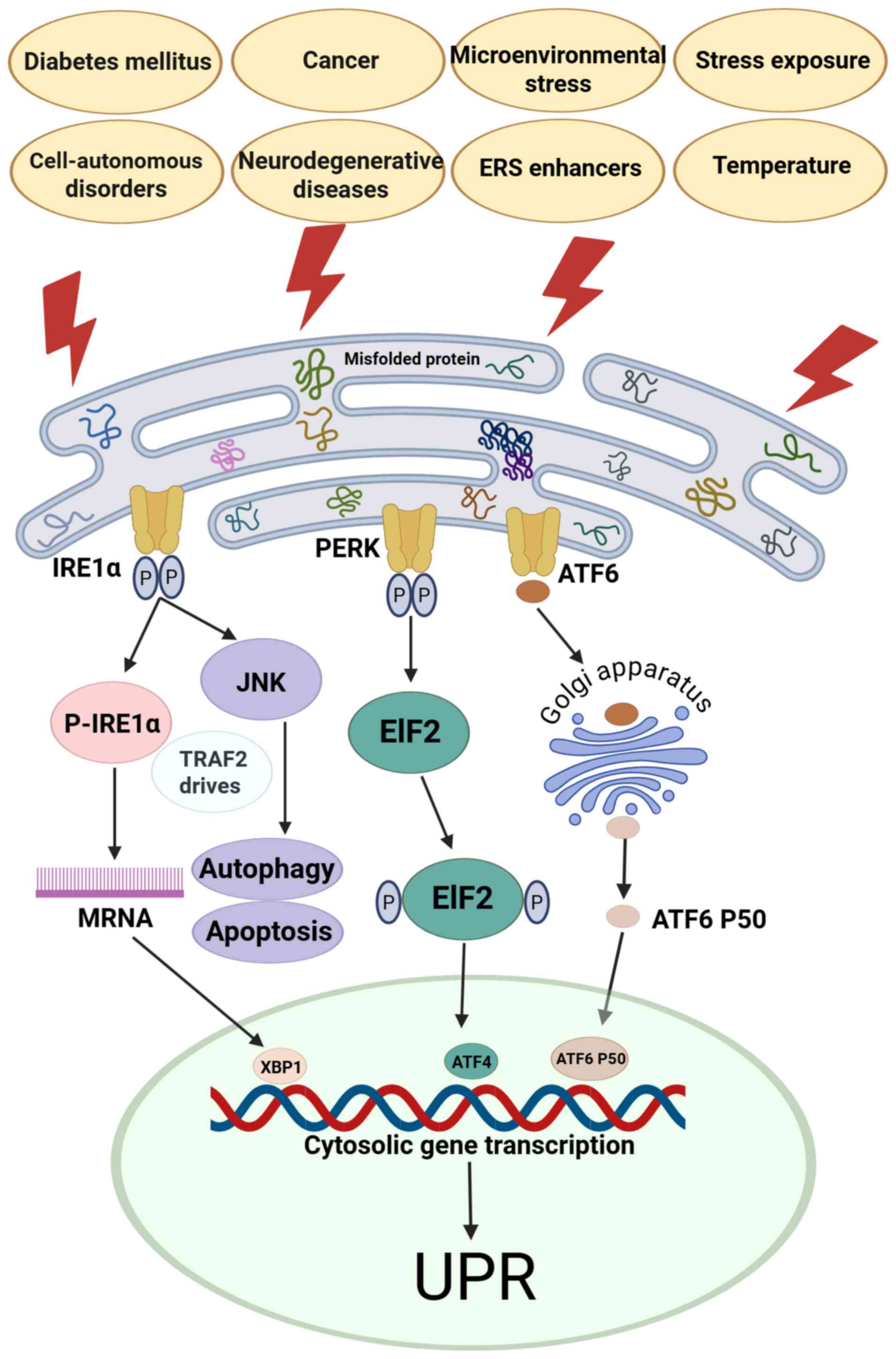
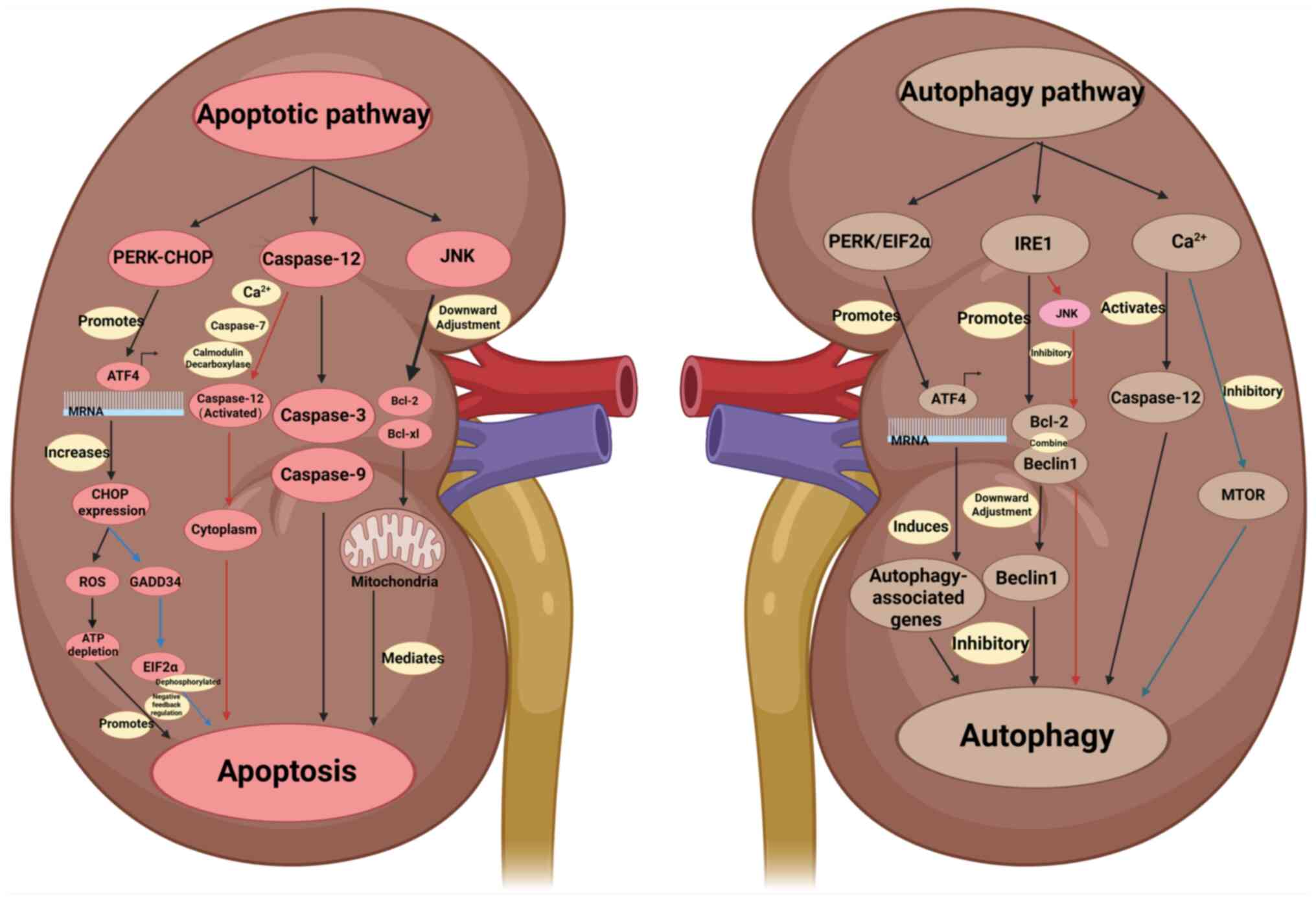
|
1
|
Zhang L, Long J, Jiang W, Shi Y, He X,
Zhou Z, Li Y, Yeung RO, Wang J, Matsushita K, et al: Trends in
chronic kidney disease in China. N Engl J Med. 375:905–906. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cybulsky AV: Endoplasmic reticulum stress,
the unfolded protein response and autophagy in kidney diseases. Nat
Rev Nephrol. 13:681–696. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
American Diabetes Association: 11
Microvascular complications and foot care: Standards of medical
care in diabetes-2020. Diabetes Care. 43 (Suppl 1):S135–S151. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pena MJ, Mischak H and Heerspink HJ:
Proteomics for prediction of disease progression and response to
therapy in diabetic kidney disease. Diabetologia. 59:1819–1831.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bustamante P, Tsering T, Coblentz J,
Mastromonaco C, Abdouh M, Fonseca C, Proença RP, Blanchard N, Dugé
CL, Andujar RAS, et al: Circulating tumor DNA tracking through
driver mutations as a liquid biopsy-based biomarker for uveal
melanoma. J Exp Clin Cancer Res. 40:1962021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tang M, Sun J and Cai Z: PCK2 inhibits
lung adenocarcinoma tumor cell immune escape through oxidative
stress-induced senescence as a potential therapeutic target. J
Thorac Dis. 15:2601–2615. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Almanza A, Carlesso A, Chintha C,
Creedican S, Doultsinos D, Leuzzi B, Luís A, McCarthy N,
Montibeller L, More S, et al: Endoplasmic reticulum stress
signalling - from basic mechanisms to clinical applications. FEBS
J. 286:241–278. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tilija Pun N, Lee N, Song SH and Jeong CH:
pitavastatin induces cancer cell apoptosis by blocking autophagy
flux. Front Pharmacol. 13:8545062022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Çiftçi YC, Yurtsever Y and Akgül B: Long
non-coding RNA-mediated modulation of endoplasmic reticulum stress
under pathological conditions. J Cell Mol Med. 28:e185612024.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kober L, Zehe C and Bode J: Development of
a novel ER stress based selection system for the isolation of
highly productive clones. Biotechnol Bioeng. 109:2599–2611. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hotamisligil GS: Endoplasmic reticulum
stress and the inflammatory basis of metabolic disease. Cell.
140:900–917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shahzad K, Ghosh S, Mathew A and Isermann
B: Methods to detect endoplasmic reticulum stress and apoptosis in
diabetic nephropathy. Methods Mol Biol. 2067:153–173. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hetz C and Papa FR: The unfolded protein
response and cell fate control. Mol Cell. 69:169–181. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guzmán Mendoza NA, Homma K, Osada H, Toda
E, Ban N, Nagai N, Negishi K, Tsubota K and Ozawa Y:
Neuroprotective effect of 4-phenylbutyric acid against photo-stress
in the Retina. Antioxidants (Basel). 10:11472021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen YY, Peng XF, Liu GY, Liu JS, Sun L,
Liu H, Xiao L and He LY: Protein arginine methyltranferase-1
induces ER stress and epithe-lial-mesenchymal transition in renal
tubular epithelial cells and contributes to diabetic nephropathy.
Biochim Biophys Acta Mol Basis Dis. 1865:2563–2575. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu H and Sun HL: LncRNA TCF7 triggered
endoplasmic reticulum stress through a sponge action with miR-200c
in patients with diabetic nephropathy. Eur Rev Med Pharmacol Sci.
23:5912–5922. 2019.PubMed/NCBI
|
|
17
|
Chen N, Song S, Yang Z, Wu M, Mu L, Zhou T
and Shi Y: ChREBP deficiency alleviates apoptosis by inhibiting
TXNIP/oxidative stress in diabetic nephropathy. J Diabetes
Complications. 35:1080502021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tervaert TW, Mooyaart AL, Amann K, Cohen
AH, Cook HT, Drachenberg CB, Ferrario F, Fogo AB, Haas M, de Heer
E, et al: Pathologic classification of diabetic nephropathy. J Am
Soc Nephrol. 21:556–563. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Oshima M, Shimizu M, Yamanouchi M, Toyama
T, Hara A, Furuichi K and Wada T: Trajectories of kidney function
in diabetes: A clinicopathological update. Nat Rev Nephrol.
17:740–750. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Garg P: A review of podocyte biology. Am J
Nephrol. 47 (Suppl 1):S3–S13. 2018. View Article : Google Scholar
|
|
21
|
O'Toole JF: Renal manifestations of
genetic mitochondrial disease. Int J Nephrol Renovasc Dis. 7:57–67.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nath KA: Tubulointerstitial changes as a
major determinant in the progression of renal damage. Am J Kidney
Dis. 20:1–17. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tang J, Yao D, Yan H, Chen X, Wang L and
Zhan H: The role of MicroRNAs in the pathogenesis of diabetic
nephropathy. Int J Endocrinol. 2019:87190602019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bazzi C, Bakoush O and Gesualdo L:
Proteinuria: From molecular to clinical applications in
glomerulonephritis. Int J Nephrol. 2012:4249682012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Edirs S, Jiang L, Xin X and Aisa HA: Kursi
Wufarikun Ziyabit improves the physiological changes by regulating
endoplasmic reticulum stress in the type 2 diabetes db/db mice.
Evid Based Complement Alternat Med. 2021:21001282021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Guo H, Cao A, Chu S, Wang Y, Zang Y, Mao
X, Wang H, Wang Y, Liu C, Zhang X and Peng W: Astragaloside IV
attenuates podocyte apoptosis mediated by endoplasmic reticulum
stress through upregulating sarco/endoplasmic reticulum Ca2+-ATPase
2 expression in diabetic nephropathy. Front Pharmacol. 7:5002016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang D, Wang YS, Zhao HM, Lu P, Li M, Li
W, Cui HT, Zhang ZY and Lv SQ: Plantamajoside improves type 2
diabetes mellitus pancreatic β-cell damage by inhibiting
endoplasmic reticulum stress through Dnajc1 up-regulation. World J
Diabetes. 16:990532025. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang HX, Yuan J and Li RS: Thalidomide
mitigates apoptosis via endoplasmic reticulum stress in diabetic
nephropathy. Endocr Metab Immune Disord Drug Targets. 22:787–794.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Van Krieken R, Mehta N, Wang T, Zheng M,
Li R, Gao B, Ayaub E, Ask K, Paton JC, Paton AW, et al: Cell
surface expression of 78-kDa glucose-regulated protein (GRP78)
mediates diabetic nephropathy. J Biol Chem. 294:7755–7768. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang MZ, Wang Y, Paueksakon P and Harris
RC: Epidermal growth factor receptor inhibition slows progression
of diabetic nephropathy in association with a decrease in
endoplasmic reticulum stress and an increase in autophagy.
Diabetes. 63:2063–2072. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen J, Hou XF, Wang G, Zhong QX, Liu Y,
Qiu HH, Yang N, Gu JF, Wang CF, Zhang L, et al: Terpene glycoside
component from Moutan Cortex ameliorates diabetic nephropathy by
regulating endoplasmic reticulum stress related inflammatory
responses. J Ethnopharmacol. 193:433–444. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yao F, Li Z, Ehara T, Yang L, Wang D, Feng
L, Zhang Y, Wang K, Shi Y, Duan H and Zhang L: Fatty acid-binding
protein 4 mediates apoptosis via endoplasmic reticulum stress in
mesangial cells of diabetic nephropathy. Mol Cell Endocrinol.
411:232–242. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ge J, Miao JJ, Sun XY and Yu JY: Huangkui
capsule, an extract from Abelmoschus manihot (L.) medic, improves
diabetic nephropathy via activating peroxisome
proliferator-activated receptor (PPAR)-α/γ and attenuating
endoplasmic reticulum stress in rats. J Ethnopharmacol.
189:238–249. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Park MJ, Han HJ and Kim DI:
Lipotoxicity-Induced PRMT1 exacerbates mesangial cell apoptosis via
endoplasmic reticulum stress. Int J Mol Sci. 18:14212017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Khoi CS, Xiao CQ, Hung KY, Lin TY and
Chiang CK: Oxidative stress-induced growth inhibitor (OSGIN1), a
Target of X-Box-Binding Protein 1, protects palmitic acid-induced
vascular lipotoxicity through maintaining autophagy. Biomedicines.
10:9922022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jeon HY, Moon CH, Kim EB, Sayyed ND, Lee
AJ and Ha KS: Simultaneous attenuation of hyperglycemic
memory-induced retinal, pulmonary, and glomerular dysfunctions by
proinsulin C-peptide in diabetes. BMC Med. 21:492023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jiao Y, Liu X, Shi J, An J, Yu T, Zou G,
Li W and Zhuo L: Unraveling the interplay of ferroptosis and immune
dysregulation in diabetic kidney disease: A comprehensive molecular
analysis. Diabetol Metab Syndr. 16:862024. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu F, Yang Z, Li J, Wu T, Li X, Zhao L,
Wang W, Yu W, Zhang G and Xu Y: Targeting programmed cell death in
diabetic kidney disease: from molecular mechanisms to
pharmacotherapy. Mol Med. 30:2652024. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cao Y, Hao Y, Li H, Liu Q, Gao F, Liu W
and Duan H: Role of endoplasmic reticulum stress in apoptosis of
differentiated mouse podocytes induced by high glucose. Int J Mol
Med. 33:809–816. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tian N, Gao Y, Wang X, Wu X, Zou D, Zhu Z,
Han Z, Wang T and Shi Y: Emodin mitigates podocytes apoptosis
induced by endoplasmic reticulum stress through the inhibition of
the PERK pathway in diabetic nephropathy. Drug Des Devel Ther.
12:2195–2211. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Garner KL, Betin VMS, Pinto V, Graham M,
Abgueguen E, Barnes M, Bedford DC, McArdle CA and Coward RJM:
Enhanced insulin receptor, but not PI3K,signalling protects
podocytes from ER stress. Sci Rep. 8:39022018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang Y, Gao X, Chen S, Zhao M, Chen J,
Liu R, Cheng S, Qi M, Wang S and Liu W: Cyclin-dependent kinase 5
contributes to endoplasmic reticulum stress induced podocyte
apoptosis via promoting MEKK1 phosphorylation at Ser280 in diabetic
nephropathy. Cell Signal. 31:31–40. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cybulsky AV: The intersecting roles of
endoplasmic reticulum stress, ubiquitin-proteasome system, and
autophagy in the pathogenesis of proteinuric kidney disease. Kidney
Int. 84:25–33. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Dong Z, Liu N and Sun M: The distinct
biological role of JAML positions it as a promising target for
treating human cancers and a range of other diseases. Front
Immunol. 16:15584882025. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Feng Z, Tang L, Wu L, Cui S, Hong Q, Cai
G, Wu D, Fu B, Wei R and Chen X: Na+/H+ exchanger-1 reduces
podocyte injury caused by endoplasmic reticulum stress via
autophagy activation. Lab Invest. 94:439–454. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Fang L, Li X, Luo Y, He W, Dai C and Yang
J: Autophagy inhibition induces podocyte apoptosis by activating
the proapoptotic pathway of endoplasmic reticulum stress. Exp Cell
Res. 322:290–301. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Guo H, Wang Y, Zhang X, Zang Y, Zhang Y,
Wang L, Wang H, Wang Y, Cao A and Peng W: Astragaloside IV protects
against podocyte injury via SERCA2-dependent ER stress reduction
and AMPKα-regulated autophagy induction in streptozotocin-induced
diabetic nephropathy. Sci Rep. 7:68522017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kato M: Intercellular transmission of
endoplasmic reticulum stress through gap junction targeted by
microRNAs as a keystep of diabetic kidney diseases? Ann Transl Med.
9:8272021. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Liang X, Duan N, Wang Y, Shu S, Xiang X,
Guo T, Yang L, Zhang S, Tang X and Zhang J: Advanced oxidation
protein products induce endothelial-to-mesenchymal transition in
human renal glomerular endothelial cellsthrough induction of
endoplasmic reticulum stress. J Diabetes Complications. 30:573–579.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ma X, Ma J, Leng T, Yuan Z, Hu T, Liu Q
and Shen T: Advances in oxidative stress in pathogenesis of
diabetic kidney disease and efficacy of TCM intervention. Ren Fail.
45:21465122023. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Cao Y, Chen Z, Hu J, Feng J, Zhu Z, Fan Y,
Lin Q and Ding G: Mfn2 regulates high glucose-induced MAMs
dysfunction and apoptosis in podocytes via PERK pathway. Front Cell
Dev Biol. 9:7692132021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Mega C, Teixeira-de-Lemos E, Fernandes R
and Reis F: Renoprotective effects of the dipeptidyl peptidase-4
inhibitor sitagliptin: A review in type 2 diabetes. J Diabetes Res.
2017:51642922017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Feng A, Yin R, Xu R, Zhang B and Yang L:
An update on renal tubular injury as related to glycolipid
metabolism in diabetic kidney disease. Front Pharmacol.
16:15590262025. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Bondue T, van den Heuvel L, Levtchenko E
and Brock R: The potential of RNA-based therapy for kidney
diseases. Pediatr Nephrol. 38:327–344. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhang J, Dong XJ, Ding MR, You CY, Lin X,
Wang Y, Wu MJ, Xu GF and Wang GD: Resveratrol decreases high
glucose-induced apoptosis in renal tubular cells via suppressing
endoplasmic reticulum stress. Mol Med Rep. 22:4367–4375.
2020.PubMed/NCBI
|
|
56
|
Zhang J, Cao P, Gui J, Wang X, Han J, Wang
Y and Wang G: Arc-tigenin ameliorates renal impairment and inhibits
endoplasmic reticulum stress in diabetic db/db mice. Life Sci.
223:194–201. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Han J, Pang X, Shi X, Zhang Y, Peng Z and
Xing Y: Ginkgo biloba extract EGB761 ameliorates the extracellular
matrix accumulation and mesenchymal transformation of renal tubules
in diabetic kidney disease by inhibiting endoplasmic reticulum
stress. Biomed Res Int. 2021:66572062021. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Huang KH, Guan SS, Lin WH, Wu CT, Sheu ML,
Chiang CK and Liu SH: Role of calbindin-D28k in diabetes-associated
advanced glycation end-products-induced renal proximal tubule cell
injury. Cells. 8:6602019. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Sun X, Sun Y, Lin S, Xu Y and Zhao D:
Histone deacetylase inhibitor valproic acid attenuates high
glucoseinduced endoplas-mic reticulum stress and apoptosis in
NRK52E cells. Mol Med Rep. 22:4041–4047. 2020.PubMed/NCBI
|
|
60
|
Shibusawa R, Yamada E, Okada S, Nakajima
Y, Bastie CC, Maeshima A. Kaira K and Yamada M: Dapagliflozin
rescues endoplasmic reticulum stress-mediated cell death. Sci Rep.
9:98872019. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Wu L, Wang Q, Guo F, Ma X, Wang J, Zhao Y,
Yan Y and Qin G: Involvement of miR-27a-3p in diabetic nephropathy
via affecting renal fibrosis, mitochondrial dysfunction, and
endoplasmic reticulum stress. J Cell Physiol. 236:1454–1468. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Fang L, Xie D, Wu X, Cao H, Su W and Yang
J: Involvement of endoplasmic reticulum stress in albuminuria
induced inflam-masome activation in renal proximal tubular cells.
PLoS One. 8:e723442013. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Kang JM, Lee HS, Kim J, Yang DH, Jeong HY,
Lee YH, Kim DJ, Park SH, Sung M, Kim J, et al: Beneficial effect of
Chloroquine and Amodiaquine on type 1 Diabetic Tubulopathy by
attenuating mitochondrial Nox4 and endoplasmic reticulum stress. J
Korean Med Sci. 35:e3052020. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Sun H, Yuan Y and Sun Z: Update on
mechanisms of renal tubule injury caused by advanced glycation end
products. Biomed Res Int. 2016:54751202019.PubMed/NCBI
|
|
65
|
Iwai T, Kume S, Chin-Kanasaki M, Kuwagata
S, Araki H, Takeda N, Sugaya T, Uzu T, Maegawa H and Araki SI:
Stearoyl-CoA Desaturase-1 protects cells against
lipotoxicity-mediated apoptosis in proximal tubular cells. Int J
Mol Sci. 17:18682016. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Liu J, Yang JR, Chen XM, Cai GY, Lin LR
and He YN: Impact of ER stress-regulated ATF4/p16 signaling on the
premature senescence of renal tubular epithelial cells in diabetic
nephropathy. Am J Physiol Cell Physiol. 308:C621–C630. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Barati MT, Powell DW, Kechavarzi BD,
Isaacs SM, Zheng S, Epstein PN, Cai L, Coventry S, Rane MJ and
Klein JB: Differential expression of endoplasmic reticulum
stress-response proteins in different renal tubule subtypes of
OVE26 diabetic mice. Cell Stress Chaperones. 21:155–166. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Liu Y, Chen DQ, Han JX, Zhao TT and Li SJ:
A review of traditional Chinese medicine in treating renal
interstitial fibrosis via endoplasmic reticulum stress-mediated
apoptosis. Biomed Res Int. 2021:66677912021. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Wang J, Lu L, Chen S, Xie J, Lu S, Zhou Y
and Jiang H: PERK overexpression-mediated Nrf2/HO-1 pathway
alleviates hypoxia/reoxygenation-induced injury in neonatal murine
cardiomyocytes via improving endoplasmic reticulum stress. Biomed
Res Int. 2020:64580602020. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Chen Z, Feng H, Peng C, Zhang Z, Yuan Q,
Gao H, Tang S and Xie C: Renoprotective effects of tanshinone IIA:
A literature review. Molecules. 28:19902023. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Nakka VP, Prakash-Babu P and Vemuganti R:
Crosstalk between endoplasmic reticulum stress, oxidative stress,
and autophagy: Potential therapeutic targets for acute CNS
injuries. Mol Neurobiol. 53:532–544. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Qiu M, Li S, Jin L, Feng P, Kong Y, Zhao
X, Lin Y, Xu Y, Li C and Wang W: Combination of chymostatin and
aliskiren attenuates ER stress induced by lipid overload in kidney
tubular cells. Lipids Health Dis. 17:1832018. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Chai H, Yao S, Gao Y, Hu Q and Su W:
Developments in the connection between epithelial-mesenchymal
transition and endoplasmic reticulum stress (Review). Int J Mol
Med. 56:1022025. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Wang WW, Liu YL, Wang MZ, Li H, Liu BH, Tu
Y, Yuan CC, Fang QJ, Chen JX, Wang J, et al: Inhibition of renal
tubular epithelial mesenchymal transition and endoplasmic reticulum
stress-induced apoptosis with shenkang injection attenuates
diabetic tubulopathy. Front Pharmacol. 12:6627062021. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Wang W, Ke B, Wang C, Xiong X, Feng X and
Yan H: Targeting ion channel networks in diabetic kidney disease:
from molecular crosstalk to precision therapeutics and clinical
innovation. Front Med (Lausanne). 12:16077012025. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Jo HJ, Yang JW, Park JH, Choi ES, Lim CS,
Lee S and Han CY: Endoplasmic reticulum stress increases DUSP5
expression via PERK-CHOP pathway, leading to hepatocyte death. Int
J Mol Sci. 20:43692019. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Hu H, Tian M, Ding C and Yu S: The C/EBP
homologous protein (CHOP) transcription factor functions in
endoplasmic reticulum Stress-Induced apoptosis and microbial
infection. Front Immunol. 9:30832019. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Jin R, Zhao A, Han S, Zhang D, Sun H, Li
M, Su D and Liang X: The interaction of S100A16 and GRP78 actives
endoplasmic reticulum stress-mediated through the IRE1α/XBP1
pathway in renal tubulointerstitial fibrosis. Cell Death Dis.
12:9422021. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Fan Y, Zhang J, Xiao W, Lee K, Li Z, Wen
J, He L, Gui D, Xue R, Jian G, et al: Rtn1a-Mediated endoplasmic
reticulum stress in podocyte injury and diabetic nephropathy. Sci
Rep. 7:3232017. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Zhang GQ, Tao YK, Bai YP, Yan ST and Zhao
SP: Inhibitory effects of simvastatin on oxidized low-density
lipoprotein-induced endoplasmic reticulum stress and apoptosis in
vascular endothelial cells. Chin Med J (Engl). 131:950–955. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Ni L, Yang L and Lin Y: Recent progress of
endoplasmic reticulum stress in the mechanism of atherosclerosis.
Front Cardiovasc Med. 11:14134412024. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Li HY, Huang LF, Huang XR, Wu D, Chen XC,
Tang JX, An N, Liu HF and Yang C: Endoplasmic reticulum stress in
systemic lupus erythematosus and lupus nephritis: Potential
therapeutic target. J Immunol Res. 2023:76258172023. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Shu S, Wang H, Zhu J, Liu Z, Yang D, Wu W,
Cai J, Chen A, Tang C and Dong Z: Reciprocal regulation between ER
stress and autophagy in renal tubular fibrosis and apoptosis. Cell
Death Dis. 12:10162021. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Li D, Zhang J, Su X, Yang Y, Lai J, Wei X,
Chen H, Liu Y, Wang H and Sun L: Calpain1 inhibition enhances
autophagy-lysosomal pathway and ameliorates tubulointerstitial
fibrosis in Nephronophthisis. Mol Med. 31:1662025. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Laorodphun P, Cherngwelling R, Panya A and
Arjinajarn P: Curcumin protects rats against gentamicin-induced
nephrotoxicity by amelioration of oxidative stress, endoplasmic
reticulum stress and apoptosis. Pharm Biol. 60:491–500. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Ma N, Xu N, Yin D, Zheng P, Liu W, Wang G,
Hui Y, Han G, Yang C and Cheng X: Levels of circulating GRP78 and
CHOP in endoplasmic reticulum stress pathways in Chinese type 2
diabetic kidney disease patients. Medicine (Baltimore).
100:e268792021. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Zhao DM, Zhong R, Wang XT and Yan ZH:
Mitochondrial dysfunction in diabetic nephropathy: Insights and
therapeutic avenues from traditional Chinese medicine. Front
Endocrinol (Lausanne). 15:14294202024. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Yan DY and Xu B: The role of autophagy in
manganese-induced neurotoxicity. Front Neurosci. 14:5747502020.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Abo-Zaid OA, Moawed FS, Taha EF, Ahmed ESA
and Kawara RS: Melissa officinalis extract suppresses endoplasmic
reticulum stress-induced apoptosis in the brain of
hypothyroidism-induced rats exposed to γ-radiation. Cell Stress
Chaperones. 28:709–720. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Kong FJ, Ma LL, Guo JJ, Xu LH, Li Y and Qu
S: Endoplasmic reticulum stress/autophagy pathway is involved in
diabetes induced neuronal apoptosis and cognitive decline in mice.
Clin Sci (Lond). 132:111–125. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Tomicic MT, Meise R, Aasland D, Berte N,
Kitzinger R, Krämer OH, Kaina B and Christmann M: Apoptosis induced
by temozolomide and nimustine in glioblastoma cells is supported by
JNK/c-Jun-mediated induction of the BH3-only protein BIM.
Oncotarget. 6:33755–33768. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Gao Z, Liu G, Hu Z, Shi W, Chen B, Zou P
and Li X: Grape seed proanthocyanidins protect against
streptozotocin-induced diabetic nephropathy by attenuating
endoplasmic reticulum stress-induced apoptosis. Mol Med Rep.
18:1447–1454. 2018.PubMed/NCBI
|
|
93
|
Sun XY, Qin HJ, Zhang Z, Xu Y, Yang XC,
Zhao DM, Li XN and Sun LK: Valproate attenuates diabetic
nephropathy through inhibition of endoplasmic reticulum
stress-induced apoptosis. Mol Med Rep. 13:661–668. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Suzuki Y, Inoue T, Murai M,
Suzuki-Karasaki M, Ochiai T and Ra C: Depolarization potentiates
TRAIL-induced apoptosis in human melanoma cells: role for
ATP-sensitive K+ channels and endoplasmic reticulum stress. Int J
Oncol. 41:465–475. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Hibi M, Lin A, Smeal T, Minden A and Karin
M: Identification of an oncoprotein- and UV-responsive protein
kinase that binds and potentiates the c-Jun activation domain.
Genes Dev. 7:2135–2148. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Shang H, Cao Z, Zhao J, Guan J, Liu J,
Peng J, Chen Y, Joseph Sferra T, Sankararaman S and Lin J: Babao
Dan induces gastric cancer cell apoptosis via regulating MAPK and
NF-κB signaling pathways. J Int Med Res. 47:5106–5119. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Liu Y, Long Y, Xing Z and Zhang D: C-Jun
recruits the NSL complex to regulate its target gene expression by
modulating H4K16 acetylation and promoting the release of the
repressive NuRD complex. Oncotarget. 6:14497–14506. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Vilas-Boas EA, Almeida DC, Roma LP, Ortis
F and Carpinelli AR: Lipotoxicity and β-Cell failure in type 2
diabetes: Oxidative stress linked to NADPH oxidase and ER stress.
Cells. 10:33282021. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Chhabra R, Dubey R and Saini N: Gene
expression profiling indicate role of ER stress in miR-23a ~ 27a ~
24 - 2 cluster induced apoptosis in HEK293T cells. RNA Biol.
8:648–664. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Rodríguez-Hernández MA, González R, de la
Rosa ÁJ, Gallego P, Ordóñez R, Navarro-Villarán E, Contreras L,
Rodríguez-Arribas M, González-Gallego J, Álamo-Martínez JM, et al:
Molecular characterisation of autophagic and apoptotic signalling
induced by sorafenib in liver cancer cells. J Cell Physiol.
234:692–708. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Zeng T, Peng L, Chao H, Xi H, Fu B, Wang
Y, Zhu Z and Wang G: IRE1α-TRAF2-ASK1 complex-mediated endoplasmic
reticulum stress and mitochondrial dysfunction contribute to
CXC195-induced apoptosis in human bladder carcinoma T24 cells.
Biochem Biophys Res Commun. 460:530–536. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Zhang J, Liang Y, Lin Y, Liu Y and YouYou
Yin W: IRE1α-TRAF2-ASK1 pathway is involved in CSTMP-induced
apoptosis and ER stress in human non-small cell lung cancer A549
cells. Biomed Pharmacother. 82:281–289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Ha J, Kang E, Seo J and Cho S:
Phosphorylation dynamics of JNK signaling: Effects of
dual-specificity phosphatases (DUSPs) on the JNK pathway. Int J Mol
Sci. 20:61572019. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Li L, Chen J, Lin L, Pan G, Zhang S, Chen
H, Zhang M, Xuan Y, Wang Y and You Z: Quzhou Fructus Aurantii
Extract suppresses inflammation via regulation of MAPK, NF-κB, and
AMPK signaling pathway. Sci Rep. 10:15932020. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Muraleva NA, Tikhonov DI, Zhdankina AA,
Plotnikov MB, Khlebnikov AI, Logvinov SV and Kolosova NG:
Alterations of JNK signaling pathway activity in the rat retina:
Effects of age, age-related macular degeneration-like pathology,
and a JNK inhibitor (IQ-1S). Cells. 14:8962025. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Bain J, Later L, Elliot M, Shpiro N,
Hastie CJ, Mclauchlan H, Klevernic I, Arthur JS, Alessi DR and
Cohen P: The selectivity of protein kinase inhibitors: A further
update. Biochem J. 408:297–315. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Bennett BL, Sasaki DT, Murray BW, O'Leary
EC, Sakata ST, Xu W, Leisten JC, Motiwala A, Pierce S, Satoh Y, et
al: SP600125, an anthrapyrazolone inhibitor of Jun N-Terminal
kinase. Proc Natl Acad Sci USA. 98:136812021. View Article : Google Scholar
|
|
108
|
Inesta-Vaquera FA, Campbell DG, Arthur JS
and Cuenda A: ERK5 pathway regulates the phosphorylation of tumour
suppressor hDlg during mitosis. Biochem Biophy Res Commun.
399:84–90. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Zhang T, Inesta-Vaquera F, Niepel M, Zhang
J, Ficarro SB, Machleidt T, Xie T, Marto JA, Kim N, Sim T, et al:
Discovery of potent and selective covalent inhibitors of JNK. Chem
Biol. 19:140–154. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Lupachyk S, Watcho P, Stavniichuk R,
Shevalye H and Obrosova IG: Endoplasmic reticulum stress plays a
key role in the pathogenesis of diabetic peripheral neuropathy.
Diabetes. 62:944–952. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Mei Y, Thompson MD, Cohen RA and Tong X:
Endoplasmic reticulum stress and related pathological processes. J
Pharmacol Biomed Anal. 1:10001072013.PubMed/NCBI
|
|
112
|
Zhang SX, Sanders E, Fliesler SJ and Wang
JJ: Endoplasmic reticulum stress and the unfolded protein responses
in retinal degeneration. Exp Eye Res. 125:30–40. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Faria JA, Reis PA, Reis MT, Rosado GL,
Pinheiro GL, Mendes GC and Fontes EP: The NAC domain-containing
protein, GmNAC6, is a downstream component of the ER stress- and
osmotic stress-induced NRP-mediated cell-death signaling pathway.
BMC Plant Biol. 11:1292011. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Tsai TC, Lai KH, Su JH, Wu YJ and Sheu JH:
7-Acetylsinumaximol B induces apoptosis and autophagy in human
gastric carcinoma cells through Mitochondria Dysfunction and
activation of the PERK/eIF2α/ATF4/CHOP signalling pathway. Mar
Drugs. 16:1042018. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Narasimhan M and Rajasekaran NS: Reductive
potential-a savior turns stressor in protein aggregation
cardiomyopathy. Biochim Biophys Acta. 1852:53–60. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Wu K, Li B, Zhang X, Fang Y, Zeng S, Hu W,
Liu X, Liu X, Lu Z, Li X, et al: Correction for Wu et al.,
‘CSFV restricts necroptosis to sustain infection by inducing
autophagy/mitophagy-targeted degradation of RIPK3’. Microbiol
Spectr. 13:e03188242025. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Koniari I, Velissaris D, Kounis NG, Koufou
E, Artopoulou E, de Gregorio C, Mplani V, Paraskevas T, Tsigkas G,
Hung MY, et al: Anti-diabetic therapy, heart failure and oxidative
stress: An update. J Clin Med. 11:46602022. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Gonen N, Sabath N, Burge CB and Shalgi R:
Widespread PERK-dependent repression of ER targets in response to
ER stress. Sci Rep. 9:43302019. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Nita M and Grzybowski A: Antioxidative
role of heterophagy, autophagy, and mitophagy in the retina and
their association with the age-related macular degeneration (AMD)
etiopathogenesis. Antioxidants (Basel). 12:13682023. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Bae D, Jones RE, Piscopo KM, Tyagi M,
Shepherd JD and Hollien J: Regulation of Blos1 by IRE1 prevents the
accumulation of Huntingtin protein aggregates. Mol Biol Cell.
33:ar1252022. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Jin B, Ishikawa T, Taniguchi M, Ninagawa
S, Okada T, Kagaya S and Mori K: Development of a rapid in vivo
assay to evaluate the efficacy of IRE1-specific inhibitors of the
unfolded protein response using medaka fish. Cell Struct Funct.
45:23–31. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Niu F, Liu W, Ren Y, Tian Y, Shi W, Li M,
Li Y, Xiong Y and Qian L: β-cell neogenesis: A rising star to
rescue diabetes mellitus. J Adv Res. 62:71–89. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Flintoaca Alexandru PR, Chiritoiu GN,
Lixandru D, Zurac S, Ionescu-Targoviste C and Petrescu SM: EDEM1
regulates the insulin mRNA level by inhibiting the endoplasmic
reticulum stress-induced IRE1/JNK/c-Jun pathway. iScience.
26:1079562023. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Flores-Santibáñez F, Medel B, Bernales JI
and Osorio F: Understanding the role of the unfolded protein
response sensor IRE1 in the biology of antigen presenting cells.
Cells. 8:15632019. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Arunagiri A, Haataja L, Cunningham CN,
Shrestha N, Tsai B, Qi L, Liu M and Arvan P: Misfolded proinsulin
in the endoplasmic reticulum during development of beta cell
failure in diabetes. Ann NY Acad Sci. 1418:5–19. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Figueroa-Juárez E, Noriega LG,
Pérez-Monter C, Alemán G, Hernández-Pando R, Correa-Rotter R,
Ramírez V, Tovar AR, Torre-Villalvazo I and Tovar-Palacio C: The
role of the unfolded protein response on renal lipogenesis in
C57BL/6 mice. Biomolecules. 11:732021. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Wu L, He S, Ye W, Shen J, Zhao K, Zhang Y,
Zhang R, Wei J, Cao S, Chen K, et al: Surf4 facilitates
reprogramming by activating the cellular response to endoplasmic
reticulum stress. Cell Prolif. 54:e131332021. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Fu YL, Han DY, Wang YJ, Di XJ, Yu HB and
Mu TW: Remodeling the endoplasmic reticulum proteostasis network
restores proteostasis of pathogenic GABAA receptors. PLoS One.
13:e02079482018. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Wang D, Qu S, Zhang Z, Tan L, Chen X,
Zhong HJ and Chong CM: Strategies targeting endoplasmic reticulum
stress to improve Parkinson's disease. Front Pharmacol.
14:12888942023. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Pérez-Martí A, Ramakrishnan S, Li J,
Dugourd A, Molenaar MR, De La Motte LR, Grand K, Mansouri A,
Parisot M, Lienkamp SS, et al: Reducing lipid bilayer stress by
monounsaturated fatty acids protects renal proximal tubules in
diabetes. Elife. 11:e743912022. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Hollien J and Weissman JS: Decay of
endoplasmic reticulum-localized mRNAs during the unfolded protein
response. Science. 313:104–107. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Huang M, Cai S and Su J: The pathogenesis
of sepsis and potential therapeutic targets. Int J Mol Sci.
20:53762019. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Wise R, Duhachek-Muggy S, Qi Y, Zolkiewski
M and Zolkiewska A: Protein disulfide isomerases in the endoplasmic
reticulum promote anchorage-independent growth of breast cancer
cells. Breast Cancer Res Treat. 157:241–252. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Schäffer DE, Iyer LM, Burroughs AM and
Aravind L: Functional innovation in the evolution of the
calcium-dependent system of the eukaryotic endoplasmic reticulum.
Front Genet. 11:342020. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
AlBashtawi J, Al-Jaber H, Ahmed S and
Al-Mansoori L: Impact of obesity-related endoplasmic reticulum
stress on cancer and associated molecular targets. Biomedicines.
12:7932024. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Park SJ, Li C and Chen YM: Endoplasmic
reticulum calcium homeostasis in kidney disease: Pathogenesis and
therapeutic targets. Am J Pathol. 191:256–265. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Krebs J, Agellon LB and Michalak M: Ca(2+)
homeostasis and endoplasmic reticulum (ER) stress: an integrated
view of calcium signaling. Biochem. Biophys Res Commun.
460:114–121. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Lim W, Yang C, Jeong M, Bazer FW and Song
G: Coumestrol induces mitochondrial dysfunction by stimulating ROS
production and calcium ion influx into mitochondria in human
placental choriocarcinoma cells. Mol Hum Reprod. 23:786–802. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Bahar E, Kim H and Yoon H: ER
stress-mediated signaling: Action potential and ca(2+) as key
players. Int J Mol Sci. 17:15582016. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Zheng Q, Chen Y, Chen D, Zhao H, Feng Y,
Meng Q, Zhao Y and Zhang H: Calcium transients on the ER surface
trigger liquid-liquid phase separation of FIP200 to specify
autophagosome initiation sites. Cell. 185:4082–4098.e22. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Høyer-Hansen M, Bastholm L, Szyniarowski
P, Campanella M, Szabadkai G, Farkas T, Bianchi K, Fehrenbacher N,
Elling F, Rizzuto R, et al: Control of macroautophagy by calcium,
calmodulin-dependent kinase kinase-beta, and Bcl-2. Mol Cell.
25:193–205. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
La Rovere RM, Roest G, Bultynck G and
Parys JB: Intracellular ca(2+) signalling and ca(2+) microdomains
in the control of cell survival, apoptosis and autophagy. Cell
Calcium. 60:74–87. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Sakaki K, Wu J and Kaufman RJ: Protein
kinase Ctheta is required for autophagy in response to stress in
the endoplasmic reticulum. J Biol Chem. 283:15370–15380. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Levin-Salomon V, Bialik S and Kimchi A:
DAP-kinase and autophagy. Apoptosis. 19:346–356. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Zhong Y, Jin C, Han J, Zhu J, Liu Q, Sun
D, Xia X, Zhang Y and Peng X: Diosgenin protects against kidney
Injury and mitochondrial apoptosis Induced by 3-MCPD through the
regulation of ER stress, Ca(2+) homeostasis, and Bcl2 expression.
Mol Nutr Food Res. 65:e20012022021. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Pu Q, Yu L, Wang X, Yan H, Xie Y, Jiang Y
and Yang Z: Immunomodulatory effect of traditional Chinese medicine
combined with systemic therapy on patients with liver cancer: A
systemic review and network meta-analysis. J Cancer. 13:3280–3296.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Wan CP, Gao LX, Hou LF, Yang XQ, He PL,
Yang YF, Tang W, Yue JM, Li J and Zuo JP: Astragaloside II triggers
T cell activation through regulation of CD45 protein tyrosine
phosphatase activity. Acta Pharmacol Sin. 34:522–530. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Shen L, Luo H, Fan L, Tian X, Tang A, Wu
X, Dong K and Su Z: Potential immunoregulatory mechanism of plant
saponins: A review. Molecules. 29:1132023. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Nalbantsoy A, Nesil T, Yılmaz-Dilsiz O,
Aksu G, Khan S and Bedir E: Evaluation of the immunomodulatory
properties in mice and in vitro anti-inflammatory activity of
cycloartane type saponins from Astragalus species. J
Ethnopharmacol. 139:574–581. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Ju Y, Su Y, Chen Q, Ma K, Ji T, Wang Z and
Li W and Li W: Protective effects of astragaloside IV on
endoplasmic reticulum stress-induced renal tubular epithelial cells
apoptosis in type 2 diabetic nephropathy rats. Biomed Pharmacother.
109:84–92. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Chen Y, Gui D, Chen J, He D, Luo Y and
Wang N: Down-regulation of PERK-ATF4-CHOP pathway by astragaloside
IV is associated with the inhibition of endoplasmic reticulum
stress-induced podocyte apoptosis in diabetic rats. Cell Physiol
Biochem. 33:1975–1987. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Wang ZS, Xiong F, Xie XH, Chen D, Pan JH
and Cheng L: Astragaloside IV attenuates proteinuria in
streptozotocin-induced diabetic nephropathy via the inhibition of
endoplasmic reticulum stress. BMC Nephrol. 16:442015. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Li M, Wang W, Xue J, Gu Y and Lin S:
Meta-analysis of the clinical value of Astragalus membranaceus in
diabetic nephropathy. J Ethnopharmacol. 133:412–419. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Xue HZ, Chen Y, Wang SD, Yang YM, Cai LQ,
Zhao JX, Huang WJ and Xiao YH: Radix astragali and its
representative extracts for diabetic nephropathy: Efficacy and
molecular mechanism. J Diabetes Res. 2024:52161132024. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Li Y and Wang J: Possible mechanism for
the protective effect of active ingredients of astragalus
membranaceus on diabetes nephropathy. J Asian Nat Prod Res.
26:1276–1284. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Zhang L, Shergis JL, Yang L, Zhang AL, Guo
X, Zhang L, Zhou S, Zeng L, Mao W and Xue CC: Astragalus
membranaceus (Huang Qi) as adjunctive therapy for diabetic kidney
disease: an updated systematic review and meta-analysis. J
Ethnopharmacol. 239:1119212019. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Guo JC, Pan HC, Yeh BY, Lu YC, Chen JL,
Yang CW, Chen YC, Lin YH and Chen HY: Associations between using
Chinese herbal medicine and long-term outcome among pre-dialysis
diabetic nephropathy patients: A retrospective population-based
cohort study. Front Pharmacol. 12:6165222021. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Cao JL, Liang LY, Liu YH, Xu ZM, Wang X,
Wei WX, Wan HJ, Lyu XH, Li WX, Zhang YX, et al: Medication rules of
Astragali Radix in ancient Chinese medical books based on
‘disease-medicine-dose’ pattern. Zhongguo Zhong Yao Za Zhi.
50:798–811. 2025.(In Chinese). PubMed/NCBI
|
|
159
|
Hassanpour Fard M, Naseh G, Lotfi N,
Hosseini SM and Hosseini M: Effects of aqueous extract of turnip
leaf (Brassica rapa) in alloxan-induced diabetic rats. Avicenna J
Phytomed. 5:148–156. 2015.PubMed/NCBI
|
|
160
|
Santos M, Fortunato RH and Spotorno VG:
Analysis of flavonoid glycosides with potential medicinal
properties on Bauhinia uruguayensis and Bauhinia forficate
subspecies pruinosa. Nat Prod Res. 33:2574–2578. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Chhatre S, Nesari T, Somani G, Kanchan D
and Sathaye S: Phytopharmacological overview of Tribulus
terrestris. Pharmacogn Rev. 8:45–51. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Abdou HM and Abd Elkader HAE: The
potential therapeutic effects of Trifolium alexandrinum extract,
hesperetin and quercetin against diabetic nephropathy via
attenuation of oxidative stress, inflammation, GSK-3β and apoptosis
in male rats. Chem Biol Interact. 352:1097812022. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Alshehri AS: Kaempferol attenuates
diabetic nephropathy in streptozotocin-induced diabetic rats by a
hypoglycaemic effect and concomitant activation of the
Nrf-2/Ho-1/antioxidants axis. Arch Physiol Biochem. 129:984–997.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Luo W, Chen X, Ye L, Chen X, Jia W, Zhao
Y, Samorodov AV, Zhang Y, Hu X, Zhuang F, et al: Kaempferol
attenuates streptozotocin-induced diabetic nephropathy by
downregulating TRAF6 expression: The role of TRAF6 in diabetic
nephropathy. J Ethnopharmacol. 268:1135532021. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Li K, Wang YJ, Chen C, Wang XJ and Li W:
Targeting pyroptosis: A novel strategy of ginseng for the treatment
of diabetes and its chronic complications. Phytomedicine.
138:1564302025. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Wu W, Hu W, Han WB, Liu YL, Tu Y, Yang HM,
Fang QJ, Zhou MY, Wan ZY, Tang RM, et al: Inhibition of
Akt/mTOR/p70S6K signaling activity with Huangkui capsule alleviates
the early glomerular pathological changes in diabetic nephropathy.
Front Pharmacol. 9:4432018. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Han W, Ma Q, Liu Y, Wu W, Tu Y, Huang L,
Long Y, Wang W, Yee H, Wan Z, et al: Huangkui capsule alleviates
renal tubular epithelial-mesenchymal transition in diabetic
nephropathy via inhibiting NLRP3 inflammasome activation and
TLR4/NF-κB signaling. Phytomedicine. 57:203–214. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Zhu Z, Luan G, Peng S, Fang Y, Fang Q,
Shen S, Wu K, Qian S, Jia W, Ye J and Wei L: Huangkui capsule
attenuates diabetic kidney disease through the induction of
mitophagy mediated by STING1/ PINK1 signaling in tubular cells.
Phytomedicine. 119:1549752023. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Shi R, Tao Y, Tang H, Wu C, Fei J, Ge H,
Gu HF and Wu J: Abelmoschus Manihot ameliorates the levels of
circulating metabolites in diabetic nephropathy by modulating gut
microbiota in non-obese diabetes mice. Microb Biotechnol.
16:813–826. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Zhang L, Li P, Xing CY, Zhao JY, He YN,
Wang JQ, Wu XF, Liu ZS, Zhang AP, Lin HL, et al: Efficacy and
safety of Abelmoschus manihot for primary glomerular disease: A
prospective, multicenter randomized controlled clinical trial. Am J
Kidney Dis. 64:57–65. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Chen X, Yu Y, Xiao C, Li S, Wu T, Wu H, Li
X, Lin C, Chen X, Guo X and Liu S: Huangkui capsules for diabetic
nephropathy: Comprehensive review of efficacy and molecular
mechanisms. Phytomedicine. 147:1572072025. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Liu X, Zhang C, Fu Y, Dai J, Lu J, Liu G
and Yang X: Huangkui capsule combined with finerenone attenuates
diabetic nephropathy by regulating the JAK2/STAT3 signaling pathway
based on network pharmacology, molecular docking, and experimental
verification. Front Pharmacol. 16:16252862025. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Wang Y, He X, Xue M, Yu H, He Q and Jin J:
Integrated 16S rRNA sequencing and metabolomic analysis reveals the
potential protective mechanism of Germacrone on diabetic
nephropathy in mice. Acta Biochim Biophys Sin (Shanghai).
56:414–426. 2024.PubMed/NCBI
|
|
174
|
Lee HS, Suh JY, Kang BC and Lee E:
Lipotoxicity dysregulates the immunoproteasome in podocytes and
kidneys in type 2 diabetes. Am J Physiol Renal Physiol.
320:F548–F558. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Nemecz M, Constantin A, Dumitrescu M,
Alexandru N, Filippi A, Tanko G and Georgescu A: The distinct
effects of palmitic and oleic acid on pancreatic beta cell
function: The elucidation of associated mechanisms and effector
molecules. Front Pharmacol. 9:15542021. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Liu Y, Jia Z, Liu S, Downton M, Liu G, Du
Y and Yang T: Combined losartan and nitro-oleic acid remarkably
improves diabetic nephropathy in mice. Am J Physiol Renal Physiol.
305:F1555–F1562. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Liu Y, Hu Z, Xing H, Kang L, Chen X, Liu B
and Niu K: Renoprotective effects of oleanolic acid and its
possible mechanisms in rats with diabetic kidney disease. Biochem
Biophys Res Commun. 636((Pt 1)): 1–9. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Lee ES, Kim HM, Kang JS, Lee EY, Yadav D,
Kwon MH, Kim YM, Kim HS and Chung CH: Oleanolic acid and
N-acetylcysteine ameliorate diabetic nephropathy through reduction
of oxidative stress and endoplasmic reticulum stress in a type 2
diabetic rat model. Nephrol Dial Transplant. 31:391–400. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Gao D, Li Q, Li Y, Liu Z, Liu Z, Fan Y,
Han Z, Li J and Li K: Antidiabetic potential of oleanolic acid from
Ligustrum lucidum Ait. Can J Physiol Pharmacol. 85:1076–1083. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Sultana N and Ata A: Oleanolic acid and
related derivatives as medicinally important compounds. J Enzyme
Inhib Med Chem. 23:739–756. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Abudoureyimu A, Chen C, Hu Y, Nuermaimaiti
D and Liu T: Quercetin alleviates diabetic nephropathy by
inhibiting M1 macrophage polarization via targeting NLRC5/NLRP3
pathway. Cell Immunol. 414:1049972025. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Yan X, Li P, Liu C, Yin F, Han J, Sun H,
Zheng Y, Chen X, Guan S and Wang X: Exploring the molecular
mechanisms for renoprotective effects of Huangkui capsule on
diabetic nephropathy mice by comprehensive serum metabolomics
analysis. J Ethnopharmacol. 340:1192232025. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Brito JCM, Lima WG, Cordeiro LPB and da
Cruz Nizer WS: Effectiveness of supplementation with quercetin-type
flavonols for treatment of viral lower respiratory tract
infections: Systematic review and meta-analysis of preclinical
studies. Phytother Res. 35:4930–4942. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Hu Q, Qu C, Xiao X, Zhang W, Jiang Y, Wu
Z, Song D, Peng X, Ma X and Zhao Y: Flavonoids on diabetic
nephropathy: Advances and therapeutic opportunities. Chin Med.
16:742021. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Hu T, Yue J, Tang Q, Cheng KW, Chen F,
Peng M, Zhou Q and Wang M: The effect of quercetin on diabetic
nephropathy (DN): A systematic review and meta-analysis of animal
studies. Food Funct. 13:4789–4803. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Lai WF and Wong WT: Design and
optimization of quercetin-based functional foods. Crit Rev Food Sci
Nutr. 62:7319–7335. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Albadrani GM, Binmowyna MN, Bin-Jumah MN,
El-Akabawy G, Aldera H and Al-Farga AM: Quercetin protects against
experimentally-induced myocardial infarction in rats by an
antioxidant potential and concomitant activation of signal
transducer and activator of transcription 3. J Physiol Pharmacol.
Apri 22–2020.(Epub ahead of print). PubMed/NCBI
|
|
188
|
Dini S, Zakeri M, Ebrahimpour S,
Dehghanian F and Esmaeili A: Quercetin-conjugated superparamagnetic
iron oxide nanoparticles modulate glucose metabolism-related genes
and miR-29 family in the hippocampus of diabetic rats. Sci Rep.
11:86182021. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Suganya N, Dornadula S, Chatterjee S and
Mohanram RK: Quercetin improves endothelial function in diabetic
rats through inhibition of endoplasmic reticulum stress-mediated
oxidative stress. Eur J Pharmacol. 819:80–88. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Zhong Y, Lee K, Deng Y, Ma Y, Chen Y, Li
X, Wei C, Yang S, Wang T, Wong J, et al: Arctigenin attenuates
diabetic kidney disease through the activation of PP2A in
podocytes. Nat Commun. 10:45232019. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Li X, Wang J, Yan J, He JC, Li Y and Zhong
Y: Additive renal protective effects between arctigenin and
puerarin in diabetic kidney disease. Biomed Pharmacother.
171:1161072024. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Medras ZJH, Mostafa YM, Ahmed AAM and
El-Sayed NM: Arctigenin improves neuropathy via ameliorating
apoptosis and modulating autophagy in streptozotocin-induced
diabetic mice. CNS Neurosci Ther. 29:3068–3080. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Wang W, Qiu L, Howard A, Solis N, Li C,
Wang X, Kopp JB and Levi M: Protective effects of aliskiren and
valsartan in mice with diabetic nephropathy. J Renin Angiotensin
Aldosterone Syst. 15:384–395. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
López V, Martin M, Cobelo C, Aranda P,
Cabello M, Sola E, Gutierrez C, Burgos D, Martínez D and Hernandez
D: Renin-angiotensin system dual blockade using angiotensin
receptor plus aliskiren decreases severe proteinuria in kidney
transplant recipients. Transplant Proc. 42:2883–2885. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Sun Y, Peng PA, Ma Y, Liu XL, Yu Y, Jia S,
Xu XH, Wu SJ and Zhou YJ: Valsartan protects against
contrast-induced acute kidney injury in rats by inhibiting
endoplasmic reticulum stress-induced apoptosis. Curr Vasc
Pharmacol. 15:174–183. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Li C, Lin Y, Luo R, Chen S, Wang F, Zheng
P, Levi M, Yang T and Wang W: Intrarenal renin-angiotensin system
mediates fatty acid-induced ER stress in the kidney. Am J Physiol
Renal Physiol. 310:F351–F363. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Kidney Disease: Improving Global Outcomes
(KDIGO) Diabetes Work Group: KDIGO 2020 clinical practice guideline
for diabetes management in chronic kidney disease. Kidney Int.
98((4S)): S1–S115. 2020.
|
|
198
|
Chang TT, Wu TC, Huang PH, Lin CP, Chen
JS, Lin LY, Lin SJ and Chen JW: Direct renin inhibition with
aliskiren improves ischemia-induced neovasculogenesis in diabetic
animals via the SDF-1 related mechanism. PLoS One. 10:e01366272015.
View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Wang Z, Yuan A, Liu C, Liu Y, Qiao L, Xu
Z, Bi S, Tian J, Yu B, Lin Z, et al: Identification of key
antifibrotic targets FPR1, TAS2R5, and LRP2BP of valsartan in
diabetic nephropathy: A transcriptomics-driven study integrating
machine learning, molecular docking, and dynamics simulations. Int
J Biol Macromol. 297:1398422025. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Zhang H, Liu G, Zhou W, Zhang W, Wang K
and Zhang J: Neprilysin inhibitor-angiotensin II receptor blocker
combination therapy (Sacubitril/valsartan) suppresses
atherosclerotic plaque formation and inhibits inflammation in
apolipoprotein E-deficient mice. Sci Rep. 9:65092019. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Ma Y, Xie D, Liu J, Han X, Xu H and Chen
Y: Angiopoietin-like protein 3 deficiency combined with valsartan
administration protects better against podocyte damage in
streptozotocin-induced diabetic nephropathy mice. Int
Immunopharmacol. 115:1097152023. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Kidney Disease: Improving Global Outcomes
(KDIGO) CKD Work Group: KDIGO 2024 clinical practice guideline for
the evaluation and management of chronic kidney disease. Kidney
Int. 105:S117–S314. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Mori K, Togo A, Ohta K, Asahi T, Nozaki C
and Kataoka K: CB1 receptor agonist ACEA Resists ER stress-mediated
apoptosis via CB1R-independent mechanism. Biol Pharm Bull.
48:769–781. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Vasincu A, Rusu RN, Ababei DC, Neamțu M,
Arcan OD, Macadan I, Beșchea Chiriac S, Bild W and Bild V:
Exploring the therapeutic potential of cannabinoid receptor
antagonists in inflammation, diabetes mellitus, and obesity.
Biomedicines. 11:16672023. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Aguirre CA, Castillo VA and Llanos MN:
Excess of the endocannabinoid anandamide during lactation induces
overweight, fat accumulation and insulin resistance in adult mice.
Diabetol Metab Syndr. 4:352012. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Kumawat VS and Kaur G: Cannabinoid
receptor 2 (CB2) agonists and L-arginine ameliorate diabetic
nephropathy in rats by suppressing inflammation and fibrosis
through NF-κβ pathway. Naunyn Schmiedebergs Arch Pharmacol.
397:381–393. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Jourdan T, Park JK, Varga ZV, Pálóczi J,
Coffey NJ, Rosenberg AZ, Godlewski G, Cinar R, Mackie K, Pacher P
and Kunos G: Cannabinoid-1 receptor deletion in podocytes mitigates
both glomerular and tubular dysfunction in a mouse model of
diabetic nephropathy. Diabetes Obes Metab. 20:698–708. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Barutta F, Piscitelli F, Pinach S, Bruno
G, Gambino R, Rastaldi MP, Salvidio G, Di Marzo V, Cavallo Perin P
and Gruden G: Protective role of cannabinoid receptor type 2 in a
mouse model of diabetic nephropathy. Diabetes. 60:2386–2396. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Yang HM, Kim J, Kim BK, Seo HJ, Kim JY,
Lee JE, Lee J, You J, Jin S, Kwon YW, et al: Resistin regulates
inflammation and insulin resistance in humans via the
endocannabinoid system. Research (Wash D C). 7:03262024.PubMed/NCBI
|
|
210
|
Lim JC, Lim SK, Park MJ, Kim GY, Han HJ
and Park SH: Cannabinoid receptor 1 mediates high glucose induced
apoptosis via endoplasmic reticulum stress in primary cultured rat
mesangial cells. Am J Physiol Renal Physiol. 301:F179–F188. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Lim JC, Lim SK, Han HJ and Park SH:
Cannabinoid receptor 1 mediates palmitic acid-induced apoptosis via
endoplasmic reticulum stress in human renal proximal tubular cells.
J Cell Physiol. 225:654–663. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
212
|
Panlilio LV, Goldberg SR and Justinova Z:
Cannabinoid abuse and addiction: Clinical and preclinical findings.
Clin Pharmacol Ther. 97:616–627. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
213
|
Cinar R, Iyer MR and Kunos G: The
therapeutic potential of second and third generation
CB1R antagonists. Pharmacol Ther. 208:1074772020.
View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Mińczuk K, Baranowska-Kuczko M, Krzyżewska
A, Schlicker E and Malinowska B: Cross-Talk between the
(Endo)cannabinoid and renin-angiotensin systems: Basic evidence and
potential therapeutic significance. Int J Mol Sci. 23:63502022.
View Article : Google Scholar : PubMed/NCBI
|
|
215
|
Jourdan T, Szanda G, Cinar R, Godlewski G,
Holovac DJ, Park JK, Nicoloro S, Shen Y, Liu J, Rosenberg AZ, et
al: Developmental role of macrophage cannabinoid-1 receptor
signaling in type 2 diabetes. Diabetes. 66:994–1007. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
216
|
Jourdan T, Szanda G, Rosenberg AZ, Tam J,
Earley BJ, Godlewski G, Cinar R, Liu Z, Liu J, Ju C, et al:
Overactive cannabinoid 1 receptor in podocytes drives type 2
diabetic nephropathy. Proc Natl Acad Sci USA. 111:E5420–E5428.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
217
|
Ghosh A, Peyot ML, Leung YH, Ravenelle F,
Madiraju SRM and Prentki M: A peripherally restricted cannabinoid-1
receptor inverse agonist promotes insulin secretion and protects
from cytokine toxicity in human pancreatic islets. Eur J Pharmacol.
944:1755892023. View Article : Google Scholar : PubMed/NCBI
|
|
218
|
Rajesh M, Bátkai S, Kechrid M,
Mukhopadhyay P, Lee WS, Horváth B, Holovac E, Cinar R, Liaudet L,
Mackie K, et al: Cannabinoid 1 receptor promotes cardiac
dysfunction, oxidative stress, inflammation, and fibrosis in
diabetic cardiomyopathy. Diabetes. 61:716–727. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
219
|
Haspula D and Clark MA: Cannabinoid
receptors: An update on cell signaling, pathophysiological roles
and therapeutic opportunities in neurological, cardiovascular, and
inflammatory diseases. Int J Mol Sci. 21:76932020. View Article : Google Scholar : PubMed/NCBI
|
|
220
|
Pointeau O, Ba AI, Geissler A, Barbosa R,
Basu A, Muhammad A, Nivot M, Loriot M, Leemput J, Passilly-Degrace
P, et al: Blockade of cannabinoid CB receptors potentiates the
anti-fibrotic effects mediated by SGLT2 inhibition in a mouse model
of diabetic nephropathy. Br J Pharmacol. 182:5355–5377. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
221
|
Dagon Y, Avraham Y, Link G, Zolotarev O,
Mechoulam R and Berry EM: The synthetic cannabinoid HU-210
attenuates neural damage in diabetic mice and hyperglycemic
pheochromocytoma PC12 cells. Neurobiol Dis. 27:174–181. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
222
|
Navas-Madroñal M, Almendra-Pegueros R,
Puertas-Umbert L, Jiménez-Altayó F, Julve J, Pérez B,
Consegal-Pérez M, Kassan M, Martínez-González J, Rodriguez C and
Galán M: Targeting mitochondrial stress with szeto-schiller 31
prevents experimental abdominal aortic aneurysm: Crosstalk with
endoplasmic reticulum stress. Br J Pharmacol. 180:2230–2249. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
223
|
Hinden L, Udi S, Drori A, Gammal A,
Nemirovski A, Hadar R, Baraghithy S, Permyakova A, Geron M, Cohen
M, et al: Modulation of renal GLUT2 by the cannabinoid-1 receptor:
Implications for the treatment of diabetic nephropathy. J Am Soc
Nephrol. 29:434–448. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
224
|
Kusaczuk M: Tauroursodeoxycholate-bile
acid with chaperoning activity: Molecular and cellular effects and
therapeutic perspectives. Cell. 8:14712019. View Article : Google Scholar
|
|
225
|
Zheng P, Lin Y, Wang F, Luo R, Zhang T, Hu
S, Feng P, Liang X, Li C and Wang W: 4-PBA improves lithiuminduced
nephrogenic diabetes insipidus by attenuating ER stress. Am J
Physiol Renal Physiol. 311:F763–F776. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
226
|
Guo Q, Xu L, Li H, Sun H, Wu S and Zhou B:
4-PBA reverses autophagic dysfunction and improves insulin
sensitivity in adipose tissue of obese mice via Akt/mTOR signaling.
Biochem Biophys Res Commun. 484:529–535. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
227
|
Ji X, Yao L, Wang M, Liu X, Peng S, Li K,
Xu M, Shen N, Luo L and Sun C: Cystatin C attenuates insulin
signaling transduction by promoting endoplasmic reticulum stress in
hepatocytes. FEBS Lett. 589((24 Part B)): 3938–3944. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
228
|
Cao AL, Wang L, Chen X, Wang YM, Guo HJ,
Chu S, Liu C, Zhang XM and Peng W: Ursodeoxycholic acid and
4-phenylbutyrate prevent endoplasmic reticulum stressinduced
podocyte apoptosis in diabetic nephropathy. Lab Invest. 96:610–622.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
229
|
Qin L, Wang Z, Tao L and Wang Y: ER stress
negatively regulates AKT/TSC/mTOR pathway to enhance autophagy.
Autophagy. 6:239–247. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
230
|
Ni L, Yuan C and Wu X: Endoplasmic
reticulum stress in diabetic nephrology: Regulation, pathological
role, and therapeutic potential. Oxid Med Cell Longev.
2021:72779662021. View Article : Google Scholar : PubMed/NCBI
|
|
231
|
Chang YC, Hee SW, Hsieh ML, Jeng YM and
Chuang LM: The role of organelle stresses in diabetes mellitus and
obesity: Implication for treatment. Anal Cell Pathol (Amst).
2015:9728912015.PubMed/NCBI
|
|
232
|
De Miguel C, Sedaka R, Kasztan M, Lever
JM, Sonnenberger M, Abad A, Jin C, Carmines PK, Pollock DM and
Pollock JS: Tauroursodeoxycholic acid (TUDCA) abolishes chronic
high salt-induced renal injury and inflammation. Acta Physiol
(Oxf). 226:e132272019. View Article : Google Scholar : PubMed/NCBI
|
|
233
|
Microvascular Complications Group of
Chinese Diabetes Society, . Clinical guideline for the prevention
and treatment of diabetic kidney disease in China (2021 edition).
Chin J Diabetes Mellitus. 13:762–784. 2021.(In Chinese).
|
|
234
|
Li Z, Li Y, Overstreet JM, Chung S, Niu A,
Fan X, Wang S, Wang Y, Zhang MZ and Harris RC: Inhibition of
epidermal growth factor receptor activation is associated with
improved diabetic nephropathy and insulin resistance in type 2
diabetes. Diabetes. 67:1847–1857. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
235
|
Rao VRA/LBV, Tan SH, Candasamy M and
Bhattamisra SK: Diabetic nephropathy: An update on pathogenesis and
drug development. Diabetes Metab Syndr. 13:754–762. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
236
|
Shih JY, Lin YW, Fisch S, Cheng JT, Kang
NW, Hong CS, Chen ZC and Chang WT: Dapagliflozin suppresses ER
stress and improves subclinical myocardial function in diabetes.
From bedside to bench. Diabetes. 70:262–267. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
237
|
Yu Y, Xia X, Li H, Zhang Y, Zhou X and
Jiang H: A new rhodopsin R135W mutation induces endoplasmic
reticulum stress and apoptosis in retinal pigment epithelial cells.
J Cell Physiol. 234:14100–14108. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
238
|
Fang Q, Zou C, Zhong P, Lin F, Li W, Wang
L, Zhang Y, Zheng C, Wang Y, Li X and Liang G: EGFR mediates
hyperlipidemia-induced renal injury via regulating inflammation and
oxidative stress: The detrimental role and mechanism of EGFR
activation. Oncotarget. 7:24361–24373. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
239
|
Martín-Pérez R, Palacios C, Yerbes R,
Cano-González A, Iglesias-Serret D, Gil J, Reginato MJ and
López-Rivas A: Activated ERBB2/HER2 licenses sensitivity to
apoptosis upon endoplasmic reticulum stress through a
PERK-dependent pathway. Cancer Res. 74:1766–1777. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
240
|
Zhou K, Zi X, Song J, Zhao Q, Liu J, Bao H
and Li L: Molecular mechanistic pathways targeted by natural
compounds in the prevention and treatment of diabetic kidney
disease. Molecules. 27:62212022. View Article : Google Scholar : PubMed/NCBI
|
|
241
|
Zhang L, Li C, Fu L, Yu Z, Xu G, Zhou J,
Shen M, Feng Z, Zhu H, Xie T, et al: Protection of catalpol against
triptolide-induced hepatotoxicity by inhibiting excessive autophagy
via the PERK-ATF4-CHOP pathway. PeerJ. 10:e127592022. View Article : Google Scholar : PubMed/NCBI
|
|
242
|
Itoh T, Hatano R, Horimoto Y, Yamada T,
Song D, Otsuka H, Shirakawa Y, Mastuoka S, Iwao N, Aune TM, et al:
IL-26 mediates epidermal growth factor receptor-tyrosine kinase
inhibitor resistance through endoplasmic reticulum stress signaling
pathway in triple-negative breast cancer cells. Cell Death Dis.
12:5202021. View Article : Google Scholar : PubMed/NCBI
|
|
243
|
Wang L, Ding S, Hu Y, Su J, Zhu G, Hong H,
Hou B, Dong Z, Xue Z, Wang J, et al: Targeting programmed cell
death pathways: Emerging therapeutic strategies for diabetic kidney
disease. Front Endocrinol (Lausanne). 16:15138952025. View Article : Google Scholar : PubMed/NCBI
|
|
244
|
Mima A: A narrative review of diabetic
kidney disease: Previous and current evidence-based therapeutic
approaches. Adv Ther. 39:3488–3500. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
245
|
Kume S and Maegawa H: Lipotoxicity,
nutrient-sensing signals, and autophagy in diabetic nephropathy.
JMA J. 3:87–94. 2020.PubMed/NCBI
|
|
246
|
Beal B, Buizen L, Yeung EK, Heath L,
Houston L, Cherney DZI, Jardine M, Pollock C, Arnott C, Kotwal SS,
et al: Effects of SGLT2 inhibition on insulin use in CKD and type 2
diabetes: Insights from the CREDENCE trial. Nephrol Dial
Transplant. 40:1727–1735. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
247
|
Kidney Disease: Improving Global Outcomes
(KDIGO) Diabetes Work Group: KDIGO 2022 clinical practice guideline
for diabetes management in chronic kidney disease. Kidney Int.
102((5S)): S1–S127. 2022.
|
|
248
|
Wanner C, Inzucchi SE, Lachin JM, Fitchett
D, von Eynatten M, Mattheus M, Johansen OE, Woerle HJ, Broedl UC
and Zinman B; EMPA-REG OUTCOME Investigators, : Empagliflozin and
progression of kidney disease in type 2 diabetes. N Engl J Med.
375:323–334. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
249
|
Neal B, Perkovic V, Mahaffey KW, de Zeeuw
D, Fulcher G, Erondu N, Shaw W, Law G, Desai M and Matthews DR;
CANVAS Program Collaborative Group, : Canagliflozin and
cardiovascular and renal events in type 2 diabetes. N Engl J Med.
377:644–657. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
250
|
Wiviott SD, Razl Bonaca MP, Mosenzon O,
Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, et
al: Dapagliflozinand cardiovascular outcomes in type 2 diabetes. N
Engl J Med. 380:347–357. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
251
|
Perkovic V, Jardine MJ, Neal B, Bompoint
S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull
S, et al: Canagliflozin and renal outcomes in type 2 diabetes and
nephropathy. N Engl J Med. 380:2295–2306. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
252
|
Barreiro E, Salazar-Degracia A,
Sancho-Muñoz A and Gea J: Endoplasmic reticulum stress and unfolded
protein response profile in quadriceps of sarcopenic patients with
respiratory diseases. J Cell Physiol. 234:11315–11329. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
253
|
Bohnert KR, McMillan JD and Kumar A:
Emerging roles of ER stress and unfolded protein response pathways
in skeletal muscle health and disease. J Cell Physiol. 233:67–78.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
254
|
Liu CY, Hsu CC, Huang TT, Lee CH, Chen JL,
Yang SH, Jiang JK, Chen WS, Lee KD and Teng HW: ER stress-related
ATF6 upregulates CIP2A and contributes to poor prognosis of colon
cancer. Mol Oncol. 12:1706–1717. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
255
|
Ryu D, Seo WY, Yoon YS, Kim YN, Kim SS,
Kim HJ, Park TS, Choi CS and Koo SH: Endoplasmic reticulum stress
promotes LIPIN2-dependent hepatic insulin resistance. Diabetes.
60:1072–1081. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
256
|
Toma L, Stancu CS and Sima AV: Endothelial
dysfunction in diabetes is aggravated by glycated lipoproteins;
Novel molecular therapies. Biomedicines. 9:182020. View Article : Google Scholar : PubMed/NCBI
|
|
257
|
Marciniak SJ, Chambers JE and Ron D:
Pharmacological targeting of endoplasmic reticulum stress in
disease. Nat Rev Drug Discov. 21:115–140. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
258
|
Anumas S and Inagi R: Mitigating
lipotoxicity: A potential mechanism to delay chronic kidney disease
progression using current pharmacological therapies. Nephrology
(Carlton). 30:e700982025. View Article : Google Scholar : PubMed/NCBI
|
|
259
|
Zheng T, Luo L, Wang X, Deng X and Xue M:
Canagliflozin ameliorates diabetic podocyte damage via enriching
mitochondria-associated endoplasmic reticulum membranes. Cell
Signal. 135:1120382025. View Article : Google Scholar : PubMed/NCBI
|
|
260
|
Wang Y, Liu S, Zhu F, Wang X, Wang H, Long
L, Xiao J and Guo C: Improved bioavailability and
anti-nephrotoxicity efficacy of polydatin on cisplatin-induced AKI
via a dual-targeting fucoidan delivery system. Int J Pharm X.
10:1004222025.PubMed/NCBI
|
|
261
|
Fang Z, Liu R, Xie J and He JC: Molecular
mechanism of renal lipid accumulation in diabetic kidney disease. J
Cell Mol Med. 28:e183642024. View Article : Google Scholar : PubMed/NCBI
|
|
262
|
Ye S, Cheng Z, Zhuo D and Liu S: Different
types of cell death in diabetic neuropathy: A focus on mechanisms
and therapeutic strategies. Int J Mol Sci. 25:81262024. View Article : Google Scholar : PubMed/NCBI
|
|
263
|
Ghemrawi R, Battaglia-Hsu SF and Arnold C:
Endoplasmic reticulum stress in metabolic disorders. Cells.
7:632018. View Article : Google Scholar : PubMed/NCBI
|
|
264
|
Chattopadhyay A, Kwartler CS, Kaw K, Li Y,
Kaw A, Chen J, LeMaire SA, Shen YH and Milewicz DM:
Cholesterol-induced phenotypic modulation of smooth muscle cells to
macrophage/fibroblast-like cells is driven by an unfolded protein
response. Arterioscler Thromb Vasc Biol. 41:302–316. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
265
|
Dong L, Xu M, Li Y, Xu W, Wu C, Zheng H,
Xiao Z, Sun G, Ding L, Li X, et al: SMURF1 attenuates endoplasmic
reticulum stress by promoting the degradation of KEAP1 to activate
NRF2 antioxidant pathway. Cell Death Dis. 14:3612023. View Article : Google Scholar : PubMed/NCBI
|
|
266
|
Rabhi N, Denechaud PD, Gromada X, Hannou
SA, Zhang H, Rashid T, Salas E, Durand E, Sand O, Bonnefond A, et
al: KAT2B is required for pancreatic beta cell adaptation to
metabolic stress by controlling the unfolded protein response. Cell
Rep. 15:1051–1061. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
267
|
Wang Y, Zhou X, Zhao D, Wang X, Gurley EC,
Liu R, Li X, Hylemon PB, Chen W and Zhou H: Berberine inhibits free
fatty acid and LPS-induced inflammation via modulating ER stress
response in macrophages and hepatocytes. PLoS One. 15:e02326302020.
View Article : Google Scholar : PubMed/NCBI
|
|
268
|
Yang M, Liu C, Jiang N, Liu Y, Luo S, Li
C, Zhao H, Han Y, Chen W, Li L, et al: Endoplasmic reticulum
homeostasis: A potential target for diabetic nephropathy. Front
Endocrinol (Lausanne). 14:11828482023. View Article : Google Scholar : PubMed/NCBI
|
|
269
|
Pennell JP: Optimizing medical management
of patients with pre-end-stage renal disease. Am J Med.
111:559–568. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
270
|
Kim SH, Yoo JH, Lee WJ and Park CY:
Gemigliptin: An update of its clinical use in the management of
type 2 diabetes mellitus. Diabetes Metab J. 40:339–353. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
271
|
Frățilă VG, Lupușoru G, Sorohan BM,
Obrișcă B, Mocanu V, Lupușoru M and Ismail G: Nephrotic Syndrome:
From pathophysiology to novel therapeutic approaches. Biomedicines.
12:5692024. View Article : Google Scholar : PubMed/NCBI
|