
content (62,63). Metformin is commonly used in
patients with diabetes. Metformin improves insulin sensitivity,
inhibits glycogenolysis, reduces hepatic sugar output and does not
result in notable weight gain (64). Metformin can alleviate T2DM-induced
liver dysfunction and improve hepatic insulin resistance in T2DM
model animals through the γ-aminobutyric acid type A
receptor-independent PI3K/Akt/GLUT4 signaling pathway (65).
Rosiglitazone (RSG), a classical insulin sensitizer,
improves lipid and glucose metabolism by activating peroxisome
proliferator-activated receptor γ (PPARγ). Combined treatment with
ursolic acid and RSG has been found to stimulate the translocation
of IRS-1, PI3K, Akt and GLUT4. The IRS-1/PI3K/Akt-dependent
signaling pathway can induce GLUT4 translocation and increase IR
expression, thereby improving the induced glucose intolerance and
insulin resistance (66). The
glucagon-like peptide agonist liraglutide, which reduces blood
glucose levels in patients with T2DM, has been found to reverse
insulin resistance in skeletal muscle cells treated with palmitic
acid (PA). The insulin-stimulated decrease in the level of glucose
transporter 4 (GLUT4) on the cell surface caused by PA, as well as
the phosphorylation phenomena of Akt, p85α-PI3K and AS160, all
these effects of PA can be reversed after treatment with
liraglutide. This indicates that liraglutide enhances
insulin-induced GLUT4 translocation by inhibiting the serine
phosphorylation of IRS-1 in muscle cells treated with PA (67).
Pioglitazone, a PPARγ agonist, can reduce insulin
resistance in peripheral tissues and the liver, improve insulin
sensitivity in patients with insulin resistance, enhance insulin
responsiveness in cells and improve glucose balance disorders in
the body. The activation of SIRT1 or PPARγ can alleviate abnormal
glucose metabolism and decrease the protein expression of
surfactant protein (SP)-B and SP-C in neonatal rats. SIRT1 enhances
the expression of PPARγ by upregulating QKI5 and activates the
PI3K/AKT pathway, thereby alleviating insulin resistance in late
preterm rats (68). Adrenomedullin
(ADM), an endogenous active peptide, is considered to be an
adipokine involved in adipocyte function. PA induces the impairment
of the insulin signaling pathway by affecting the PI3K/Akt axis and
GLUT4 levels. ADM has been shown to reverse the effect of PA on the
insulin signaling pathway, decrease the levels of pro-inflammatory
factors TNF-α, IL-1β and IL-6, and alleviate oxidative stress
(69). A summary can be found in
Table II.
 | Table II.Research on the regulation of the
IRS/PI3K/Akt signaling pathway by traditional drugs to improve
insulin resistance. |
Table II.
Research on the regulation of the
IRS/PI3K/Akt signaling pathway by traditional drugs to improve
insulin resistance.
| First author/s,
year | Medicine | In
vitro/in vivo | Experimental
subject | Dosages | Remarks | (Refs.) |
|---|
| Liu et al,
2015 |
1-deoxynojirimycin | In vivo | db/db mice | 20, 40 and 80
mg/kg | By activating the
insulin signaling PI3K/AKT pathway in the skeletal muscles of db/db
mice, insulin sensitivity was significantly enhanced. | (62) |
| Kang et al,
2022 |
1-deoxynojirimycin | In vivo | db/db mice | 40 mM/kg | Regulated the
insulin signaling pathway in the skeletal muscle of db/db mice to
improve insulin resistance. | (63) |
| Garabadu and
Krishnamurthy, 2017 | Metformin | In vivo | Type 2 diabetes
mellitus rats | 25 mg/kg | Alleviated the
diabetes-induced reduction of phosphorylated Akt and GLUT4
translocation in the liver and improved insulin resistance. | (65) |
| Sundaresan et
al, 2016 | Ursolic acid and
rosiglitazone | In vivo | C57/BL/6J mice | Ursolic acid (5
mg/kg); rosiglitazone (4 mg/kg) | Increased the
expression of insulin receptors and improved high fat diet-induced
glucose intolerance and insulin resistance. | (66) |
| Li et al,
2018 | Liraglutide | In
vitro | C2C12-GLUT4
myc-tagged cells | 100 nM | Inhibited the
phosphorylation of IRS-1 serine in muscle cells treated with PA to
enhance insulin-induced GLUT4 translocation. | (67) |
| He et al,
2023 | Pioglitazone | In vivo | Wistar rats | 5 mg/kg | Upregulation of
quaking-5 promoted the expression of peroxisome
proliferator-activated receptor γ, activated the PI3K/Akt pathway
and improved insulin resistance. | (68) |
| He et al,
2023 | Pioglitazone | In
vitro | AT-II cells | 100 nM | Activation of SIRT1
alleviates abnormal glucose metabolism, reduces the expression of
SP-B and SP-C, activates the PI3K/AKT pathway, and decreases
cellular inflammation and apoptosis. | (68) |
| Dai et al,
2022 | Adrenomedullin | In vivo | Obese rats | 300 ng/kg | Improved insulin
resistance in obese rats, restored insulin signal transduction and
reduced inflammation and oxidative stress. | (69) |
| Dai et al,
2022 | Adrenomedullin | In
vitro | 3T3-L1
adipocytes | 10 nM | Regulating the
PI3K/Akt pathway improved insulin resistance, inflammation and
oxidative stress induced by PA in adipocytes. | (69) |
Modern methods of treatment
Vascular endothelial growth factor B (VEGFB) is a
member of the vascular endothelial growth factor family and plays a
role in the balance of glucose and lipid metabolism (70). Experiments have demonstrated that
VEGFB/VEGFR1 activates PI3K/Akt signaling by increasing the levels
of phosphorylated-IRS-1Ser307, and inhibits the
expression of phosphorylated-forkhead box O1pS256 and
phosphorylated-GSK3Ser9, thereby reducing
gluconeogenesis and glycogen synthesis in the liver (71). RSG is a traditional drug that
alleviates insulin resistance, but its clinical application is
limited due to the risk of adverse reactions. A co-crystal of RSG
and berberine (RB) has been synthesized from RSG and RB at an molar
ratio of 1:1 (72). RB was found
to improve glucose and lipid metabolism, insulin resistance, and
diabetes-induced liver and pancreatic lesions in high glucose and
PA-stimulated KK-Ay mice, as well as in C2C12 and HepG2 cell lines.
The upregulation of p-PI3K and p-Akt in KK-Ay mice, and HepG2 and
C2C12 cells may be associated with regulation of the PI3K/Akt
signaling pathway (73).
Tetrahedral framework nucleic acid is a DNA
nanomaterial that has been shown to increase glucose uptake and
improve insulin resistance via the IRS-1/PI3K/Akt pathway (74). Dysregulation of negative regulators
of insulin signaling, such as PTEN, induces insulin resistance
through a mechanism associated with hyperactivation (75). It has been found that human
umbilical cord mesenchymal stem cells (HUC-MSCs) can effectively
control the development of diabetes by restoring islet function and
improving insulin resistance (76). This may be associated with PTEN
regulating the PI3K/Akt pathway and reducing inflammatory release.
HUC-MSCs stimulate glucose uptake and improve insulin action;
melatonin increases the proliferation, migration and
differentiation of HUC-MSCs by regulating the PI3K/Akt signaling
pathway, thereby alleviating impaired glucose control and insulin
resistance (77).
MicroRNA (miR)-27a has been shown to be involved in
the signaling pathways associated with glucose metabolism in
insulin resistance. PPARγ is the direct target of miR-27a, and has
been shown to improve insulin resistance and mediate glucose
metabolism. The mechanism of miR-27a activity is associated with
regulation of the PPARγ-PI3K/Akt-GLUT4 signaling axis (78). It has also been confirmed that
miR-506-3p expression is associated with insulin sensitivity and
regulates the expression of S6K1, which participates in the protein
expression of key genes in the PI3K/Akt insulin signaling pathway
and alleviates insulin resistance in adipocytes, by binding to its
3′ untranslated region (79). The
RNA-binding protein Grb10-interacting GYF protein 2 has been shown
to mediate obesity-induced insulin resistance by upregulating
double-stranded RNA-binding protein Staufen homolog 1/PTEN and
disrupting the PI3K/Akt signaling axis (80). A summary can be found in Table III.
 | Table III.Research on the improvement of
insulin resistance by modern technology regulating the insulin
receptor substrate/PI3K/Akt signaling pathway. |
Table III.
Research on the improvement of
insulin resistance by modern technology regulating the insulin
receptor substrate/PI3K/Akt signaling pathway.
| First author/s,
year | Modern
technology | In
vitro/in vivo | Experimental
subject | Dosages | Remarks | (Refs.) |
|---|
| Li et al,
2024 | VEGFB gene | In vivo | VEGFB knockout
mice | - | Activate the
PI3K/AKT signaling pathway in mice, inhibit glucose production and
promote glycogen synthesis, thereby improving insulin resistance
and hepatic steatosis. | (71) |
| Li et al,
2024 | VEGFB gene | In
vitro | HepG2 cells | - | Activate the
PI3K/AKT signaling pathway in HepG2 cells induced by PA, and
improve the levels of glucose and lipids. | (71) |
| He et al,
2022 | Co-crystal of
rosiglitazone with berberine | In vivo | KK-Ay mice | 0.7, 2.11 and 6.33
mg/kg | The mechanism of
improving insulin resistance and metabolic disorders may involve
regulation of the PI3K/Akt/thioredoxin-interacting protein
signaling pathway. | (73) |
| Li et al,
2021 | Tetrahedral
framework nucleic acids | In
vitro | HepG2 cells | - | Reduced blood
glucose levels and improved insulin resistance in hepatocytes
through the PI3K/Akt pathway. | (74) |
| Chen et al,
2020 | HUC-MSCs | In vivo | db/db mice | 1×107
HUC-MSCs (in 0.7 ml saline) or 1×108 HUC-MSCs (in 2 ml
aline) | By regulating the
PI3K/Akt and ERK/MAPK signaling pathways through PTEN, insulin
resistance is alleviated. | (76) |
| Aierken et
al, 2022 | Melatonin and
HUC-MSCs | In vivo | Kunming mice | 1×106
HUC-MSCs (in 0.2 ml of 0.9% NaCl) | Regulating the
PI3K/AKT pathway leads to hUC-MSC stimulating glucose uptake and
improving insulin action. | (77) |
| Chen et al,
2019 | AD-miR-27a | In vivo | Obese mice | 1.0×108
plaque-forming units (in 0.2 ml PBS) | miR-27a was
involved in the PPARγ-PI3K/Akt-GLUT4 signaling axis, increasing
glucose uptake and reducing insulin resistance. | (78) |
| Chen et al,
2019 | AD-miR-27a | In
vitro | 3T3-L1
adipocytes | 10 nM | MiR-27a activates
through PPAR-γ and activates the PI3K/Akt signaling pathway to
regulate insulin sensitivity. | (78) |
| Zhong et al,
2021 | miR-506-3p
mimic | In
vitro | Human
preadipocytes | - | Insulin resistance
of adipocytes was altered by regulating the activation of the
ribosomal protein S6kinase β-1-mediated PI3K/Akt pathway. | (79) |
| Lv et al,
2024 | RNA-binding protein
GIGYF2 | In vivo | C57BL/6J mice | 1×109
plaque-forming units/100 µl (GIGYF2 lentivirus) | GIGYF2 disrupted
the PI3K/Akt signaling axis by upregulating double- stranded
RNA-binding protein staufen homolog 1/PTEN, thereby mediating
obesity-related insulin resistance. | (80) |
| Lv et al,
2024 | RNA-binding protein
GIGYF2 | In
vitro | HepG2 and 293T
cells | - | GIGYF2 promotes
insulin resistance through PTEN-mediated inactivation of the
PI3K/AKT pathway in hepatocytes. | (80) |
Other
Notably, certain components of everyday foods have
also been found to alleviate insulin resistance. Tomato pectin, a
potential active ingredient in tomato processing residues, can
reduce insulin resistance and inflammatory factor expression in the
liver of model mice by regulating the PI3K/Akt pathway (81). Consumption of dietary chokeberry
and dried jujube alters the protein expression of IRS, PI3K, Akt
and catalase in the liver, all of which have been implicated in
insulin resistance (82).
Theabrownin is a bioactive component in dark tea that regulates
glucose and lipid metabolism. Theabrownin has been shown to reverse
insulin resistance in HepG2 cells through the IRS/PI3K/Akt
signaling pathway, to regulate GSK3β, G6Pase, glucokinase, PEPCK1
and other related indicators, reduce oxidative stress (83). Pterostilbene effectively rescues
advanced glycation end-product (AGE)-induced phenotypes and
enhances IRS-1/PI3K/Akt insulin signaling in a dose-dependent
manner in both Lo2 and HepG2 cell lines. The results of animal
experiments are consistent with in vitro results, revealing
a reduction of AGE accumulation in the liver and serum (84). Vitamin D deficiency can also cause
insulin resistance; however, vitamin D supplementation cannot
markedly mediate the increase of acute inflammation and insulin
resistance in obesity (85).
Discussion
Insulin resistance is a key pathological feature
affecting the development of T2DM. Therefore, effective control of
insulin resistance is important for the prevention and treatment of
diabetes (86). The
insulin-related signaling pathway is the molecular basis of insulin
regulation of metabolism. The binding of insulin to its receptor
activates the PI3K/Akt pathway, thereby improving glucose metabolic
transformation, including glucose uptake and glycogenesis (87).
In the field of diabetic insulin resistance
research, further research on the IRS/PI3K/Akt signaling pathway
needs to address the following issues. The core flaws can be
summarized as two major research gaps: i) Focusing on the basic
regulatory mechanism of the IRS/PI3K/Akt signaling pathway in
insulin resistance during diabetes; or ii) only focusing on the
research progress of a single treatment method, such as the use of
natural drugs alone or traditional treatment regimens. The core
flaws are manifested in two aspects: i) There is a disconnect
between mechanism explanation and treatment application, failing to
establish a connection between the basic mechanism and clinical
intervention; and ii) there remain only sporadic mentions of the
abnormality of a single molecule, such as IRS-1 or Akt, and its
influence on insulin resistance, without systematically sorting out
the abnormal interaction patterns among molecules within the
pathway, leaving research gaps. These two aspects are mutually
related and jointly restrict the breakthrough from basic mechanism
elucidation to clinical translation in this field.
Since insulin resistance plays an important role in
the pathogenesis of diabetes, and IRS/PI3K/Akt signaling pathway is
an important pathway of insulin resistance, this review elaborates
the relationship between IRS and PI3K/Akt, and explores the role of
IRS/PI3K/Akt in insulin resistance in general. The regulatory value
and importance of insulin resistance in the early stage of diabetes
are reviewed. Primarily, the present review clarified the cascade
effect of IRS phosphorylation imbalance leading to PI3K activation
blockage, which in turn results in Akt signal silencing in the
insulin signaling pathway under pathological conditions, and its
core driving role in the occurrence and development of insulin
resistance. For example, an increase in Ser307 phosphorylation of
IRS-1 will prevent the p85 subunit of PI3K from binding, thereby
reducing the phosphorylation level of Thr308 or Ser473 in Akt and
ultimately inhibiting the membrane translocation of GLUT4 and the
activation of glycogen synthase (88). Furthermore, the present review
simultaneously clarified how targeted intervention against this
cascade effect, such as regulating the balance of serine
phosphorylation of insulin receptor molecules, drug
co-crystallization technology and tetrahedral framework nucleic
acid nanomaterials, can directly promote glucose transport by
increasing the expression level of GLUT4 in the cell membrane,
accelerate glycogen synthesis and maintain the lipid metabolism
balance (89). In summary, the
present review outlined how the IRS/PI3K/Akt pathway is the key
intervention target for improving insulin resistance in
diabetes.
Drug therapy can improve insulin resistance by
upregulating IRS-1, activating the PI3K/Akt pathway, promoting
glycogen synthesis, and regulating glucose and lipid metabolism
(90). Furthermore, drug therapies
reduce oxidative stress and inflammation, increase the insulin
sensitivity index, increase glucose uptake, glucose consumption and
glycogen storage, decrease plasma glucose levels (91,92).
These therapies include the isolation of active ingredients from
natural medicines and research involves the establishment of animal
or cell models to discover the effectiveness of extracts that serve
a role in the treatment of T2DM (93–96).
Traditional drugs and monomeric active ingredients can alleviate
the inflammatory response, improve hepatic insulin resistance, and
enhance glucose uptake and utilization in peripheral tissues by
regulating the IRS/PI3K/Akt pathway, thereby improving abnormal
lipid metabolism function and protecting islet β cells (97,98).
Certain drugs have multi-target synergistic effects, which can not
only regulate the IRS/PI3K/Akt and AMPK pathways, but also reduce
the accumulation of AGEs and receptor for AGEs (99), upregulate IRS-1 and increase the
phosphorylation of p85-PI3K and Akt, as well as improve related
complications, such as polydipsia, polyphagia, polyuria and body
wasting caused by diabetes (100).
The present review discusses how the intestinal
microbiota of patients with T2DM not only show a higher
concentration of flora, but are also structurally and functionally
different from those of non-diabetic individuals. Acidophilus,
Blautia, Desulfovibrio, Dorea and Faecalibacterium, as
well as agglomerates and anaerobic bacteria, have been found to be
nominally associated with T2DM (101). Gut bacteria have been shown to be
associated with insulin resistance and sensitivity, to exhibit a
distinct carbohydrate metabolism signature and, in the case of
insulin sensitivity-related bacteria, to ameliorate the phenotype
of host insulin resistance in mouse models (102). However, the molecular mechanisms
of how gut microbiota directly regulate the IRS/PI3K/Akt pathway,
such as the short-chain fatty acid/G-protein coupled receptor
43/PI3K axis, remain to be fully elucidated, and human intervention
studies are limited. At present, the clinical treatment mainly
focuses on targeted therapy, which is superior to traditional drug
therapy in terms of efficacy, safety and precision. Novel drug
therapies use co-crystals formed by the non-covalent bond between
one active pharmaceutical ingredient and another, or in some cases
multiple co-crystal forming agents. Co-crystals have previously
been used in the field of medicine and show great potential in the
treatment of type 2 diabetes, with co-crystal formation overcoming
the adverse physicochemical properties of the parent drug by
forming eutectic structures (72,73).
Tetrahedral frame nucleic acids improve hepatic insulin resistance
and alleviate type 2 diabetes (74). These novel technologies are of
notable value in the treatment of diabetes. Furthermore, HUC-MSCs
can restore the function of insulin. Melatonin can increase the
proliferation, migration and differentiation of HUC-MSCs by
regulating the PI3K/Akt signaling pathway, thereby controlling
blood glucose levels and alleviating insulin resistance (76). However, there are few studies on
the effect of stem cells on insulin resistance, which will have
notable value in the future treatment of diabetes.
At present, the study of the IRS/PI3K/Akt pathway in
insulin resistance still faces a number of challenges. The
traditional view was that the IRS-1/PI3K/Akt pathway is inhibited
when insulin resistance occurs, but this pathway is abnormally
activated in specific tissues or stages (103). For example, although serine
phosphorylation of IRS-1 in the liver inhibits PI3K activity, Akt
may be activated through PI3K-independent pathways such as the
RAS/MAPK pathway, leading to the upregulation of gluconeogenic
genes (104). This partial
activation is associated with tissue-specific regulation.
Furthermore, studies predominantly focus on liver and adipose
tissue, while the mechanistic analysis of skeletal muscle is
relatively weak (105–107). Studies on different organizations
should receive more attention in future research. In addition, the
role of the p110α and p110β isoforms of PI3K in insulin resistance
remains controversial. p110α serves a dominant role in hepatic
glucose metabolism, while p110β may affect muscle insulin
sensitivity by regulating GLUT4 transport (108,109). However, the majority of existing
studies use pan-PI3K inhibitors such as LY294002, which leads to
the masking of subtype-specific mechanisms (110).
In addition, due to insulin resistance being a
progressive disease, the majority of studies use cross-sectional
designs in animal experiments. For example, in the study on obesity
and insulin resistance caused by high-fat diet (HFD), it was found
that after 3 days of feeding with HFD, the phosphorylation of AMPK
significantly decreased compared to mice fed with regular diet.
After 14 days of feeding with HFD, systemic insulin resistance
occurred, and the phosphorylation of Akt significantly decreased
(111). This dynamic change
suggests that there is a key point in pathway regulation, but
existing models lack time series analysis. Furthermore, the
translational dilemma between animal models and human studies is
that the HFD-induced mouse model of insulin resistance shows a
decreased expression of IRS-1 (112); however, in humans, a study
focused on obese individuals without diabetes. Through skeletal
muscle biopsy, it was found that the total protein level of IRS-1
in the skeletal muscles of obese individuals did not show
significant fluctuations. However, different dietary interventions
would cause obvious abnormalities in the phosphorylation of Ser307.
For example, in the high-fat and low-carbohydrate diet group, the
phosphorylation of Ser307 significantly increased, while in the
low-fat and high-carbohydrate diet group, it showed a decreasing
trend (113). In addition, ADM
causes insulin resistance by interfering with endothelial insulin
signaling in mouse models (114),
but clinical evidence for this mechanism in humans is insufficient,
suggesting that interspecies pathway differences may be
underestimated.
The IRS/PI3K/Akt pathway can provide novel
strategies and methods for the treatment of diabetes. However, the
regulatory mechanism of this pathway and existing problems in
diabetes research require further study in order to provide an
improved theoretical basis and novel drug targets for the
prevention and treatment of diabetes.
Acknowledgements
Not applicable.
Funding
The present review was supported by the Talented Research Grant
of Huyang from Tarim University (grant nos. TDZKBS202574,
TDZKBS202575 and TDZKBS202576).
Availability of data and materials
Not applicable.
Authors' contributions
CG and SD made substantial contributions to
conception, design and funding support. WT drafted the manuscript.
HL and XL revised it critically for important intellectual content.
Data authentication is not applicable. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Saeedi P, Petersohn I, Salpea P, Malanda
B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA,
Ogurtsova K, et al: Global and regional diabetes prevalence
estimates for 2019 and projections for 2030 and 2045: Results from
the International diabetes federation diabetes atlas, 9th edition.
Diabetes Res Clin Pract. 157:1078432019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
DeFronzo RA and Tripathy D: Skeletal
muscle insulin resistance is the primary defect in type 2 diabetes.
Diabetes Care. 32 (Suppl 2):S157–S163. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Karagözoğlu F, Şahin E, Melek Ş, Bediz
Şahin S, Koca RH, Yüksel H and Güngören A: Effects of apilarnil on
type 2 diabetes-induced IRS-1/PI3K/Akt mediated insulin resistance
in male rats. ACS Omega. 10:25027–25038. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hong S, Lee J, Choi SY, Park JH and Lee
YG: Ginsenoside Rf improves glucose metabolism via the IRS/PI3K/Akt
and PPARα/PGC1α signaling pathways in insulin-resistant AML12
cells. BMC Complement Med Ther. 25:3402025. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang Y, Zhang L, He M, Chen X, Pei L and
Xi S: Cobalt exposure increases fasting plasma glucose by
inhibiting hepatic glycogen synthesis and enhancing
gluconeogenesis. J Hazard Mater. 499:1401322025. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Luo Z, Xu W, Yuan T, Shi C, Jin T, Chong
Y, Ji J, Lin L, Xu J, Zhang Y, et al: Platycodon grandiflorus root
extract activates hepatic PI3K/PIP3/Akt insulin signaling by
enriching gut Akkermansia muciniphila in high fat diet fed mice.
Phytomedicine. 109:1545952025. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Horii N, Hasegawa N, Fujie S, Uchida M and
Iemitsu M: Resistance exercise-induced increase in muscle
5α-dihydrotestosterone contributes to the activation of muscle
Akt/mTOR/p70S6K- and Akt/AS160/GLUT4-signaling pathways in type 2
diabetic rats. FASEB J. 34:11047–11057. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li Q, Su J, Jin SJ, Wei W, Cong XD, Li XX
and Xu M: Argirein alleviates vascular endothelial insulin
resistance through suppressing the activation of Nox4-dependent O2-
production in diabetic rats. Free Radic Biol Med. 121:169–179.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chadt A and Al-Hasani H: Glucose
transporters in adipose tissue, liver, and skeletal muscle in
metabolic health and disease. Pflugers Arch. 472:1273–1298. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang G, Liu Z, Liang D, Yu J, Wang T, Zhou
F and Chen W: Aqueous extract of Polygonatum sibiricum ameliorates
glucose and lipid metabolism via PI3K/AKT signaling pathway in
high-fat diet and streptozotocin-induced diabetic mice. J Food
Biochem. 46:e144022022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu X, Cai S, Yi J and Chu C: Chinese
sumac fruits (Rhus chinesis Mill.) alleviate type 2 diabetes in
C57BL/6 mice through repairing islet cell functions, regulating
IRS-1/PI3K/AKT pathways and promoting the entry of Nrf2 into the
nucleus. Nutrients. 15:40802023. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Escribano O, Beneit N, Rubio-Longás C,
López-Pastor AR and Gómez-Hernández A: The role of insulin receptor
isoforms in diabetes and its metabolic and vascular complications.
J Diabetes Res. 2017:14032062017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li J, Park J, Mayer JP, Webb KJ, Uchikawa
E, Wu J, Liu S, Zhang X, Stowell MHB, Choi E and Bai XC:
Synergistic activation of the insulin receptor via two distinct
sites. Nat Struct Mol Biol. 29:357–368. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yuan YL, Lin BQ, Zhang CF, Cui LL, Ruan
SX, Yang ZL, Li F and Ji D: Timosaponin B-II ameliorates
palmitate-induced insulin resistance and inflammation via
IRS-1/PI3K/Akt and IKK/NF-[Formula: see text]B pathways. Am J Chin
Med. 44:755–769. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Franko A, Kunze A, Böse M, von
Kleist-Retzow JC, Paulsson M, Hartmann U and Wiesner RJ: Impaired
insulin signaling is associated with hepatic mitochondrial
dysfunction in IR+/-IRS-1+/- double heterozygous (IR-IRS1dh) mice.
Int J Mol Sci. 18:11562017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Draznin B: Molecular mechanisms of insulin
resistance: serine phosphorylation of insulin receptor substrate-1
and increased expression of p85alpha: The two sides of a coin.
Diabetes. 55:2392–2397. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Krisnamurti DGB, Louisa M, Poerwaningsih
EH, Tarigan TJE, Soetikno V, Wibowo H and Nugroho CMH: Vitamin D
supplementation alleviates insulin resistance in prediabetic rats
by modifying IRS-1 and PPARγ/NF-κB expressions. Front Endocrinol
(Lausanne). 14:10892982023. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Granata R, Settanni F, Gallo D, Trovato L,
Biancone L, Cantaluppi V, Nano R, Annunziata M, Campiglia P,
Arnoletti E, et al: Obestatin promotes survival of pancreatic
beta-cells and human islets and induces expression of genes
involved in the regulation of beta-cell mass and function.
Diabetes. 57:967–979. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sharfi H and Eldar-Finkelman H: Sequential
phosphorylation of insulin receptor substrate-2 by glycogen
synthase kinase-3 and c-Jun NH2-terminal kinase plays a role in
hepatic insulin signaling. Am J Physiol Endocrinol Metab.
294:E307–E315. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Luo Q, Ling Z, Huang X and Zuo Y:
Association of IRS-1 and IRS-2 polymorphisms with predisposition to
type-2 diabetes (T2D): A meta-analysis and trial sequential
analysis. Nucleosides Nucleotides Nucleic Acids. 42:837–851. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Escribano O, Fernández-Moreno MD, Zueco
JA, Menor C, Fueyo J, Ropero RM, Diaz-Laviada I, Román ID and
Guijarro LG: Insulin receptor substrate-4 signaling in quiescent
rat hepatocytes and in regenerating rat liver. Hepatology.
37:1461–1469. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Boura-Halfon S and Zick Y: Phosphorylation
of IRS proteins, insulin action, and insulin resistance. Am J
Physiol Endocrinol Metab. 296:E581–E591. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou K, Chen Q, Chen J, Liang D, Feng W,
Liu M, Wang Q, Wang R, Ouyang Q, Quan C and Chen S: Spatiotemporal
regulation of insulin signaling by liquid-liquid phase separation.
Cell Discov. 8:642022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cantley LC: The phosphoinositide 3-kinase
pathway. Science. 296:1655–1657. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Van Gerwen J, Shun-Shion AS and Fazakerley
DJ: Insulin signalling and GLUT4 trafficking in insulin resistance.
Biochem Soc Trans. 51:1057–1069. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cao Y, Sun W and Xu G: Fuzhu jiangtang
granules combined with metformin reduces insulin resistance in
skeletal muscle of diabetic rats via PI3K/Akt signaling. Pharm
Biol. 57:660–668. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen H, Li J, Zhang Y, Zhang W, Li X, Tang
H, Liu Y, Li T, He H, Du B, et al: Bisphenol F suppresses
insulin-stimulated glucose metabolism in adipocytes by inhibiting
IRS-1/PI3K/AKT pathway. Ecotoxicol Environ Saf. 231:1132012022.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhu S, Sun F, Li W, Cao Y, Wang C, Wang Y,
Liang D, Zhang R, Zhang S, Wang H and Cao F: Apelin stimulates
glucose uptake through the PI3K/Akt pathway and improves insulin
resistance in 3T3-L1 adipocytes. Mol Cell Biochem. 353:305–313.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Malone JI and Hansen BC: Does obesity
cause type 2 diabetes mellitus (T2DM)? Or is it the opposite?
Pediatr Diabetes. 20:5–9. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Watt MJ, Miotto PM, De Nardo W and
Montgomery MK: The liver as an endocrine organ-linking NAFLD and
insulin resistance. Endocr Rev. 40:1367–1393. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Petersen MC and Shulman GI: Mechanisms of
insulin action and insulin resistance. Physiol Rev. 98:2133–2223.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Eckstein SS, Weigert C and Lehmann R:
Divergent roles of IRS (Insulin Receptor Substrate) 1 and 2 in
liver and skeletal muscle. Curr Med Chem. 24:1827–1852. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fan Y, He Z, Wang W, Li J, Hu A, Li L, Yan
L, Li Z and Yin Q: Tangganjian decoction ameliorates type 2
diabetes mellitus and nonalcoholic fatty liver disease in rats by
activating the IRS/PI3K/AKT signaling pathway. Biomed Pharmacother.
106:733–737. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee J and Kim MS: The role of GSK3 in
glucose homeostasis and the development of insulin resistance.
Diabetes Res Clin Pract. 77 (Suppl 1):S49–S57. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Guo X, Sun W, Luo G, Wu L, Xu G, Hou D,
Hou Y, Guo X, Mu X, Qin L and Liu T: Panax notoginseng saponins
alleviate skeletal muscle insulin resistance by regulating the
IRS1-PI3K-AKT signaling pathway and GLUT4 expression. FEBS Open
Bio. 9:1008–1019. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang J, He Y, Yu D, Jin L, Gong X and
Zhang B: Perilla oil regulates intestinal microbiota and alleviates
insulin resistance through the PI3K/AKT signaling pathway in type-2
diabetic KKAy mice. Food Chem Toxicol. 135:1109652020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang LY, Wang Y, Xu DS, Ruan KF, Feng Y
and Wang S: MDG-1, a polysaccharide from Ophiopogon japonicus
exerts hypoglycemic effects through the PI3K/Akt pathway in a
diabetic KKAy mouse model. J Ethnopharmacol. 143:347–354. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu N, Cui X, Guo T, Wei X, Sun Y, Liu J,
Zhang Y, Ma W, Yan W and Chen L: Baicalein ameliorates insulin
resistance of HFD/STZ mice through activating PI3K/AKT signal
pathway of liver and skeletal muscle in a GLP-1R-dependent manner.
Antioxidants (Basel). 13:12462024. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cui X, Qian DW, Jiang S, Shang EX, Zhu ZH
and Duan JA: Scutellariae radix and coptidis rhizoma improve
glucose and lipid metabolism in T2DM rats via regulation of the
metabolic profiling and MAPK/PI3K/Akt signaling pathway. Int J Mol
Sci. 19:36342018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang S, Zhang S, Zhang Y, Wang H, Chen Y
and Lu H: Activation of NRF2 by epiberberine improves oxidative
stress and insulin resistance in T2DM mice and IR-HepG2 cells in an
AMPK dependent manner. J Ethnopharmacol. 327:1179312024. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liu M, Liu C, Zhaxi P, Kou X, Liu Y and
Xue Z: Research progress on hypoglycemic effects and molecular
mechanisms of flavonoids: A review. Antioxidants (Basel).
14:3782025. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yang Z, Huang W, Zhang J, Xie M and Wang
X: Baicalein improves glucose metabolism in insulin resistant HepG2
cells. Eur J Pharmacol. 854:187–193. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li QP, Dou YX, Huang ZW, Chen HB, Li YC,
Chen JN, Liu YH, Huang XQ, Zeng HF, Yang XB, et al: Therapeutic
effect of oxyberberine on obese non-alcoholic fatty liver disease
rats. Phytomedicine. 85:1535502021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Entezari M, Hashemi D, Taheriazam A,
Zabolian A, Mohammadi S, Fakhri F, Hashemi M, Hushmandi K,
Ashrafizadeh M, Zarrabi A, et al: AMPK signaling in diabetes
mellitus, insulin resistance and diabetic complications: A
pre-clinical and clinical investigation. Biomed Pharmacother.
146:1125632022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lee YW and Pyo YH: Monascus-fermented
grain vinegar enhances glucose homeostasis through the
IRS-1/PI3K/Akt and AMPK signaling pathways in HepG2 cell and db/db
mice. Food Sci Biotechnol. 31:1583–1591. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Xia X, Xu J, Wang X, Wang H, Lin Z, Shao
K, Fang L, Zhang C and Zhao Y: Jiaogulan tea (Gpostemma
pentaphyllum) potentiates the antidiabetic effect of white tea via
the AMPK and PI3K pathways in C57BL/6 mice. Food Funct.
11:4339–4355. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang P, Liu Y, Kang SY, Lyu C, Han X, Ho
T, Lee KJ, Meng X, Park YK and Jung HW: Clean-DM1, a Korean
polyherbal formula, improves high fat diet-induced diabetic
symptoms in mice by regulating IRS/PI3K/AKT and AMPK expressions in
pancreas and liver tissues. Chin J Integr Med. 30:125–134. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sharma BR, Kim HJ and Rhyu DY: Caulerpa
lentillifera extract ameliorates insulin resistance and regulates
glucose metabolism in C57BL/KsJ-db/db mice via PI3K/AKT signaling
pathway in myocytes. J Transl Med. 13:622015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Arden C, Hampson LJ, Huang GC, Shaw JA,
Aldibbiat A, Holliman G, Manas D, Khan S, Lange AJ and Agius L: A
role for PFK-2/FBPase-2, as distinct from fructose
2,6-bisphosphate, in regulation of insulin secretion in pancreatic
beta-cells. Biochem J. 411:41–51. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Nie Q, Chen H, Hu J, Fan S and Nie S:
Dietary compounds and traditional Chinese medicine ameliorate type
2 diabetes by modulating gut microbiota. Crit Rev Food Sci Nutr.
59:848–863. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Gong X, Xiong L, Bi C and Zhang B:
Diosmetin ameliorate type 2 diabetic mellitus by up-regulating
Corynebacterium glutamicum to regulate IRS/PI3K/AKT-mediated
glucose metabolism disorder in KK-Ay mice. Phytomedicine.
87:1535822021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Gong P, Xiao X, Wang S, Shi F, Liu N, Chen
X, Yang W, Wang L and Chen F: Hypoglycemic effect of astragaloside
IV via modulating gut microbiota and regulating AMPK/SIRT1 and
PI3K/AKT pathway. J Ethnopharmacol. 281:1145582021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Tan Y, Miao L, Xiao J and Cheang WS:
3,3′,4,5′- Tetramethoxy-trans-stilbene improves insulin resistance
by activating the IRS/PI3K/Akt pathway and inhibiting oxidative
stress. Curr Issues Mol Biol. 44:2175–2185. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Feng M, Liu F, Xing J, Zhong Y and Zhou X:
Anemarrhena saponins attenuate insulin resistance in rats with
high-fat diet-induced obesity via the IRS-1/PI3K/AKT pathway. J
Ethnopharmacol. 277:1142512021. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yang Q, Wen YM, Shen J, Chen MM, Wen JH,
Li ZM, Liang YZ and Xia N: Guava leaf extract attenuates insulin
resistance via the PI3K/Akt signaling pathway in a type 2 diabetic
mouse model. Diabetes Metab Syndr Obes. 13:713–718. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Xiao H, Sun X, Lin Z, Yang Y, Zhang M, Xu
Z, Liu P, Liu Z and Huang H: Gentiopicroside targets PAQR3 to
activate the PI3K/AKT signaling pathway and ameliorate disordered
glucose and lipid metabolism. Acta Pharm Sin B. 12:2887–2904. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Xu S, Chen Y and Gong Y: Improvement of
theaflavins on glucose and lipid metabolism in diabetes mellitus.
Foods. 13:17632024. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yaribeygi H, Sathyapalan T, Atkin SL and
Sahebkar A: Molecular Mechanisms Linking Oxidative Stress and
Diabetes Mellitus. Oxid Med Cell Longev. 2020:86092132020.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wang GY, Yan PY, Liu W, Liu LK, Li JP and
Zeng Y: Potentilla bifurca flavonoids effectively improve insulin
resistance. Eur Rev Med Pharmacol Sci. 26:8358–8369.
2022.PubMed/NCBI
|
|
60
|
Jiang Z, Zhao M, Voilquin L, Jung Y, Aikio
MA, Sahai T, Dou FY, Roche AM, Carcamo-Orive I, Knowles JW, et al:
Isthmin-1 is an adipokine that promotes glucose uptake and improves
glucose tolerance and hepatic steatosis. Cell Metab.
33:1836–1852.e11. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Kong WH, Oh SH, Ahn YR, Kim KW, Kim JH and
Seo SW: Antiobesity effects and improvement of insulin sensitivity
by 1-deoxynojirimycin in animal models. J Agric Food Chem.
56:2613–2619. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Liu Q, Li X, Li C, Zheng Y and Peng G:
1-Deoxynojirimycin alleviates insulin resistance via activation of
insulin signaling PI3K/AKT pathway in skeletal muscle of db/db
mice. Molecules. 20:21700–21714. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Kang CW, Park M and Lee HJ: Mulberry
(Morus alba L.) leaf extract and 1-deoxynojirimycin improve
skeletal muscle insulin resistance via the activation of
IRS-1/PI3K/Akt pathway in db/db mice. Life (Basel).
12:16302022.PubMed/NCBI
|
|
64
|
LaMoia TE and Shulman GI: Cellular and
molecular mechanisms of metformin action. Endocr Rev. 42:77–96.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Garabadu D and Krishnamurthy S: Metformin
attenuates hepatic insulin resistance in type-2 diabetic rats
through PI3K/Akt/GLUT-4 signalling independent to
bicuculline-sensitive GABAA receptor stimulation. Pharm Biol.
55:722–728. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Sundaresan A, Radhiga T and Pugalendi KV:
Ursolic acid and rosiglitazone combination improves insulin
sensitivity by increasing the skeletal muscle insulin-stimulated
IRS-1 tyrosine phosphorylation in high-fat diet-fed C57BL/6J mice.
J Physiol Biochem. 72:345–352. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Li Z, Zhu Y, Li C, Tang Y, Jiang Z, Yang
M, Ni CL, Li D, Chen L and Niu W: Liraglutide ameliorates
palmitate-induced insulin resistance through inhibiting the IRS-1
serine phosphorylation in mouse skeletal muscle cells. J Endocrinol
Invest. 41:1097–1102. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
He J, Fan F, Li J, Han Y, Song Y, Zhang R,
Xu Y, Wu H and Fan R: SIRT1 alleviates insulin resistance and
respiratory distress in late preterm rats by activating
QKI5-mediated PPARγ/PI3K/AKT pathway. Cell Cycle. 22:2449–2466.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Dai HB, Wang HY, Wang FZ, Qian P, Gao Q,
Zhou H and Zhou YB: Adrenomedullin ameliorates palmitic
acid-induced insulin resistance through PI3K/Akt pathway in
adipocytes. Acta Diabetol. 59:661–673. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Luo X, Li RR, Li YQ, Yu HP, Yu HN, Jiang
WG and Li YN: Reducing VEGFB expression regulates the balance of
glucose and lipid metabolism in mice via VEGFR1. Mol Med Rep.
26:2852022. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Li Y, Li W, Zhu X, Xu N, Meng Q, Jiang W,
Zhang L, Yang M, Xu F and Li Y: VEGFB ameliorates insulin
resistance in NAFLD via the PI3K/AKT signal pathway. J Transl Med.
22:9762024. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Guan X, Jiang L, Cai L, Zhang L and Hu X:
A new co-crystal of synthetic drug rosiglitazone with natural
medicine berberine: Preparation, crystal structures, and
dissolution. Molecules. 25:42882020. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
He Q, Chen B, Wang G, Zhou D, Zeng H, Li
X, Song Y, Yu X, Liang W, Chen H, et al: Co-crystal of
rosiglitazone with berberine ameliorates hyperglycemia and insulin
resistance through the PI3K/AKT/TXNIP pathway in vivo and in vitro.
Front Pharmacol. 13:8428792022. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Li Y, Tang Y, Shi S, Gao S, Wang Y, Xiao
D, Chen T, He Q, Zhang J and Lin Y: Tetrahedral framework nucleic
acids ameliorate insulin resistance in type 2 diabetes mellitus via
the PI3K/Akt pathway. ACS Appl Mater Interfaces. 13:40354–40364.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Li YZ, Di Cristofano A and Woo M:
Metabolic role of PTEN in insulin signaling and resistance. Cold
Spring Harb Perspect Med. 10:a0361372020. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Chen G, Fan XY, Zheng XP, Jin YL, Liu Y
and Liu SC: Human umbilical cord-derived mesenchymal stem cells
ameliorate insulin resistance via PTEN-mediated crosstalk between
the PI3K/Akt and Erk/MAPKs signaling pathways in the skeletal
muscles of db/db mice. Stem Cell Res Ther. 11:4012020. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Aierken A, Li B, Liu P, Cheng X, Kou Z,
Tan N, Zhang M, Yu S, Shen Q, Du X, et al: Melatonin treatment
improves human umbilical cord mesenchymal stem cell therapy in a
mouse model of type II diabetes mellitus via the PI3K/AKT signaling
pathway. Stem Cell Res Ther. 13:1642022. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Chen T, Zhang Y, Liu Y, Zhu D, Yu J, Li G,
Sun Z, Wang W, Jiang H and Hong Z: MiR-27a promotes insulin
resistance and mediates glucose metabolism by targeting
PPAR-γ-mediated PI3K/AKT signaling. Aging (Albany NY).
11:7510–7524. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Zhong FY, Li J, Wang YM, Chen Y, Song J,
Yang Z, Zhang L, Tian T, Hu YF and Qin ZY: MicroRNA-506 modulates
insulin resistance in human adipocytes by targeting S6K1 and
altering the IRS1/PI3K/AKT insulin signaling pathway. J Bioenerg
Biomembr. 53:679–692. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Lv Z, Ren Y, Li Y, Niu F, Li Z, Li M, Li
X, Li Q, Huang D, Yu Y, et al: RNA-binding protein GIGYF2
orchestrates hepatic insulin resistance through STAU1/PTEN-mediated
disruption of the PI3K/AKT signaling cascade. Mol Med. 30:1242024.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Sun J, Wu K, Wang P, Wang Y, Wang D, Zhao
W, Zhao Y, Zhang C and Zhao X: Dietary tomato pectin attenuates
hepatic insulin resistance and inflammation in high-fat-diet mice
by regulating the PI3K/AKT pathway. Foods. 13:4442024. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Jeong O and Kim HS: Dietary chokeberry and
dried jujube fruit attenuates high-fat and high-fructose
diet-induced dyslipidemia and insulin resistance via activation of
the IRS-1/PI3K/Akt pathway in C57BL/6 J mice. Nutr Metab (Lond).
16:382019. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Liu J, Wang X, Zhu Y, Deng H, Huang X,
Jayavanth P, Xiao Y, Wu J and Jiao R: Theabrownin from dark tea
ameliorates insulin resistance via attenuating oxidative stress and
modulating IRS-1/PI3K/Akt pathway in HepG2 cells. Nutrients.
15:38622023. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Yu W, Fan L, Wang M, Cao B and Hu X:
pterostilbene improves insulin resistance caused by advanced
glycation end products (AGEs) in hepatocytes and mice. Mol Nutr
Food Res. 65:e21003212021. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Mutt SJ, Raza GS, Mäkinen MJ,
Keinänen-Kiukaanniemi S, Järvelin MR and Herzig KH: Vitamin D
deficiency induces insulin resistance and re-supplementation
attenuates hepatic glucose output via the PI3K-AKT-FOXO1 mediated
pathway. Mol Nutr Food Res. 64:e19007282020. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Huang X, Liu G, Guo J and Su Z: The
PI3K/AKT pathway in obesity and type 2 diabetes. Int J Biol Sci.
14:1483–1496. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Tremblay F, Brûlé S, Hee Um S, Li Y,
Masuda K, Roden M, Sun XJ, Krebs M, Polakiewicz RD, Thomas G and
Marette A: Identification of IRS-1 Ser-1101 as a target of S6K1 in
nutrient- and obesity-induced insulin resistance. Proc Natl Acad
Sci USA. 104:14056–14061. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Wu YS, Li ZM, Chen YT, Dai SJ, Zhou XJ,
Yang YX, Lou JS, Ji LT, Bao YT, Xuan L, et al: Berberine improves
inflammatory responses of diabetes mellitus in zucker diabetic
fatty rats and insulin-resistant HepG2 cells through the PPM1B
pathway. J Immunol Res. 2020:21415082020. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Flores-Opazo M, McGee SL and Hargreaves M:
Exercise and GLUT4. Exerc Sport Sci Rev. 48:110–118. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Jayaraman S, Krishnamoorthy K, Prasad M,
Veeraraghavan VP, Krishnamoorthy R, Alshuniaber MA, Gatasheh MK and
Elrobh M Gunassekaran: Glyphosate potentiates insulin resistance in
skeletal muscle through the modulation of IRS-1/PI3K/Akt mediated
mechanisms: An in vivo and in silico analysis. Int J Biol Macromol.
242((Pt 2)): 1249172023. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Zhong RF, Liu CJ, Hao KX, Fan XD and Jiang
JG: Polysaccharides from Flos Sophorae Immaturus ameliorates
insulin resistance in IR-HepG2 cells by co-regulating signaling
pathways of AMPK and IRS-1/PI3K/AKT. Int J Biol Macromol. 280((Pt
4)): 1360882024. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Wang DS, Wang JM, Zhang FR, Lei FJ, Wen X,
Song J, Sun GZ and Liu Z: Ameliorative effects of malonyl
ginsenoside from panax ginseng on glucose-lipid metabolism and
insulin resistance via IRS1/PI3K/Akt and AMPK signaling pathways in
type 2 diabetic mice. Am J Chin Med. 50:863–882. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Hassan MA, Elmageed GMA, El-Qazaz IG,
El-Sayed DS, El-Samad LM and Abdou HM: The synergistic influence of
polyflavonoids from citrus aurantifolia on diabetes treatment and
their modulation of the PI3K/AKT/FOXO1 signaling pathways:
Molecular docking analyses and in vivo investigations.
Pharmaceutics. 15:23062023. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Yan J, Wang C, Jin Y, Meng Q, Liu Q, Liu
Z, Liu K and Sun H: Catalpol ameliorates hepatic insulin resistance
in type 2 diabetes through acting on AMPK/NOX4/PI3K/AKT pathway.
Pharmacol Res. 130:466–480. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Xu Z, Cai K, Su SL, Zhu Y, Liu F and Duan
JA: Salvianolic acid B and tanshinone IIA synergistically improve
early diabetic nephropathy through regulating PI3K/Akt/NF-κB
signaling pathway. J Ethnopharmacol. 319((Pt 3)): 1173562024.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Alaaeldin R, Abdel-Rahman IAM, Hassan HA,
Youssef N, Allam AE, Abdelwahab SF, Zhao QL and Fathy M:
Carpachromene ameliorates insulin resistance in HepG2 cells via
modulating IR/IRS1/PI3k/Akt/GSK3/FoxO1 pathway. Molecules.
26:76292021. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Savova MS, Mihaylova LV, Tews D, Wabitsch
M and Georgiev MI: Targeting PI3K/AKT signaling pathway in obesity.
Biomed Pharmacother. 159:1142442023. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Zhou YJ, Xu N, Zhang XC, Zhu YY, Liu SW
and Chang YN: Chrysin improves glucose and lipid metabolism
disorders by regulating the AMPK/PI3K/AKT signaling pathway in
insulin-resistant HepG2 Cells and HFD/STZ-Induced C57BL/6J Mice. J
Agric Food Chem. 69:5618–5627. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Feng SY, Wu SJ, Chang YC, Ng LT and Chang
SJ: Stimulation of GLUT4 Glucose uptake by anthocyanin-rich extract
from black rice (Oryza sativa L.) via PI3K/Akt and AMPK/p38 MAPK
signaling in C2C12 cells. Metabolites. 12:8562022. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Zhao H, Zhai BW, Zhang MY, Huang H, Zhu
HL, Yang H, Ni HY and Fu YJ: Phlorizin from Lithocarpus
litseifolius [Hance] Chun ameliorates FFA-induced insulin
resistance by regulating AMPK/PI3K/AKT signaling pathway.
Phytomedicine. 130:1557432024. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Zhang H, Ma L, Peng W, Wang B and Sun Y:
Association between gut microbiota and onset of type 2 diabetes
mellitus: A two-sample Mendelian randomization study. Front Cell
Infect Microbiol. 14:13270322024. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Takeuchi T, Kubota T, Nakanishi Y, Tsugawa
H, Suda W, Kwon ATJ, Yazaki J, Ikeda K, Nemoto S, Mochizuki Y, et
al: Gut microbial carbohydrate metabolism contributes to insulin
resistance. Nature. 621:389–395. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Zhao SL, Liu D, Ding LQ, Liu GK, Yao T, Wu
LL, Li G, Cao SJ, Qiu F and Kang N: Schisandra chinensis lignans
improve insulin resistance by targeting TLR4 and activating
IRS-1/PI3K/AKT and NF-κB signaling pathways. Int Immunopharmacol.
142((Pt A)): 1130692024. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Zhang Y, Yang S, Zhang M, Wang Z, He X,
Hou Y and Bai G: Glycyrrhetinic acid improves insulin-response
pathway by regulating the balance between the Ras/MAPK and PI3K/Akt
pathways. Nutrients. 11:6042019. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Zhou J, Shi Y, Yang C, Lu S, Zhao L, Liu
X, Zhou D, Luo L and Yin Z: γ-glutamylcysteine alleviates insulin
resistance and hepatic steatosis by regulating adenylate cyclase
and IGF-1R/IRS1/PI3K/Akt signaling pathways. J Nutr Biochem.
119:1094042023. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Guo X, Yin T, Chen D, Xu S, Ye R and Zhang
Y: Astragaloside IV regulates insulin resistance and inflammatory
response of adipocytes via modulating MIR-21/PTEN/PI3K/AKT
signaling. Endocr Metab Immune Disord Drug Targets. 23:1538–1547.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Deng A, Wang Y, Huang K, Xie P, Mo P, Liu
F, Chen J, Chen K, Wang Y and Xiao B: Artichoke (Cynara scolymus
L.) water extract alleviates palmitate-induced insulin resistance
in HepG2 hepatocytes via the activation of IRS1/PI3K/AKT/FoxO1 and
GSK-3β signaling pathway. BMC Complement Med Ther. 23:4602023.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Rathinaswamy MK and Burke JE: Class I
phosphoinositide 3-kinase (PI3K) regulatory subunits and their
roles in signaling and disease. Adv Biol Regul. 75:1006572019.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Kim Y, Rouse M, González-Mariscal I, Egan
JM and O'Connell JF: Dietary curcumin enhances insulin clearance in
diet-induced obese mice via regulation of hepatic PI3K-AKT axis and
IDE, and preservation of islet integrity. Nutr Metab (Lond).
16:482019. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Chen Z, Liu H, Lei S, Zhao B and Xia Z:
LY294002 prevents lipopolysaccharide-induced hepatitis in a murine
model by suppressing IκB phosphorylation. Mol Med Rep. 13:811–816.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Shiwa M, Yoneda M, Okubo H, Ohno H, Kobuke
K, Monzen Y, Kishimoto R, Nakatsu Y, Asano T and Kohno N: Distinct
time course of the decrease in hepatic AMP-Activated protein kinase
and Akt phosphorylation in mice fed a high fat diet. PLoS One.
10:e01355542015. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Lalli CA, Pauli JR, Prada PO, Cintra DE,
Ropelle ER, Velloso LA and Saad MJ: Statin modulates insulin
signaling and insulin resistance in liver and muscle of rats fed a
high-fat diet. Metabolism. 57:57–65. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Wang CC, Adochio RL, Leitner JW, Abeyta
IM, Draznin B and Cornier MA: Acute effects of different diet
compositions on skeletal muscle insulin signalling in obese
individuals during caloric restriction. Metabolism. 62:595–603.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Cho H, Lai CC, Bonnavion R, Alnouri MW,
Wang S, Roquid KA, Kawase H, Campos D, Chen M, Weinstein LS, et al:
Endothelial insulin resistance induced by adrenomedullin mediates
obesity-associated diabetes. Science. 387:674–682. 2025. View Article : Google Scholar : PubMed/NCBI
|