Introduction
Overactive bladder (OAB) is a common, benign urinary
disorder. It causes lower urinary tract symptoms (LUTS). It is
characterized by urinary urgency, often accompanied by increased
frequency and nocturia, with or without urinary incontinence,
excluding urinary tract infections and other pathological changes
(1–3). The primary distinction between the
diagnosis of OAB and other lower urinary tract disorders causing
LUTS is the presence of urinary urgency. In the Asia-Pacific
region, the overall prevalence of OAB is as high as 20.8% and
prevalence rises with age (4).
Among Chinese women aged 31–40, the rate already exceeds 20%
(5). OAB, often referred to as
‘social cancer’, markedly affects the physical and mental
well-being of affected individuals.
The etiology of OAB is incompletely understood, with
neurogenic, myogenic, epitheliogenic and urogenic hypotheses
proposed. Established risk factors include advanced age, elevated
BMI, pelvic surgery, vaginal delivery, diabetes and chronic
constipation (6,7). Psychological factors such as
depression and anxiety also play a role (8,9).
Research on the OAB-anxiety link is scarce and inconclusive. The
underlying mechanisms of OAB remain inadequately understood,
leading to limited efficacy of various treatment modalities,
including behavioral therapy, pharmacotherapy and sacral
neuromodulation. Consequently, investigating the mechanisms
underlying OAB is of substantial clinical importance for the
prevention and treatment of this condition.
Anxiety is one of the commonest mental disorders. It
can both trigger and worsen OAB, and severe OAB can intensify
anxiety (10–12). An in vivo study conducted by
Tanyeri et al (13)
demonstrated that administration of mirabegron, a first-line
treatment for OAB, to anxious mice resulted in a significant
reduction in anxiety levels. This finding suggested the potential
relationship between anxiety and OAB. Anxiety also provoked
oxidative stress. It activated the hypothalamic pituitary adrenal
axis, raising glucocorticoids and damaging mitochondria, leading to
malondialdehyde (MDA) rise, while superoxide dismutase (SOD) and
glutathione peroxidase (GSH-Px) fell (14,15).
Furthermore, oxidative stress can induce neuroinflammation and the
release of pro-inflammatory cytokines, including IL-6, IL-1β and
TNF-α, which influence neuronal excitability and synaptic
transmission, thereby exacerbating anxiety (16,17).
In colitis, multiple sclerosis and Alzheimer models, oxidative
stress turns on the NF-κB pathway, amplifying inflammation and
tissue injury (18–20). Although research suggests a
correlation between anxiety, oxidative stress and NF-κB activation,
the relationship between these factors and OAB remains unclear.
At present, no clinical research has investigated
the causal relationship between anxiety and OAB, nor the potential
mechanisms involved in anxiety-induced OAB in vivo. The
mechanism of anxiety-induced OAB is categorized under the
aforementioned neurogenic hypothesis. The present study aimed to
explore the possibility of anxiety-induced OAB and identify key
genes in vivo, with the objective of offering new
perspectives and foundational research for the diagnosis of this
condition.
Materials and methods
Research design and clinical data
collection
The present study was conducted at Fujian Provincial
Hospital and received approval from the Ethics Committee (approval
no. K2024-10-019). Informed written consent was obtained from all
participants. Inclusion criteria: All patients who meet the
diagnostic criteria for OAB and healthy volunteers. Patients with
any one of the following conditions were ineligible: urinary tract
infection, various cystitis, urinary urogenital cancer, urinary
tract stones, neurogenic bladder, pelvic organ prolapse, pregnancy
and reluctance to participate in the present study. Between July
2023 and July 2024, a total of 83 patients presenting with LUTS and
40 healthy volunteers serving as controls were recruited from
Fujian Provincial Hospital. Of the 83 patients, 43 were excluded
due to LUTS resulting from other causes, leaving 40 patients who
were diagnosed with OAB and included in the disease cohort.
Comprehensive clinical data were successfully collected for 80
participants, encompassing the overactive bladder symptom scores
(OABSS; Table SI) and the
generalized anxiety disorder scale-7 (GAD-7; Table SII). Based on the OABSS scale, a
diagnosis of OAB is made when urinary urgency has a score of ≥2 and
the overall OABSS score is ≥3. When OABSS with a total score range
of 3–5, mild OAB; 6–11, moderate OAB; ≥12, severe OAB. When GAD-7
with a total score range of 0–4, normal; 5–9, mild anxiety; 10–13,
moderate anxiety; 14–18, moderately severe anxiety; 19–21 points,
severe anxiety. These assessment tools are primarily utilized for
the screening and evaluation of OAB severity and anxiety levels,
respectively (21). Based on the
OABSS and GAD-7 results, participants were categorized into four
groups: OAB with anxiety (n=33), OAB without anxiety (n=7), control
with anxiety (n=5), and control without anxiety (n=35). The
recruitment process for the study participants is illustrated in
Fig. 1.
Animal anxiety model
Animal experiments were conducted at the Nanfang
Hospital's Animal Research Center, approved by its Institutional
Animal Ethical Care Committee (approval no. NFYY-2021-0572) and
followed the National Institutes of Health Guide for the Care and
Use of Laboratory Animals (22). A
total of 60 female C57BL/6 mice (5–8 weeks old, 16–19 g) were
obtained from SPF Biotechnology Co., Ltd. Mice were maintained
under specific pathogen-free conditions in individually ventilated
cages with 0.22-µm filter tops at 21–23°C, 40–55% humidity, under a
12-h light/dark cycle, with autoclaved food and water available
ad libitum. All handling was performed under HEPA-filtered
air conditions to maintain barrier integrity. Before the
establishment of the model, all the animals were first marked on
their ears for identification and then randomly assigned to groups.
For three weeks, mice underwent chronic restraint stress (CRS) by
being immobilized in a transparent cylinder for six hours daily,
from 09:00 to 15:00, without access to food or water (n=6). The
control group could move, eat, and drink freely (n=6) (23). Prior to the tests, the groups were
randomly shuffled and unrelated staff were invited to perform the
operations. All behavioral tests were repeated three times per
mouse. Humane endpoints were predetermined and approved by the
Institutional Animal Care and Use Committee, including >20%
weight loss, severe dehydration, persistent hunched posture,
inability to ambulate, or any signs of restraint-induced injury.
Animals were monitored twice daily; no animals reached these
endpoints. Mice were deeply anesthetized with pentobarbital (60–80
mg/kg, intraperitoneal) and subsequently euthanized by cervical
dislocation.
Elevated plus maze (EPM) test
The EPM test consists of two open and two closed
arms, each measuring 50×10 cm, elevated 50 cm above the ground. The
closed arms have 30 cm high opaque walls, and the intersection of
the arms forms a 10×10 cm center area. Mice are placed in the
center facing an open arm, and their movements are recorded for 5
min using a video system (Shanghai Jiliang Software Science &
Technology Co., Ltd.). Anxiety-like behavior is indicated by
increased entries and prolonged time spent in the closed arms.
Open field (OF) test
The OF test involves a 60×60×40 cm open box with a
video system (Shanghai Jiliang Software Science & Technology
Co., Ltd.) to track movement. The box is divided into 25 squares,
with 9 central and 16 peripheral squares. During the 5-min test,
the total distance traveled, distance in the central area, and time
spent in the central area are recorded. A significant reduction in
these measures indicates anxiety in mice.
Cystometry
Mice were anesthetized with isoflurane (3.0–4.0% for
induction, 1.0–1.5% for maintenance) and placed in a supine
position. A midline incision exposed the lower abdomen, and a
0.6×15 mm needle was inserted into the bladder to remove residual
urine. Bladder pressure was measured using the BL-420N BioSignal
Acquisition System (Chengdu Techman Software Co., Ltd.), with
sterile saline infused at 1 ml/h. Once a stable micturition
waveform was achieved, intravesical pressure data were recorded for
30 min or at least five voiding cycles. Urine outflow was
monitored, and bladder contractions were identified by increased
intravesical pressure, indicating micturition. Bladder contractions
without urination were labeled as non-micturition contractions, it
reflects the involuntary contraction of the bladder and is one of
the core indicators for diagnosing and describing OAB (24). The peak intravesical pressure
during urination was measured and maximum bladder capacity was
determined by multiplying infusion time by infusion rate.
RNA-seq analysis
Bladder tissues were stored at −80°C. Total RNA was
extracted with the Eastep Super Kit (cat. no. LS1040; Promega
Corporation). Poly-A mRNA was selected on oligo(dT) beads and
converted to cDNA with the NEBNext Ultra kit (cat. no. 7530; New
England Biolabs, Inc.). The cDNA was amplified and sequenced on the
Illumina Novaseq6000 instrument (Illumina, Inc.), which was owned
by Gene Denovo Biotechnology Co. The specific parameters can be
found in Appendix S1.
To identify differential gene expression, a
comparative analysis was performed using the DESeq2 software
package v 1.48.1 (https://bioconductor.org/packages/release/bioc/html/DESeq2.html).
Genes with FDR ≤0.05 and |fold change|≥1.5 were called significant
(25).
Analysis of gene ontology (GO) and
Kyoto encyclopedia of genes and genomes (KEGG)
This part of the analysis was conducted using
clusterProfiler software v 3.81 (26).GO enrichment analysis connects
differentially expressed genes (DEGs) to significant biological
functions by identifying enriched GO terms compared with the genome
background (27).
After Bonferroni correction, P-values were adjusted
using FDR correction, with a threshold of FDR ≤0.05. KEGG aids in
understanding gene functions through pathway-based analysis. After
using the same multiple testing corrections, the Q-value, which is
the P-value after FDR correction, defined as the markedly enriched
pathways.
Generation of protein-protein
interaction (PPI) and discovery of key genes
The PPI network was built with STRING v10
(http://string-db.org), with genes as nodes and
interactions as edges (28).
Cytoscape v3.7.1 visualized the network, highlighting core and hub
genes (www.cytoscape.org) (29). Bioinformatics analyses were
conducted using Omicsmart, an online platform for data analysis
(http://www.omicsmart.com). The details
of analysis parameters are listed in Appendix S2.
Reverse transcription-quantitative
(RT-q) PCR
The primers used in the present study are listed in
Table SIII. cDNA synthesis and
qPCR were performed according to the manufacturer's protocols. RNA
was extracted from the bladder using the Animal Total RNA Isolation
Kit (Foregene Co., Ltd.) following the manufacturer's instructions.
cDNA was synthesized from total RNA with HiScript III RT SuperMix
for qPCR (Vazyme Biotech Co., Ltd.) at 50°C for 15 min and 85°C for
5 sec. RT-qPCR was conducted on a LightCycler480 system (Roche
Diagnostics, Ltd.) using ChamQ SYBR qPCR Master Mix (Vazyme Biotech
Co., Ltd.) at 95°C for 30 sec; followed by 40 cycles at 95°C for 10
sec and 60°C for 30 sec; and a final melt curve analysis at
60–95°C. GAPDH was the internal reference, and gene
expression was measured using the 2−∆∆Cq method
(30).
Determining the levels of oxidative
stress and pro-inflammatory cytokines
Each mouse had a blood sample of ~1 ml collected by
cardiac puncture. Serum and bladder levels of MDA (cat. no.
S0131S), total superoxide dismutase (T-SOD; cat. no. S0101S) and
GSH (cat. no. S0052) were measured using specific assay kits from
Beyotime Biotechnology. ELISA kits from R&D Systems, Inc. were
used to assess IL-6 (cat. no. M6000B), IL-1β (cat. no. MLB00C) and
TNF-α (cat. no. MTA00B) levels. All procedures followed the
provided instructions.
Immunohistochemistry
Bladder tissues were immersed in 4% paraformaldehyde
for fixation at 4°C for 8 h, and sequentially underwent dehydration
with an ethanol gradient (25°C, 5 h), permeabilization with 0.3%
Triton X (25°C, 20 min), paraffin dipping (58°C, 1.5 h) and
embedding (58°C to 4°C, 30 min); after which, the tissues were cut
into 4-µm sections. The sections were cleaned and sealed with drops
of goat serum (cat. no. BL210A; Biosharp Life Sciences) for 30 min
at room temperature. Subsequently, the blocking solution was
discarded and diluted rabbit anti-Hsp90aa1 (1:200; cat. no.
BF0084-50; Affinity Biosciences), anti-Hsp90ab1 (1:200; cat. no.
BF0215; Affinity Biosciences), anti-Hsp90b1 (1:200; cat. no.
AF5287; Affinity Biosciences) and anti-NF-κB (1:200; cat. no.
YM8209; ImmunoWay Biotechnology Company) antibodies were added
dropwise to the sections and incubated overnight at 4°C. The next
day, goat anti-rabbit secondary antibody (1:200; cat. no. LF102;
Epizyme; Ipsen Pharma) was dropped into the sections and incubated
at 37°C for 30 min. DAB chromogen concentrate was mixed with
diluent to prepare a working solution according to the instructions
of the DAB kit (cat. no. BL732A; Biosharp Life Sciences) and
applied to the tissue sections, which were washed, re-stained with
hematoxylin at room temperature for 3 min and washed again.
Finally, the sections were dehydrated, permeabilized with xylene
and sealed. Scanning and viewing of the images was performed using
NanoZoomer Digital slide scanner (Hamamatsu Photonics K.K.) and NDP
View2 Plus Image viewing software (version U12388-01; Hamamatsu
Photonics K.K.). The average optical density was quantified using
Image-Pro Plus software (version 6.0; Media Cybernetics, Inc.),
with measurements taken from three randomly selected fields of view
at ×10 magnification for every sample.
Hematoxylin and eosin staining
Paraffin-embedded sections (4 µm) were dewaxed in
xylene (3×5 min) and rehydrated through a descending ethanol series
(100, 95 and 70%, 2 min each). Nuclei were stained with Mayer's
hematoxylin (cat. no. MHS16-100ML; MilliporeSigma) for 90 sec at
room temperature, differentiated in 1% acid alcohol (1 sec), blued
in 0.2% ammonia water (30 sec) and counterstained with 1% eosin Y
(cat. no. 318906-25G; MilliporeSigma) for 45 sec. After dehydration
in ethanol (95 and 100%, 2 min each) and permeabilization in xylene
(2×3 min), slides were mounted with neutral balsam (cat. no.
10050041; Sinopharm Chemical Reagent Co., Ltd.). Digital images
were acquired at ×20 magnification using a NanoZoomer S60 scanner
(Hamamatsu Photonics K.K.).
Statistical analysis
Continuous variables were summarized as mean ±
standard deviation or median (Q1-Q3) for normal and non-normal
distributions, respectively, while categorical variables were shown
as frequency and percentage (GraphPad Prism software version 10;
Dotmatics). When comparing the difference in means between two
samples, the unpaired Student's t-test was used when data had a
normal distribution, whereas the Mann-Whitney U test was employed
when data had a non-normal distribution. χ2 or Fisher's
exact tests compared categorical variables. Correlations were
analyzed using Pearson's or Spearman's coefficients. Stepwise
multivariate logistic regression identified key predictors,
reporting odds ratios (OR) with 95% confidence intervals (CI).
Model fit was checked with the Hosmer-Lemeshow test, and predictive
ability was evaluated using the ROC curve. All candidate variables
with P<0.10 in univariate analysis were entered into the initial
model. Multicollinearity was assessed by variance inflation factor
(VIF); all retained variables showed VIF <5. The final model
exhibited good calibration (Hosmer-Lemeshow test P=0.616). All the
data underwent normality tests before being subjected to
statistical analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
Demographic characteristics of the
participants
Demographic characteristics of participants were
assessed in a study involving 40 patients with OAB and 40 healthy
controls (CON). As shown in Table
I, there were no significant differences in age, BMI, sex,
history of pelvic surgery, diabetes, or hypertension between the
groups. OABSS were markedly higher in the OAB group; intriguingly,
GAD-7 was notably elevated at 7.18±3.56 compared with 2.00 (1.00,
3.00) in the CON group, indicating a potential link between anxiety
and OAB.
 | Table I.Comparisons of characteristics
between OAB group and CON group. |
Table I.
Comparisons of characteristics
between OAB group and CON group.
| Characteristic | OAB (n=40) | CON (n=40) | P-value |
|---|
| Age | 35.90±7.65 | 37.45±12.70 | 0.51 |
| BMI | 21.74±2.43 | 22.65±2.72 | 0.12 |
| Sex |
|
| 0.82 |
|
Male | 21 (53) | 20 (50) |
|
|
Female | 19 (47) | 20 (50) |
|
| History of pelvic
surgery | 8 (20) | 6 (15) | 0.56 |
| Diabetes | 7 (17.5) | 8 (20) | 0.76 |
| Hypertension | 9 (22.5) | 8 (20) | 0.79 |
| GAD-7 | 7.18±3.56 | 2.00 (1.00,
3.00) | <0.001 |
| OABSS | 7.00 (4.25,
8.00) | 1.00 (0.00,
2.00) | <0.001 |
The relationship between anxiety and
OAB
To elucidate the relationship between anxiety and
OAB, and to determine whether anxiety can induce OAB, the present
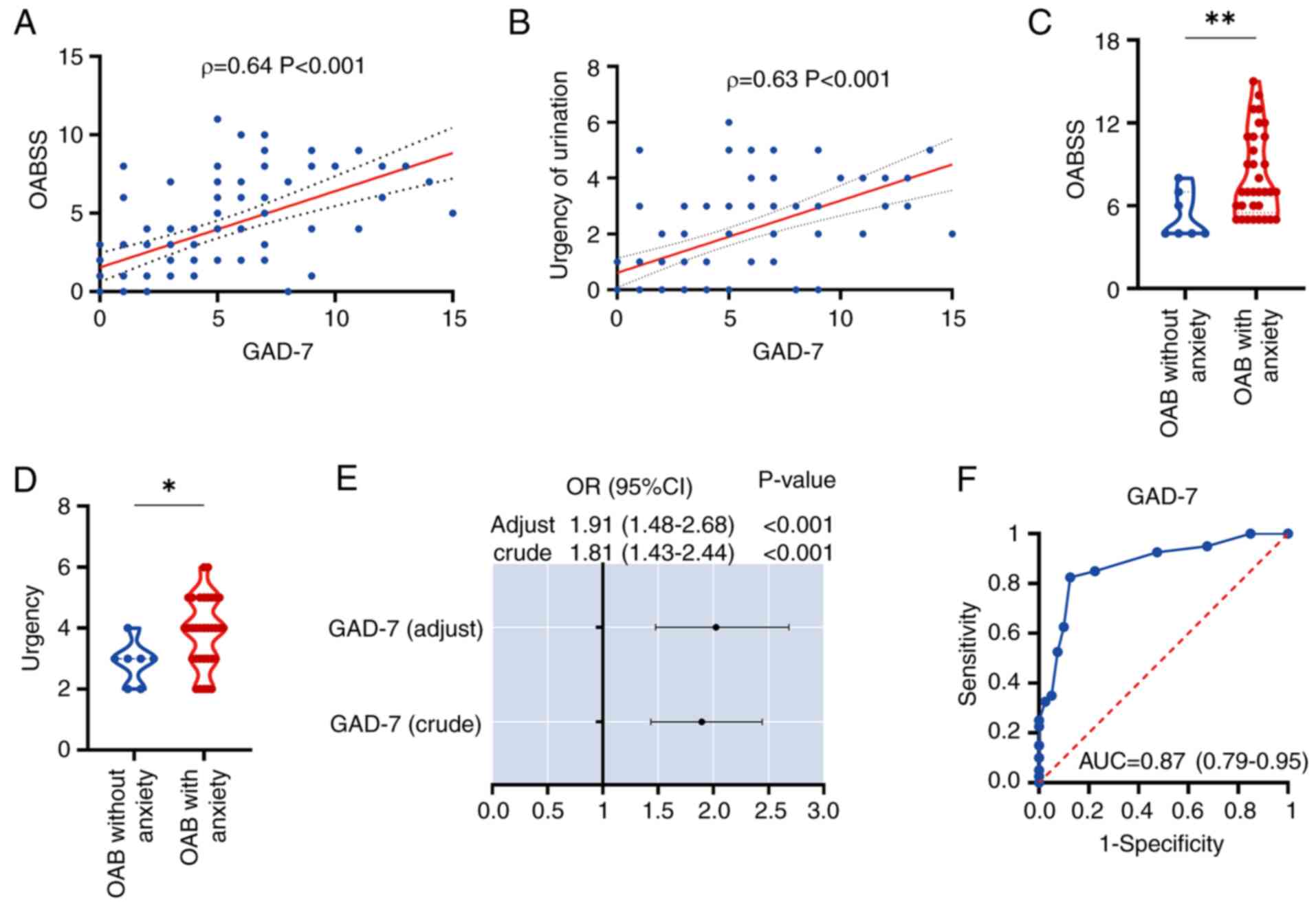
study conducted correlation and regression analyses. The findings
indicated a positive correlation between OABSS and the GAD-7
(Fig. 2A). Additionally, urinary
urgency, a key symptom of OAB, was positively correlated with GAD-7
(Fig. 2B). Subsequently, all OAB
patients were categorized into two groups based on the presence or
absence of anxiety and compared their OABSS and urinary urgency
scores. The results demonstrated that OAB patients with anxiety
exhibited markedly higher OABSS and urinary urgency levels compared
with those without anxiety (Fig. 2C
and D). To further explore the causal relationship between
anxiety and OAB, the present study initially performed a univariate
logistic regression analysis on GAD-7, which yielded an odds ratio
(OR) of 1.81 (95% CI: 1.43–2.44), with a crude P-value of
<0.001. This was followed by a multivariate logistic regression
analysis, incorporating all baseline variables, which resulted in
an adjusted OR of 1.91 (95% CI: 1.48–2.68), with an adjusted
P-value of <0.001 (Fig. 2E). In
conclusion, receiver operating characteristic (ROC) curves were
used to evaluate the potential of anxiety as an independent risk
factor for predicting the occurrence of OAB. The GAD-7 demonstrated
a notably high predictive accuracy, with an area under the curve
(AUC) of 0.87 (95% CI: 0.79–0.95) (Fig. 2F), the specificity was 0.875, the
sensitivity was 0.825 and the Youden's index was 1.7. This aspect
of the present study effectively demonstrated, at a clinical level,
that anxiety can be employed as an independent risk factor for
forecasting the onset of OAB.
Anxiety can induce OAB-like symptoms
in vivo
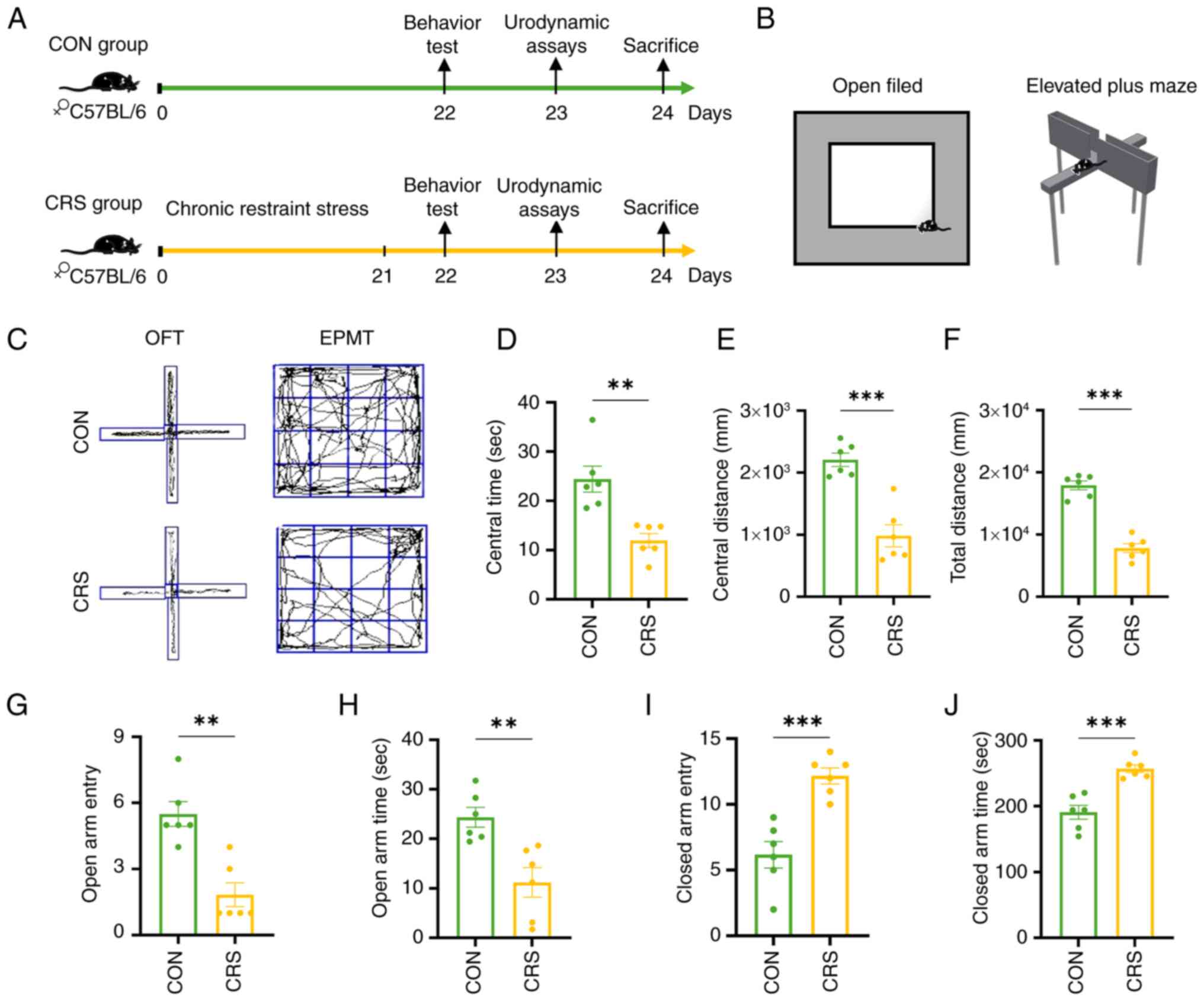
In the present study, an anxiety model was developed
using 21 days of CRS (Fig.
3A).
In the open-field (OF) test (Fig. 3B and C), the time spent (11.98±1.41
sec) by the CRS mice in the central area, the distance traveled
(983±179 mm) during activity, and the total distance traveled
(7,803±727.10 mm) were all markedly lower than those of the control
group (24.41±2.63 sec), (2,209±106.80 mm), (1,7926±722.10 mm),
(Fig. 3D-F). In the elevated-plus
maze (EPM), The number of times the CRS mice entered the open arm
(1.83±0.54)and the duration they stayed (11.20±2.97 sec) there were
markedly lower than those of the control group (5.50±0.56),
(24.35±2.00 sec) while the number of times they entered the closed
arm (12.17±0.60) and the duration they stayed (257.10± 5.83 sec)
there were markedly higher than those of the control group
(6.16±1.01), (190.90±10.60 sec), (Fig.
3G-J). These changes confirmed that CRS induced anxiety-like
behavior, as the mice exhibited behaviors resembling anxiety, such
as reduced exploration desire and avoidance of conflicts.
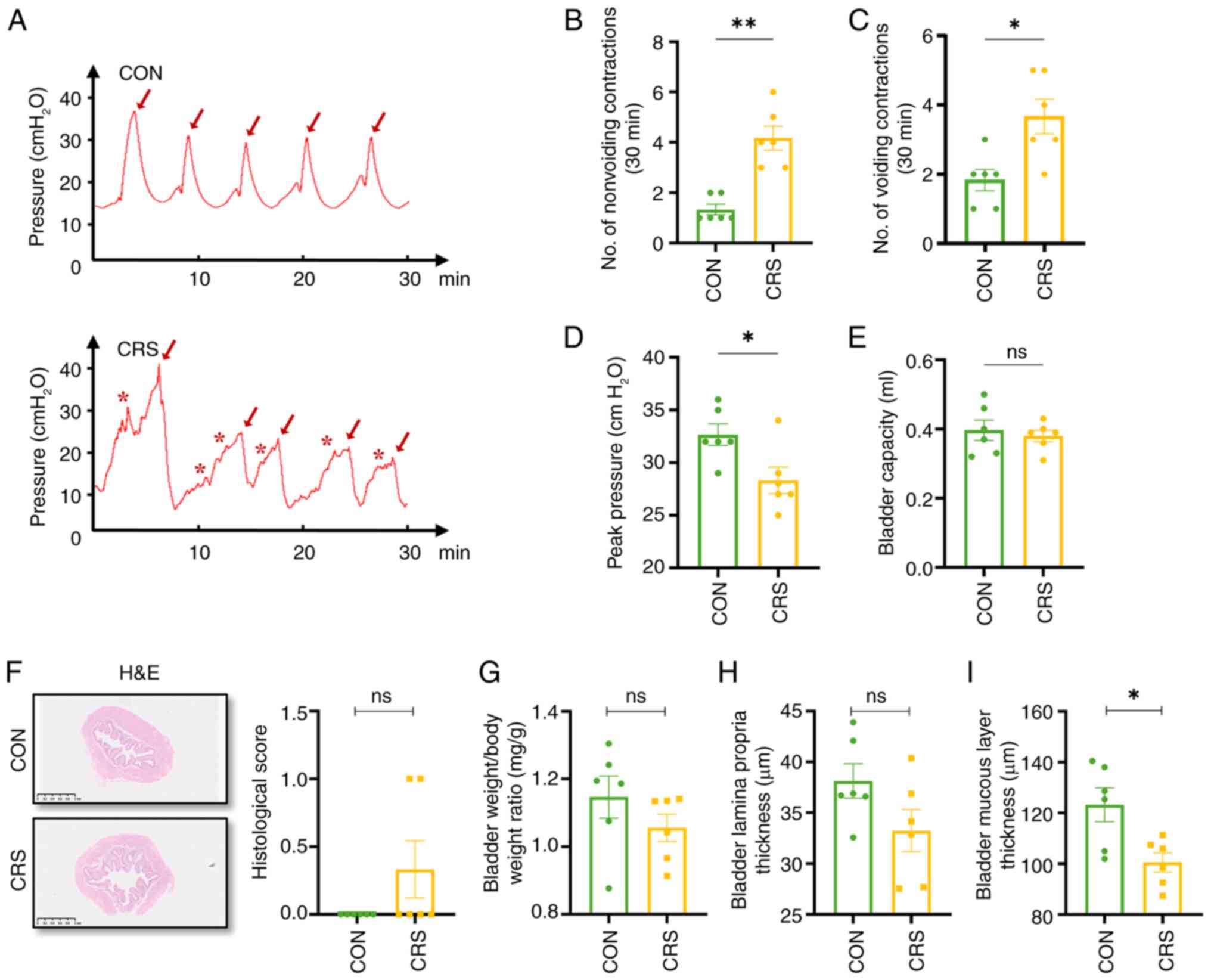
Furthermore, cystometry was conducted to assess
bladder function (Fig. 4A) and it
was and found that CRS mice had more non-voiding (4.16±0.47 vs.
1.33±0.21), voiding contractions (3.67±0.95 vs. 1.83±0.31) and
lower peak pressure (28.33±1.26 vs. 32.67±1.02 cm H2O),
whereas bladder capacity was unchanged (0.38±0.02 vs. 0.39±0.03
ml), (Fig. 4B-E). These results
indicate that CRS-induced anxiety may cause OAB-like symptoms in
mice through involuntary contractions of the detrusor muscle.
Subsequently, the present study conducted hematoxylin and eosin
staining on bladders and calculated the bladder-to-body weight
ratio, finding no difference in bladder-to-body weight ratio or
lamina propria thickness between groups, mirroring human OAB
(31) (Fig. 4F-H). The bladder mucosal layer was
thinner in CRS mice compared with controls (Fig. 4I), suggesting that mucosal layer
changes might contribute to OAB symptoms.
Exploration of differentially
expressed genes (DEGs)
The present study conducted RNA-seq analysis to
identify DEGs between CRS mice and control mice. After processing
and normalizing the raw data, 551 DEGs were found, with 58
upregulated and 493 downregulated (Fig. S1). This meant that certain
pathways are activated, while numerous others are suppressed.
Enrichment analysis of function and
pathways in DEGs
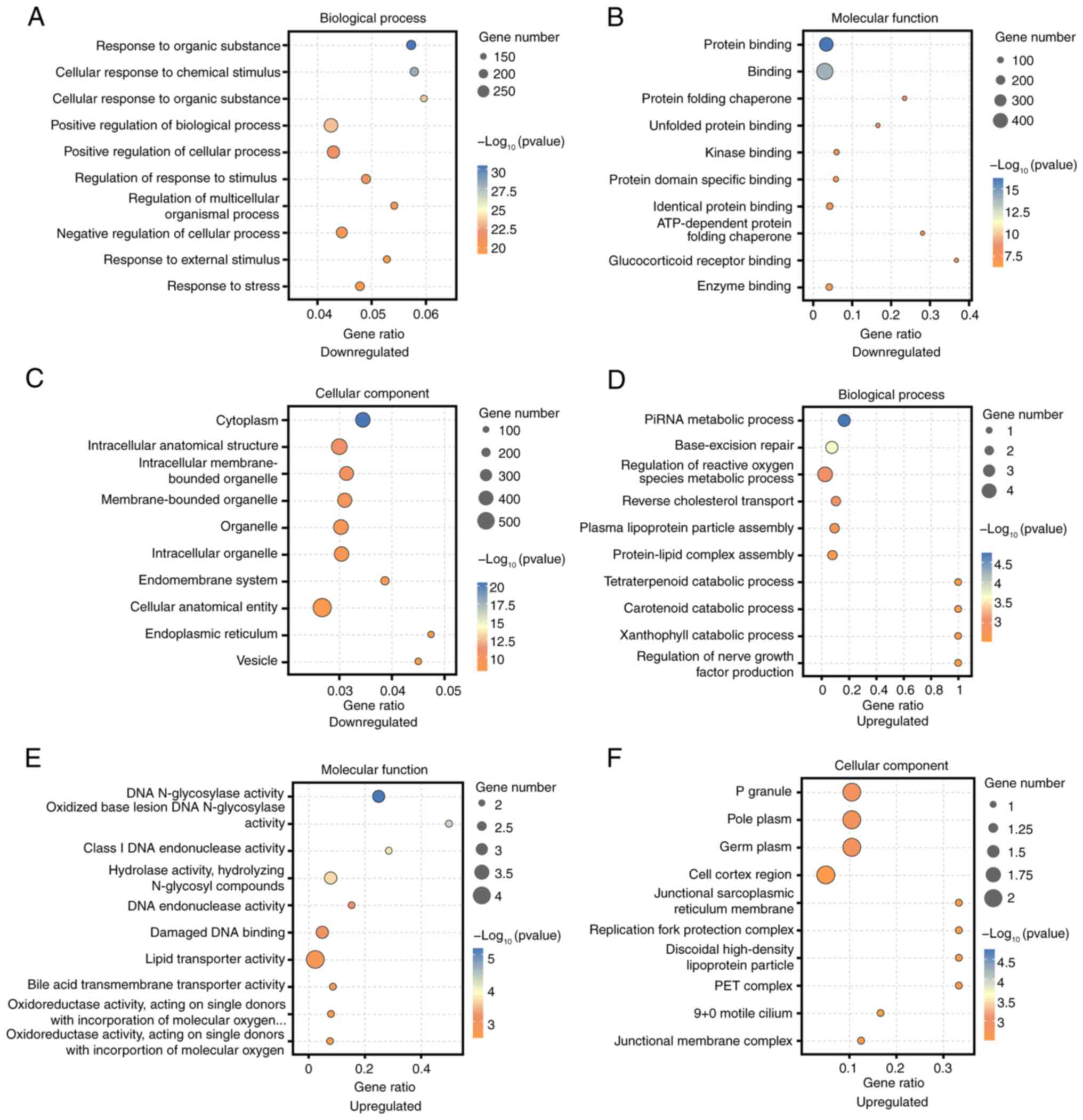
GO analysis identified the most enriched categories
among downregulated DEGs as ‘positive regulation of biological
process’, ‘Binding’, and ‘Cellular anatomical entity’ (Fig. 5A-C). For upregulated DEGs, the top
categories were ‘Regulation of reactive oxygen species metabolic
process’, ‘lipid transporter activity’, and ‘P granule’ (Fig. 5D-F). KEGG pathway analysis revealed
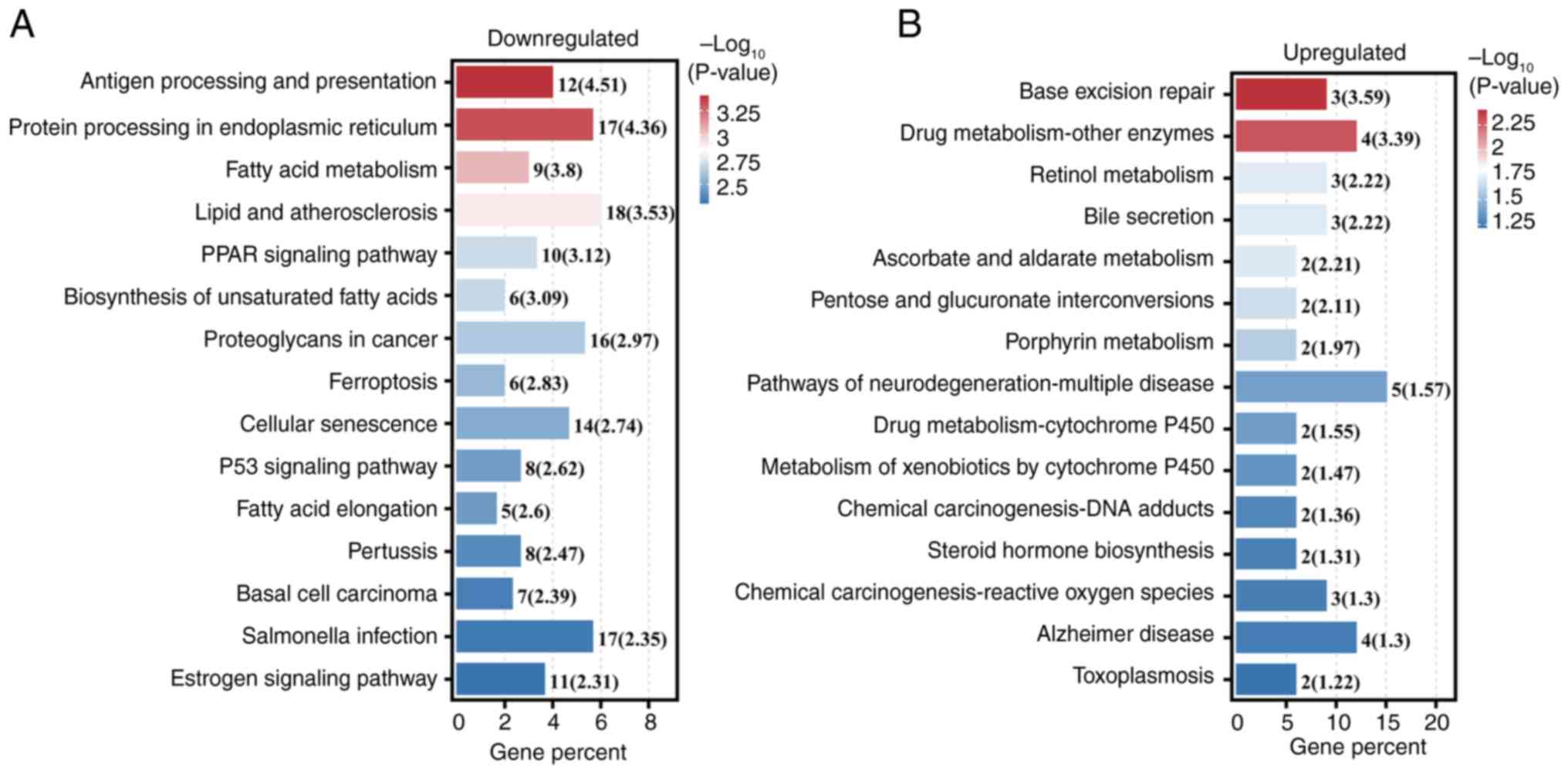
‘Antigen processing and presentation’ as the most markedly
associated with downregulated DEGs (Fig. 6A), while ‘Base excision repair’ was
most significant for upregulated DEGs (Fig. 6B).
Construction of a PPI network and
identification of key hub genes
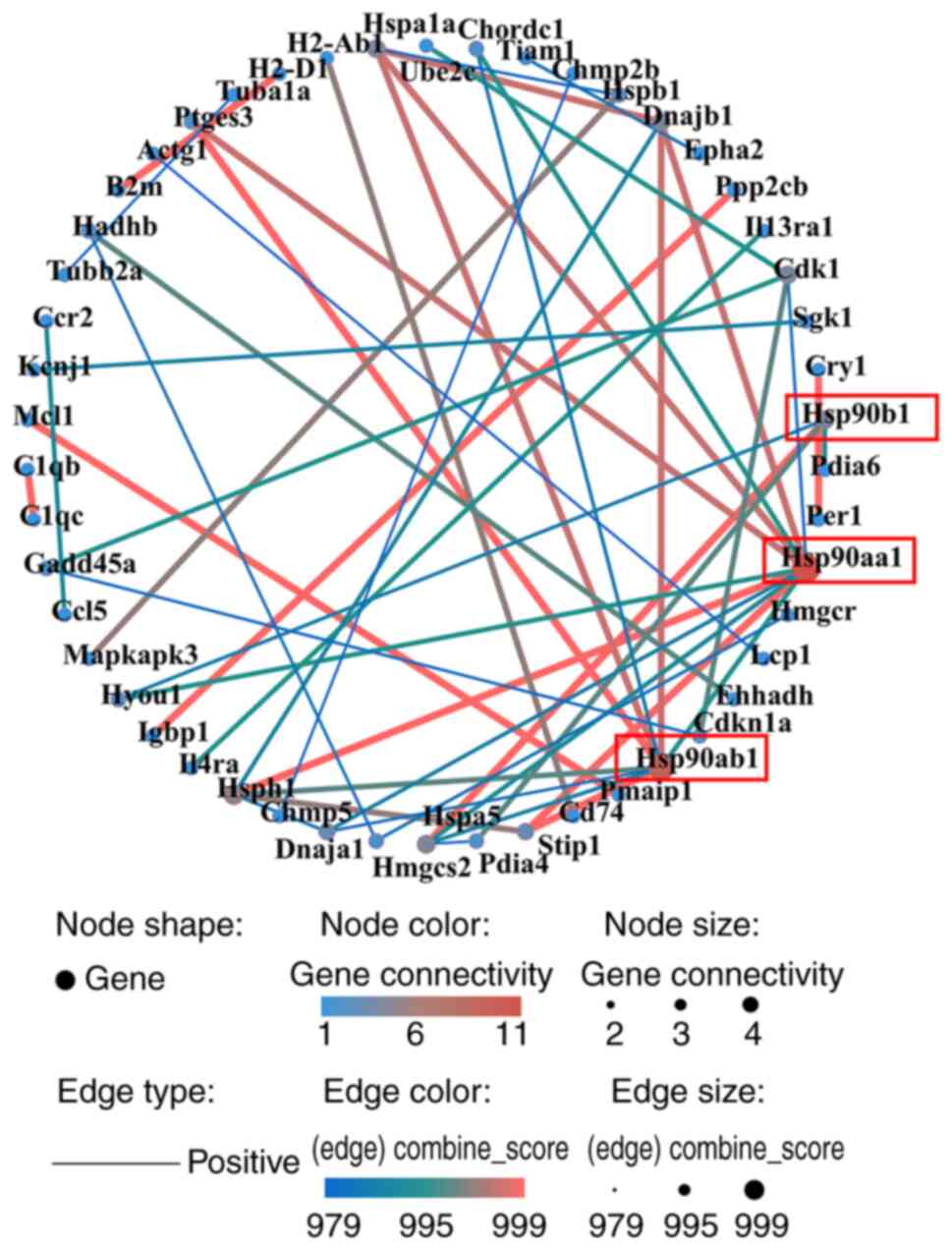
The PPI network comprised 50 nodes and 533 edges
(Fig. 7). The top three hub genes,
identified by combined score, were Hsp90aa1, Hsp90ab1, and
Hsp90b1, all from upregulated DEGs.
Anxiety-induced OAB activated
oxidative stress and NF-κB pathways in vivo
Based on GO analysis and previous studies, it was
hypothesized that the disease mechanism might involve oxidative
stress, which could activate the NF-κB pathway (32,33).
To investigate, the present study measured serum levels of MDA,
GSH, and T-SOD in mice, as these indicate oxidative stress
activation. Serum MDA was higher in CRS mice, whereas GSH and T-SOD
were lower (Fig. 8A-C). The same
pattern was seen in bladder tissue (Fig. 8G-I). Next, we examined downstream
inflammation. Serum IL-6, IL-1β and TNF-α were all elevated
(Fig. 8D-F), and identical
increases were found in the bladder (Fig. 8J-L).
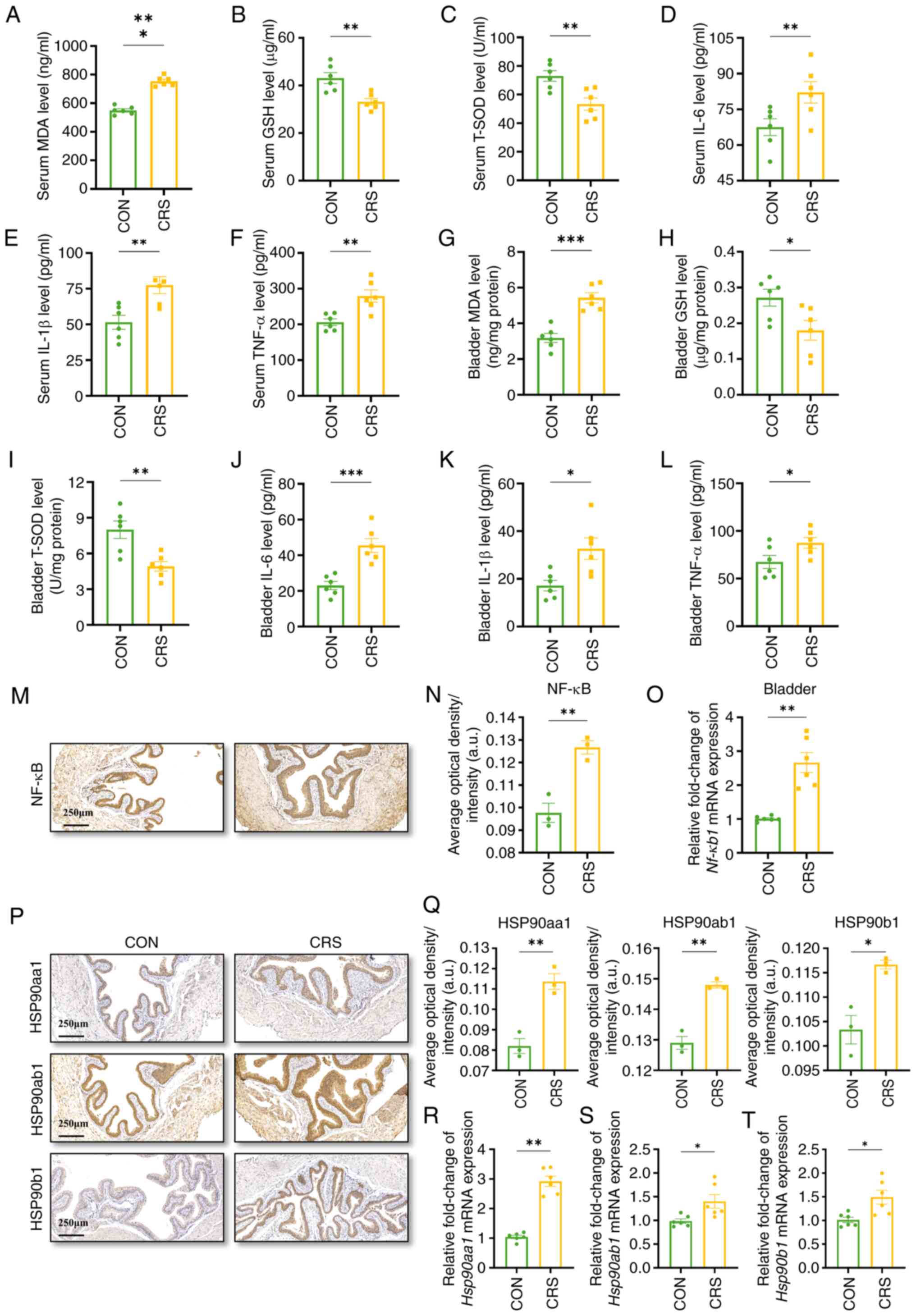 | Figure 8.Anxiety active oxidative stress and
NF-κB signaling. The concentration of (A) MDA, (B) GSH, (C) T-SOD,
(D) IL-6, (E) IL-1β and (F) TNF-α in the serum. The concentration
of (G) MDA, (H) GSH, (I) T-SOD, (J) IL-6, (K) IL-1β and (L) TNF-α
in the bladder. (M) Representative images of NF-κB in the bladder,
scale bar, 250 µm. (N) Average optical density of NF-κB in the
bladder. (O) Relative mRNA level of Nf-κb1 in the
bladder. (P) Representative immunohistochemistry of Hsp90aa1,
Hsp90ab1 and Hsp90b1 in the bladder, scale bar, 250 µm. (Q) The
average optical density analysis results of Hsp90aa1, Hsp90ab1 and
Hsp90b1 in the bladder. (R) Relative mRNA levels of Hsp90aa1
in bladder tissues. (S) Relative mRNA levels of Hsp90ab1 in
bladder tissues. (T) Relative mRNA levels of Hsp90b1 in
bladder tissues. Data were presented as the mean ± SD. n=3
in M, N, P and Q, n=6 in A-L, O and R-T for each group.
P-values were calculated using a two-tailed unpaired Student's
t-test (*P<0.05, **P<0.01, ***P<0.001). MDA,
malondialdehyde; GSH, glutathione; T-SOD, total superoxide
dismutase. |
To further verify the status of NF-κB in the
bladder, the gene and protein expression of NF-κB in the bladder of
each group were examined and the results showed that the gene
expression of Nf-κb1 and the protein expression levels of
NF-κB as well as IL-6, IL-1β and TNF-α were markedly increased in
the CRS group compared with the control group (Fig. 8M-O). These findings might indicate
that anxiety could trigger the NF-κB pathway through oxidative
stress and pro-inflammatory mediators, causing bladder
overactivity.
Identification of key genes and
proteins in bladder
Hsp90 has been linked to NF-κB in various
disease models (34,35). In the present study, Hsp90
genes were identified during PPI network construction. The present
study used qPCR and IHC on mouse bladder tissue to confirm Hsp90
mRNA and protein levels. Results (Fig.
8P-T) showed significant upregulation of Hsp90aa1, Hsp90ab1,
and Hsp90b1 in the OAB group compared with controls, with protein
expression mainly in the mucosal layer.
Discussion
OAB is a common cause of LUTS. It markedly affects
patients' quality of life, leading to mobility issues, urinary
tract infections, anxiety, depression and loss of self-confidence
(36). The unclear pathogenesis of
OAB results in a lack of targeted treatments. Thus, understanding
OAB's underlying mechanisms is crucial for effective prevention and
treatment.
Studies have established a positive correlation
between OAB and factors such as age and BMI (37–39).
Additionally, conditions such as diabetes mellitus, hypertension,
history of pelvic surgery and reproductive history can lead to the
onset or progression of OAB (40,41).
Regarding psychological factors, limited research suggests a
correlation between the severity of OAB and anxiety (42–44).
Nonetheless, there is insufficient evidence to conclude whether
anxiety independently induces OAB or serves as a standalone risk
factor for its development. The current study identified a positive
correlation between the level of anxiety and the severity of OAB
and urinary urgency, corroborating previous findings. Furthermore,
through multivariate logistic regression analysis and the
construction of ROC curves, it established that anxiety serves as
an independent risk factor for the development of OAB.
To explore anxiety-induced OAB mechanisms, the
present study conducted in vivo experiments after analyzing
clinical data. OAB modeling methods include drug injection and
bladder outlet obstruction (BOO), with BOO being the most common
(45–47). BOO can lead to issues such as
collagen redistribution, muscle hypertrophy and changes in bladder
capacity, potentially skewing OAB mechanism studies (48–50).
The present study uniquely applied CRS, typically used for anxiety
models, to induce OAB in mice (51). This method minimized surgical
impact on bladder physiology, reducing confounding factors and
realistically replicated anxiety-induced OAB showed in clinical
settings.
Having confirmed anxiety as an independent OAB risk
factor and generated a murine anxiety-OAB model, the present study
performed bladder transcriptomics to dissect mechanisms, biomarkers
and targets. RNA-seq revealed 551 DEGs, with 58 upregulated and 493
downregulated. It was hypothesized that this transcriptional
imbalance represents a cellular ‘functional trade-off’, where the
extensive downregulation of homeostatic pathways serves to conserve
energy under chronic anxiety stress. Concurrently, the selective
upregulation of genes related to ROS metabolism and base excision
repair indicated a prioritization of essential survival mechanisms
over routine physiological functions, such as antigen presentation.
GO analysis highlighted enriched terms such as ‘regulation of
reactive oxygen species metabolic process’, ‘lipid transporter
activity’, and ‘P granule’ among the upregulated genes. Reactive
oxygen species (ROS) are highly reactive compounds involved in
various biological processes (52). The excessive accumulation of ROS
induces oxidative stress within cells, resulting in damage to DNA,
proteins and lipids. This damage is implicated in the pathogenesis
of various diseases, including cancer, cardiovascular diseases and
neurodegenerative disorders. Furthermore, ROS accumulation has been
associated with NF-κB signaling and ferroptosis, as documented in
several studies (53–55). These findings establish a molecular
framework for mechanistic inquiry. Notably, KEGG pathway analysis
revealed a significant enrichment of upregulated genes in the ‘Base
Excision Repair’ pathway. Base excision repair is a crucial
mechanism by which cells address DNA damage and preserve genomic
stability, thereby playing an essential role in maintaining
cellular health and preventing disease progression (56).
Through GO analysis and literature review, the
present study concentrated on oxidative stress and the NF-κB
signaling pathway. Anxiety boosts ROS by stimulating the
neuroendocrine, autonomic and inflammatory systems (57). Excess ROS damages DNA, proteins and
mitochondria, depletes SOD/GSH and raises MDA, IL-6, IL-1β and
TNF-α (58–60). Oxidative stress switches on NF-κB,
releasing inflammatory mediators and neurotransmitters that amplify
anxiety. In bladder ischemia-reperfusion models, the same ROS burst
sensitives bladder nerves and triggered overactivity (61). Our previous study found that both
oxidative stress and NF-κB are involved in OAB models induced by a
high-salt diet (62). The present
study confirmed oxidative stress and NF-κB pathway activation in an
anxiety-induced OAB model, aligning with previous findings.
To identify DEGs, a PPI network was constructed
through data mining and integration. The present study found that
the upregulated DEGs Hsp90aa1, Hsp90ab1, and
Hsp90b1 were markedly elevated in the bladder tissues of the
CRS group, confirmed by q-PCR, with increased protein levels.
Heat shock protein 90 (Hsp90) is a key molecular
chaperone involved in protein folding, stability, and signal
transduction. The main homologs in the Hsp90 family are Hsp90aa1,
Hsp90ab1, and Hsp90b1 (63).
Hsp90aa1, found on chromosome 14, encodes a chaperone that forms a
homodimer to assist in protein folding using ATPase activity
(64,65). Hsp90ab1 stabilizes various proteins
in the cytoplasm, maintaining cell homeostasis under stress
(66). Hsp90b1, located in the
endoplasmic reticulum (ER), aids in protein folding and secretion,
crucial during ER stress for maintaining protein balance (67). In cancers such as osteosarcoma and
breast cancer, NF-κB, JAK/STAT and autophagy pathways often
upregulate Hsp90 and fuel tumor growth (68–72).
However, few studies connect Hsp90 to urologic diseases such as
bladder cancer and acute kidney injury, with unclear mechanisms
(73,74). While HSP90 is important in various
diseases, its connection to OAB has not been reported until now.
The present study is the first, to the best of the authors'
knowledge, to show increased Hsp90 expression in an anxiety-induced
OAB model, potentially linked to oxidative stress and the NF-κB
signaling pathway. Perhaps in the future, we may be able to predict
the occurrence and severity of OAB by detecting the content of
HSP90 in the patient's bladder tissue or develop drugs that inhibit
the expression of HSP90 to treat OAB.
The present study successfully identified anxiety as
an independent risk factor for the onset of OAB for the first time
to the best of the authors' knowledge, developed an in vivo
model of anxiety-induced OAB, and made preliminary discoveries
regarding the underlying mechanisms. However, several limitations
must be acknowledged. First, the sample sizes collected from
clinical settings were relatively small, limiting the
generalizability of the findings. Although the overall sample
(n=80) provided 88% power to detect a medium-to-large main effect
of anxiety on OAB (partial ρ2 ≥0.20, α=0.05), post-hoc
analysis revealed that pairwise comparisons within the smallest
subgroups (OAB without anxiety, n=7; controls with anxiety, n=5)
remain under-powered (<50%). Consequently, negative findings in
these strata should be interpreted cautiously and future
prospective studies with pre-specified balanced allocation are
warranted. Second, the conclusions derived from the animal model
may not accurately represent the human condition, and it is
recommended that tissue samples be obtained via cystoscopic biopsy
to further validate these findings. Additionally, the results of
the present study cannot conclusively prove the causality between
anxiety and OAB. Further research using pharmacologic inhibition or
genetic knockdown are needed to confirm causal relationship.
The present study was the first, to the best of the
authors' knowledge, to identify anxiety as an independent risk
factor for OAB, using in vivo experiments to create an
anxiety-induced OAB model. Bioinformatic analyses, qPCR and IHC
confirmed increased Hsp90 expression in the bladder. The study also
suggested that anxiety-induced OAB activates oxidative stress and
the NF-κB pathway. Hsp90 could become a potential biomarker
and target for diagnosing and treating anxiety-induced OAB, pending
clinical trial confirmation.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was funded by the Natural Science Foundation
of Fujian Province, China (grant no. 2024J011023) and the Startup
Fund for Scientific Research at Fujian Medical University (grant
no. 2022QH1057).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author. The raw sequence data
generated in the present study may be found in the Genome Sequence
Archive (Genomics, Proteomics & Bioinformatics 2025), National
Genomics Data Center (Nucleic Acids Res 2025), China National
Center for Bioinformation/Beijing Institute of Genomics, Chinese
Academy of Sciences under accession number GSA: CRA034777 or at the
following URL: https://ngdc.cncb.ac.cn/gsa/browse/CRA034777.
Authors' contributions
HH conceived and designed the study. ZZ and LX
conducted experiments and prepared samples, with ZZ and HW handling
RNA-seq analysis. ZZ and HW confirmed the authenticity of all the
raw data. HW verified the data. ZZ and LX wrote the paper, while HH
and HW offered critical advice and revised it extensively. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study received ethical clearance from
the Institutional Review Board of Fujian Provincial Hospital, with
the protocol number K2024-10-019. Informed consent was obtained in
writing from all participants. Animal experiments were performed at
the Animal Research Center of the Nanfang hospital, following
approval from the Institutional Animal Care and Use Committee
(approval no. NFYY-2021-0572).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nambiar AK, Arlandis S, Bø K,
Cobussen-Boekhorst H, Costantini E, de Heide M, Farag F, Groen J,
Karavitakis M, Lapitan MC, et al: European association of urology
guidelines on the diagnosis and management of female non-neurogenic
lower urinary tract symptoms. Part 1: Diagnostics, overactive
bladder, stress urinary incontinence, and mixed urinary
incontinence. Eur Urol. 82:49–59. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Peyronnet B, Mironska E, Chapple C,
Cardozo L, Oelke M, Dmochowski R, Amarenco G, Gamé X, Kirby R, Van
Der Aa F and Cornu JN: A comprehensive review of overactive bladder
pathophysiology: On the way to tailored treatment. Eur Urol.
75:988–1000. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen LC and Kuo HC: Current management of
refractory overactive bladder. Low Urin Tract Symptoms. 12:109–116.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chow PM, Liu SP, Chuang YC, Lee KS, Yoo
TK, Liao L, Wang JY, Liu M, Sumarsono B and Jong JJ: The prevalence
and risk factors of nocturia in China, South Korea, and Taiwan:
Results from a cross-sectional, population-based study. World J
Urol. 36:1853–1862. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang S, Guo C, Tai S, Ding H, Mao D,
Huang J and Qian B: Prevalence of overactive bladder in Chinese
women: A systematic review and meta-analysis. PLoS One.
18:e02903962023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ninomiya S, Naito K, Nakanishi K and
Okayama H: Prevalence and risk factors of urinary incontinence and
overactive bladder in Japanese Women. Low Urin Tract Symptoms.
10:308–314. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mckellar K, Bellin E, Schoenbaum E and
Abraham N: Prevalence, risk factors, and treatment for overactive
bladder in a racially diverse population. Urology. 126:70–75. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu L: Exploring the association between
overactive bladder (OAB) and Cognitive decline: Mediation by
depression in elderly adults, a NHANES weighted analysis. Sci Rep.
15:36692025. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Van den Ende M, Apostolidis A, Sinha S,
Kheir GB, Mohamed-Ahmed R, Selai C, Abrams P and Vrijens D: Should
We Be treating affective symptoms, like anxiety and depression
which may be related to LUTD in patients with OAB? ICI-RS 2024.
Neurourol Urodyn. 44:661–667. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Akkoç Y, Bardak AN, Yıldız N, Özlü A,
Erhan B, Yürü B, Öztekin SNS, Türkoğlu MB, Paker N, Yumuşakhuylu Y,
et al: The relationship between severity of overactive bladder
symptoms and cognitive dysfunction, anxiety and depression in
female patients with multiple sclerosis. Mult Scler Relat Disord.
70:1044762023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zuluaga L, Caicedo JI, Mogollón MP,
Santander J, Bravo-Balado A, Trujillo CG, Diaz Ritter C, Rondón M
and Plata M: Anxiety and depression in association with lower
urinary tract symptoms: Results from the COBaLT study. World J
Urol. 41:1381–1388. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sakakibara R, Ito T, Yamamoto T, Uchiyama
T, Yamanishi T, Kishi M, Tsuyusaki Y, Tateno F, Katsuragawa S and
Kuroki N: Depression, anxiety and the bladder. Low Urin Tract
Symptoms. 5:109–120. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tanyeri MH, Buyukokuroglu ME, Tanyeri P,
Mutlu O, Ozturk A, Yavuz K and Kaya RK: Effects of mirabegron on
depression, anxiety, learning and memory in mice. An Acad Bras
Cienc. 93 (Suppl 4):e202106382021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bouayed J and Soulimani R: Evidence that
hydrogen peroxide, a component of oxidative stress, induces
high-anxiety-related behaviour in mice. Behav Brain Res.
359:292–297. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Showraki M, Showraki T and Brown K:
Generalized anxiety disorder: Revisited. Psychiatr Q. 91:905–914.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sheng L, Yao X, Ye J, Wang Z, Chen Y, Li J
and Li M: Eriocitrin alleviates inflammation and oxidative stress
in subarachnoid hemorrhage by regulating DUSP14. Discov Med.
35:1134–1146. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Song J, Han K, Wang Y, Qu R, Liu Y, Wang
S, Wang Y, An Z, Li J, Wu H and Wu W: Microglial activation and
oxidative stress in PM2.5-induced neurodegenerative disorders.
Antioxidants (Basel). 11:14822022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Y, Tang Q, Duan P and Yang L:
Curcumin as a therapeutic agent for blocking NF-κB activation in
ulcerative colitis. Immunopharmacol Immunotoxicol. 40:476–482.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bai R, Guo J, Ye XY, Xie Y and Xie T:
Oxidative stress: The core pathogenesis and mechanism of
Alzheimer's disease. Ageing Res Rev. 77:1016192022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sanabria-Castro A, Alape-Girón A,
Flores-Díaz M, Echeverri-McCandless A and Parajeles-Vindas A:
Oxidative stress involvement in the molecular pathogenesis and
progression of multiple sclerosis: A literature review. Rev
Neurosci. 35:355–371. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Toussaint A, Hüsing P, Gumz A, Wingenfeld
K, Härter M, Schramm E and Löwe B: Sensitivity to change and
minimal clinically important difference of the 7-item Generalized
Anxiety Disorder Questionnaire (GAD-7). J Affect Disord.
265:395–401. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
National Research Council, . Committee for
the Update of the Guide for the Care and Use of Laboratory Animals:
Guide for the Care and Use of Laboratory Animals. 8th edition.
National Academies Press; Washington, DC: 2011
|
|
23
|
Campos AC, Fogaça MV, Aguiar DC and
Guimarães FS: Animal models of anxiety disorders and stress. Braz J
Psychiatry. 35 (Suppl 2):S101–S111. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Q, Wu Q, Wang J, Chen Y, Zhang G,
Chen J, Zhao J and Wu P: Ketamine analog methoxetamine induced
inflammation and dysfunction of bladder in rats. Int J Mol Sci.
18:1172017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Love MI, Huber W and Anders S: Moderated
estimation of fold change and dispersion for RNA-seq data with
DESeq2. Genome Biol. 15:5502014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yu G, Wang LG, Han Y and He QY:
clusterProfiler: An R package for comparing biological themes among
gene clusters. OMICS. 16:284–287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ashburner M, Ball CA, Blake JA, Botstein
D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT,
et al: Gene Ontology: Tool for the unification of biology. The gene
ontology consortium. Nat Genet. 25:25–29. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Szklarczyk D, Franceschini A, Wyder S,
Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos
A, Tsafou KP, et al: STRING v10: Protein-protein interaction
networks, integrated over the tree of life. Nucleic Acids Res.
43:D447–D452. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shannon P, Markiel A, Ozier O, Baliga NS,
Wang JT, Ramage D, Amin N, Schwikowski B and Ideker T: Cytoscape: A
software environment for integrated models of biomolecular
interaction networks. Genome Res. 13:2498–2504. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Abrams P, Cardozo L, Fall M, Griffiths D,
Rosier P, Ulmsten U, Van Kerrebroeck P, Victor A and Wein A;
Standardisation Sub-Committee of the International Continence
Society, : The standardisation of terminology in lower urinary
tract function: Report from the standardisation sub-committee of
the International Continence Society. Urology. 61:37–49. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Obaidul Islam M, Bacchetti T, Berrougui H,
Abdelouahed Khalil and Ferretti G: Effect of glycated HDL on
oxidative stress and cholesterol homeostasis in a human bladder
cancer cell line, J82. Exp Mol Pathol. 126:1047772022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen J, Li Q, Hong Y, Zhou X, Yu C, Tian
X, Zhao J, Long C, Shen L, Wu S and Wei G: Inhibition of the NF-κB
signaling pathway alleviates pyroptosis in bladder epithelial cells
and neurogenic bladder fibrosis. Int J Mol Sci. 24:111602023.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fang T, Xiong J, Huang X, Fang X, Shen X,
Jiang Y and Lu H: Extracellular Hsp90 of Candida albicans
contributes to the virulence of the pathogen by activating the
NF-κB signaling pathway and inducing macrophage pyroptosis.
Microbiol Res. 290:1279642025. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cheng HM, Xing M, Zhou YP, Zhang W, Liu Z,
Li L, Zheng Z, Ma Y, Li P, Liu X, et al: HSP90β promotes
osteoclastogenesis by dual-activation of cholesterol synthesis and
NF-κB signaling. Cell Death Differ. 30:673–686. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Knight S, Luft J, Nakagawa S and Katzman
WB: Comparisons of pelvic floor muscle performance, anxiety,
quality of life and life stress in women with dry overactive
bladder compared with asymptomatic women. BJU Int. 109:1685–1689.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bou Kheir G, Verbakel I, Hervé F, Bauters
W, Abou Karam A, Holm-Larsen T, Van Laecke E and Everaert K: OAB
supraspinal control network, transition with age, and effect of
treatment: A systematic review. Neurourol Urodyn. 41:1224–1239.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhu J, Hu X, Dong X and Li L: Associations
between risk factors and overactive bladder: A Meta-analysis.
Female Pelvic Med Reconstr Surg. 25:238–246. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhong M and Wang Z: The Association
between anthropometric indices and overactive bladder (OAB): A
Cross-sectional study from the NHANES 2005–2018. Neurourol Urodyn.
44:345–359. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Karjalainen PK, Tolppanen A-M, Mattsson
NK, Wihersaari OAE, Jalkanen JT and Nieminen K: Pelvic organ
prolapse surgery and overactive bladder symptoms-a population-based
cohort (FINPOP). Int Urogynecol J. 33:95–105. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Palma T, Raimondi M, Souto S, Fozzatti C,
Palma P and Riccetto C: Prospective study of prevalence of
overactive bladder symptoms and child-bearing in women of
reproductive age. J Obstet Gynaecol. 39:1324–1329. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lai HH, Rawal A, Shen B and Vetter J: The
relationship between anxiety and overactive bladder or urinary
incontinence symptoms in the clinical population. Urology.
98:50–57. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Melotti IGR, Juliato CRT, Tanaka M and
Riccetto CLZ: Severe depression and anxiety in women with
overactive bladder. Neurourol Urodyn. 37:223–228. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mehr AA, Kreder KJ, Lutgendorf SK, Ten
Eyck P, Greimann ES and Bradley CS: Daily symptom associations for
urinary urgency and anxiety, depression and stress in women with
overactive bladder. Int Urogynecol J. 33:841–850. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sezginer EK, Yilmaz-Oral D, Lokman U,
Nebioglu S, Aktan F and Gur S: Effects of varying degrees of
partial bladder outlet obstruction on urinary bladder function of
rats: A novel link to inflammation, oxidative stress and hypoxia.
Low Urin Tract Symptoms. 11:O193–O201. 2019.PubMed/NCBI
|
|
46
|
Dunton CL, Purves JT, Hughes FM and
Nagatomi J: BOO induces fibrosis and EMT in urothelial cells which
can be recapitulated in vitro through elevated storage and voiding
pressure cycles. Int Urol Nephrol. 53:2007–2018. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Niemczyk G, Fus L, Czarzasta K, Jesion A,
Radziszewski P, Gornicka B and Cudnoch-Jedrzejewska A: Expression
of toll-like receptors in the animal model of bladder outlet
obstruction. Biomed Res Int. 2020:66323592020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wishahi M, Hassan S, Kamal N, Badawy M and
Hafiz E: Is bladder outlet obstruction rat model to induce
overactive bladder (OAB) has similarity to human OAB? Research on
the events in smooth muscle, collagen, interstitial cell and
telocyte distribution. BMC Res Notes. 17:222024. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kang YJ, Jin LH, Park CS, Shin HY, Yoon SM
and Lee T: Early sequential changes in bladder function after
partial bladder outlet obstruction in awake Sprague-Dawley rats:
Focus on the decompensated bladder. Korean J Urol. 52:835–841.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Shen JD, Chen SJ, Chen HY, Chiu KY, Chen
YH and Chen WC: Review of animal models to study urinary bladder
function. Biology (Basel). 10:13162021.PubMed/NCBI
|
|
51
|
Ye F, Dong MC, Xu CX, Jiang N, Chang Q,
Liu XM and Pan RL: Effects of different chronic restraint stress
periods on anxiety- and depression-like behaviors and
tryptophan-kynurenine metabolism along the brain-gut axis in
C57BL/6N mice. Eur J Pharmacol. 965:1763012024. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Brieger K, Schiavone S, Miller FJ and
Krause KH: Reactive oxygen species: From health to disease. Swiss
Med Wkly. 142:w136592012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Rauf A, Khalil AA, Awadallah S, Khan SA,
Abu-Izneid T, Kamran M, Hemeg HA, Mubarak MS, Khalid A and
Wilairatana P: Reactive oxygen species in biological systems:
Pathways, associated diseases, and potential inhibitors-A review.
Food Sci Nutr. 12:675–693. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wang P, Liu WC, Han C, Wang S, Bai MY and
Song CP: Reactive oxygen species: Multidimensional regulators of
plant adaptation to abiotic stress and development. J Integr Plant
Biol. 66:330–367. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ahola S and Langer T: Ferroptosis in
mitochondrial cardiomyopathy. Trends Cell Biol. 34:150–160. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Gohil D, Sarker AH and Roy R: Base
excision repair: Mechanisms and impact in biology, disease, and
medicine. Int J Mol Sci. 24:141862023. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Murphy MP, Bayir H, Belousov V, Chang CJ,
Davies KJA, Davies MJ, Dick TP, Finkel T, Forman HJ,
Janssen-Heininger Y, et al: Guidelines for measuring reactive
oxygen species and oxidative damage in cells and in vivo. Nat
Metab. 4:651–662. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Burtscher J, Niedermeier M, Hüfner K, van
den Burg E, Kopp M, Stoop R, Burtscher M, Gatterer H and Millet GP:
The interplay of hypoxic and mental stress: Implications for
anxiety and depressive disorders. Neurosci Biobehav Rev.
138:1047182022. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Martinez-Moral MP and Kannan K: Analysis
of 19 urinary biomarkers of oxidative stress, nitrative stress,
metabolic disorders, and inflammation using liquid
chromatography-tandem mass spectrometry. Anal Bioanal Chem.
414:2103–2116. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Chaudhary P, Janmeda P, Docea AO,
Yeskaliyeva B, Abdull Razis AF, Modu B, Calina D and Sharifi-Rad J:
Oxidative stress, free radicals and antioxidants: Potential
crosstalk in the pathophysiology of human diseases. Front Chem.
10:11581982023. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Wu YH, Chueh KS, Chuang SM, Long CY, Lu JH
and Juan YS: Bladder hyperactivity induced by oxidative stress and
bladder ischemia: A review of treatment strategies with
antioxidants. Int J Mol Sci. 22:60142021. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Xue J, Zhou Z, Zhu Z, Sun Q, Zhu Y and Wu
P: A high salt diet impairs the bladder epithelial barrier and
activates the NLRP3 and NF-κB signaling pathways to induce an
overactive bladder in vivo. Exp Ther Med. 28:3622024.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Chen B, Piel WH, Gui L, Bruford E and
Monteiro A: The HSP90 family of genes in the human genome: Insights
into their divergence and evolution. Genomics. 86:627–637. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Yang S, Nie T, She H, Tao K, Lu F, Hu Y,
Huang L, Zhu L, Feng D, He D, et al: Regulation of TFEB nuclear
localization by HSP90AA1 promotes autophagy and longevity.
Autophagy. 19:822–838. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Zuehlke AD, Beebe K, Neckers L and Prince
T: Regulation and function of the human HSP90AA1 gene. Gene.
570:8–16. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Sharma S and Kumar P: Dissecting the
functional significance of HSP90AB1 and other heat shock proteins
in countering glioblastomas and ependymomas using omics analysis
and drug prediction using virtual screening. Neuropeptides.
102:1023832023. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Huang X, Zhang W, Yang N, Zhang Y, Qin T,
Ruan H, Zhang Y, Tian C, Mo X, Tang W, et al: Identification of
HSP90B1 in pan-cancer hallmarks to aid development of a potential
therapeutic target. Mol Cancer. 23:192024. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Liu C, Zhao W, Su J, Chen X, Zhao F, Fan
J, Li X, Liu X, Zou L, Zhang M, et al: HSP90AA1 interacts with CSFV
NS5A protein and regulates CSFV replication via the JAK/STAT and
NF-κB signaling pathway. Front Immunol. 13:10318682022. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Liu H, Zhang Z, Huang Y, Wei W, Ning S, Li
J, Liang X, Liu K and Zhang L: Plasma HSP90AA1 predicts the risk of
breast cancer onset and distant metastasis. Front Cell Dev Biol.
9:6395962021. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Tang F, Li Y, Pan M, Wang Z, Lu T, Liu C,
Zhou X and Hu G: HSP90AA1 promotes lymphatic metastasis of
hypopharyngeal squamous cell carcinoma by regulating
epithelial-mesenchymal transition. Oncol Res. 31:787–803. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Xiao X, Wang W, Li Y, Yang D, Li X, Shen
C, Liu Y, Ke X, Guo S and Guo Z: HSP90AA1-mediated autophagy
promotes drug resistance in osteosarcoma. J Exp Clin Cancer Res.
37:2012018. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Wei D, Tian X, Zhu L, Wang H and Sun C:
USP14 governs CYP2E1 to promote nonalcoholic fatty liver disease
through deubiquitination and stabilization of HSP90AA1. Cell Death
Dis. 14:5662023. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Li S, Zhou H, Liang Y, Yang Q, Zhang J,
Shen W and Lei L: Integrated analysis of transcriptome-wide
m6A methylation in a Cd-induced kidney injury rat model.
Ecotoxicol Environ Saf. 256:1149032023. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Pichler R, Diem G, Hackl H, Koutník J,
Mertens LS, D Andrea D, Pradere B, Soria F, Mari A, Laukhtina E, et
al: Intravesical BCG in bladder cancer induces innate immune
responses against SARS-CoV-2. Front Immunol. 14:12021572023.
View Article : Google Scholar : PubMed/NCBI
|