Introduction
Prostate cancer (PCa) is one of the most prevalent
malignancies among men worldwide, making it a significant public
health concern (1). According to
the Global Cancer Observatory, PCa accounted for ~1.4 million new
cases and 375,000 deaths in 2020, ranking it as the second leading
cause of cancer-related mortality in men (1). The incidence of PCa varies by
geographic region, with higher rates observed in North America and
Europe compared with Asia and Africa (2). Despite advances in early detection
and treatment strategies, such as radical prostatectomy and
androgen deprivation therapy (ADT), the pathophysiology of PCa
remains complex and multifactorial and a deeper understanding of
its underlying mechanisms is needed to improve therapeutic outcomes
(3).
Recent studies have illuminated the importance of
the gut microbiota in human health, highlighting its role in
various physiological and pathological processes (4,5). The
gut microbiota, defined as the collective community of
microorganisms residing in the gastrointestinal tract, includes
bacteria, archaea, viruses and fungi (6). A diverse gut microbiota plays a
crucial role in the synthesis of nutrients, metabolism of drugs,
regulation of immune responses and protection against pathogenic
organisms (7). Dysbiosis, or an
imbalance in gut microbiota composition, has been implicated in a
wide range of diseases, including obesity, diabetes, inflammatory
bowel disease and even various forms of cancer (8). The gut-brain and gut-liver axes
further underscore the systemic effects of the microbiota,
suggesting that microbial composition can influence distant organ
systems (9,10).
The rationale for exploring the role of gut
microbiota in PCa stems from emerging evidence linking gut health
with cancer risk and progression (11). Several studies have identified a
distinctive microbial signature in patients with PCa compared with
healthy individuals, suggesting that alterations in gut microbiota
may influence cancer development (12,13).
The mechanisms through which gut microbiota may exert their
influence on prostate carcinogenesis are multifaceted, encompassing
modulation of local and systemic inflammation, alteration of
hormone metabolism and impacts on drug metabolism and efficacy
(14). Given that inflammation and
hormonal factors are well-established contributors to PCa
development, understanding how microbial populations affect these
pathways is critical (15,16).
Furthermore, the interplay between diet, lifestyle
factors and the gut microbiota presents an intriguing opportunity
for research (17). Dietary
patterns have been shown to markedly alter gut microbiota
composition, with Western diets, characterized by high fat and low
fiber intake, being associated with increased dysbiosis (18). This suggests a potential link
between dietary habits, gut health and PCa risk that warrants
further investigation. Additionally, the growing interest in
microbiome-targeted therapies, such as probiotics and dietary
interventions, raises the prospect of novel prevention and
treatment strategies in PCa (19).
Despite the promising associations established
between gut microbiota and PCa, the field remains nascent, with
significant gaps in our understanding. Studies on this topic often
present conflicting results, stemming from variations in
methodology, sample sizes and population diversity (20). Some research suggests specific
microbial taxa may be associated with increased cancer risk, while
others highlight protective microbial profiles. Such discrepancies
highlight the need for a critical appraisal of existing literature
to elucidate consistent findings and bounding contradictions
(21). The present review aimed to
synthesize the current evidence and propose directions for future
research, with the hope of fostering an improved understanding of
how gut microbiota may serve as both a modulator of PCa risk and a
potential target for therapeutic intervention.
Gut microbiota and cancer
Description of gut microbiota
composition and diversity
The gut microbiota is an intricate consortium of
microorganisms, including bacteria, archaea, fungi and virus, that
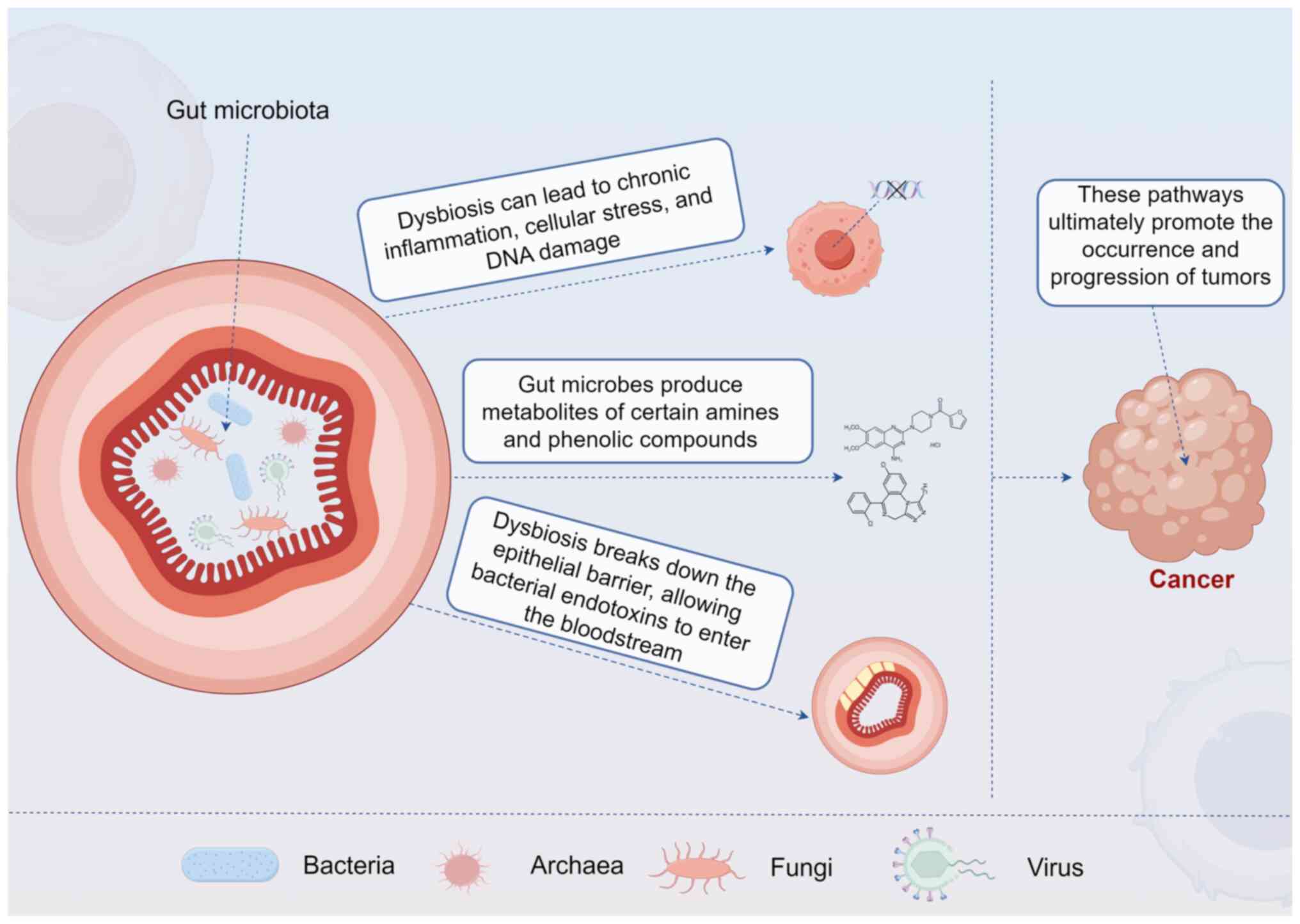
inhabit the gastrointestinal tract (22) (Fig.
1). The human gut harbors ~100 trillion microbial cells,
outnumbering human cells by a factor of ten (23). These microorganisms predominantly
belong to two major phyla: Firmicutes and Bacteroidetes, along with
smaller representations from Actinobacteria, Proteobacteria and
Verrucomicrobia (23). The
composition and diversity of the gut microbiota can be influenced
by various factors, including diet, age, genetics and antibiotic
use (24).
A diverse gut microbiota is indicative of a healthy
gastrointestinal ecosystem, playing a crucial role in nutrient
metabolism, barrier integrity and immune system function (25). Dysbiosis, or an imbalance in
microbiota composition, has been associated with various diseases,
including inflammatory bowel disease, obesity and cancer (8,26,27).
Recent advances in high-throughput sequencing technologies have
enabled a more nuanced understanding of microbial communities and
have revealed specific microbial signatures associated with
specific health conditions, including malignancies (28).
Variability in gut microbiota profiles has been
observed across populations, suggesting environmental and lifestyle
factors play significant roles in shaping these communities
(29). For instance, populations
consuming a Western diet rich in fats and sugars often exhibit
decreased microbial diversity, which has been linked to increased
cancer risk (30,31). Comparatively, traditional diets
that emphasize plant-based foods are associated with a higher
diversity of beneficial gut microbes, which may provide protective
effects against various cancers (32).
Mechanisms by which gut microbiota may
influence cancer pathogenesis
The influence of gut microbiota on cancer
pathogenesis occurs through several mechanisms, which can markedly
affect the initiation, promotion and progression of tumors
(33). Among these mechanisms are
immunomodulation, metabolic processes and the production of
bioactive compounds (33).
Immunomodulation: The gut microbiota plays a pivotal
role in shaping the immune system, promoting both innate and
adaptive immune responses (34).
Commensal bacteria stimulate the production of immune cells,
including regulatory T cells (Tregs) and dendritic cells, which in
turn influence systemic immune responses (34). Dysbiosis can lead to an altered
immune landscape, characterized by chronic inflammation, which is
recognized as a risk factor for cancer development (35). For instance, chronic inflammation
driven by microbial imbalances can result in cellular stress, DNA
damage and ultimately tumorigenesis (35) (Fig.
1).
Metabolism of xenobiotics and nutrients: The gut
microbiota is crucial in metabolizing dietary and environmental
xenobiotics, converting them into bioactive compounds that can
influence cancer risk (7). For
example, certain gut bacteria can metabolize dietary fiber into
short-chain fatty acids (SCFAs), such as butyrate, which possesses
anti-cancer properties (36).
Butyrate has been shown to inhibit tumor cell proliferation and
promote apoptosis in colorectal cancer models (37). Conversely, the metabolism of
certain amines and phenolic compounds by gut microbes can yield
carcinogenic metabolites, increasing cancer susceptibility
(38) (Fig. 1).
Alteration of Gut Barrier Function: A healthy gut
microbiota helps maintain intestinal barrier integrity, preventing
translocation of pathogens and toxins into the systemic circulation
(39). Dysbiosis can disrupt the
epithelial barrier, allowing bacterial endotoxins to enter the
bloodstream and provoke systemic inflammation, contributing to
cancer risk (40) (Fig. 1).
The association of gut microbiota with tumors,
including PCa. Significant evidence suggests that gut microbiota is
implicated in the pathogenesis of various cancers, including
colorectal, breast and liver cancers (41). A notable study by Kang et al
(42) observed distinct microbial
profiles in colorectal cancer patients characterized by a reduction
in butyrate-producing bacteria. Similarly, research has indicated
that specific phylotypes, such as Fusobacterium nucleatum,
are enriched in colorectal tumors and may promote tumor growth
through inflammatory pathways (43). In the context of breast cancer,
several studies have reported associations between gut microbiota
composition and tumor development. Caleça et al (44) found that women with breast cancer
exhibited specific dysbiotic features compared with healthy
controls. Furthermore, gut microbiota modulation through dietary
interventions showed promise in altering the risk of breast cancer
through immune system reprogramming (45).
The relevance of gut microbiota to PCa is a
burgeoning area of research. Wang et al (46) reported a significant correlation
between gut microbiota composition and PCa risk, indicating that
distinctive microbial communities may be associated with tumor
presence. Notably, studies employing fecal microbiota
transplantation have highlighted the potential for gut microbiota
to influence host tumor immunology and responses to therapeutic
interventions (47,48). Despite these findings, there are
inconsistencies and limitations in the current literature linking
gut microbiota to PCa. Some studies report significant
associations, while others fail to demonstrate a clear connection,
suggesting that variations in methodology, sample size and
environmental factors may influence outcomes (49,50).
Furthermore, the geographic and dietary contexts in which studies
are conducted may contribute to discrepancies in microbial
composition and cancer susceptibility (51,52).
Clinical evidence linking gut microbiota to
PCa
Accumulating clinical evidence supports the
association between gut microbiota and PCa pathogenesis and
progression (53–63). Key studies have identified distinct
microbial signatures in patients with PCa compared with healthy
controls, with variations observed across disease stages and
treatment modalities. These findings are summarized in Table I, which highlights study designs,
patient cohorts and principal outcomes.
 | Table I.Clinical studies of gut microbiota
and PCa. |
Table I.
Clinical studies of gut microbiota
and PCa.
| First author/s,
year | Study type | Number of
patients | Patient type | Findings | (Refs.) |
|---|
| Golombos et
al, 2018 | Prospective pilot
study | 32 | Men diagnosed with
PCa vs. healthy controls | Significant
differences in gut microbiota composition | (53) |
| Liu and Jiang,
2020 | Retrospective
study | 21 | Hormone-sensitive
vs. castration-resistant patients with PCa | Compositional
differences with enrichment in specific taxa in
castration-resistant disease | (54) |
| Newton et
al, 2019 | Randomized
controlled trial | 60 | Individuals
undergoing ADT for PCa | Exercise
intervention led to modifications in the gut microbiota | (55) |
| Matsushita et
al, 2021 | Case-control
study | 152 | Patients with
high-Gleason PCa | Unique microbial
signature associated with aggressive cancer phenotypes | (56) |
| Li et al,
2021 | Cross-sectional
study | 86 | Patients with PCa
undergoing prostatectomy or ADT | Significant changes
in gut microbiota profiles with more pronounced dysbiosis in ADT
patients | (57) |
| Takezawa et
al, 2021 | Case-control
study | 128 | Patients with
prostate enlargement | Potential
association between gut microbiota composition and urological
conditions | (58) |
| Matsushita et
al, 2022 | Cohort study | 54 | Elderly men with
PCa | Firmicutes
correlations with blood testosterone levels | (59) |
| Fernandes et
al, 2022 | Pilot case series
study | 5 | Patients with PCa
treated with Radium-223 | Significant shifts
in microbial populations post-treatment | (60) |
| Huang et al,
2024 | Systematic review
and meta-analysis | 442 | Patients with PCa
vs. healthy individuals | Significant
differences in gut microbiota profiles | (61) |
| Smith et al,
2021 | Randomised
controlled trial | 44 | Breast and PCa
cases vs. matched cancer-free controls | Gut microbial
differences | (62) |
| Reichard et
al, 2022 | Prospective
analysis of a cohort | 692 | Patients at risk of
lethal PCa | Gut
microbiota-dependent metabolic pathways and risk of lethal PCa | (63) |
The role of the gut microbiota in PCa pathology has
gained traction, as illustrated by Golombos et al (53) in their prospective pilot study. The
authors observed significant differences in gut microbiota
composition among men diagnosed with PCa compared with healthy
controls, highlighting alterations in specific microbial profiles
that may be relevant to cancer development (53). However, the small sample size and
the lack of diverse demographics limit the generalizability of
these findings. In another study conducted by Liu and Jiang
(54), compositional differences
were noted between hormone-sensitive and castration-resistant
patients with PCa. This study found that specific microbial taxa
were enriched in patients with castration-resistant disease,
suggesting that gut microbiota may influence disease progression
and therapeutic resistance. However, the design was retrospective
and causality could not be firmly established.
A randomized controlled trial has also provided
valuable insights. Newton et al (55) explored the effect of exercise on
gut microbiota composition in individuals undergoing ADT for PCa.
The authors reported that the exercise intervention led to
modifications in the gut microbiota, which may correlate with
improved patient outcomes. While this study provides promising
evidence on lifestyle interventions, the results must be
interpreted with caution due to potential confounding factors
related to exercise types and patient adherence. Moreover,
Matsushita et al (56)
assessed gut microbiota in relation to high-Gleason PCa. The
authors identified a unique microbial signature associated with
aggressive cancer phenotypes, thus emphasizing the potential of
microbiota profiling for risk stratification in clinical practice.
The findings contribute to our understanding but warrant further
validation in larger cohorts.
Li et al (57) conducted a cross-sectional study
among patients with PCa undergoing prostatectomy or ADT,
demonstrating significant changes in gut microbiota profiles. The
study revealed that patients undergoing ADT experienced more
pronounced dysbiosis, which correlated with therapy outcomes. While
these findings are useful, the cross-sectional nature of the study
limits causal inferences. Takezawa et al (58) also investigated the
Firmicutes/Bacteroidetes ratio in connection with prostate
enlargement, establishing a potential association between gut
microbiota composition and urological conditions. However, the
specific relevance to cancer remains unclear and needs to be
contextualized within a broader oncological framework.
Emerging longitudinal studies have further
illuminated the relationship between gut microbiota and PCa.
Matsushita et al (59)
conducted a detailed examination of firmicutes correlations and
blood testosterone levels in elderly men with PCa, supporting the
concept that microbiota could influence hormonal pathways that are
critical in cancer progression. While insightful, the study's
retrospective nature poses challenges to establishing direct
causative relationships. The case series exploring the effects of
Radium-223 on gut microbiota reported significant shifts in
microbial populations post-treatment, suggesting that certain
therapies might modulate the microbiome, which in turn could
influence cancer dynamics (60).
The limited sample size and lack of controls temper the broader
applicability of these findings, highlighting the need for further
robust studies.
A systematic review and meta-analysis by Huang et
al (61) synthesized data from
various studies, confirming that significant differences exist in
gut microbiota profiles between patients with PCa and healthy
individuals. This comprehensive review underscores the
reproducibility of observed dysbiosis across different populations
and study designs. Nevertheless, the heterogeneity of study
methodologies and microbiota analyses complicates direct
comparisons. Moreover, a Mendelian randomization study by Wang
et al (46) suggested a
causal relationship between gut microbiota and PCa. The authors'
findings advocate for the gut-prostate axis by demonstrating
potential microbiome-driven mechanisms influencing prostate disease
pathology. This strengthens the biologic plausibility of the gut
microbiota's role in PCa, although the models require rigorous
validation. In addition, prospective cohort analyses have shown
that gut microbiome-dependent metabolic pathways may be associated
with the risk of developing lethal prostate cancer, further
supporting the clinical relevance of microbial metabolism in PCa
pathogenesis (63).
In summary, clinical evidence consistently reports a
state of gut microbiota dysbiosis in patients with PCa compared
with healthy controls (53,61).
Key findings include distinct microbial signatures associated with
PCa presence (53,56), differences between disease states
such as hormone-sensitive and castration-resistant PCa (54) and modulations induced by therapies
such as ADT (57) or Radium-223
(60). Furthermore, interventions
such as exercise have been shown to alter the gut microbiota in
patients with PCa (55). Despite
this accumulation of evidence, significant inconsistencies remain
regarding the specific microbial taxa associated with increased or
decreased risk. For instance, the roles of Bacteroides,
Streptococcus and Faecalibacterium prausnitzii, as well as the
Firmicutes/Bacteroidetes ratio, are not consistently reported
across studies (58,62,64,65).
These discrepancies are likely attributable to methodological
variations (such as sequencing techniques, bioinformatic pipelines)
and profound inter-individual and geographic heterogeneity in
microbiome composition (49,51).
Therefore, while a consensus confirms the involvement of gut
microbiota in PCa, future multi-center studies with standardized
protocols are essential to identify robust, generalizable microbial
signatures for clinical application.
Mechanisms of action
The gut microbiota influences PCa through multiple
interconnected mechanisms, including metabolic regulation, immune
modulation and hormonal signaling. Major pathways involve microbial
metabolites such as short-chain fatty acids (SCFAs),
inflammation-related axes (such as NF-κB-IL6-STAT3) and androgen
metabolism (66), and key
mechanistic pathways are illustrated in Fig. 2. A summary of these mechanisms and
supporting studies is provided in Table II.
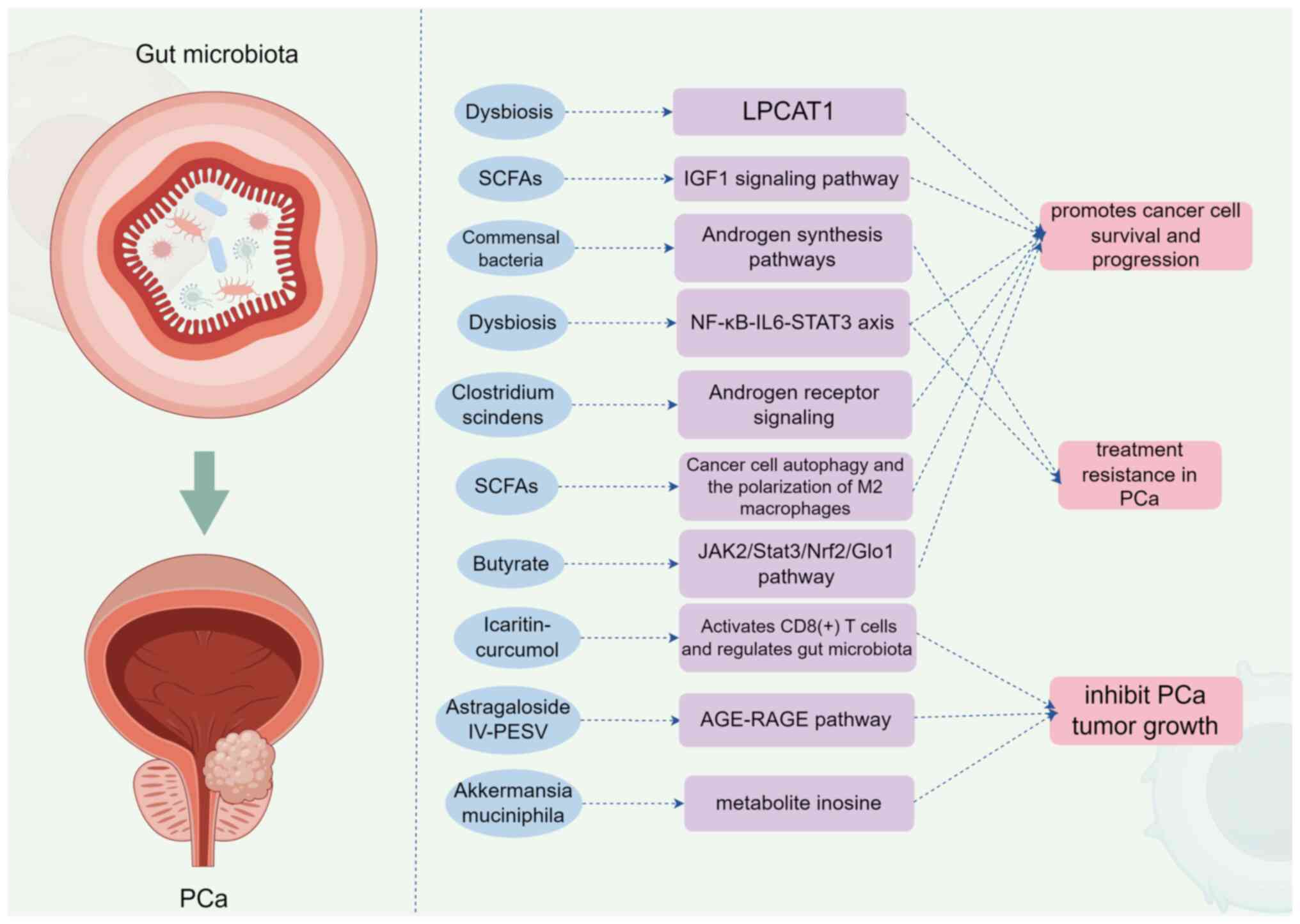 | Figure 2.Mechanism of action of gut microbiota
on prostate cancer (generated with Figdraw; www.figdraw.com; ID: RWTIA8dd77). SCFAs, short-chain
fatty acids; LPCAT1, lysophosphatidylcholine acyltransferase; IGF1,
insulin-like growth factor 1; NF-κB, nuclear factor
kappa-light-chain-enhancer of activated B cells; IL-6,
interleukin-6; STAT3, signal transducer and activator of
transcription 3; JAK2, Janus kinase 2; Nrf2, nuclear factor
erythroid 2-related factor 2; Glo1, glyoxalase 1; LPCAT1,
lysophosphatidylcholine acyltransferase 1; AGE, advanced glycation
end-product; RAGE, receptor for advanced glycation end-products;
PCa, prostate cancer; CRPC, castration-resistant prostate
cancer. |
 | Table II.Key mechanisms of gut microbiota
influence on PCa progression. |
Table II.
Key mechanisms of gut microbiota
influence on PCa progression.
| First author/s,
year | Mechanism
category | Key
elements/microbial factors | Proposed effect on
PCa | (Refs.) |
|---|
| Matsushita et
al, 2021; Liu et al, 2023 | Metabolic
Regulation | SCFAs | Activate IGF1
signaling, promoting cell proliferation and survival. Promote
autophagy and M2 macrophage polarization, facilitating a
tumor-promoting environment. | (68,74) |
| Hsia et al,
2024 | Metabolic
Regulation | Microbial
modulation of methylglyoxal | Butyrate increases
methylglyoxal production via JAK2/Stat3/Nrf2/Glo1 pathway in
CRPC. | (75) |
| Zhong et al,
2022 | Immune Modulation
and Inflammation | NF-κB-IL6-STAT3
axis activation | Dysbiosis activates
this inflammatory axis, promoting PCa progression and docetaxel
resistance. | (72) |
| Xu et al,
2024 | Immune Modulation
and Inflammation | CD8 (+) T cell
activation | Compounds such as
Icaritin-curcumol can activate CD8 (+) T cells and regulate gut
microbiota, suppressing cancer. | (76) |
| Pernigoni et
al, 2021 | Hormonal
Signaling | Androgen
biosynthesis | Commensal bacteria
can alter androgen synthesis pathways, promoting endocrine
resistance. | (69) |
| Bui et al,
2023 | Hormonal
Signaling | Androgen receptor
signaling | Metabolites from
Clostridium scindens can trigger PCa progression via
androgen receptor signaling. | (73) |
| Liu et al,
2021 | Other Pathways | LPCAT1
upregulation | Gut dysbiosis can
increase LPCAT1 expression, enhancing DNA repair pathways and
promoting cancer cell survival. | (67) |
| You et al,
2024 | Other Pathways | AGE-RAGE
pathway | Restoration of gut
microbiota and metabolic homeostasis via this pathway can inhibit
tumor growth (such as Astragaloside IV-PESV). | (77) |
| Yu et al,
2024 | Other Pathways | Bacterial
metabolites (e.g., Inosine) | Akkermansia
muciniphila metabolite inosine inhibits castration
resistance. | (78) |
Dysbiosis and PCa progression
A growing body of evidence suggests that alterations
in gut microbiota composition, referred to as dysbiosis, are
closely associated with PCa progression (61,67).
Liu et al (67) found that
specific taxa, when disrupted, lead to increased levels of LPCAT1,
a lysophosphatidylcholine acyltransferase involved in membrane
repair, which promotes cancer cell survival and progression. In
this context, the study highlights a critical intersection between
microbial metabolism and cancer biology. Another study by
Matsushita et al (68)
emphasizes the role of SCFAs derived from gut microbiota in
promoting PCa growth. The authors' research revealed that SCFAs
activate the insulin-such as growth factor 1 (IGF1) signaling
pathway, which is well-known for its role in cell proliferation and
survival . Hence, the microbial production of SCFAs offers a
potential oncogenic pathway that merits further exploration.
Androgen biosynthesis and resistance
mechanisms
Studies have illuminated the role of gut microbiota
in modulating androgen biosynthesis, a crucial factor in PCa
development. Pernigoni et al (69) demonstrated that commensal bacteria
could promote endocrine resistance by altering androgen synthesis
pathways, potentially leading to treatment resistance in PCa. This
finding underlines the necessity of considering microbial
influences in both hormonal regulation and therapeutic strategies
against PCa. Furthermore, Matsushita et al (70) also reported that high-fat diets,
which can alter gut microbiota composition, enhance histamine
signaling and foster an environment conducive to PCa progression.
The dual role of diet and gut microbiota in cancer dynamics
highlights the complexity of interactions and the importance of
holistic approaches in cancer management.
Inflammatory pathways involved in PCa
progression
Dysbiosis has been linked to chronic inflammation,
which is a precursor in a number of cancers, including PCa
(71). Zhong et al
(72) established that gut
microbiota dysbiosis activates the NF-κB-IL6-STAT3 axis, promoting
not only PCa progression but also docetaxel resistance. This
finding suggests that therapies targeting the gut microbiota could
enhance the efficacy of conventional treatments by modulating the
inflammatory milieu associated with PCa. Moreover, Bui et al
(73) highlighted Clostridium
scindens metabolites as triggers of PCa progression through
androgen receptor signaling. This indicates that specific microbial
metabolites can directly affect cancer biology, suggesting possible
intervention strategies aimed at microbial pathway
manipulation.
Autophagy and immune response
modulation
Gut microbiota-derived molecules have also been
implicated in regulating autophagy and immune responses in PCa. Liu
et al (74) reported that
SCFAs promote cancer cell autophagy and the polarization of M2
macrophages, facilitating a tumor-promoting environment. As immune
evasion is a critical hallmark of cancer, understanding how gut
microbiota influences immune modulation could pave the way for
innovative therapeutic strategies (34). In a related study, Hsia et
al (75) demonstrated that
butyrate, a major SCFAs, increases methylglyoxal production via the
JAK2/Stat3/Nrf2/Glo1 pathway in castration-resistant PCa cells,
further implicating metabolic interplay in cancer progression.
These findings emphasize the multifaceted roles of gut-derived
metabolites in shaping tumor biology.
Mechanisms of cancer suppression via
gut microbiota
Notably, research has also shown that certain
natural compounds can restore gut microbiota homeostasis,
potentially suppressing PCa progression (61). Xu et al (76) explored the action of
Icaritin-curcumol, which activates CD8 (+) T cells and regulates
gut microbiota, demonstrating a dual effect on immune enhancement
and cancer suppression. This highlights promising avenues for
therapeutic strategies that incorporate microbiota modulation
alongside conventional treatments. Furthermore, You et al
(77) presented evidence
indicating that Astragaloside IV-PESV can inhibit PCa tumor growth
through gut microbiota restoration and metabolic homeostasis, which
is mediated via the AGE-RAGE pathway. Such findings contribute to a
growing body of literature supporting the significance of gut
microbiota in cancer therapeutics. Lastly, Yu et al
(78) investigated Akkermansia
muciniphila and its metabolite inosine, which inhibits
castration resistance in PCa. This research reinforces the notion
that specific microbial taxa could serve as therapeutic targets or
biomarkers, further integrating gut microbiota research into PCa
clinical practice.
The intricate interplay between gut microbiota and
PCa progression underscores the necessity for further research in
this burgeoning field (79).
Studies consistently highlight that dysbiosis not only contributes
to the pathogenesis of PCa but also influences the efficacy of
various treatment modalities (52,72).
As we continue to unravel these complex relationships, it is vital
to incorporate gut microbiota considerations into PCa management
strategies, paving the way for personalized therapeutic approaches
that target the microbiome for improved outcomes. The ongoing
exploration of microbial metabolites, signaling pathways and
dietary factors will undoubtedly provide new insights into the
mechanisms of PCa progression and resistance, ultimately benefiting
patient care and treatment efficacy.
Microbiota as a therapeutic target
Modulating gut microbiota presents a promising
avenue for influencing PCa pathogenesis and treatment outcomes
(11). Interventions, including
probiotics, prebiotics and dietary changes, aim to restore
microbiota balance and enhance immune function (19). However, the variability in
individual microbiome compositions and the specificities of
intervention effects necessitate careful evaluation of available
studies and clinical trials to establish effective and personalized
therapeutic strategies (52).
Role of microbiota in modulating
responses to PCa treatments
Emerging evidence suggests that the composition of
gut microbiota can markedly influence the effectiveness of various
PCa treatments, specifically immunotherapies and chemotherapies
(52,72). For instance, it has been shown that
specific gut bacteria can enhance the efficacy of immune checkpoint
inhibitors by modulating systemic immune responses (80). A study found that patients with
colorectal cancer who had a higher abundance of certain gut
bacteria experienced improved responses to immunotherapy, raising
the possibility that similar principles could apply to PCa
treatment (81). Conversely,
dysbiosis may lead to decreased effectiveness of chemotherapy.
Antibiotic exposure during treatment can disrupt microbiota
balance, potentially diminishing therapeutic outcomes and
increasing susceptibility to adverse effects (67). Thus, understanding how gut
microbiota interact with therapeutic modalities could inform
approaches to enhance treatment efficacy and minimize toxicity.
Exploration of potential interventions
targeting gut microbiota
Probiotics, defined as live microorganisms that
confer health benefits, have been shown to restore gut microbiota
balance (82). Specific strains,
such as Lactobacillus rhamnosus, have been demonstrated to
enhance immune modulation, which may inhibit tumor growth and
progression. However, the efficacy of probiotics can vary markedly
by strain and individual response due to unique host microbiota
compositions (83). For instance,
while certain strains may exert beneficial effects, others may not
confer the same advantages, raising questions about strain
specificity and the need for personalized approaches (84).
Prebiotics, which are non-digestible food components
that promote beneficial bacteria growth, have also gained attention
(85). They can selectively
increase populations of SCFAs-producing bacteria, such as
Faecalibacterium, linked to anti-inflammatory properties
(86). Dietary modifications
focused on increasing fiber intake may support these beneficial
communities while simultaneously mitigating dysbiosis and
inflammation (87). Nevertheless,
the challenge lies in establishing standardized guidelines on
effective prebiotic intake, given the variability in individual gut
microbiota responses.
Further, dietary interventions are increasingly seen
as viable approaches to alter gut microbiota (88). Studies suggest that Mediterranean
diets rich in fruits, vegetables and whole grains can enhance
microbial diversity and reduce cancer progression risk (89,90).
However, a number of these studies are observational and require
causal evidence to substantiate claims regarding dietary impact on
PCa outcomes.
Emerging clinical trials and
studies
The gut microbiota has emerged as a potential
therapeutic target in the management of PCa, with recent studies
highlighting its role in disease progression and response to
treatment (91). The modulation of
gut microbiota through dietary interventions, prebiotics,
probiotics and fecal microbiota transplantation (FMT) has shown
promise in enhancing treatment efficacy and mitigating adverse
effects associated with standard therapies such as chemotherapy,
immunotherapy and hormone therapy (19,92).
Critical evaluation of the literature reveals a
growing body of evidence supporting the impact of gut microbiota on
PCa. For instance, Liss et al (93) identified specific bacterial species
associated with PCa, suggesting a role for microbiota in disease
pathogenesis. Furthermore, Matsushita et al (56) reported that gut microbiota
metabolites, particularly SCFAs, promote PCa growth, indicating a
potential therapeutic avenue through dietary fiber manipulation.
The therapeutic potential of the gut microbiota is further
supported by studies showing that probiotics can enhance the
response to treatment and reduce postoperative infections,
suggesting their utility as an adjuvant therapy (94). FMT has also demonstrated potential
in modifying the gut microbiota to improve treatment responses,
although the clinical efficacy of such interventions requires
further exploration (69).
Despite the promising findings, there is a noted
inconsistency in the results across studies, which may be
attributed to variations in study design, population demographics
and methodology (53). For
example, while some studies report an increase in
Bacteroides and Streptococcus in patients with PCa,
others find a decrease in Faecalibacterium prausnitzii,
highlighting the complexity of the gut microbiota's role in PCa
(64,65).
Notwithstanding the promising potential of
microbiota-targeted therapies, several critical limitations must be
acknowledged. The efficacy of probiotics is highly strain-specific
and beneficial effects observed with one strain, such as
Lactobacillus rhamnosus GG (83), cannot be generalized to other
strains or probiotic formulations (84). Furthermore, substantial
inter-individual variability in baseline gut microbiota
composition, driven by factors such as genetics, diet and prior
antibiotic exposure (52),
markedly influences responses to interventions, complicating the
development of universal therapeutic recommendations. Patient
heterogeneity also extends to disease status and prior treatments,
which may alter the gut ecosystem and modulate intervention
outcomes (69). Safety
considerations are equally paramount. While probiotics and
prebiotics are generally regarded as safe, the long-term
consequences of microbiota manipulation, especially in
immunocompromised cancer patients, remain inadequately studied.
More invasive approaches, such as FMT, carry risks of pathogen
transmission, unintended microbial engraftment and unpredictable
shifts in microbial communities that could potentially exacerbate
inflammation or promote resistance pathways (92,95).
Therefore, future clinical applications must incorporate rigorous
safety monitoring, strain-level characterization and personalized
strategies that account for individual microbiome profiles and
clinical contexts.
Current limitations and challenges
The gut microbiota has emerged as a significant
player in the pathogenesis and progression of PCa, yet several
limitations and challenges hinder its clinical application as a
therapeutic target (50). One of
the primary challenges is the complexity and variability of the gut
microbiota across individuals. Factors such as diet, genetics, age
and environmental influences contribute to this variability, making
it difficult to establish a standardized microbiome profile that
correlates with PCa risk or treatment response (14,19).
This heterogeneity complicates the interpretation of microbiome
studies and the generalization of findings across diverse
populations.
The interpretation of gut microbiota studies in PCa
is heavily influenced by the diversity of research designs
employed, including observational, interventional, cross-sectional
and longitudinal approaches. Observational studies, such as
case-control and cohort designs, have been instrumental in
identifying associations between specific microbial taxa and PCa
risk or progression (53,56). However, these studies are prone to
confounding factors, including diet, lifestyle and comorbidities,
and cannot establish causality. Interventional studies, such as
randomized controlled trials (RCTs) investigating probiotics or
dietary modifications (55), offer
stronger evidence for causal relationships but are often limited by
small sample sizes, short durations and heterogeneous intervention
protocols. Cross-sectional studies (57) provide a snapshot of microbial
composition at a single time point, which is useful for hypothesis
generation but cannot capture dynamic changes in the microbiome
over time or in response to disease progression or treatment. By
contrast, longitudinal studies allow for the assessment of temporal
changes and are more suited for evaluating the microbiome's role in
disease progression or treatment response (59). Nevertheless, they are
resource-intensive and may still be affected by unmeasured
confounders. The variability in study designs contributes markedly
to the inconsistency of reported findings and complicates the
comparison and synthesis of results across studies. Therefore,
future research should prioritize well-designed, prospective
longitudinal studies and RCTs with standardized methodologies to
enhance the reliability and generalizability of conclusions.
Moreover, the methodologies employed in microbiome
research often vary markedly, leading to inconsistent results. For
instance, studies utilizing different sampling techniques (for
example, stool vs. urine vs. tissue) and analytical methods (for
example, 16S rRNA sequencing vs. shotgun metagenomics) can yield
divergent microbiome compositions and associations with PCa
(53,56). Such discrepancies highlight the
need for standardized protocols to ensure comparability and
reproducibility of results. Furthermore, the lack of consensus on
the definition of ‘healthy’ compared with ‘dysbiotic’ microbiomes
poses additional challenges in interpreting the implications of
microbiome alterations in patients with PCa (96).
Another significant limitation is the current
understanding of the mechanisms by which gut microbiota influences
PCa. While certain bacterial taxa, such as Bacteroides
massiliensis and Akkermansia muciniphila, have been
implicated in PCa progression, the precise pathways and
interactions involved remain poorly characterized (14,92).
For example, the role of microbial metabolites, such as SCFAs, in
modulating inflammation and hormone levels in the prostate is still
under investigation, necessitating further research to elucidate
these mechanisms (69).
Additionally, the therapeutic potential of
modulating the gut microbiota through interventions such as
probiotics, prebiotics and FMT faces several obstacles (95). The efficacy of these treatments is
often inconsistent, with some studies reporting beneficial effects
while others show no significant impact on PCa outcomes (97,98).
This inconsistency may stem from variations in individual
microbiome composition and the specific strains used in probiotic
formulations, underscoring the need for tailored approaches based
on individual microbiome profiles. Furthermore, the safety and
long-term effects of manipulating the gut microbiota in patients
with PCa are not well understood. Concerns regarding the potential
for adverse effects, such as the introduction of pathogenic
bacteria or the exacerbation of existing conditions, necessitate
careful consideration and monitoring in clinical settings (92).
Future directions
The exploration of the gut microbiota as a potential
modulator of PCa pathogenesis and progression represents an
exciting frontier in cancer research. To translate recent
associative findings into actionable clinical strategies, future
investigations must prioritize several key areas through
coordinated and rigorous scientific approaches.
A primary imperative is the establishment of
large-scale, multiethnic prospective cohorts. Such studies are
essential to account for the profound heterogeneity in gut
microbiota composition influenced by genetics, diet, geography and
lifestyle (14,29,51).
These initiatives should employ standardized methodologies for
sample collection, DNA sequencing (preferentially shotgun
metagenomics) and bioinformatic analysis to minimize technical
variability and enable robust cross-study comparisons. This will
help identify consistent microbial signatures for PCa risk
stratification across diverse populations and clarify the role of
the racial disparities that have been highlighted in preliminary
research (80).
Moreover, a deeper mechanistic dissection of the
gut-prostate axis is crucial. While microbial metabolites such as
SCFAs and their involvement in pathways such as IGF1 signaling and
chronic inflammation (such as the NF-κB-IL6-STAT3 axis) have been
implicated (68,72,99),
the precise cause-and-effect relationships remain incompletely
defined. Future work should utilize gnotobiotic animal models,
organoids and multi-omics integrations (metagenomics, metabolomics,
proteomics) to definitively link specific microbial taxa and their
metabolic outputs (such as SCFAs, polyamines and secondary bile
acids) to molecular events driving PCa initiation, progression and
therapy resistance. The role of bacterial components in modulating
intratumoral immune responses represents a particularly promising
yet underexplored avenue (52).
The therapeutic potential of microbiota modulation
demands rigorous evaluation through well-designed interventional
trials. Promising strategies include targeted probiotic and
prebiotic formulations, personalized dietary interventions (such as
Mediterranean, high-fiber, or polyphenol-rich diets) and FMT
(19,69,88,92).
Future clinical trials must move beyond observational correlations
and focus on randomized controlled designs that assess the efficacy
of these interventions in improving responses to standard therapies
(such as ADT, immunotherapy and chemotherapy) and mitigating
treatment-related adverse effects. A critical aspect will be to
develop personalized approaches that consider an individual's
baseline microbiome, ensuring that interventions are tailored for
maximal efficacy (52,84).
Finally, fostering interdisciplinary collaboration
among oncologists, microbiologists, immunologists, nutritionists
and bioinformaticians is paramount. Such synergy is necessary to
unravel the complex interactions between diet, microbiota and host
physiology. The integration of artificial intelligence and machine
learning with microbiome data holds significant promise for
developing predictive models of disease progression and treatment
response (19). By systematically
addressing these priorities, including standardized large-scale
cohorts, mechanistic elucidation, targeted interventional trials
and interdisciplinary collaboration, the field can accelerate the
translation of gut microbiota research into novel diagnostic tools
and personalized therapeutic strategies for PCa, ultimately
improving patient outcomes.
In summary, the gut microbiota represents a
promising, yet complex, modulator in PCa pathogenesis. Future
research should focus on addressing the existing gaps in
understanding microbial diversity, elucidating mechanisms of action
and translating insights into clinical applications. By
prioritizing these future directions, we enhance our potential to
develop innovative strategies that leverage the gut microbiota in
the prevention and treatment of PCa, ultimately contributing to
improved patient outcomes.
Conclusions
The present review highlighted the emerging role of
gut microbiota as a potential modulator in PCa pathogenesis and
progression, underscoring significant associations between
microbial composition and cancer outcomes. While current studies
demonstrate the promise of gut microbiota modulation as a novel
strategy for PCa prevention and treatment, inconsistencies in
methodologies and findings warrant caution. Further investigations
are necessary to establish causative relationships and identify
actionable therapeutic pathways. Continued research will be crucial
in integrating gut microbiota insights into clinical practice,
ultimately enhancing patient management and outcomes in PCa.
Acknowledgements
Not applicable.
Funding
The present study was supported by Baiyin Science and Technology
Plan Project (Clinical Application Research on Early Screening of
Prostate Cancer in Baiyin Area: Grant no. 2022-3-6Y) and Baiyin
First People's Hospital Science and Technology Plan Project (grant
no. 2023-2-16Y).
Availability of data and materials
Not applicable.
Authors' contributions
ZH, YX and QZ contributed to the manuscript
conception. ZH, KC and RZ were involved in drafting the manuscript
and revising it critically for important intellectual content. HS,
LL, YL, JH and JW have revised the article for content and
language. Data authentication is not applicable. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI
|
|
2
|
Almeeri MNE, Awies M and Constantinou C:
Prostate cancer, pathophysiology and recent developments in
management: A narrative review. Curr Oncol Rep. 26:1511–1519. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wasim S, Lee SY and Kim J: Complexities of
prostate cancer. Int J Mol Sci. 23:142572022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Perler BK, Friedman ES and Wu GD: The role
of the gut microbiota in the relationship between diet and human
health. Annu Rev Physiol. 85:449–468. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang MY, Sang LX and Sun SY: Gut
microbiota and female health. World J Gastroenterol. 30:1655–1662.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Matijašić M, Meštrović T, Paljetak HC,
Perić M, Barešić A and Verbanac D: Gut microbiota beyond
bacteria-mycobiome, virome, archaeome, and eukaryotic parasites in
IBD. Int J Mol Sci. 21:26682020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pant A, Maiti TK, Mahajan D and Das B:
Human gut microbiota and drug metabolism. Microb Ecol. 86:97–111.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang Q, Wang B, Zheng Q, Li H, Meng X,
Zhou F and Zhang L: A review of gut microbiota-derived metabolites
in tumor progression and cancer therapy. Adv Sci (Weinh).
10:e22073662023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Aburto MR and Cryan JF: Gastrointestinal
and brain barriers: unlocking gates of communication across the
microbiota-gut-brain axis. Nat Rev Gastroenterol Hepatol.
21:222–247. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hsu CL and Schnabl B: The gut-liver axis
and gut microbiota in health and liver disease. Nat Rev Microbiol.
21:719–733. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pernigoni N, Guo C, Gallagher L, Yuan W,
Colucci M, Troiani M, Liu L, Maraccani L, Guccini I, Migliorini D,
et al: The potential role of the microbiota in prostate cancer
pathogenesis and treatment. Nat Rev Urol. 20:706–718. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gut microbiota differs significantly
between men with and without prostate cancer. Cancer. 129:169–170.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kalinen S, Kallonen T, Gunell M, Ettala O,
Jambor I, Knaapila J, Syvänen KT, Taimen P, Poutanen M, Aronen HJ,
et al: Differences in gut microbiota profiles and microbiota
steroid hormone biosynthesis in men with and without prostate
cancer. Eur Urol Open Sci. 62:140–150. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zha C, Peng Z, Huang K, Tang K, Wang Q,
Zhu L, Che B, Li W, Xu S, Huang T, et al: Potential role of gut
microbiota in prostate cancer: Immunity, metabolites, pathways of
action? Front Oncol. 13:11962172023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Porter CM, Shrestha E, Peiffer LB and
Sfanos KS: The microbiome in prostate inflammation and prostate
cancer. Prostate Cancer Prostatic Dis. 21:345–354. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cereda V, Falbo PT, Manna G, Iannace A,
Menghi A, Corona M, Semenova D, Calò L, Carnevale R, Frati G and
Lanzetta G: Hormonal prostate cancer therapies and cardiovascular
disease: A systematic review. Heart Fail Rev. 27:119–134. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Beam A, Clinger E and Hao L: Effect of
diet and dietary components on the composition of the gut
microbiota. Nutrients. 13:27952021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
García-Montero C, Fraile-Martínez O,
Gómez-Lahoz AM, Pekarek L, Castellanos AJ, Noguerales-Fraguas F,
Coca S, Guijarro LG, García-Honduvilla N, Asúnsolo A, et al:
Nutritional components in western diet versus mediterranean diet at
the gut microbiota-immune system interplay. implications for health
and disease. Nutrients. 13:6992021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pei X, Liu L and Han Y: Advances in human
microbiome and prostate cancer research. Front Immunol.
16:15766792025. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sha S, Ni L, Stefil M, Dixon M and
Mouraviev V: The human gastrointestinal microbiota and prostate
cancer development and treatment. Investig Clin Urol. 61 (Suppl
1):S43–S50. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Han EJ, Ahn JS, Choi YJ, Kim DH, Choi JS
and Chung HJ: Exploring the gut microbiome: A potential biomarker
for cancer diagnosis, prognosis, and therapy. Biochim Biophys Acta
Rev Cancer. 1880:1892512025. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ling Z, Liu X, Cheng Y, Yan X and Wu S:
Gut microbiota and aging. Crit Rev Food Sci Nutr. 62:3509–3534.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Adak A and Khan MR: An insight into gut
microbiota and its functionalities. Cell Mol Life Sci. 76:473–493.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang Y, Zhou M, Zhou Y and Guan X:
Dietary components regulate chronic diseases through gut
microbiota: A review. J Sci Food Agric. 103:6752–6766. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
McCallum G and Tropini C: The gut
microbiota and its biogeography. Nat Rev Microbiol. 22:105–118.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Weingarden AR and Vaughn BP: Intestinal
microbiota, fecal microbiota transplantation, and inflammatory
bowel disease. Gut Microbes. 8:238–252. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Boulangé CL, Neves AL, Chilloux J,
Nicholson JK and Dumas ME: Impact of the gut microbiota on
inflammation, obesity, and metabolic disease. Genome Med. 8:422016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang Y, Zhu Y, Guo Q, Wang W and Zhang L:
High-throughput sequencing analysis of the characteristics of the
gut microbiota in aged patients with sarcopenia. Exp Gerontol.
182:1122872023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang X, Zhong H, Li Y, Shi Z, Ren H,
Zhang Z, Zhou X, Tang S, Han X, Lin Y, et al: Sex- and age-related
trajectories of the adult human gut microbiota shared across
populations of different ethnicities. Nat Aging. 1:87–100. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fajstova A, Galanova N, Coufal S, Malkova
J, Kostovcik M, Cermakova M, Pelantova H, Kuzma M, Sediva B,
Hudcovic T, et al: Diet rich in simple sugars promotes
pro-inflammatory response via gut microbiota alteration and TLR4
signaling. Cells. 9:27012020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Francescangeli F, De Angelis ML and Zeuner
A: Dietary factors in the control of gut homeostasis, intestinal
stem cells, and colorectal cancer. Nutrients. 11:29362019.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li C and Hu Y: Align resistant starch
structures from plant-based foods with human gut microbiome for
personalized health promotion. Crit Rev Food Sci Nutr.
63:2509–2520. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu Y, Cao Y, Liu P, Zhai S, Liu Y, Tang
X, Lin J, Shi M, Qi D, Deng X, et al: ATF3-induced activation of
NF-κB pathway results in acquired PARP inhibitor resistance in
pancreatic adenocarcinoma. Cell Oncol (Dordr). 47:939–950. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhou CB, Zhou YL and Fang JY: Gut
microbiota in cancer immune response and immunotherapy. Trends
Cancer. 7:647–660. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu Q, Yang Y, Pan M, Yang F, Yu Y and
Qian Z: Role of the gut microbiota in tumorigenesis and treatment.
Theranostics. 14:2304–2328. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sivaprakasam S, Prasad PD and Singh N:
Benefits of short-chain fatty acids and their receptors in
inflammation and carcinogenesis. Pharmacol Ther. 164:144–151. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kaźmierczak-Siedlecka K, Marano L, Merola
E, Roviello F and Połom K: Sodium butyrate in both prevention and
supportive treatment of colorectal cancer. Front Cell Infect
Microbiol. 12:10238062022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cheng WY, Wu CY and Yu J: The role of gut
microbiota in cancer treatment: friend or foe? Gut. 69:1867–1876.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jandhyala SM, Talukdar R, Subramanyam C,
Vuyyuru H, Sasikala M and Nageshwar Reddy D: Role of the normal gut
microbiota. World J Gastroenterol. 21:8787–8803. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu L, Wang H, Chen X, Zhang Y, Zhang H
and Xie P: Gut microbiota and its metabolites in depression: from
pathogenesis to treatment. EBioMedicine. 90:1045272023. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chen Y, Wang X, Ye Y and Ren Q: Gut
microbiota in cancer: Insights on microbial metabolites and
therapeutic strategies. Med Oncol. 41:252023. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kang J, Sun M, Chang Y, Chen H, Zhang J,
Liang X and Xiao T: Butyrate ameliorates colorectal cancer through
regulating intestinal microecological disorders. Anticancer Drugs.
34:227–237. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kostic AD, Chun E, Robertson L, Glickman
JN, Gallini CA, Michaud M, Clancy TE, Chung DC, Lochhead P, Hold
GL, et al: Fusobacterium nucleatum potentiates intestinal
tumorigenesis and modulates the tumor-immune microenvironment. Cell
Host Microbe. 14:207–215. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Caleça T, Ribeiro P, Vitorino M, Menezes
M, Sampaio-Alves M, Mendes AD, Vicente R, Negreiros I, Faria A and
Costa DA: Breast cancer survivors and healthy women: Could gut
microbiota make a difference?-‘biotacancersurvivors’: A
case-control study. Cancers (Basel). 15:5942023. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Arnone AA, Wilson AS, Soto-Pantoja DR and
Cook KL: Diet modulates the gut microbiome, metabolism, and mammary
gland inflammation to influence breast cancer risk. Cancer Prev Res
(Phila). 17:415–428. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang L, Zheng YB, Yin S, Li KP, Wang JH,
Bao EH and Zhu PY: Causal relationship between gut microbiota and
prostate cancer contributes to the gut-prostate axis: Insights from
a Mendelian randomization study. Discov Oncol. 15:582024.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ooijevaar RE, Terveer EM, Verspaget HW,
Kuijper EJ and Keller JJ: Clinical application and potential of
fecal microbiota transplantation. Annu Rev Med. 70:335–351. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chen D, Wu J, Jin D, Wang B and Cao H:
Fecal microbiota transplantation in cancer management: Current
status and perspectives. Int J Cancer. 145:2021–2031. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yin Z, Liu B, Feng S, He Y, Tang C, Chen
P, Wang X and Wang K: A large genetic causal analysis of the gut
microbiota and urological cancers: A bidirectional mendelian
randomization study. Nutrients. 15:40862023. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Matsushita M, Fujita K, Hatano K, De
Velasco MA, Tsujimura A, Uemura H and Nonomura N: Emerging
relationship between the gut microbiome and prostate cancer. World
J Mens Health. 41:759–768. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Fujimoto S, Hatano K, Banno E, Motooka D,
De Velasco MA, Kura Y, Toyoda S, Hashimoto M, Adomi S, Minami T, et
al: Comparative analysis of gut microbiota in hormone-sensitive and
castration-resistant prostate cancer in Japanese men. Cancer Sci.
116:462–469. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Shyanti RK, Greggs J, Malik S and Mishra
M: Gut dysbiosis impacts the immune system and promotes prostate
cancer. Immunol Lett. 268:1068832024. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Golombos DM, Ayangbesan A, O'Malley P,
Lewicki P, Barlow L, Barbieri CE, Chan C, DuLong C, Abu-Ali G,
Huttenhower C and Scherr DS: The role of gut microbiome in the
pathogenesis of prostate cancer: A prospective, pilot study.
Urology. 111:122–128. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Liu Y and Jiang H: Compositional
differences of gut microbiome in matched hormone-sensitive and
castration-resistant prostate cancer. Transl Androl Urol.
9:1937–1944. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Newton RU, Christophersen CT, Fairman CM,
Hart NH, Taaffe DR, Broadhurst D, Devine A, Chee R, Tang CI, Spry N
and Galvão DA: Does exercise impact gut microbiota composition in
men receiving androgen deprivation therapy for prostate cancer? A
single-blinded, two-armed, randomised controlled trial. BMJ Open.
9:e0248722019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Matsushita M, Fujita K, Motooka D, Hatano
K, Fukae S, Kawamura N, Tomiyama E, Hayashi Y, Banno E, Takao T, et
al: The gut microbiota associated with high-Gleason prostate
cancer. Cancer Sci. 112:3125–3135. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Li JKM, Wang LL, Wong CYP, Chiu PKF, Teoh
JYC, Kwok HSW, Leung SCH, Wong SH, Tsui SKW and Ng CF: A
cross-sectional study on gut microbiota in prostate cancer patients
with prostatectomy or androgen deprivation therapy. Prostate Cancer
Prostatic Dis. 24:1063–1072. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Takezawa K, Fujita K, Matsushita M,
Motooka D, Hatano K, Banno E, Shimizu N, Takao T, Takada S, Okada
K, et al: The Firmicutes/Bacteroidetes ratio of the human gut
microbiota is associated with prostate enlargement. Prostate.
81:1287–1293. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Matsushita M, Fujita K, Motooka D, Hatano
K, Hata J, Nishimoto M, Banno E, Takezawa K, Fukuhara S, Kiuchi H,
et al: Firmicutes in gut microbiota correlate with blood
testosterone levels in elderly men. World J Mens Health.
40:517–525. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Fernandes A, Oliveira A, Guedes C,
Fernandes R, Soares R and Barata P: Effect of radium-223 on the gut
microbiota of prostate cancer patients: A pilot case series study.
Curr Issues Mol Biol. 44:4950–4959. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Huang H, Liu Y, Wen Z, Chen C, Wang C, Li
H and Yang X: Gut microbiota in patients with prostate cancer: A
systematic review and meta-analysis. BMC Cancer. 24:2612024.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Smith KS, Frugé AD, van der Pol W, Caston
NE, Morrow CD, Demark-Wahnefried W and Carson TL: Gut microbial
differences in breast and prostate cancer cases from two randomised
controlled trials compared to matched cancer-free controls. Benef
Microbes. 12:239–248. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Reichard CA, Naelitz BD, Wang Z, Jia X, Li
J, Stampfer MJ, Klein EA, Hazen SL and Sharifi N: Gut
microbiome-dependent metabolic pathways and risk of lethal prostate
cancer: Prospective analysis of a PLCO cancer screening trial
cohort. Cancer Epidemiol Biomarkers Prev. 31:192–199. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Alanee S, El-Zawahry A, Dynda D, Dabaja A,
McVary K, Karr M and Braundmeier-Fleming A: A prospective study to
examine the association of the urinary and fecal microbiota with
prostate cancer diagnosis after transrectal biopsy of the prostate
using 16sRNA gene analysis. Prostate. 79:81–87. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Hurst R, Meader E, Gihawi A, Rallapalli G,
Clark J, Kay GL, Webb M, Manley K, Curley H, Walker H, et al:
Microbiomes of urine and the prostate are linked to human prostate
cancer risk groups. Eur Urol Oncol. 5:412–419. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Li D, Wang P, Wang P, Hu X and Chen F: The
gut microbiota: A treasure for human health. Biotechnol Adv.
34:1210–1224. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Liu Y, Yang C, Zhang Z and Jiang H: Gut
microbiota dysbiosis accelerates prostate cancer progression
through increased LPCAT1 expression and enhanced DNA repair
pathways. Front Oncol. 11:6797122021. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Matsushita M, Fujita K, Hayashi T, Kayama
H, Motooka D, Hase H, Jingushi K, Yamamichi G, Yumiba S, Tomiyama
E, et al: Gut microbiota-derived short-chain fatty acids promote
prostate cancer growth via IGF1 signaling. Cancer Res.
81:4014–4026. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Pernigoni N, Zagato E, Calcinotto A,
Troiani M, Mestre RP, Calì B, Attanasio G, Troisi J, Minini M,
Mosole S, et al: Commensal bacteria promote endocrine resistance in
prostate cancer through androgen biosynthesis. Science.
374:216–224. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Matsushita M, Fujita K, Hatano K, Hayashi
T, Kayama H, Motooka D, Hase H, Yamamoto A, Uemura T, Yamamichi G,
et al: High-fat diet promotes prostate cancer growth through
histamine signaling. Int J Cancer. 151:623–636. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Biragyn A and Ferrucci L: Gut dysbiosis: A
potential link between increased cancer risk in ageing and
inflammaging. Lancet Oncol. 19:e295–e304. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Zhong W, Wu K, Long Z, Zhou X, Zhong C,
Wang S, Lai H, Guo Y, Lv D, Lu J and Mao X: Gut dysbiosis promotes
prostate cancer progression and docetaxel resistance via activating
NF-κB-IL6-STAT3 axis. Microbiome. 10:942022. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Bui NN, Li CY, Wang LY, Chen YA, Kao WH,
Chou LF, Hsieh JT, Lin H and Lai CH: Clostridium scindens
metabolites trigger prostate cancer progression through androgen
receptor signaling. J Microbiol Immunol Infect. 56:246–256. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Liu Y, Zhou Q, Ye F, Yang C and Jiang H:
Gut microbiota-derived short-chain fatty acids promote prostate
cancer progression via inducing cancer cell autophagy and M2
macrophage polarization. Neoplasia. 43:1009282023. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Hsia YJ, Lin ZM, Zhang T and Chou TC:
Butyrate increases methylglyoxal production through regulation of
the JAK2/Stat3/Nrf2/Glo1 pathway in castration-resistant prostate
cancer cells. Oncol Rep. 51:712024. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Xu W, Li Y, Liu L, Xie J, Hu Z, Kuang S,
Fu X, Li B, Sun T, Zhu C, et al: Icaritin-curcumol activates CD8(+)
T cells through regulation of gut microbiota and the DNMT1/IGFBP2
axis to suppress the development of prostate cancer. J Exp Clin
Cancer Res. 43:1492024. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
You X, Qiu J, Li Q, Zhang Q, Sheng W, Cao
Y and Fu W: Astragaloside IV-PESV inhibits prostate cancer tumor
growth by restoring gut microbiota and microbial metabolic
homeostasis via the AGE-RAGE pathway. BMC Cancer. 24:4722024.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Yu Y, Li L, Yang Q, Xue J, Wang B, Xie M,
Shangguan W, Zhu Z and Wu P: Akkermansia muciniphila metabolite
inosine inhibits castration resistance in prostate cancer.
Microorganisms. 12:16532024. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Li S, Liu R, Hao X and Liu X: The role of
gut microbiota in prostate cancer progression: A Mendelian
randomization study of immune mediation. Medicine (Baltimore).
103:e388252024. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Miya TV, Marima R, Damane BP, Ledet EM and
Dlamini Z: Dissecting microbiome-derived SCFAs in prostate cancer:
Analyzing gut microbiota, racial disparities, and epigenetic
mechanisms. Cancers (Basel). 15:40862023. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Zhang SL, Cheng LS, Zhang ZY, Sun HT and
Li JJ: Untangling determinants of gut microbiota and tumor
immunologic status through a multi-omics approach in colorectal
cancer. Pharmacol Res. 188:1066332023. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Legesse Bedada T, Feto TK, Awoke KS,
Garedew AD, Yifat FT and Birri DJ: Probiotics for cancer
alternative prevention and treatment. Biomed Pharmacother.
129:1104092020. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Banna GL, Torino F, Marletta F, Santagati
M, Salemi R, Cannarozzo E, Falzone L, Ferraù F and Libra M:
Lactobacillus rhamnosus GG: An overview to explore the rationale of
its use in cancer. Front Pharmacol. 8:6032017. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Kaźmierczak-Siedlecka K,
Skonieczna-Żydecka K, Hupp T, Duchnowska R, Marek-Trzonkowska N and
Połom K: Next-generation probiotics - do they open new therapeutic
strategies for cancer patients? Gut Microbes. 14:20356592022.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Yadav MK, Kumari I, Singh B, Sharma KK and
Tiwari SK: Probiotics, prebiotics and synbiotics: Safe options for
next-generation therapeutics. Appl Microbiol Biotechnol.
106:505–521. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Mahdavi M, Laforest-Lapointe I and Massé
E: Preventing colorectal cancer through prebiotics. Microorganisms.
9:13252021. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Holscher HD: Dietary fiber and prebiotics
and the gastrointestinal microbiota. Gut Microbes. 8:172–184. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Fernandes MR, Aggarwal P, Costa RGF, Cole
AM and Trinchieri G: Targeting the gut microbiota for cancer
therapy. Nat Rev Cancer. 22:703–722. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Jain MG, Hislop GT, Howe GR and Ghadirian
P: Plant foods, antioxidants, and prostate cancer risk: Findings
from case-control studies in Canada. Nutr Cancer. 34:173–184. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Miller PE, Morey MC, Hartman TJ, Snyder
DC, Sloane R, Cohen HJ and Demark-Wahnefried W: Dietary patterns
differ between urban and rural older, long-term survivors of
breast, prostate, and colorectal cancer and are associated with
body mass index. J Acad Nutr Diet. 112:824–31. 831.e12012.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Lachance G, Robitaille K, Laaraj J,
Gevariya N, Varin TV, Feldiorean A, Gaignier F, Julien IB, Xu HW,
Hallal T, et al: The gut microbiome-prostate cancer crosstalk is
modulated by dietary polyunsaturated long-chain fatty acids. Nat
Commun. 15:34312024. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Yadav A, Kaushik M, Tiwari P and Dada R:
From microbes to medicine: Harnessing the gut microbiota to combat
prostate cancer. Microb Cell. 11:187–197. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Liss MA, White JR, Goros M, Gelfond J,
Leach R, Johnson-Pais T, Lai Z, Rourke E, Basler J, Ankerst D and
Shah DP: Metabolic Biosynthesis Pathways Identified from Fecal
Microbiome Associated with Prostate Cancer. Eur Urol. 74:575–582.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Sfanos KS, Markowski MC, Peiffer LB, Ernst
SE, White JR, Pienta KJ, Antonarakis ES and Ross AE: Compositional
differences in gastrointestinal microbiota in prostate cancer
patients treated with androgen axis-targeted therapies. Prostate
Cancer Prostatic Dis. 21:539–548. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Matzaras R, Nikopoulou A, Protonotariou E
and Christaki E: Gut microbiota modulation and prevention of
dysbiosis as an alternative approach to antimicrobial resistance: A
narrative review. Yale J Biol Med. 95:479–494. 2022.PubMed/NCBI
|
|
96
|
Lv J, Jin S, Zhang Y, Zhou Y, Li M and
Feng N: Equol: A metabolite of gut microbiota with potential
antitumor effects. Gut Pathog. 16:352024. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Daisley BA, Chanyi RM, Abdur-Rashid K, Al
KF, Gibbons S, Chmiel JA, Wilcox H, Reid G, Anderson A, Dewar M, et
al: Abiraterone acetate preferentially enriches for the gut
commensal Akkermansia muciniphila in castrate-resistant prostate
cancer patients. Nat Commun. 11:48222020. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Kim SJ, Park M, Choi A and Yoo S:
Microbiome and prostate cancer: Emerging diagnostic and therapeutic
opportunities. Pharmaceuticals (Basel). 17:1122024. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Duan H, Wang L, Huangfu M and Li H: The
impact of microbiota-derived short-chain fatty acids on macrophage
activities in disease: Mechanisms and therapeutic potentials.
Biomed Pharmacother. 165:1152762023. View Article : Google Scholar : PubMed/NCBI
|