Introduction
Chronic obstructive pulmonary disease (COPD) is a
progressive, largely irreversible lung disorder driven by genetic
or environmental factors that is characterized by a complex
inflammatory response and the destruction of lung parenchyma,
leading to emphysema formation (1). From a genetic standpoint, a single
amino acid substitution in the Z allele of the SERPINA1 gene
results in reduced levels of α-1 antitrypsin (AAT) in the
bloodstream of homozygous or loss-of-function homozygous
individuals, which constitutes the primary pathogenesis of COPD
(2). Environmental risk factors,
particularly prolonged exposure to cigarette smoke and harmful
particulate matter (PM), are key contributors, with cigarette smoke
identified as a notable environmental risk factor for COPD
(3).
The inhalation of carbon monoxide, nicotine and
other components of cigarette smoke damages lung epithelial cells,
thereby triggering the release of various inflammatory mediators.
These mediators subsequently recruit circulating monocytes,
mesophilic granulocytes and T cells into lung tissue, thereby
initiating a complex inflammatory response (4). The recruited immune cells further
release inflammatory mediators, which increase pulmonary vascular
permeability, induce pulmonary edema and secrete elastases, which
damage lung tissue and promote airway remodeling (5). As COPD advances, patients face an
increased risk of cor pulmonale (also known as pulmonary heart
disease) and respiratory failure (6). Current treatments are primarily
symptomatic, comprising inhaled bronchodilators, glucocorticoids or
oxygen therapy to alleviate dyspnea in individuals with persistent
airflow limitation (7).
Consequently, the molecular mechanisms underlying COPD and the
development of higher-quality drug delivery systems require further
research to improve the current the treatment of COPD.
Extracellular vesicles (EVs) are nanoscale lipid
bilayer vesicles secreted by living cells, which are present in
various body fluids, including blood, urine and ascites (8). Upon release into the extracellular
space, EVs facilitate the efficient transfer of bioactive
molecules, including proteins, microRNAs (miRNAs/miRs) and DNA,
making them important mediators of intercellular communication
(9). In previous years, EVs have
been increasingly associated with the progression of various
diseases, including malignant tumors, diabetes mellitus, stroke and
lung inflammation (10,11). Furthermore, based on their
widespread presence in biofluids, high biocompatibility, small
particle size and other physiological properties, EVs have both
been applied as a marker for disease diagnosis and developed as a
platform for drug-targeted therapeutic carriers (12). The present review aims to explore
the molecular mechanisms underlying COPD pathogenesis, with a focus
on the potential role of EVs in COPD development. The present
review also summarizes current approaches to using EVs as
biomarkers for COPD prediction. Finally, the present review
highlights previous advances that have been made in the development
of EV-based therapies and their potential as drug delivery systems
for COPD treatment, with the aim of offering novel research avenues
for the diagnosis, treatment and prevention of COPD. Literature
searches were conducted in PubMed (https://pubmed.ncbi.nlm.nih.gov/), Scopus (https://www.scopus.com/), and Web of Science
(https://www.webofscience.com/) using the
following search terms: ‘Chronic obstructive pulmonary disease’ AND
‘Extracellular vesicles’. After further filtering based on article
content, 97 relevant articles were ultimately retained.
Molecular mechanisms of COPD
pathogenesis
The pathogenesis of COPD is both complex and
multifactorial, involving airway epithelial cell (AEC) damage from
cigarette smoke inhalation, a dysregulated inflammatory response
driven by an imbalance between oxidation and antioxidation, and the
recruitment of immune cells to lung tissue that release various
proteases, leading to protease/antiprotease imbalance and
subsequent airway remodeling.
Imbalance of oxidation and
anti-oxidation
An important factor in the pathogenesis of COPD is
the imbalance between oxidant sources and sinks in the airways.
Patients with COPD are exposed to exogenous oxidants, such as
cigarette smoke and airborne PM, which trigger an endogenous
inflammatory response (13). This
response recruits neutrophils and mononuclear macrophages to the
lungs, where they release superoxide anions, hydrogen peroxide and
reactive oxygen species (ROS), thereby inducing oxidative stress in
the airways (14). These ROS
further amplify the inflammatory response by regulating
redox-sensitive transcription factors, including nuclear factor κB
(NF-κB), activator protein-1 and c-Jun N-terminal kinase (JNK)
(15). Additionally, ROS promote
mucous membrane metaplasia and the expression of the mucin genes
MUC5AC and MUC5B, leading to increased mucus
production (16). Notably,
oxidative stress can damage DNA, and patients with COPD often fail
to repair double-stranded DNA breaks promptly, which has the effect
of raising their susceptibility to lung cancer (17).
Nuclear factor erythroid-2 counteracts oxidative
reactions by regulating related factors, such as nuclear factor
erythroid-2-related factor-2 (Nrf2), reducing cellular oxidative
damage. In oxidative stress responses, Nrf2 undergoes
phosphorylation and decouples from Kelch-like ECH-associated
protein 1, subsequently entering the cell nucleus to participate in
gene transcription, where it can activate the transcription of
various antioxidant factors (including heme oxygenase 1,
N-acetyl-L-tyrosine oxidoreductase and glutathione S-transferase)
(18). However, in patients with
COPD, reduced levels of this factor lead to an impairment of
endogenous antioxidant production, thereby weakening the
self-protective mechanisms of the body (19). Collectively, the residual effects
of oxidative stress contribute to lung cell damage, excessive mucus
secretion, protease inactivation and enhanced lung inflammation via
activation of redox-sensitive transcription factors, thereby
facilitating COPD progression. Additionally, cigarette smoke
induces mitophagy in AECs in COPD, which both impairs oxidative
phosphorylation, and leads to decreases in intracellular ATP levels
and increases in mitochondrial ROS (mtROS) production. This
mitochondrial dysfunction contributes to cellular senescence,
further exacerbating the progression of COPD (20).
Chronic inflammation
COPD is characterized by chronic inflammation, with
patients exhibiting distinctive inflammatory profiles in the lungs
marked by increases in the numbers of macrophages, neutrophils,
eosinophils and T lymphocytes (21). Inhalation of cigarette smoke, PM or
other oxidants activates AECs and macrophages, stimulating them to
release a range of inflammatory mediators, including leukotriene B4
(LTB4), interleukin (IL)-6, granulocyte-macrophage
colony-stimulating factor, CXCL8, CXCL1, ROS and tumor necrosis
factor α (TNF-α) (22). Even after
smoking cessation, the inflammatory response persists, suggesting
that COPD inflammation is maintained by self-sustaining mechanisms
(21). The aforementioned
inflammatory mediators not only exacerbate lung tissue damage and
amplify the inflammatory response, but also serve to recruit
monocytes, neutrophils and lymphocytes from the peripheral blood to
the lungs (23). CXCL1 primarily
recruits monocytes, whereas LTB4, CXCL1, CXCL5 and CXCL8 facilitate
the recruitment of neutrophils. The recruited neutrophils and
macrophages, activated by oxidative stress, contribute to the
metaplasia of goblet cells and increased mucus secretion (24). The release of proteases, including
matrix metalloproteinases (MMPs), tissue proteases and neutrophil
elastase, degrades the extracellular matrix and alveolar elastin,
leading to localized and generalized alveolar emphysema (25). Notably, collagen and elastin
breakdown products act as chemotactic factors for inflammatory
cells, further perpetuating the inflammatory cycle in patients with
COPD (26).
Protease-antiprotease imbalance
In response to oxidative stress and inflammatory
mediators, macrophages and neutrophils are recruited to the lungs,
where they secrete various proteases (such as inherited deficiency
of α1-antitrypsin, neutrophil elastase and MMPs) that degrade the
structural components of the lungs, contributing to tissue
remodeling (26). For example,
macrophage secretions of MMP-2 and MMP-9 disrupt the elastic
framework of the alveoli, leading to emphysema formation (27). MMP-12 is involved in pulmonary
vascular remodeling through regulating the migration, proliferation
and release of mitogens and growth factors from smooth muscle cells
(28). In COPD, the endogenous MMP
inhibitor, tissue inhibitor of metalloproteinases, is cleaved by
neutrophil elastase and MMPs, resulting in elevated MMP-2 levels in
lung tissue (29). Additionally,
mutations in the AAT gene, particularly the Z allele, have been
found to cause a marked deficiency in AAT, leading to the
accumulation of the misfolded protein in hepatocytes (30). In addition, a deficiency of
circulating AAT results in an imbalance between proteases and
inhibitors in the lung tissue, promoting airway remodeling and
airflow limitation (31).
EV involvement in COPD pathogenesis
General overview of EVs
EVs are non-replicating, nanoscale vesicles
originally recognized for their role in maintaining homeostasis and
eliminating waste, which has attracted notable research interest
(31). During biogenesis, EVs
effectively encapsulate nucleic acids, proteins, lipids and other
cellular components from the donor cell (32). On the basis of their biogenesis
pathways and particle sizes, EVs are classified into three
categories: Exosomes (30–150 nm), microvesicles (MVs; 100 nm-1 µm)
and apoptotic bodies (50 nm-5 µm) (33,34).
Exosome biogenesis begins with the formation of plasma membrane
indentations that encapsulate cell proteins and soluble proteins
from the extracellular environment, creating a cup-shaped
structure. These early endosomes undergo further sorting,
development and maturation into late endosomes, which invaginate to
form multivesicular bodies (MVBs). MVBs subsequently fuse with
lysosomes or autophagosomes, releasing exosomes (35). The biogenesis of MVs involves
localized accumulation of actin filaments and the flipping of
phosphatidylserine from the inner to the outer leaflet of the
membrane, altering membrane curvature and inducing vesicle budding
(36). Apoptotic bodies are
generated through the rupture of the plasma membrane in apoptotic
cells, followed by continuous volume reduction and actinin-driven
contraction, which causes the accumulation of cellular contents and
an increase in hydrostatic pressure, ultimately leading to vesicle
formation. The nucleus then fragments, resulting in the formation
of apoptotic bodies (37). Once
released into the extracellular space, EVs can be internalized by
recipient cells via mechanisms such as granzyme-independent
endocytosis, macropinocytosis and phagocytosis, where they release
their cargo, including nucleic acids, proteins and other molecules,
thereby facilitating intercellular communication (38) (Fig.
1).
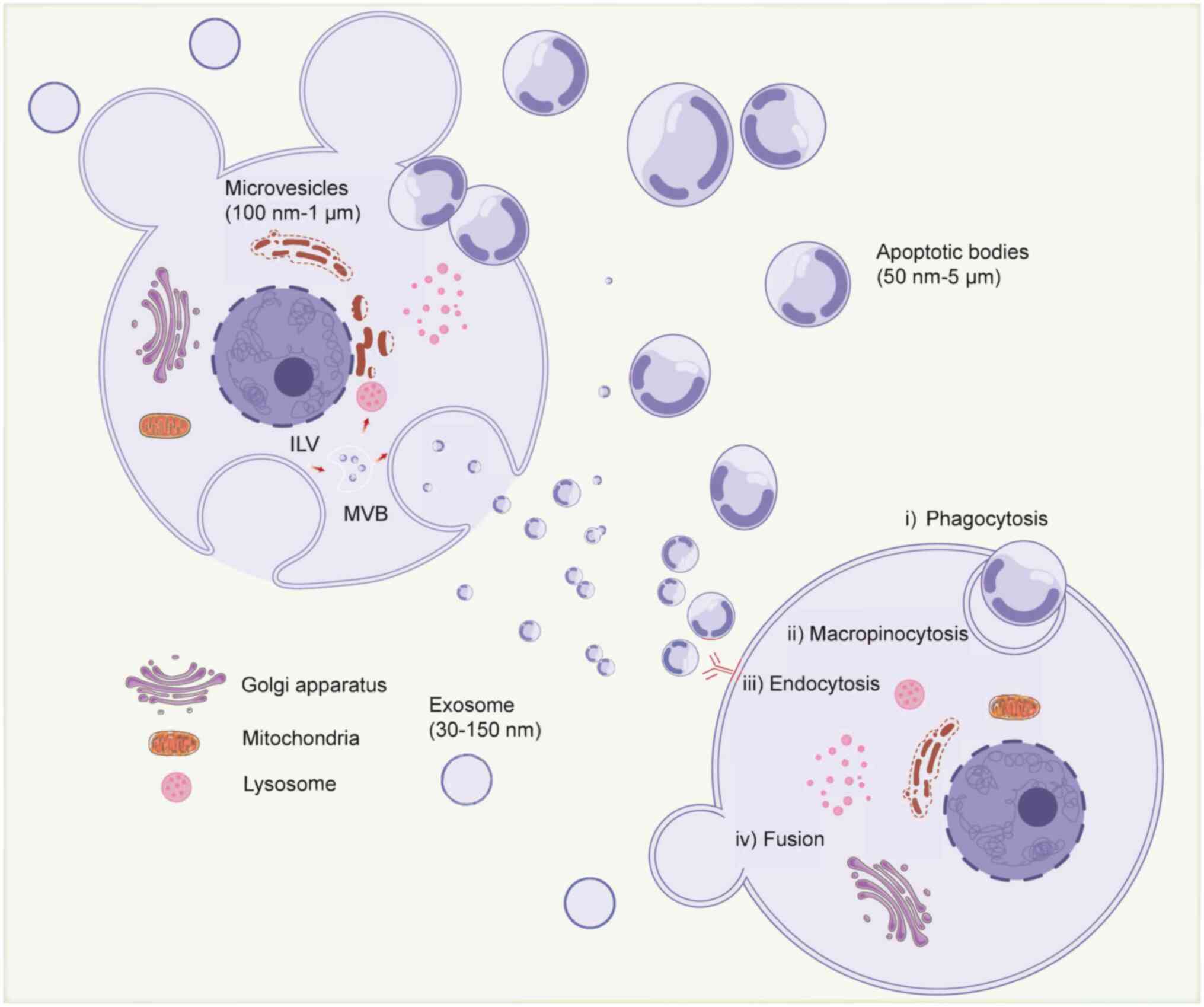 | Figure 1.Biogenesis, secretion and cell entry
of EVs. EVs are categorized into exosomes, apoptotic bodies and MVs
based on their biological origin and size. Exosomes produce ILVs
through inward budding of early endosomes to form MVBs, which
subsequently fuse with lysosomes or cell membranes and are released
into the extracellular environment of the exosome. MVs are produced
by outward budding from the plasma membrane. Apoptotic bodies are
released during cell death through plasma membrane vesicles. EVs
can deliver cargo to recipient cells through phagocytosis,
megakaryocytosis, endocytosis, direct fusion and other means, and
subsequently modulate biological behavior. Created with
BioRender.com (https://app.biorender.com/illustrations/692ee7fb97717aa47f30567d?slideId=76ff231c-5a96-4f30-a8e7-0972073ada7c).
EVs, extracellular vesicles; MVs, microvesicles; MVBs,
multivesicular bodies; ILV, intraluminal vesicle. |
Role of EVs in the pathogenesis of
COPD
Prolonged exposure to irritants such as cigarette
smoke leads to the destruction of the structural integrity of the
lungs and their dysfunction (39).
In response to such irritants, AECs secrete a variety of EVs,
containing components such as miR-210, miR-21 and cellular
communication network factor 1 (CCN1) (40). Among these, CCN1-containing EVs
activate the Wnt signaling pathway, which induces the secretion of
pro-inflammatory cytokines, such as IL-8 and monocyte
chemoattractant protein 1, by epithelial cells. This activation
promotes the recruitment of circulating T lymphocytes, neutrophils
and monocytes to the lungs, further exacerbating the inflammatory
response (41). Neutrophils and
monocytes release matrix proteases that degrade the structural
integrity of alveoli, contributing to the development of pulmonary
emphysema. Furthermore, T lymphocytes secrete EVs that, through a
positive feedback mechanism, stimulate epithelial cells to release
IL-6 and inhibit the secretion of the anti-inflammatory cytokine
IL-10, further damaging the airways (42).
Additionally, miR-210-containing EVs target the
autophagy-related 7 gene in lung fibroblasts (LFs), inhibiting
autophagosome formation (42).
This impairment in autophagy leads to the increased expression of
type I collagen and α-smooth muscle actin (α-SMA) in LFs, promoting
myofibroblast differentiation and airway wall thickening (43). Similarly, miR-21-containing EVs
promote myofibroblast differentiation by targeting von
Hippel-Lindau protein (pVHL), stabilizing hypoxia-inducible factor
1α, thus facilitating airway remodeling (44). EVs from inhaled bacteria or viruses
also serve a role in COPD pathogenesis. For example, bacterial
outer-membrane vesicles (OMVs) from Moraxella catarrhalis
contribute to pulmonary inflammation (45). OMVs derived from Pseudomonas
aeruginosa inhibit chloride secretion mediated by cystic
fibrosis transmembrane conductance regulator, reducing pathogen
clearance through mucociliary action and amplifying the
inflammatory response (46).
Although the exact mechanisms via which EVs influence COPD have yet
to be fully elucidated, their involvement in the pathogenesis of
COPD is notable (Fig. 2).
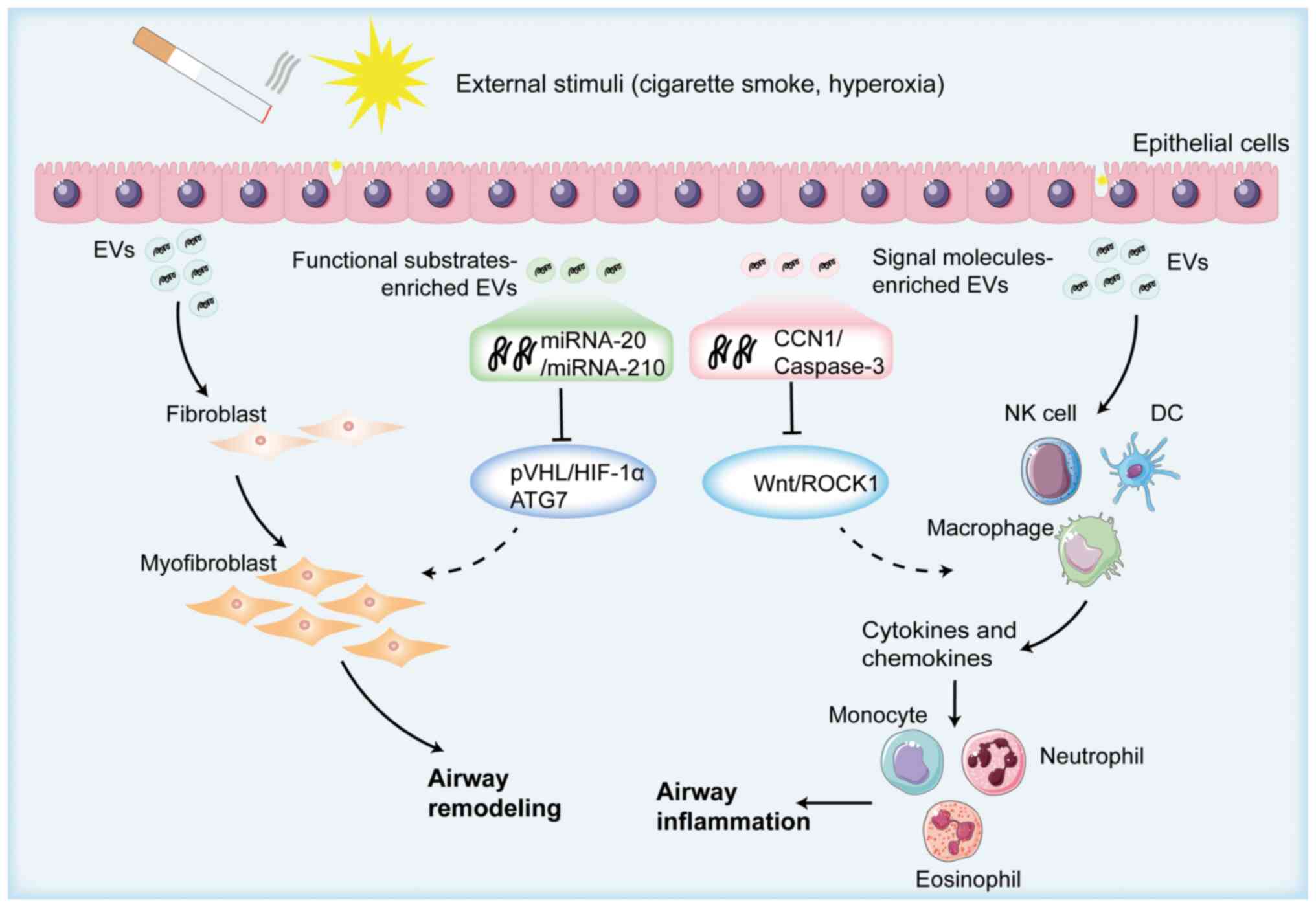 | Figure 2.Schematic overview of epithelial
EV-induced airway remodeling. Epithelial cells secrete a large
number of EVs after being stimulated by the external environment,
and miR-210, miR-21, cellular communication network factor 1 and
other components contained in EVs induce the increased expression
of pro-inflammatory cytokines, such as IL-8 and monocyte
chemoattractant protein-1, secreted by activated epithelial cells.
This is achieved by activating the Wnt signaling pathway/hypoxia
inducible factor-1α pathway, which enhances the differentiation of
fibroblasts into myofibroblasts, the contraction of smooth muscle
cells and the accumulation of extracellular matrix proteins,
ultimately promoting airway remodeling. Created with BioRender.com
(https://app.biorender.com/illustrations/692eed2c2bdb071cce996f3d?slideId=f9661b16-c75e-4ba9-a385-974bcfbc0a16).
COPD, chronic obstructive pulmonary disease; EVs, extracellular
vesicles; CCN1, cellular communication network factor 1; miRNA,
microRNA; NK, natural killer; DC, dendritic cell; ROCK1,
Rho-associated protein kinase 1; PVHL, von Hippel-Lindau tumor
suppressor; HIF-1α, hypoxia inducible factor-1α; ATG7, autophagy
related 7. |
EVs in the diagnosis and treatment of
COPD
Application of EVs in COPD
diagnosis
Patients with COPD typically present with one or
more symptoms such as exertional dyspnea, cough, sputum production,
chest tightness or fatigue (47).
Clinically, COPD diagnosis involves assessing lung function [using
the ratio of forced expiratory volume in 1 sec (FEV1) to forced
vital capacity (FVC) post-bronchodilator, with a ratio <0.70
indicating a positive COPD diagnosis], along with chest X-rays,
chest CT scans, and serum markers such as soluble receptors for
advanced glycation end products (48,49).
The heterogeneity of EVs is associated with the type and state of
the cells from which they originate. Analyzing the number or
specific content of EVs in peripheral blood, bronchoalveolar lavage
fluid (BALF) or sputum offers new diagnostic possibilities for COPD
(50) (Fig. 3).
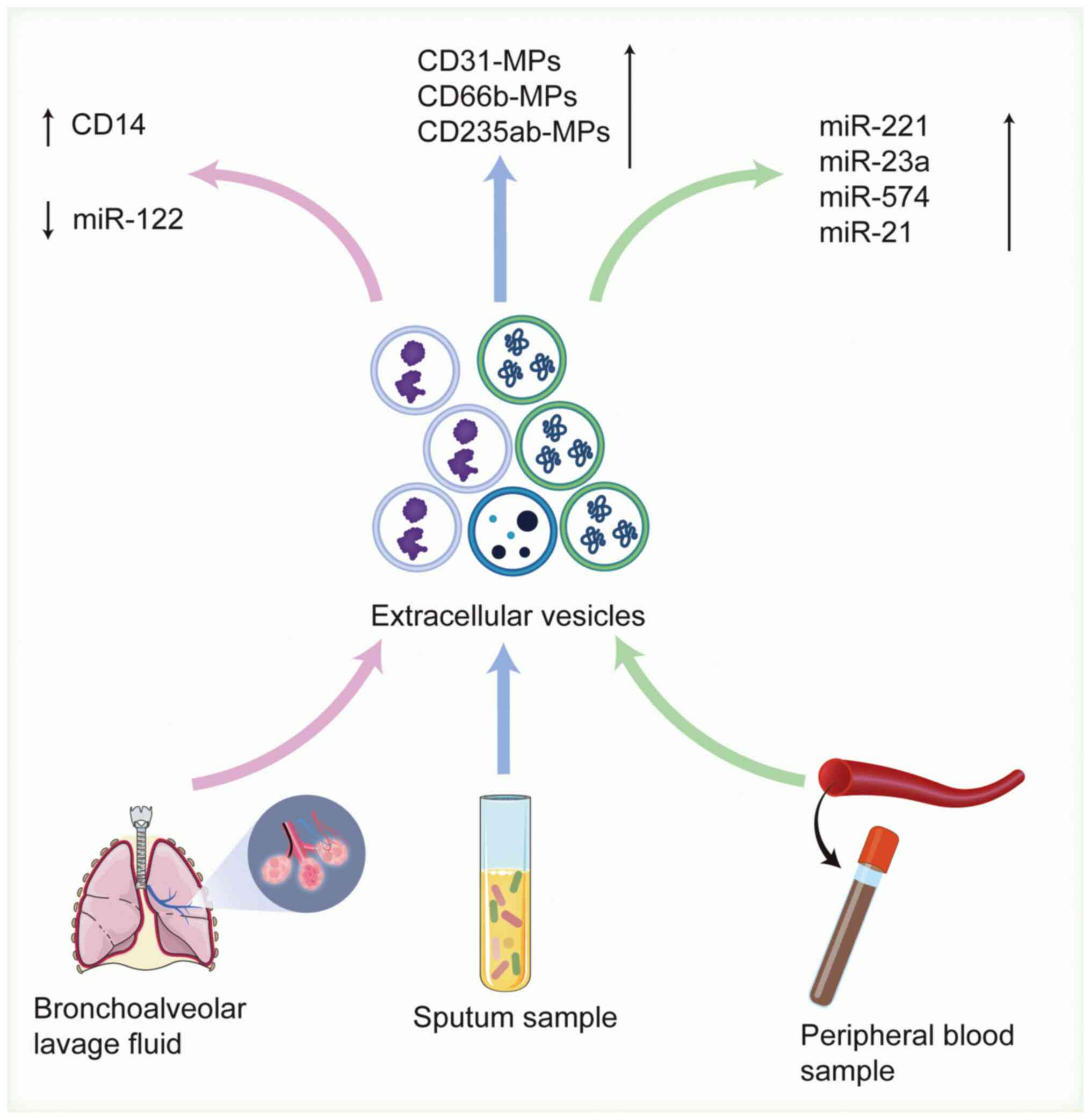 | Figure 3.Schematic overview of EV-based
biomarkers in COPD diagnosis. By detecting sputum, bronchoalveolar
lavage fluid and EVs in blood samples as markers for the diagnosis
of chronic obstructive pulmonary disease, the concentrations of
CD31-MPs, CD66b-MPs, CD235ab-MPs and total protein in sputum
sample-derived EVs are notably increased, the levels of miR-122 in
bronchoalveolar lavage fluid are decreased, and the expression
levels of EV miR-221, miR-23a and miR-574 in blood samples are also
notably increased. Created with BioRender.com (https://app.biorender.com/illustrations/692eeccc235f309bfe04b81d?slideId=44a0ee63-27a9-433b-8188-676b7956b82a).
COPD, chronic obstructive pulmonary disease; miR, microRNA; EVs,
extracellular vesicles; MPs, microparticles. |
EVs derived from sputum
Sputum, a secretion from the respiratory tract, is a
vital diagnostic tool for lung diseases such as COPD, lung cancer,
tuberculosis and chronic pneumonia. The analysis of sputum
supernatant media or sputum cells can provide important diagnostic
information (51). For example,
miR-21, miR-155 and miR-210 levels are markedly elevated in sputum
from patients with lung cancer, while detecting Mycobacterium
tuberculosis in sputum is key for diagnosing pulmonary
tuberculosis (52). However,
spontaneous sputum samples often have poor quality, and hypertonic
or isotonic saline containing salbutamol is typically used to
induce sputum production (53).
Sputum testing is less invasive and easier to perform compared with
testing BALF (54). Recent
research has successfully isolated and purified EVs from sputum
samples of 20 patients with COPD, revealing a notable increase in
the general protein concentration in COPD sputum samples
(106.70±60.56 µg/ml) compared with the healthy control group
(56.82±11.99 µg/ml) (55). Another
study identified that EVs containing CD31, CD66b and CD235 in
sputum are associated with COPD progression (56). Although few studies have been
published on the physicochemical properties of sputum EVs, these
findings suggest that analyzing EVs in sputum components could be a
valuable diagnostic approach for COPD.
BALF
BALF is a standard clinical method for diagnosing
infectious diseases, non-infectious immune diseases and malignant
conditions in the lungs (57). The
procedure involves introducing saline into the alveoli via a
bronchoscope, and then aspirating alveolar cells and biochemical
components under negative pressure (58). Compared with more invasive
procedures, such as lung tissue or bronchial biopsies, BALF is less
invasive and has fewer complications. BALF detects a variety of
components, including cells, proteins, genes, microorganisms and
EVs, making this procedure a more comprehensive reflection of lung
pathology (59). Given the
heterogeneity of EVs and their close association with the biology
of their donor cells, analyzing EVs in BALF can provide a valuable
basis for diagnosing COPD. For example, one study found that the
expression of miR-122-5p in pulmonary EVs from patients with COPD
was significantly downregulated compared with that in healthy
smokers and non-smokers (57). A
metabolomics database of BALF comprising 117 individuals, including
non-smokers, smokers and patients diagnosed with COPD, revealed the
presence of >11,000 lipids and ~650 water-soluble substances in
BALF. Among these, one-tenth of the substances were present in all
samples (60).
Blood-derived EVs
Peripheral blood contains millions of EVs, and the
nucleic acids, proteins and other components within these vesicles
can reflect the biological behavior of their donor cells (58). Consequently, analyzing the
expression levels of nucleic acids and proteins in blood-derived
EVs from patients with COPD compared with healthy individuals
offers a promising foundation for diagnosing COPD. miRNAs are key
regulators in gene expression networks, having roles in cell
differentiation, apoptosis, inflammatory responses, tissue
remodeling and angiogenesis. Profiling miRNA expression using miRNA
microarrays or panel-based quantitative PCR technologies holds
potential as a biomarker for COPD (61). A study comparing circulating miRNA
and cytokine levels between 103 patients with COPD and 25 control
patients found that miR-1 and miR-499 levels were 2.5 and 1.5 times
higher, respectively, in the COPD group compared with in controls
(62). However, one challenge of
this detection method is that miRNAs in circulation are susceptible
to degradation by endogenous RNA enzymes, which could lead to an
inaccurate reflection of the miRNA expression levels in the donor
cells (63). By contrast, EVs, as
natural carriers of signal molecules, are encased in a phospholipid
bilayer that protects miRNAs and proteins from degradation by
proteases and nucleases in the blood. Therefore, analyzing miRNAs
and proteins in blood-derived EVs offers a more reliable approach
for COPD diagnosis (61). For
example, a previous study found that the levels of miR-221, miR-23a
and miR-574 in blood-derived EVs from 41 patients with COPD and 29
healthy individuals were negatively correlated with the FEV1/FVC
ratio, with 95% confidence intervals of 0.776, 0.688 and 0.842,
respectively (64). Another study
identified a notable increase in miR-21 levels in blood-derived EVs
from cigarette smoke-induced COPD, where miR-21 targets pVHL to
regulate α-SMA gene expression, thereby contributing to airway
remodeling. Furthermore, during acute COPD exacerbations, the
number of plasma exosomes, along with the levels of plasma
C-reactive protein and soluble TNF receptor-1, have been found to
increase (65). Although
differential detection of blood-derived EVs is not yet a routine
diagnostic tool in clinical practice, their potential as a
prospective biomarker for predicting COPD is increasingly evident
(Table I) (55–57,61,62,64,65).
 | Table I.Potential markers for the diagnosis
of EV-based chronic obstructive pulmonary disease. |
Table I.
Potential markers for the diagnosis
of EV-based chronic obstructive pulmonary disease.
| First author,
year | EV test sample
source | Detection
indicators | Trends in detection
indicators | (Refs.) |
|---|
| Liu et al,
2025 | Sputum | EVs containing
CD31, CD66b and CD235 | Upregulated | (56) |
| Kotsiou et
al, 2024 | Sputum | Total protein
concentration | Upregulated | (55) |
| Kaur et al,
2021 | Bronchoalveolar
lavage fluid | miR-122-5p | Downregulated | (57) |
| Gon et al,
2020 | Bronchoalveolar
lavage fluid | Microvesicles
containing CD14 | Upregulated | (61) |
| Donaldson et
al, 2013 | Blood | miR-1 and
miR-499 | Upregulated | (62) |
| Shen et al,
2021 | Blood | miR-221, miR-23a
and miR-574 | Upregulated | (64) |
| Tan et al,
2017 | Blood | EV number and blood
plasma C-reactive protein and soluble tumor necrosis factor
receptor-1 | Upregulated | (65) |
Application of EVs in COPD
treatment
In addition to the promising potential of liquid
biopsy technology for diagnosing COPD, EVs also offer a new avenue
for treatment. There are two current strategies for utilizing EVs
in COPD treatment. Primarily, the removal of EVs containing nucleic
acids or proteins associated with disease pathogenesis can be
achieved by: i) Inhibiting the secretion of EVs that promote COPD
progression; ii) blocking the effective binding of EVs to recipient
cells; or iii) capturing circulating EVs that contribute to disease
progression (66). For example,
treatment with the EV-production inhibitor GW4869 has been shown to
reduce miR-125a-5p expression in cigarette smoke-induced EV
secretion from AECs. miR-125a-5p promotes M1 macrophage
polarization by downregulating IL-1 receptor antagonist protein,
thereby activating the Toll-like receptor (TLR)4/myeloid
differentiation primary response 88/TNF receptor-associated factor
6/NF-κB signaling pathway and enhancing its pro-inflammatory
effects (67). Alternatively, EVs
can be used as therapeutic agents or as vehicles for delivering
therapeutic agents to the lungs of patients with COPD. Mesenchymal
stem cell (MSC)-derived EVs, in particular, have been explored for
their therapeutic potential in COPD (Fig. 4) (68).
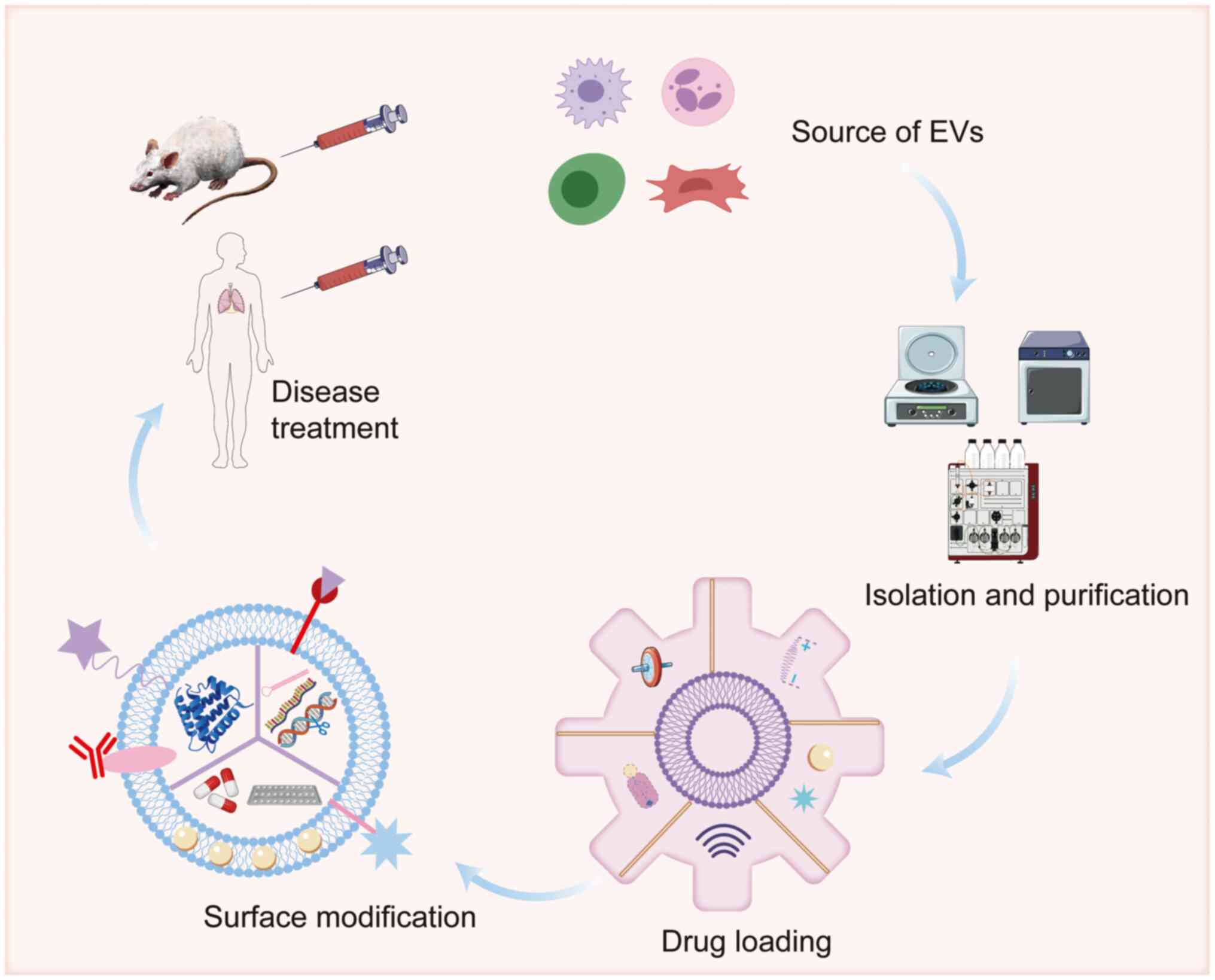 | Figure 4.Therapeutic applications of
engineered extracellular vesicles in COPD. EVs extracted from
different cells, including mesenchymal stem cells, macrophages and
DCs, by methods such as differential centrifugation, size exclusion
chromatography, immunoaffinity capture, microfluidics or
precipitation, can be used in the treatment of COPD. These EVs are
loaded with drugs endogenously and/or exogenously, and their
membrane surfaces are modified to improve lung tissue targeting.
Created with BioRender.com (https://app.biorender.com/illustrations/692ee91969efa5da6646019a?slideId=15732dc8-116e-49fa-a545-6343ae7d7599).
miR, microRNA; EVs, extracellular vesicles. |
Application of MSC-derived EVs in
therapy
MSCs are multipotent adult stem cells that can be
isolated from various human tissues, including bone marrow (BM),
adipose tissue, umbilical cord and placenta (69). MSCs secrete numerous
anti-inflammatory cytokines, including IL-13, neurotrophin 3,
ciliary neurotrophic factor and IL-10, and growth factors that aid
tissue repair (70). Due to their
anti-inflammatory and immunomodulatory properties, MSCs have been
investigated for COPD treatment. A previous study has shown that
BM-derived MSCs are able to inhibit p38 mitogen-activated protein
kinase (MAPK) and extracellular signal-regulated kinase
phosphorylation and activation, thereby reducing the production of
prostaglandin E2 and cyclooxygenase 2 in macrophages and
alleviating airway inflammation and emphysema in a COPD mouse model
(71). Adipose-derived stem cells
have been found to reduce chronic cigarette smoke-induced
activation of JNK1 and protein kinase B (AKT) by eliminating p38
MAPK phosphorylation, which, in turn, mitigates lung inflammation
and cysteine aspartate activity, protecting alveolar structural
integrity in patients with COPD (72). A clinical trial assessing the
systemic administration of allogeneic MSCs in patients with
moderate-to-severe COPD evaluated inflammatory markers, including
TNF-α, IFN-γ, IL-2 and TGF-β, pulmonary function and
electrocardiographic parameters. The study confirmed the safety of
systemic MSC infusion in individuals with impaired lung function,
and demonstrated that MSCs may inhibit inflammation and reduce or
reverse emphysematous changes (73). However, the clinical application of
MSCs is limited by challenges such as the difficulty in obtaining
high-quality MSCs, issues with proper storage, potential
tumorigenicity and the ethical complexities of the infusion
process. These factors constrain the broader therapeutic use of
MSCs for patients with COPD (74).
Consequently, there is a growing need to develop MSC-based
cell-free therapies that overcome these limitations.
EVs derived from MSCs retain the anti-inflammatory,
immunomodulatory and tissue-regenerative properties of their parent
cells, effectively transporting proteins, lipids, DNA fragments,
mRNA and other molecules to recipient cells, thereby facilitating
intercellular communication and regulating the biological functions
of these cells (75). Compared
with the donor cells, MSC-derived EVs are enriched with functional
cargo, including signaling molecules and growth factors, and offer
advantages such as long-term stability for storage and transport,
ethical acceptance for infusion, deep-tissue penetration and
versatile administration methods (76). Furthermore, c-Myc gene
transfection, hydrogen peroxide and lipopolysaccharide pretreatment
can further enhance EV secretion from MSCs (77,78).
Over time, MSC-derived EVs have emerged as a promising cell-free
therapeutic alternative.
The bioactive substances contained within EVs
contribute towards COPD treatment through promoting lung cell
proliferation and reducing systemic inflammatory-mediator
production (79). Chronic exposure
to harmful particles or gases triggers a complex inflammatory
response, driving the characteristic pathological features of COPD,
including emphysematous destruction, alveolar remodeling and
narrowing of small airways (80).
A key event in this process is endothelial cell apoptosis in the
lung parenchyma, which is closely associated with emphysema
development. Notably, patients with COPD exhibit reduced expression
levels of vascular endothelial growth factor (VEGF) and VEGF
receptor 2 (VEGFR2) in their bodies, and this leads to extensive
endothelial cell death and microvascular damage upon exposure to
cigarette smoke extract, accelerating emphysema progression
(81). MSC-derived EVs retain ~70%
of the beneficial effects of MSCs and can effectively deliver
signaling molecules and growth factors to lung tissue, reducing
inflammation and promoting the restoration of damaged lung function
(82). Human umbilical cord MSC
exosomes (hUCMSC-Exos) contain hsa-miR-10a-5p and hsa-miR-146a-5p,
which inhibit endothelial cell apoptosis by facilitating the
specific binding of VEGF to VEGFR2 on endothelial cells, thereby
activating the phosphoinositide 3-kinase/AKT pathway (83). Additionally, hUCMSC-Exo treatment
can reduce the lung tissue inflammation score in COPD model mice,
decreasing the influx of peripheral blood neutrophils, eosinophils,
lymphocytes and macrophages into the lungs, while also reducing the
number of mucus-secreting goblet cells. Mechanistically,
hUCMSC-Exos exert their therapeutic effects by inhibiting the
phosphorylation of the pro-inflammatory transcription factor NF-κB
subunit p65 (84).
Mesenchymal stem cells, potent mitochondrial donors,
exhibit elevated levels of the mitochondrial fission protein
dynamin-related protein 1 (DRP1) upon exposure to cigarette smoke,
leading to impaired mitochondrial function. Prolonged exposure
reduces the expression of fusion proteins such as mitofusin (MFN)1,
MFN2 and optic atrophy 1 (OPA1), causing mitochondrial damage, and
ultimately triggers mitophagy (85). Disrupted mitophagy results in the
accumulation of perinuclear mitochondria in lung epithelial cells
and fibroblasts, impaired oxidative phosphorylation and an increase
in mtROS, all of which exacerbate COPD progression (86). To address COPD induced by
mitochondrial damage, a previous study employed BM-derived MSCs and
exosomes (MSC + EXO) in a COPD mitochondrial Keima mouse model
induced by acute cigarette smoke exposure over a period of 10 days
(87). The combination therapy
markedly upregulated the expression of MFN1, MFN2 and OPA1, whereas
the levels of damage-associated molecular pattern (DAMP) markers,
including MMP9 and high mobility group box 1, were markedly
increased, supporting the hypothesis that MSC + EXO therapy
protects against lung mitochondrial dysfunction. By contrast, MSC
or EXO treatments alone exhibited limited effects (87). These results highlight MSC-derived
EVs as promising cell-free therapeutic agents capable of addressing
COPD through multiple mechanisms.
Treatment of COPD with EVs as a drug
delivery vehicle
The current standard of care for COPD involves
triple therapy, combining inhaled corticosteroids, long-acting β-2
agonists and long-acting muscarinic receptor antagonists (88). However, steroid resistance in a
number of patients necessitates the administration of higher doses,
which increase the risk of pneumonia and a range of side effects
that negatively impact the function of normal cells, including
osteoporosis, metabolic disorders, cardiac arrhythmias and
hyperglycemia (89). This
underscores the notable need for the development of a high-quality
drug-delivery platform that targets lung tissue to enhance COPD
treatment efficacy.
EVs have been engineered to deliver a wide range of
drugs for the treatment of various diseases (90). For example, EVs loaded with
miR-451a can target MMP10 to inhibit the proliferation, migration
and invasion of hepatocellular carcinoma (91). EVs carrying gemcitabine (GEM)
enhance the therapeutic effect of GEM treatment on pancreatic
ductal carcinoma, while reducing GEM-induced toxicity to the liver
and kidneys (92). Curcumin-loaded
EVs mitigate ischemic stroke by suppressing the expression of
phosphorylated-p65 and caspase-3 (93). Similarly, EVs as a drug delivery
platform show notable potential in COPD treatment. Drug-loaded EVs
can regulate key signaling pathways involved in COPD pathogenesis
to inhibit disease progression. For example, CD24-overexpressing
EVs can block the activation of the NF-κB pathway by binding to
DAMPs, preventing their interaction with pattern recognition
receptors, such as TLRs and NOD-like receptors, thereby dampening
the excessive inflammatory response in COPD (94). Furthermore, nebulized human
platelet-derived exosome products have been shown to reduce
smoke-induced emphysema in mice by inhibiting NF-κB activation,
inflammatory cytokine production and apoptotic protein expression,
while promoting an increase in CD4+/forkhead box
P3+ regulatory T cells in lung tissue, thereby
alleviating oxidative lung injury, inflammation and apoptotic
alveolar epithelial cell death (95). In addition, artificial nanovesicles
derived from adipose-derived stem cells expressing fibroblast
growth factor 2 have been shown to enhance the differentiation
potential of type II alveolar cells into type I alveolar cells,
thereby inhibiting emphysema formation (96). Some practical applications of
EV-based treatments for COPD are summarized in Table II (83,84,87,95,96).
 | Table II.Practical application of EV-based
treatment of chronic obstructive pulmonary disease. |
Table II.
Practical application of EV-based
treatment of chronic obstructive pulmonary disease.
| First author,
year | Sources of EVs | Cargo | Mechanism of
action | Effect of
action | (Refs.) |
|---|
| Chen et al,
2022 | Human umbilical
cord mesenchymal stem cells | miR-10a and
miR-146a | Promotes specific
binding of VEGF to VEGF receptor 2 of endothelial cells to activate
the phosphoinositide 3-kinase/protein kinase B pathway | Inhibition of
endothelial cell apoptosis | (83) |
| Ridzuan et
al, 2021 | Human umbilical
cord mesenchymal stem cells | Not applicable | Inhibits
phosphorylation of the pro-inflammatory transcription factor NF-κB
subunit p65 | Reduces influx of
neutrophils, eosinophils, lymphocytes and macrophages into lung
tissue | (84) |
| Maremanda et
al, 2019 | Bone marrow
mesenchymal stem cells | Not applicable | Increases the
expression levels of MFN1, MFN2 and optic atrophy 1 | Strengthens
protection against pulmonary mitochondrial dysfunction | (87) |
| Xuan et al,
2024 | Platelets | Not applicable | Inhibits NF-κB
activation, inflammatory cytokine production and apoptotic protein
expression | Inhibits oxidative
lung injury, inflammation and apoptotic alveolar epithelial cell
death | (95) |
| Kim et al,
2017 | Adipose-derived
stem cells | Fibroblast growth
factor 2 | Enhances type II
alveolar cell proliferation and differentiation potential to type I
alveolar cells | Inhibits the
formation of emphysema | (96) |
Achieving the therapeutic effects of drug-loaded EVs
targeting lung tissue involves several important steps: i)
Isolation and purification of EVs; ii) loading of therapeutic
agents into EVs; and iii) artificial modification of EVs to enhance
targeting efficiency. The first step, the isolation and
purification of EVs, primarily utilizes methods based on the
physicochemical properties of EVs such as size, density and surface
marker proteins (97). Techniques
used include differential centrifugation, size-exclusion
chromatography, immunoadsorption capture, microfluidics and
precipitation (98). Among these,
differential centrifugation is considered the ‘gold standard’ for
EV isolation in laboratories, and remains the predominant method
for extracting EVs from biofluids. The second step is the effective
loading of therapeutic drugs into EVs, which can occur either
before or after EV isolation (98,99).
The third important step is modifying EVs to improve their
targeting capability. Natural EVs typically exhibit poor targeting
properties and are rapidly cleared from circulation, primarily
accumulating in the liver and kidneys following intravenous
administration (100). To address
this limitation, artificial modification of EVs is necessary to
enhance their tissue- or cell-specific delivery. A key approach
involves using genetic engineering to transfect plasmids encoding
targeting peptides or proteins, such as lysosomal-associated
membrane protein 2B (LAMP2B) and tetraspanins, into EVs. These
targeting peptides are either incorporated into the biogenesis
pathway of the EVs or directly loaded onto the EV membrane surface,
improving the ability of the EVs to target and deliver cargo to
specific tissues or cells (101).
For example, the fusion of neuron-specific rabies virus
glycoprotein with the N-terminus of LAMP2B enables the targeted
delivery of GAPDH small interfering RNA (siRNA) to neurons,
microglia and oligodendrocytes in the brain. Furthermore, other
tissues do not absorb GAPDH siRNA in this treatment (102).
The route of administration for EVs targeting lung
tissue has garnered notable interest from researchers. Currently,
the primary methods of EV-based COPD treatment involve intravenous
administration and intratracheal delivery. Intravenous
administration is the most widely used method (103), with therapeutic EVs reaching the
lungs through the circulatory system to exert their effects. In
studies performed in isolated cells, typical injection doses of EV
therapeutics range from 2–40 µg/ml (104). However, intravenous
administration carries a risk of microcirculation aggregation,
potentially leading to mutagenicity and carcinogenicity (105). Intratracheal delivery, which
directly targets the airways, reduces systemic side effects
compared with intravenous administration, although the complexity
of the procedure markedly increases (106).
Previous review summaries and novelty of the
present review
A recent review summarized the current understanding
of EV-miRNAs in conditions such as COPD, asthma, lung cancer and
COVID-19, highlighting their potential as both biomarkers and
therapeutic targets in these respiratory diseases (107). The previous work emphasized the
crucial role of EV-miRNAs in regulating immune cell function,
chronic airway inflammation and disease progression. Additionally,
the previous work largely focused on EVs as biomarkers and
therapeutic targets, whereas the present review provides a more
detailed analysis of bacteria-derived OMVs and their impact on
COPD, linking impaired mucociliary clearance and inflammation.
Furthermore, the current review uniquely addresses EV engineering
strategies for targeted drug delivery in COPD, including pre- and
post-isolation drug-loading methods and genetic modifications to
enhance targeting efficiency, topics not as extensively covered in
previous reviews (1,8). A previous study also emphasized the
importance of EVs in regulating immune cell function, chronic
inflammation and disease progression (108). However, the present review
introduces novel insights by exploring mitochondrial dysfunction as
a key mechanism in COPD progression, a topic not deeply covered in
the prior study. Furthermore, while the previous review discussed
EVs as biomarkers detected in biological fluids, such as blood and
sputum, the current review offers a more detailed quantitative
analysis of miRNA expression in various fluids. Additionally, this
review expands on EV engineering strategies for targeted drug
delivery in COPD, including both pre- and post-isolation
drug-loading methods and genetic modifications, which is a more
focused exploration compared with the broader discussions in the
previous review.
Furthermore, another review highlighted the
involvement of EVs in COPD pathogenesis, particularly their
influence on inflammation, immune responses and airway remodeling
(109). However, the present
review introduces novel insights by exploring mitochondrial
dysfunction as a key mechanism in COPD progression, specifically
through mitochondrial fission/fusion proteins, which were not
extensively covered in the referenced review. Furthermore, while
both reviews have discussed EVs as biomarkers, the current review
provides a more detailed quantitative analysis of miRNAs in various
biological fluids, offering deeper insights into their diagnostic
potential. Additionally, this review expands on EV engineering
strategies for targeted drug delivery in COPD, which is more
thoroughly explored than in the previous review, which provided a
broader discussion of EVs as biomarkers and therapeutic
targets.
The present review has provided novel insights into
EV-mediated mitochondrial dysfunction, characterized by imbalances
in key regulators such as DRP1/MFN/OPA1 and increased mtROS
production, as a key driver of COPD progression, a mechanism
frequently overlooked in previous reviews (107–109). Furthermore, the present review
has broadened the perspective of current EV-based research by
linking bacteria-derived OMVs, for example from Moraxella
and Pseudomonas, to impaired mucociliary clearance and
inflammatory responses, thereby establishing a notable association
between microbiology and EV biology.
In terms of diagnostic value, the present review has
systematically compared and evaluated potential EV biomarkers in
blood, BALF and sputum, supplemented with quantitative data. For
example: i) The general protein concentration of sputum EVs in
patients with COPD is higher compared with that in healthy controls
(55); ii) the expression levels
of miR-122-5p in EVs from the lungs of patients with COPD are
significantly downregulated, and a structured dataset of
BALF-derived EV particles, encompassing particle size distribution
and concentration, has been established and analyzed (57,60);
and iii) the expression levels of miR-1 and miR-499 are higher in
the blood of patients with COPD, and the diagnostic confidence
intervals of miR-23a, miR-221 and miR-574-5p have been analyzed
(62,64). These have filled in several gaps in
previous studies.
Regarding therapeutic applications, the present
review has detailed EV engineering strategies for targeted drug
delivery in COPD, including both pre-isolation and post-isolation
drug-loading methods, modification technologies such as genetically
engineered fusion targeting peptides, and their specific processes.
The present review has also objectively analyzed the limitations
and challenges of using EVs as drug delivery systems, and discussed
the therapeutic potential of stem cell-derived EVs, such as the
effects of hUCMSC-Exos and combined therapy with BM-derived MSC +
EXO treatment.
Conclusion
In conclusion, EVs represent a ‘double-edged sword’
in COPD treatment. On one hand, the substances they carry can
contribute to the progression of COPD by regulating key signaling
pathways. On the other hand, liquid biopsy technologies that detect
EVs in sputum, blood and alveolar lavage fluid, along with their
associated cargo, may offer valuable diagnostic markers for early
COPD detection. Additionally, EVs derived from MSCs or used as drug
delivery vehicles for COPD treatment present a promising new
cell-free therapeutic strategy. Therefore, further research into
the role of EVs in COPD progression is important for advancing
preventative measures. Simultaneously, extensive preclinical
studies and clinical trials are required to validate the
therapeutic applications of EVs or therapeutic vectors in COPD.
Acknowledgements
Not applicable.
Funding
The present work was supported by the National Natural Science
Foundation of China (grant no. 8180150490).
Availability of data and materials
Not applicable.
Authors' contributions
YZ, ZG and CF conceived and designed the review. TR,
JX and YY retrieved the relevant literature, acquired relevant
references, screened data, and analyzed and organized reference
data. XZ, XH and WY critically revised and edited the manuscript.
YZ, ZG and CF created figures and reviewed the article. Data
authentication is not applicable. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
COPD
|
chronic obstructive pulmonary
disease
|
|
EVs
|
extracellular vesicles
|
|
AAT
|
α-1 antitrypsin
|
|
PM
|
particulate matter
|
|
ROS
|
reactive oxygen species
|
|
NF-κB
|
nuclear factor κB
|
|
JNK
|
c-Jun N-terminal kinase
|
|
IL
|
interleukin
|
|
MMPs
|
matrix metalloproteinases
|
|
MVB
|
multivesicular bodies
|
|
LFs
|
lung fibroblasts
|
|
HIF-1α
|
hypoxia-inducible factor 1α
|
|
OMVs
|
outer-membrane vesicles
|
|
FVC
|
forced vital capacity
|
|
BALF
|
bronchoalveolar lavage fluid
|
|
AECs
|
airway epithelial cells
|
|
MSCs
|
mesenchymal stem cells
|
|
BM
|
bone marrow
|
|
DAMPs
|
damage-associated molecular
patterns
|
References
|
1
|
Wang C, Zhou J, Wang J, Li S, Fukunaga A,
Yodoi J and Tian H: Progress in the mechanism and targeted drug
therapy for COPD. Signal Transduct Target Ther. 5:2482020.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Silverman EK: Genetics of COPD. Annu Rev
Physiol. 82:413–431. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Christenson SA, Smith BM, Bafadhel M and
Putcha N: Chronic obstructive pulmonary disease. Lancet.
399:2227–2242. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Abbaszadeh H, Ghorbani F, Abbaspour-Aghdam
S, Kamrani A, Valizadeh H, Nadiri M, Sadeghi A, Shamsasenjan K,
Jadidi-Niaragh F, Roshangar L and Ahmadi M: Chronic obstructive
pulmonary disease and asthma: Mesenchymal stem cells and their
extracellular vesicles as potential therapeutic tools. Stem Cell
Res Ther. 13:2622022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wei YY, Chen TT, Zhang DW, Zhang Y, Li F,
Ding YC, Wang MY, Zhang L, Chen KG and Fei GH: Microplastics
exacerbate ferroptosis via mitochondrial reactive oxygen
species-mediated autophagy in chronic obstructive pulmonary
disease. Autophagy. 21:1717–1743. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dumas G, Arabi YM, Bartz R, Ranzani O,
Scheibe F, Darmon M and Helms J: Diagnosis and management of
autoimmune diseases in the ICU. Intensive Care Med. 50:17–35. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Billington CK, Penn RB and Hall IP:
β2 agonists. Handb Exp Pharmacol. 237:23–40. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Trappe A, Donnelly SC, McNally P and
Coppinger JA: Role of extracellular vesicles in chronic lung
disease. Thorax. 76:1047–1056. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kalita-de Croft P, Sharma S, Sobrevia L
and Salomon C: Extracellular vesicle interactions with the external
and internal exposome in mediating carcinogenesis. Mol Aspects Med.
87:1010392022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Akhmerov A and Parimon T: Extracellular
vesicles, inflammation, and cardiovascular disease. Cells.
11:22292022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Marar C, Starich B and Wirtz D:
Extracellular vesicles in immunomodulation and tumor progression.
Nat Immunol. 22:560–570. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tian J, Han Z, Song D, Peng Y, Xiong M,
Chen Z, Duan S and Zhang L: Engineered exosome for drug delivery:
Recent development and clinical applications. Int J Nanomedicine.
18:7923–7940. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schaberg T, Klein U, Rau M, Eller J and
Lode H: Subpopulations of alveolar macrophages in smokers and
nonsmokers: Relation to the expression of CD11/CD18 molecules and
superoxide anion production. Am J Respir Crit Care Med.
151:1551–1558. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen J, Wang T, Li X, Gao L, Wang K, Cheng
M, Zeng Z, Chen L, Shen Y and Wen F: DNA of neutrophil
extracellular traps promote NF-κB-dependent autoimmunity via
cGAS/TLR9 in chronic obstructive pulmonary disease. Signal
Transduct Target Ther. 9:1632024. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Barnes PJ: Oxidative stress-based
therapeutics in COPD. Redox Biol. 33:1015442020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nwozor KO, Hackett TL, Chen Q, Yang CX,
Aguilar Lozano SP, Zheng X, Al-Fouadi M, Kole TM, Faiz A, Mahbub
RM, et al: Effect of age, COPD severity, and cigarette smoke
exposure on bronchial epithelial barrier function. Am J Physiol
Lung Cell Mol Physiol. 328:L724–Ll737. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vestbo J, Hurd SS, Agustí AG, Jones PW,
Vogelmeier C, Anzueto A, Barnes PJ, Fabbri LM, Martinez FJ,
Nishimura M, et al: Global strategy for the diagnosis, management,
and prevention of chronic obstructive pulmonary disease: GOLD
executive summary. Am J Respir Crit Care Med. 187:347–365. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cui W, Zhang Z, Zhang P, Qu J, Zheng C, Mo
X, Zhou W, Xu L, Yao H and Gao J: Nrf2 attenuates inflammatory
response in COPD/emphysema: Crosstalk with Wnt3a/β-catenin and AMPK
pathways. J Cell Mol Med. 22:3514–3525. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guo P, Li R, Piao TH, Wang CL, Wu XL and
Cai HY: Pathological mechanism and targeted drugs of COPD. Int J
Chron Obstruct Pulmon Dis. 17:1565–1575. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Barnes PJ, Burney PG, Silverman EK, Celli
BR, Vestbo J, Wedzicha JA and Wouters EF: Chronic obstructive
pulmonary disease. Nat Rev Dis Primers. 1:150762015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Barnes PJ: Inflammatory mechanisms in
patients with chronic obstructive pulmonary disease. J Allergy Clin
Immunol. 138:16–27. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Brightling C and Greening N: Airway
inflammation in COPD: Progress to precision medicine. Eur Respir J.
54:19006512019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu J, Zeng Q, Li S, Su Q and Fan H:
Inflammation mechanism and research progress of COPD. Front
Immunol. 15:14046152024. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Upadhyay P, Wu CW, Pham A, Zeki AA, Royer
CM, Kodavanti UP, Takeuchi M, Bayram H and Pinkerton KE: Animal
models and mechanisms of tobacco smoke-induced chronic obstructive
pulmonary disease (COPD). J Toxicol Environ Health B Crit Rev.
26:275–305. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
DI Stefano A, Gnemmi I, Dossena F,
Ricciardolo FL, Maniscalco M, Lo Bello F and Balbi B: Pathogenesis
of COPD at the cellular and molecular level. Minerva Med.
113:405–423. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shapiro SD: Proteolysis in the lung. Eur
Respir J Suppl. 44:30s–32s. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Abboud RT and Vimalanathan S: Pathogenesis
of COPD. Part I. The role of protease-antiprotease imbalance in
emphysema. Int J Tuberc Lung Dis. 12:361–367. 2008.PubMed/NCBI
|
|
28
|
Haq I, Chappell S, Johnson SR, Lotya J,
Daly L, Morgan K, Guetta-Baranes T, Roca J, Rabinovich R, Millar
AB, et al: Association of MMP-2 polymorphisms with severe and very
severe COPD: A case control study of MMPs-1, 9 and 12 in a European
population. BMC Med Genet. 11:72010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
D'Armiento JM, Goldklang MP, Hardigan AA,
Geraghty P, Roth MD, Connett JE, Wise RA, Sciurba FC, Scharf SM,
Thankachen J, et al: Increased matrix metalloproteinase (MMPs)
levels do not predict disease severity or progression in emphysema.
PLoS One. 8:e563522013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lomas DA: Does protease-antiprotease
imbalance explain chronic obstructive pulmonary disease? Ann Am
Thorac Soc. 13 (Suppl 2):S130–S137. 2016.PubMed/NCBI
|
|
31
|
Xin XF, Zhao M, Li ZL, Song Y and Shi Y:
Metalloproteinase-9/tissue inhibitor of metalloproteinase-1 in
induced sputum in patients with asthma and chronic obstructive
pulmonary disease and their relationship to airway inflammation and
airflow limitation. Zhonghua Jie He He Hu Xi Za Zhi. 30:192–196.
2007.(In Chinese). PubMed/NCBI
|
|
32
|
Wickman G, Julian L and Olson MF: How
apoptotic cells aid in the removal of their own cold dead bodies.
Cell Death Differ. 19:735–742. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Du S, Guan Y, Xie A, Yan Z, Gao S, Li W,
Rao L, Chen X and Chen T: Extracellular vesicles: A rising star for
therapeutics and drug delivery. J Nanobiotechnology. 21:2312023.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tenchov R, Sasso JM, Wang X, Liaw WS, Chen
CA and Zhou QA: Exosomes-nature's lipid nanoparticles, a rising
star in drug delivery and diagnostics. ACS Nano. 16:17802–17846.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu J, Ren L, Li S, Li W, Zheng X, Yang Y,
Fu W, Yi J, Wang J and Du G: The biology, function, and
applications of exosomes in cancer. Acta Pharm Sin B. 11:2783–2797.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Antonyak MA and Cerione RA: Microvesicles
as mediators of intercellular communication in cancer. Methods Mol
Biol. 1165:147–173. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xu X, Lai Y and Hua ZC: Apoptosis and
apoptotic body: Disease message and therapeutic target potentials.
Biosci Rep. 39:BSR201809922019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu Q, Li D, Pan X and Liang Y: Targeted
therapy using engineered extracellular vesicles: Principles and
strategies for membrane modification. J Nanobiotechnology.
21:3342023. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hallstrand TS, Hackett TL, Altemeier WA,
Matute-Bello G, Hansbro PM and Knight DA: Airway epithelial
regulation of pulmonary immune homeostasis and inflammation. Clin
Immunol. 151:1–15. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yang Y, Yuan L, Du X, Zhou K, Qin L, Wang
L, Yang M, Wu M, Zheng Z, Xiang Y, et al: Involvement of
epithelia-derived exosomes in chronic respiratory diseases. Biomed
Pharmacother. 143:1121892021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xu H, Ling M, Xue J, Dai X, Sun Q, Chen C,
Liu Y, Zhou L, Liu J, Luo F, et al: Exosomal microRNA-21 derived
from bronchial epithelial cells is involved in aberrant
epithelium-fibroblast cross-talk in COPD induced by cigarette
smoking. Theranostics. 8:5419–5433. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Qiu Q, Dan X, Yang C, Hardy P, Yang Z, Liu
G and Xiong W: Increased airway T lymphocyte microparticles in
chronic obstructive pulmonary disease induces airway epithelial
injury. Life Sci. 261:1183572020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Fujita Y, Araya J, Ito S, Kobayashi K,
Kosaka N, Yoshioka Y, Kadota T, Hara H, Kuwano K and Ochiya T:
Suppression of autophagy by extracellular vesicles promotes
myofibroblast differentiation in COPD pathogenesis. J Extracell
Vesicles. 4:283882015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hernández-Díazcouder A, Romero-Nava R,
Del-Río-Navarro BE, Sánchez-Muñoz F, Guzmán-Martín CA,
Reyes-Noriega N, Rodríguez-Cortés O, Leija-Martínez JJ,
Vélez-Reséndiz JM, Villafaña S, et al: The Roles of MicroRNAs in
asthma and emerging insights into the effects of vitamin
D3 supplementation. Nutrients. 16:3412024. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Augustyniak D, Roszkowiak J, Wiśniewska I,
Skała J, Gorczyca D and Drulis-Kawa Z: Neuropeptides SP and CGRP
diminish the Moraxella catarrhalis outer membrane
vesicle-(OMV-) triggered inflammatory response of human a549
epithelial cells and neutrophils. Mediators Inflamm.
2018:48472052018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bomberger JM, Ye S, Maceachran DP, Koeppen
K, Barnaby RL, O'Toole GA and Stanton BA: A Pseudomonas
aeruginosa toxin that hijacks the host ubiquitin proteolytic
system. PLoS Pathog. 7:e10013252011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Riley CM and Sciurba FC: Diagnosis and
outpatient management of chronic obstructive pulmonary disease: A
review. JAMA. 321:786–797. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ferrera MC, Labaki WW and Han MK: Advances
in chronic obstructive pulmonary disease. Annu Rev Med. 72:119–134.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kahnert K, Jörres RA, Behr J and Welte T:
The diagnosis and treatment of COPD and its comorbidities. Dtsch
Arztebl Int. 120:434–444. 2023.PubMed/NCBI
|
|
50
|
Beetler DJ, Di Florio DN, Bruno KA, Ikezu
T, March KL, Cooper LT Jr, Wolfram J and Fairweather D:
Extracellular vesicles as personalized medicine. Mol Aspects Med.
91:1011552023. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Brightling CE: Clinical applications of
induced sputum. Chest. 129:1344–1348. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Jin Y, Chen Z, Chen Q, Sha L and Shen C:
Role and significance of bioactive substances in sputum in the
diagnosis of lung cancer. Zhongguo Fei Ai Za Zhi. 24:867–873.
2021.(In Chinese). PubMed/NCBI
|
|
53
|
Guiot J, Demarche S, Henket M, Paulus V,
Graff S, Schleich F, Corhay JL, Louis R and Moermans C: Methodology
for sputum induction and laboratory processing. J Vis Exp.
566122017.PubMed/NCBI
|
|
54
|
Pastor L, Vera E, Marin JM and Sanz-Rubio
D: Extracellular vesicles from airway secretions: New insights in
lung diseases. Int J Mol. 22:5832021. View Article : Google Scholar
|
|
55
|
Kotsiou OS, Katsanaki K, Tsiggene A,
Papathanasiou S, Rouka E, Antonopoulos D, Gerogianni I, Balatsos
NAA, Gourgoulianis KI and Tsilioni I: Detection and
characterization of extracellular vesicles in sputum samples of
COPD patients. J Pers Med. 14:8202024. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Liu Y, Huang J, Li E, Xiao Y, Li C, Xia M,
Ke J, Xiang L and Lei M: Analysis of research trends and hot spots
on COPD biomarkers from the perspective of bibliometrics. Respir
Med. 240:1080302025. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kaur G, Maremanda KP, Campos M, Chand HS,
Li F, Hirani N, Haseeb MA, Li D and Rahman I: Distinct exosomal
miRNA profiles from BALF and lung tissue of COPD and IPF patients.
Int J Mol Sci. 22:118302021. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wu J, Ma Y and Chen Y: Extracellular
vesicles and COPD: Foe or friend? J Nanobiotechnology. 21:1472023.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Roth K, Hardie JA, Andreassen AH, Leh F
and Eagan TM: Predictors of diagnostic yield in bronchoscopy: A
retrospective cohort study comparing different combinations of
sampling techniques. BMC Pulm Med. 8:22008. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Walmsley S, Cruickshank-Quinn C, Quinn K,
Zhang X, Petrache I, Bowler RP, Reisdorph R and Reisdorph N: A
prototypic small molecule database for bronchoalveolar lavage-based
metabolomics. Sci Data. 5:1800602018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Gon Y, Shimizu T, Mizumura K, Maruoka S
and Hikichi M: Molecular techniques for respiratory diseases:
MicroRNA and extracellular vesicles. Respirology. 25:149–160. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Donaldson A, Natanek SA, Lewis A, Man WD,
Hopkinson NS, Polkey MI and Kemp PR: Increased skeletal
muscle-specific microRNA in the blood of patients with COPD.
Thorax. 68:1140–1149. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
McDonald JS, Milosevic D, Reddi HV, Grebe
SK and Algeciras-Schimnich A: Analysis of circulating microRNA:
Preanalytical and analytical challenges. Clin Chem. 57:833–840.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Shen Y, Wang L, Wu Y, Ou Y, Lu H and Yao
X: A novel diagnostic signature based on three circulating exosomal
mircoRNAs for chronic obstructive pulmonary disease. Exp Ther Med.
22:7172021. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Tan DBA, Armitage J, Teo TH, Ong NE, Shin
H and Moodley YP: Elevated levels of circulating exosome in COPD
patients are associated with systemic inflammation. Respir Med.
132:261–264. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Park KS, Lässer C and Lötvall J:
Extracellular vesicles and the lung: from disease pathogenesis to
biomarkers and treatments. Physiol Rev. 105:1733–1821. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Wang R, Zhu Z, Peng S, Xu J, Chen Y, Wei S
and Liu X: Exosome microRNA-125a-5p derived from epithelium
promotes M1 macrophage polarization by targeting IL1RN in chronic
obstructive pulmonary disease. Int Immunopharmacol. 137:1124662024.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Hade MD, Suire CN and Suo Z: Mesenchymal
stem cell-derived exosomes: applications in regenerative medicine.
Cells. 10:19592021. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Chen L, Qu J, Kalyani FS, Zhang Q, Fan L,
Fang Y, Li Y and Xiang C: Mesenchymal stem cell-based treatments
for COVID-19: Status and future perspectives for clinical
applications. Cell Mol Life Sci. 79:1422022. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Pierro M, Ionescu L, Montemurro T, Vadivel
A, Weissmann G, Oudit G, Emery D, Bodiga S, Eaton F, Péault B, et
al: Short-term, long-term and paracrine effect of human umbilical
cord-derived stem cells in lung injury prevention and repair in
experimental bronchopulmonary dysplasia. Thorax. 68:475–484. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Gu W, Song L, Li XM, Wang D, Guo XJ and Xu
WG: Mesenchymal stem cells alleviate airway inflammation and
emphysema in COPD through down-regulation of cyclooxygenase-2 via
p38 and ERK MAPK pathways. Sci Rep. 5:87332015. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Schweitzer KS, Johnstone BH, Garrison J,
Rush NI, Cooper S, Traktuev DO, Feng D, Adamowicz JJ, Van Demark M,
Fisher AJ, et al: Adipose stem cell treatment in mice attenuates
lung and systemic injury induced by cigarette smoking. Am J Respir
Crit Care Med. 183:215–225. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Weiss DJ, Casaburi R, Flannery R,
LeRoux-Williams M and Tashkin DP: A placebo-controlled, randomized
trial of mesenchymal stem cells in COPD. Chest. 143:1590–1598.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Lowenthal J and Sugarman J: Ethics and
policy issues for stem cell research and pulmonary medicine. Chest.
147:824–834. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Mohammadipoor A, Antebi B, Batchinsky AI
and Cancio LC: Therapeutic potential of products derived from
mesenchymal stem/stromal cells in pulmonary disease. Respir Res.
19:2182018. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Tan F, Li X, Wang Z, Li J, Shahzad K and
Zheng J: Clinical applications of stem cell-derived exosomes.
Signal Transduct Target Ther. 9:172024. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Sung DK, Chang YS, Sung SI, Ahn SY and
Park WS: Thrombin preconditioning of extracellular vesicles derived
from mesenchymal stem cells accelerates cutaneous wound healing by
boosting their biogenesis and enriching cargo content. J Clin Med.
8:5332019. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Lai RC, Yeo RW, Padmanabhan J, Choo A, de
Kleijn DP and Lim SK: Isolation and characterization of exosome
from human embryonic stem cell-derived C-Myc-immortalized
mesenchymal stem cells. Methods Mol Biol. 1416:477–494. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Miethe S, Potaczek DP, Bazan-Socha S,
Bachl M, Schaefer L, Wygrecka M and Garn H: The emerging role of
extracellular vesicles as communicators between adipose tissue and
pathologic lungs with a special focus on asthma. Am J Physiol Cell
Physiol. 324:C1119–C1125. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Manisalidis I, Stavropoulou E,
Stavropoulos A and Bezirtzoglou E: Environmental and health impacts
of air pollution: A review. Front Public Health. 8:142020.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Chen YT, Miao K, Zhou L and Xiong WN: Stem
cell therapy for chronic obstructive pulmonary disease. Chin Med J
(Engl). 134:1535–1545. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Ji HL, Liu C and Zhao RZ: Stem cell
therapy for COVID-19 and other respiratory diseases: Global trends
of clinical trials. World J Stem Cells. 12:471–480. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Chen Q, Lin J, Deng Z and Qian W: Exosomes
derived from human umbilical cord mesenchymal stem cells protect
against papain-induced emphysema by preventing apoptosis through
activating VEGF-VEGFR2-mediated AKT and MEK/ERK pathways in rats.
Regen Ther. 21:216–224. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Ridzuan N, Zakaria N, Widera D, Sheard J,
Morimoto M, Kiyokawa H, Mohd Isa SA, Chatar Singh GK, Then KY, Ooi
GC and Yahaya BH: Human umbilical cord mesenchymal stem
cell-derived extracellular vesicles ameliorate airway inflammation
in a rat model of chronic obstructive pulmonary disease (COPD).
Stem Cell Res Ther. 12:542021. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Las G and Shirihai OS: Miro1: New wheels
for transferring mitochondria. EMBO J. 33:939–941. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Lerner CA, Sundar IK and Rahman I:
Mitochondrial redox system, dynamics, and dysfunction in lung
inflammaging and COPD. Int J Biochem Cell Biol. 81:294–306. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Maremanda KP, Sundar IK and Rahman I:
Protective role of mesenchymal stem cells and mesenchymal stem
cell-derived exosomes in cigarette smoke-induced mitochondrial
dysfunction in mice. Toxicol Appl Pharmacol. 385:1147882019.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Calzetta L, Matera MG, Rogliani P and
Cazzola M: The role of triple therapy in the management of COPD.
Expert Rev Clin Pharmacol. 13:865–874. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Wang N, Wang Q, Du T, Gabriel ANA, Wang X,
Sun L, Li X, Xu K, Jiang X and Zhang Y: The potential roles of
exosomes in chronic obstructive pulmonary disease. Front Med
(Lausanne). 7:6185062021. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Yang Y, Hong Y, Cho E, Kim GB and Kim IS:
Extracellular vesicles as a platform for membrane-associated
therapeutic protein delivery. J Extracell Vesicles. 7:14401312018.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Xu Y, Lai Y, Cao L, Li Y, Chen G, Chen L,
Weng H, Chen T, Wang L and Ye Y: Human umbilical cord mesenchymal
stem cells-derived exosomal microRNA-451a represses
epithelial-mesenchymal transition of hepatocellular carcinoma cells
by inhibiting ADAM10. RNA Biol. 18:1408–1423. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Li YJ, Wu JY, Wang JM, Hu XB, Cai JX and
Xiang DX: Gemcitabine loaded autologous exosomes for effective and
safe chemotherapy of pancreatic cancer. Acta Biomater. 101:519–530.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Tian T, Zhang HX, He CP, Fan S, Zhu YL, Qi
C, Huang NP, Xiao ZD, Lu ZH, Tannous BA and Gao J: Surface
functionalized exosomes as targeted drug delivery vehicles for
cerebral ischemia therapy. Biomaterials. 150:137–149. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Shapira S, Schwartz R, Tsiodras S,
Bar-Shai A, Melloul A, Borsekofsky S, Peer M, Adi N, MacLoughlin R
and Arber N: Inhaled CD24-enriched exosomes (EXO-CD24) as a novel
immune modulator in respiratory disease. Int J Mol Sci. 25:772023.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Xuan W, Wang S, Alarcon-Calderon A,
Bagwell MS, Para R, Wang F, Zhang C, Tian X, Stalboerger P,
Peterson T, et al: Nebulized platelet-derived extracellular
vesicles attenuate chronic cigarette smoke-induced murine
emphysema. Transl Res. 269:76–93. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Kim YS, Kim JY, Cho R, Shin DM, Lee SW and
Oh YM: Adipose stem cell-derived nanovesicles inhibit emphysema
primarily via an FGF2-dependent pathway. Exp Mol Med. 49:e2842017.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Staubach S, Bauer FN, Tertel T, Börger V,
Stambouli O, Salzig D and Giebel B: Scaled preparation of
extracellular vesicles from conditioned media. Adv Drug Deliv Rev.
177:1139402021. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Jia Y, Yu L, Ma T, Xu W, Qian H, Sun Y and
Shi H: Small extracellular vesicles isolation and separation:
Current techniques, pending questions and clinical applications.
Theranostics. 12:6548–6575. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Araldi RP, Delvalle DA, da Costa VR,
Alievi AL, Teixeira MR, Dias Pinto JR and Kerkis I: Exosomes as a
nano-carrier for chemotherapeutics: A new era of oncology. Cells.
12:21442023. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Liang Y, Duan L, Lu J and Xia J:
Engineering exosomes for targeted drug delivery. Theranostics.
11:3183–3195. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Bauer D, Cornejo MA, Hoang TT, Lewis JS
and Zeglis BM: Click chemistry and radiochemistry: An update.
Bioconjug Chem. 34:1925–1950. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Alvarez-Erviti L, Seow Y, Yin H, Betts C,
Lakhal S and Wood MJ: Delivery of siRNA to the mouse brain by
systemic injection of targeted exosomes. Nat Biotechnol.
29:341–345. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Bari E, Ferrarotti I, Torre ML, Corsico AG
and Perteghella S: Mesenchymal stem/stromal cell secretome for lung
regeneration: The long way through ‘pharmaceuticalization’ for the
best formulation. J Control Release. 309:11–24. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Ma Y, Liu X, Long Y and Chen Y: Emerging
therapeutic potential of mesenchymal stem cell-derived
extracellular vesicles in chronic respiratory diseases: An overview
of recent progress. Front Bioeng Biotechnol. 10:8450422022.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Brave H and MacLoughlin R: State of the
art review of cell therapy in the treatment of lung disease, and
the potential for aerosol delivery. Int J Mol Sci. 21:64352020.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Rogliani P, Calzetta L, Coppola A, Cavalli
F, Ora J, Puxeddu E, Matera MG and Cazzola M: Optimizing drug
delivery in COPD: The role of inhaler devices. Respir Med.
124:6–14. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Lv J and Xiong X: Extracellular vesicle
microRNA: A promising biomarker and therapeutic target for
respiratory diseases. Int J Mol Sci. 25:91472024. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Neri T, Celi A, Tinè M, Bernardinello N,
Cosio MG, Saetta M, Nieri D and Bazzan E: The emerging role of
extracellular vesicles detected in different biological fluids in
COPD. Int J Mol Sci. 23:51362022. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Reid LV, Spalluto CM, Watson A, Staples KJ
and Wilkinson TMA: The Role of Extracellular vesicles as a shared
disease mechanism contributing to multimorbidity in patients with
COPD. Front Immunol. 12:7540042021. View Article : Google Scholar : PubMed/NCBI
|


















