Introduction
Infertility is a prevalent global reproductive
health problem, affecting ~15% of couples of reproductive ages,
with ~50% of these cases attributed to male factors (1). Idiopathic asthenozoospermia (iAZS)
represents a primary cause of male infertility, yet no optimal
therapeutic strategies for iAZS have been established. Currently,
the non-invasive management of male infertility, including iAZS,
primarily involves changes in lifestyle, oxidative stress therapy,
prebiotic and probiotic supplements, hormone therapy and
enhancement of male gonadal function; however, the efficiency of
these approaches remains unsatisfactory (2–4).
Furthermore, ideal treatment methods (high efficacy with a
favorable safety profile) for asthenozoospermia (AZS) have not been
established. Thus, investigating novel therapeutic methods for male
reproductive disorders is required. The application of traditional
Chinese herbal medicine in reproductive health has garnered notable
attention (5,6). Echinacoside (ECH), a naturally
occurring compound with potentially beneficial biological activity,
has been increasingly recognized for its role in improving sperm
quality (5).
ECH, the most active component extracted from
Cistanche tubulosa (Schrenk) Wight which belongs to the
Asteraceae family, has exhibited extensive biological activities,
such as anti-inflammatory, antioxidant and antitumor effects
(6,7). Notably, ECH positively influences
male reproductive health by enhancing glutathione peroxidase
(GSH-PX) levels and the antioxidant capacity of testicular tissue,
thereby mitigating reproductive toxicity induced by bisphenol A or
lead acetate in male rats (5,8,9).
However, the molecular mechanisms underlying ECH-induced
improvements in sperm quality, particularly sperm motility, remain
poorly understood.
The cation channel of sperm (CatSper), which
comprises a minimum of 11 subunits, including four α subunits that
form the pore region, is specifically expressed in the testis and
localized in the principal section of sperm flagellum (10,11).
This channel mediates Ca2+ influx into the sperm,
thereby promoting capacitation, hyperactivation and participation
in sperm chemotaxis, making CatSper important for male fertility
and human fertilization (12–14).
In human sperm, the expression and function of CatSper channels are
closely linked to progressive motility and may contribute to the
pathogenesis of AZS (15,16). An association has been observed
between single nucleotide polymorphisms in CatSper1 and iAZS, as
well as between CatSper1 protein expression and progressive, total
and hyperactivated sperm motility (17,18).
Similarly, CatSper1 and CatSper3 mRNA expression levels are also
reduced in sperm from asthenoteratozoospermic males (19). In a previous study, we demonstrated
that decreased expression and function of CatSper resulted in
decreased sperm motility, while transcutaneous electrical acupoint
stimulation and electroacupuncture (EA) at a frequency of 2 Hz
exerted therapeutic effects on iAZS by inducing the functional
upregulation of CatSper channels in sperm (16). However, the roles and mechanisms of
CatSper channels in the therapeutic effects of ECH on iAZS remain
unclear.
The sex-determining region Y (Sry)-related
high-mobility-group (HMG)-box (Sox) family comprises a group of
transcription factors that are intricately involved in sex
determination and embryonic development (20). Specifically, Sox4, 8, 9 and 12 are
highly expressed in Sertoli cells, whereas Sox5, 6 and 30 are
predominantly expressed in spermatocytes and spermatozoa. These
transcription factors serve notable roles at distinct stages of
male reproductive development (21). Sox5, 6, 13, 30 and 32 coordinate
the regulation of gene expression within the testes, thereby
promoting spermatogenesis in adult males (22–25).
Sox5 is an important transcription factor implicated in
spermatogenesis and maturation (24). The mutation or aberrant expression
of Sox5 can result in spermatogenic dysfunction, ultimately
contributing to male infertility, and Sox5 has been identified as a
susceptibility gene for non-obstructive azoospermia (26–29).
In addition, the CatSper1 promoter contains four transcriptional
start sites (TSSs) and three functional Sox-binding sites (20). In vivo studies have
demonstrated that Sox5 can enhance the transactivation of the
CatSper1 promoter, suggesting that CatSper1 may serve as a target
gene of Sox5 (30).
The present study aimed to investigate whether ECH
treatment exerted its effects on AZS rats and patients with iAZS
through CatSper channels, as well as whether ECH treatment
upregulated CatSper1 protein expression through the activation of
Sox5.
Materials and methods
Chemicals, reagents and
antibodies
All chemicals, reagents and antibodies used are
listed in Table SI.
High-performance liquid chromatography
(HPLC) analysis
The HPLC was performed using an Agilent 1260
Infinity II system (Agilent Technologies). Separation was achieved
on a Phenomenex Luna 5 µm C18(A) (250.0×4.6 mm, 5 µm) maintained at
30°C. A sample volume of 10 µl was injected into the system. The
mobile phase consisted of two solvents: solvent A (0.1% formic acid
in water) and solvent B (acetonitrile). A gradient elution program
was applied with a flow rate of 1.0 ml/min: solvent B was increased
from 10 to 20% over 14 min.
Nuclear magnetic resonance (NMR)
spectroscopic analyses
NMR spectra were measured on a Varian INOVA-500 NMR
spectrometer (Varian Medical Systems Inc., USA), using
methanol-d4 as solvent, and the chemical shifts
were referenced to the solvent residual peak.
Animals
Sexually mature male Sprague-Dawley rats (age, 8
weeks; initial body weight, 200–230 g) were obtained from the
Department of Experimental Animal Sciences at Peking University
Health Science Center (Beijing, China). The rats were individually
kept in a climate-controlled environment at a temperature of 22±2°C
and a relative humidity of 50±10%, with a 12-h light/dark cycle and
free access to food and water. The health and behavior of rats were
monitored every day. A total of 59 rats were used in the present
study. The duration of the experiment was 30 days. Humane endpoints
were as follows: Complete anorexia or signs of depression
accompanied by hypothermia, observable as a body temperature
<37°C, without anesthesia or sedation. The rats were euthanized
by intraperitoneal injection of an overdose of 1% pentobarbital
sodium (300 mg/kg). Animal death was confirmed by respiratory and
cardiac arrest and pupil dilation was observed for ≥10 min. There
were no rats that reached the humane endpoints of the study. All
experimental procedures involving animals were approved by the
Animal Care and Use Committee of Peking University (Beijing, China)
prior to the initiation of the study (approval no. J2024179).
Animal model of AZS
A rat model of AZS was established through
intragastric administration of ornidazole (ORN) to rats, as
previously described with specific modifications (16). In brief, ORN was administered
intragastrically at a dose of 320 mg/kg body weight once daily for
30 consecutive days. Control rats received an equivalent volume of
0.2% carboxymethylcellulose sodium solution, the vehicle of ORN,
throughout the experimental period. On day 32, eight rats were
euthanized by intraperitoneal injection of an overdose of 1%
pentobarbital sodium (300 mg/kg), before their testes and
epididymides were immediately excised for further examination. The
development of the AZS rat model was confirmed by evaluating
epididymal sperm motility and count (16).
ECH treatment for animals
ECH (provided by Professor Yong Jiang) was stored at
room temperature prior to use (6,7). The
AZS model rats (n=35) were intragastrically administered low-dose
ECH (L-ECH; 6 mg/kg/day, 5 rats), middle-dose ECH (M-ECH; 18
mg/kg/day, 4 rats), high-dose ECH (H-ECH; 54 mg/kg/day, 13 rats) or
equal amounts of normal saline (NS; vehicle of ECH, 13 rats) once a
day on days 11–31. Meanwhile, both ECH- and NS-treated AZS model
rats were intragastrically administered ORN (320 mg/kg/d) once a
day to maintain the pathological state of iAZS. On day 32, the rats
were euthanized by intraperitoneal injection of an overdose of 1%
pentobarbital sodium (300 mg/kg).
Sperm motility and count
Cauda epididymal sperm were collected and processed
as previously described (31). In
brief, both caudal epididymides were excised and placed in a
modified N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid
(HEPES)-buffered medium with the following composition: 120 mM
NaCl, 2 mM KCl, 1.2 mM MgSO4, 0.36 mM
NaH2PO4, 25 mM NaHCO3, 10 mM
HEPES, 5.6 mM glucose and 1.1 mM sodium pyruvate. The medium was
supplemented with 100 IU/ml penicillin and 100 µg/ml streptomycin,
and the pH was adjusted to 7.4 using NaOH. Each cauda epididymis
was carefully dissected into three segments and incubated at 37°C
for 10 min in a humidified atmosphere containing 5% CO2.
Following incubation, the sperm suspension was filtered through a
nylon mesh to remove tissue debris, centrifuged at 2,000 × g for 5
min at room temperature and resuspended in 1 ml of pre-warmed
(37°C) NS. Sperm motility and kinematic parameters were analyzed
using a computer-assisted semen analysis (CASA) system with Olympus
CX33 light microscope (cat. no. WLJY-9000; Beijing Weili New
Century Science and Technology Development Co., Ltd.). Parameters
assessed included the percentage of rapid progressive motile sperm
(grade A; %), progressive motility (grades A + B; %), straight-line
velocity (VSL; µm/s), curve-line velocity (VCL; µm/s), average path
velocity (VAP; µm/s), amplitude of lateral head displacement (ALH;
µm), linearity (LIN; %), straightness (STR; %) and sperm viability
(31). Sperm concentration was
determined using the hemocytometer method with Olympus CX33 light
microscope and expressed as ×106/ml, based on two
independent semen sample preparations (16).
Assessment of sperm hyperactivation
and acrosome reaction
Rat sperm were capacitated in a modified
HEPES-buffered saline solution (HBSS) containing the following: 135
mM NaCl, 5 mM KCl, 1 mM MgSO4, 2 mM CaCl2, 20
mM HEPES, 5 mM glucose, 10 mM lactic acid, 1 mM Na-pyruvate, 5
mg/ml bovine serum albumin (BSA, Sigma-Aldrich; Merck KGaA), 15 mM
NaHCO3 and 30 mM NH4Cl. The solution was
adjusted to pH 7.4 with NaOH and sperm were incubated for 90 min at
37°C in a 5% CO2 incubator. Sperm motility was assessed
using a CASA system, with each analysis based on a minimum of 200
spermatozoa per sample. Sperm hyperactivation was defined as
follows: VCL >100 µm/s, ALH ≥2.0 µm, LIN ≤38.0% and wobble ≥16%
(32).
The NH4Cl-induced acrosome reaction in
rat sperm was evaluated using a previously described method
(33). Briefly, following
capacitation, sperm were pelleted using centrifugation at 2,000 × g
for 5 min at room temperature, washed twice with HBSS buffer and
then spread onto clean glass slides. The samples were air-dried and
fixed with 4% formaldehyde at room temperature for 15 min.
Acrosomes were labeled with 1 µM fluorescein
isothiocyanate-conjugated peanut agglutinin (PNA-FITC;
Sigma-Aldrich; Merck KGaA) and nuclei were counterstained with
4′,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich; Merck KGaA) for
60 min at room temperature. The acrosome reaction was identified by
the absence of PNA-FITC fluorescence in the sperm head region.
Stained samples were analyzed using a confocal laser scanning
microscope (Leica TCS SP8; Leica Microsystems, Inc.) (33). The percentage of sperm undergoing
the acrosome reaction was determined by analyzing a minimum of 200
sperm cells per sample.
Oxidative stress assessments
Commercial kits procured from the Nanjing Jiancheng
Bioengineering Institute were used to evaluate oxidative stress in
rat testicular tissues and assays were performed according to the
manufacturer's instructions and as previously described (34). The following assays were conducted:
GSH-PX activity was measured using a total GSH-PX assay kit with
NADPH (cat. no. A005); superoxide dismutase (SOD) activity was
assessed with a total SOD assay kit utilizing the water-soluble
tetrazolium salt method (cat. no. A001-3); and malondialdehyde
(MDA) levels, an indicator of lipid peroxidation, were determined
using a lipid peroxidation MDA assay kit (cat. no. A003-1)
(34).
ELISA
ELISA kits were used to determine the concentrations
of testosterone (T; cat. no. ml059506, Shanghai Enzyme-linked
Biotechnology Co., Ltd.), luteinizing hormone (LH; cat. no.
ml064293, Shanghai Enzyme-linked Biotechnology Co., Ltd.),
estradiol (E2; cat. no. mlc4525, Shanghai Enzyme-linked
Biotechnology Co., Ltd.) and follicle-stimulating hormone (FSH;
cat. no. ml059034, Shanghai Enzyme-linked Biotechnology Co., Ltd.)
in the plasma of rats according to the instructions of
manufacturer. Optical density (OD) values of each well were
measured at 450 nm using a Multiskan GO Microplate
Spectrophotometer (Thermo Fisher Scientific, Inc.). Subsequently,
the concentrations of the analytes in each well were calculated by
referencing their respective OD values to a standard curve derived
from serially diluted standard samples, fitted to a regression
model (35).
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from rat testicular tissues
using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). First-strand cDNA was synthesized from total RNA
using oligo(dT) primers and PrimeScript™ RT reagent kit (Takara
Corporation), according to the instructions of the manufacturers.
Each 20 µl reaction contained 1 µl dNTP Mixture, 1 µl Oligo dT
Primer, 1 µl Random 6 Primer, 1 µg Total RNA and was brought to
final volume using diethylpyrocarbonate-treated water. RT-qPCR was
performed using GoTaq qPCR Master Mix (Promega Corporation) on an
ABI 7500 Fast Real-Time PCR Detection System (Applied Biosystems;
Thermo Fisher Scientific, Inc.). Each 20 µl reaction contained 1 µl
cDNA template, 10 µl GoTaq qPCR Master Mix and 0.2 µM of each
primer and was brought to final volume using
diethylpyrocarbonate-treated water. β-actin was amplified
concurrently as an endogenous reference gene for normalization.
Primer sequences were described in a previous study (16). Thermal cycling conditions were as
follows: Initial denaturation at 95°C for 3 min; followed by 40
cycles of 95°C for 10 sec, 58°C for 20 sec and 72°C for 10 sec.
Relative mRNA expression levels were calculated using the
2−ΔΔCq method (16).
Western blotting
Rat sperm suspensions or testicular tissue fragments
were immediately homogenized in ice-cold lysis buffer [50 mM Tris
(pH 8.0); 150 mM NaCl; 1% NP-40; 0.5% sodium deoxycholate; 0.1%
SDS; and 1 mM PMSF]. Protein concentrations were determined using a
BCA assay kit (Pierce; Thermo Fisher Scientific, Inc.). Equal
protein aliquots (60 µg) were denatured, separated by 10% SDS-PAGE
and transferred onto PVDF membranes (Bio-Rad Laboratories, Inc.).
Membranes were blocked with 5% non-fat milk in Tris-buffered saline
with Tween 20 [20 mM Tris-HCl (pH 7.5); 150 mM NaCl; and 0.05%
Tween 20] for 1 h at room temperature, followed by overnight
incubation at 4°C with primary antibodies: Rabbit anti-rat
phosphotyrosine (1:1,000; cat. no. ab179530; Abcam), rabbit
anti-rat CatSper1 (1:100; cat. no. sc-33153; Santa Cruz
Biotechnology, Inc.), rabbit anti-rat CatSper2 (1:100; cat. no.
sc-98539; Santa Cruz Biotechnology, Inc.), rabbit anti-rat CatSper3
(1:100; cat. no. sc-98818; Santa Cruz Biotechnology, Inc.), goat
anti-rat CatSper4 (1:100; cat. no. sc-83126; Santa Cruz
Biotechnology, Inc.), mouse anti-rat Sox5 (1:100; cat. no.
sc-293215; Santa Cruz Biotechnology, Inc.), mouse anti-β-actin
(1:2,000; cat. no. sc-47778; Santa Cruz Biotechnology, Inc.) and
mouse anti-α-tubulin (1:2,000; cat. no. sc-32293; Santa Cruz
Biotechnology, Inc.). Membranes were then incubated for 1 h at room
temperature with HRP-conjugated secondary antibodies: Goat
anti-rabbit IgG (1:2,000; cat. no. sc-2004; Santa Cruz
Biotechnology, Inc.), goat anti-mouse IgG (1:2,000; cat. no.
sc-2005; Santa Cruz Biotechnology, Inc.) and rabbit anti-goat IgG
(1:2,000; cat. no. sc-2768; Santa Cruz Biotechnology, Inc.).
Protein bands were visualized using ECL Western Blotting Substrate
(Thermo Fisher Scientific, Inc.), captured by autoradiography
(Hyperfilm MP; GE Healthcare) and quantified with ImageJ software
2.16.0 (National Institutes of Health) (16,36).
Calcium imaging analysis
Sperm calcium imaging was performed as described
(16); the protocol was performed
at 37°C. Rat sperm were isolated from cauda epididymides by
swim-out in HBSS, washed and resuspended in HBSS supplemented with
5 mg/ml BSA (Sigma-Aldrich; Merck KGaA) (HBSS+). Human sperm were
purified using discontinuous Percoll® density gradients
(40–80% in Earle's balanced salt solution, Thermo Fisher
Scientific, Inc.) centrifuged at 600 × g for 20 min (room
temperature), then washed and resuspended in human tubal fluid
(HTF+) [97.8 mM NaCl, 4.69 mM KCl, 4 mM NaHCO3, 0.37 mM
KH2PO4, 2.04 mM CaCl2, 0.2 mM
MgCl2, 21.4 mM lactic acid, 21 mM HEPES, 2.78 mM glucose
and 0.33 mM Na-pyruvate (pH 7.3)] containing 3 mg/ml human serum
albumin (Sigma-Aldrich; Merck KGaA). Sperm were loaded with 5 µM
fura-2-acetoxymethyl ester (Fura-2-AM) and 0.05%
Pluronic® F-127 for 30 min in 5% CO2 in the
dark, washed and resuspended in HBSS (rat) or HTF (human;
Sigma-Aldrich; Merck KGaA) and adhered for 20 min to Cell-Tak™
(Corning, Inc.)-coated glass-bottom dishes (Wuxi Nice Life Science
& Technology Co., Ltd.). Rat sperm were pre-incubated in HBSS+
with or without 10 µM NNC 55–0396 for 5 min; human sperm were
incubated in HTF+ with or without 0.06 mg/ml ECH for 30 min.
Imaging was performed using a Polychrome V
monochromator at 340 nm excitation on an Olympus IX-71 inverted
microscope with ×40 (rat) or ×100 (human) objectives. Emissions
(515–565 nm) were filtered using a HQ540/50 filter and captured
every 100 msec at 5-sec intervals using a CoolSNAP HQ CCD camera. F
was monitored before and after the application of 30 mM
NH4Cl. Fura-2-AM signals were expressed as
background-subtracted F340/F380 ratios normalized to the baseline
(Fbaseline, mean intensity pre-treatment).
Ftreatment and Fbefore represented
intensities after and before NH4Cl application. Images
were analyzed using MetaFluor v7 software (Molecular Devices, LCC)
(16,37,38).
Immunofluorescent staining
To prepare testis tissues for immunofluorescence
staining, rats were anesthetized using 1% pentobarbital sodium (50
mg/kg) and underwent intracardiac perfusion with 300 ml of 0.1 M
phosphate buffer (PBS) followed by 300 ml 4% paraformaldehyde.
Testes were harvested and post-fixed in 4% paraformaldehyde (in 0.1
M PBS; pH 7.4) at 4°C for 18 h (16). Following fixation, tissues were
paraffin-embedded and sectioned (5 µm). Paraffin-embedded samples
were dewaxed by xylene followed by immersing in 100% ethanol for
three times (5 min/time). Later, slides were sequentially immersed
in 95, 80, and 70% ethanol and finally washed by double distilled
water (36). Sections underwent
antigen retrieval by heating at 95°C in EDTA buffer (cat. no.
ZLI-9071; Beijing Zhongshan Jinqiao Biotechnology Co., Ltd.) for 30
min, followed by cooling to room temperature. Subsequently, samples
were blocked with 10% donkey serum (Sigma-Aldrich; Merck KGaA)
containing 0.3% Triton X-100 in 0.1 M PBS for 1 h at room
temperature, sections were incubated overnight at 4°C with rabbit
anti-rat CatSper1 (1:100) and mouse anti-rat Sox5 (1:100) primary
antibodies diluted in 1% donkey serum and PBS. Following three PBS
washes, sections were incubated for 1 h at room temperature with
secondary antibodies: Cy™3-conjugated donkey anti-mouse IgG (H+L)
and Alexa Fluor 488-conjugated donkey anti-rabbit IgG (H+L) (1:500;
Jackson ImmunoResearch Laboratories, Inc.) (34). Details of secondary antibodies are
listed in Table SI. Nuclei were
counterstained with DAPI (100 ng/ml; Sigma-Aldrich) at room
temperature for 10 min. After three final PBS washes, slides were
mounted in Gel-Mount medium. Imaging was performed using a confocal
microscope (Zeiss LSM880; Zeiss AG) with excitation wavelengths of
488 (green), 555 (red) and 405 nm (blue) (34).
Sequence analysis
Briefly, multiple CatSper1 promoter sequences from
different species were aligned using T-coffee
(tcoffee.crg.eu/apps/tcoffee/do:regular). Sox motifs were then
identified using ConSite (http://consite.genereg.net/) under default settings,
revealing putative binding sites for transcription factors
containing HMG DNA-binding domains. For each promoter, predicted
binding sites were filtered using a transcription factor score
cutoff of 80% to ensure specificity and reliability.
Chromatin immunoprecipitation
(ChIP)
ChIP was performed as previously described (39,40)
with minor modifications. Briefly, DNA and associated proteins were
cross-linked in homogenized rat testicular tissues by incubation
with 1% formaldehyde at room temperature for 30 min. The reaction
was quenched by adding glycine to a final concentration of 125 mM.
Following washing twice with ice-cold PBS containing protease
inhibitors (cOmplete™ ULTRA tablets, Mini, EASYpack, Sigma-Aldrich;
Merck KGaA), the samples were pelleted by centrifugation (12,000 ×
g for 10 min at 4°C) and resuspended in 1X SDS lysis buffer [1%
SDS, 10 mM EDTA, 50 mM Tris-HCl (pH 8.1), 10 µl/ml protease
inhibitor cocktail and 10 µl/ml phosphatase inhibitor (Thermo
Fisher Scientific, Inc.)]. The lysates were incubated for 15 min at
4°C (39,40). Chromatin was sheared by sonication
(6×10-sec pulses) to generate 250–1,000 bp DNA fragments, confirmed
by agarose gel electrophoresis. Following centrifugation at 3,000 ×
g for 10 min at 4°C, the chromatin-containing supernatant was
collected and diluted 10-fold in ChIP dilution buffer [1% Triton
X-100, 2 mM EDTA, 150 mM NaCl, 20 mM Tris-HCl (pH 8.1), 10 µl/ml
protease inhibitor cocktail and 10 µl/ml phosphatase inhibitor] and
an aliquot was saved as input DNA (39,40).
Samples were pre-cleared with protein G agarose at
4°C overnight and then incubated with the corresponding antibodies
(10 µg each; 1:50 or 1:200 mouse anti-Sox5 or IgG, respectively.
Mouse anti-Sox5: Cat. no. sc-293215, Santa Cruz Biotechnology,
Inc.; IgG: Cat. no. A7028, Beyotime Biotechnology, Shanghai, China)
on a rocker at 4°C overnight. The complexes were washed three times
with lysis buffer [50 mM Tris (pH 7.4), 1 M NaCl, 1 mM EDTA, 0.1%
SDS, 1% NP-40 and 0.5% sodium deoxycholate]. The beads were then
resuspended in lysis buffer and digested with proteinase K at 45°C
for 45 min. Co-precipitated DNA was purified using the TIANquick
Maxi Purification Kit (Tiangen Biotech Co., Ltd.) and eluted in 50
µl nuclease-free water (39,40).
For normalization, 10% of total chromatin was
retained as input material prior to immunoprecipitation.
Immunoprecipitated DNA was quantified using RT-qPCR as
aforementioned following elution and purification, normalizing all
values to the input. For ChIP-qPCR, primers targeting the CatSper1
promoter region (Table SII) were
used under standard RT-qPCR protocol. PCR product quality was
assessed using 1.5% agarose gel electrophoresis and data was
analyzed using the percent input method, with normal IgG used as
the negative control (41).
Participants
A total of 6 infertile male patients with iAZS (age
range, 25–35 years old; median age, 29.5 years old) and 7 healthy
control subjects (age range, 25–30 years old; median age, 28 years
old) with normal sperm quality and a successful reproductive
history within the past 2 years were enrolled from the Reproductive
Medicine Center of Peking University Third Hospital (Beijing,
China) from January 2023 to January 2024. The diagnosis of iAZS was
established according to the following criteria (42): i) A sexually active,
non-contracepting couple failing to achieve pregnancy after 12
months due to male factor infertility; ii) two or more semen
analyses, with an abstinence period of 3–7 days each time,
demonstrating AZS: Progressive motility (grade A + B sperm) <32%
or total motility (grade A + B + C sperm) <40%; iii) sperm
concentration >15×106 sperm/ml; iv) proportion of
morphologically normal sperm ≥4%; v) no identifiable underlying
causes of infertility, including congenital testicular or genital
dysplasia or deformity, reproductive system infections, positive
serum anti-sperm antibodies, drug exposure, abnormal sex hormone
levels, abnormal seminal plasma biochemistry or a family history of
fertility issues; and vi) a physical examination revealing no
abnormalities in height, weight, secondary sexual characteristics,
testicular size, external genitalia, spermatic veins or mental
status. The present study was approved by the Institutional Review
Board of Peking University Third Hospital (approval no.
IRB00006761-M2022692). All participants provided written voluntary
informed consent prior to enrollment.
Semen analysis for patients
The semen parameters, including semen volume, sperm
motility, sperm concentration and sperm morphology, were evaluated
in human participants according to pre-established guidelines
(42). To evaluate the direct
effect of ECH on human sperm motility in vitro, 1 ml human
semen was incubated with 0.06 mg/ml ECH at 37°C in a water bath and
sperm motility parameters were analyzed using CASA at four time
points: Immediately (0 min) and at 10-, 20- and 30-min
post-incubation with ECH (42).
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 9.0 for Windows (Dotmatics). All quantitative biochemical
data were representative of at least three independent experiments.
A two-tailed paired or unpaired Student's t-test was used to
compare differences between two groups, while one-way ANOVA
followed by Sidak's post-hoc test was used for multiple
comparisons. Data are presented as the mean ± standard error of the
mean and P<0.05 was considered to indicate a statistically
significant difference.
Results
Improvement of sperm quality in AZS
rats via ECH treatment
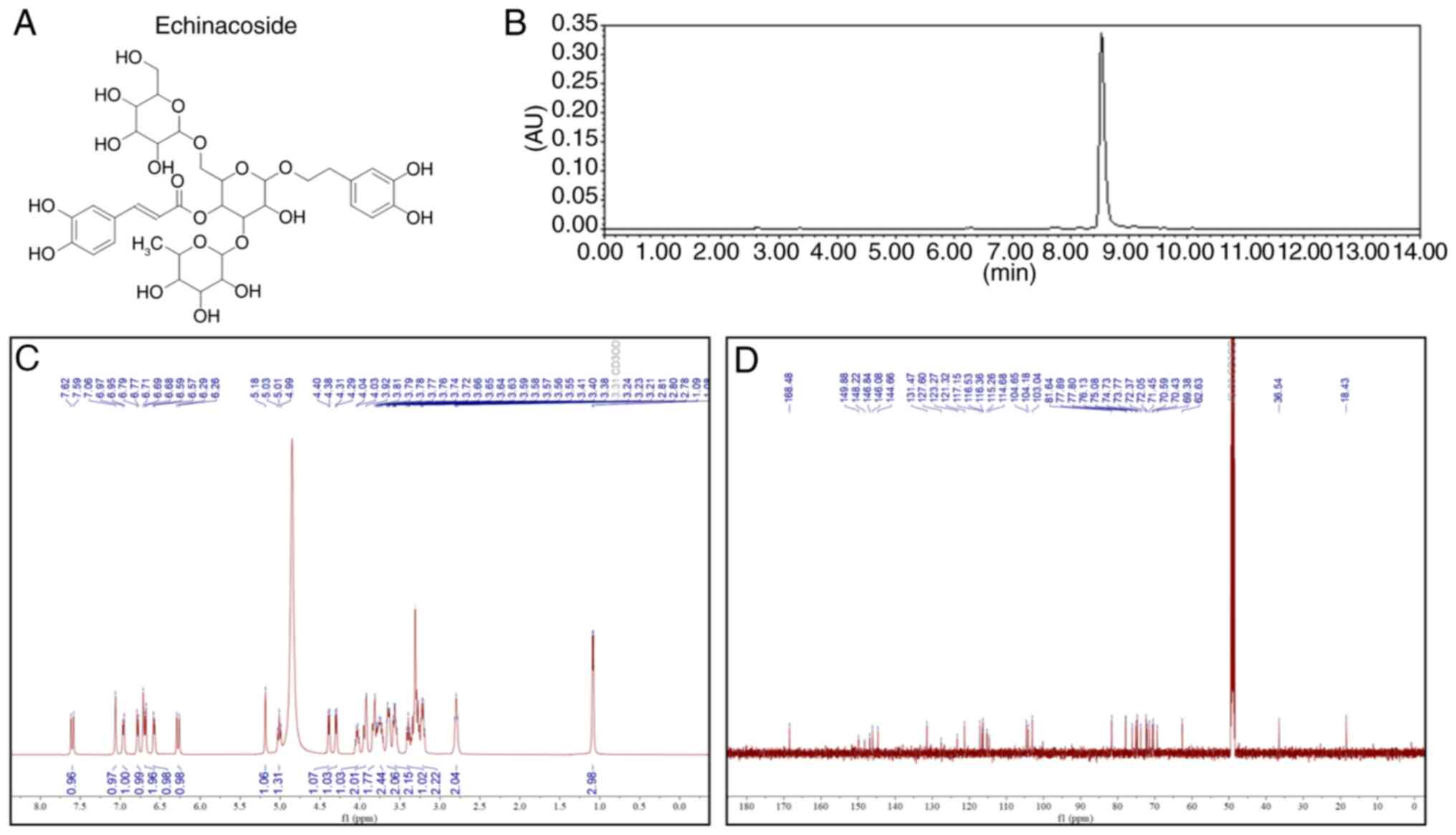
The chemical structure of ECH is illustrated in
Fig. 1A. Subsequently,
high-performance liquid chromatography (Fig. 1B) was used to assess the purity of
ECH. 1H and 13C nuclear magnetic resonance
spectroscopic analyses (Fig. 1C and
D) were performed to identify the structure of ECH. As
previously reported (8,9), the effective dosage and treatment
protocol of ECH was first determined via sperm quality in AZS rats
(Fig. 2A). The results
demonstrated that L-ECH treatment had no significant effect on
sperm quality in AZS rats (Fig.
2B-J). By contrast, M-ECH treatment significantly increased the
motility of grade A + B sperm in AZS rats (Fig. 2C). Notably, H-ECH treatment,
hereafter referred to as ECH treatment, significantly improved
sperm quality in AZS rats, as evidenced by enhanced sperm motility,
evident in both grade A and grade A + B sperm groupings, and
significant increases in other parameters of sperm motility such as
VSL, VAP and ALH, as well as sperm viability (Fig. 2B-J). Collectively, these results
suggest that high-dose ECH treatment in AZS rats improves the
motility of epididymal sperm.
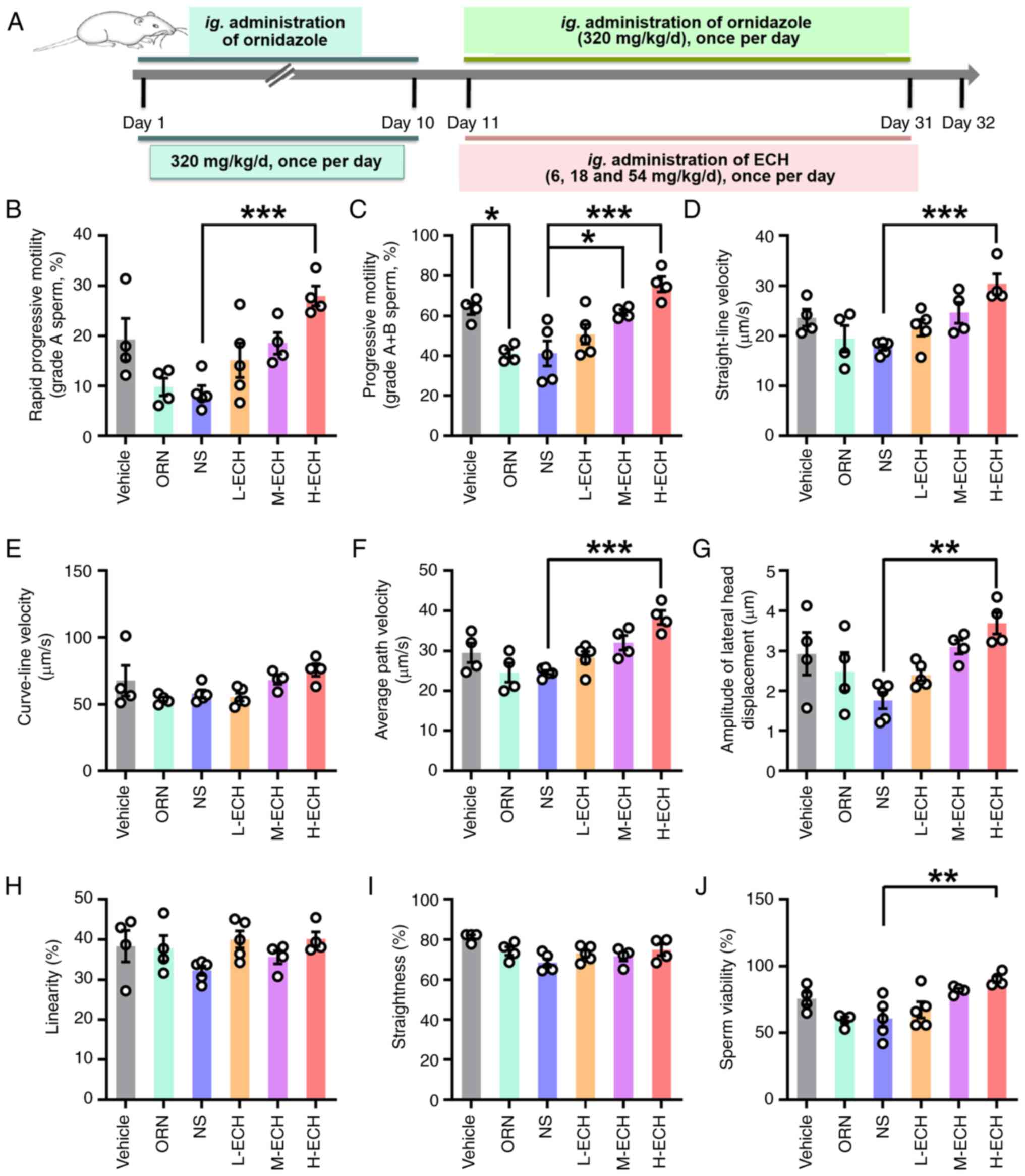 | Figure 2.ECH treatment improves sperm motility
in AZS rats. (A) Experimental protocol for ECH administration in
AZS rats. Sperm motility parameters, including percentage of (B)
grade A and (C) grade A + B sperm, as well as (D) straight-line
velocity, (E) VCL, (F) average path velocity, (G) amplitude of
lateral head displacement, (H) linearity, (I) straightness and (J)
sperm viability. Vehicle group: 0.2% carboxymethylcellulose sodium;
NS group: Ornidazole + normal saline; L-ECH group: Ornidazole +
low-dose ECH; M-ECH group: Ornidazole + medium-dose ECH; H-ECH
group: Ornidazole + high-dose ECH. Data are presented as mean ±
standard error of the mean. *P<0.05, **P<0.01 and
***P<0.001. One-way ANOVA with Sidak's post hoc test; n=4–5 rats
per group. ECH, echinacoside; AZS, asthenozoospermia; VSL,
straight-line velocity; VCL, curve-line velocity; VAP, average path
velocity; ALH, amplitude of lateral head displacement; LIN,
linearity; STR, straightness; ORN, ornidazole; ig.,
intragastric. |
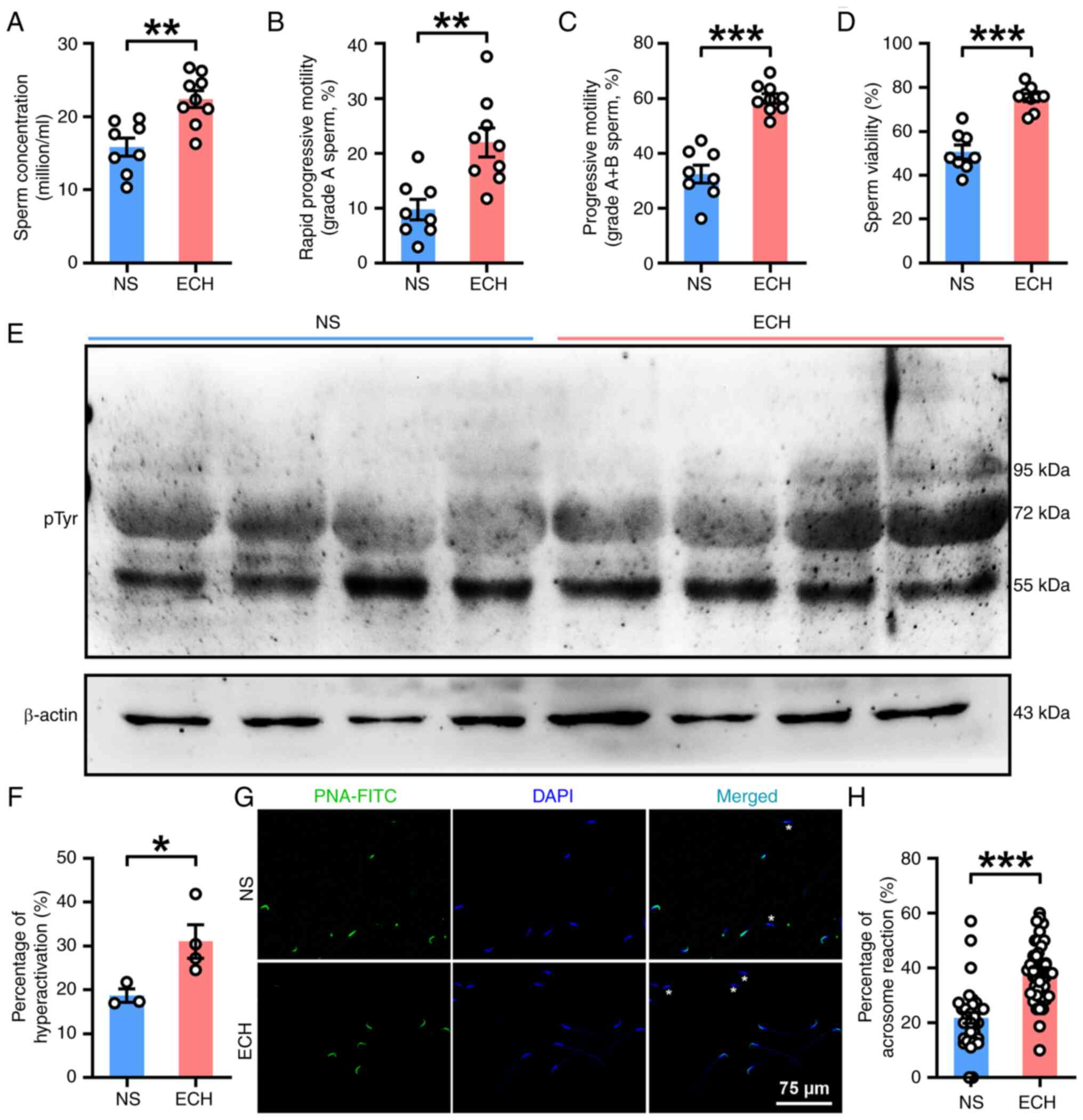
Improvement of sperm function of AZS
rats by ECH treatment
As shown in the aforementioned data (Fig. 2), H-ECH treatment improved sperm
motility in AZS rats. In the present study, the effects of H-ECH
treatment on sperm quality was first determined in AZS rats. ECH
treatment significantly improved sperm quality, as exhibited by
significant increases in sperm concentration and motility in both
grade A and grade A + B sperm (Fig.
3A-C), as well as other sperm motility parameters such as VSL,
VAP, LIN and STR (Figs. S1A-F).
Additionally, ECH treatment demonstrated significantly elevated
sperm viability (Fig. 3D).
Subsequently, the effects of ECH treatment on sperm function were
investigated. The results demonstrated that ECH treatment
effectively reversed the impaired sperm functionality observed in
AZS rats, including a significant increase in protein tyrosine
phosphorylation, increased hyperactivation and increased acrosome
reaction in epididymal sperm, as assessed by PNA-FITC staining
(Fig. 3E-H). In combination, these
results suggest that ECH treatment in AZS rats improved the sperm
quality and functional characteristics of epididymal sperm.
Functional upregulation of CatSper
channels in the sperm of AZS rats by ECH treatment
To investigate how ECH improved sperm quality in AZS
rats, testicular oxidative stress was first assessed. ECH treatment
significantly increased GSH-PX levels compared with vehicle
controls (Fig. 4A), while SOD and
MDA levels showed no statistically significant differences between
experimental groups (Fig. 4B and
C). This indicates partial
alleviation of testicular oxidative stress by ECH. The analysis of
plasma hormones revealed no significant changes in E2, T and FSH
levels (Figs. 4D, E and S1G), but significant reduction of LH in
ECH-treated rats (Fig. S1H).
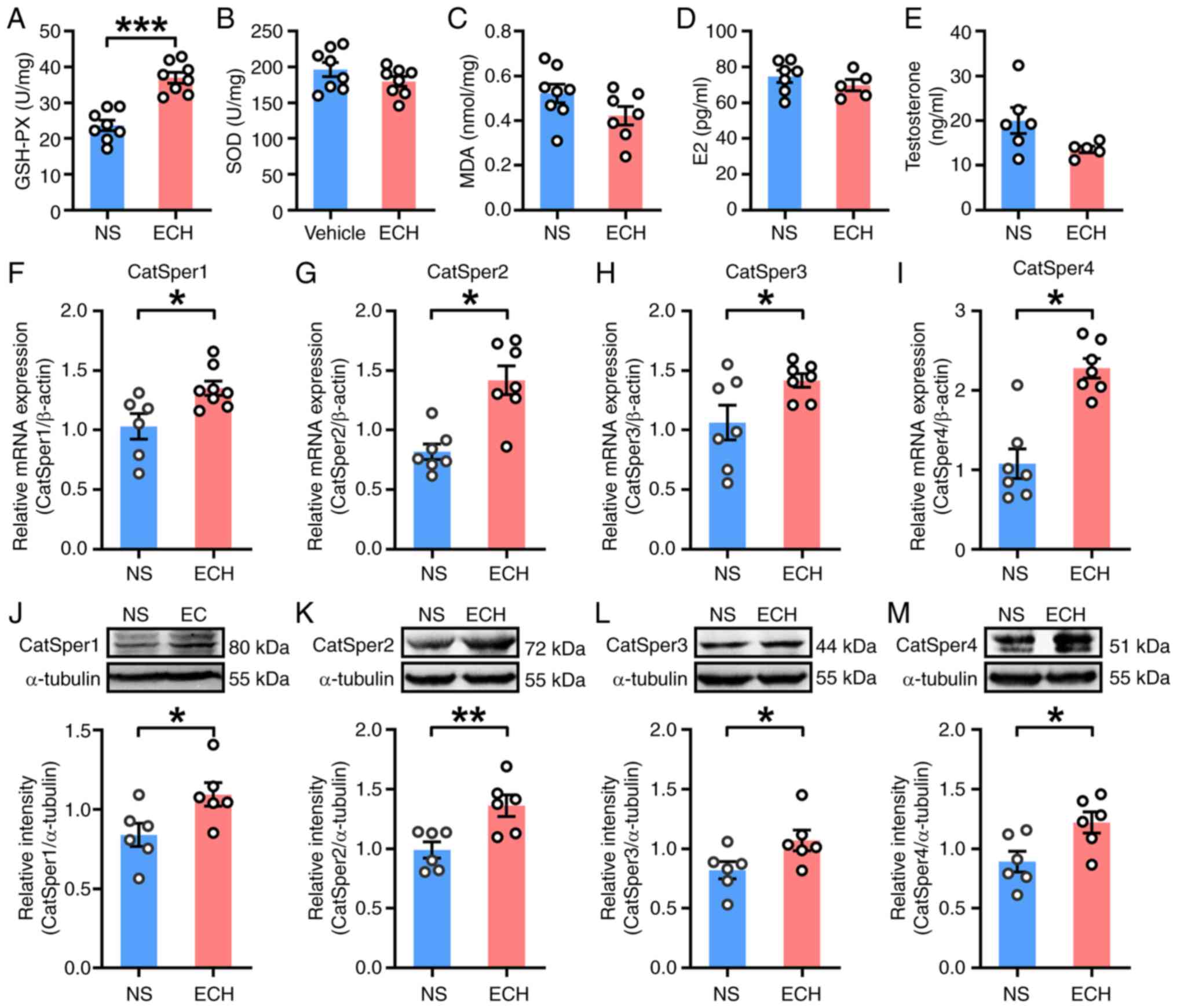 | Figure 4.ECH treatment enhances CatSper
channel expression in testis tissues of rats with
asthenozoospermia. Levels of oxidative stress markers in testis
tissues: (A) GSH-PX, (B) SOD and (C) MDA; n=7–8 rats per group.
Plasma levels of (D) E2 and (E) testosterone; n=5–7 rats per group.
mRNA expression of (F) CatSper1, (G) CatSper2, (H) CatSper3, (I)
CatSper4. Protein expression levels of (J) CatSper1, (K) CatSper2,
(L) CatSper3, (M) CatSper4 in testis tissues; n=6–8 rats per group.
NS group: Ornidazole + normal saline; ECH group: Ornidazole +
high-dose ECH. Data are presented as mean ± standard error of the
mean. *P<0.05, **P<0.01 and ***P<0.001. NS, normal saline;
ECH, echinacoside; GSH-PX, glutathione peroxidase; SOD, superoxide
dismutase; MDA, malondialdehyde; E2, estradiol; CatSper, cation
channel of sperm. |
Due to the ECH-mediated enhancement of sperm
motility, CatSper channel expression was examined. In testicular
tissues, ECH significantly increased mRNA (Fig. 4F-I) and protein (Fig. 4J-M) levels of CatSper1-4, as
compared with vehicle controls. Epididymal sperm also showed
significantly increased CatSper1-4 protein expression (Fig. 5A-D). CatSper-mediated
Ca2+ influx was subsequently evaluated (Figs. 5E-K). ECH treatment significantly
enhanced NH4Cl-evoked intracellular calcium levels
[Ca2+]i responses in the sperm of AZS rats at
the single-cell (Fig. 5I),
population (Fig. 5J) and mean
intensity levels (Fig. 5K)
compared with the NS group. Notably, the CatSper inhibitor NNC
55–0396 blocked NH4Cl-induced Ca2+ signals in
both groups (Fig. 5I-K),
supporting CatSper mediation. Collectively, these findings
demonstrate that the functional upregulation of CatSper channels
markedly mediated ECH-induced sperm motility improvement in AZS
rats.
![ECH treatment upregulates functional
expression of CatSper channels in spermatozoa from rats with
asthenozoospermia. (A) CatSper1, (B) CatSper2, (C) CatSper3 and (D)
CatSper4 protein abundance in sperm; n=5–6 rats per group.
Representative fura-2-acetoxymethyl ester fluorescence images of
sperm before and after 30 mM NH4Cl stimulation in (E)
NS, (F) ECH, (G) NS + NNC and (H) ECH + NNC group. Arrows indicate
[Ca2+]i fluorescent signals in response to
NH4Cl. Scale bar, 10 µm. (I) Representative single-sperm
fluorescence traces. (J) Normalized [Ca2+]i
responses in all tested sperm. (K) Summary plot of normalized
[Ca2+]i signals after NH4Cl
treatment (n=27–33 sperm per group from 5–6 rats). NS group:
Ornidazole + normal saline; ECH group: Ornidazole + ECH. Data are
presented as mean ± standard error of the mean. *P<0.05,
**P<0.01 and ***P<0.001; Unpaired t-test. ECH, echinacoside;
NS, normal saline; NNC, NNC 55-0396; CatSper, cation channel of
sperm; [Ca2+]i, intracellular calcium levels;
F340, fluorescence at 340 nm; F380, fluorescence at 380 nm.](/article_images/mmr/33/3/mmr-33-03-13794-g04.jpg) | Figure 5.ECH treatment upregulates functional
expression of CatSper channels in spermatozoa from rats with
asthenozoospermia. (A) CatSper1, (B) CatSper2, (C) CatSper3 and (D)
CatSper4 protein abundance in sperm; n=5–6 rats per group.
Representative fura-2-acetoxymethyl ester fluorescence images of
sperm before and after 30 mM NH4Cl stimulation in (E)
NS, (F) ECH, (G) NS + NNC and (H) ECH + NNC group. Arrows indicate
[Ca2+]i fluorescent signals in response to
NH4Cl. Scale bar, 10 µm. (I) Representative single-sperm
fluorescence traces. (J) Normalized [Ca2+]i
responses in all tested sperm. (K) Summary plot of normalized
[Ca2+]i signals after NH4Cl
treatment (n=27–33 sperm per group from 5–6 rats). NS group:
Ornidazole + normal saline; ECH group: Ornidazole + ECH. Data are
presented as mean ± standard error of the mean. *P<0.05,
**P<0.01 and ***P<0.001; Unpaired t-test. ECH, echinacoside;
NS, normal saline; NNC, NNC 55-0396; CatSper, cation channel of
sperm; [Ca2+]i, intracellular calcium levels;
F340, fluorescence at 340 nm; F380, fluorescence at 380 nm. |
ECH treatment enhances the
Sox5-mediated transcriptional activation of CatSper1 channels in
the testes of AZS rats
Numerous studies have indicated that the Sox family
can coordinate the regulation of gene expression within the testes
(22–25). Specifically, Sox5 has been shown to
enhance the transactivation of the Catsper1 promoter in vivo
(30). To investigate whether Sox5
activated the transcription of CatSper1 in the rat testis, the
co-localization of Sox5 and CatSper1 was examined in testicular
tissues. As expected, immunofluorescent staining revealed that Sox5
co-localized with CatSper1 in testicular tissues (Fig. 6A). In the testis, Sox5 was
predominantly located in cell nuclei, whereas CatSper1 exhibited a
broader distribution, being present in both cytoplasm and nuclei
(Fig. 6A). Subsequently, RT-qPCR
and western blotting were performed on testicular tissues from
vehicle- and ECH-treated rats. Consistently, a significant increase
in the mRNA levels of Sox5 was observed in the ECH-treated group
compared with the control group (Fig.
6B). Notably, the short isoform of Sox5 (S-Sox5), rather than
the long isoform, was identified as the predominant form of Sox5
expressed in the testis. ECH treatment selectively enhanced the
protein expression of S-Sox5 in the testes of AZS rats (Fig. 6C). These findings provide notable
evidence supporting the involvement of Sox5-mediated
transcriptional activation of CatSper1 within the testis.
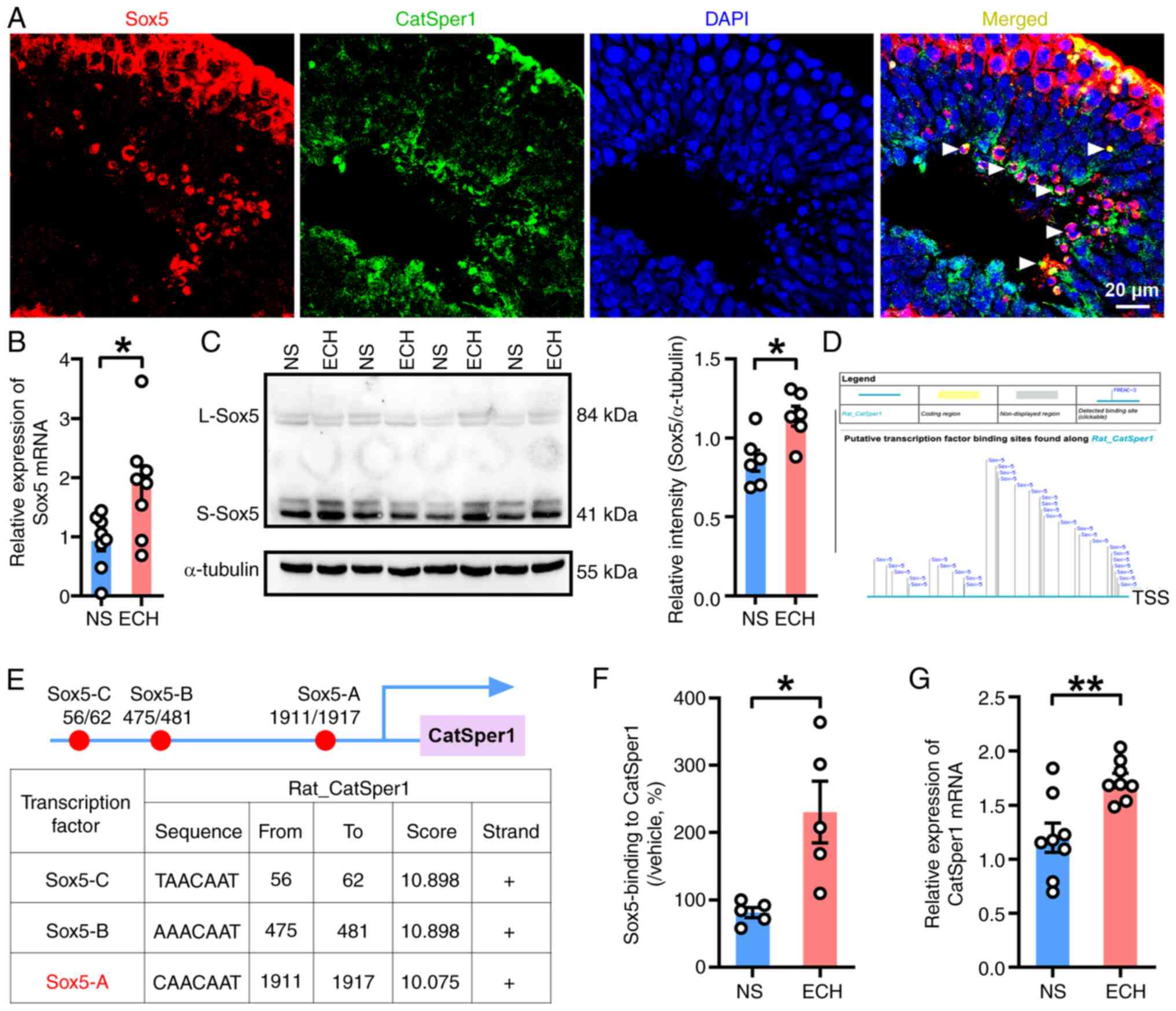 | Figure 6.ECH treatment enhances Sox5-mediated
transcriptional activation of CatSper1 in testis tissues of AZS
rats. (A) Representative immunofluorescence images showing
co-localization of Sox5 (red) and CatSper1 (green) in testis
tissues of naïve rats (n=3 rats per group). Merged signals are
indicated by triangles. Scale bar, 20 µm. (B) Sox5 mRNA and (C)
S-Sox5 protein expression in testis tissues of ECH-treated AZS
rats. (D and E) In silico analysis of Sox5 binding sites in
the CatSper1 promoter: Sox5-A (−1911 to −1917 bp), Sox5-B (−475 to
−481 bp) and Sox5-C (−56 to −62 bp) relative to the transcriptional
start site. (F) ChIP-qPCR analysis of Sox5 binding at the Sox5-A
site in the CatSper1 promoter. (G) CatSper1 mRNA expression; n=5–8
rats per group. NS group: Ornidazole + normal saline; ECH group:
Ornidazole + high-dose ECH. Data are presented as mean ± standard
error of the mean. *P<0.05 and **P<0.01. Unpaired t-test.
ECH, echinacoside; AZS, asthenozoospermia; ChIP, chromatin
immunoprecipitation; qPCR, quantitative PCR; Sox5, sex-determining
region Y-related high-mobility-group box family, member 5; CatSper,
cation channel of sperm; TSS, transcriptional start site; L-Sox5,
long isoform-Sox5; S-Sox5, short isoform Sox5. |
Subsequently, in silico analysis was employed
to predict potential binding sites of Sox5 in the promoter region
of the CatSper1 gene. The analysis revealed three distinct
Sox5-binding sites in the CatSper1 gene promoter: i) Sox5-A, −1911
to −1917 bp upstream of the TSS; ii) Sox5-B, −475 to −481 bp
upstream of the TSS; and iii) Sox5-C, located −56 to −62 bp
upstream of the TSS (Fig. 6D and
E). To validate these predictions, ChIP-qPCR was performed to
assess the binding of Sox5 to the CatSper1 gene promoter at the
Sox5-A site. A significant increase in the relative enrichment of
Sox5 was observed at the Sox5-A site in the testicular tissues of
the ECH-treated group compared with the vehicle-treated group
(Fig. 6F). Furthermore, RT-qPCR
analysis demonstrated a significant increase in CatSper1 mRNA
expression in the ECH-treated group compared with the control group
(Fig. 6G). These results suggest
that ECH treatment enhances Sox5 expression, thereby promoting
Sox5-mediated transcriptional activation of CatSper1 in the
testicular tissues of AZS rats. Taken together, this cascade
ultimately improved sperm motility and acrosome reaction capability
in AZS rats.
Functional upregulation of CatSper
channels in the human sperm by ECH treatment
To validate the findings obtained from AZS rats, the
present study investigated whether ECH treatment induced the
functional upregulation of CatSper channels in human sperm. In
vitro experiments were first performed to determine whether
sperm motility was increased when ECH was directly added to human
semen. Spermatozoa from healthy subjects (HS) or patients with iAZS
were incubated with ECH prior to sperm motility analysis at 0-,
10-, 20- and 30-min post-incubation. The results demonstrated that
ECH incubation significantly increased the progressive motility
(grade A + B sperm) at 20 min and total motility (grade A + B + C
sperm) at 30 min in sperm from HS (Fig. 7A-C). Notably, 20-min ECH incubation
significantly improved sperm motility in samples from patients with
iAZS, enhancing rapid progressive motility (grade A sperm),
progressive motility and total motility (Fig. 7D-F). Subsequently, the effects of
ECH incubation on CatSper-mediated Ca2+ influx in human
sperm were examined. Notably, a marked increase in
NH4Cl-evoked [Ca2+]i fluorescent
signals was observed in sperm from both HS and patients with iAZS
after 30-min incubation with ECH (Fig.
7G-J). In summary, a consistent enhancement of
NH4Cl-evoked [Ca2+]i fluorescent
signals was observed in the ECH-treated groups compared with their
control counterparts in single spermatozoa (Fig. 7K) and in all tested sperm (Fig. 7L), as well as significant increases
in the mean fluorescence intensity of all tested sperm
post-NH4Cl exposure (Fig.
7M). These findings suggest that ECH incubation may have
increased intracellular calcium levels
[Ca2+]i in human sperm, thereby enhancing
sperm motility.
![Functional activation of CatSper
channels in human sperm by ECH treatment. Sperm motility parameters
in healthy subjects: (A) Grade A, (B) grade A + B and (C) grade A +
B + C sperm. Sperm motility parameters in patients with AZS: (D)
Grade A, (E) grade A + B and (F) grade A + B + C sperm. n=6–7
subjects per group. (G-J) Representative fura-2-acetoxymethyl ester
fluorescence images of human sperm before and after 30 mM
NH4Cl stimulation. Arrows indicate
[Ca2+]i responses. Scale bar, 10 µm. (K)
Representative single-sperm fluorescence traces. (L) Normalized
[Ca2+]i responses in all tested sperm. (M)
Summary plot of normalized [Ca2+]i signals
after NH4Cl treatment (n=33–36 sperm per group from 5–6
subjects). Data are presented as mean ± standard error of the mean.
*P<0.05, **P<0.01 and ***P<0.001. Paired t-test for (A-F),
unpaired t-test for (M). ECH, echinacoside; AZS, asthenozoospermia;
[Ca2+]i, intracellular calcium levels; NS,
normal saline; HS, healthy subjects; F340, fluorescence at 340 nm;
F380, fluorescence at 380 nm.](/article_images/mmr/33/3/mmr-33-03-13794-g06.jpg) | Figure 7.Functional activation of CatSper
channels in human sperm by ECH treatment. Sperm motility parameters
in healthy subjects: (A) Grade A, (B) grade A + B and (C) grade A +
B + C sperm. Sperm motility parameters in patients with AZS: (D)
Grade A, (E) grade A + B and (F) grade A + B + C sperm. n=6–7
subjects per group. (G-J) Representative fura-2-acetoxymethyl ester
fluorescence images of human sperm before and after 30 mM
NH4Cl stimulation. Arrows indicate
[Ca2+]i responses. Scale bar, 10 µm. (K)
Representative single-sperm fluorescence traces. (L) Normalized
[Ca2+]i responses in all tested sperm. (M)
Summary plot of normalized [Ca2+]i signals
after NH4Cl treatment (n=33–36 sperm per group from 5–6
subjects). Data are presented as mean ± standard error of the mean.
*P<0.05, **P<0.01 and ***P<0.001. Paired t-test for (A-F),
unpaired t-test for (M). ECH, echinacoside; AZS, asthenozoospermia;
[Ca2+]i, intracellular calcium levels; NS,
normal saline; HS, healthy subjects; F340, fluorescence at 340 nm;
F380, fluorescence at 380 nm. |
Discussion
The present study provides evidence that ECH exerts
therapeutic effects on iAZS by functionally upregulating CatSper
channels in sperm. This mechanism was mediated through increased
expression of the testicular transcription factor Sox5, which
transcriptionally activated CatSper1. These findings suggest that
CatSper represents a promising therapeutic target for iAZS. In
addition, ECH may serve as a potential complementary and
alternative medicine (CAM) for treating male infertility associated
with iAZS in clinical settings.
ECH is the primary bioactive compound derived from
Cistanche deserticola (Schrenk) Wight. and exhibits a wide
range of pharmacological effects. It suppresses the P2X
purinoceptor 7/fractalkine/C-X3-C motif chemokine receptor 1
pathway to exert neuroprotective effects (43,44),
inhibits the microglial α-synuclein/toll like receptor 2/NF-κB/NLR
family pyrin domain containing 3 axis for antinociception (43), activates the brain derived
neurotrophic factor (BDNF)/neurotrophic receptor tyrosine kinase 2
or BDNF/cAMP responsive element binding protein 1 pathways for
antidepressant effects (45,46)
and blocks the activin A receptor type 2A-mediated TGF-β1/Smad
signaling pathway to exert anti-hepatic fibrosis effects (47). ECH also suppresses the PI3K/AKT
pathway to inhibit tumor metastasis (48) and targets the Janus kinase 1/signal
transducer and activator of transcription 1/interferon regulatory
factor 1 pathway for antitumor activity (49,50).
Additionally, ECH reduces the levels of pro-inflammatory cytokines
such as TNF-α, IL-1β and IL-6 (50), demonstrating potent
anti-inflammatory properties. ECH also protects against testicular
injury by restoring T synthesis pathways (51–53).
However, the molecular mechanisms through which ECH improves sperm
quality, particularly motility, remain to be fully elucidated.
In addition to Sheng-Jing-San (SJS) and EA, several
Traditional Chinese Medicine (TCM) formulations have been shown to
enhance sperm motility in AZS rats (16,31).
In the present study, the administration of a high-dose of ECH (54
mg/kg) for 21 consecutive days significantly improved sperm
motility, hyperactivation and acrosome reaction in AZS rats.
Previous studies have shown that ECH mitigates oxidative stress by
activating antioxidant enzymes and MAPK signaling components, such
as p38 and JNK, thereby increasing sperm count, reducing deformity
rates, improving progressive motility and alleviating
spermatogenesis dysfunction in mouse testes (9,12).
Furthermore, ECH prevents oligospermia and AZS by inhibiting
hypothalamic androgen receptor activity (8). In combination, these findings support
the conclusion that ECH effectively enhances sperm motility and
increases sperm count in AZS rats.
Human fertilization, both in vivo and in
vitro, relies on the CatSper channel to trigger sperm
hyperactivation (14). The CatSper
channel consists of at least 11 subunits, including four
pore-forming α subunits, and is specifically expressed in the
testis, localized to the principal piece of the sperm flagellum
(10,11). This channel serves an important
role in sperm functions such as hyperactivation and the acrosome
reaction, which are important for male fertility (13). Microarray analyses have revealed
that CatSper1 and CatSper3 mRNA levels are reduced in men with AZS,
and mRNA levels of CatSper1 and CatSper3 are positively associated
with sperm motility, mitochondrial membrane potential,
capacitation, fertilization rate, cleavage rate and embryo quality
(19). In human sperm, CatSper
expression and function have been shown to be closely associated
with progressive motility and may contribute to the pathogenesis of
AZS (15,16). Notably, TCM interventions such as
SJS and EA have been shown to improve sperm motility in AZS rats by
upregulating CatSper channels (16,31).
Consistent with these findings, the present study demonstrated that
ECH enhanced both CatSper mRNA and protein expression and increases
antioxidant capacity in the testicular tissue of AZS rats. In
addition, CatSper function was upregulated in sperm from
ECH-treated AZS rats and in human sperm incubated with ECH.
Furthermore, the indole derivative
N'-(4-dimethylaminobenzylidene)-2-1-(4-(methylsulfinyl)
benzylidene)-5-fluoro-2-methyl-1H-inden-3-yl) acetohydrazide has
been shown to restore impaired sperm motility and concentration in
cis-diamminedichloroplatinum (II)-treated rats by upregulating
CatSper expression and reducing oxidative stress and inflammation
(54). Taxifolin and the hexane
fraction of Prunus japonica seed also enhance sperm motility
in boars by increasing the expression of α-2-glycoprotein 1,
zinc-binding, protein kinase A), CatSper and ERK phosphorylated ERK
(55–59). These findings, together with the
present results, indicate that natural compounds such as ECH and
taxifolin could improve sperm quality across various animal
models.
Transcription factors regulate gene expression by
binding to specific DNA sequences in response to intracellular
signals (60,61). The Sox family, a group of
evolutionarily conserved transcription factors, serves a number of
important roles in cell fate determination, tissue homeostasis and
embryonic development (62). The
term ‘Sox’ stands for ‘Sry-related HMG box’, referring to the
shared DNA-binding domain among these proteins (62). Based on sequence homology and
structural conservation, the Sox family is classified into 11
subfamilies from SoxA to SoxK (22). Sox proteins are important for male
embryonic development, particularly in sex determination and
Sertoli cell differentiation (24). Among the Sox family members, Sox30,
Sox32 and Sox5 are involved in testicular development and
spermatogenesis, serving key roles in maintaining fertility
(22–25). Sox5 is an important transcription
factor in spermatogenesis and maturation (24), and its mutation or dysregulation
can lead to spermatogenic dysfunction and male infertility
(27,29). The present findings showed that ECH
significantly increased Sox5 protein expression in the testes of
AZS rats. Sox5 has been shown to enhance CatSper1 promoter activity
in vivo (30) and elevated
Sox5 levels were shown to regulate transcription by binding to the
promoter regions of genes such as CatSper1. Therefore, ECH may have
enhanced Sox5 expression and functional activity, promoting the
expression of key genes such as CatSper1 and thereby improving
sperm motility and fertilization capacity in male rats. Notably,
data from healthy individuals and patients with iAZS suggested that
ECH may have also enhanced sperm motility in clinical settings.
These findings position ECH as a promising CAM candidate for
improving sperm function and managing iAZS in clinical
practice.
The present study had limitations merit
consideration. First, species differences may limit direct
translation from the rat model to humans with AZS. Second, key
clinical parameters, such as ECH dosages and treatment duration,
remain undefined. Thus, well-designed clinical trials are needed to
validate the translational potential of the ECH/Sox5/CatSper
pathways in male subfertility.
In conclusion, the data in the present study
indicated that ECH improved sperm motility and fertilization
capacity through multiple mechanisms, including enhanced
antioxidant activity, as demonstrated by elevated GSH-PX levels,
upregulated CatSper mRNA and protein expression and increased
Sox5-mediated transactivation of the CatSper gene in AZS rats
(Fig. S2). These findings provide
novel insights into the role of plant-derived compounds in
enhancing male reproductive health and have identified potential
candidates for the development of new therapies for male
infertility.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The present work was supported by grants from the National
Natural Science Foundation of China (grant nos. 82371227, 82171226,
81974169, 82450110, 82101676 and 82104543), the Natural Science
Foundation of Beijing Municipality (grant nos. 7222105 and
L256061), the National Key Research and Development Program of
China (grant no. 2019YFC1712104) and the Key Research and
Development Project of Xinjiang (grant nos. 2022B02012 and
2022E02122).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
GGX, ZRJ and YJ conceived and designed the
experiments. ZRJ and YWH performed the experiments. ZRJ, HJ and JC
revised the manuscript. HJ and JC made substantial contributions to
conception and design. BHL, HT, SY, YT and KX analyzed and
interpreted the data. YWH wrote the manuscript. All authors read
and approved the final manuscript. GGX and YJ confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
All experimental procedures involving animals were
approved by the Animal Care and Use Committee of Peking University
(Beijing, China; approval no. J2024179). The present study was
approved by the Institutional Review Board of Peking University
(approval no. IRB00006761-M2022692). All participants provided
voluntary written informed consent.
Patient consent for publication
All participants provided voluntary written
informed consent for publication.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ALH
|
amplitude of lateral head
displacement
|
|
AZS
|
asthenozoospermia
|
|
CAM
|
complementary and alternative
medicine
|
|
CASA
|
computer-assisted semen analysis
|
|
CatSper
|
cation channel of sperm
|
|
ChIP-qPCR
|
chromatin
immunoprecipitation-quantitative PCR
|
|
ECH
|
echinacoside
|
|
EA
|
electroacupuncture
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
E2
|
estradiol
|
|
PNA-FITC
|
fluorescein isothiocyanate-conjugated
peanut agglutinin
|
|
FSH
|
follicle-stimulating hormone
|
|
GSH-PX
|
glutathione peroxidase
|
|
HS
|
healthy subjects
|
|
HTF
|
human tubal fluid
|
|
LIN
|
linearity
|
|
LH
|
luteinizing hormone
|
|
MDA
|
malondialdehyde
|
|
NS
|
normal saline solution
|
|
OD
|
optical density
|
|
ORN
|
ornidazole
|
|
RT-qPCR
|
reverse transcription-quantitative
PCR
|
|
Sox5
|
sex-determining region Y-related
high-mobility-group-box family, member 5
|
|
STR
|
straightness
|
|
SOD
|
superoxide dismutase
|
|
T
|
testosterone
|
|
TCM
|
Traditional Chinese Medicine
|
|
VAP
|
average path velocity
|
|
VCL
|
curve-line velocity
|
|
VSL
|
straight-line velocity
|
References
|
1
|
Cox CM, Thoma ME, Tchangalova N, Mburu G,
Bornstein MJ, Johnson CL and Kiarie J: Infertility prevalence and
the methods of estimation from 1990 to 2021: A systematic review
and meta-analysis. Human Reprod Open. 12:hoac0512022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Minhas S, Boeri L, Capogrosso P, Cocci A,
Corona G, Dinkelman-Smit M, Falcone M, Jensen CF, Gül M, Kalkanli
A, et al: European association of urology guidelines on male sexual
and reproductive health: 2025 Update on male infertility. Eur Urol.
87:601–615. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang C, Li P and Li Z: Clinical
application of aromatase inhibitors to treat male infertility. Hum
Reprod Update. 28:30–50. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shahrokhi SZ, Salehi P, Alyasin A,
Taghiyar S and Deemeh MR: Asthenozoospermia: Cellular and molecular
contributing factors and treatment strategies. Andrologia.
52:e134632020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li Z, Li J, Li Y, Guo L, Xu P, Du H, Lin N
and Xu Y: The role of Cistanches Herba and its ingredients in
improving reproductive outcomes: A comprehensive review.
Phytomedicine. 129:1556812024. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jiang Y and Tu PF: Analysis of chemical
constituents in Cistanche species. J Chromatogr A. 1216:1970–1979.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Song Y, Zeng K, Jiang Y and Tu P:
Cistanches Herba, from an endangered species to a big brand of
Chinese medicine. Med Res Rev. 41:1539–1577. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jiang Z, Zhou B, Li X, Kirby GM and Zhang
X: Echinacoside increases sperm quantity in rats by targeting the
hypothalamic androgen receptor. Sci Rep. 8:38392018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao G, Wang Y, Lai Z, Zheng L and Zhao D:
Echinacoside protects against dysfunction of spermatogenesis
through the MAPK signaling pathway. Reprod Sci. 29:1586–1596. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cavarocchi E, Whitfield M, Saez F and
Touré A: Sperm ion transporters and channels in human
asthenozoospermia: Genetic etiology, lessons from animal models,
and clinical perspectives. Int J Mol Sci. 23:39262022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hwang JY and Chung JJ: CatSper calcium
channels: 20 years on. Physiology (Bethesda). 38:02023.PubMed/NCBI
|
|
12
|
Zhang X, Liang M, Song D, Huang R, Chen C,
Liu X, Chen H, Wang Q, Sun X, Song J, et al: Both protein and
non-protein components in extracellular vesicles of human seminal
plasma improve human sperm function via CatSper-mediated calcium
signaling. Hum Reprod. 39:658–673. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hwang JY, Wang H, Lu Y, Ikawa M and Chung
JJ: C2cd6-encoded CatSperτ targets sperm calcium channel to Ca(2+)
signaling domains in the flagellar membrane. Cell Rep.
38:1102262022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Young S, Schiffer C, Wagner A, Patz J,
Potapenko A, Herrmann L, Nordhoff V, Pock T, Krallmann C,
Stallmeyer B, et al: Human fertilization in vivo and in vitro
requires the CatSper channel to initiate sperm hyperactivation. J
Clin Invest. 134:e1735642024. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tamburrino L, Marchiani S, Minetti F,
Forti G, Muratori M and Baldi E: The CatSper calcium channel in
human sperm: Relation with motility and involvement in
progesterone-induced acrosome reaction. Hum Reprod. 29:418–428.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jin ZR, Fang D, Liu BH, Cai J, Tang WH,
Jiang H and Xing GG: Roles of CatSper channels in the pathogenesis
of asthenozoospermia and the therapeutic effects of
acupuncture-like treatment on asthenozoospermia. Theranostics.
11:2822–2844. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shu F, Zhou X, Li F, Lu D, Lei B, Li Q,
Yang Y, Yang X, Shi R and Mao X: Analysis of the correlation of
CATSPER single nucleotide polymorphisms (SNPs) with idiopathic
asthenospermia. J Assist Reprod Genet. 32:1643–1649. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tamburrino L, Marchiani S, Vicini E,
Muciaccia B, Cambi M, Pellegrini S, Forti G, Muratori M and Baldi
E: Quantification of CatSper1 expression in human spermatozoa and
relation to functional parameters. Hum Reprod. 30:1532–1544. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jalalabadi FN, Cheraghi E, Janatifar R and
Momeni HR: The detection of CatSper1 and CatSper3 expression in men
with normozoospermia and asthenoteratozoospermia and its
association with sperm parameters, fertilization rate, embryo
quality. Reprod Sci. 31:704–713. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Daigle M, Roumaud P and Martin LJ:
Expressions of Sox9, Sox5, and Sox13 transcription factors in mice
testis during postnatal development. Mol Cell Biochem. 407:209–221.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Roumaud P, Haché J and Martin LJ:
Expression profiles of Sox transcription factors within the
postnatal rodent testes. Mol Cell Biochem. 447:175–187. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang M, Ding H, Liu M, Gao Y, Li L, Jin C,
Bao Z, Wang B and Hu J: Genome wide analysis of the sox32 gene in
germline maintenance and differentiation in leopard coral grouper
(Plectropomus leopardus). Comp Biochem Physiol Part D Genomics
Proteomics. 54:1014022024. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Han F, Yin L, Jiang X, Zhang X, Zhang N,
Yang JT, Ouyang WM, Hao XL, Liu WB, Huang YS, et al: Identification
of SRY-box 30 as an age-related essential gatekeeper for male
germ-cell meiosis and differentiation. Aging Cell. 20:e133432021.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Diawara M and Martin LJ: Regulatory
mechanisms of SoxD transcription factors and their influences on
male fertility. Reprod Biol. 23:1008232023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wei L, Tang Y, Zeng X, Li Y, Zhang S, Deng
L, Wang L and Wang D: The transcription factor Sox30 is involved in
Nile tilapia spermatogenesis. J Genet Genomics. 49:666–676. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cerván-Martín M, Bossini-Castillo L,
Rivera-Egea R, Garrido N, Luján S, Romeu G, Santos-Ribeiro S;
IVIRMA Group and Lisbon Clinical Group and Castilla JA, ; et al:
Effect and in silico characterization of genetic variants
associated with severe spermatogenic disorders in a large Iberian
cohort. Andrology. 9:1151–1165. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gu X, Li H, Chen X, Zhang X, Mei F, Jia M
and Xiong C: PEX10, SIRPA-SIRPG, and SOX5 gene polymorphisms are
strongly associated with nonobstructive azoospermia susceptibility.
J Assist Reprod Genet. 36:759–768. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tu W, Liu Y, Shen Y, Yan Y, Wang X, Yang
D, Li L, Ma Y, Tao D, Zhang S and Yang Y: Genome-wide Loci linked
to non-obstructive azoospermia susceptibility may be independent of
reduced sperm production in males with normozoospermia. Biol
Reprod. 92:412015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zou S, Li Z, Wang Y, Chen T, Song P, Chen
J, He X, Xu P, Liang M, Luo K, et al: Association study between
polymorphisms of PRMT6, PEX10, SOX5, and nonobstructive azoospermia
in the Han Chinese population. Biol Reprod. 90:962014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mata-Rocha M, Hernández-Sánchez J,
Guarneros G, de la Chesnaye E, Sánchez-Tusié AA, Treviño CL, Felix
R and Oviedo N: The transcription factors Sox5 and Sox9 regulate
Catsper1 gene expression. FEBS Lett. 588:3352–3360. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang YN, Wang B, Liang M, Han CY, Zhang B,
Cai J, Sun W and Xing GG: Down-regulation of CatSper1 channel in
epididymal spermatozoa contributes to the pathogenesis of
asthenozoospermia, whereas up-regulation of the channel by
Sheng-Jing-San treatment improves the sperm motility of
asthenozoospermia in rats. Fertil Steril. 99:579–587. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Aguirre-Arias MV, Velarde V and Moreno RD:
Effects of ascorbic acid on spermatogenesis and sperm parameters in
diabetic rats. Cell Tissue Res. 370:305–317. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang T, Yin Q, Ma X, Tong MH and Zhou Y:
Ccdc87 is critical for sperm function and male fertility. Biol
Reprod. 99:817–827. 2018.PubMed/NCBI
|
|
34
|
Jin Z, Yang Y, Cao Y, Wen Q, Xi Y, Cheng
J, Zhao Q, Weng J, Hong K, Jiang H, et al: The gut metabolite
3-hydroxyphenylacetic acid rejuvenates spermatogenic dysfunction in
aged mice through GPX4-mediated ferroptosis. Microbiome.
11:2122023. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jin Z, Cao Y, Wen Q, Zhang H, Fang Z, Zhao
Q, Xi Y, Luo Z, Jiang H, Zhang Z and Hang J: Dapagliflozin
ameliorates diabetes-induced spermatogenic dysfunction by
modulating the adenosine metabolism along the gut microbiota-testis
axis. Sci Rep. 14:6412024. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lin SC, Lee HC, Hsu CT, Huang YH, Li WN,
Hsu PL, Wu MH and Tsai SJ: Targeting Anthrax toxin receptor 2
ameliorates endometriosis progression. Theranostics. 9:620–632.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kelly MC, Brown SG, Costello SM,
Ramalingam M, Drew E, Publicover SJ, Barratt CLR and Martins Da
Silva S: Single-cell analysis of [Ca2+]i signalling in sub-fertile
men: Characteristics and relation to fertilization outcome. Hum
Reprod. 33:1023–1033. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yin C, Liu B, Li Y, Li X, Wang J, Chen R,
Tai Y, Shou Q, Wang P, Shao X, et al: IL-33/ST2 induces
neutrophil-dependent reactive oxygen species production and
mediates gout pain. Theranostics. 10:12189–12203. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Song C, Han Y, Luo H, Qin Z, Chen Z, Liu
Y, Lu S, Sun H and Zhou C: HOXA10 induces BCL2 expression, inhibits
apoptosis, and promotes cell proliferation in gastric cancer.
Cancer Med. 8:5651–5661. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang ZX, Tian Y, Li S, Jing HB, Cai J, Li
M and Xing GG: Involvement of HDAC2-mediated kcnq2/kcnq3 genes
transcription repression activated by EREG/EGFR-ERK-Runx1 signaling
in bone cancer pain. Cell Commun Signal. 22:4162024. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kim TH and Dekker J: ChIP-quantitative
polymerase chain reaction (ChIP-qPCR). Cold Spring Harb Protoc. May
1–2018.(Epub ahead of print). View Article : Google Scholar
|
|
42
|
World Health Organization, . WHO
laboratory manual for the examination and processing of human
semen. Fifth Edition. World Health Organization; Geneva: pp. 26–44.
2010, https://iris.who.int/server/api/core/bitstreams/6fcf020b-c7f9-48ea-b3ee-c13402d7328e/contentFebruary
16–2023
|
|
43
|
Liu N, Zhang GX, Zhu CH, Lan XB, Tian MM,
Zheng P, Peng XD, Li YX and Yu JQ: Antinociceptive and
neuroprotective effect of echinacoside on peripheral neuropathic
pain in mice through inhibiting P2X7R/FKN/CX3CR1 pathway. Biomed
Pharmacother. 168:1156752023. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yang XP, Huang JH, Ye FL, Yv QY, Chen S,
Li WW and Zhu M: Echinacoside exerts neuroprotection via
suppressing microglial α-synuclein/TLR2/NF-κB/NLRP3 axis in
Parkinsonian models. Phytomedicine. 123:1552302024. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yang Z, Zhao Y, Wang Y, Liu X, Jiang Y,
Jiang Y, Liu T, Hu Y and Chang H: Echinacoside ameliorates
post-stroke depression by activating BDNF signaling through
modulation of Nrf2 acetylation. Phytomedicine. 128:1554332024.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lu R, Zhang L, Wang H, Li M, Feng W and
Zheng X: Echinacoside exerts antidepressant-like effects through
enhancing BDNF-CREB pathway and inhibiting neuroinflammation via
regulating microglia M1/M2 polarization and JAK1/STAT3 pathway.
Front Pharmacol. 13:9934832022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Liang J, Chen T, Xu H, Wang T, Gong Q, Li
T, Liu X, Wang J, Wang Y and Xiong L: Echinacoside exerts
antihepatic fibrosis effects in high-fat mice model by modulating
the ACVR2A-smad pathway. Mol Nutr Food Res. 68:e23005532024.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wei J, Zheng Z, Hou X, Jia F, Yuan Y, Yuan
F, He F, Hu L and Zhao L: Echinacoside inhibits colorectal cancer
metastasis via modulating the gut microbiota and suppressing the
PI3K/AKT signaling pathway. J Ethnopharmacol. 318:1168662024.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang X, Tan B, Liu J, Wang J, Chen M, Yang
Q, Zhang X, Li F, Wei Y, Wu K, et al: Echinacoside inhibits tumor
immune evasion by downregulating inducible PD-L1 and reshaping
tumor immune landscape in breast and colorectal cancer.
Phytomedicine. 135:1561882024. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yi Q, Sun M, Jiang G, Liang P, Chang Q and
Yang R: Echinacoside promotes osteogenesis and angiogenesis and
inhibits osteoclast formation. Eur J Clin Invest. 54:e141982024.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Jiang Z, Wang J, Li X and Zhang X:
Echinacoside and Cistanche tubulosa (Schenk) R. Wight ameliorate
bisphenol A-induced testicular and sperm damage in rats through
gonad axis regulated steroidogenic enzymes. J Ethnopharmacol.
193:321–328. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kong ZL, Johnson A, Ko FC, He JL and Cheng
SC: Effect of cistanche tubulosa extracts on male reproductive
function in streptozotocin-nicotinamide-induced diabetic rats.
Nutrients. 10:15622018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Guo Y, Wang L, Li Q, Zhao C, He P and Ma
X: Enhancement of kidney invigorating function in mouse model by
cistanches herba dried rapidly at a medium high temperature. J Med
Food. 22:1246–1253. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Afsar T, Razak S, Trembley JH, Khan K,
Shabbir M, Almajwal A, Alruwaili NW and Ijaz MU: Prevention of
testicular damage by indole derivative MMINA via upregulated StAR
and CatSper channels with coincident suppression of oxidative
stress and inflammation: In silico and in vivo validation.
Antioxidants (Basel). 11:20632022. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Cooray A, Chae MR, Wijerathne TD, Kim DG,
Kim J, Kim CY, Lee SW and Lee KP: Hexane fraction of Prunus
japonica thunb. Seed extract enhances boar sperm motility via
CatSper ion channel. Heliyon. 9:e136162023. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhou Y, Chen L, Han H, Xiong B, Zhong R,
Jiang Y, Liu L, Sun H, Tan J, Cheng X, et al: Taxifolin increased
semen quality of Duroc boars by improving gut microbes and blood
metabolites. Front Microbiol. 13:10206282022. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Mohammadi S, Jalali M, Nikravesh MR, Fazel
A, Ebrahimzadeh A, Gholamin M and Sankian M: Effects of vitamin-E
treatment on CatSper genes expression and sperm quality in the
testis of the aging mouse. Iran J Reprod Med. 11:989–998.
2013.PubMed/NCBI
|
|
58
|
Mohammadi S, Movahedin M and Mowla SJ:
Up-regulation of CatSper genes family by selenium. Reprod Biol
Endocrinol. 7:1262009. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Park EH, Kim DR, Kim HY, Park SK and Chang
MS: Panax ginseng induces the expression of CatSper genes and sperm
hyperactivation. Asian J Androl. 16:845–851. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Lambert SA, Jolma A, Campitelli LF, Das
PK, Yin Y, Albu M, Chen X, Taipale J, Hughes TR and Weirauch MT:
The human transcription factors. Cell. 172:650–665. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zeng L, Zhu Y, Moreno CS and Wan Y: New
insights into KLFs and SOXs in cancer pathogenesis, stemness, and
therapy. Semin Cancer Biol. 90:29–44. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Jasim SA, Farhan SH, Ahmad I, Hjazi A,
Kumar A, Jawad MA, Pramanik A, Altalbawy MAF, Alsaadi SB and
Abosaoda MK: A cutting-edge investigation of the multifaceted role
of SOX family genes in cancer pathogenesis through the modulation
of various signaling pathways. Funct Integ Genomics. 25:62025.
View Article : Google Scholar : PubMed/NCBI
|

















![ECH treatment upregulates functional
expression of CatSper channels in spermatozoa from rats with
asthenozoospermia. (A) CatSper1, (B) CatSper2, (C) CatSper3 and (D)
CatSper4 protein abundance in sperm; n=5–6 rats per group.
Representative fura-2-acetoxymethyl ester fluorescence images of
sperm before and after 30 mM NH4Cl stimulation in (E)
NS, (F) ECH, (G) NS + NNC and (H) ECH + NNC group. Arrows indicate
[Ca2+]i fluorescent signals in response to
NH4Cl. Scale bar, 10 µm. (I) Representative single-sperm
fluorescence traces. (J) Normalized [Ca2+]i
responses in all tested sperm. (K) Summary plot of normalized
[Ca2+]i signals after NH4Cl
treatment (n=27–33 sperm per group from 5–6 rats). NS group:
Ornidazole + normal saline; ECH group: Ornidazole + ECH. Data are
presented as mean ± standard error of the mean. *P<0.05,
**P<0.01 and ***P<0.001; Unpaired t-test. ECH, echinacoside;
NS, normal saline; NNC, NNC 55-0396; CatSper, cation channel of
sperm; [Ca2+]i, intracellular calcium levels;
F340, fluorescence at 340 nm; F380, fluorescence at 380 nm.](/article_images/mmr/33/3/mmr-33-03-13794-g04.jpg)

![Functional activation of CatSper
channels in human sperm by ECH treatment. Sperm motility parameters
in healthy subjects: (A) Grade A, (B) grade A + B and (C) grade A +
B + C sperm. Sperm motility parameters in patients with AZS: (D)
Grade A, (E) grade A + B and (F) grade A + B + C sperm. n=6–7
subjects per group. (G-J) Representative fura-2-acetoxymethyl ester
fluorescence images of human sperm before and after 30 mM
NH4Cl stimulation. Arrows indicate
[Ca2+]i responses. Scale bar, 10 µm. (K)
Representative single-sperm fluorescence traces. (L) Normalized
[Ca2+]i responses in all tested sperm. (M)
Summary plot of normalized [Ca2+]i signals
after NH4Cl treatment (n=33–36 sperm per group from 5–6
subjects). Data are presented as mean ± standard error of the mean.
*P<0.05, **P<0.01 and ***P<0.001. Paired t-test for (A-F),
unpaired t-test for (M). ECH, echinacoside; AZS, asthenozoospermia;
[Ca2+]i, intracellular calcium levels; NS,
normal saline; HS, healthy subjects; F340, fluorescence at 340 nm;
F380, fluorescence at 380 nm.](/article_images/mmr/33/3/mmr-33-03-13794-g06.jpg)