|
1
|
Cox CM, Thoma ME, Tchangalova N, Mburu G,
Bornstein MJ, Johnson CL and Kiarie J: Infertility prevalence and
the methods of estimation from 1990 to 2021: A systematic review
and meta-analysis. Human Reprod Open. 12:hoac0512022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Minhas S, Boeri L, Capogrosso P, Cocci A,
Corona G, Dinkelman-Smit M, Falcone M, Jensen CF, Gül M, Kalkanli
A, et al: European association of urology guidelines on male sexual
and reproductive health: 2025 Update on male infertility. Eur Urol.
87:601–615. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang C, Li P and Li Z: Clinical
application of aromatase inhibitors to treat male infertility. Hum
Reprod Update. 28:30–50. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shahrokhi SZ, Salehi P, Alyasin A,
Taghiyar S and Deemeh MR: Asthenozoospermia: Cellular and molecular
contributing factors and treatment strategies. Andrologia.
52:e134632020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li Z, Li J, Li Y, Guo L, Xu P, Du H, Lin N
and Xu Y: The role of Cistanches Herba and its ingredients in
improving reproductive outcomes: A comprehensive review.
Phytomedicine. 129:1556812024. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jiang Y and Tu PF: Analysis of chemical
constituents in Cistanche species. J Chromatogr A. 1216:1970–1979.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Song Y, Zeng K, Jiang Y and Tu P:
Cistanches Herba, from an endangered species to a big brand of
Chinese medicine. Med Res Rev. 41:1539–1577. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jiang Z, Zhou B, Li X, Kirby GM and Zhang
X: Echinacoside increases sperm quantity in rats by targeting the
hypothalamic androgen receptor. Sci Rep. 8:38392018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao G, Wang Y, Lai Z, Zheng L and Zhao D:
Echinacoside protects against dysfunction of spermatogenesis
through the MAPK signaling pathway. Reprod Sci. 29:1586–1596. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cavarocchi E, Whitfield M, Saez F and
Touré A: Sperm ion transporters and channels in human
asthenozoospermia: Genetic etiology, lessons from animal models,
and clinical perspectives. Int J Mol Sci. 23:39262022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hwang JY and Chung JJ: CatSper calcium
channels: 20 years on. Physiology (Bethesda). 38:02023.PubMed/NCBI
|
|
12
|
Zhang X, Liang M, Song D, Huang R, Chen C,
Liu X, Chen H, Wang Q, Sun X, Song J, et al: Both protein and
non-protein components in extracellular vesicles of human seminal
plasma improve human sperm function via CatSper-mediated calcium
signaling. Hum Reprod. 39:658–673. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hwang JY, Wang H, Lu Y, Ikawa M and Chung
JJ: C2cd6-encoded CatSperτ targets sperm calcium channel to Ca(2+)
signaling domains in the flagellar membrane. Cell Rep.
38:1102262022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Young S, Schiffer C, Wagner A, Patz J,
Potapenko A, Herrmann L, Nordhoff V, Pock T, Krallmann C,
Stallmeyer B, et al: Human fertilization in vivo and in vitro
requires the CatSper channel to initiate sperm hyperactivation. J
Clin Invest. 134:e1735642024. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tamburrino L, Marchiani S, Minetti F,
Forti G, Muratori M and Baldi E: The CatSper calcium channel in
human sperm: Relation with motility and involvement in
progesterone-induced acrosome reaction. Hum Reprod. 29:418–428.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jin ZR, Fang D, Liu BH, Cai J, Tang WH,
Jiang H and Xing GG: Roles of CatSper channels in the pathogenesis
of asthenozoospermia and the therapeutic effects of
acupuncture-like treatment on asthenozoospermia. Theranostics.
11:2822–2844. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shu F, Zhou X, Li F, Lu D, Lei B, Li Q,
Yang Y, Yang X, Shi R and Mao X: Analysis of the correlation of
CATSPER single nucleotide polymorphisms (SNPs) with idiopathic
asthenospermia. J Assist Reprod Genet. 32:1643–1649. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tamburrino L, Marchiani S, Vicini E,
Muciaccia B, Cambi M, Pellegrini S, Forti G, Muratori M and Baldi
E: Quantification of CatSper1 expression in human spermatozoa and
relation to functional parameters. Hum Reprod. 30:1532–1544. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jalalabadi FN, Cheraghi E, Janatifar R and
Momeni HR: The detection of CatSper1 and CatSper3 expression in men
with normozoospermia and asthenoteratozoospermia and its
association with sperm parameters, fertilization rate, embryo
quality. Reprod Sci. 31:704–713. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Daigle M, Roumaud P and Martin LJ:
Expressions of Sox9, Sox5, and Sox13 transcription factors in mice
testis during postnatal development. Mol Cell Biochem. 407:209–221.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Roumaud P, Haché J and Martin LJ:
Expression profiles of Sox transcription factors within the
postnatal rodent testes. Mol Cell Biochem. 447:175–187. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang M, Ding H, Liu M, Gao Y, Li L, Jin C,
Bao Z, Wang B and Hu J: Genome wide analysis of the sox32 gene in
germline maintenance and differentiation in leopard coral grouper
(Plectropomus leopardus). Comp Biochem Physiol Part D Genomics
Proteomics. 54:1014022024. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Han F, Yin L, Jiang X, Zhang X, Zhang N,
Yang JT, Ouyang WM, Hao XL, Liu WB, Huang YS, et al: Identification
of SRY-box 30 as an age-related essential gatekeeper for male
germ-cell meiosis and differentiation. Aging Cell. 20:e133432021.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Diawara M and Martin LJ: Regulatory
mechanisms of SoxD transcription factors and their influences on
male fertility. Reprod Biol. 23:1008232023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wei L, Tang Y, Zeng X, Li Y, Zhang S, Deng
L, Wang L and Wang D: The transcription factor Sox30 is involved in
Nile tilapia spermatogenesis. J Genet Genomics. 49:666–676. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cerván-Martín M, Bossini-Castillo L,
Rivera-Egea R, Garrido N, Luján S, Romeu G, Santos-Ribeiro S;
IVIRMA Group and Lisbon Clinical Group and Castilla JA, ; et al:
Effect and in silico characterization of genetic variants
associated with severe spermatogenic disorders in a large Iberian
cohort. Andrology. 9:1151–1165. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gu X, Li H, Chen X, Zhang X, Mei F, Jia M
and Xiong C: PEX10, SIRPA-SIRPG, and SOX5 gene polymorphisms are
strongly associated with nonobstructive azoospermia susceptibility.
J Assist Reprod Genet. 36:759–768. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tu W, Liu Y, Shen Y, Yan Y, Wang X, Yang
D, Li L, Ma Y, Tao D, Zhang S and Yang Y: Genome-wide Loci linked
to non-obstructive azoospermia susceptibility may be independent of
reduced sperm production in males with normozoospermia. Biol
Reprod. 92:412015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zou S, Li Z, Wang Y, Chen T, Song P, Chen
J, He X, Xu P, Liang M, Luo K, et al: Association study between
polymorphisms of PRMT6, PEX10, SOX5, and nonobstructive azoospermia
in the Han Chinese population. Biol Reprod. 90:962014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mata-Rocha M, Hernández-Sánchez J,
Guarneros G, de la Chesnaye E, Sánchez-Tusié AA, Treviño CL, Felix
R and Oviedo N: The transcription factors Sox5 and Sox9 regulate
Catsper1 gene expression. FEBS Lett. 588:3352–3360. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang YN, Wang B, Liang M, Han CY, Zhang B,
Cai J, Sun W and Xing GG: Down-regulation of CatSper1 channel in
epididymal spermatozoa contributes to the pathogenesis of
asthenozoospermia, whereas up-regulation of the channel by
Sheng-Jing-San treatment improves the sperm motility of
asthenozoospermia in rats. Fertil Steril. 99:579–587. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Aguirre-Arias MV, Velarde V and Moreno RD:
Effects of ascorbic acid on spermatogenesis and sperm parameters in
diabetic rats. Cell Tissue Res. 370:305–317. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang T, Yin Q, Ma X, Tong MH and Zhou Y:
Ccdc87 is critical for sperm function and male fertility. Biol
Reprod. 99:817–827. 2018.PubMed/NCBI
|
|
34
|
Jin Z, Yang Y, Cao Y, Wen Q, Xi Y, Cheng
J, Zhao Q, Weng J, Hong K, Jiang H, et al: The gut metabolite
3-hydroxyphenylacetic acid rejuvenates spermatogenic dysfunction in
aged mice through GPX4-mediated ferroptosis. Microbiome.
11:2122023. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jin Z, Cao Y, Wen Q, Zhang H, Fang Z, Zhao
Q, Xi Y, Luo Z, Jiang H, Zhang Z and Hang J: Dapagliflozin
ameliorates diabetes-induced spermatogenic dysfunction by
modulating the adenosine metabolism along the gut microbiota-testis
axis. Sci Rep. 14:6412024. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lin SC, Lee HC, Hsu CT, Huang YH, Li WN,
Hsu PL, Wu MH and Tsai SJ: Targeting Anthrax toxin receptor 2
ameliorates endometriosis progression. Theranostics. 9:620–632.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kelly MC, Brown SG, Costello SM,
Ramalingam M, Drew E, Publicover SJ, Barratt CLR and Martins Da
Silva S: Single-cell analysis of [Ca2+]i signalling in sub-fertile
men: Characteristics and relation to fertilization outcome. Hum
Reprod. 33:1023–1033. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yin C, Liu B, Li Y, Li X, Wang J, Chen R,
Tai Y, Shou Q, Wang P, Shao X, et al: IL-33/ST2 induces
neutrophil-dependent reactive oxygen species production and
mediates gout pain. Theranostics. 10:12189–12203. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Song C, Han Y, Luo H, Qin Z, Chen Z, Liu
Y, Lu S, Sun H and Zhou C: HOXA10 induces BCL2 expression, inhibits
apoptosis, and promotes cell proliferation in gastric cancer.
Cancer Med. 8:5651–5661. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang ZX, Tian Y, Li S, Jing HB, Cai J, Li
M and Xing GG: Involvement of HDAC2-mediated kcnq2/kcnq3 genes
transcription repression activated by EREG/EGFR-ERK-Runx1 signaling
in bone cancer pain. Cell Commun Signal. 22:4162024. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kim TH and Dekker J: ChIP-quantitative
polymerase chain reaction (ChIP-qPCR). Cold Spring Harb Protoc. May
1–2018.(Epub ahead of print). View Article : Google Scholar
|
|
42
|
World Health Organization, . WHO
laboratory manual for the examination and processing of human
semen. Fifth Edition. World Health Organization; Geneva: pp. 26–44.
2010, https://iris.who.int/server/api/core/bitstreams/6fcf020b-c7f9-48ea-b3ee-c13402d7328e/contentFebruary
16–2023
|
|
43
|
Liu N, Zhang GX, Zhu CH, Lan XB, Tian MM,
Zheng P, Peng XD, Li YX and Yu JQ: Antinociceptive and
neuroprotective effect of echinacoside on peripheral neuropathic
pain in mice through inhibiting P2X7R/FKN/CX3CR1 pathway. Biomed
Pharmacother. 168:1156752023. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yang XP, Huang JH, Ye FL, Yv QY, Chen S,
Li WW and Zhu M: Echinacoside exerts neuroprotection via
suppressing microglial α-synuclein/TLR2/NF-κB/NLRP3 axis in
Parkinsonian models. Phytomedicine. 123:1552302024. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yang Z, Zhao Y, Wang Y, Liu X, Jiang Y,
Jiang Y, Liu T, Hu Y and Chang H: Echinacoside ameliorates
post-stroke depression by activating BDNF signaling through
modulation of Nrf2 acetylation. Phytomedicine. 128:1554332024.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lu R, Zhang L, Wang H, Li M, Feng W and
Zheng X: Echinacoside exerts antidepressant-like effects through
enhancing BDNF-CREB pathway and inhibiting neuroinflammation via
regulating microglia M1/M2 polarization and JAK1/STAT3 pathway.
Front Pharmacol. 13:9934832022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Liang J, Chen T, Xu H, Wang T, Gong Q, Li
T, Liu X, Wang J, Wang Y and Xiong L: Echinacoside exerts
antihepatic fibrosis effects in high-fat mice model by modulating
the ACVR2A-smad pathway. Mol Nutr Food Res. 68:e23005532024.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wei J, Zheng Z, Hou X, Jia F, Yuan Y, Yuan
F, He F, Hu L and Zhao L: Echinacoside inhibits colorectal cancer
metastasis via modulating the gut microbiota and suppressing the
PI3K/AKT signaling pathway. J Ethnopharmacol. 318:1168662024.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang X, Tan B, Liu J, Wang J, Chen M, Yang
Q, Zhang X, Li F, Wei Y, Wu K, et al: Echinacoside inhibits tumor
immune evasion by downregulating inducible PD-L1 and reshaping
tumor immune landscape in breast and colorectal cancer.
Phytomedicine. 135:1561882024. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yi Q, Sun M, Jiang G, Liang P, Chang Q and
Yang R: Echinacoside promotes osteogenesis and angiogenesis and
inhibits osteoclast formation. Eur J Clin Invest. 54:e141982024.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Jiang Z, Wang J, Li X and Zhang X:
Echinacoside and Cistanche tubulosa (Schenk) R. Wight ameliorate
bisphenol A-induced testicular and sperm damage in rats through
gonad axis regulated steroidogenic enzymes. J Ethnopharmacol.
193:321–328. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kong ZL, Johnson A, Ko FC, He JL and Cheng
SC: Effect of cistanche tubulosa extracts on male reproductive
function in streptozotocin-nicotinamide-induced diabetic rats.
Nutrients. 10:15622018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Guo Y, Wang L, Li Q, Zhao C, He P and Ma
X: Enhancement of kidney invigorating function in mouse model by
cistanches herba dried rapidly at a medium high temperature. J Med
Food. 22:1246–1253. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Afsar T, Razak S, Trembley JH, Khan K,
Shabbir M, Almajwal A, Alruwaili NW and Ijaz MU: Prevention of
testicular damage by indole derivative MMINA via upregulated StAR
and CatSper channels with coincident suppression of oxidative
stress and inflammation: In silico and in vivo validation.
Antioxidants (Basel). 11:20632022. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Cooray A, Chae MR, Wijerathne TD, Kim DG,
Kim J, Kim CY, Lee SW and Lee KP: Hexane fraction of Prunus
japonica thunb. Seed extract enhances boar sperm motility via
CatSper ion channel. Heliyon. 9:e136162023. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhou Y, Chen L, Han H, Xiong B, Zhong R,
Jiang Y, Liu L, Sun H, Tan J, Cheng X, et al: Taxifolin increased
semen quality of Duroc boars by improving gut microbes and blood
metabolites. Front Microbiol. 13:10206282022. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Mohammadi S, Jalali M, Nikravesh MR, Fazel
A, Ebrahimzadeh A, Gholamin M and Sankian M: Effects of vitamin-E
treatment on CatSper genes expression and sperm quality in the
testis of the aging mouse. Iran J Reprod Med. 11:989–998.
2013.PubMed/NCBI
|
|
58
|
Mohammadi S, Movahedin M and Mowla SJ:
Up-regulation of CatSper genes family by selenium. Reprod Biol
Endocrinol. 7:1262009. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Park EH, Kim DR, Kim HY, Park SK and Chang
MS: Panax ginseng induces the expression of CatSper genes and sperm
hyperactivation. Asian J Androl. 16:845–851. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Lambert SA, Jolma A, Campitelli LF, Das
PK, Yin Y, Albu M, Chen X, Taipale J, Hughes TR and Weirauch MT:
The human transcription factors. Cell. 172:650–665. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zeng L, Zhu Y, Moreno CS and Wan Y: New
insights into KLFs and SOXs in cancer pathogenesis, stemness, and
therapy. Semin Cancer Biol. 90:29–44. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Jasim SA, Farhan SH, Ahmad I, Hjazi A,
Kumar A, Jawad MA, Pramanik A, Altalbawy MAF, Alsaadi SB and
Abosaoda MK: A cutting-edge investigation of the multifaceted role
of SOX family genes in cancer pathogenesis through the modulation
of various signaling pathways. Funct Integ Genomics. 25:62025.
View Article : Google Scholar : PubMed/NCBI
|















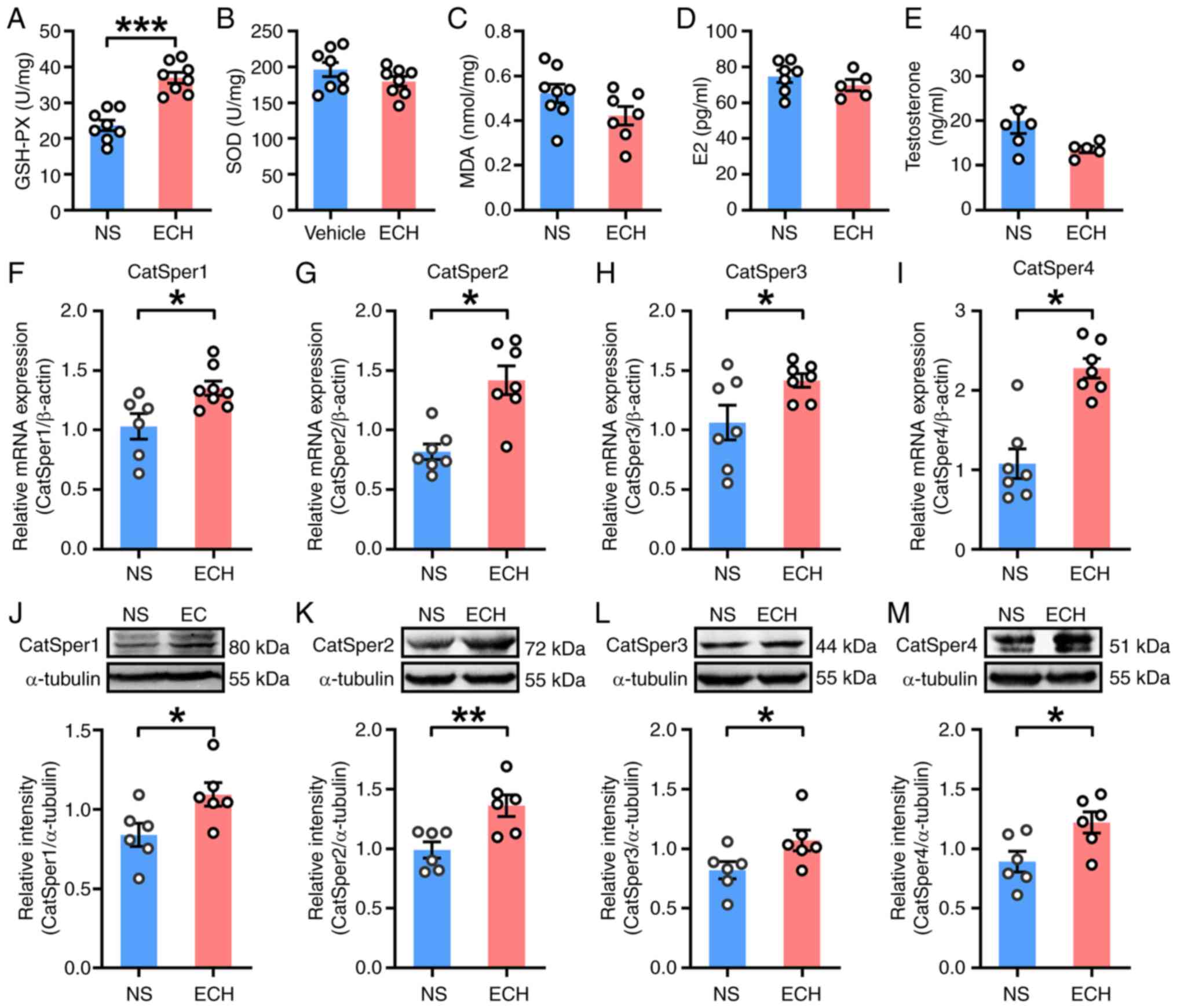
![ECH treatment upregulates functional
expression of CatSper channels in spermatozoa from rats with
asthenozoospermia. (A) CatSper1, (B) CatSper2, (C) CatSper3 and (D)
CatSper4 protein abundance in sperm; n=5–6 rats per group.
Representative fura-2-acetoxymethyl ester fluorescence images of
sperm before and after 30 mM NH4Cl stimulation in (E)
NS, (F) ECH, (G) NS + NNC and (H) ECH + NNC group. Arrows indicate
[Ca2+]i fluorescent signals in response to
NH4Cl. Scale bar, 10 µm. (I) Representative single-sperm
fluorescence traces. (J) Normalized [Ca2+]i
responses in all tested sperm. (K) Summary plot of normalized
[Ca2+]i signals after NH4Cl
treatment (n=27–33 sperm per group from 5–6 rats). NS group:
Ornidazole + normal saline; ECH group: Ornidazole + ECH. Data are
presented as mean ± standard error of the mean. *P<0.05,
**P<0.01 and ***P<0.001; Unpaired t-test. ECH, echinacoside;
NS, normal saline; NNC, NNC 55-0396; CatSper, cation channel of
sperm; [Ca2+]i, intracellular calcium levels;
F340, fluorescence at 340 nm; F380, fluorescence at 380 nm.](/article_images/mmr/33/3/mmr-33-03-13794-g04.jpg)
![Functional activation of CatSper
channels in human sperm by ECH treatment. Sperm motility parameters
in healthy subjects: (A) Grade A, (B) grade A + B and (C) grade A +
B + C sperm. Sperm motility parameters in patients with AZS: (D)
Grade A, (E) grade A + B and (F) grade A + B + C sperm. n=6–7
subjects per group. (G-J) Representative fura-2-acetoxymethyl ester
fluorescence images of human sperm before and after 30 mM
NH4Cl stimulation. Arrows indicate
[Ca2+]i responses. Scale bar, 10 µm. (K)
Representative single-sperm fluorescence traces. (L) Normalized
[Ca2+]i responses in all tested sperm. (M)
Summary plot of normalized [Ca2+]i signals
after NH4Cl treatment (n=33–36 sperm per group from 5–6
subjects). Data are presented as mean ± standard error of the mean.
*P<0.05, **P<0.01 and ***P<0.001. Paired t-test for (A-F),
unpaired t-test for (M). ECH, echinacoside; AZS, asthenozoospermia;
[Ca2+]i, intracellular calcium levels; NS,
normal saline; HS, healthy subjects; F340, fluorescence at 340 nm;
F380, fluorescence at 380 nm.](/article_images/mmr/33/3/mmr-33-03-13794-g06.jpg)