Introduction
Neuroblastoma (NB), the most common extracranial
solid tumor in infants and young children, accounting for 8–10% of
childhood malignancies (1).
Childhood NB is difficult to treat and has a poor prognosis with
single treatment. Although the 5-year survival rate of
neuroblastoma patients has increased from <20–50% in the past
few decades, it still accounts for ~15% of all childhood cancer
deaths (2). Current treatment
approaches aim to improve the effectiveness of treatment by adding
immunotherapy and targeted therapy to standard regimens (3).
Autophagy is the main intracellular material
transport mechanism, responsible for transporting various
intracellular substances to lysosomes for degradation and
recycling. Recent studies have focused not only on the function of
autophagy in tumor cells themselves, but also on the role of
autophagy in the tumor microenvironment and the functions of
related immune cells. There is increasing evidence showing how
autophagy and its related processes affect the development and
progression of cancer, which helps guide the design of anticancer
therapeutics based on inhibiting or promoting autophagy (4). In the tumor microenvironment,
autophagy of tumor cells can be induced by a combination of
intracellular and extracellular stress signals, including metabolic
stress, hypoxia, redox stress and immune signals (5). In response to metabolic stress, tumor
cells rewire their own metabolic pathways by upregulating nutrient
transporters and activating autophagy (6). Mechanistically, 5′-AMP-activated
protein kinase (AMPK) and mTOR complex 1 (mTORC1) are two opposing
regulatory kinases. After target phosphorylation in the
pre-autophagy initiation complex, AMPK is located at the activation
site and mTORC1 is located at the inhibition site (7,8). To
initiate autophagy, AMPK phosphorylates six different sites of
unc-51-like kinase 1 (ULK1) and S91/S94 of Beclin 1 (9). Wang et al (10) predicted a risk signature of four
autophagy-related genes for neuroblastoma survival that was
associated with tumor immunity. Bishayee et al (11) demonstrated that the RNA-binding
protein HuD promotes autophagy and tumor stress survival by
inhibiting mTORC1 activity and increasing ARL6IP1 levels.
Ugun-Klusek et al (12)
found that monoamine oxidase-A promotes protective autophagy in
human SH-SY5Y neuroblastoma cells through Bcl-2 phosphorylation.
These results suggest that autophagy can promote apoptosis of
neuroblastoma cells.
The endoplasmic reticulum (ER) is involved in a
number of cellular functions, including protein synthesis, calcium
homeostasis or phospholipid synthesis. Under stressful situations,
the ER environment is disrupted and protein maturation is impaired,
leading to the accumulation of misfolded proteins and a
characteristic stress response called the unfolded protein
response. ER stress and the unfolded protein response (UPR) can
mediate toxic consequences through various pathogenic mechanisms
(including cell death, fibrosis and inflammatory signaling
pathways) (13). Activation of
PerK can also promote the production of pro-inflammatory IL-6 and
IL-8 by increasing p38 and PerK signaling pathways, while CHOP can
regulate the transcription of cytokines including IL-6. In certain
types of cancer, ER stress leads to the release of proinflammatory
cytokines, including IL-6 and TNFα, each of which contains XBP1s
binding sites in its promoter. These cytokines can promote
pathology by driving inflammation and, in some cases, cancer cell
proliferation (14). A number of
studies have demonstrated that ER stress can trigger the autophagy
process (15). Celesia et
al (16) demonstrated that
reactive oxygen species (ROS)-dependent ER stress and autophagy
play an important role in the mechanism of action of TBT-F in colon
cancer cells. The antitumor drug ABTL0812 impairs the growth of
neuroblastoma cells through ER stress-mediated autophagy and
apoptosis (17). Therefore, the
relationship between autophagy and altered oxidative stress is
evident, following the pathway of ER stress and/or mitochondrial
changes.
Melatonin regulates circadian rhythms and is
associated with improved sleep, scavenging of ROS, anti-aging
effects, and seasonal and circadian rhythms. There is a mutual
relationship between melatonin and autophagy. Ge et al
(18) found that autophagy has a
potential role in regulating melatonin synthesis in rat pineal
cells. At the same time, the therapeutic potential of melatonin in
cancer, neurodegenerative diseases, viral infections and obesity is
related to its role as an autophagy regulator, in which melatonin
regulates ER stress, autophagy and apoptosis (19,20).
Zhang et al (21) found
that melatonin increased cardiomyocyte autophagy by regulating the
VEGF-BGRP78PERK signaling pathway, thereby alleviating diabetic
cardiomyopathy. In preclinical studies, fetal hypoxia caused
autophagy and mitochondrial damage in ovarian granulosa cells,
which was alleviated by melatonin supplementation (22). In terms of anti-inflammatory
effects, melatonin alleviates sepsis-induced small intestinal
damage by upregulating SIRT3-mediated oxidative stress, inhibiting
mitochondrial protection and inducing autophagy (23). However, the therapeutic effect of
melatonin on neuroblastoma and its underlying mechanism remain to
be elucidated.
The present study investigated whether melatonin
reduces ER stress and impairs neuroblastoma tumor growth, enabling
cytotoxic autophagy and apoptosis. Specifically, it investigated
whether melatonin induces the transcription of glucose-regulated
protein (GRP)78 and GRP94 and CHOP in neuroblasts and alleviates ER
stress. In addition, it also explored how melatonin regulates
p21-activated kinase 2 (Pak2) and its mediated signaling pathway to
alleviate ER stress in neuroblastoma. This provided a new treatment
option for patients with high-risk neuroblastoma.
Materials and methods
Cell recovery and passaging
Neuro-2a cells (N2a cells; cat. no. CL0383; Hunan
Fenghui Biotechnology Co., Ltd.) were used for cell studies. A tube
of N2a cells were placed on −80°C ice and the cryopreservation tube
placed in a 37°C water bath until the cryopreservation solution
melted into liquid. Then, 1 ml of the solution was transferred to a
15 ml centrifuge tube, and 1 ml of 10% DMEM was added, mixed and
centrifuged (room temperature, 1,000 × g for 5 min). After the
cells were resuspended, they were transferred to a T25 culture
flask and 5 ml DMEM was added. The cells were observed under a
microscope to be single cells and evenly distributed. The cells
were then transferred to a cell culture incubator at 37°C, and 5%
CO2 for culture. At 24 h after cell recovery, the
adhesion and cell density of N2a cells were observed and fresh 5 ml
DMEM was replaced. At this time, it was observed that 70–80% of the
cells were round, with a small number of spindle-shaped cells, and
the cell density accounted for ~60% of the T25 flask. When the cell
density in the T25 flask reached ~90%, the cell culture was
passaged using the 1-to-2 method to avoid growth inhibition.
Cell proliferation
Since N2a cells proliferate rapidly, neuroblastoma
cells were plated at 3×105 cells/well in 6-well plates
with 1.5 ml of culture medium added, so that the cell confluence of
each well was 60–70% and observed after 24 h. After the cells
adhered, new culture medium was replaced and designated drugs were
used for intervention treatment.
Reagents and instruments
Melatonin reagent, ER stress inhibitor
4-phenylbutyric acid (4-PBA) and AMPK inhibitor Dorsomorphin
(Dorso) were purchased from Beijing Solarbio Science &
Technology Co., Ltd.; ER-related protein GRP78 was purchased from
Wuhan Servicebio Technology Co., Ltd.; Beclin1 was purchased from
BIOSS; GRP94, CHOP, autophagy-related protein P62,
autophagy-related protein 5 (ATG5), LC3, β-Actin, mTOR,
phosphorylated (p)-mTOR, ULK1, phosphorylated (p)-ULK1 and Pak2
were provided by Wuhan Sanying Biotechnology. The PAK2
overexpression lentivirus packaging and PAK2 interference
lentivirus packaging were provided by Beyotime Biotechnology. The
experimental instruments and equipment are as follows: fluorescence
microscope (Olympus Corporation), transmission electron microscope
(Hitachi High-Technologies Corporation), chemiluminescence imager
(Tanon4800 Multi; Tanon Science and Technology Co., Ltd.), and
constant temperature cell culture incubator (Thermo Scientific
Forma Series II; Thermo Fisher Scientific, Inc.).
PAK2 overexpression and knockdown
The PAK2 overexpression lentivirus packaging and
PAK2 interference lentivirus packaging were provided by Beyotime
Institute of Biotechnology (3rd-generation lentiviral packaging
system). PAK2 overexpression lentivirus: The mouse PAK2
interference lentivirusAK2 gene sequence (accession no.
NM_008779.3; http://www.ncbi.nlm.nih.gov/nuccore/NM_008779.3/)
is available in the National Center for Biotechnology Information
database. The PAK2 overexpression lentivirus was packaged using the
pLOV-UbiC-EGFP vector and then 293T cells (cat. no. CRL-3216;
American Type Culture Collection) were transfected with the
expression vectors. To construct stable PAK2 overexpression and
knockdown cell lines, lentiviruses were packaged using a
three-plasmid system. In brief, HEK293T cells were co-transfected
with 8 µg pLOV-UbiC-EGFP plasmid, 6 µg psPAX2 plasmid and 2 µg
pMD2.G plasmid at a mass ratio of 4:3:1. Viral supernatant was
collected 48 and 72 h after transfection and concentrated to obtain
high titer virus solution. The optimal multiplicity of infection
(MOI) was determined to be 20. Screening was performed 48 h after
infection, using puromycin at a concentration of 2 µg/ml for 7 days
to remove uninfected cells. Subsequently, the concentration of
puromycin was reduced to 0.5 µg/ml for maintenance culture, and
stable cell lines were finally obtained. The PAK2 overexpression
lentivirus was harvested, filtered and concentrated. To establish
short hairpin (sh)RNA-mediated PAK2 knockdown, pLKO.1-puro
lentiviral plasmids were used containing the validated mouse PAK2
shRNA Seq [TRCN0000023619; mRNA Target: GCUGAUGAAGUUGCUGAGUAU.
Constructing the full shRNA Transcript with U bases:
5′-(GCUGAUGAAGUUGCUGAGUAU)-[CUCGAG)-(AUACUCAGCAACUUCAUCAGC)-3′].
The PAK2 shRNA lentiviral plasmid was co-transfected with the
packaging constructs psPAX2 and pMD2.G by Lipofectamine®
2000 (Invitrogen; Thermo Fisher Scientific, Inc.) into the human
293T cells. After 24 and 48 h, virus particles were collected,
filtered through a 0.45-µm PES filter and then used to infect the
N2a cells. The lentivirus without the transgene was used as the
negative control (NC) and was produced in the same manner as the
inhibitor vector. Transfection efficiency was confirmed by RT-qPCR
and western blot analysis.
Melatonin intervention and protein
extraction
Different concentrations of melatonin, 1, 5, 10, 20
and 0 µm (control group) were placed into the proliferating N2a
cells and cultured in a cell culture incubator at 37°C and 5%
CO2 for 48 h. Then the old culture medium was discarded,
each well washed with 1 ml PBS, 300 ml RIPA lysis buffer + PMSF
protease inhibitor (100:1) mixture added and lysed on ice for 5
min. Then, the cells in the well plate were scraped and transferred
into a new 1.5 ml tube for further lysis on ice for 30 min with
shaking every 10 min. The tube was placed at 4°C and 10,000 × g for
15 min, and 280 µl of the supernatant was aspirated. After
quantification of sample proteins by BCA analysis, each sample was
mixed with loading buffer and denatured in a high-temperature water
bath. Finally, divide into 3 tubes and store at −20°C.
Western blotting
After collecting cells from each group for protein
extraction, the denatured proteins were loaded onto the gel (40 µg
per well) for electrophoresis using separation and concentration
gels. After electrophoresis, the proteins were transferred to a
PVDF membrane of the appropriate size. Each antibody was detected
using a constant current of 290 mA, followed by a quick blocking
solution (cat. no. G2052-500ML; Wuhan Servicebio Technology Co.,
Ltd.) for 10 min, washed twice with TBS +0.05% Tween (TBST) at room
temperature for 3 min each and incubated with the primary antibody
after blocking. Membranes were incubated overnight at 4°C, then the
secondary antibody was applied at room temperature for 1 h and
washed three times with TBST for 5 min each. An ECL
chemiluminescence substrate kit (ultra-sensitive; cat. no. BL520A;
Biosharp Life Science) was used, and the developer was added.
Luminol and HRP were used to form luminous images. Developer was to
allow chemiluminescence imaging (Tanon4800 Multi; Tanon Science and
Technology Co., Ltd). The membranes were washed with antibody
eluent (cat. no. G2079-100M; Wuhan Servicebio Technology Co., Ltd)
to remove bound primary antibody at room temperature, and then
washed twice with TBST, each time for 5 min. The membranes were
incubated with the internal control antibody at room temperature in
2 h, using mouse β-actin (1:10,000; cat. no. 66009-1-Ig; Wuhan
Sanying Biotechnology). Following which the membranes were
incubated with a secondary antibody and developed in room
temperature 1 h again. The experiment was repeated twice. The
antibodies used in this experiment were as follows: Rabbit resist
grp94 (1:8,000; cat. no. 14700-1-AP; Wuhan Sanying Biotechnology),
CHOP (1:20,000; cat. no. 15204-1-AP; Wuhan Sanying Biotechnology),
LC3 (1:20,000; cat. no. 14600-1-AP; Wuhan Sanying Biotechnology),
ATG5 (1:5,000; cat. no. 10181-2-AP; Wuhan Sanying Biotechnology),
Beclin1 (1:5,000; cat. no. bs-1353R; BIOSS), P62(1:5,000; cat. no.
18420-1-AP; Wuhan Sanying Biotechnology), PAK2 (1:10,000; cat. no.
21401-1-AP; Wuhan Sanying Biotechnology), ULK1 (1:10,000; cat. no.
68445-1-Ig; Wuhan Sanying Biotechnology), p-ULK1(1:50,000; cat. no.
80218-1-RR; Wuhan Sanying Biotechnology), mouse resist grp78
(1:10,000; cat. no. GB15098; Wuhan Servicebio Technology Co., Ltd),
mTOR (1:10,000; cat. no. 66888-1-Ig; Wuhan Sanying Biotechnology)
and p-mTOR (1:5,000; cat. no. 67778-1-Ig; Wuhan Sanying
Biotechnology). Secondary antibodies were used as follows:
HRP-conjugated Goat Anti-Rabbit IgG (H+L) (1:6,000; cat. no.
SA00001-2; Wuhan Sanying Biotechnology) and HRP-conjugated Goat
Anti-Mouse IgG (H+L) (1:6,000; cat. no. SA00001-1; Wuhan Sanying
Biotechnology).
Immunofluorescence
N2a cells were seeded into 24-well plates, with
1×105 cells per well. After 24 h, cells were treated
with the indicated treatments and 48 h later, immunofluorescence
was performed. First, cells were washed twice with pre-cooled PBS,
then fixed with anhydrous ethanol in room temperature for 15 min,
washed three times for 5 min each time with PBST and then blocked
with 10% blocking solution, 20 µl bovine serum (cat. no.
S12012-100g; Shanghai Yuanye Biotechnology Co., Ltd.) + 180 µl
0.25% Triton X-100, at room temperature for 45 min. The blocking
solution was discarded and the primary antibody, 200 µl rabbit LC3
(1:200; cat. no. 14600-1-AP; Wuhan Sanying Biotechnology) was added
and incubated at 4°C overnight, followed by secondary antibody
CoraLite488-conjugated Goat Anti-Mouse IgG (H+L) (1:200; cat. no.
SA00013-2; Wuhan Sanying Biotechnology) at room temperature in the
dark for 1 h. The cell nuclei were stained with Hoechst 33342 (5
mg/ml) at room temperature for 10 min, washed and mounted with an
anti-fluorescence quencher, and then images captured with a
fluorescent microscope (200X).
Transmission electron microscopy
observation of autophagosomes
Culture medium was discarded, 1 ml of PBS was added
to each well for washing, and then 1 ml of trypsin was added for
digestion, followed by the addition of 10% DMEM to terminate the
digestion and the cells incubated at 300 × g for 5 min at room
temperature. After discarding the supernatant, when the cell pellet
was ~5 mm3, 2.5% glutaraldehyde fixative was used at
room temperature in the dark for 30 min and then transferred to 4°C
for storage. The cells were stained with 2% uranyl acetate
saturated alcohol solution for 8 min in the dark; 2.6% lead citrate
solution was used to avoid carbon dioxide staining for a further 8
min. Following which infiltration embedding was performed as
follows: i) Acetone:812 embedding agent (1:1) at 37°C for 2–4 h;
ii) acetone:812 embedding agent (1:2) at 37°C for overnight
infiltration; and iii) pure 812 embedding agent at 37°C for 5–8 h.
The pure 812 embedding agent was poured into the embedding plate,
and the samples were inserted into the embedding plate and oven at
37°C overnight. Subsequently, the embedding plate was transferred
to the oven at 60°C for polymerization for 48 h and the resin block
was removed. The fixed precipitates were sectioned (thickness, 60
nm) and images captured using a transmission electron microscope
(cat. no. HT7800; Hitachi, Ltd.).
Construction of PAK2 overexpression
and inhibition N2a cell model
N2a cells were digested with 0.25% trypsin digestion
solution (cat. no. G4004-100 ml; Servicebio) for 1 min, centrifuged
at 300 × g for 5 min, and resuspended. The resuspended N2a cells
were added to 6-well plates, and 3×105 N2a cells were
added to each well. After 24 h, when the cell confluence reached
50–70%, 6 µl of PAK2 overexpression lentivirus stock solution
(1×109 TU/ml) was added. When MOI=20, 6 µl of PAK2
inhibition lentivirus stock solution and polybrene were added to
make the final concentration of 5 µg/ml. After 48 h, 1, 5, 10 and
20 µm of melatonin were added to the corresponding groups, and cell
protein was extracted after 48 h.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from homogenized the cell
pellets using an RNA extraction buffer (AgBio, Inc.) following the
manufacturer's guidelines. Subsequently, mRNA was reverse
transcribed into cDNA using a cDNA synthesis kit (Takara Bio,
Inc.). A mixture of 1 µl of synthetic cDNA and specific primers was
used to amplify inflammatory cytokine target genes by SYBR Premix
Ex Taq2 (Takara Bio, Inc.). The primers used in the present study
were as follows: Pak2 forward (F), 5′-ACACCAGCACTGAACACCAA-3′, and
reverse (R), 5′-CAATCTGCGCTTCGTCCATG-3′; and GAPDH F,
5′-AGGTCGGTGTGAACGGATTTG-3′ and R, 5′-TGTAGACCATGTAGTTGAGGTCA-3′.
The following thermocycling conditions were used for qPCR: initial
step at 50°C for 2 min; 95°C for 10 min; 45 cycles of 95°C for 10
sec, 60°C for 10 sec and 72°C for 15 sec. GAPDH was used as a
housekeeping and internal control gene to assess the relative
expression of target genes. The comparative Cq method
(2−ΔΔCq) was used to calculate the expression change of
the target gene relative to the reference gene (24). Data analysis was performed using
the software CFX Manager™ (BioRadCFXManager; version 2.2; Bio-Rad
Laboratories, Inc.).
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 8.0 (Dotmatics). All values are presented as mean ± SD.
Multiple comparisons were performed with one-way analysis of
variance followed by Bonferroni post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Melatonin reduces ER stress in N2a
neuroblastoma cells
Melatonin has been shown to play a role in ER stress
in various diseases and can directly or indirectly interfere with
ER-related sensors and downstream targets of UPR, but there are
currently no studies on ER stress in N2a neuroblastoma cells
(25). The present study used the
UPR to drive the increased expression of the ER chaperones GRP78
and GRP94 and the Perk downstream protein CHOP to verify that
melatonin alleviates ER stress in N2a neuroblastoma cells (26,27).
After N2a cells were treated with melatonin at different
concentrations for 48 h, western blotting showed that the
expression of GRP94, GRP78 and CHOP proteins increased (Fig. 1A). The rapid proliferation of N2a
cells caused the accumulation of misfolded and unfolded proteins in
the ER lumen, triggering the ER stress response. At the N2a cell
level, after melatonin treatment, the expression of CHOP (Fig. 1B), GRP78 (Fig. 1C) and GRP94 (Fig. 1D) proteins increased, which can
help accelerate the processing of misfolded and unfolded proteins
in the ER, thereby alleviating ER stress and maintaining the
homeostasis of the intracellular environment.
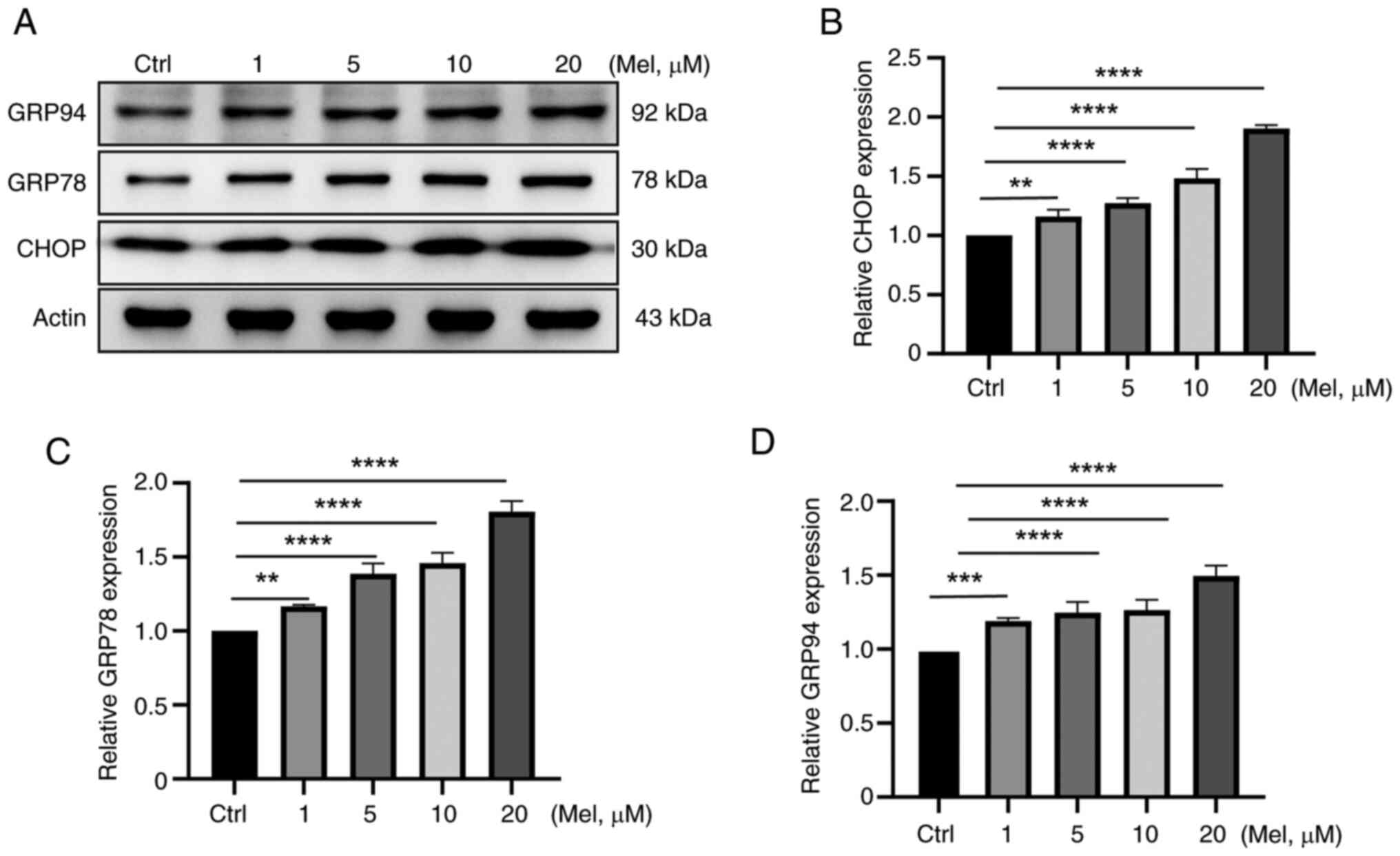 | Figure 1.Melatonin reduces ER stress in N2a
neuroblastoma cells. N2a cells were treated with melatonin at 1, 5,
10, 20 µM concentrations for 48 h. (A) Western blotting detection
showed that the expression of (B) CHOP, (C) GRP78 and (D)
GRP94proteins increased. **P<0.01, ***P<0.001,
****P<0.0001, ns, not significant; ER, endoplasmic reticulum;
N2a, Neuro-2a; GRP, glucose-regulated protein; Mel, melatonin. |
Melatonin can induce autophagy in N2a
neuroblastoma cells
To evaluate the effect of melatonin on autophagy in
N2a neuroblastoma cells, the present study used western blotting to
detect autophagy-related proteins ATG5, P62, BECLIN1, LC3BI/LC3BII
and electron microscopy to observe the formation of autophagosomes
and immunofluorescence for verification (28). The western blotting results of N2a
cells treated with melatonin for 48 h showed that the ratio of
protein LC3II/LC3I increased, the expression of ATG5 and Beclin1
proteins increased, and the expression of p62 protein decreased.
There was a negative association between p62 and autophagy levels,
and a positive association between Beclin1 and autophagy levels
(Fig. 2A-E). The results showed
that as the concentration of melatonin increased, the expression of
autophagy-related proteins also increased, indicating that cellular
autophagy was enhanced.
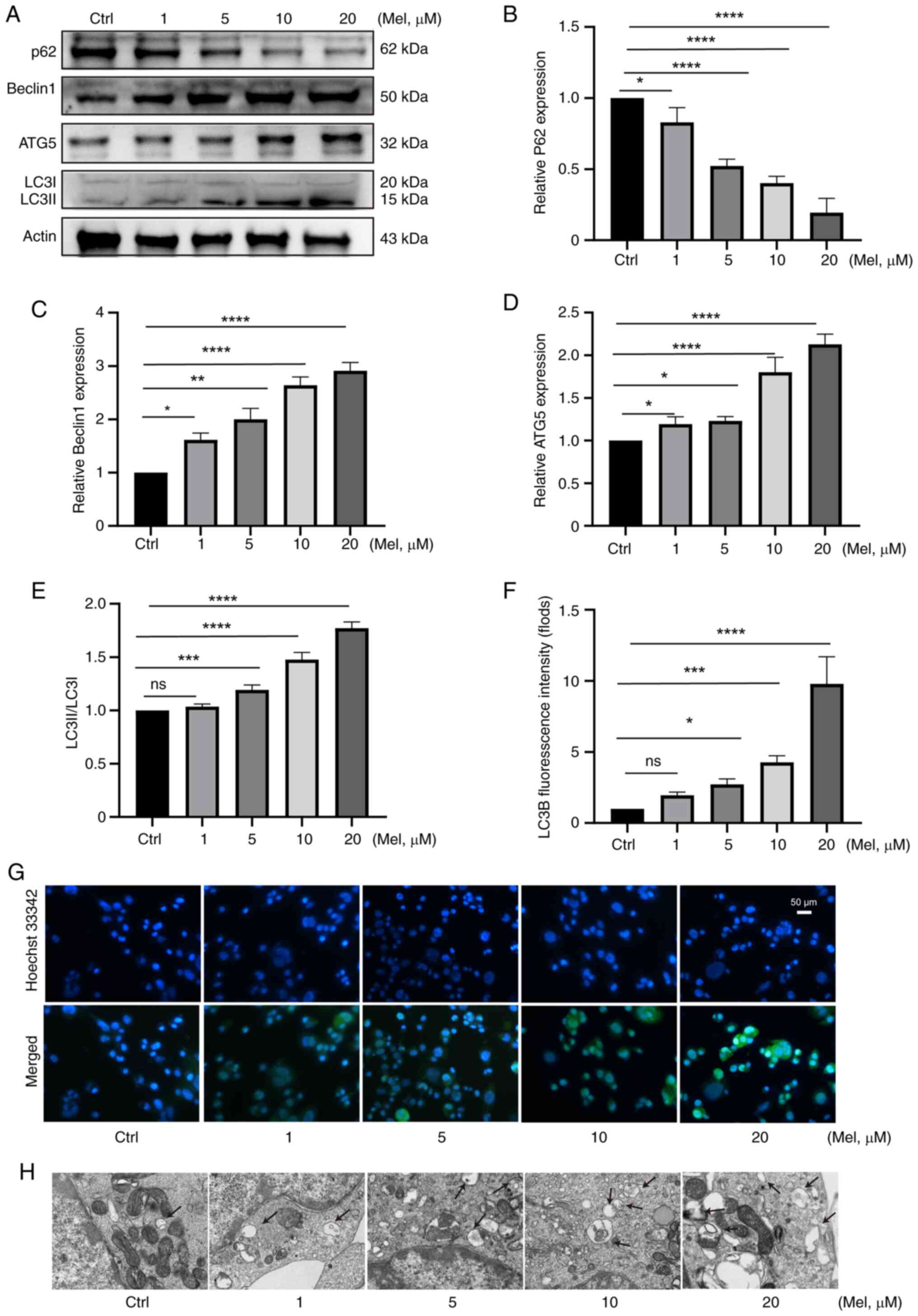 | Figure 2.Melatonin can induce autophagy in N2a
neuroblastoma cells. N2a cells were treated with melatonin at 1, 5,
10, 20 µM concentrations for 48 h. (A) western blotting detection
showed (B) the expression of p62 protein decreased, (C) the
expression of Beclin1 proteins and (D) ATG5 increased and (E) the
ratio of protein LC3II/LC3I increased. (F and G) As the
concentration of melatonin increased, the intensity of LC3 staining
(green) gradually increased. (H) As the concentration of melatonin
increased, the number of autophagosomes gradually increased.
*P<0.05, **P<0.01, ***P<0.001, ****P<0.0001; ns, not
significant; N2a, Neuro-2a; ATG5, autophagy-related protein 5; Mel,
melatonin. |
Regarding LC3B-green fluorescence, the fluorescence
signal of positive samples should show a bright and uniform
morphology, which indicates strong autophagic activity. Conversely,
weak or absent fluorescence signal represent that autophagy
activity is weak or not occurring. As the concentration of
melatonin increased, the LC3B-green fluorescence intensity
gradually increased (Fig. 2F and
G).
The cell pellets collected after melatonin treatment
were examined by transmission electron microscopy. There are three
types of autophagy markers: Isolation membrane, autophagosome and
autolysosome. The present study experiment mainly observed the
autophagosome. As the concentration of melatonin increased, the
number of autophagosomes gradually increased (Fig. 2H).
Therefore, through western blotting detection,
transmission electron microscopy and immunofluorescence, it can be
concluded that as the concentration of melatonin increased, the
ability of N2a neuroblastoma to undergo autophagy increased.
Effects of different concentrations of
melatonin on autophagy in N2a neuroblastoma cells via ER
stress
In order to further verify the relationship between
melatonin and ER stress and autophagy in neuroblastoma cells, the
effects of different concentrations of melatonin on autophagy in
neuroblastoma cells were detected by western blotting after
treatment with the ER stress inhibitor 4-PBA (Fig. 3A). The expression of p62 protein is
inversely proportional to the intensity of autophagy, so after the
inhibitor treatment, its expression increased, indicating that the
intensity of autophagy was weakened (Fig. 3B). Compared with the group treated
with the same concentration of melatonin, the expression of
autophagy-related proteins (ATG5, BECLIN1, LC3B) in the group
treated with 4-PBA was relatively reduced (Fig. 3C-E).
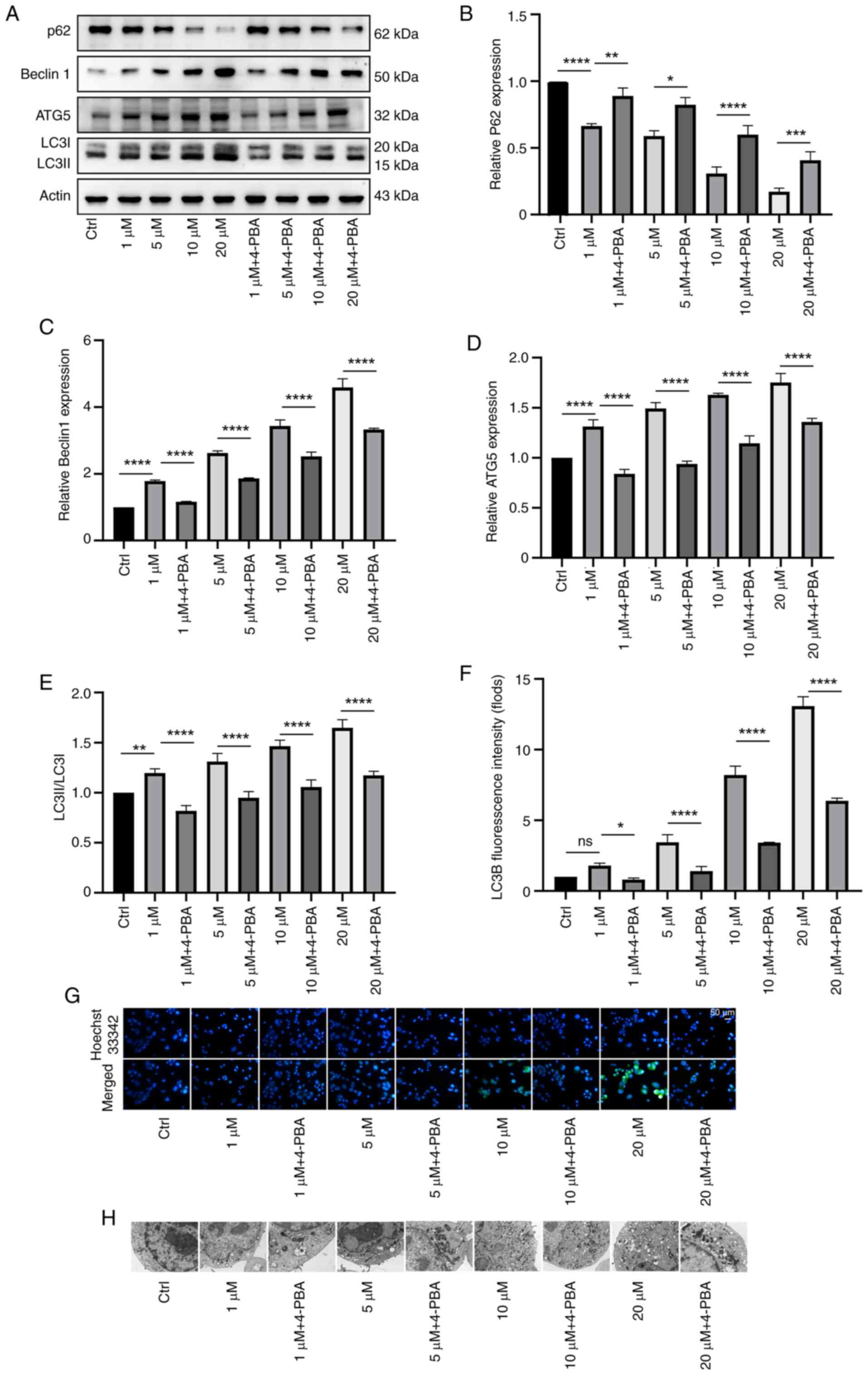 | Figure 3.Effects of different concentrations
of melatonin on autophagy in N2a neuroblastoma cells via ER stress.
(A) N2a cells were treated with melatonin at 1, 5, 10 and 20 µM
concentration and with the ER stress inhibitor 4-PBA. (B) The
expression of p62 protein was inversely proportional to the
intensity of autophagy, its expression increases. Compared with the
group treated with the same concentration of melatonin, the
expression of autophagy-related proteins (C) ATG5, (D) Beclin1, (E)
LC3B in the group treated with 4-PBA was relatively reduced. (F and
G) Immunofluorescence of LC3B (green) revealed that autophagy was
weakened after treatment with 4-PBA, and the green fluorescence was
reduced compared with that of the same concentration of melatonin.
(H) Electron microscopy revealed that the number of vacuolar
autophagosomes after treatment with 4-PBA was reduced compared with
that after treatment with the same concentration of melatonin.
*P<0.05, **P<0.01, ***P<0.001, ****P<0.0001. N2a,
Neuro-2a; ER, endoplasmic reticulum; 4-PBA, 4-phenylbutyric acid;
ATG5, autophagy-related protein 5; Mel, melatonin. |
Similarly, immunofluorescence (LC3B) revealed that
autophagy was weakened after treatment with 4-PBA, and the green
fluorescence was reduced compared with that of the same
concentration of melatonin (Fig. 3F
and G).
Electron microscopy revealed that the number of
vacuolar autophagosomes after treatment with 4-PBA was also reduced
compared with that after treatment with the same concentration of
melatonin (Fig. 3H).
Melatonin regulates Pak2 expression
and detects the activation of the downstream AMPK signaling pathway
of Pak2
Pak2 was tested by western blotting to detect
whether melatonin could regulate Pak2 expression, and the
activation of the AMPK signaling pathway downstream of Pak2 was
detected by western blotting. With the increase of melatonin
concentration, the expression of PAK2 protein gradually increased,
indicating that melatonin can regulate Pak2 expression. For all
shRNA and overexpression vector transfections, it was confirmed
that the transfection was successful (Fig. S1). In the detection of AMPK
signaling pathway: the total protein of MTOR and ULK1 remained
basically unchanged, but the expression of phosphorylated protein
of MTOR decreased, and the expression of phosphorylated protein of
ULK1 increased. This indicated that after melatonin treatment, the
MTOR activity in the AMPK signaling pathway was inhibited,
resulting in a decrease in the MTOR phosphorylation level. It
promoted the activity of ULK1, indicating that the expression of
Pak2 can affect the activation of the downstream AMPK signaling
pathway, thereby participating in regulating the initiation and
progress of cellular autophagy (Fig.
4A-D).
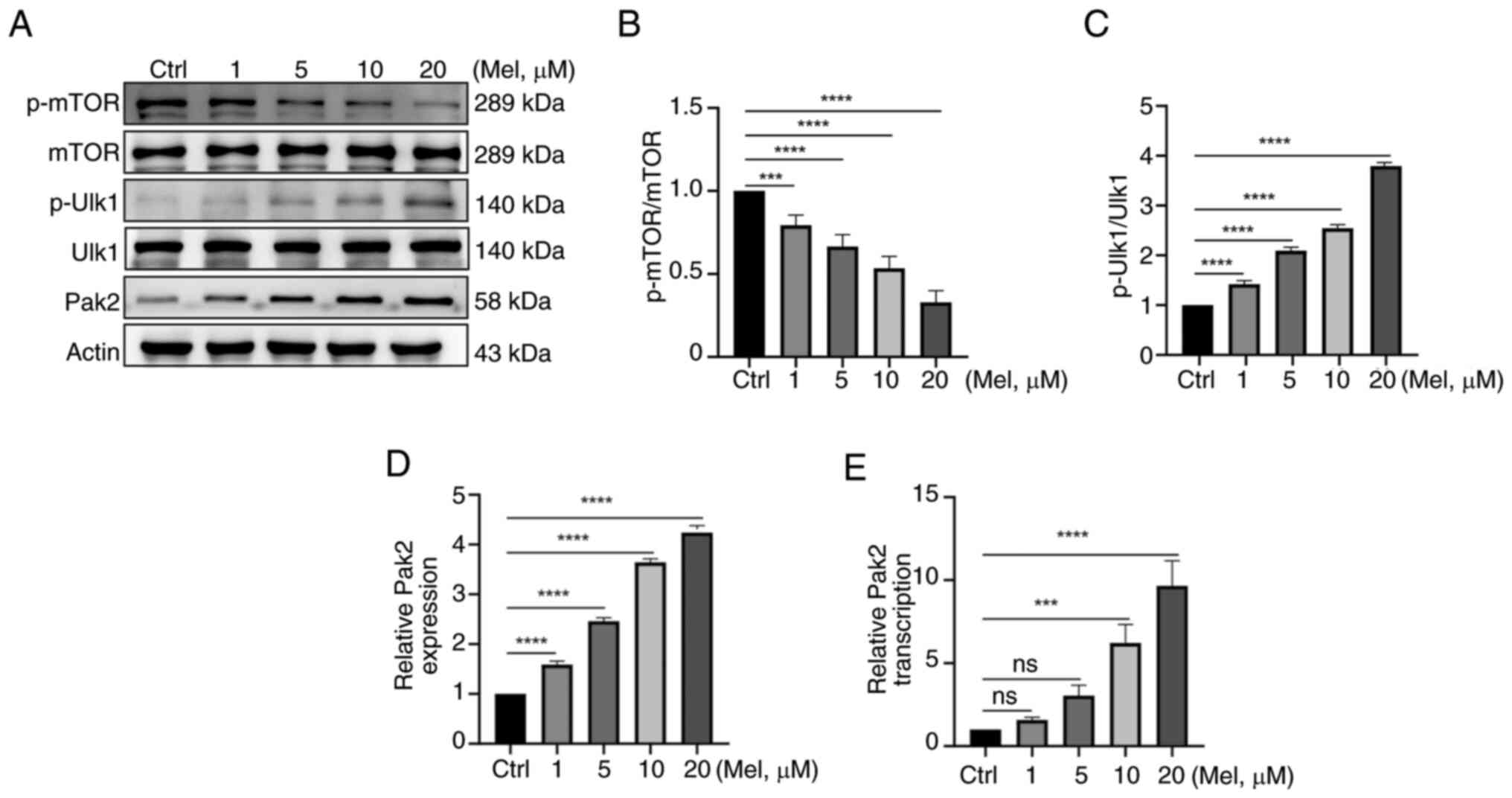 | Figure 4.Melatonin regulates Pak2 expression
and detects the activation of the downstream AMPK signaling pathway
of Pak2. (A) Pak2 and AMPK signaling pathway was tested by western
blotting. The total protein of mTOR and ULK1 remained unchanged;
(B) however, the expression of phosphorylated protein of mTOR
decreased and (C) the expression of phosphorylated protein of ULK1
increased. This indicated that after melatonin treatment, the mTOR
activity in the AMPK signaling pathway is inhibited. (D) With the
increase of melatonin concentration (1, 5, 10 and 20 µM), the
expression of Pak2 protein gradually increased. (E) Quantitative
PCR was used to detect the ability to regulate Pak2 expression
increases with the enhancement of melatonin concentration.
***P<0.001, ****P<0.0001. ns, not significant; Pak2,
p21-activated kinase 2; AMPK, 5′-AMP-activated protein kinase;
ULK1, unc-51-like kinase 1; Mel, melatonin. |
RNA was isolated from N2a cells treated with
different concentrations of melatonin and the ability to express
Pak2 was measured by qRT-PCR analysis. As the dose of melatonin
increased, especially when the melatonin concentration was ≥10 µm,
the ability of relative Pak2 transcription was significantly
increased (Fig. 4E).
Effects of different concentrations of
melatonin on ER stress and autophagy in N2a neuroblastoma
cells
A neuroblastoma cell model of Pak2 overexpression
and expression inhibition was constructed and western blotting was
used to detect the effects of different concentrations of melatonin
on ER stress in neuroblastoma cells. After constructing the Pak2
overexpression model, different concentrations of melatonin were
added and it was found that the expression of GRP94, GRP78 and CHOP
proteins increased compared with the Pak2 overexpression and Pak2
inhibition groups with the same concentration of melatonin. This
indicated that overexpression of Pak2 increased the level of
melatonin in alleviating ER stress in neuroblastoma; conversely,
inhibition of Pak2 expression reduces the level of melatonin in
alleviating ER stress in neuroblastoma (Fig. 5A-D).
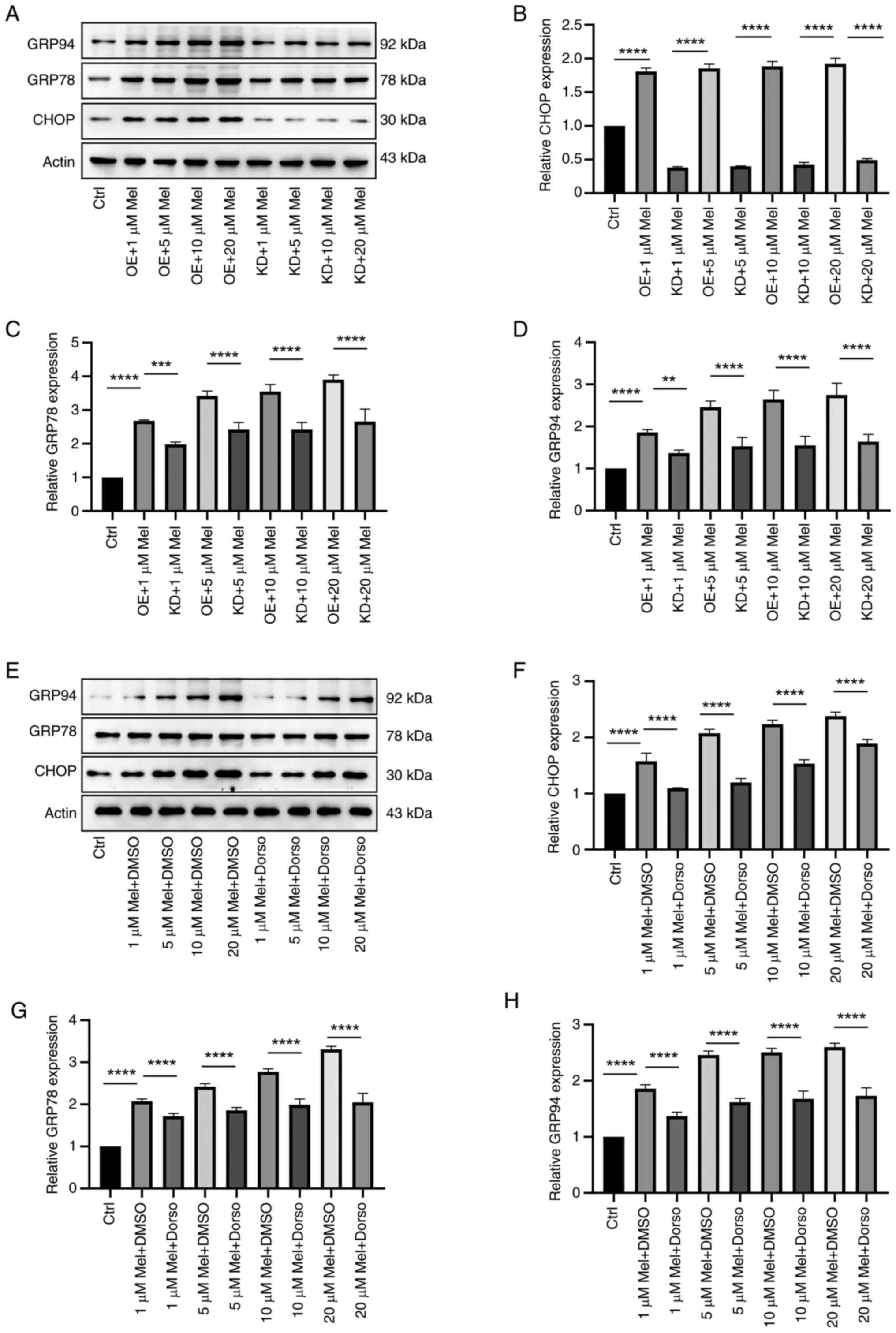 | Figure 5.Effects of different concentrations
of melatonin on ER stress and autophagy in N2a neuroblastoma cells.
(A) The model of Pak2 overexpression and expression inhibition was
constructed, and western blotting was used to detect the effects of
different concentrations of melatonin on ER stress in neuroblastoma
cells. (B-D) The expression of GRP94, GRP78 and CHOP proteins
increased compared with the Pak2 overexpression and Pak2 inhibition
groups with the same concentration of melatonin. (E) For the groups
of AMPK signaling pathway inhibitors and DMSO solvent at the same
melatonin concentration, the protein levels of GRP94, GRP78 and
CHOP were detected via Western blot analysis. Compared with the
AMPK signaling pathway inhibitor and DMSO solvent groups with the
same concentration of melatonin, the expression of (F) CHOP, (G)
GRP78 and (H) GRP94 proteins was weakened (F-H). **P<0.01,
***P<0.001, ****P<0.0001. ER, endoplasmic reticulum; N2a,
Neuro-2a; Pak2, p21-activated kinase 2; GRP, glucose-regulated
protein; AMPK, 5′-AMP-activated protein kinase; Mel, melatonin; OE,
Pak2 overexpression; KD, Pak2 knockdown; Dorso, dorsomorphin; DMSO,
solvent control group. |
To verify the accuracy, AMPK signaling pathway
inhibitors were added into neuroblastoma cells and western blotting
used to detect the effects of different concentrations of melatonin
on ER stress in neuroblastoma cells. The expression of GRP94, GRP78
and CHOP was compared by different concentrations of melatonin in
Dorso and DMSO. This indicates that inhibition of the AMPK
signaling pathway reduces the level of melatonin in alleviating ER
stress in neuroblastoma (Fig.
5E-H).
Discussion
Melatonin is an important immunomodulatory molecule
and exhibits inhibitory effects on cancer growth. Its anti-tumor
mechanism is complex and extensive, mainly including the regulation
of the estrogen action pathway, affecting the cell cycle,
regulating growth factors, interfering with calmodulin and tubulin
functions, increasing intercellular gap junctions, affecting cell
metabolism and antioxidant and immune-enhancing effects (29). The present study explored whether
melatonin could reduce ER stress in N2a neuroblastoma cells and
induce autophagy in N2a cells. It studied the relationship between
ER stress and autophagy in N2a cells under the action of melatonin,
observed the regulation of Pak2 expression by melatonin, detected
the activation of AMPK signaling pathway downstream of Pak2 and
constructed N2a cell models of Pak2 overexpression and expression
inhibition. In contrast to Xing et al (30), who applied the
hypoxia-reoxygenation (HR) model, proven that melatonin alleviates
ER stress in HR injury through the AMPK-Pak2 pathway, the present
study applied the tumor microenvironment stress model; the end
point of Xing et al (30)
was cell survival/apoptosis inhibition, whereas the present study
focused on autophagy activation. Xing et al (30) focused their analysis on apoptosis
inhibition, whereas the present study revealed autophagy
initiation. Hence, the present study aimed to demonstrate that
melatonin induces neuroblastoma autophagy by alleviating
Pak2-mediated ER stress, thereby inhibiting the growth of N2a
neuroblastoma cells.
Early studies have found that physiological
concentrations of melatonin can inhibit human neuroblastoma cells,
suggesting that melatonin has anti-proliferation and
pro-differentiation effects (31,32).
These studies support the present findings that low concentrations
of melatonin have the function of promoting differentiation and
inducing autophagy. High concentrations of melatonin could induce
cell cycle arrest at G2/M phase, activate caspase-3 and
lead to 75% cell apoptosis (33).
In a study of Alzheimer's disease, Singrang et al (34) found that melatonin was able to
inhibit hypoxia-induced amyloid-producing pathway, thereby
alleviating Alzheimer's disease-related pathological changes in
SH-SY5Y cells. This evidence confirms that melatonin triggers
downstream autophagy or apoptosis by alleviating ER stress and that
Pak2 is a key regulator. Melatonin upregulates Pak2 through AMPK,
which in turn inhibits mTOR and activates ULK1 to determine cell
fate (autophagy or apoptosis). In particular, Lee et al
(32) found that melatonin
promoted differentiation through HAS3-mitophagy, while the present
study found that melatonin inhibited tumor growth through Pak2-ER
stress-autophagy, both pointing to autophagy as a bridge between
differentiation and tumor suppression.
In the tumor microenvironment, multiple stressors
are enriched to dynamically perturb the protein folding capacity of
the ER of malignant tumor cells and stromal cells. In addition to
the adverse environmental conditions created by tumors, genetic
alterations in cancer cells can exacerbate ER stress and promote
sustained activation of the UPR pathway. Co-ordination of the ER
stress response is a highly dynamic process that can result in both
pro-survival and pro-apoptotic outputs. The intensity and duration
of UPR have decisive effects on the fate of cells and a previous
study has revealed the role of the ER stress response pathway in
the occurrence and development of cancer (35). GRP94 and GRP78 are UPR-driven ER
molecular chaperones whose purpose is to clear unfolded proteins
and restore ER homeostasis. A previous study has demonstrated that
melatonin reduces ER stress by activating ER-associated protein
degradation (36). Melatonin
inhibits the expression of GRP78 and GRP94 through
receptor-mediated mechanisms, blocks the excessive activation of
the ER stress signaling pathway and ultimately alleviates
BPA-induced testicular cell apoptosis and ER homeostasis imbalance
(37). Under conditions of
myocardial ischemia-reperfusion injury or HR stress, the protein
and mRNA levels of GRP78 are markedly upregulated. Melatonin can
markedly reduce the expression of GRP78 by activating Pak2
(38). Based on previous studies,
the present study used different concentrations of melatonin to act
on N2a cells and found that the expression of GRP94, GRP78 and CHOP
proteins increased, proving for the first time to the best of the
authors' knowledge that melatonin is also effective in controlling
ER stress response in N2a neuroblasts.
Autophagy is a key part of the way tumor cells
metabolize. Liu et al (39)
observed that autophagic degradation of CDK4 was responsible for
G0G1 cell cycle arrest in NVP-BEZ235-treated neuroblastoma cells,
and Liu et al (40) found
that Aβ(1–42) ginsenosides Rg1 and Rg2 activated autophagy and
alleviated oxidative stress in neuroblastoma cells overexpressing
Aβ(1–42). Melatonin also induces autophagy in neuroblastoma cells.
Lee et al (32) found that
melatonin promotes neuroblastoma cell differentiation by activating
hyaluronan synthase 3-induced mitochondrial autophagy. The present
study detected autophagy-related proteins ATG5, P62, BECLIN1 and
LC3BI/LC3BII by western blotting and observed the formation of
autophagosomes and immunofluorescence by electron microscopy and
found that autophagic activity increased with the increase of
melatonin concentration. At the same time, it used western blotting
to detect the effect of different concentrations of melatonin on
autophagy of neuroblastoma cells after treatment with the ER stress
inhibitor 4-PBA, and found that melatonin induces autophagy of
neuroblastoma cells by alleviating ER stress.
Pak2 is a novel stress response kinase localized to
the ER membrane. A study found that Pak2 is a new therapeutic
target for ER stress response (41). Xing et al (30) found that melatonin regulates the
expression of Pak2 through the AMPK pathway and that inhibition of
the AMPK pathway can inhibit melatonin-mediated Pak2 upregulation
and promote N2a cell death. The present study observed that with
the increase of melatonin concentration, the expression of PAK2
protein gradually increased and after melatonin treatment, although
the total protein of MTOR and ULK1 remained basically unchanged,
the expression of MTOR phosphorylated protein decreased and the
expression of ULK1 phosphorylated protein increased. The
aforementioned confirms that in neuroblastoma cells, the expression
of Pak2 following melatonin treatment can affect the activation of
the downstream AMPK signaling pathway, thereby participating in
regulating the initiation and progress of cellular autophagy. The
present study simultaneously constructed neuroblastoma cell models
with Pak2 overexpression and expression inhibition and added AMPK
signaling pathway inhibitors to neuroblastoma cells. It
demonstrated for the first time that inhibition of the AMPK
signaling pathway reduced the level of melatonin in alleviating ER
stress in neuroblastoma.
The present study is a preliminary exploration of
the effects of melatonin on autophagy in neuroblastoma cells. It
revealed for the first time its novel therapeutic effects in in
vitro experiments on neuroblastoma cells and delineated its
mechanism by alleviating the ER stress pathway. However, the
present study has its limitations. The results were only verified
in in vitro tumor cell experiments and further related
research is needed to confirm its effectiveness and safety in
clinical applications. In addition, although the present study
confirmed the therapeutic effect of melatonin on neuroblastoma
cells, the specific therapeutic mechanism still needs to be
verified through further research in animal models and clinical
trials.
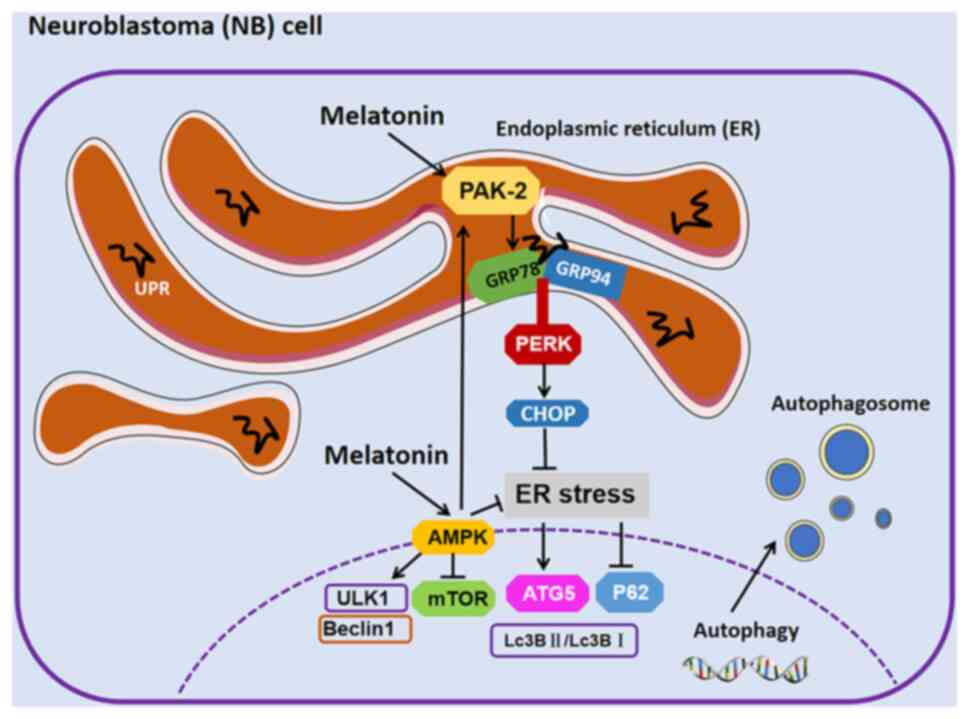
Taken together, the present study concluded that
melatonin induced autophagy in neuroblastoma by alleviating
Pak2-mediated ER stress (Fig.
6).
In conclusion, the present study demonstrated for
the first time to the best of the authors' knowledge that melatonin
has a therapeutic effect on neuroblastoma by alleviating
Pak2-mediated ER stress and inducing autophagy. Therefore, the
present study hypothesized that melatonin is a promising new drug
candidate for the treatment of neuroblastoma. In the future, more
effective combined treatment methods can be explored by combining
melatonin with other drugs or treatments, thereby improving the
comprehensive treatment effect of neuroblastoma.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by Guangzhou Health Science and
Technology Project (grant no. 20241A011036) and The Key R&D
Program of Guangzhou (grant no. 202206010006).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
QQ and XS designed the study. QQ, NZ, YX, JQ, GY and
XS performed the literature review, experiments and statistical
analysis. QQ, NZ and XS edited the manuscript. QQ and XS confirm
the authenticity of all the raw data. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Del Bufalo F, De Angelis B, Caruana I, Del
Baldo G, De Ioris MA, Serra A, Mastronuzzi A, Cefalo MG, Pagliara
D, Amicucci M, et al: GD2-CART01 for relapsed or refractory
High-risk neuroblastoma. N Engl J Med. 388:1284–1295. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Qiu B and Matthay KK: Advancing therapy
for neuroblastoma. Nat Rev Clin Oncol. 19:515–533. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Abbasi J: Mixed findings in pediatric
neuroblastoma CAR-T therapy trial. JAMA. 325:1212021. View Article : Google Scholar
|
|
4
|
Debnath J, Gammoh N and Ryan KM: Autophagy
and autophagy-related pathways in cancer. Nat Rev Mol Cell Biol.
24:560–575. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xia H, Green DR and Zou W: Autophagy in
tumour immunity and therapy. Nat Rev Cancer. 21:281–297. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bian Y, Li W, Kremer DM, Sajjakulnukit P,
Li S, Crespo J, Nwosu ZC, Zhang L, Czerwonka A, Pawłowska A, et al:
Cancer SLC43A2 alters T cell methionine metabolism and histone
methylation. Nature. 585:277–282. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim J, Kundu M, Viollet B and Guan KL:
AMPK and mTOR regulate autophagy through direct phosphorylation of
Ulk1. Nat Cell Biol. 13:132–141. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Egan DF, Shackelford DB, Mihaylova MM,
Gelino S, Kohnz RA, Mair W, Vasquez DS, Joshi A, Gwinn DM, Taylor
R, et al: Phosphorylation of ULK1 (hATG1) by AMP-activated protein
kinase connects energy sensing to mitophagy. Science. 331:456–461.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim J, Kim YC, Fang C, Russell RC, Kim JH,
Fan W, Liu R, Zhong Q and Guan KL: Differential regulation of
distinct Vps34 complexes by AMPK in nutrient stress and autophagy.
Cell. 152:290–303. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang Y, Zhao W, Xiao Z, Guan G, Liu X and
Zhuang M: A risk signature with four autophagy-related genes for
predicting survival of glioblastoma multiforme. J Cell Mol Med.
24:3807–3821. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bishayee K, Habib K, Nazim UM, Kang J,
Szabo A, Huh SO, Sadra A, et al: RNA binding protein HuD promotes
autophagy and tumor stress survival by suppressing mTORC1 activity
and augmenting ARL6IP1 levels. J Exp Clin Cancer Res. 41:182022.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ugun-Klusek A, Theodosi TS, Fitzgerald JC,
Burté F, Ufer C, Boocock DJ, Yu-Wai-Man P, Bedford L and Billett
EE: Monoamine oxidase-A promotes protective autophagy in human
SH-SY5Y neuroblastoma cells through Bcl-2 phosphorylation. Redox
Biol. 20:167–181. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Marciniak SJ, Chambers JE and Ron D:
Pharmacological targeting of endoplasmic reticulum stress in
disease. Nat Rev Drug Discov. 21:115–140. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Clarke HJ, Chambers JE, Liniker E and
Marciniak SJ: Endoplasmic reticulum stress in malignancy. Cancer
Cell. 25:563–573. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang R, Ma S, Zhuo R, Xu L, Jia S, Yang P,
Yao Y, Cao H, Ma L, Pan J and Wang J: Suppression of endoplasmic
reticulum stress-dependent autophagy enhances cynaropicrin-induced
apoptosis via attenuation of the P62/Keap1/Nrf2 pathways in
neuroblastoma. Front Pharmacol. 13:9776222022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Celesia A, Morana O, Fiore T, Pellerito C,
D'Anneo A, Lauricella M, Carlisi D, De Blasio A, Calvaruso G,
Giuliano M and Emanuele S: ROS-Dependent ER stress and autophagy
mediate the anti-tumor effects of tributyltin (IV) ferulate in
colon cancer cells. Int J Mol Sci. 21:81352020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
París-Coderch L, Soriano A, Jiménez C,
Erazo T, Muñoz-Guardiola P, Masanas M, Antonelli R, Boloix A, Alfón
J, Pérez-Montoyo H, et al: The antitumour drug ABTL0812 impairs
neuroblastoma growth through endoplasmic reticulum stress-mediated
autophagy and apoptosis. Cell Death Dis. 11:7732020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ge W, Yan ZH, Wang L, Tan SJ, Liu J,
Reiter RJ, Luo SM, Sun QY and Shen W: A hypothetical role for
autophagy during the day/night rhythm-regulated melatonin synthesis
in the rat pineal gland. J Pineal Res. 71:e127422021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Boga JA, Caballero B, Potes Y,
Perez-Martinez Z, Reiter RJ, Vega-Naredo I and Coto-Montes A:
Therapeutic potential of melatonin related to its role as an
autophagy regulator: A review. J Pineal Res. 66:e125342029.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fernández A, Ordóñez R, Reiter RJ,
González-Gallego J and Mauriz JL: Melatonin and endoplasmic
reticulum stress: Relation to autophagy and apoptosis. J Pineal
Res. 59:292–307. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang S, Tian W, Duan X, Zhang Q, Cao L,
Liu C, Li G, Wang Z, Zhang J, Li J, et al: Melatonin attenuates
diabetic cardiomyopathy by increasing autophagy of cardiomyocytes
via regulation of VEGF-B/GRP78/PERK signaling pathway. Cardiovasc
Diabetol. 23:192024. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang L, Liu K, Liu Z, Tao H, Fu X, Hou J,
Jia G and Hou Y: In pre-clinical study fetal hypoxia caused
autophagy and mitochondrial impairment in ovary granulosa cells
mitigated by melatonin supplement. J Adv Res. 64:15–30. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu S, Li L, Wu J, An S, Fang H, Han Y,
Huang Q, Chen Z and Zeng Z: Melatonin attenuates sepsis-induced
small-intestine injury by upregulating SIRT3-Mediated
oxidative-stress inhibition, mitochondrial protection, and
autophagy induction. Front Immunol. 12:6256272021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2002.
View Article : Google Scholar
|
|
25
|
De Almeida Chuffa LG, Seiva FRF, Silveira
HS, Cesário RC, da Silva Tonon K, Simão VA, Zuccari DAPC and Reiter
RJ: Melatonin regulates endoplasmic reticulum stress in diverse
pathophysiological contexts: A comprehensive mechanistic review. J
Cell Physiol. 239:e313832024. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lebeau PF, Wassef H, Byun JH, Platko K,
Ason B, Jackson S, Dobroff J, Shetterly S, Richards WG, Al-Hashimi
AA, et al: The loss-of-function PCSK9Q152H variant increases ER
chaperones GRP78 and GRP94 and protects against liver injury. J
Clin Invest. 131:e1286502021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cubillos-Ruiz JR, Bettigole SE and
Glimcher LH: Tumorigenic and immunosuppressive effects of
endoplasmic reticulum stress in cancer. Cell. 168:692–706. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vargas JNS, Hamasaki M, Kawabata T, Youle
RJ and Yoshimori T: The mechanisms and roles of selective autophagy
in mammals. Nat Rev Mol Cell Biol. 24:167–185. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li M, Hao B, Zhang M, Reiter RJ, Lin S,
Zheng T, Chen X, Ren Y, Yue L, Abay B, et al: Melatonin enhances
radiofrequency-induced NK antitumor immunity, causing cancer
metabolism reprogramming and inhibition of multiple pulmonary tumor
development. Signal Transduct Target Ther. 6:3302021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xing J, Xu H, Liu C, Wei Z, Wang Z, Zhao L
and Ren L: Melatonin ameliorates endoplasmic reticulum stress in
N2a neuroblastoma cell hypoxia-reoxygenation injury by activating
the AMPK-Pak2 pathway. Cell Stress Chaperones. 24:621–633. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cos S, Verduga R, Fernández-Viadero C,
Megías M and Crespo D: Effects of melatonin on the proliferation
and differentiation of human neuroblastoma cells in culture.
Neurosci Lett. 216:113–136. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee WJ, Chen LC, Lin JH, Cheng TC, Kuo CC,
Wu CH, Chang HW, Tu SH and Ho YS: Melatonin promotes neuroblastoma
cell differentiation by activating hyaluronan synthase 3-induced
mitophagy. Cancer Med. 8:4821–4835. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
García-Santos G, Antolín I, Herrera F,
Martín V, Rodriguez-Blanco J, del Pilar Carrera M and Rodriguez C:
Melatonin induces apoptosis in human neuroblastoma cancer cells. J
Pineal Res. 41:130–135. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Singrang N, Nopparat C, Panmanee J and
Govitrapong P: Melatonin inhibits Hypoxia-induced Alzheimer's
disease pathogenesis by regulating the amyloidogenic pathway in
human neuroblastoma cells. Int J Mol Sci. 25:52252024. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen X and Cubillos-Ruiz JR: Endoplasmic
reticulum stress signals in the tumour and its microenvironment.
Nat Rev Cancer. 21:71–88. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Choi SI, Lee E, Akuzum B, Jeong JB, Maeng
YS, Kim TI and Kim EK: Melatonin reduces endoplasmic reticulum
stress and corneal dystrophy-associated TGFBIp through activation
of endoplasmic reticulum-associated protein degradation. J Pineal
Res. 632017.doi: 10.1111/jpi.12426.
|
|
37
|
Qi Q, Feng L, Liu J, Xu D, Wang G and Pan
X: Melatonin alleviates BPA-induced testicular apoptosis and
endoplasmic reticulum stress. Front Biosci (Landmark Ed).
29:952024. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang S, Bian W, Zhen J, Zhao L and Chen W:
Melatonin-mediated Pak2 activation reduces cardiomyocyte death
through suppressing hypoxia reoxygenation Injury-induced
endoplasmic reticulum stress. J Cardiovasc Pharmacol. 74:20–29.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu Z, Wang XY, Wang HW, Liu SL, Zhang C,
Liu F, Guo Y and Gao FH: Autophagic degradation of CDK4 is
responsible for G0/G1 cell cycle arrest in NVP-BEZ235-treated
neuroblastoma. Cancer Biol Ther. 25:23855172024. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu Z, Cecarini V, Cuccioloni M, Bonfili
L, Gong C, Angeletti M and Eleuteri AM: Ginsenosides Rg1 and Rg2
activate autophagy and attenuate oxidative stress in neuroblastoma
cells overexpressing Aβ(1–42). Antioxidants (Basel). 13:3102024.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Binder P, Binder P, Wang S, Radu M, Zin M,
Collins L, Khan S, Li Y, Sekeres K, Humphreys N, et al: Pak2 as a
novel therapeutic target for cardioprotective endoplasmic reticulum
stress response. Circ Res. 124:696–711. 2019. View Article : Google Scholar : PubMed/NCBI
|