Allergic rhinitis (AR) is a type I hypersensitivity
reaction mediated by immunoglobulin (Ig)-E, driven by T helper 2
(Th2) cells and induced by allergen exposure in susceptible
individuals (1). The prevalence of
AR is increasing annually due to increasing environmental
pollution. Currently, its global prevalence is 5–50%, affecting
~500 million individuals and exhibiting a constant upward trend
(2). As a global health issue, AR
is a burdensome condition with a notable socioeconomic impact due
to its high prevalence, direct medical costs (3) and reduced productivity of affected
individuals (4,5). Typical AR symptoms include profuse
watery rhinorrhea, paroxysmal sneezing, nasal obstruction and
itching of the eyes and nose, with olfactory impairment also noted
in certain cases (6). AR involves
more than the classic symptoms, which are associated with daily
functioning impairments (1).
Inadequately controlled AR markedly impacts the sleep, daily
activities and work productivity of affected individuals, leading
to psychological stress and economic burden (7). It has been reported that 10–20% of
individuals in the United States are affected by AR, posing a
burden on the healthcare system. Indirect costs that are associated
with lost work time, missed diagnosis, over-prescription and
secondary effects further increase this burden (8).
Chronic and extensive occurrence of AR leads to
various complications and coexistence of multiple diseases,
including asthma (1,9), sleep disorders (10), chronic sinusitis (11) and olfactory dysfunction (12). Asthma has become a notable concern
in recent years (1). Several
studies support the ‘one airway, one disease’ concept, emphasizing
the interrelation and co-occurrence of diseases affecting the upper
and lower respiratory tracts due to their anatomical continuity and
similarity in disease mechanisms (13–15).
The World Health Organization and other notable guidelines have
highlighted the importance of combined treatment strategies for
such conditions (16–18).
AR can be treated both pharmacologically and
non-pharmacologically. Environmental control minimizing allergen
exposure is theoretically optimal (19). Various interventions, such as those
controlling dust mites, pets, air quality and humidity, reduce
allergen exposure (20,21). However, comprehensive and sustained
environmental control is often expensive and difficult to maintain,
particularly in patients with multiple allergies who may not be
able to completely prevent allergen exposure (22). Therefore, pharmacological
interventions are often required to control symptoms of AR. Current
first-line treatments include intranasal corticosteroids (INCSs),
antihistamines and leukotriene receptor antagonists. INCSs, which
reduce nasal inflammation, are typically recommended as topical
monotherapies for moderate-to-severe AR (23). However, despite providing temporary
symptom relief, INCSs can induce dependence, resistance and adverse
effects, including epistaxis (4–8% incidence with short-term use
and 20–28% incidence with 1-year use) (24). Further concerns include potential
growth suppression in children and increased risk of osteopenia,
osteoporosis, glaucoma and cataracts (25). Although immunotherapy exhibits
long-term efficacy, multiyear treatment limits its extensive use
(26).
Considering its high morbidity and limitations of
current treatments, novel therapies are urgently needed for AR
(26). Natural active ingredients
derived from medicinal plants have garnered notable interest for
their multi-target pharmacological activities and favorable safety
profiles (27–29). Specifically, saponins, a class of
bioactive plant-derived compounds present in herbs such as ginseng,
Panax notoginseng and Platycodon grandiflorus, exert
promising anti-allergic and immunomodulatory effects (30–32).
Their efficacies and mechanisms of action have been extensively
investigated in vivo and in vitro (27–32).
However, to the best of our knowledge, the latest advancements in
the use of saponins for AR treatment have not yet been
comprehensively reviewed.
In the present review, the therapeutic potential of
saponins for AR was comprehensively evaluated, particularly in
cases of asthma comorbidity. The underlying pathophysiology was
systematically analyzed, the multi-target mechanisms for AR pathway
modulation were elucidated and translational challenges and future
research directions were assessed, thereby providing a scientific
foundation for the development of next-generation AR
therapeutics.
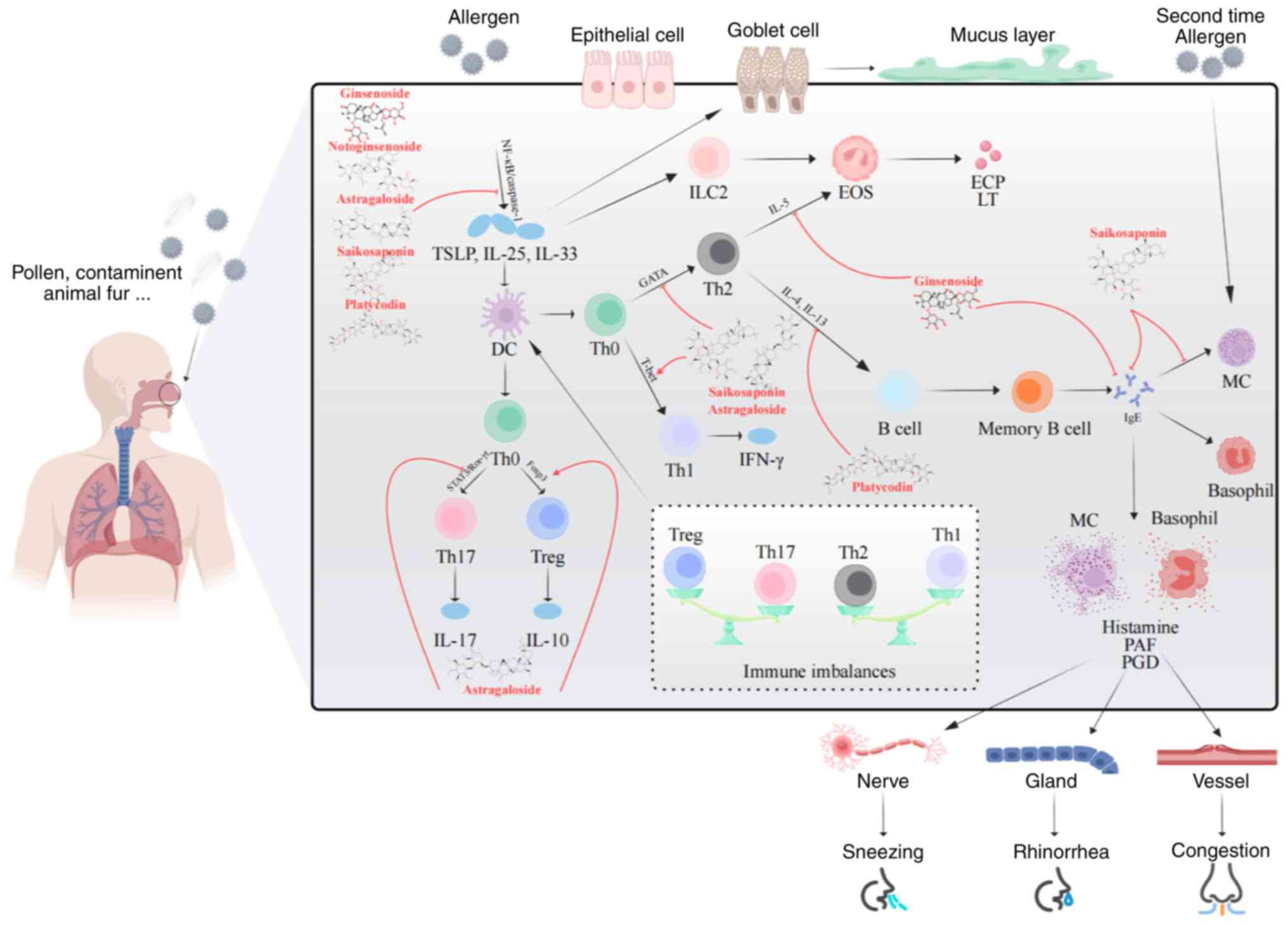
AR pathogenesis involves complex immunoregulatory
processes that are broadly categorized into sensitization and
elicitation phases. During sensitization, allergens are presented
to naive T (Th0) cells by antigen-presenting cells, such as
epithelial and dendritic cells, driving their differentiation into
Th2 cells (33). These Th2 cells
release cytokines (for example, IL-4, IL-5 and IL-13) stimulating B
cells to produce allergen-specific IgE antibodies (34), which bind to mast cells (MCs) and
basophils, priming the immune system (35). Elicitation involves both immediate-
and late-phase reactions. The immediate phase is characterized by
MC degranulation, release of mediators, such as histamine and
bradykinin, and triggering of symptoms, such as nasal itching,
sneezing and congestion (34). The
late phase, driven by mediators (for example, TNF-α, leukotriene B4
and IL-5) released from MCs, involves eosinophil and basophil
infiltration, leading to further tissue damage and worsening
symptoms such as nasal congestion and rhinorrhea (34,35).
Dysregulation of the immune system, including an imbalance in
Th1/Th2 responses (increased Th2 cell proportions), impaired
regulatory T cell (Treg)/Th17 balance (decreased Treg cell
proportions and increased Th17 cell proportions and IL-17 levels)
(36–39) and activation of type 2 innate
lymphoid cells (ILC2s), contributes to inflammation. ILC2s are
activated by cytokines, such as thymic stromal lymphopoietin, IL-25
and IL-33 from epithelial cells, which release IL-5 and IL-13 and
promote Th2 cell differentiation and eosinophil recruitment,
linking innate and adaptive immunity (40–42).
These dysregulated immune responses collectively contribute to the
development of AR (Fig. 1).
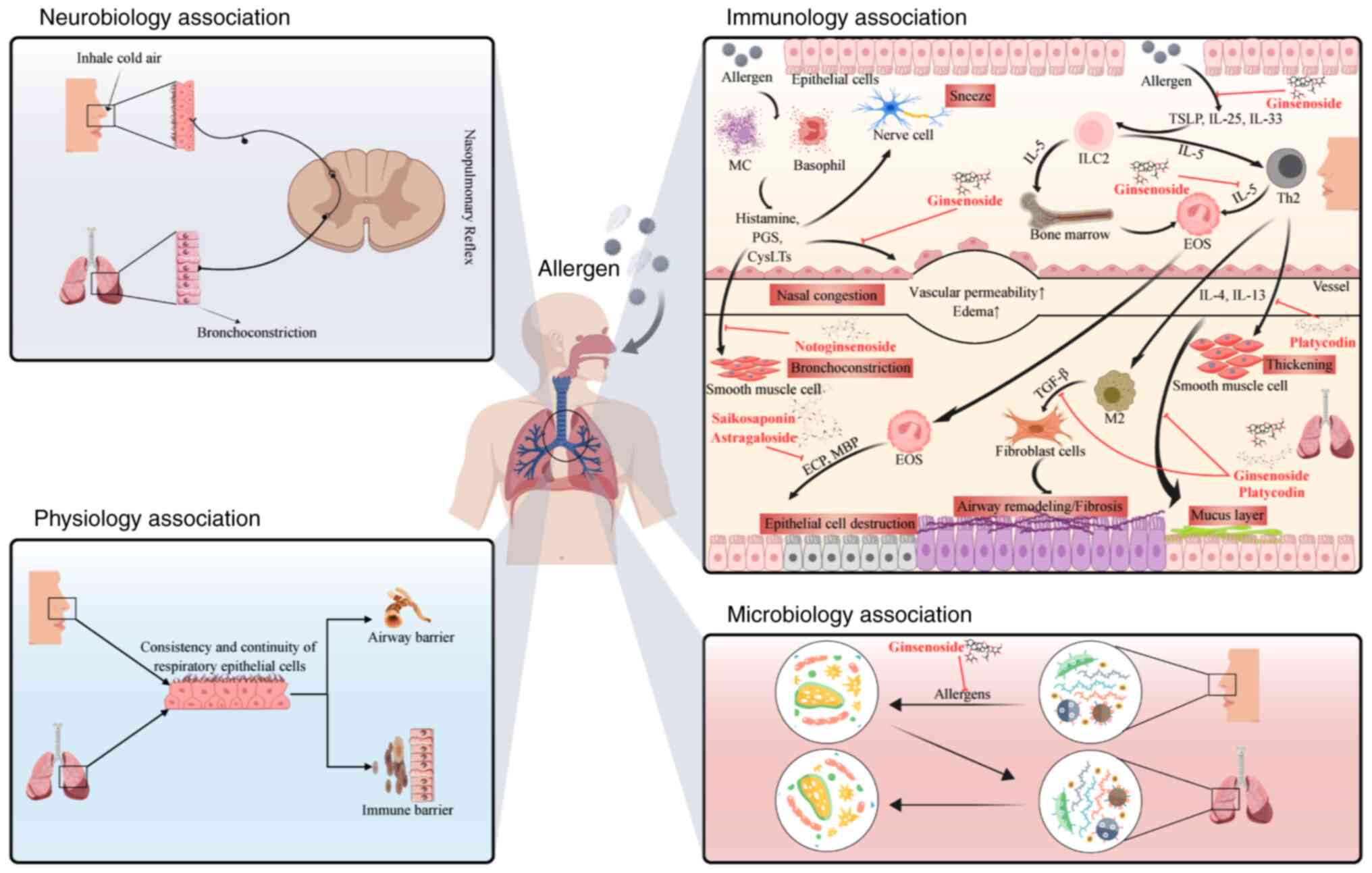
AR and asthma frequently coexist and exhibit a
bidirectional relationship, often conceptualized under the ‘one
airway, one disease’ paradigm (20–22).
Uncontrolled AR exacerbates asthma (42) and asthma worsens upper airway
inflammation (43). The present
review focuses on the mechanistic pathways by which AR is
complicated by asthma, a disease involving multiple interconnected
mechanisms (Fig. 2).
Anatomically, the respiratory tract, from the nose
to the bronchi, is a continuous mucosal surface. Inflammation
initiated in the nasal mucosa leads to systemic dissemination of
inflammatory mediators and cells, which subsequently affect the
lower airways, thereby inducing or worsening asthma (44). Physiologically, the nasobronchial
reflex provides a neural association and nasal irritation or
inflammation enhances reflex-mediated bronchoconstriction (45). Emerging evidence suggests that
dysbiosis of the nasal microbiome influences the lung
microenvironment via the circulation of immune cells or mediators,
potentially exacerbating asthmatic inflammation in AR (46,47).
AR and asthma share a core pathogenic mechanism
driven by Th2-type immune responses (19). Systemic inflammation in AR,
characterized by elevated Th2 cytokine levels in the circulation,
primes the lungs for inflammation, providing a fundamental
association with comorbidity (48). Even subclinical nasal inflammation
in patients with AR can signify generalized airway susceptibility
(49). This shared pathophysiology
is the reason for asthma being the most common comorbidity among
patients with AR, affecting >80% of cases (1) and highlights the necessity of
concurrently managing both conditions (50,51).
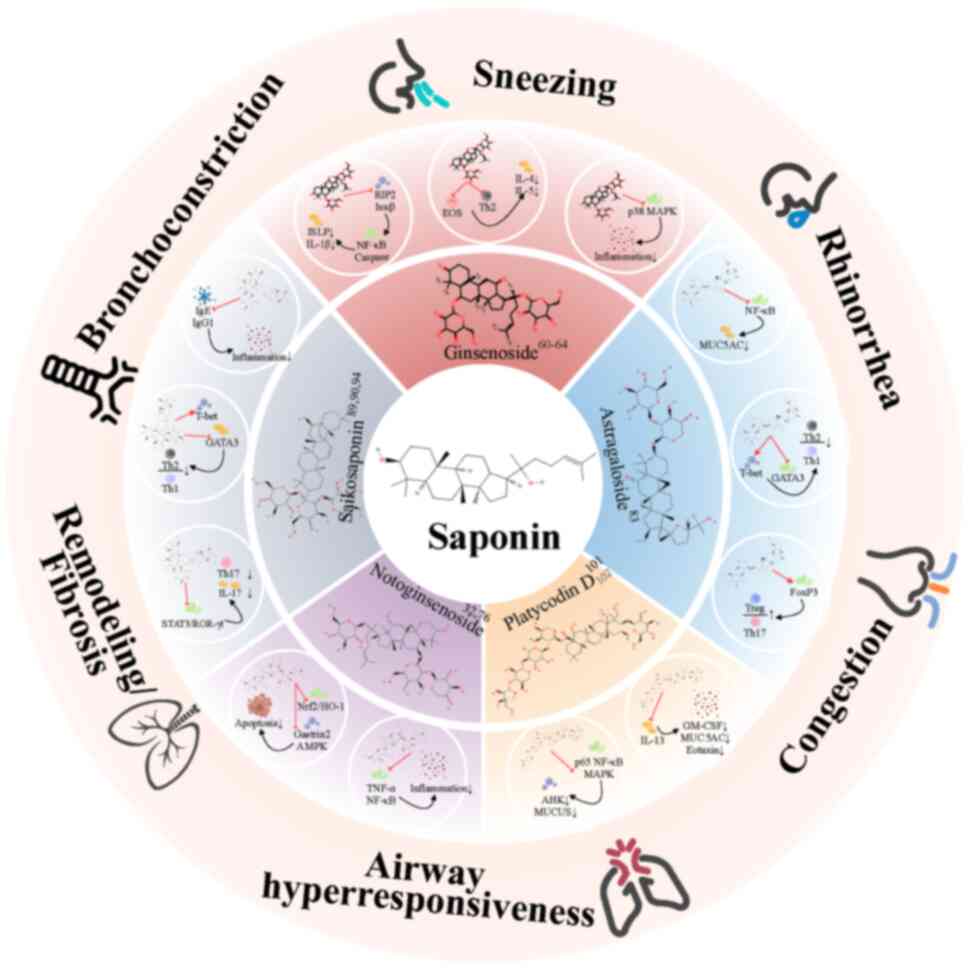
Saponins are a class of amphipathic glycosides
extensively distributed in various plants, marine organisms and
certain lower animals, such as ginseng, starfish and soft corals
(27–29). Their structure comprises a
hydrophobic aglycone (genin) coupled to ≥1 hydrophilic sugar
moieties via glycosidic bonds (52). Based on the aglycone structure,
saponins are primarily classified into two major types: Steroids
and triterpenoids (52). They
exhibit various pharmacological activities, including
immunomodulatory, anti-inflammatory, antifungal and antiviral
activities (30–32). The present review focuses on the
therapeutic effects and mechanisms of action of saponins derived
from five Chinese herbal medicines (CHMs) against AR (Fig. 1, Fig.
2, Fig. 3; Table SI).
One mechanism involves the inhibition of the release
of inflammatory mediators. For example, ginsenoside Rg3 reduces
cyclooxygenase-2 expression in IL-1β-stimulated adenocarcinoma
human alveolar basal epithelial (A549) cells and reduces C-C motif
chemokine ligand (CCL)-24, CCL11, CCL5, monocyte chemoattractant
protein-1, IL-6, IL-8, intercellular cell adhesion molecule-1 and
reactive oxygen species production in human bronchial epithelioid
(BEAS-2B) cells stimulated by IL-4 and TNF-α (57–59).
Kim et al (60) reported
that fermented red ginseng and ginsenoside Rd alleviate ovalbumen
(OVA)-induced AR in mice by inhibiting IgE, IL-4 and IL-5
expression. Ginsenosides also alleviate allergic reactions by
inhibiting the activation of inflammatory cells. Treatment of
inflammatory BEAS-2B cells with ginsenoside Rg3 reduces the
expression levels of CCL and pro-inflammatory cytokines and
adhesion of monocytes to BEAS-2B cells (58), thereby markedly reducing eosinophil
infiltration, oxidative stress, airway inflammation and airway
hyperresponsiveness in the lungs of asthmatic mice. Ginsenosides
also modulate key signaling pathways. For example, Rg1 reduces the
expression levels of receptor-interacting serine/threonine-protein
kinase 2 and inhibitor of κB kinase β and accumulation of NF-κB,
thereby inhibiting the production of thymic stromal lymphopoietin
in AR model mice (61). Li et
al (62) revealed that
ginsenoside Rh2 alleviates allergic airway inflammation by
modulating NF-κB activation and p38 mitogen-activated protein
kinase (MAPK) phosphorylation. Overall, ginsenosides act on
multiple targets and affect multiple pathological pathways to
improve AR symptoms. This has been validated by an in vivo
study integrating metabolomics and transcriptomics (63), which reported the downregulation of
the expression levels of three AR-related pro-inflammatory genes
are downregulated and upregulation of the expression levels of nine
anti-inflammatory genes. These expression patterns have also been
validated by other studies and revealed to be closely associated
with asthma (64–69). These studies revealed the potential
cause of AR with asthma and highlighted the therapeutic mechanisms
of ginsenosides via multi-target anti-AR and asthma effects. A
previous study identified the upregulation of COP9 signalosome
subunit 3 is potentially associated with Lactobacillus
helveticus and microbiome modulation (70). Collectively, these findings
underscore the potential of ginsenosides to treat both AR and
concurrent asthma via multitarget therapeutic effects (Fig. 2; Table SI).
NF-κB signaling pathway serves a key role in
inflammatory responses by regulating the expression levels of
various inflammatory genes (such as IL6, IL1B and
TNF) (90). Saikosaponins
markedly inhibit the activation of inflammatory pathways. A
previous study demonstrated the ability of saikosaponin to inhibit
NF-κB activation and reduce the expression levels of target genes
in macrophages (87).
Saikosaponins exert therapeutic effects against AR by inhibiting
inflammatory cell activation in the nasal mucosa and
pro-inflammatory factor production. Piao et al (88) reported that SSA inhibits the
activation of the IL-6/ STAT3/ROR-γt/IL-17 and NF-κB pathways,
thereby alleviating rhinitis symptoms, including nose rubbing and
sneezing, and suppressing nasal mucosal remodeling in an
OVA-induced AR mouse model. STAT3/ROR-γt pathway is closely
associated with Th17 cell differentiation and IL-17 production,
whereas T-bet/GATA3 pathway is associated with the balance of Th2
and Th1 cells (91,92). SSD alleviates inflammatory
reactions by modulating the T-bet/GATA3 and NF-κB pathway and
restoring the Th1/Th2 balance in AR model mice (89). SSD also alleviates allergic
reactions by downregulating IgE and IgG1 production in AR model
mice. Furthermore, SSA inhibits MC activation by targeting the MC
activation targets (for example, zyxin and A-23187), thereby
alleviating allergic asthma (93,94)
(Fig. 2; Table SI).
Previous studies have suggested the potential
therapeutic mechanisms of saponins against AR and asthma involve
multiple immunomodulatory pathways (including NF-κB, MAPK, Th1/Th2
and Th17/Treg pathways). Although relevant research on combined
treatment strategies for concurrent AR and asthma is currently
lacking, to the best of our knowledge, existing research suggests
that saponin components, with their multi-pathway and multi-target
immunomodulatory characteristics, synergistically regulate the
complex comorbid mechanisms of AR and concurrent asthma,
facilitating the unified treatment of the upper and lower
respiratory tracts and alleviating inflammation and symptoms of AR
and its complications (32,59,63,75,88,93,100,101)
(Fig. 2; Table SI). Consistently, He et al
(102) reported that Jieyu
Gubentang, a herbal formula containing saponin-based drugs,
simultaneously inhibits inflammatory cell infiltration and damage
in the nasal mucosa and lung tissues, reducing the overall allergic
reaction in the respiratory tract of rats, highlighting its
potential for the synergistic regulation of comorbidities. However,
further studies, including in-depth mechanistic studies and
clinical trials, are necessary to validate the efficacy and safety
of saponin-based therapies for AR complicated by asthma in the
future.
Saponins offer unique advantages over traditional
multiherbal Chinese medicinal formulas and conventional
pharmacotherapies for AR. There has been a resurgence of interest
in TCM, with increased research on the use of CHMs to treat complex
diseases, including AR (54–56,102,103). However, treatment often faces
challenges, such as complex herbal compositions, variable efficacy
and dependence on clinician expertise. The notable success of
artemisinin derived from Artemisia annua in treating malaria
provided a novel avenue for CHMs (104,105), with reduced safety concerns
associated with complex herbal mixtures and markedly enhanced
efficacy. Complex TCM formulas, for example, Biminne (106), Yu Ping Feng San (composed of
Astragalus membranaceus, Atractylodis macrocephalae rhizoma and
Saposhnikoviae radix) (107),
antiasthma simplified herbal medicine intervention (composed of
Ganoderma lucidum, Radix Sophorae flavescentis and Radix
Glycyrrhiza) (108) and Jieyu
Gubentang (composed of Bupleurum chinense, Angelica sinensis,
Paeonia lactiflora, Radix Glycyrrhiza, Magnolia biondii,
Cryptotympana pustulata, Perilla frutescens, Citrus reticulata,
Astragalus membranaceus, Atractylodes macrocephala, Saposhnikoviae
Radix and Xanthium strumarium) (102) exhibit variable efficacies,
multifaceted yet unclear mechanisms and standardization limitations
(36,55,102,109,110). By contrast, isolated saponins
(for example, ginsenosides, AS-IV, SSA, SSD and PLD) exhibit
defined chemical structures, facilitating improved standardization,
quality control, targeted mechanistic studies (for example,
modulation of NF-κB and MAPK pathways and Th1/Th2 and Treg/Th17
balance), potentially more predictable pharmacokinetics and a
reduced risk of toxicity or unknown interactions compared with
whole extracts or multi-herb mixtures. Unlike several
pharmaceutical drugs [for example, INCSs and antihistamines
(23,24,103)], antileukotrienes (111–113), which often focus on single
targets primarily for symptomatic relief and possibly lead to side
effects (such as epistaxis and growth concerns) or dependency on
long-term use (114), saponins
exert pleiotropic effects. Their potential to simultaneously
modulate multiple inflammatory and immune pathways (including
NF-κB, MAPK, Th1/Th2 and Th17/Treg pathways) offers a more holistic
approach to manage the complex pathophysiology of AR and concurrent
asthma, addressing systemic inflammation and neuroimmune
interactions more effectively compared with single-target agents,
while also possessing a favorable safety profile inherent to
natural products (Table SII).
The potential of saponins in more holistic and
multi-faceted approaches arises directly from their ability to
inhibit the occurrence and development of AR and its complications,
such as asthma, through multiple mechanisms. Unlike single-target
conventional drugs, they regulate Th1/Th2 and Th17/Treg imbalance,
reduce inflammatory factor levels in the serum, alleviate systemic
inflammation caused by AR and potentially improve gut microbiota
dysbiosis. In addition to regulating immune mechanisms, saponins
potentially alleviate AR and asthma symptoms by regulating
neuroimmune interactions. The nasobronchial reflex is a key neural
pathway connecting the upper and lower respiratory tracts (45); attenuation of local inflammation in
the nasal mucosa reduces its sensitivity, thereby alleviating the
lung symptoms resulting from this reflex. SSA exerts
neuroprotective effects, such as inhibiting neuronal apoptosis,
attenuating oxidative stress and suppressing neuroinflammation
(83). And by inhibiting
neuroinflammation, it possibly also reduces airway hyperreactivity.
Notably, pathological mechanisms of AR are heterogeneous, with
effective treatment requiring individualized approaches based on
specific biomarkers. Different saponins improve AR symptoms through
distinct therapeutic mechanisms, providing the option of selecting
suitable drugs for individualized and precise treatment. For
example, saponins inhibiting ILC2 activity (for example,
ginsenoside Rg1) can be selected for patients with primary ILC2
activation, whereas those regulating the Th17/Treg balance (for
example, SSA) can be used for patients with primary Th17 cell
activation (61,89). Furthermore, compared with
conventional treatments, saponins are typically derived from
natural resources, are renewable and offer various advantages in
terms of drug economics (27–29).
Despite promising preclinical findings, therapeutic
application of saponins for AR remains challenging. Low
bioavailability, resulting from their high molecular weight, high
glycosylation, poor water solubility and low oral absorption hinder
their effectiveness (115).
Nanotechnology offers a potential solution by enhancing
bioavailability via encapsulation (37,116–119). However, extensive application of
nanotechnology has limitations and research on the majority of
nanodrug formulations for AR treatment remains in the animal
experimental and in vitro research stages (37,119). Therefore, further research is key
to clarifying their safety and efficacy after application.
Furthermore, the existing research on saponins for AR treatment is
limited to animal experiments. Animal experimental results cannot
be used to accurately predict drug efficacy in humans (120). The majority of AR and asthma
mouse models are induced using histamine or OVA, with no comorbid
models for concurrent AR and asthma currently available. Therefore,
study results cannot be generalized to patients with simple AR or
AR complicated by asthma who are allergic to specific allergens
(for example, dust mites and pollen) (121). Therefore, development of suitable
comorbidity models and promotion of clinical studies are key
research directions for the future.
Currently, various drugs are used to treat AR;
however, their limited efficacy and adverse reactions pose major
concerns. Natural active ingredients, particularly saponins,
exhibit notable potential for individualized treatment of complex
AR because of their multi-target multi-pathway effects.
Furthermore, saponins from different sources provide therapeutic
advantages via distinct mechanisms. Additionally,
nanotechnology-based drug delivery systems exhibit the potential to
overcome the bioavailability limitations of saponins, further
enhancing their efficacy and targeting capacity. However, clinical
translation of saponins is limited by various challenges, including
low bioavailability, complex action mechanisms and a lack of
clinical evidence. Therefore, future studies should conduct
in-depth analyses of the mechanisms of action of saponins, develop
novel delivery systems and perform high-quality clinical trials to
potentially identify more effective and safer natural drug options
for AR treatment.
Not applicable.
The present review was funded by grants from the National
Natural Science Foundation of China (grant no. 82171127).
Not applicable.
YCL, BYL, ZYF and PTZ designed the present review.
FFL, KH, ZHX, YXH, YZ and SWC contributed to data collection,
analysis and interpretation. YHL, HFP and YCL supervised the
project and revised the manuscript. All authors read and approved
the final manuscript. Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Bousquet J, Van Cauwenberge P and Khaltaev
N; Aria Workshop Group; World Health Organization, : Allergic
rhinitis and its impact on asthma. J Allergy Clin Immunol. 108
(Suppl 5):S147–S334. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cox L: The role of allergen immunotherapy
in the management of allergic rhinitis. Am J Rhinol Allergy.
30:48–53. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hellgren J, Cervin A, Nordling S, Bergman
A and Cardell LO: Allergic rhinitis and the common cold-high cost
to society. Allergy. 65:776–783. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Devillier P, Bousquet J, Salvator H,
Naline E, Grassin-Delyle S and de Beaumont O: In allergic rhinitis,
work, classroom and activity impairments are weakly related to
other outcome measures. Clin Exp Allergy. 46:1456–1464. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vandenplas O, Vinnikov D, Blanc PD, Agache
I, Bachert C, Bewick M, Cardell LO, Cullinan P, Demoly P, Descatha
A, et al: Impact of rhinitis on work productivity: A systematic
review. J Allergy Clin Immunol Pract. 6:1274–1286.e9. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Seidman MD, Gurgel RK, Lin SY, Schwartz
SR, Baroody FM, Bonner JR, Dawson DE, Dykewicz MS, Hackell JM, Han
JK, et al: Clinical practice guideline: allergic rhinitis executive
summary. Otolaryngol Head Neck Surg. 152:197–206. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Juniper EF, Thompson AK, Ferrie PJ and
Roberts JN: Validation of the standardized version of the
rhinoconjunctivitis quality of life questionnaire. J Allergy Clin
Immunol. 104:364–369. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Katial R: Primary care: Clinics in office
practice. Preface. Prim Care. 35:xi–xii. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu Z, Fan Y, Nguyen T, Piao CH, Lee BH,
Lee SY, Shin HS, Kim TG, Song CH and Chai OH: Undaria pinnatifida
extract attenuates combined allergic rhinitis and asthma syndrome
by the modulation of epithelial cell dysfunction and oxidative
stress. Acta Biochim Biophys Sin (Shanghai). 57:792–804. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pagel JML and Mattos JL: Allergic rhinitis
and its effect on sleep. Otolaryngol Clin North Am. 57:319–328.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Grimm D, Hwang PH and Lin YT: The link
between allergic rhinitis and chronic rhinosinusitis. Curr Opin
Otolaryngol Head Neck Surg. 31:3–10. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang X, Zhou Y, Liu Z and Liu Y:
Olfactory dysfunction in allergic rhinitis. Clin Rev Allergy
Immunol. 68:32024. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bousquet J, Jacot W, Vignola AM, Bachert C
and Van Cauwenberge P: Allergic rhinitis: A disease remodeling the
upper airways? J Allergy Clin Immunol. 113:43–49. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bachert C, Vignola AM, Gevaert P, Leynaert
B, Van Cauwenberge P and Bousquet J: Allergic rhinitis,
rhinosinusitis, and asthma: One airway disease. Immunol Allergy
Clin North Am. 24:19–43. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Grossman J: One airway, one disease.
Chest. 111 (Suppl 2):11S–16S. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brozek JL, Bousquet J, Baena-Cagnani CE,
Bonini S, Canonica GW, Casale TB, van Wijk RG, Ohta K, Zuberbier T,
Schünemann HJ, et al: Allergic rhinitis and its impact on asthma
(ARIA) guidelines: 2010 Revision. J Allergy Clin Immunol.
126:466–476. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wise SK, Damask C, Roland LT, Ebert C,
Levy JM, Lin S, Luong A, Rodriguez K, Sedaghat AR, Toskala E, et
al: International consensus statement on allergy and rhinology:
Allergic rhinitis-2023. Int Forum Allergy Rhinol. 13:293–859. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Brożek JL, Bousquet J, Agache I, Agarwal
A, Bachert C, Bosnic-Anticevich S, Brignardello-Petersen R,
Canonica GW, Casale T, Chavannes NH, et al: Allergic rhinitis and
its impact on asthma (ARIA) guidelines-2016 revision. J Allergy
Clin Immunol. 140:950–958. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nappi E, Paoletti G, Malvezzi L, Ferri S,
Racca F, Messina MR, Puggioni F, Heffler E and Canonica GW:
Comorbid allergic rhinitis and asthma: Important clinical
considerations. Expert Rev Clin Immunol. 18:747–758. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Eggleston PA, Butz A, Rand C,
Curtin-Brosnan J, Kanchanaraksa S, Swartz L, Breysse P, Buckley T,
Diette G, Merriman B and Krishnan JA: Home environmental
intervention in inner-city asthma: A randomized controlled clinical
trial. Ann Allergy Asthma Immunol. 95:518–524. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nurmatov U, van Schayck CP, Hurwitz B and
Sheikh A: House dust mite avoidance measures for perennial allergic
rhinitis: An updated Cochrane systematic review. Allergy.
67:158–165. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bousquet J, Anto JM, Bachert C, Baiardini
I, Bosnic-Anticevich S, Walter Canonica G, Melén E, Palomares O,
Scadding GK, Togias A and Toppila-Salmi S: Allergic rhinitis. Nat
Rev Dis Primers. 6:952020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dykewicz MS, Wallace DV, Amrol DJ, Baroody
FM, Bernstein JA, Craig TJ, Dinakar C, Ellis AK, Finegold I, Golden
DBK, et al: Rhinitis 2020: A practice parameter update. J Allergy
Clin Immunol. 146:721–767. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bernstein JA, Bernstein JS, Makol R and
Ward S: Allergic rhinitis: A review. JAMA. 331:866–877. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Czech EJ, Overholser A and Schultz P:
Allergic rhinitis. Prim Care. 50:159–178. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pavón-Romero GF, Parra-Vargas MI,
Ramírez-Jiménez F, Melgoza-Ruiz E, Serrano-Pérez NH and Teran LM:
Allergen immunotherapy: Current and future trends. Cells.
11:2122022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen H, He Y, Chen S, Qi S and Shen J:
Therapeutic targets of oxidative/nitrosative stress and
neuroinflammation in ischemic stroke: Applications for natural
product efficacy with omics and systemic biology. Pharmacol Res.
158:1048772020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Islam MR, Islam F, Nafady MH, Akter M,
Mitra S, Das R, Urmee H, Shohag S, Akter A, Chidambaram K, et al:
Natural small molecules in breast cancer treatment: Understandings
from a therapeutic viewpoint. Molecules. 27:21652022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Katz L and Baltz RH: Natural product
discovery: Past, present, and future. J Ind Microbiol Biotechnol.
43:155–176. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gao H, Kang N, Hu C, Zhang Z, Xu Q, Liu Y
and Yang S: Ginsenoside Rb1 exerts anti-inflammatory effects in
vitro and in vivo by modulating toll-like receptor 4 dimerization
and NF-kB/MAPKs signaling pathways. Phytomedicine. 69:1531972020.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guo H and Liu MP: Mechanism of traditional
Chinese medicine in the treatment of allergic rhinitis. Chin Med J
(Engl). 126:756–760. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang Y, Song Y, Wang C, Jiang J, Liu S,
Bai Q, Li L, Jin H, Jin Y and Yan G: Panax notoginseng
saponin R1 attenuates allergic rhinitis through AMPK/Drp1 mediated
mitochondrial fission. Biochem Pharmacol. 202:1151062022.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Iinuma T, Kiuchi M, Hirahara K, Kurita J,
Kokubo K, Yagyu H, Yoneda R, Arai T, Sonobe Y, Fukuyo M, et al:
Single-cell immunoprofiling after immunotherapy for allergic
rhinitis reveals functional suppression of pathogenic
TH2 cells and clonal conversion. J Allergy Clin Immunol.
150:850–860.e5. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Galli SJ, Tsai M and Piliponsky AM: The
development of allergic inflammation. Nature. 454:445–454. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cheng L, Chen J, Fu Q, He S, Li H, Liu Z,
Tan G, Tao Z, Wang D, Wen W, et al: Chinese society of allergy
guidelines for diagnosis and treatment of allergic rhinitis.
Allergy Asthma Immunol Res. 10:300–353. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Park H, Li Z, Yang XO, Chang SH, Nurieva
R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q and Dong C: A distinct
lineage of CD4 T cells regulates tissue inflammation by producing
interleukin 17. Nat Immunol. 6:1133–1141. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shahgordi S, Sankian M, Yazdani Y,
Mashayekhi K, Hasan Ayati S, Sadeghi M, Saeidi M and Hashemi M:
Immune responses modulation by curcumin and allergen encapsulated
into PLGA nanoparticles in mice model of rhinitis allergic through
sublingual immunotherapy. Int Immunopharmacol. 84:1065252020.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wei P, Hu GH, Kang HY, Yao HB, Kou W, Liu
H, Zhang C and Hong SL: An aryl hydrocarbon receptor ligand acts on
dendritic cells and T cells to suppress the Th17 response in
allergic rhinitis patients. Lab Invest. 94:528–535. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xuekun H, Qintai Y, Yulian C and Gehua Z:
Correlation of gammadelta-T-cells, Th17 cells and IL-17 in
peripheral blood of patients with allergic rhinitis. Asian Pac J
Allergy Immunol. 32:235–239. 2014.PubMed/NCBI
|
|
40
|
Halim TY, Hwang YY, Scanlon ST, Zaghouani
H, Garbi N, Fallon PG and McKenzie AN: Group 2 innate lymphoid
cells license dendritic cells to potentiate memory TH2 cell
responses. Nat Immunol. 17:57–64. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Price AE, Liang HE, Sullivan BM, Reinhardt
RL, Eisley CJ, Erle DJ and Locksley RM: Systemically dispersed
innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad
Sci USA. 107:11489–11494. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lin L, Chen Z, Dai F, Wei JJ, Tang XY and
Sun GB: CD4+ T cells induce productions of IL-5 and
IL-13 through MHCII on ILC2s in a murine model of allergic
rhinitis. Auris Nasus Larynx. 46:533–541. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Marriott H, Duchesne M, Moitra S, Okoye I,
Gerla L, Mayers I, Moolji J, Adatia A and Lacy P: Upper airway
alarmin cytokine expression in asthma of different severities. J
Clin Med. 13:37212024. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Simons FE: Allergic rhinobronchitis: The
asthma-allergic rhinitis link. J Allergy Clin Immunol. 104:534–540.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Fontanari P, Burnet H, Zattara-Hartmann MC
and Jammes Y: Changes in airway resistance induced by nasal
inhalation of cold dry, dry, or moist air in normal individuals. J
Appl Physiol (1985). 81:1739–1743. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bousquet J, Khaltaev N, Cruz AA, Denburg
J, Fokkens WJ, Togias A, Zuberbier T, Baena-Cagnani CE, Canonica
GW, van Weel C, et al: Allergic rhinitis and its impact on asthma
(ARIA) 2008 update (in collaboration with the World Health
Organization, GA(2)LEN and AllerGen). Allergy. 63 (Suppl
86):S8–S160. 2008. View Article : Google Scholar
|
|
47
|
Chen M, He S, Miles P, Li C, Ge Y, Yu X,
Wang L, Huang W, Kong X, Ma S, et al: Nasal bacterial microbiome
differs between healthy controls and those with asthma and allergic
rhinitis. Front Cell Infect Microbiol. 12:8419952022. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Paiva Ferreira LKD, Paiva Ferreira LAM,
Monteiro TM, Bezerra GC, Bernardo LR and Piuvezam MR: Combined
allergic rhinitis and asthma syndrome (CARAS). Int Immunopharmacol.
74:1057182019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ciprandi G, Buscaglia S, Pesce G, Pronzato
C, Ricca V, Parmiani S, Bagnasco M and Canonica GW: Minimal
persistent inflammation is present at mucosal level in patients
with asymptomatic rhinitis and mite allergy. J Allergy Clin
Immunol. 96:971–979. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Adams RJ, Fuhlbrigge AL, Finkelstein JA
and Weiss ST: Intranasal steroids and the risk of emergency
department visits for asthma. J Allergy Clin Immunol. 109:636–642.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Crystal-Peters J, Neslusan C, Crown WH and
Torres A: Treating allergic rhinitis in patients with comorbid
asthma: The risk of asthma-related hospitalizations and emergency
department visits. J Allergy Clin Immunol. 109:57–62. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hamdi A, Viera-Alcaide I, Jiménez-Araujo
A, Rodríguez-Arcos R and Guillén-Bejarano R: Applications of
saponin extract from asparagus roots as functional ingredient.
Foods. 13:2742024. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Guan W and Qi W: Ginsenoside Rh2: A
shining and potential natural product in the treatment of human
nonmalignant and malignant diseases in the near future.
Phytomedicine. 118:1549382023. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Cheng L, Luo W, Ye A, Zhang Y, Li L and
Xie H: How to more effectively obtain ginsenoside Rg5:
Understanding pathways of conversion. Molecules. 28:73132023.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Li Q, Zhai C, Wang G, Zhou J, Li W, Xie L
and Shi Z: Ginsenoside Rh1 attenuates ovalbumin-induced asthma by
regulating Th1/Th2 cytokines balance. Biosci Biotechnol Biochem.
85:1809–1817. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Xu W, Lyu W, Duan C, Ma F, Li X and Li D:
Preparation and bioactivity of the rare ginsenosides Rg3 and Rh2:
An updated review. Fitoterapia. 167:1055142023. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Bae HM, Cho OS, Kim SJ, Im BO, Cho SH, Lee
S, Kim MG, Kim KT, Leem KH and Ko SK: Inhibitory effects of
ginsenoside re isolated from ginseng berry on histamine and
cytokine release in human mast cells and human alveolar epithelial
cells. J Ginseng Res. 36:369–374. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Huang WC, Huang TH, Yeh KW, Chen YL, Shen
SC and Liou CJ: Ginsenoside Rg3 ameliorates allergic airway
inflammation and oxidative stress in mice. J Ginseng Res.
45:654–664. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Lee IS, Uh I, Kim KS, Kim KH, Park J, Kim
Y, Jung JH, Jung HJ and Jang HJ: Anti-inflammatory effects of
ginsenoside Rg3 via NF-κB pathway in A549 cells and human asthmatic
lung tissue. J Immunol Res. 2016:75216012016. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Kim HI, Kim JK, Kim JY, Han MJ and Kim DH:
Fermented red ginseng and ginsenoside Rd alleviate
ovalbumin-induced allergic rhinitis in mice by suppressing IgE,
interleukin-4, and interleukin-5 expression. J Ginseng Res.
43:635–644. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Oh HA, Seo JY, Jeong HJ and Kim HM:
Ginsenoside Rg1 inhibits the TSLP production in allergic rhinitis
mice. Immunopharmacol Immunotoxicol. 35:678–686. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Li LC, Piao HM, Zheng MY, Lin ZH, Choi YH
and Yan GH: Ginsenoside Rh2 attenuates allergic airway inflammation
by modulating nuclear factor-κB activation in a murine model of
asthma. Mol Med Rep. 12:6946–6954. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Liu J, Yang N, Yi X, Wang G, Wang C, Lin
H, Sun L, Wang F and Zhu D: Integration of transcriptomics and
metabolomics to reveal the effect of ginsenoside Rg3 on allergic
rhinitis in mice. Food Funct. 14:2416–2431. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Bean CJ, Boulet SL, Ellingsen D, Pyle ME,
Barron-Casella EA, Casella JF, Payne AB, Driggers J, Trau HA, Yang
G, et al: Heme oxygenase-1 gene promoter polymorphism is associated
with reduced incidence of acute chest syndrome among children with
sickle cell disease. Blood. 120:3822–3828. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Emsley J, Knight CG, Farndale RW, Barnes
MJ and Liddington RC: Structural basis of collagen recognition by
integrin alpha2beta1. Cell. 101:47–56. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Islam T, McConnell R, Gauderman WJ, Avol
E, Peters JM and Gilliland FD: Ozone, oxidant defense genes, and
risk of asthma during adolescence. Am J Respir Crit Care Med.
177:388–395. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Liu B, Wang J and Ren Z: SKP2-Promoted
ubiquitination of FOXO3 promotes the development of asthma. J
Immunol. 206:2366–2375. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Yao X, Dai C, Fredriksson K, McCoy JP, Qu
X, Yu ZX, Keeran KJ, Zywicke GJ, Amar MJ, Remaley AT and Levine SJ:
5A, an apolipoprotein A-I mimetic peptide, attenuates the induction
of house dust mite-induced asthma. J Immunol. 186:576–583. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Wang M, Tang S, Yang X, Xie X, Luo Y, He
S, Li X and Feng X: Identification of key genes and pathways in
chronic rhinosinusitis with nasal polyps and asthma comorbidity
using bioinformatics approaches. Front Immunol. 13:9415472022.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Yamashita M, Miyoshi M, Iwai M, Takeda R,
Ono T and Kabuki T: Lactobacillus helveticus SBT2171
alleviates perennial allergic rhinitis in japanese adults by
suppressing eosinophils: A randomized, double-blind,
placebo-controlled study. Nutrients. 12:36202020. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Wang Y, Sun X, Xie Y, Du A, Chen M, Lai S,
Wei X, Ji L and Wang C: Panax notoginseng saponins alleviate
diabetic retinopathy by inhibiting retinal inflammation:
Association with the NF-κB signaling pathway. J Ethnopharmacol.
319:1171352024. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Yang H, Liu Z, Hu X, Liu X, Gui L, Cai Z
and Dai C: Protective effect of Panax notoginseng saponins
on apolipoprotein-E-deficient atherosclerosis-prone mice. Curr
Pharm Des. 28:671–677. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Uzayisenga R, Ayeka PA and Wang Y:
Anti-diabetic potential of Panax notoginseng saponins (PNS):
A review. Phytother Res. 28:510–516. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Xu C, Wang W, Wang B, Zhang T, Cui X, Pu Y
and Li N: Analytical methods and biological activities of Panax
notoginseng saponins: Recent trends. J Ethnopharmacol.
236:443–465. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Xue K, Ruan L, Hu J, Fu Z, Tian D and Zou
W: Panax notoginseng saponin R1 modulates TNF-α/NF-κB
signaling and attenuates allergic airway inflammation in asthma.
Int Immunopharmacol. 88:1068602020. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Zhang Y, Zhao Y, Ran Y, Guo J, Cui H and
Liu S: Notoginsenoside R1 attenuates sevoflurane-induced
neurotoxicity. Transl Neurosci. 11:215–226. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Li L, Hou X, Xu R, Liu C and Tu M:
Research review on the pharmacological effects of astragaloside IV.
Fundam Clin Pharmacol. 31:17–36. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Ren S, Zhang H, Mu Y, Sun M and Liu P:
Pharmacological effects of astragaloside IV: A literature review. J
Tradit Chin Med. 33:413–416. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Zhang J, Wu C, Gao L, Du G and Qin X:
Astragaloside IV derived from Astragalus membranaceus: A
research review on the pharmacological effects. Adv Pharmacol.
87:89–112. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Chen Z, Liu L, Gao C, Chen W, Vong CT, Yao
P, Yang Y, Li X, Tang X, Wang S and Wang Y: Astragali Radix
(Huangqi): A promising edible immunomodulatory herbal medicine. J
Ethnopharmacol. 258:1128952020. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Li K, Chen Y, Jiang R, Chen D, Wang H,
Xiong W, Li D, Liu Z, Li X, Li J and Yuan K: Protective effects of
astragaloside IV against ovalbumin-induced allergic rhinitis are
mediated by T-box protein expressed in T cells/GATA-3 and forkhead
box protein 3/retinoic acid-related orphan nuclear receptor γt. Mol
Med Rep. 16:1207–1215. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Guo J and Xu S: Astragaloside IV
suppresses histamine-induced inflammatory factors and mucin 5
subtype AC overproduction in nasal epithelial cells via regulation
of inflammation-related genes. Bioengineered. 12:6045–6056. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Tong Y, Zhao G, Shuang R, Wang H and Zeng
N: Saikosaponin a activates tet1/dll3/notch1 signalling and
promotes hippocampal neurogenesis to improve depression-like
behavior in mice. J Ethnopharmacol. 319:1172892024. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Luo H, Chen J, Su C and Zha L: Advances in
the bioactivities of phytochemical saponins in the prevention and
treatment of atherosclerosis. Nutrients. 14:49982022. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Chen MF, Huang CC, Liu PS, Chen CH and
Shiu LY: Saikosaponin a and saikosaponin d inhibit proliferation
and migratory activity of rat HSC-T6 cells. J Med Food. 16:793–800.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Peng D, Chen Y, Sun Y, Zhang Z, Cui N,
Zhang W, Qi Y, Zeng Y, Hu B, Yang B, et al: Saikosaponin A and its
epimers alleviate LPS-induced acute lung injury in mice. Molecules.
28:9672023. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Lu CN, Yuan ZG, Zhang XL, Yan R, Zhao YQ,
Liao M and Chen JX: Saikosaponin a and its epimer saikosaponin d
exhibit anti-inflammatory activity by suppressing activation of
NF-κB signaling pathway. Int Immunopharmacol. 14:121–126. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Piao CH, Song CH, Lee EJ and Chai OH:
Saikosaponin A ameliorates nasal inflammation by suppressing
IL-6/ROR-γt/STAT3/IL-17/NF-κB pathway in OVA-induced allergic
rhinitis. Chem Biol Interact. 315:1088742020. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Piaoa CH, Zou SC, Bui TT, Song CH and Chai
OH: Saikosaponin D inhibits nasal inflammation by regulating the
transcription factors T-box protein expressed in T cells/GATA-3 and
retinoic acid-related orphan nuclear receptor γt in a murine model
of allergic rhinitis. Heliyon. 9:e173192023. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Hayden MS and Ghosh S: Shared principles
in NF-kappaB signaling. Cell. 132:344–362. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Fu Y, Hu X, Cao Y, Zhang Z and Zhang N:
Saikosaponin a inhibits lipopolysaccharide-oxidative stress and
inflammation in human umbilical vein endothelial cells via
preventing TLR4 translocation into lipid rafts. Free Radic Biol
Med. 89:777–785. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Subbanna M, Shivakumar V, Talukdar PM,
Narayanaswamy JC, Venugopal D, Berk M, Varambally S,
Venkatasubramanian G and Debnath M: Role of IL-6/RORC/IL-22 axis in
driving Th17 pathway mediated immunopathogenesis of schizophrenia.
Cytokine. 111:112–118. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Bai H, Zhang Y, Zhang X, Li C, Ma M, Gao
J, Deng T, Gao C and Wang N: Zyxin-a novel detrimental target, is
inhibited by saikosaponin A during allergic asthma. Phytomedicine.
138:1564342025. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Park KH, Park J, Koh D and Lim Y: Effect
of saikosaponin-A, a triterpenoid glycoside, isolated from
Bupleurum falcatum on experimental allergic asthma. Phytother Res.
16:359–363. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Bailly C and Vergoten G: Proposed
mechanisms for the extracellular release of PD-L1 by the anticancer
saponin platycodin D. Int Immunopharmacol. 85:1066752020.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Wu JT, Yang GW, Qi CH, Zhou L, Hu JG and
Wang MS: Anti-inflammatory activity of platycodin D on
alcohol-induced fatty liver rats via TLR4-MYD88-NF-κB signal path.
Afr J Tradit Complement Altern Med. 13:176–183. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Song Y, Lv P and Yu J: Platycodin D
inhibits diabetic retinopathy via suppressing TLR4/MyD88/NF-κB
signaling pathway and activating Nrf2/HO-1 signaling pathway. Chem
Biol Drug Des. 103:e144192024. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Liu H, Xu L, Lu E, Tang C, Zhang H, Xu Y,
Yu Y, Ong N, Yang XD, Chen Q and Zheng Y: Platycodin D facilitates
antiviral immunity through inhibiting cytokine storm via targeting
K63-linked TRAF6 ubiquitination. J Leukoc Biol. 117:qiae0752025.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Peng F, Xiao F and Lin L: Protective
effects of platycodin D3 on airway remodeling and inflammation via
modulating MAPK/NF-κB signaling pathway in asthma mice. Evid Based
Complement Alternat Med. 2022:16128292022. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Wang B, Gao Y, Zheng G, Ren X, Sun B, Zhu
K, Luo H, Wang Z and Xu M: Platycodin D inhibits
interleukin-13-induced the expression of inflammatory cytokines and
mucus in nasal epithelial cells. Biomed Pharmacother. 84:1108–1112.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Zhang T, Yang S, Du J, Jinfu Y and Shumin
W: Platycodin D attenuates airway inflammation in a mouse model of
allergic asthma by regulation NF-κB pathway. Inflammation.
38:1221–1228. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
He Y, Liang Y, Fan M, Zhang J and Miao Q:
Jieyu Guben decoction alleviates combined allergic rhinitis and
asthma syndrome by balancing Th17/Treg expression and restoring
PPARD. Phytomedicine. 139:1565082025. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Lim CY, Moon JM, Kim BY, Lim SH, Lee GS,
Yu HS and Cho SI: Comparative study of Korean White Ginseng and
Korean Red Ginseng on efficacies of OVA-induced asthma model in
mice. J Ginseng Res. 39:38–45. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Tu Y: Artemisinin-A gift from traditional
Chinese medicine to the world (nobel lecture). Angew Chem Int Ed
Engl. 55:10210–10226. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Ma N, Zhang Z, Liao F, Jiang T and Tu Y:
The birth of artemisinin. Pharmacol Ther. 216:1076582020.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Zhao J, Yan X, Gai J, Han J, Zhang H, Luo
H, Huang S and Wang J: Efficacy of Bimin decoction for patients
with perennial allergic rhinitis: An open-label non-inferiority
randomized controlled trial. Trials. 20:8022019. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Nie J, Jiang X, Wang G, Xu Y, Pan R, Yu W,
Li Y and Wang J: Yu-Ping-Feng-San alleviates inflammation in atopic
dermatitis mice by TLR4/MyD88/NF-κB pathway. J Ethnopharmacol.
329:1180922024. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Busse PJ, Schofield B, Birmingham N, Yang
N, Wen MC, Zhang T, Srivastava K and Li XM: The traditional Chinese
herbal formula ASHMI inhibits allergic lung inflammation in
antigen-sensitized and antigen-challenged aged mice. Ann Allergy
Asthma Immuno. 104:236–246. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Chan HHL and Ng T: Traditional Chinese
medicine (TCM) and allergic diseases. Curr Allergy Asthma Rep.
20:672020. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Qin Z, Xie L, Li W, Wang C and Li Y: New
insights into mechanisms traditional Chinese Medicine for allergic
rhinitis by regulating inflammatory and oxidative stress pathways.
J Asthma Allergy. 17:97–112. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Cobanoğlu B, Toskala E, Ural A and Cingi
C: Role of leukotriene antagonists and antihistamines in the
treatment of allergic rhinitis. Curr Allergy Asthma Rep.
13:203–208. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Nayak A: A review of montelukast in the
treatment of asthma and allergic rhinitis. Expert Opin
Pharmacother. 5:679–686. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Zyryanov SK and Vozzhaev AV: Modern
approaches to rational combination pharmacotherapy of allergic
rhinitis. Vestn Otorinolaringol. 89:68–77. 2024.(In Russian).
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
McDonnell J, Weller K and Pien LC: Safety
of intranasal steroids: An updated perspective. Curr Allergy Asthma
Rep. 20:692020. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Yu K, Chen F and Li C: Absorption,
disposition, and pharmacokinetics of saponins from Chinese
medicinal herbs: What do we know and what do we need to know more?
Curr Drug Metab. 13:577–598. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Fan PS, Sun MJ, Qin D, Yuan CS, Chen XG
and Liu Y: Nanosystems as curative platforms for allergic disorder
management. J Mater Chem B. 9:1729–1744. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Ren Y, Yao D, Wu F, Xiao J, Ma L, Zhang Y,
Zhang Z, He G, Deng W, Qin B, et al: Tolerogenic nanovaccines for
the treatment of type I allergic diseases. J Control Release.
380:664–685. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Teng Z, Yang J, Chen X and Liu Y:
Intranasal morphology transformation nanomedicines for long-term
intervention of allergic rhinitis. ACS Nano. 17:25322–25334. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Ansari B, Abbaspour MR, Estajy A,
Haghnavaz N, Pordel S, Rezaee M, Shobeiri SS, Moghadam M, Hashemi M
and Sankian M: Development of fast-dissolving sublingual nanofibers
containing allergen and curcumin for immune response modulation in
a mouse model of allergic rhinitis. Naunyn Schmiedebergs Arch
Pharmacol. 397:7839–7856. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Cao F, Cheng MH, Hu LQ, Shen HH, Tao JH,
Li XM, Pan HF and Gao J: Natural products action on pathogenic cues
in autoimmunity: Efficacy in systemic lupus erythematosus and
rheumatoid arthritis as compared to classical treatments. Pharmacol
Res. 160:1050542020. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Skoner DP: Allergic rhinitis: Definition,
epidemiology, pathophysiology, detection, and diagnosis. J Allergy
Clin Immunol. 108 (Suppl 1):S2–S8. 2001. View Article : Google Scholar : PubMed/NCBI
|