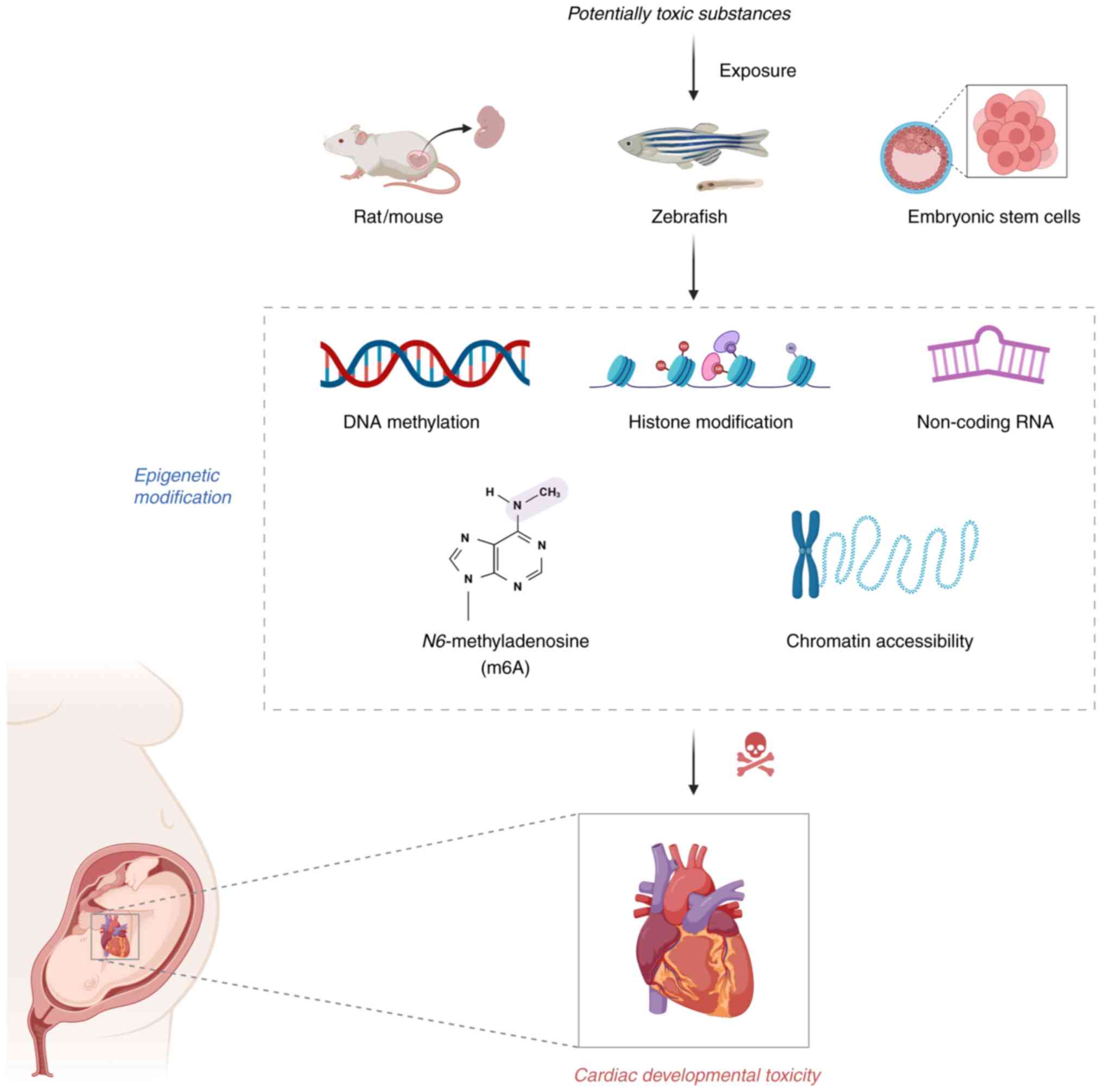
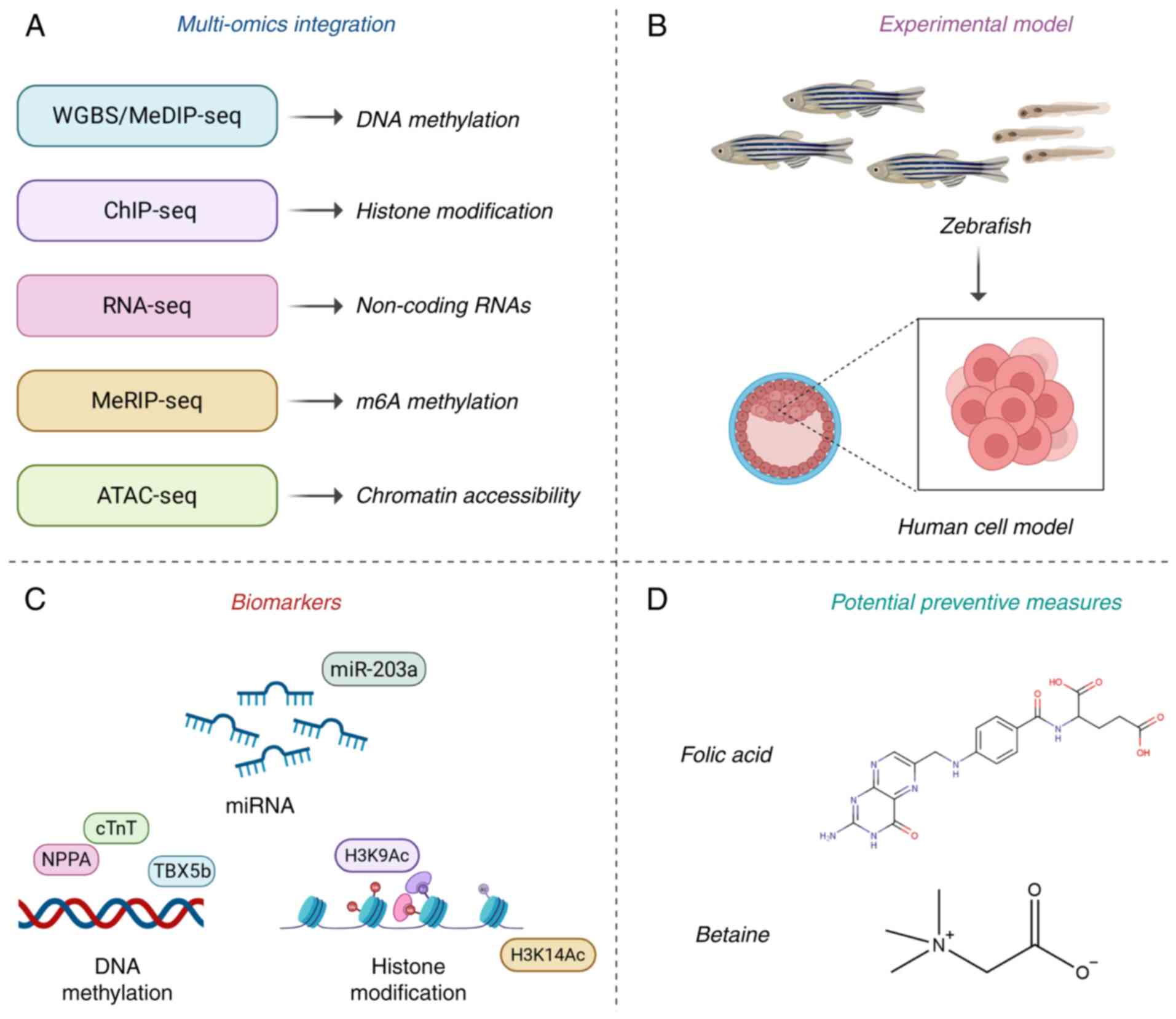
|
1
|
Mahler GJ and Butcher JT: Cardiac
developmental toxicity. Birth Defects Res C Embryo Today.
93:291–297. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hoffman JI and Kaplan S: The incidence of
congenital heart disease. J Am Coll Cardiol. 39:1890–1900. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hoffman JI: The global burden of
congenital heart disease. Cardiovasc J Afr. 24:141–145. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hoffman JI: Incidence of congenital heart
disease: II. Prenatal incidence. Pediatr Cardiol. 16:155–165. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sun R, Liu M, Lu L, Zheng Y and Zhang P:
Congenital heart disease: Causes, diagnosis, symptoms, and
treatments. Cell Biochem Biophys. 72:857–860. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jenkins KJ, Correa A, Feinstein JA, Botto
L, Britt AE, Daniels SR, Elixson M, Warnes CA and Webb CL; American
Heart Association Council on Cardiovascular Disease in the Young, :
Noninherited risk factors and congenital cardiovascular defects:
Current knowledge: A scientific statement from the American heart
association council on cardiovascular disease in the young:
Endorsed by the American academy of pediatrics. Circulation.
115:2995–3014. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
de Oliveira DT and Guerra-Sá R: Uncovering
epigenetic landscape: A new path for biomarkers identification and
drug development. Mol Biol Rep. 47:9097–9122. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Maher N, Maiellaro F, Ghanej J, Rasi S,
Moia R and Gaidano G: Unraveling the epigenetic landscape of mature
B cell neoplasia: Mechanisms, biomarkers, and therapeutic
opportunities. Int J Mol Sci. 26:81322025. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Leduque B, Edera A, Vitte C and Quadrana
L: Simultaneous profiling of chromatin accessibility and DNA
methylation in complete plant genomes using long-read sequencing.
Nucleic Acids Res. 52:6285–6297. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Choi SW and Friso S: Epigenetics: A new
bridge between nutrition and health. Adv Nutr. 1:8–16. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Samanta S, Rajasingh S, Cao T, Dawn B and
Rajasingh J: Epigenetic dysfunctional diseases and therapy for
infection and inflammation. Biochim Biophys Acta Mol Basis Dis.
1863:518–528. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Smith ZD and Meissner A: DNA methylation:
Roles in mammalian development. Nat Rev Genet. 14:204–220. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang H, Lang Z and Zhu JK: Dynamics and
function of DNA methylation in plants. Nat Rev Mol Cell Biol.
19:489–506. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gowher H, Liebert K, Hermann A, Xu G and
Jeltsch A: Mechanism of stimulation of catalytic activity of Dnmt3A
and Dnmt3B DNA-(cytosine-C5)-methyltransferases by Dnmt3L. J Biol
Chem. 280:13341–13348. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Okano M, Bell DW, Haber DA and Li E: DNA
methyltransferases Dnmt3a and Dnmt3b are essential for de novo
methylation and mammalian development. Cell. 99:247–257. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hermann A, Goyal R and Jeltsch A: The
Dnmt1 DNA-(cytosine-C5)-methyltransferase methylates DNA
processively with high preference for hemimethylated target sites.
J Biol Chem. 279:48350–48359. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bansal A and Pinney SE: DNA methylation
and its role in the pathogenesis of diabetes. Pediatr Diabetes.
18:167–177. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jones PA: Functions of DNA methylation:
Islands, start sites, gene bodies and beyond. Nat Rev Genet.
13:484–492. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bochtler M, Kolano A and Xu GL: DNA
demethylation pathways: Additional players and regulators.
Bioessays. 39:1–13. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Strasenburg W, Borowczak J, Piątkowska D,
Jóźwicki J and Grzanka D: The role of DNA methylation and
demethylation in bladder cancer: A focus on therapeutic strategies.
Front Oncol. 15:15672422025. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu F, Li K, Li S, Liu J, Zhang Y, Zhou M,
Zhao H, Chen H, Wu N, Liu Z and Su J: CFEA: A cell-free epigenome
atlas in human diseases. Nucleic Acids Res. 48((D1)): D40–D44.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu SC and Zhang Y: Active DNA
demethylation: Many roads lead to rome. Nat Rev Mol Cell Biol.
11:607–620. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li X, Cui J, Zhang X, Wu Y, Xu J, Zhao Y,
Hussain S and Chen L: Integrated analysis of methylomic and
transcriptomic profiles in fetal mouse hypothalamus in response to
maternal gestational exposure to arsenic. Ecotoxicol Environ Saf.
299:1184002025. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Demond H, Khan S, Castillo-Fernandez J,
Hanna CW and Kelsey G: Transcriptome and DNA methylation profiling
during the NSN to SN transition in mouse oocytes. BMC Mol Cell
Biol. 26:22025. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jiang Y, Li J, Ren F, Ji C, Aniagu S and
Chen T: PM2.5-induced extensive DNA methylation changes in the
heart of zebrafish embryos and the protective effect of folic acid.
Environ Pollut. 255:1133312019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Qiu M, Chen J, Liu M, Shi Y, Nie Z, Dong
G, Li X, Chen J, Ou Y and Zhuang J: Reprogramming of DNA
methylation patterns mediates perfluorooctane sulfonate-induced
fetal cardiac dysplasia. Sci Total Environ. 919:1709052024.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cai J, Zhao Y, Liu P, Xia B, Zhu Q, Wang
X, Song Q, Kan H and Zhang Y: Exposure to particulate air pollution
during early pregnancy is associated with placental DNA
methylation. Sci Total Environ. 607–608. 1103–1108. 2017.
|
|
28
|
Heudorf U, Mersch-Sundermann V and Angerer
J: Phthalates: Toxicology and exposure. Int J Hyg Environ Health.
210:623–634. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang Y and Qian H: Phthalates and their
impacts on human health. Healthcare (Basel). 9:6032021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mu X, Chen X, Liu J, Yuan L, Wang D, Qian
L, Qian Y, Shen G, Huang Y, Li X, et al: A multi-omics approach
reveals molecular mechanisms by which phthalates induce cardiac
defects in zebrafish (danio rerio). Environ Pollut. 265((Pt B)):
1138762020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Thangavel P, Park D and Lee YC: Recent
insights into particulate matter (PM2.5)-mediated
toxicity in humans: An overview. Int J Environ Res Public Health.
19:75112022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cai J, Shen Y, Meng X, Zhao Y, Niu Y, Chen
R, Du W, Quan G, Barnett AL, Jones G, et al: Association of
developmental coordination disorder with early-life exposure to
fine particulate matter in chinese preschoolers. Innovation (Camb).
4:1003472022.PubMed/NCBI
|
|
33
|
Lin CA, Pereira LA, Nishioka DC, Conceição
GM, Braga AL and Saldiva PH: Air pollution and neonatal deaths in
São Paulo, Brazil. Braz J Med Biol Res. 37:765–770. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gouveia N, Bremner SA and Novaes HM:
Association between ambient air pollution and birth weight in Sao
Paulo, Brazil. J Epidemiol Community Health. 58:11–17. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li L, Zhang B, Li L and Borthwick AGL:
Microbial selenate detoxification linked to elemental sulfur
oxidation: Independent and synergic pathways. J Hazard Mater.
422:1269322022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lin JH, Liu CC, Liu CY, Hsu TW, Yeh YC,
How CK, Hsu HS and Hung SC: Selenite selectively kills lung
fibroblasts to treat bleomycin-induced pulmonary fibrosis. Redox
Biol. 72:1031482024. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hariharan S, Chauhan S, Marcharla E,
Alphonse CRW, Rajaretinam RK and Ganesan S: Developmental toxicity
and neurobehavioral effects of sodium selenite and selenium
nanoparticles on zebrafish embryos. Aquat Toxicol. 266:1067912024.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Imai T, Kurihara T, Esaki N and Mihara H:
Glutathione contributes to the efflux of selenium from hepatoma
cells. Biosci Biotechnol Biochem. 78:1376–1380. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ma Y, Wu M, Li D, Li XQ, Li P, Zhao J, Luo
MN, Guo CL, Gao XB, Lu CL and Ma X: Embryonic developmental
toxicity of selenite in zebrafish (Danio rerio) and prevention with
folic acid. Food Chem Toxicol. 50:2854–2863. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ganie SY, Javaid D, Hajam YA and Reshi MS:
Arsenic toxicity: Sources, pathophysiology and mechanism. Toxicol
Res (Camb). 13:tfad1112023. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cantoni O, Zito E, Fiorani M and
Guidarelli A: Arsenite impinges on endoplasmic
reticulum-mitochondria crosstalk to elicit mitochondrial ROS
formation and downstream toxicity. Semin Cancer Biol. 76:132–138.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li D, Lu C, Wang J, Hu W, Cao Z, Sun D,
Xia H and Ma X: Developmental mechanisms of arsenite toxicity in
zebrafish (Danio rerio) embryos. Aquat Toxicol. 91:229–237. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hrelia P, Vigagni F, Maffei F, Morotti M,
Colacci A, Perocco P, Grilli S and Cantelli-Forti G: Genetic safety
evaluation of pesticides in different short-term tests. Mutat Res.
321:219–228. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jiao F, Zhao Y, Limbu SM, Kong L, Zhang D,
Liu X, Yang S, Gui W and Rong H: Cyhexatin causes developmental
toxic effects by disrupting endocrine system and inducing
behavioral inhibition, apoptosis and DNA hypomethylation in
zebrafish (Danio rerio) larvae. Chemosphere. 339:1397692023.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kim J and Choi J: Trans- and
multigenerational effects of isothiazolinone biocide CMIT/MIT on
genotoxicity and epigenotoxicity in daphnia magna. Toxics.
11:3882023. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lee H, Kim Y, Cho Y, Jeon EJ, Jeong SH,
Lee JH and Kim S: Nociceptive effects and gene alterations of
CMIT/MIT mixture in zebrafish embryos and larvae. J Hazard Mater.
493:1383922025. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Molinari F, Tranchida N, Inferrera F,
Fusco R, Faggio C, Impellitteri F, Cuzzocrea S, Cordaro M and Di
Paola R: Biocide mixture (CMIT/MIT) induces neurotoxicity through
the upregulation of the MAPKs signaling pathways. J Neurophysiol.
134:183–192. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chatterjee N, Lee H, Kim J, Kim D, Lee S
and Choi J: Critical window of exposure of CMIT/MIT with respect to
developmental effects on zebrafish embryos: multi-level endpoint
and proteomics analysis. Environ Pollut. 268((Pt A)): 1157842021.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kausar S, Abbas MN, Gul I, Liu R, Li Q,
Zhao E, Lv M and Cui H: Molecular identification of two DNA
methyltransferase genes and their functional characterization in
the anti-bacterial immunity of antheraea pernyi. Front Immunol.
13:8558882022. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Castillo-Aguilera O, Depreux P, Halby L,
Arimondo PB and Goossens L: DNA methylation targeting: The DNMT/HMT
crosstalk challenge. Biomolecules. 7:32017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Liu L, Ni S, Zhang L, Chen Y, Xie M and
Huang X: Molecular insights and clinical implications of DNA
methylation in sepsis-associated acute kidney injury: A narrative
review. BMC Nephrol. 26:2532025. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhang Y, Zhang Q, Zhang Y and Han J: The
role of histone modification in DNA replication-coupled nucleosome
assembly and cancer. Int J Mol Sci. 24:49392023. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Fagerli E, Escobar I, Ferrier FJ, Jackson
CW, Perez-Lao EJ and Perez-Pinzon MA: Sirtuins and cognition:
implications for learning and memory in neurological disorders.
Front Physiol. 13:9086892022. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zhang J, Liu H, Liu Y, Luo E and Liu S:
Unlocking the potential of histone modification in regulating bone
metabolism. Biochimie. 227:286–298. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Qi Q, Li L, Liang H and Zeng Y: Role and
research progress of histone modification in cardiovascular
diseases (Review). Exp Ther Med. 30:1322025. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Yang Y and Luan Y, Yuan RX and Luan Y:
Histone methylation related therapeutic challenge in cardiovascular
diseases. Front Cardiovasc Med. 8:7100532021. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Mao W, Wang B, Huang R, Sun Z, Yan M and
Dong P: Histone modifications in head and neck squamous cell
carcinoma. Front Oncol. 14:14277252024. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wang K, Li Y, Qiang T, Chen J and Wang X:
Role of epigenetic regulation in myocardial ischemia/reperfusion
injury. Pharmacol Res. 170:1057432021. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zhang J, Huang H, Ding B, Liu Z, Chen D,
Li S, Shen T and Zhu Q: Histone demethylase KDM4A mediating
macrophage polarization: A potential mechanism of trichloroethylene
induced liver injury. Cell Biol Int. 48:1148–1159. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Kim J and Choi J: Histone
methylation-mediated reproductive toxicity to consumer product
chemicals in caenorhabditis elegans: An epigenetic adverse outcome
pathway (AOP). Environ Sci Technol. 58:19604–19616. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Mehta I, Verma M, Quasmi MN, Kumar D and
Jangra A: Emerging roles of histone modifications in environmental
toxicants-induced neurotoxicity. Toxicology. 515:1541642025.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Wang Q, Sun S, Sun G, Han B, Zhang S,
Zheng X and Chen L: Histone modification inhibitors: an emerging
frontier in thyroid cancer therapy. Cell Signal. 131:1117032025.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Liu M, Chen B, Pei L, Zhang Q, Zou Y, Xiao
H, Zhou J, Chen L and Wang H: Decreased H3K9ac level of StAR
mediated testicular dysplasia induced by prenatal dexamethasone
exposure in Male offspring rats. Toxicology. 408:1–10. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zhou J, Liu F, Yu L, Xu D, Li B, Zhang G,
Huang W, Li L, Zhang Y, Zhang W and Wang H: nAChRs-ERK1/2-egr-1
signaling participates in the developmental toxicity of nicotine by
epigenetically down-regulating placental 11β-HSD2. Toxicol Appl
Pharmacol. 344:1–12. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Pei LG, Zhang Q, Yuan C, Liu M, Zou YF, Lv
F, Luo DJ, Zhong S and Wang H: The GC-IGF1 axis-mediated testicular
dysplasia caused by prenatal caffeine exposure. J Endocrinol.
242:M17–M32. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Li Y, Dong F, Liu X, Xu J, Li J, Kong Z,
Chen X and Zheng Y: Environmental behavior of the chiral triazole
fungicide fenbuconazole and its chiral metabolites:
Enantioselective transformation and degradation in soils. Environ
Sci Technol. 46:2675–2683. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Wu Y, Yang Q, Chen M, Zhang Y, Zuo Z and
Wang C: Fenbuconazole exposure impacts the development of zebrafish
embryos. Ecotoxicol Environ Saf. 158:293–299. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Zhang Y, Guo J, Tang C, Xu K, Li Z and
Wang C: Early life stage exposure to fenbuconazole causes
multigenerational cardiac developmental defects in zebrafish and
potential reasons. Environ Pollut. 349:1239382024. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Madrid JV, Vera-Colón MKM and zur Nieden
NI: Perturbations in osteogenic cell fate following exposure to
constituents present in tobacco: A combinatorial study. Toxics.
11:9982023. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Prasad S, Kaisar MA and Cucullo L:
Unhealthy smokers: Scopes for prophylactic intervention and
clinical treatment. BMC Neurosci. 18:702017. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Sabbagh HJ, Baghlaf KK, Jamalellail HMH,
Bakhuraybah AS, AlGhamdi SM, Alharbi OA, AlHarbi KM and Hassan MHA:
Environmental tobacco smoke exposure and non-syndromic orofacial
cleft: Systematic review and meta-analysis. Tob Induc Dis.
21:762023. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Kharrazi M, DeLorenze GN, Kaufman FL,
Eskenazi B, Bernert JT Jr, Graham S, Pearl M and Pirkle J:
Environmental tobacco smoke and pregnancy outcome. Epidemiology.
15:660–670. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Deng C, Pu J, Deng Y, Xie L, Yu L, Liu L,
Guo X, Sandin S, Liu H and Dai L: Association between maternal
smoke exposure and congenital heart defects from a case-control
study in China. Sci Rep. 12:149732022. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Macdonald-Wallis C, Tobias JH, Davey Smith
G and Lawlor DA: Parental smoking during pregnancy and offspring
bone mass at age 10 years: Findings from a prospective birth
cohort. Osteoporos Int. 22:1809–1819. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Cheng W, Zhou R, Feng Y and Wang Y:
Mainstream smoke and sidestream smoke affect the cardiac
differentiation of mouse embryonic stem cells discriminately.
Toxicology. 357–358. 1–10. 2016.PubMed/NCBI
|
|
76
|
Rahbari A, Esmaielpour B, Azarmi R, Fatemi
H, Lajayer HM, Panahirad S, Gohari G and Vita F: Symbiotic fungus
serendipita indica as a natural bioenhancer against cadmium
toxicity in chinese cabbage. Plants (Basel). 14:27732025.PubMed/NCBI
|
|
77
|
Zhu Y, Guan H, Zhu X, Cai J, Jiao X, Shan
J, Li Y, Wu Q and Zhang Z: Astilbin antagonizes developmental
cardiotoxicity after cadmium exposure in chicken embryos by
inhibiting endoplasmic reticulum stress and maintaining calcium
homeostasis. Ecotoxicol Environ Saf. 270:1158472024. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Vasanthakumaran M, Ramesh M, Murugan K,
Hema T, Rajaganesh R and Hwang JS: Developmental toxicity,
biochemical and biomarker in the zebrafish (Danio rerio) embryo
exposed to biosynthesized cadmium oxide nanoparticles. Chemosphere.
369:1438512024. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Wu X, Chen Y, Luz A, Hu G and Tokar EJ:
Cardiac development in the presence of cadmium: An in vitro study
using human embryonic stem cells and cardiac organoids. Environ
Health Perspect. 130:1170022022. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Dasgupta A, Nandi S, Gupta S, Roy S and
Das C: To ub or not to ub: The epic dilemma of histones that
regulate gene expression and epigenetic cross-talk. Biochim Biophys
Acta Gene Regul Mech. 1867:1950332024. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Ghate NB, Nadkarni KS, Barik GK, Tat SS,
Sahay O and Santra MK: Histone ubiquitination: role in genome
integrity and chromatin organization. Biochim Biophys Acta Gene
Regul Mech. 1867:1950442024. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Shu Q, Liu Y and Ai H: The emerging role
of the histone H2AK13/15 ubiquitination: mechanisms of writing,
reading, and erasing in DNA damage repair and disease. Cells.
14:3072025. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Zambrano-Carrasco J, Zou J, Wang W, Sun X,
Li J and Su H: Emerging roles of cullin-RING ubiquitin ligases in
cardiac development. Cells. 13:2352024. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Zhao X, Jiang B, Hu H, Mao F, Mi J, Li Z,
Liu Q, Shao C and Gong Y: Zebrafish cul4a, but not cul4b, modulates
cardiac and forelimb development by upregulating tbx5a expression.
Hum Mol Genet. 24:853–864. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Zhang L, Xu X and Su X: Noncoding RNAs in
cancer immunity: Functions, regulatory mechanisms, and clinical
application. Mol Cancer. 19:482020. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Song Z, Suo C, Liu Y, Jin L, Xie X, Liu J,
Yu B, Wang Y, Zhang Z and Xie D: Comprehensive evaluation of
non-coding RNA-mediated autophagy regulation in myocardial
ischemia-reperfusion injury. Front Pharmacol. 16:15813412025.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Anastasiadou E, Jacob LS and Slack FJ:
Non-coding RNA networks in cancer. Nat Rev Cancer. 18:5–18. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Liu Y, Yuan J, Zhang Y, Ma T, Ji Q, Tian S
and Liu C: Non-coding RNA as a key regulator and novel target of
apoptosis in diabetic cardiomyopathy: Current status and future
prospects. Cell Signal. 128:1116322025. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Aghaei-Zarch SM, Alipourfard I,
Rasoulzadeh H, Najafi S, Aghaei-Zarch F, Partov S, Movafagh A,
Jahanara A, Toolabi A, Sheikhmohammadi A, et al: Non-coding RNAs:
an emerging player in particulate matter 2.5-mediated toxicity. Int
J Biol Macromol. 235:1237902023. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Sanjari Nia AH, Reyhani Ardabili M,
Sheikhvand M, Bagheri-Mohammadi S, Niknejad H, Rasoulzadeh H,
Movafagh A, Kharazi Neghad S, Baniasadi M, Ashrafi Asgarabad A, et
al: Non-coding RNAs: A new frontier in benzene-mediated toxicity.
Toxicology. 500:1536602023. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Huang Y, Jiang B, Xia Y, Wang J, Ji C,
Tong J, Chen T and Jiang Y: Downregulation of miR-133a contributes
to the cardiac developmental toxicity of trichloroethylene in
zebrafish. Chemosphere. 251:1266102020. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Smirnova L, Block K, Sittka A,
Oelgeschläger M, Seiler AE and Luch A: MicroRNA profiling as tool
for in vitro developmental neurotoxicity testing: The case of
sodium valproate. PLoS One. 9:e988922014. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Xiao H, Zhang F, Zou Y, Li J, Liu Y and
Huang W: The function and mechanism of long non-coding RNA-ATB in
cancers. Front Physiol. 9:3212018. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
D'Angelo E and Agostini M: Long non-coding
RNA and extracellular matrix: The hidden players in cancer-stroma
cross-talk. Noncoding RNA Res. 3:174–177. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Ghorbanzadeh S, Poor-Ghassem N, Afsa M,
Nikbakht M and Malekzadeh K: Long non-coding RNA NR2F2-AS1: Its
expanding oncogenic roles in tumor progression. Hum Cell.
35:1355–1363. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Ye D, Bao Z, Yu Y, Han Z, Yu Y, Xu Z, Ma
W, Yuan Y, Zhang L, Xu Y, et al: Inhibition of cardiomyocyte
differentiation of human induced pluripotent stem cells by
ribavirin: Implication for its cardiac developmental toxicity.
Toxicology. 435:1524222020. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Ronzoni L, Aghemo A, Rumi MG, Prati G,
Colancecco A, Porretti L, Monico S, Colombo M and Cappellini MD:
Ribavirin suppresses erythroid differentiation and proliferation in
chronic hepatitis C patients. J Viral Hepat. 21:416–423. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Vishnoi A and Rani S: miRNA biogenesis and
regulation of diseases: An updated overview. Methods Mol Biol.
2595:1–12. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Ye J, Xu M, Tian X, Cai S and Zeng S:
Research advances in the detection of miRNA. J Pharm Anal.
9:217–226. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Li W, Mu J, Ni S, Pei W, Wan L, Wu X, Zhu
J, Zhang Z and Li L: Pentachlorophenol exposure delays the recovery
of colitis in association with altered gut microbiota and purine
metabolism. Environ Toxicol. 40:101–110. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Xu EG, Khursigara AJ, Li S, Esbaugh AJ,
Dasgupta S, Volz DC and Schlenk D: mRNA-miRNA-seq reveals
neuro-cardio mechanisms of crude oil toxicity in red drum
(Sciaenops ocellatus). Environ Sci Technol. 53:3296–3305. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Xu EG, Magnuson JT, Diamante G, Mager E,
Pasparakis C, Grosell M, Roberts AP and Schlenk D: Changes in
microRNA-mRNA signatures agree with morphological, physiological,
and behavioral changes in larval mahi-mahi treated with deepwater
horizon oil. Environ Sci Technol. 52:13501–13510. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Deng H, Bai Z, Huangfu C, Wang N, Chen M,
Li G, Huang C, Ao T, Tang X, Xia T, et al: Gremlin1
repression-mediated mitochondrial network hyperfunction contributes
to TCE-induced zebrafish cardiac defects. Cell Commun Signal.
23:3182025. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Drake VJ, Koprowski SL, Lough J, Hu N and
Smith SM: Trichloroethylene exposure during cardiac valvuloseptal
morphogenesis alters cushion formation and cardiac hemodynamics in
the avian embryo. Environ Health Perspect. 114:842–847. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Jin H, Ji C, Ren F, Aniagu S, Tong J,
Jiang Y and Chen T: AHR-mediated oxidative stress contributes to
the cardiac developmental toxicity of trichloroethylene in
zebrafish embryos. J Hazard Mater. 385:1215212020. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Johnson PD, Goldberg SJ, Mays MZ and
Dawson BV: Threshold of trichloroethylene contamination in maternal
drinking waters affecting fetal heart development in the rat.
Environ Health Perspect. 111:289–292. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Long SM and Holdway DA: Acute toxicity of
crude and dispersed oil to octopus pallidus (Hoyle, 1885)
hatchlings. Water Res. 36:2769–2776. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Sørhus E, Donald CE, Nakken CL, Perrichon
P, Durif CMF, Shema S, Browman HI, Skiftesvik AB, Lie KK, Rasinger
JD, et al: Co-exposure to UV radiation and crude oil increases
acute embryotoxicity and sublethal malformations in the early life
stages of atlantic haddock (Melanogrammus aeglefinus). Sci Total
Environ. 859:1600802023. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Khursigara AJ, Ackerly KL and Esbaugh AJ:
Oil toxicity and implications for environmental tolerance in fish.
Comp Biochem Physiol C Toxicol Pharmacol. 220:52–61. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Magnuson JT, Qian L, McGruer V, Cheng V,
Volz DC and Schlenk D: Relationship between miR-203a inhibition and
oil-induced toxicity in early life stage zebrafish (danio rerio).
Toxicol Rep. 9:373–381. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Guo M, Li X, Choi M, Zhang J, Yan S, Ma D,
Zeng J, Ding W, Wen Y, Li D, et al: Microcystin-LR prenatal
exposure induces coronary heart disease through macrophage
polarization imbalance mediated by trophoblast-derived
extracellular vesicles. Sci Total Environ. 948:1749792024.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Desrosiers R, Friderici K and Rottman F:
Identification of methylated nucleosides in messenger RNA from
Novikoff hepatoma cells. Proc Natl Acad Sci USA. 71:3971–3975.
1974. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
An Y and Duan H: The role of m6A RNA
methylation in cancer metabolism. Mol Cancer. 21:142022. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Qin Y, Li L, Luo E, Hou J, Yan G, Wang D,
Qiao Y and Tang C: Role of m6A RNA methylation in cardiovascular
disease (Review). Int J Mol Med. 46:1958–1972. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Fan Y, Lv X, Chen Z, Peng Y and Zhang M:
m6A methylation: Critical roles in aging and neurological diseases.
Front Mol Neurosci. 16:11021472023. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Liu J, Yue Y, Han D, Wang X, Fu Y, Zhang
L, Jia G, Yu M, Lu Z, Deng X, et al: A METTL3-METTL14 complex
mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem
Biol. 10:93–95. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Zheng G, Dahl JA, Niu Y, Fedorcsak P,
Huang CM, Li CJ, Vågbø CB, Shi Y, Wang WL, Song SH, et al: ALKBH5
is a mammalian RNA demethylase that impacts RNA metabolism and
mouse fertility. Mol Cell. 49:18–29. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Jia G, Fu Y, Zhao X, Dai Q, Zheng G, Yang
Y, Yi C, Lindahl T, Pan T, Yang YG and He C: N6-Methyladenosine in
nuclear RNA is a major substrate of the obesity-associated FTO. Nat
Chem Biol. 7:885–887. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Zou S, Toh JD, Wong KH, Gao YG, Hong W and
Woon EC: N(6)-Methyladenosine: A conformational marker that
regulates the substrate specificity of human demethylases FTO and
ALKBH5. Sci Rep. 6:256772016. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Yang L, Qi G, Rao W, Cen Y, Chen L, Li W
and Pang Y: Aluminum causes irreversible damage to the development
of hippocampal neurons by regulating m6A RNA methylation. Toxicol
Lett. 399:34–42. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Qian Q, Pu Q, Li X, Liu X, Ni A, Han X,
Wang Z, Wang X, Yan J and Wang H: Acute/chronic triclosan exposure
induces downregulation of m6A-RNA methylation modification via
mettl3 suppression and elicits developmental and immune toxicity to
zebrafish. Chemosphere. 352:1413952024. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Gao Y, Li A, Zhang W, Pang S, Liang Y and
Song M: Assessing the toxicity of bisphenol A and its six
alternatives on zebrafish embryo/larvae. Aquat Toxicol.
246:1061542022. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Rezg R, El-Fazaa S, Gharbi N and Mornagui
B: Bisphenol A and human chronic diseases: Current evidences,
possible mechanisms, and future perspectives. Environ Int.
64:83–90. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Liu J, Lin J, Chen J, Maimaitiyiming Y, Su
K, Sun S, Zhan G and Hsu CH: Bisphenol C induces developmental
defects in liver and intestine through mTOR signaling in zebrafish
(Danio rerio). Chemosphere. 322:1381952023. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Su K, Liu J, Chen J, Wu H, Tang W, Sun S,
Lin J, Zhan G and Hsu CH: Bisphenol C induces cardiac developmental
defects by disrupting m6A homeostasis. Environ Sci Technol.
58:17259–17269. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Ji C, Tao Y, Li X, Wang J, Chen J, Aniagu
S, Jiang Y and Chen T: AHR-mediated m6A RNA methylation contributes
to PM2.5-induced cardiac malformations in zebrafish larvae. J
Hazard Mater. 457:1317492023. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Wang Y, Zou J and Zhou H: N6-methyladenine
RNA methylation epigenetic modification and diabetic microvascular
complications. Front Endocrinol (Lausanne). 15:14621462024.
View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Chen Y, Liang R, Li Y, Jiang L, Ma D, Luo
Q and Song G: Chromatin accessibility: Biological functions,
molecular mechanisms and therapeutic application. Signal Transduct
Target Ther. 9:3402024. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Fyodorov DV, Zhou BR, Skoultchi AI and Bai
Y: Emerging roles of linker histones in regulating chromatin
structure and function. Nat Rev Mol Cell Biol. 19:192–206. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Routh A, Sandin S and Rhodes D: Nucleosome
repeat length and linker histone stoichiometry determine chromatin
fiber structure. Proc Natl Acad Sci USA. 105:8872–8877. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
131
|
McBryant SJ, Adams VH and Hansen JC:
Chromatin architectural proteins. Chromosome Res. 14:39–51. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Eustermann S, Patel AB, Hopfner KP, He Y
and Korber P: Energy-driven genome regulation by ATP-dependent
chromatin remodellers. Nat Rev Mol Cell Biol. 25:309–332. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Struhl K: Non-canonical functions of
enhancers: Regulation of RNA polymerase III transcription, DNA
replication, and V(D)J recombination. Trends Genet. 40:471–479.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Wilkinson AL, Zorzan I and Rugg-Gunn PJ:
Epigenetic regulation of early human embryo development. Cell Stem
Cell. 30:1569–1584. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Cao L, Luo Y, Guo X, Liu S, Li S, Li J,
Zhang Z, Zhao Y, Zhang Q, Gao F, et al: SAFA facilitates chromatin
opening of immune genes through interacting with anti-viral host
RNAs. PLoS Pathog. 18:e10105992022. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Lopes-Paciencia S and Ferbeyre G:
Increased chromatin accessibility underpins senescence. FEBS J.
292:4112–4132. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Grandi FC, Modi H, Kampman L and Corces
MR: Chromatin accessibility profiling by ATAC-seq. Nat Protoc.
17:1518–1552. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Tang L, Song S, Hu C, Liu M, Lam PKS, Zhou
B, Lam JCW and Chen L: Parental exposure to perfluorobutane
sulfonate disturbs the transfer of maternal transcripts and
offspring embryonic development in zebrafish. Chemosphere.
256:1271692020. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Liu Q, Van Bortle K, Zhang Y, Zhao MT,
Zhang JZ, Geller BS, Gruber JJ, Jiang C, Wu JC and Snyder MP:
Disruption of mesoderm formation during cardiac differentiation due
to developmental exposure to 13-cis-retinoic acid. Sci Rep.
8:129602018. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Guan G, Abulaiti A, Qu C, Chen CC, Gu Z,
Yang J, Zhang T and Chen X, Zhou Z, Lu F and Chen X: Multi-omics
panoramic analysis of HBV integration, transcriptional regulation,
and epigenetic modifications in PLC/PRF/5 cell line. J Med Virol.
96:e296142024. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Qi C, Jin X, Wang H and Xu D: Crosstalk
between N6-methyladenosine and other epigenetic mechanisms in
central nervous system development and disorders. Biomolecules.
15:10922025. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Tan RZ, Jia J, Li T, Wang L and Kantawong
F: A systematic review of epigenetic interplay in kidney diseases:
Crosstalk between long noncoding RNAs and methylation, acetylation
of chromatin and histone. Biomed Pharmacother. 176:1169222024.
View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Li W, Luo P, Chen Q, Cheng L, Gan L, Zhang
F, Zhong H, Zheng L and Qian B: Epigenetic modifications in bladder
cancer: Crosstalk between DNA methylation and miRNAs. Front
Immunol. 16:15181442025. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Buscariollo DL, Fang X, Greenwood V, Xue
H, Rivkees SA and Wendler CC: Embryonic caffeine exposure acts via
A1 adenosine receptors to alter adult cardiac function and DNA
methylation in mice. PLoS One. 9:e875472014. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Buscariollo DL, Breuer GA, Wendler CC and
Rivkees SA: Caffeine acts via A1 adenosine receptors to disrupt
embryonic cardiac function. PLoS One. 6:e282962011. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Fang X, Mei W, Barbazuk WB, Rivkees SA and
Wendler CC: Caffeine exposure alters cardiac gene expression in
embryonic cardiomyocytes. Am J Physiol Regul Integr Comp Physiol.
307:R1471–1487. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Rohweder R, de Oliveira Schmalfuss T, Dos
Santos Borniger D, Ferreira CZ, Zanardini MK, Lopes GPTF, Barbosa
CP, Moreira TD, Schuler-Faccini L, Sanseverino MTV, et al: Caffeine
intake during pregnancy and adverse outcomes: An integrative
review. Reprod Toxicol. 123:1085182024. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Rodriguez-Martinez A, Vuorinen EM,
Shcherban A, Uusi-Mäkelä J, Rajala NKM, Nykter M and Kallioniemi A:
Novel ZNF414 activity characterized by integrative analysis of
ChIP-exo, ATAC-seq and RNA-seq data. Biochim Biophys Acta Gene
Regul Mech. 1865:1948112022. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Eisa-Beygi S, Benslimane FM, El-Rass S,
Prabhudesai S, Abdelrasoul MKA, Simpson PM, Yalcin HC, Burrows PE
and Ramchandran R: Characterization of endothelial cilia
distribution during cerebral-vascular development in zebrafish
(Danio rerio). Arterioscler Thromb Vasc Biol. 38:2806–2818. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Zhang L and Zhou J: Zebrafish: A smart
tool for heart disease research. J Fish Biol. 105:1487–1500. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Stainier DY, Kontarakis Z and Rossi A:
Making sense of anti-sense data. Dev Cell. 32:7–8. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Poon KL and Brand T: The zebrafish model
system in cardiovascular research: A tiny fish with mighty
prospects. Glob Cardiol Sci Pract. 2013:9–28. 2013.PubMed/NCBI
|
|
153
|
Glickman NS and Yelon D: Cardiac
development in zebrafish: Coordination of form and function. Semin
Cell Dev Biol. 13:507–513. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Salman HE and Yalcin HC: Computational
modeling of blood flow hemodynamics for biomechanical investigation
of cardiac development and disease. J Cardiovasc Dev Dis.
8:142021.PubMed/NCBI
|
|
155
|
Wafer LN, Jensen VB, Whitney JC, Gomez TH,
Flores R and Goodwin BS: Effects of environmental enrichment on the
fertility and fecundity of zebrafish (Danio rerio). J Am Assoc Lab
Anim Sci. 55:291–294. 2016.PubMed/NCBI
|
|
156
|
Ducharme NA, Reif DM, Gustafsson JA and
Bondesson M: Comparison of toxicity values across zebrafish early
life stages and mammalian studies: Implications for chemical
testing. Reprod Toxicol. 55:3–10. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Khabib MNH, Sivasanku Y, Lee HB, Kumar S
and Kue CS: Alternative animal models in predictive toxicology.
Toxicology. 465:1530532022. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Bambino K and Chu J: Zebrafish in
toxicology and environmental health. Curr Top Dev Biol.
124:331–367. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Evans T: Embryonic stem cells as a model
for cardiac development and disease. Drug Discov Today Dis Models.
5:147–155. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Keller G: Embryonic stem cell
differentiation: Emergence of a new era in biology and medicine.
Genes Dev. 19:1129–1155. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Mills RJ, Titmarsh DM, Koenig X, Parker
BL, Ryall JG, Quaife-Ryan GA, Voges HK, Hodson MP, Ferguson C,
Drowley L, et al: Functional screening in human cardiac organoids
reveals a metabolic mechanism for cardiomyocyte cell cycle arrest.
Proc Natl Acad Sci USA. 114:E8372–E8381. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Mills RJ, Parker BL, Quaife-Ryan GA, Voges
HK, Needham EJ, Bornot A, Ding M, Andersson H, Polla M, Elliott DA,
et al: Drug screening in human PSC-cardiac organoids identifies
pro-proliferative compounds acting via the mevalonate pathway. Cell
Stem Cell. 24:895–907.e6. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Aronson JK and Ferner RE: Biomarkers-A
general review. Curr Protoc Pharmacol. Mar 17–2017.(Epub ahead of
print). View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Imbard A, Benoist JF and Blom HJ: Neural
tube defects, folic acid and methylation. Int J Environ Res Public
Health. 10:4352–4389. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Movendane Y, Sipalo MG and Chan LCZ:
Advances in folic acid biosensors and their significance in
maternal, perinatal, and paediatric preventive medicine. Biosensors
(Basel). 13:9122023. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Ma Y, Zhang C, Gao XB, Luo HY, Chen Y, Li
HH, Ma X and Lu CL: Folic acid protects against arsenic-mediated
embryo toxicity by up-regulating the expression of Dvr1. Sci Rep.
5:160932015. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Zhao G, He F, Wu C, Li P, Li N, Deng J,
Zhu G, Ren W and Peng Y: Betaine in inflammation: Mechanistic
aspects and applications. Front Immunol. 9:10702018. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Hoffmann L, Brauers G, Gehrmann T,
Häussinger D, Mayatepek E, Schliess F and Schwahn BC: Osmotic
regulation of hepatic betaine metabolism. Am J Physiol Gastrointest
Liver Physiol. 304:G835–G846. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Barak AJ, Beckenhauer HC and Tuma DJ:
Betaine, ethanol, and the liver: A review. Alcohol. 13:395–398.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Chen X, Luo J, Yang L, Guo Y, Fan Y, Liu
J, Sun J, Zhang Y, Jiang Q, Chen T and Xi Q: miR-143-mediated
responses to betaine supplement repress lipogenesis and hepatic
gluconeogenesis by targeting MAT1a and MAPK11. J Agric Food Chem.
70:7981–7992. 2022. View Article : Google Scholar : PubMed/NCBI
|