Introduction
Hepatocellular carcinoma (HCC) is a major global
health burden, representing the most common primary liver cancer
and the third leading cause of cancer-related death worldwide
(1). Most cases are diagnosed at
an intermediate or advanced stage due to the insidious onset of the
disease, and high post-treatment relapse rates further contribute
to a poor overall prognosis (2,3).
While conventional therapies, such as surgical resection, ablation,
transarterial chemoembolization (TACE) and kinase inhibitors,
remain central to HCC management, the emergence of cancer
immunotherapy has introduced new therapeutic options. Immune-based
treatments, particularly immune checkpoint inhibitors (ICIs)
targeting programmed cell death protein 1 (PD-1)/programmed
death-ligand 1 (PD-L1) or cytotoxic T-lymphocyte-associated protein
4 (CTLA-4), demonstrate durable clinical responses in several types
of solid tumors, including efficacy in HCC (4,5). For
example, it has been shown that in advanced HCC, single-agent ICIs
can induce objective responses in 15–20% of patients (6).
However, most patients with HCC do not respond
adequately to immunotherapy (7).
This has been attributed, in part, to the distinct tumor immune
microenvironment of HCC, which often renders the tumor
immunologically ‘cold’ or non-inflamed, thereby diminishing
immune-mediated antitumor activity (5,8). The
concept of ‘hot’ and ‘cold’ tumors refers to the degree of immune
cell infiltration and activity in the tumor microenvironment (TME).
Hot HCC tumors are characterized by high densities of
CD8+ T cells infiltrating the tumor the core and margin,
a strong interferon-γ (IFN-γ) gene expression signature indicative
of an active T helper 1 immune response, and the upregulated
expression of checkpoint molecules such as PD-L1 (9,10).
Conversely, cold tumors in general exhibit sparse T-cell
infiltration, diminished levels of IFN-γ-associated inflammatory
cytokines, and minimal checkpoint expression, reflecting an
immunologically inactive microenvironment (9,10).
Several quantitative approaches have been developed
to distinguish between cold and hot tumors in HCC and other solid
cancers. These include immunohistochemical analysis of intratumoral
CD8+T-cell density, commonly using multiplex
immunohistochemistry (11),
IFN-γ-associated immune gene expression profiling, for example
using RNA sequencing or targeted Nanostring panels to generate a
T-cell-inflamed or tumor inflammation signature (12), and spatial immune cell profiling
(13). Composite immune scoring
systems such as the Immunoscore, which quantifies CD3+
and CD8+ T cells in situ, are also used to
stratify tumors immunologically (14).
HCC typically arise in the context of chronic
inflammation, such as that caused by hepatitis B or C infection or
non-alcoholic steatohepatitis (NASH), but paradoxically often
develops in an immunosuppressive microenvironment (15). Chronic viral antigen exposure and
liver-specific tolerogenic mechanisms contribute to T-cell
exhaustion and immune evasion in HCC (8). Notably, 25–30% of HCC tumors exhibit
an ‘immune-excluded’ phenotype, associated with Wnt/β-catenin
pathway activation and characterized by a lack of T-cell
infiltration, which predicts resistance to ICIs (10).
Converting an immunologically cold HCC into a hot
tumor is a promising strategy to overcome immune resistance and
improve patient responses. In the present review, a condensed
overview of the HCC tumor immune landscape is provided and the
mechanisms underlying its immunologically cold phenotype, including
the roles of various immune cells, cytokines and tumor-intrinsic
pathways in suppressing antitumor immunity are presented. The
established and emerging therapeutic strategies aimed at
reprogramming the HCC microenvironment to a more inflamed,
T-cell-permissive state are also discussed.
Tumor immune landscape in HCC
HCC develops within a complex TME composed of tumor
cells, stromal elements and a diverse array of immune cells. The
baseline immune landscape of HCC is often immunosuppressive and
heterogeneous, contributing to variable immune surveillance and
inconsistent therapeutic responses (16). Chronic liver inflammation,
resulting from viral hepatitis, alcohol or fatty liver disease,
serves as the foundation for immune dysfunction even before
malignant transformation occurs (17). As HCC develops, the intrinsic
tolerogenic milieu of the liver, which normally prevents
overactivation by persistent exposure to gut-derived antigens, is
co-opted by the tumor to escape immune elimination (18). The roles of different cell types in
the TME of HCC are summarized in Table
I (8,16,19–27).
 | Table I.Properties of the tumor immune
microenvironment in HCC. |
Table I.
Properties of the tumor immune
microenvironment in HCC.
| Cell type | Marker | Role in HCC tumor
microenvironment | (Refs.) |
|---|
| TAMs | CD68; M2 markers
(CD163, CD206); M1 markers (CD80, CD86) | Often polarized to
immunosuppressive M2 phenotype; secrete IL-10, TGF-β and other
factors that suppress T cell activity. High TAM density is
associated with reduced CD8+ T cell infiltration and
poor prognosis | (8,19) |
| TANs | CD15, CD66b; high
PD-L1 and arginase-1 expression | Can inhibit CTL
function by releasing reactive oxygen species and expressing
immunosuppressive molecules, such as PD-L1. TANs blunt
CD8+ T cell cytotoxicity in HCC and are associated with
aggressive disease | (20) |
| cDCs | cDC1:
CLEC9A+ XCR1+;cDC2:
CD1c+CD11b+ | Primary
antigen-presenting cells that prime T cells. In HCC, cDCs are often
functionally impaired or present in insufficient numbers, limiting
effective anti-tumor T-cell priming | (16) |
| pDCs | BDCA-2, CD303 | Tend to accumulate
in HCC and promote tolerance. pDC infiltration is associated with
Treg expansion and poorer outcomes, suggesting pDCs contribute to
immunosuppressive networks in HCC | (8) |
| NK cells and
ILCs | NK:
CD56+CD16+ (cytotoxic NK); ILCs:
CD127+ (ILC1-3) | NK cells can
directly kill HCC cells, and are associated with improved survival,
but often functionally suppressed in HCC. Certain ILC subsets (ILC2
and ILC3) mimic Th2/Th17 functions and secrete IL-13, IL-17,
promoting tumor growth and suppressing immunity | (21) |
| CTLs | CD8, granzyme B,
IFN-γ | Essential effectors
for tumor clearance. Active, granzyme B-positive CD8 T cells
indicate a hot tumor and are associated with a favorable prognosis.
However, numerous HCCs exhibit low CD8 infiltration or
dysfunctional CTLs due to chronic antigen stimulation and
inhibitory checkpoint signaling | (22) |
| Exhausted T
cells |
PD-1+TIM-3+CTLA-4+
CD8 T cells | High levels of
exhausted T cells (expressing markers such as PD-1 and TIM-3) are
characteristic of HCC. They have impaired effector function and
their enrichment is associated with shorter progression-free and
overall survival | (19). |
| TRM cells | CD69, CD103 | TRM cells reside in
liver tissue and provide local immune surveillance. In HCC,
CD69+CD103+ TRM cells are associated with
improved response to ICIs and superior outcomes, suggesting a
pre-existing local immunity | (22) |
| Tregs |
CD4+FOXP3+, often
CTLA-4+ high | Abundant in HCC and
the surrounding liver. Tregs suppress anti-tumor T-cell responses
via IL-10, TGF-β and checkpoint molecules. Elevated intratumoral
Tregs are associated with CD8 dysfunction and poor survival | (23) |
| Th cells | Th1: IFN-γ, IL-2;
Th2: IL-4, IL-5; Th17: IL-17 | Th1 cells support
antitumor immunity by producing IFN-γ, whereas Th2 and Th17-skewed
responses can aid tumor progression. HCC with a dominant Th2/Th17
cytokine profile tends to have poor immune-mediated control | (24) |
| B cells | CD19, CD20 (plasma
cells: CD138) | Can form TLS in
HCC. TLS with B cells and follicular helper T cells are associated
with an enhanced immune response and improved prognosis. Some B
cells, however, may act as regulatory B cells producing IL-10 | (25) |
| CAFs | α-SMA, collagen I,
FAP | Create dense stroma
and physical barriers to immune cells. CAFs secrete TGF-β and other
factors that foster Treg differentiation and exclude T cells. They
directly interact with immune cells to mediate immune evasion and
are associated with poor immunotherapy responses | (26) |
| ECs |
VEGFR+CD31+
(VEGF-responsive vasculature) | Abnormal tumor
endothelium limits lymphocyte infiltration. VEGF from HCC drives
angiogenesis and suppresses DC and T-cell function. Anti-VEGF
therapy normalizes vessels and improves T-cell entry. ECs in HCC
can express immune checkpoint ligands such as PD-L1, contributing
to local T-cell suppression | (27) |
One hallmark of the HCC immune landscape is the
abundance of immunosuppressive and tumor-promoting immune cells
relative to effector cells (28).
Tumor-associated macrophages (TAMs), which are often skewed toward
an M2-like phenotype, are typically the most abundant immune
infiltrates in HCC and are associated with a poor prognosis
(29). TAMs promote tumor growth
by secreting immunosuppressive cytokines, such as IL-10 and
transforming growth factor-β (TGF-β), promoting angiogenesis and
directly inhibiting T cell activity (30).
Similarly, tumor-associated neutrophils in HCC can
suppress T cell cytotoxicity by expressing PD-L1 and arginase-1,
which contribute to the exhaustion of local T cells (20). Dendritic cells (DCs) are present in
the HCC microenvironment but are often functionally impaired:
Conventional DCs may be reduced in number or exhibit dysfunctional
antigen-presenting capacity, whereas plasmacytoid DCs tend to
induce immune tolerance and are associated with the accumulation of
regulatory T cells (Tregs) (8).
Crucially, cytotoxic T lymphocytes, the primary
effectors targeted by immunotherapies, are often excluded from HCC
tumor nests or rendered anergic or functionally exhausted (31). While a subset of HCCs exhibit an
‘immune-active’ phenotype characterized by substantial
CD8+ T-cell infiltration and IFN signaling, most HCC
tumors are categorized as ‘immune-exhausted’ or ‘immune-excluded’.
These are characterized by high expression of checkpoint receptors,
including PD-1 and T cell immunoglobulin and mucin-domain
containing protein 3 (TIM-3), on T cells, or by the physical
exclusion of T cells from the tumor parenchyma (10,32).
Cancer-associated fibroblasts (CAFs) and endothelial
cells in HCC further shape the immune landscape. CAFs produce
extracellular matrix proteins and desmoplastic stroma, which act as
physical barriers to immune cell infiltration (33,34).
They also secrete TGF-β and cytokines including IL-6, IL-8, IL-10,
CXCL12, prostaglandin E2 and vascular endothelial growth factor
(VEGF), which promote Treg differentiation and inhibit effector T
cell activity, thereby facilitating immune evasion (33,34).
The tumor endothelium in HCC is often abnormal, expressing
molecules such as PD-L1, Fas ligand and vascular cell adhesion
molecule-1, which can impair lymphocyte trafficking or induce
T-cell apoptosis, a phenomenon termed the endothelial checkpoint
(35,36). Elevated vascular endothelial growth
factor (VEGF) levels in HCC not only drive angiogenesis but also
directly suppress DC maturation and T-cell responses, contributing
further to immune exclusion (37).
Despite the generally immunosuppressive landscape,
HCC retains a degree of immunogenicity, as evidenced by occasional
spontaneous tumor regression and the observed efficacy of donor
lymphocyte infusions following liver transplantation (38). It is reported that 15–25% of HCC
tumors exhibit a more inflamed, hot phenotype, characterized by
higher CD8+ T-cell infiltration and IFN signaling. These
tumors are associated with an improved prognosis and greater
responsiveness to immunotherapy (5).
Mechanisms of immune ‘coldness’ in HCC
Multiple interrelated mechanisms contribute to the
immunologically cold phenotype of HCC tumors, acting at the tumor
cell-intrinsic level, within the broader liver microenvironment,
and at the systemic level.
Tumor-intrinsic immune evasion
pathways
Genetic and epigenetic alterations in HCC cells can
contribute to an immune-deserted microenvironment. Notably,
activation of the Wnt/β-catenin signaling pathway is observed in
30–50% of HCCs and is strongly associated with the exclusion of T
cells from the tumor (10,39). β-catenin activation in tumor cells
downregulates the expression of C-C motif chemokine ligand (CCL) 4,
which impairs DC recruitment and thereby prevents the effective
priming of anti-tumor T cells. This creates an immunologically cold
tumor niche. Clinically, HCC tumors with catenin β-1 gene mutations
that activate β-catenin rarely respond to ICIs, making this pathway
a recognized driver of immune resistance (40).
Other oncogenic pathways frequently altered in HCC
also contribute to immunosuppression; for example, MYC oncogene
overexpression, which is detected in ~50% of HCCs, upregulates
PD-L1 in tumor cells and modulates cell metabolism to favor an
immunosuppressive milieu (39).
Similarly, the loss-of-function of phosphatase and tensin homologs
or activation of PI3K-AKT signaling can promote an immunoresistant
environment by increasing the expression of inhibitory molecules
and recruiting suppressive myeloid cells (41).
HCC cells actively upregulate immune checkpoint
ligands and other immunoinhibitory molecules. HCCs frequently
express PD-L1 on their surfaces of their cells, especially those
with upregulated MYC expression or enriched in progenitor-like
traits. PD-L1 can directly bind to PD-1 on T cells, thereby
promoting T-cell exhaustion (16).
In addition, some HCC cells express galectin-9, an
immunosuppressive lectin that interacts with TIM-3 on T cells,
further contributing to T-cell exhaustion (42).
Immunosuppressive cellular
constituents
The immune infiltrate in HCC is skewed toward cell
types that sustain an immunosuppressive, cold TME (28). Tregs are often enriched in the
blood and tumor tissues of patients with HCC, where they suppress
antitumor immunity by secreting IL-10 and TGF-β, and by consuming
IL-2, which limits the proliferation of effector T cells (43,44).
TAMs of the M2 phenotype produce high levels of
IL-10 and prostaglandins, and express immune checkpoint molecules
such as PD-1, which suppress phagocytosis and innate immune
responses (45). These TAMs also
recruit Tregs via CCL22 and promote tissue remodeling, which
impairing or inactivating effector T cell functions.
Myeloid-derived suppressor cells (MDSCs), a heterogeneous
population of immature monocytes and neutrophils with
immunosuppressive activity, are also present in HCC. MDSCs release
arginase and inducible nitric oxide synthase, which metabolically
starve T cells of L-arginine and produce nitric oxide, thereby
causing T-cell dysfunction (44).
Cytokine and metabolic milieu
The TME of HCC is enriched with immunosuppressive
cytokines (40,46). TGF-β, often abundant due to
cirrhosis and CAF activity, is a key driver of immune exclusion. It
inhibits the proliferation and effector functions of T cells and
natural killer (NK) cells, while promoting the differentiation of
Tregs and M2 macrophages (46). A
high-TGF-β signature in HCC is associated with an immune-excluded
phenotype and resistance to immune checkpoint blockade (40).
HCC tumors are frequently hypoxic due to their rapid
growth and abnormal vasculature (47). Hypoxia induces hypoxia-inducible
factor-1α, which upregulates adenosine production and VEGF
expression, both of which suppress antitumor immunity. Adenosine
accumulates in the TME via CD39/CD73 ectonucleotidase activity in
cancer and stromal cells. It potently inhibits T- and NK-cell
activity through A2A adenosime receptors, while promoting the
expansion and functional activation of Tregs and the polarization
of macrophages to the M2 phenotype (48).
Liver-specific tolerogenic
factors
The unique immune environment of the liver plays a
central role in the immune evasion of HCC. Under normal conditions,
the liver is constantly exposed to food antigens and microbial
products from the gut via the portal circulation. Therefore,
non-immunogenic (tolerogenic) mechanisms have evolved in the liver
to prevent unnecessary immune activation. Kupffer cells, which are
liver-resident macrophages, and liver sinusoidal endothelial cells
(LSECs) constitutively express inhibitory molecules, including
PD-L1 and Fas ligand, and secrete anti-inflammatory cytokines such
as IL-10 and TGF-β, which induce T-cell tolerance (49–51).
Naïve T cells encountering antigens in the liver often become
anergic or differentiate into Tregs (52,53).
HCC exploits these tolerogenic pathways: Kupffer
cells may present tumor antigens in a tolerogenic manner, while
LSECs can induce the deletion of tumor-reactive T cells, thereby
preventing effective antitumor immune priming (54). In addition, cirrhotic livers
exhibit expanded populations of immunosuppressive myeloid and
stellate cells, contributing to an immunosuppressive fibrotic
microenvironment, even before malignant transformation (55).
Immune editing and antigen loss
During HCC development, immune editing can occur,
whereby the adaptive immune system initially eliminates highly
immunogenic tumor cell clones, leading to the selection of tumor
cells that are less detectable by T cells (56). Over time, HCC tumors may exhibit
the downregulation or loss of certain tumor-associated antigens.
For example, tumor variants that no longer express common antigens,
such as α-fetoprotein (AFP) or glypican-3 (GPC3), may evade T-cell-
or antibody-based therapies targeting these antigens (57). Similarly, the loss of major
histocompatibility complex (MHC) class I molecule expression due to
β2-microglobulin mutations or defects in the antigen presentation
machinery can impair cytotoxic T-cell recognition, although this
may potentially increase the susceptibility of the tumor to NK
cells (58,59).
Strategies to reprogram the immune
microenvironment in HCC
Given the array of mechanisms that render HCC an
immunologically cold tumor, various therapeutic strategies have
been developed to modulate the TME and warm it up, thereby
enhancing the efficacy of immunotherapies. These strategies involve
combining ICIs with other treatments, targeting specific
immunosuppressive pathways or cell populations, and introducing
proinflammatory stimuli into the tumor milieu. A summary of these
strategies is presented in Fig. 1.
In addition, a flowchart illustrating key combination therapies
used to convert an immunologically cold HCC tumor into a hot tumor
is shown in Fig. 2.
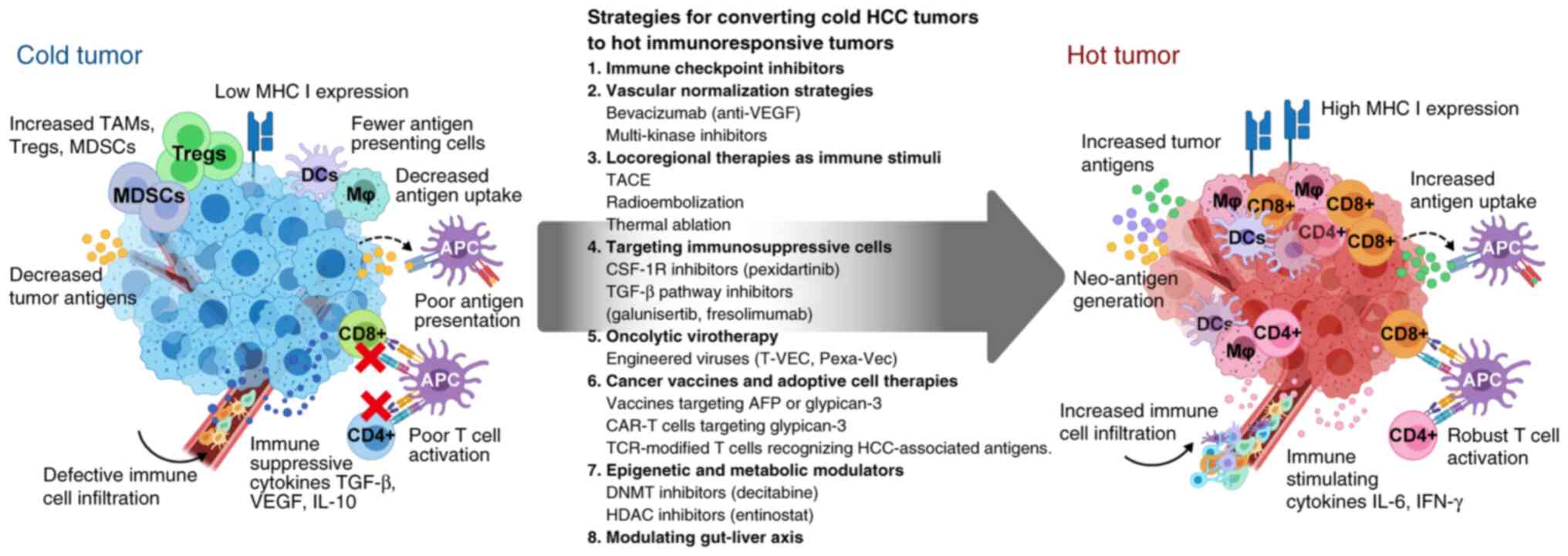 | Figure 1.Transformation of immunologically
cold HCC tumors into hot immunoresponsive tumors by various
therapeutic strategies. The left panel depicts a cold tumor
microenvironment characterized by low MHC I expression, low levels
of tumor antigens, defective immune cell infiltration and
immunosuppressive features, including abundant TAMs, Tregs and
MDSCs. These tumors show poor antigen presentation, decreased
antigen uptake by DCs, and ineffective T-cell activation due to
immunosuppressive cytokines TGF-β, VEGF and IL-10. The right panel
shows a successfully converted hot tumor with high MHC I
expression, increased tumor antigen levels and uptake, and robust
immune cell infiltration. This environment features activated
CD8+ and CD4+ T cells, functional APCs, and
immune-stimulating cytokines IL-6 and IFN-γ. The central arrow
outlines eight key strategic approaches for this conversion. Each
strategy includes specific therapeutic examples currently being
investigated for HCC treatment. AFP, α-fetoprotein; APC,
antigen-presenting cell; CAR-T, chimeric antigen receptor-T;
CSF-1R, colony stimulating factor-1 receptor; DC, dendritic cell;
DNMT, DNA methyltransferase; HCC, hepatocellular carcinoma; HDAC,
histone deacetylase; Mj, macrophage; MDSC, myeloid-derived
suppressor cell; MHC, major histocompatibility complex; Pexa-Vec,
pexastimogene devacirepvec; T-VEC, talimogene laherparepvec; TACE,
transarterial chemoembolization; TAMs, tumor-associated
macrophages; TCR, T cell receptor; TGF-β, transforming growth
factor-β; Tregs, regulatory T cells; VEGF, vascular endothelial
growth factor. |
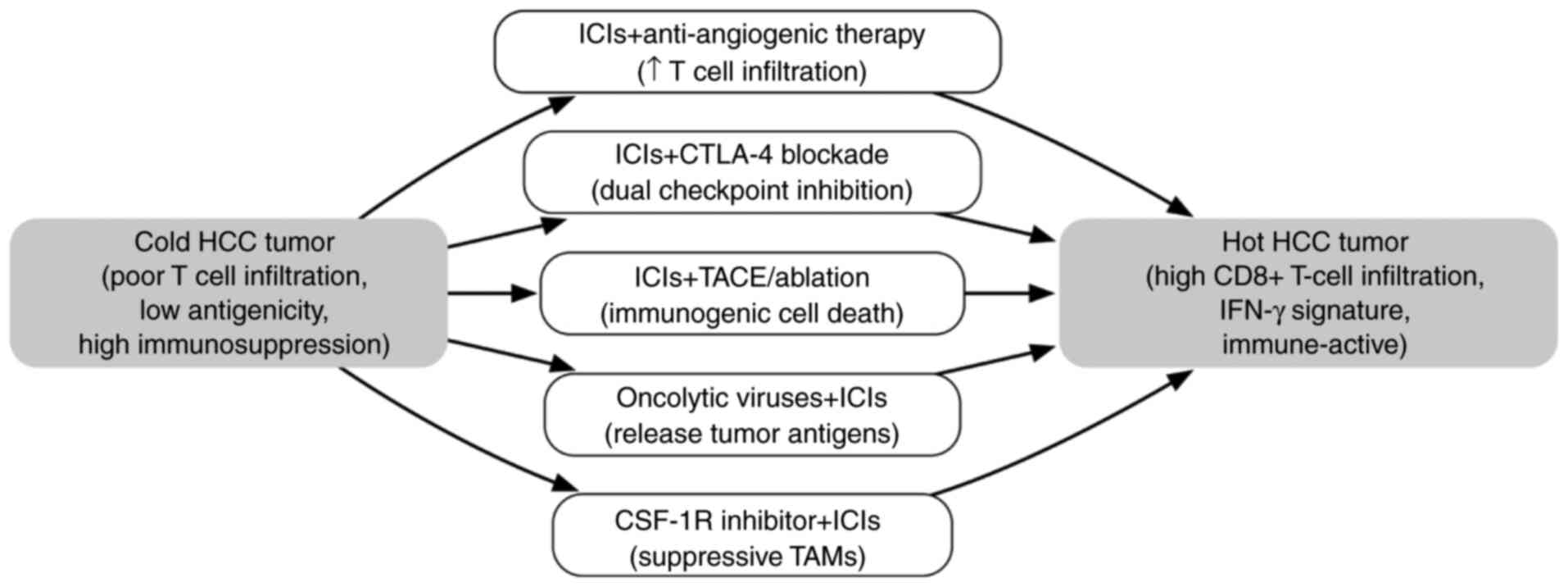 | Figure 2.Flowchart illustrating key
combination therapies to convert an immunologically cold HCC tumor
into a hot tumor. Cold tumors exhibit low T-cell infiltration and
strong immunosuppressive factors, whereas hot tumors have abundant
CD8+ T cells and an inflamed microenvironment. Each
arrow represents a combination strategy designed to overcome a
specific barrier to antitumor immunity. ICIs (e.g., PD-1/PD-L1
inhibitor) + anti-angiogenic therapy (e.g., bevacizumab): Targeting
angiogenesis normalizes VEGF-driven abnormal tumor vasculature and
reduces VEGF-mediated immunosuppression, thereby increasing T-cell
infiltration into the tumor. ICIs (e.g., PD-1/PD-L1 inhibitor) +
CTLA-4 inhibitor: Dual checkpoint blockade enhances T-cell priming
and reverses T-cell exhaustion, thereby reducing Treg-mediated
suppression. ICI + TACE or ablation: Locoregional treatments such
as TACE or ablation induce immunogenic cell death, the release of
neoantigens and antigen presentation, thereby promoting T-cell
priming and infiltration. Oncolytic viruses + ICIs: Viral oncolysis
triggers innate immune sensing, tumor antigen release and
inflammatory signals, which are sustained and amplified by ICIs.
CSF-1R inhibitor + ICIs: CSF-1R inhibitors deplete or reprogram
immunosuppressive tumor-associated macrophages, relieving
macrophage-induced T cell suppression. In combination with
anti-PD-1/PD-L1 therapy, this increases cytotoxic T cell activity.
CSF-1R, colony stimulating factor-1 receptor; CTLA-4, cytotoxic
T-lymphocyte-associated protein 4; HCC, hepatocellular carcinoma;
ICIs, immune checkpoint inhibitors; IFN-γ, interferon-γ; PD-1,
programmed cell death protein 1; PD-L1, programmed death-ligand 1;
TACE, transarterial chemoembolization; TAMs, tumor-associated
macrophages; Treg, regulatory T cell; VEGF, vascular endothelial
growth factor. |
ICIs: Foundation for combination
therapies
ICIs include anti-PD-1 antibodies, such as nivolumab
and pembrolizumab, anti-PD-L1 antibodies, such as atezolizumab and
durvalumab, and anti-CTLA-4 antibodies, such as ipilimumab and
tremelimumab. These agents form the foundation of immunotherapy
strategies in HCC, and are able to activate T-cell responses if
primed T cells are already present in the TME. However,
single-agent ICIs have shown limited overall response rates in HCC
(~15%), indicating that numerous tumors lack preexisting active T
cells (60,61). Therefore, ICIs are commonly
combined with other agents that can initiate or amplify antitumor
immunity. One such approach is dual checkpoint blockade, such as
PD-1 plus CTLA-4 inhibition, where CTLA-4 blockade primarily acts
on the lymphoid organs to expand T-cell clonal diversity and reduce
Treg-mediated suppression, whereas PD-1/PD-L1 blockade reactivates
exhausted T cells in the tumor (62).
In HCC, the combination of nivolumab and ipilimumab
has demonstrated manageable toxicity and a notably higher response
rate compared with nivolumab monotherapy, indicating that dual
blockage can convert a subset of immunologically cold tumors into
responsive ones (6). Similarly, a
combination of tremelimumab and durvalumab was evaluated in the
phase III HIMALAYA trial, using a single priming dose of
tremelimumab followed by ongoing durvalumab treatment. This regimen
induced an immunogenic boost in patients with unresectable HCC that
improved survival compared with that of patients treated with
sorafenib (63).
Anti-angiogenic and vascular
normalization strategies
Abnormal blood vessels in HCC contribute to immune
evasion by promoting hypoxia and serving as physical barriers to
immune cell trafficking. Anti-angiogenic therapies, including the
anti-VEGF antibody bevacizumab and multi-kinase inhibitors, such as
sorafenib, lenvatinib, cabozantinib and regorafenib, not only
inhibit tumor angiogenesis but also modulate the TME (37). VEGF blockade has immunomodulatory
effects; it can normalize tumor vasculature, thereby enhancing
lymphocyte infiltration and alleviating the VEGF-mediated
suppression of DCs and T cells (37).
The combination of PD-L1 inhibitor atezolizumab with
bevacizumab in the IMbrave150 phase III trial significantly
improved response as well as overall survival (OS) and
progression-free survival (PFS) in patients with unresectable HCC
compared with those of patients treated with sorafenib (60). This exemplifies how combining
immune checkpoint blockade with angiogenesis inhibition can convert
a cold tumor into a more immune-active one. However, the results of
the IMbrave050 trial, a follow-up study of adjuvant treatment with
atezolizumab plus bevacizumab after resection or ablation in
high-risk HCC showed that the benefit in recurrence-free survival
was not sustained, and the effect on OS remained non-significant
(64). The IMbrave150 trial
succeeded because advanced HCC presents abundant neoantigens and
ongoing immune activity that respond to PD-L1 and VEGF blockade,
whereas the adjuvant IMbrave050 setting involved minimal residual
disease and immune quiescence, limiting checkpoint and angiogenesis
inhibition efficacy in preventing recurrence. It is suggested that
bevacizumab enhances T-cell access to the tumor and reduces
VEGF-induced immunosuppression, thereby allowing
atezolizumab-activated T cells to function more effectively
(65).
Locoregional therapies as immune
stimuli
Traditional locoregional treatments for HCC,
including TACE, radioembolization with yttrium-90 and thermal
ablation, including radiofrequency or microwave ablation, can exert
immunological effects that may be exploited to ignite antitumor
immunity (66). These treatments
cause tumor cell death and promote the release of tumor antigens
and damage-associated molecular patterns into the environment, a
process known as immunogenic cell death (66).
TACE induces ischemic and chemotherapeutic stress in
tumor cells, potentially triggering a surge in neoantigen and
proinflammatory cytokine release that attracts immune cells
(67). Evidence suggests that TACE
or ablation can lead to transient increases in T-cell activation
and clonal expansion targeting tumor antigens (68). Radiation therapy upregulates MHC
class I expression on tumor cells and increases chemokine levels
for T-cell recruitment, while also inducing an abscopal effect,
whereby localized radiation leads to systemic antitumor immunity
(69).
Clinical trials have combined ICIs with locoregional
therapies. For example, tremelimumab combined with ablation has
been shown to enhance T-cell responses and induce objective tumor
regression in patients with advanced HCC (67). Ongoing trials, such as EMERALD-1
and −2, are evaluating the PD-L1 inhibitor durvalumab in
combination with TACE for patients with intermediate-stage HCC
(70,71). Selected clinical trials and
combination immunotherapy strategies for advanced HCC are
summarized in Table II (6,60,67,72–77).
 | Table II.Selected clinical trials and
combination immunotherapy strategies in advanced HCC. |
Table II.
Selected clinical trials and
combination immunotherapy strategies in advanced HCC.
| Therapy/trial | Regimen | Key outcomes | Implications | (Refs.) |
|---|
| IMbrave150, phase
III | Atezolizumab
(anti-PD-L1) + bevacizumab (anti-VEGF) vs. sorafenib in
unresectable HCC, 1st line | Improved median OS
(19.2 vs. 13.4 months), ORR (27 vs. 12%) and 1-year OS (~67 vs.
55%). Achieved first regulatory approval for an ICI combination in
HCC. | VEGF inhibition +
PD-L1 blockade synergistically converts HCCs to responsive tumors;
new standard of care for front-line therapy. However, updated
results in patients with more advanced disease showed that the
initial benefit in recurrence-free survival was not sustained, and
the change in OS remained non-significant | (60) |
| HIMALAYA, phase
III | Durvalumab
(anti-PD-L1) + tremelimumab (anti-CTLA-4). Single high-dose
tremelimumab + durvalumab maintenance vs. sorafenib, 1st line | Improved median OS
(16.4 vs. 13.8 months) with 3-year OS ~30% and ORR ~20%. Durvalumab
monotherapy was non-inferior to sorafenib. FDA-approved in Oct
2022 | Short CTLA-4
blockade pulse + PD-L1 inhibition can induce durable immunity;
offers an alternative 1st-line regimen, particularly for patients
unsuitable for bevacizumab | (72) |
| CheckMate-040,
phase II | Nivolumab +
ipilimumab in advanced HCC post-sorafenib; 3 dosing arms | Best arm (nivolumab
1 mg/kg + ipilimumab 3 mg/kg at 3 weekly intervals, 4 doses in
total) had an ORR of 32%, CR rate of 8% and median OS of 22 months.
Other arms with a lower ipilimumab dose had an ORR of 27%, and OS
of 12 months. Accelerated FDA approval (second-line setting) | Dual checkpoint
blockade yields high response rates and some cures, at cost of
increased immune toxicity. CTLA-4 dose intensity appears critical
for maximal efficacy | (6) |
| KEYNOTE-240, phase
III | Pembrolizumab
(anti-PD-1) vs. placebo in advanced HCC post-sorafenib, 2nd
line | Did not meet
P<0.0175, despite a trend of increased OS (13.9 vs. 10.6 months;
P=0.023) and improved PFS. ORR was 18 vs. 4% for placebo. Generally
well tolerated | Single-agent PD-1
blockade shows activity but under-powered survival benefit;
indicates requirement for combination in most patients. Led to
exploration of pembro-lizumab in combinations, e.g., with
lenvatinib | (60) |
| LEAP-002, phase
III | Pembrolizumab +
lenvatinib vs. lenvatinib alone, 1st line | Median OS increase
(21.2 vs. 19.0 months; HR, 0.84; P=0.0227), did not meet required
P<0.017. ORR (26.1 vs. 17.5%) and PFS (8.2 vs. 8.1 months; HR,
0.87). No new safety signals observed | No significant
improvement in OS, likely due to the efficacy of lenvatinib
monotherapy. However, higher response rate with the combination
suggests certain patients derived added benefit; highlights the
challenge of improving upon strong TKI performance | (73) |
| RESCUE, phase
III | Camrelizumab
(anti-PD-1) + apatinib (VEGFR2 TKI) vs. sorafenib, 1st line | Median OS (22.1 vs.
15.2 months; HR, ~0.62), ORR (25 vs. 5%) and PFS (5.6 vs. 3.7
months). Manageable toxicities, including hand-foot skin reaction
and hypertension. Approved in China | Reinforces class
effect of PD-1 + anti-angiogenic synergy in HCC. Achieved one of
the longest OS reported, albeit in a selected population | (74) |
| Locoregional + ICI,
phase Ib/II | Tremelimumab
(single dose) + computed tomography-guided tumor ablation (30%
tumor volume) in advanced HCC | ORR 26%
(unirradiated lesions) with disease control 89%. Post-treatment,
increased CD8+ T cells and PD-1+ T cells in
blood; 20% had >30% tumor necrosis in non-ablated lesions
(abscopal effect). | Feasible and
promising immunogenic synergy between ablation and CTLA-4 blockade,
priming T cells that attack distant tumors. Supports larger trials
of ICI + RFA/TACE | (67) |
| KEYNOTE-524, phase
Ib | Pembrolizumab +
lenvatinib in advanced unresectable HCC with no prior systemic
treatment | ORR 36% according
to modified RECIST, including 1 CR. Median PFS 9.7 months and OS 22
months. No additive toxicity beyond known profiles | Early sign that
PD-1 + multi-kinase inhibitor yields a high response rate,
justifying phase III (LEAP-002). PFS/OS were encouraging in the
single-arm setting | (75) |
| Oncolytic
virotherapy + ICI, phase Ib | Intratumoral T-VEC
(modified HSV-1 GM-CSF virus) + pembrolizumab in advanced liver
tumors, including HCC | Among patients with
HCC, responses were observed in injected and non-injected lesions.
Overall response rate ~23% across the cohort. Treatment well
tolerated, with no grade 4 or 5 events | Provides clinical
evidence that an oncolytic virus can inflame the TME and improve
responses to PD-1 blockade. Injection approach is viable in liver
tumors | (76) |
| TIL therapy,
pilot | Autologous TIL
infusion + IL-2 after partial hepatectomy, adjuvant setting | Feasibility
demonstrated: TILs were expanded from resected tumor and infused.
1-year recurrence rate appeared lower than the historical value
(~33 vs. 50%), but the sample was very small | Suggests TIL
therapy is feasible for HCC and may help eliminate microscopic
disease. Larger studies are necessary, and combining TILs with ICIs
may enhance the persistence of infused TILs | (77) |
Targeting immunosuppressive cell
populations
Another approach to convert cold tumors into more
immunogenic ones is to deplete or reprogram immunosuppressive cells
in the TME. Colony stimulating factor-1 receptor (CSF-1R)
inhibitors, such as pexidartinib, can reduce TAM numbers or alter
TAMs from an immunosuppressive M2-like phenotype toward a more
proinflammatory, antitumor M1-like phenotype (78). In HCC mouse models, the inhibition
of CSF-1/CSF-1R signaling, which is critical for
monocyte-to-macrophage differentiation and survival, decreases TAM
infiltration and promotes a shift toward a pro-inflammatory
environment, thereby sensitizing tumors to anti-PD-1 therapy
(79).
C-C motif chemokine receptor 2 (CCR2) antagonists
also show promise by preventing the recruitment of monocytes with
high expression of lymphocyte antigen 6 complex, locus C, a key
surface glycoprotein marker used to distinguish functional subsets
of monocytes, that are precursors for MDSCs and TAMs (80). The inhibition of CCL2-CCR2
signaling reduces the accumulation of intratumoral macrophages and
leads to T-cell-dependent tumor regression in preclinical HCC
models (81).
Tregs are more challenging to target selectively,
but low-dose cyclophosphamide or anti-CD25 antibodies have been
used in solid tumors such as ovarian, breast cancer, prostate
cancer, renal cell carcinoma, and melanoma to transiently deplete
Treg populations (82).
Finally, TGF-β pathway inhibitors are another class
of compounds used to remove a major suppressive influence.
Inhibitors such as galunisertib, a TGF-β receptor kinase inhibitor,
or fresolimumab, an anti-TGF-β antibody, have been investigated in
patients with HCC (83,84). Although these agents have not yet
been tested in phase III trials in combination with ICIs,
preclinical analysis suggests that blocking TGF-β signaling
enhances the penetration of T cells into tumors and is a promising
mechanism for use in combinations that aim to convert cold tumors
into hot ones (85).
Oncolytic virotherapy
Oncolytic viruses (OVs) are engineered or naturally
occurring viruses that selectively replicate in and lyse cancer
cells while stimulating an antitumor immune response (86). This lytic process releases tumor
antigens and viral pathogen signals, effectively transforming the
tumor into a vaccine depot. Several OVs have been evaluated for the
treatment of HCC.
Talimogene laherparepvec (T-VEC), a modified herpes
simplex virus type-1 that encodes granulocyte-macrophage (GM)-CSF,
has been approved for melanoma treatment and tested via
intratumoral injection in liver tumors (87). Early trials combining T-VEC with
ICIs have shown increased local immune cell infiltration in HCC and
demonstrated the feasibility of generating a localized autitumor
immune response (88).
Pexastimogene devacirepvec (Pexa-Vec; JX-594), an
oncolytic vaccinia virus expressing GM-CSF, has also been evaluated
in HCC. A phase II trial showed signs of improved survival in a
subset of patients (89), In
addition, a phase III trial (PHOCUS), evaluating Pexa-Vec combined
with sorafenib, was conducted in advanced HCC (86). The PHOCUS phase III trial showed
that adding Pexa-Vec to sorafenib does not improve overall survival
in advanced HCC, leading to early study termination, though
Pexa-Vec was well tolerated with manageable safety (90).
Cancer vaccines and adoptive cell
therapies
Active immunization strategies aim to stimulate the
immune system to recognize HCC-specific antigens, thereby enhancing
T-cell priming against the tumor. Although past vaccine trials in
HCC targeting antigens such as AFP (91), GPC3 (92) or multi-peptide mixtures (93) have shown only modest success, they
all have demonstrated immunogenicity, indicating that T cells
targeting these antigens can be expanded in patients. A summary of
emerging immunotherapeutic approaches for HCC is provided in
Table III (21,23,35,67,79,89,94–101). Certain approaches-particularly
cancer vaccines, oncolytic viruses, and certain adoptive cell
therapies-have shown evidence of inducing tumor-specific immune
responses in clinical or preclinical studies, whereas others
(metabolic, microbiome, and TAM-targeting strategies) are still at
the investigational or early-trial stage and remain to be validated
for immunogenic effects.
 | Table III.Emerging immunotherapeutic approaches
in HCC. |
Table III.
Emerging immunotherapeutic approaches
in HCC.
| Strategy | Description | Evidence | Status | (Refs.) |
|---|
| TAM targeting | CSF-1R inhibitors,
including pexidartinib and cabiralizumab | CSF-1R blockade
decreases M2 TAMs and synergized with PD-L1 inhibition, increasing
T cell activity | Phase I trials
ongoing with CSF-1R mAb + nivolumab; biomarkers required for
TAM-high tumors | (79) |
| Treg targeting | CTLA-4 inhibitors;
anti-CD25 antibodies | Tremelimumab
reduced Tregs and increased effector T cells; improved CD8:Treg
ratios are associated with tumor control | CTLA-4 inhibitors
are included in approved combinations; cyclophosphamideanti-CD25
trials underway; however, autoimmunity is a risk factor | (23,67) |
| Neutrophil/MDSC
targeting | CXCR2 inhibitors;
ARG1 inhibitors; IL-8 blockers | CXCR2 antagonist +
PD-1 blockade reduced TANs, and improved the T-cell response; high
neutrophils predict poor ICI outcomes | CXCR2 inhibitor +
durvalumab/bevacizumab trial ongoing; IL-8 antibodies in ongoing
clinical trials | (60,94) |
| OVs | JX-594
(vaccinia-GM-CSF); T-VEC (HSV-GM-CSF); AFP-targeting HDV | JX-594 increased
immune infiltration in tumors. OVs upregulate IFN genes and enhance
antigen presentation | Pexastimogene
devacirepvec + sorafenib, phase III, negative; OV + ICI
combinations in phase I/II; T-VEC + pembrolizumab, ~20%
response | (89,101) |
| CAR T/NK cells | Engineered cells
targeting GPC3, AFP and EpCAM admini- stered intravenously or via
the hepatic artery | GPC3-CAR T induced
tumor necrosis, IFN-γ secretion; PD-1 knockout CAR-Ts overcame
exhaustion | Early trials,
GPC3-CAR T/ NK and AFP-TCR T cells; next-generation, IL-15
secretion and TGF-β resistance | (35,100) |
| Cancer
vaccines | Peptide/neoantigen
vaccines; DC vaccines; oncolytic vaccines | DC vaccine improved
recurrence-free survival post-surgery; neoantigen vaccines induced
tumor- infiltrating T cells | OVAX + nivolumab,
GPC3 peptide + ICI trials ongoing; optimum effect for minimal
disease or with checkpoints | (92,99) |
| Epigenetic
modulators + ICIs | DNMT inhibitors;
HDAC inhibitors + ICIs | DNMT inhibition
increased MHC-I and antigen presentation; HDAC blockade
reprogrammed macrophages and enhanced ICI efficacy | Durvalumab +
tremelimumab + guadecitabine and nivolumab + entinostat trials
ongoing to ‘unmask’ tumors | (98) |
| Metabolic
modulation | A2A antagonists;
IDO inhibitors; lactate modulators | A2A blockade
restored T cell function in hypoxia; IDO inhibition + PD-1 blockade
showed synergy in preclinical models | A2A antagonist
trial (CPI-444) in phase I; metformin showing promise in
NASH-HCC | (21,97) |
| Microbiome
modulation | Probiotics; FMT;
antibiotic management | Akkermansia
and Bifidobacterium are associated with improved outcomes;
FMT from responders improved response in resistant models | Early FMT trials
promising; HCC-specific probiotic interventions in development | (95,96) |
Combining cancer vaccines with ICIs or other TME
modulators is a rational strategy. For example, DC vaccines pulsed
with HCC tumor antigens can generate a wave of tumor-specific T
cells, and concurrent PD-1 blockade can prevent their exhaustion,
allowing them to penetrate and exert cytotoxic activity within
tumors (102).
Adoptive cell therapy supplies effector cells
directly to patients. In HCC, efforts have focused on engineering T
cells to target tumor antigens. Chimeric antigen receptor (CAR) T
cells specific for GPC3, an oncofetal antigen expressed in HCC
cells, have been tested in early-phase trials (102). A phase I study of CAR-GPC3 T
cells in advanced HCC showed some partial responses; however, it
also revealed that the immunosuppressive TME limited CAR-T
persistence and function (100).
A preclinical study used murine and xenograft HCC
models to show that CAR-T cells engineered to secrete IL-12 upon
antigen engagement could remodel the TME and enhance systemic
antitumor activity (103).
Researchers isolated and characterized high-affinity TCRs specific
for AFP peptides presented by HLA-A*02:01. These engineered TCR-T
cells recognize AFP-expressing HCC cells and exhibited potent,
antigen-specific cytotoxicity in vitro and in mouse
xenograft models. Follow-up studies using the same AFP TCR
construct led to a first-in-human phase I trial (NCT03971747) in
HCC patients (104). These TCR-T
cells can recognize intracellular antigens presented on MHC
molecules, potentially broadening targetable proteins beyond
surface markers.
Epigenetic and metabolic
modulators
An emerging strategy to enhance antitumor immunity
involves modulating epigenetic or metabolic pathways to render
tumor and immune cells more immunogenic. Epigenetic drugs,
including DNA methyltransferase inhibitors such as decitabine or
histone deacetylase (HDAC) inhibitors such as entinostat, can
restore the expression of silenced tumor-associated antigens and
increase MHC molecule presentation on cancer cells, thereby
enhancing their recognition by T cells (105).
In addition to their effects on tumor cells,
epigenetic modulators can also alter the differentiation of immune
cells. For example, HDAC inhibition has been shown to promote a
pro-inflammatory macrophage phenotype and reduce the accumulation
of MDSCs, effectively boosting immune responses (106). Furthermore, in HCC models,
combining HDAC inhibitors with PD-1 blockade increased intratumoral
T-cell infiltration and led to tumor regression (106).
Modulating the gut-liver axis
The gut microbiome has a major influence on systemic
immunity and has been shown to affect the response to ICIs in
melanoma, non-small cell lung cancer and renal cell carcinoma.
Therefore, researchers are interested in modulating the gut
microbiota to shift the TME in HCC to a hotter immune state
(95). Certain bacterial
metabolites in the gut can enhance antitumor immunity. Fecal
microbiota transplantation from ICI-responsive patients has been
shown to improve therapeutic responses in melanoma and may hold
promise for application in HCC (95).
Clinical and preclinical evidence
ICI combinations in advanced HCC
The first wave of phase III trials of single-agent
ICIs for advanced HCC has yielded mixed results. In the
CheckMate-459 trial, nivolumab showed durable responses in a subset
of patients when used as a first-line treatment compared with
sorafenib, but it did not significantly improve OS in the primary
analysis (107). Similarly, in
the second-line setting of the KEYNOTE-240 trial, pembrolizumab
yielded a modest improvement in survival compared with placebo in
the second-line setting, however this was not significant (108).
Atezolizumab + bevacizumab (anti-PD-L1
+ anti-VEGF)
The IMbrave150 trial demonstrated that combining
atezolizumab with bevacizumab significantly improved OS and the
objective response rate (ORR) in treatment-naïve patients with
advanced HCC. The median OS was 19.2 months in the combination arm
compared with 13.4 months in patients treated with sorafenib, while
the ORR was ~ 27% compared with ~12%, respectively (60). Numerous responders showed
substantial tumor shrinkage, and some achieved durable remission
(60).
Durvalumab + tremelimumab (anti-PD-L1
+ anti-CTLA-4)
In the phase III HIMALAYA trial, a single high dose
of tremelimumab (300 mg) combined with chronic dosing of durvalumab
achieved a superior OS compared with that of sorafenib in
front-line advanced HCC (63).
This Single Tremelimumab Regular Interval Durvalumab (STRIDE)
regimen capitalized on an initial wave of broad T-cell activation
from CTLA-4 blockade, followed by sustained PD-L1 blockade. The
median OS was 16.4 months compared with 13.8 months in patients
treated with sorafenib. A subset of patients treated with the
STRIDE regimen achieved long-term survival >2 years (63).
Nivolumab + ipilimumab (anti-PD-1 +
anti-CTLA-4)
In the CheckMate-040 cohort 4 phase II study, three
different regimens combining nivolumab with ipilimumab were
evaluated in patients who had progressed on sorafenib. An ORR of
32% and median OS of 22 months was obtained in the arm with higher
ipilimumab dosage (6).
Approximately 8% of patients achieved a CR (6).
Locoregional and multimodal therapy
evidence
A pilot trial combining the CTLA-4 inhibitor
tremelimumab with subtotal radiofrequency ablation in patients with
advanced HCC whose tumors were suitable for partial ablation
reported that 26% of patients had a partial response in non-ablated
lesions, and disease control was achieved in 89% of patients
(67). Notably, an increase in
intratumoral CD8+ T cells and a reduction in viral load
in hepatitis B virus (HBV)-infected patients were observed after
treatment, indicating a systemic immune effect (67). These findings support the concept
that local tumor destruction can synergize with checkpoint
blockade.
Retrospective analyses also suggest that patients
receiving radiotherapy or TACE shortly before or during nivolumab
therapy have improved response rates compared with those receiving
nivolumab alone (109). For
example, one study found an abscopal response in ~20% of patients
who received radiotherapy for a single lesion while receiving
nivolumab treatment compared with <10% in those treated with
nivolumab alone (110).
Adoptive cell therapy and
vaccines
CAR T cells
A phase I trial of CAR T cells targeting GPC3 in
patients with advanced HCC reported a partial response in 2 of 13
patients, with disease stabilization observed in seven patients
(100). Although the clinical
response was modest, tumor biopsies following CAR T infusion
demonstrated the accumulation of CAR T cells at tumor sites, and
some patients exhibited substantial tumor necrosis. These findings
indicate that CAR T cells are able to traffic to and attack HCC
lesions (111). However, the
expansion and persistence of CAR T cells in vivo is limited,
possibly due to immunosuppressive checkpoints and the dense
TME.
TCR-engineered T cells
A first-in-human study of T cells engineered with a
TCR against an AFP peptide presented by HLA-A2 in patients with
AFP-positive HCC showed that the treatment was safe and resulted in
transient reductions in serum AFP levels and tumor shrinkage in
certain patients (112). Although
no objective responses were observed according to RECIST criteria,
one patient exhibited a measurable decrease in tumor burden that
does not meet the threshold for a partial response (PR), and others
achieved stable disease (112).
DC vaccines
A randomized phase II trial of patients with
early-stage HCC compared a tumor lysate-activated autologous DC
vaccine with a control group of patients treated with best
supportive care following curative resection or ablation (113). The vaccinated group experienced a
significantly longer median time to recurrence than the control
group (36 vs. 25 months, respectively) and a higher recurrence-free
survival at 1 year (75.6 vs. 61.1%, respectively) (113).
Preclinical insights
Several mouse model studies have directly
demonstrated the benefits of reprogramming the HCC
microenvironment:
NASH-HCC model
A study of mice with diet-induced NASH revealed that
PD-1 blockade did not protect against liver cancer and instead
promoted the incidence and progression of HCC due to activated, but
dysfunctional, CD8+ T cells producing TNF-α. However,
when those CD8+ T cells were depleted or TNF-α was
neutralized, checkpoint blockade efficacy improved (15).
Osteopontin (OPN)/CSF-1R axis
In a chemically-induced HCC mouse model, it was
revealed that tumor-derived OPN recruits TAMs through the CSF-1
pathway. The blockade of CSF-1R not only reduced TAM infiltration
but also led to increased CD8+ T-cell activity and
responsiveness to anti-PD-L1 therapy (79).
Fibroblast growth factor receptor 4
(FGFR4) and Tregs
A study of lenvatinib demonstrated that the
inhibition of FGFR4, a protein that is overexpressed in some HCC
tissues, not only affected tumor cell growth but also reduced
intratumoral Treg accumulation in mouse xenografts and PD-L1
expression in tumor cells. This dual effect was associated with
improved anti-PD-1 antibody efficacy and tumor rejection in mouse
models (114).
CAR T cells with checkpoint
knockout
In an orthotopic HCC model in mice, PD-1
gene-deleted CAR T cells targeting GPC3 outperformed conventional
CAR T cells, as they were more persistent and infiltrated the
tumors more deeply. In addition, these modified CAR T cells
resulted in complete tumor eradication in a higher proportion of
the mice (35).
Other therapeutical targets
Preclinical findings have revealed new targets,
including CSF-1R (78), CXCR2
(115) and adenosine (116), and confirmed mechanisms such as
Wnt-mediated exclusion, that can be therapeutically targeted
(116).
Future directions
The field of HCC immunotherapy is rapidly evolving,
and the paradigm of converting cold tumors into hot ones will
continue to guide future research and clinical practice. Several
key directions are anticipated.
Personalized immunotherapy and
biomarker-driven treatment
As our understanding of HCC immune biology deepens,
there is growing recognition that not all patients should receive
the same immunotherapy regimen. Future treatment algorithms will
likely incorporate biomarkers to stratify patients. For example,
tumors with Wnt/β-catenin mutations or an immune-excluded phenotype
may be prioritized for trials evaluating Wnt pathway inhibitors or
other T-cell-recruiting strategies (39).
A robust immune score for HCC could be developed,
analogous to the Immunoscore used in colorectal cancer (117). This could help to determine the
required intensity of immunotherapy. Liquid biopsies, including
circulating tumor DNA and immune cell profiling assays, may enable
the early assessment of whether a tumor is becoming hotter or
remains immunologically cold, allowing for adaptive treatment
adjustments.
Next-generation checkpoint
targets
While PD-1/PD-L1 and CTLA-4 are the current targets,
several other inhibitory pathways are being explored in clinical
trials and could be integrated into HCC treatment (5). Antibodies targeting
lymphocyte-activation gene 3 (LAG-3), known as relatlimab, were
recently approved by the US Food and Drug Administration for the
treatment of melanoma and are of interest in HCC, as LAG-3 is
upregulated in exhausted T cells in the liver (118).
T-cell immunoreceptor with Ig and ITIM domains
(TIGIT) is another checkpoint protein expressed on T and NK cells;
anti-TIGIT antibodies, such as tiragolumab, combined with anti-PD-1
therapy are in trials for HCC following promising results in other
cancer types (119). These
emerging checkpoint inhibitors may help to revive exhausted T cells
in HCC when PD-1 blockade alone is inadequate.
Triplet therapies and beyond
Building on the success of doublet regimens, such as
atezolizumab + bevacizumab and durvalumab + tremelimumab, the next
wave of studies is testing triplet combinations to further convert
cold tumors into a hot state. For example, the COSMIC-312 trial has
evaluated a triplet of PD-1 + CTLA-4 + tyrosine kinase inhibitors,
namely nivolumab + ipilimumab + cabozantinib, to simultaneously
target T cells, Tregs and tumor angiogenesis (120). The COSMIC-312 phase III trial
reported that cabozantinib + atezolizumab significantly improved
progression-free survival vs. sorafenib (6.8 vs 4.2 months; HR
0.63) but did not improve overall survival (121). However, the nivolumab +
ipilimumab + cabozantinib triplet remains under investigation, with
no published efficacy results yet for this regimen.
However, as toxicity and cost increase with each
added agent, future research must identify which patient subsets
truly require multilayered therapy. Sequential approaches, such as
an induction phase followed by a maintenance phase, rather than
concurrent triple therapy, may also mitigate toxicity.
Immunotherapy in earlier-stage
HCC
Although most immunotherapy trials have focused on
advanced HCC, there is a strong rationale for applying these
strategies in earlier stages of the disease, when immune function
is less impaired and the tumor burden is lower. Neoadjuvant
immunotherapy prior to surgery is being investigated, and small
studies administering ICIs or combinations prior to resection have
shown that some patients achieve significant tumor necrosis and
robust pathologic responses, which might translate to a lower
recurrence risk (40).
In the adjuvant setting, after curative resection or
ablation, the goal is to eradicate residual disease and prevent
recurrence. The CheckMate-9DX phase III trial of adjuvant nivolumab
did not meet the primary endpoint of recurrence-free survival
(122), possibly due to trial
design considerations or insufficient treatment duration. However,
other adjuvant trials are ongoing, including the KEYNOTE-937 trial
of pembrolizumab and the EMERALD-2 trial of durvalumab ±
bevacizumab (123,124).
Addressing etiology-specific immune
contexts
As already noted, NASH-related HCC has a suppressive
immune milieu that may require tailored therapeutic strategies
(15). One future approach is to
treat underlying liver conditions in parallel with the cancer
itself. In NASH, this could involve therapies that reduce hepatic
inflammation, such as farnesoid X receptor agonists, acetyl-CoA
carboxylase inhibitors or anti-IL-1β agents, administered in
conjunction with immunotherapy, to prevent the non-productive
activation of T cells. For HBV-associated HCC, continuing antiviral
therapy is crucial, and additional strategies, such as therapeutic
HBV vaccines or TCR-based approaches targeting HBV antigens, may
boost antitumor immunity, given that some HCC tumor cells express
HBV-derived proteins.
Novel delivery systems
Accessibility of the liver via the hepatic artery
allows for innovative strategies for the delivery of
immunotherapies directly to the tumor or liver, thereby maximizing
local immune activation effects while minimizing systemic toxicity.
Ongoing trials are currently examining the locoregional delivery of
IL-12 plasmids (125), toll-like
receptor 9 agonists (126), and
CAR T cells through hepatic artery infusion (127). Nanotechnology-based approaches
may enable nanoparticles carrying immune stimulants, such as
stimulators of IFN gene agonists, or small interfering RNAs
targeting immune checkpoints, to be delivered selectively to liver
tumors (96). Such approaches can
convert the tumor site into an immune-reactive zone without
exposing the whole body to high cytokine or drug levels, thereby
potentially reducing systemic side effects.
Monitoring and managing immune-related
toxicity
As immunotherapy strategies are intensified to
convert cold tumors into hot ones, the risk of collateral damage to
normal liver tissue increases. Future protocols will require robust
monitoring procedures for immune-related adverse events and
potentially prophylactic measures. For example, patients with
cirrhosis are at risk of decompensation if immunotherapy triggers
autoimmune hepatitis (128,129). Gut microbiome manipulation is
being considered as a means of improving efficacy and also
mitigating toxicity, since certain microbiome profiles are
associated with the risk of colitis (130,131). Researchers are also exploring
selective immunosuppressants capable of controlling toxicity
without completely compromising antitumor immunity (132,133).
Integration of artificial intelligence
and systems biology
As data from genomic, transcriptomic, imaging and
clinical sources accumulate, artificial intelligence and machine
learning will likely play a role in the identification of features
that define cold and hot tumors and in guiding treatment selection.
For example, deep learning applied to histological images can
quantify immune infiltrates and their spatial distribution, thereby
potentially predicting outcomes (115). Multiomics analysis may identify
new therapeutic targets, for example, by identifying that a certain
chemokine is the key factor limiting T-cell infiltration in a
subset of patients, which could be targeted by drugs. Systems
biology approaches may be used to model complex interactions within
the HCC immune ecosystem, simulate the performance of a given
combination and guide the rational design of treatment
regimens.
Conclusions
HCC has long been considered an immunologically
cold tumor, posing a formidable challenge for immunotherapy. In the
present review, numerous factors, including immunosuppressive cell
populations, inhibitory cytokines, tumor-intrinsic pathways and
liver-induced tolerance are described that are able to blunt
antitumor immunity in HCC. Crucially, it is emphasized that these
barriers are not insurmountable. Through various strategies,
researchers and clinicians have demonstrated that the active
transformation of cold HCC tumors into hot, immune-reactive tumors
is possible.
Combination therapies combining ICIs with agents
such as anti-angiogenic drugs or CTLA-4 blockers have already
yielded significantly improved clinical outcomes, validating the
principle that immune silencing in HCC can be reversed. Emerging
modalities, including locoregional therapy combined with
immunotherapy, OVs, CAR T/NK-cell approaches, and metabolic or
epigenetic modulators, have further expanded the therapeutic
options for reprogramming the TME.
As the immune landscape of HCC is progressively
understood and manipulated, it is anticipated that what was once
considered a non-immunogenic cancer may become routinely
manageable, and potentially even curable, by harnessing the immune
system of the patient. The journey from cold to hot HCC (Fig. 1) exemplifies a paradigm shift in
oncology: Rather than treating the tumor alone, the TME and the
host immune response are also targeted, creating a situation in
which the immune system contributes to HCC eradication.
Acknowledgements
Not applicable.
Funding
This study was funded by the Chang Gung Memorial Hospital (grant
nos. CMRPG8Q0181 and CORPG8N0241).
Availability of data and materials
Not applicable.
Authors' contributions
CHH was responsible for study conceptualization and
writing the original draft of the manuscript. PCC contributed to
study methodology, and the review and editing of the manuscript.
Data authentication is not applicable. Both authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AFP
|
α-fetoprotein
|
|
CAF
|
cancer-associated fibroblast
|
|
CAR
|
chimeric antigen receptor
|
|
CCL
|
C-C motif chemokine ligand
|
|
CCR
|
C-C motif chemokine receptor
|
|
CR
|
complete response
|
|
CSF
|
colony stimulating factor
|
|
CTL
|
cytotoxic T lymphocyte
|
|
CTLA-4
|
cytotoxic T-lymphocyte-associated
protein 4
|
|
DC
|
dendritic cell
|
|
FGFR
|
fibroblast growth factor receptor
|
|
GM-CSF
|
granulocyte-macrophage
colony-stimulating factor
|
|
GPC3
|
glypican-3
|
|
HBV
|
hepatitis B virus
|
|
HCC
|
hepatocellular carcinoma
|
|
HDAC
|
histone deacetylase
|
|
HLA
|
human leukocyte antigen
|
|
ICI
|
immune checkpoint inhibitor
|
|
IFN-γ
|
interferon-γ
|
|
IL
|
interleukin
|
|
LAG-3
|
lymphocyte-activation gene 3
|
|
LSEC
|
liver sinusoidal endothelial cell
|
|
MDSC
|
myeloid-derived suppressor cell
|
|
MHC
|
major histocompatibility complex
|
|
NASH
|
non-alcoholic steatohepatitis
|
|
NK
|
natural killer
|
|
OPN
|
osteopontin
|
|
ORR
|
objective response rate
|
|
OS
|
overall survival
|
|
OV
|
oncolytic virus
|
|
PD-1
|
programmed cell death protein 1
|
|
PD-L1
|
programmed death-ligand 1
|
|
T-VEC
|
talimogene laherparepvec
|
|
TACE
|
transarterial chemoembolization
|
|
TAM
|
tumor-associated macrophage
|
|
TCR
|
T cell receptor
|
|
TGF-β
|
transforming growth factor-β
|
|
TIGIT
|
T cell immunoreceptor with Ig and
ITIM domains
|
|
TIM-3
|
T cell immunoglobulin and
mucin-domain containing protein 3
|
|
TME
|
tumor microenvironment
|
|
Treg
|
regulator T cell
|
|
VEGF
|
vascular endothelial growth
factor
|
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI
|
|
2
|
Villanueva A: Hepatocellular carcinoma. N
Engl J Med. 380:1450–1462. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ker CG: Hepatobiliary surgery in Taiwan:
The past, present, and future. Part I; biliary surgery. Formos J
Surg. 57:1–10. 2024. View Article : Google Scholar
|
|
4
|
Sangro B, Gomez-Martin C, de la Mata M,
Iñarrairaegui M, Garralda E, Barrera P, Riezu-Boj JI, Larrea E,
Alfaro C, Sarobe P, et al: A clinical trial of CTLA-4 blockade with
tremelimumab in patients with hepatocellular carcinoma and chronic
hepatitis C. J Hepatol. 59:81–88. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Llovet JM, Castet F, Heikenwalder M, Maini
MK, Mazzaferro V, Pinato DJ, Pikarsky E, Zhu AX and Finn RS:
Immunotherapies for hepatocellular carcinoma. Nat Rev Clin Oncol.
19:151–172. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yau T, Kang YK, Kim TY, El-Khoueiry AB,
Santoro A, Sangro B, Melero I, Kudo M, Hou MM, Matilla A, et al:
Efficacy and safety of nivolumab plus ipilimumab in patients with
advanced hepatocellular carcinoma previously treated with
sorafenib: The checkmate 040 randomized clinical trial. JAMA Oncol.
6:e2045642020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Childs A, Aidoo-Micah G, Maini MK and
Meyer T: Immunotherapy for hepatocellular carcinoma. JHEP Rep.
6:1011302024. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ho DW, Tsui YM, Chan LK, Sze KM, Zhang X,
Cheu JW, Chiu YT, Lee JM, Chan AC, Cheung ET, et al: Single-cell
RNA sequencing shows the immunosuppressive landscape and tumor
heterogeneity of HBV-associated hepatocellular carcinoma. Nat
Commun. 12:36842021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Galon J and Bruni D: Approaches to treat
immune hot, altered and cold tumours with combination
immunotherapies. Nat Rev Drug Discov. 18:197–218. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pinyol R, Sia D and Llovet JM: Immune
exclusion-Wnt/CTNNB1 class predicts resistance to immunotherapies
in HCC. Clin Cancer Res. 25:2021–2023. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Giraud J, Chalopin D, Blanc JF and Saleh
M: Hepatocellular carcinoma immune landscape and the potential of
immunotherapies. Front Immunol. 12:6556972021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
De Marchi P, Leal LF, da Silva LS, Cavagna
RO, da Silva FAF, da Silva VD, da Silva EC, Saito AO, de Lima VCC
and Reis RM: Gene expression profiles (GEPs) of immuno-oncologic
pathways as predictors of response to checkpoint inhibitors in
advanced NSCLC. Transl Oncol. 39:1018182024. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hernandez S, Lazcano R, Serrano A, Powell
S, Kostousov L, Mehta J, Khan K, Lu W and Solis LM: Challenges and
opportunities for immunoprofiling using a spatial high-plex
technology: The NanoString GeoMx(®) digital spatial
profiler. Front Oncol. 12:8904102022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bruni D, Angell HK and Galon J: The immune
contexture and Immunoscore in cancer prognosis and therapeutic
efficacy. Nat Rev Cancer. 20:662–680. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pfister D, Núñez NG, Pinyol R, Govaere O,
Pinter M, Szydlowska M, Gupta R, Qiu M, Deczkowska A, Weiner A, et
al: NASH limits anti-tumour surveillance in immunotherapy-treated
HCC. Nature. 592:450–456. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang Q, He Y, Luo N, Patel SJ, Han Y, Gao
R, Modak M, Carotta S, Haslinger C, Kind D, et al: Landscape and
dynamics of single immune cells in hepatocellular carcinoma. Cell.
179:829–845.e20. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yi Q, Yang J, Wu Y, Wang Y, Cao Q and Wen
W: Immune microenvironment changes of liver cirrhosis: Emerging
role of mesenchymal stromal cells. Front Immunol. 14:12045242023.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Montella L, Sarno F, Ambrosino A, Facchini
S, D'antò M, Laterza M, Fasano M, Quarata E, Ranucci RAN, Altucci
L, et al: The role of immunotherapy in a tolerogenic environment:
Current and future perspectives for hepatocellular carcinoma.
Cells. 10:19092021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shu QH, Ge Y, Hua X, Gao XQ, Pan JJ, Liu
DB, Xu GL, Ma JL and Jia WD: Prognostic value of polarized
macrophages in patients with hepatocellular carcinoma after
curative resection. J Cell Mol Med. 20:1024–1035. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xue R, Zhang Q, Cao Q, Kong R, Xiang X,
Liu H, Feng M, Wang F, Cheng J, Li Z, et al: Liver tumour immune
microenvironment subtypes and neutrophil heterogeneity. Nature.
612:141–147. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ghaedi M and Ohashi P: ILC
transdifferentiation: Roles in cancer progression. Cell Res.
30:562–563. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim HD, Song G, Park S, Jung M, Kim MH,
Kang H, Yoo C, Yi K, Kim KH, Eo S, et al: Association between
expression level of PD1 by tumor-infiltrating CD8+ T cells and
features of hepatocellular carcinoma. Gastroenterology.
155:1936–1950. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fu J, Xu D, Liu Z, Shi M, Zhao P, Fu B,
Zhang Z, Yang H, Zhang H, Zhou C, et al: Increased regulatory T
cells correlate with CD8 T-cell impairment and poor survival in
hepatocellular carcinoma patients. Gastroenterology. 132:2328–2239.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yan J, Liu XL, Xiao G, Li NL, Deng YN, Han
L, Yin LC, Ling LJ and Liu LX: Prevalence and clinical relevance of
T-Helper cells, Th17 and Th1, in hepatitis B virus-related
hepatocellular carcinoma. PLoS One. 9:e960802014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hu C, You W, Kong D, Huang Y, Lu J, Zhao
M, Jin Y, Peng R, Hua D, Kuang DM and Chen Y: tertiary lymphoid
structure-associated B cells enhance CXCL13+CD103+CD8+
tissue-resident memory T-Cell response to programmed cell death
protein 1 blockade in cancer immunotherapy. Gastroenterology.
166:1069–1084. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ford K, Hanley C, Mellone M, Szyndralewiez
C, Heitz F, Wiesel P, Wood O, Machado M, Lopez MA, Ganesan AP, et
al: NOX4 inhibition potentiates immunotherapy by overcoming
cancer-associated fibroblast-mediated CD8 T-cell exclusion from
tumors. Cancer Res. 80:1846–1860. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Deng HJ, Kan A, Lyu N, Mu L, Han Y, Liu L,
Zhang Y, Duan Y, Liao S, Li S, et al: Dual vascular endothelial
growth factor receptor and fibroblast growth factor receptor
inhibition elicits antitumor immunity and enhances programmed cell
death-1 checkpoint blockade in hepatocellular carcinoma. Liver
Cancer. 9:338–357. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yin Y, Feng W, Chen J, Chen X, Wang G,
Wang S, Xu X, Nie Y, Fan D, Wu K and Xia L: Immunosuppressive tumor
microenvironment in the progression, metastasis, and therapy of
hepatocellular carcinoma: From bench to bedside. Exp Hematol Oncol.
13:722024. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yeung O, Lo C, Ling C, Qi X, Geng W, Li C,
Ng KT, Forbes SJ, Guan XY, Poon RT, et al: Alternatively activated
(M2) macrophages promote tumour growth and invasiveness in
hepatocellular carcinoma. J Hepatol. 62:607–616. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mantovani A, Allavena P, Marchesi F and
Garlanda C: Macrophages as tools and targets in cancer therapy. Nat
Rev Drug Discov. 21:799–820. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhu Y, Tan H, Wang J, Zhuang H, Zhao H and
Lu X: Molecular insight into T cell exhaustion in hepatocellular
carcinoma. Pharmacol Res. 203:1071612024. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sia D, Jiao Y, Martinez-Quetglas I, Kuchuk
O, Villacorta-Martin C, de Moura MC, Putra J, Camprecios G,
Bassaganyas L, Akers N, et al: Identification of an immune-specific
class of hepatocellular carcinoma, based on molecular features.
Gastroenterology. 153:812–826. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Guo T and Xu J: Cancer-associated
fibroblasts: A versatile mediator in tumor progression, metastasis,
and targeted therapy. Cancer Metastasis Rev. 43:1095–1116. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gao D, Fang L, Liu C, Yang M, Yu X, Wang
L, Zhang W, Sun C and Zhuang J: Microenvironmental regulation in
tumor progression: Interactions between cancer-associated
fibroblasts and immune cells. Biomed Pharmacother. 167:1156222023.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu ZL, Zhu LL, Liu JH, Pu ZY, Ruan ZP and
Chen J: Vascular endothelial growth factor receptor-2 and its
association with tumor immune regulatory gene expression in
hepatocellular carcinoma. Aging (Albany NY). 12:25172–25188. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Allen E, Jabouille A, Rivera LB,
Lodewijckx I, Missiaen R, Steri V, Feyen K, Tawney J, Hanahan D,
Michael IP and Bergers G: Combined antiangiogenic and anti-PD-L1
therapy stimulates tumor immunity through HEV formation. Sci Transl
Med. 9:eaak96792017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Apte RS, Chen DS and Ferrara N: VEGF in
signaling and disease: Beyond discovery and development. Cell.
176:1248–1264. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Singh K: Spontaneous regression of
hepatocellular carcinoma from autoinfarction and implications on
liver transplantation. ACG Case Rep J. 9:e008252022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Luke JJ, Bao R, Sweis RF, Spranger S and
Gajewski TF: WNT/β-catenin pathway activation correlates with
immune exclusion across human cancers. Clin Cancer Res.
25:3074–3083. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Harding JJ, Nandakumar S, Armenia J,
Khalil DN, Albano M, Ly M, Shia J, Hechtman JF, Kundra R, El Dika
I, et al: Prospective genotyping of hepatocellular carcinoma:
Clinical implications of next-generation sequencing for matching
patients to targeted and immune therapies. Clin Cancer Res.
25:2116–2126. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gao X, Qiao X, Xing X, Huang J, Qian J,
Wang Y, Zhang Y, Zhang X, Li M, Cui J and Yang Y: Matrix
stiffness-upregulated MicroRNA-17-5p attenuates the intervention
effects of metformin on HCC invasion and metastasis by targeting
the PTEN/PI3K/Akt pathway. Front Oncol. 10:15632020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li H, Wu K, Tao K, Chen L, Zheng Q, Lu XM,
Liu J, Shi L, Liu C, Wang G and Zou W: Tim-3/galectin-9 signaling
pathway mediates T-cell dysfunction and predicts poor prognosis in
patients with hepatitis B virus-associated hepatocellular
carcinoma. Hepatology. 56:1342–1351. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Trehanpati N and Vyas AK: Immune
regulation by T regulatory cells in hepatitis B virus-related
inflammation and cancer. Scand J Immunol. 85:175–181. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hoechst B, Voigtlaender T, Ormandy L,
Gamrekelashvili J, Zhao F, Wedemeyer H, Lehner F, Manns MP, Greten
TF and Korangy F: Myeloid derived suppressor cells inhibit natural
killer cells in patients with hepatocellular carcinoma via the
NKp30 receptor. Hepatology. 50:799–807. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yeung W, Liu L, Chen Z, Lo C and Man K:
Delta PD-1 expressed M2 macrophages promote liver tumor recurrence
after transplantation. Transplantation. 102 (Suppl 7):S250–S251.
2018. View Article : Google Scholar
|
|
46
|
Xie Q, Zhang P, Wang Y, Mei W and Zeng C:
Overcoming resistance to immune checkpoint inhibitors in
hepatocellular carcinoma: Challenges and opportunities. Front
Oncol. 12:9587202022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Argentiero A, Delvecchio A, Fasano R,
Andriano A, Caradonna IC, Memeo R and Desantis V: The complexity of
the tumor microenvironment in hepatocellular carcinoma and emerging
therapeutic developments. J Clin Med. 12:74692023. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Vaupel P and Multhoff G: Adenosine can
thwart antitumor immune responses elicited by radiotherapy.
Strahlenther Onkol. 192:279–287. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Karimi M, Geramizadeh B and Malek-Hosseini
S: Tolerance induction in liver. Int J Organ Transplant Med.
6:45–54. 2015.PubMed/NCBI
|
|
50
|
Karrar A, Broomé U, Uzunel M, Qureshi AR
and Sumitran-Holgersson S: Human liver sinusoidal endothelial cells
induce apoptosis in activated T cells: A role in tolerance
induction. Gut. 56:243–252. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Casey L, Hughes K, Saunders M, Miller S,
Pearson R and Shea L: Mechanistic contributions of Kupffer cells
and liver sinusoidal endothelial cells in nanoparticle-induced
antigen-specific immune tolerance. Biomaterials. 283:1214572022.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wang Y, Liu TT, Tang W, Deng B, Chen Y,
Zhu JM and Shen X: Hepatocellular carcinoma cells induce regulatory
T cells and lead to poor prognosis via production of transforming
growth factor-β1. Cell Physiol Biochem. 38:306–318. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kakita N, Kanto T, Itose I, Kuroda S,
Inoue M, Matsubara T, Higashitani K, Miyazaki M, Sakakibara M,
Hiramatsu N, et al: Comparative analyses of regulatory T cell
subsets in patients with hepatocellular carcinoma: A crucial role
of CD25-FOXP3- T cells. Int J Cancer. 131:2573–2783. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Liu Y, Yang H, Li T and Zhang N:
Immunotherapy in liver cancer: Overcoming the tolerogenic liver
microenvironment. Front Immunol. 15:14602822024. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Patseas D, El-Masry A, Liu Z, Ramachandran
P and Triantafyllou E: Myeloid cells in chronic liver inflammation.
Cell Mol Immunol. 22:1237–1261. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Chakravarthy A, Pasini E, Zhao X, To J,
Shen SYR, Fischer S, Ghanekar A, Vogel A, Grant RC, Knox J, et al:
Evolutionary dynamics of recurrent hepatocellular carcinoma under
divergent immune selection pressures. Front Oncol. 15:15370872025.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Yang Z, Cheng C, Li Z, Wang H, Zhang M,
Xie E, He X, Liu B, Sun H, Wang J, et al: Advancing liver cancer
treatment with dual-targeting CAR-T therapy. J Nanobiotechnol.
23:4622025. View Article : Google Scholar
|
|
58
|
Taylor BC and Balko JM: Mechanisms of
MHC-I downregulation and role in immunotherapy response. Front
Immunol. 13:8448662022. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Hazini A, Fisher K and Seymour L:
Deregulation of HLA-I in cancer and its central importance for
immunotherapy. J Immunother Cancer. 9:e0028992021. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Finn RS, Qin S, Ikeda M, Galle PR, Ducreux
M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, et al: Atezolizumab
plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J
Med. 382:1894–1905. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Nishida N and Kudo M: Immune checkpoint
blockade for the treatment of human hepatocellular carcinoma.
Hepatol Res. 48:622–634. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Wei SC, Levine JH, Cogdill AP, Zhao Y,
Anang NAS, Andrews MC, Sharma P, Wang J, Wargo JA, Pe'er D and
Allison JP: Distinct cellular mechanisms underlie Anti-CTLA-4 and
Anti-PD-1 checkpoint blockade. Cell. 170:1120–1133.e17. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Abou-Alfa GK, Lau G, Kudo M, Chan SL,
Kelley RK, Furuse J, Sukeepaisarnjaroen W, Kang YK, Dao TV, De Toni
EN, et al: Plain language summary of the HIMALAYA study:
Tremelimumab and durvalumab for unresectable hepatocellular
carcinoma (liver cancer). Future Oncol. 19:2505–2516. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Taddei TH, Brown DB, Yarchoan M,
Mendiratta-Lala M and Llovet JM: Critical update: AASLD practice
guidance on prevention, diagnosis, and treatment of hepatocellular
carcinoma. Hepatology. 82:272–274. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Brackenier C, Kinget L, Cappuyns S,
Verslype C, Beuselinck B and Dekervel J: Unraveling the synergy
between atezolizumab and bevacizumab for the treatment of
hepatocellular carcinoma. Cancers (Basel). 15:3482023. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Hatzidakis A, Müller L, Krokidis M and
Kloeckner R: Local and regional therapies for hepatocellular
carcinoma and future combinations. Cancers (Basel). 14:24692022.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Duffy AG, Ulahannan SV, Makorova-Rusher O,
Rahma O, Wedemeyer H, Pratt D, Davis JL, Hughes MS, Heller T,
ElGindi M, et al: Tremelimumab in combination with ablation in
patients with advanced hepatocellular carcinoma. J Hepatol.
66:545–551. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Leuchte K, Staib E, Thelen M, Gödel P,
Lechner A, Zentis P, Garcia-Marquez M, Waldschmidt D, Datta RR,
Wahba R, et al: Microwave ablation enhances tumor-specific immune
response in patients with hepatocellular carcinoma. Cancer Immunol
Immunother. 70:893–907. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Zhang Z, Liu X, Chen D and Yu J:
Radiotherapy combined with immunotherapy: The dawn of cancer
treatment. Signal Transduct Target Ther. 7:2582022. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Sangro B, Kudo M, Erinjeri JP, Qin S, Ren
Z, Chan SL, Arai Y, Heo J, Mai A, Escobar J, et al: Durvalumab with
or without bevacizumab with transarterial chemoembolisation in
hepatocellular carcinoma (EMERALD-1): A multiregional, randomised,
double-blind, placebo-controlled, phase 3 study. Lancet.
405:216–232. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
AstraZeneca. A phase III, randomized,
double-blind, placebo-controlled, multi center study of durvalumab
monotherapy or in combination with bevacizumab as adjuvant therapy
in patients with hepatocellular carcinoma who are at high risk of
recurrence after curative hepatic resection or ablation.
ClinicalTrialsgov Identifier: NCT04276941. Available from:.
https://clinicaltrialsgov/study/NCT038474282019.
|
|
72
|
Sangro B, Galle PR, Kelley RK, Charoentum
C, De Toni EN, Ostapenko Y, Heo J, Cheng AL, Woods AW, Gupta C, et
al: Patient-reported outcomes from the phase III HIMALAYA study of
tremelimumab plus durvalumab in unresectable hepatocellular
carcinoma. J Clin Oncol. 42:2790–2799. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Llovet JM, Kudo M, Merle P, Meyer T, Qin
S, Ikeda M, Xu R, Edeline J, Ryoo BY, Ren Z, et al: Lenvatinib plus
pembrolizumab versus lenvatinib plus placebo for advanced
hepatocellular carcinoma (LEAP-002): A randomised, double-blind,
phase 3 trial. Lancet Oncol. 24:1399–1410. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Qin S, Chan S, Gu S, Bai Y, Ren Z, Lin X,
Chen Z, Jia W, Jin Y, Guo Y, et al: Camrelizumab plus rivoceranib
versus sorafenib as first-line therapy for unresectable
hepatocellular carcinoma (CARES-310): A randomised, open-label,
international phase 3 study. Lancet. 402:1133–1146. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Kudo M, Finn R, Ikeda M, Sung M, Baron A,
Okusaka T, Kobayashi M, Kumada H, Kaneko S, Pracht M, et al: A
phase 1b study of lenvatinib plus pembrolizumab in patients with
unresectable hepatocellular carcinoma: Extended analysis of study
116. Liver Cancer. 13:451–458. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Jenkins F, Johnson JE, Collichio F and
Ollila D: Talimogene laherparepvec and novel injectable oncolytic
viruses in the management of metastatic melanoma. J Transl Genet
Genom. 5:396–413. 2021.
|
|
77
|
Ridolfi R, Flamini E, Riccobon A, De Paola
F, Maltoni R, Gardini A, Ridolfi L, Medri L, Poletti G and Amadori
D: Adjuvant adoptive immunotherapy with tumour-infiltrating
lymphocytes and modulated doses of interleukin-2 in 22 patients
with melanoma, colorectal and renal cancer, after radical
metastasectomy, and in 12 advanced patients. Cancer Immunol
Immunother. 46:185–193. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Fujiwara T, Yakoub MA, Chandler A, Christ
AB, Yang G, Ouerfelli O, Rajasekhar VK, Yoshida A, Kondo H, Hata T,
et al: CSF1/CSF1R signaling inhibitor pexidartinib (PLX3397)
reprograms tumor-associated macrophages and stimulates T-cell
infiltration in the sarcoma microenvironment. Mol Cancer Ther.
20:1388–1399. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Zhu Y, Yang J, Xu D, Gao XM, Zhang Z, Hsu
JL, Li CW, Lim SO, Sheng YY, Zhang Y, et al: Disruption of
tumour-associated macrophage trafficking by the osteopontin-induced
colony-stimulating factor-1 signalling sensitises hepatocellular
carcinoma to anti-PD-L1 blockade. Gut. 68:1653–1666. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Flores-Toro JA, Luo D, Gopinath A,
Sarkisian MR, Campbell JJ, Charo IF, Singh R, Schall TJ, Datta M,
Jain RK, et al: CCR2 inhibition reduces tumor myeloid cells and
unmasks a checkpoint inhibitor effect to slow progression of
resistant murine gliomas. Proc Natl Acad Sci USA. 117:1129–1138.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Li X, Yao W, Yuan Y, Chen P, Li B, Li JQ,
Chu R, Song H, Xie D, Jiang X and Wang H: Targeting of
tumour-infiltrating macrophages via CCL2/CCR2 signalling as a
therapeutic strategy against hepatocellular carcinoma. Gut.
66:157–167. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Shan F, Somasundaram A, Bruno TC, Workman
CJ and Vignali DAA: Therapeutic targeting of regulatory T cells in
cancer. Trends Cancer. 8:944–961. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Faivre S, Santoro A, Kelley RK, Gane E,
Costentin CE, Gueorguieva I, Smith C, Cleverly A, Lahn MM, Raymond
E, et al: Novel transforming growth factor beta receptor I kinase
inhibitor galunisertib (LY2157299) in advanced hepatocellular
carcinoma. Liver Int. 39:1468–1477. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Morris JC, Tan AR, Olencki TE, Shapiro GI,
Dezube BJ, Reiss M, Hsu FJ, Berzofsky JA and Lawrence DP: Phase I
study of GC1008 (fresolimumab): A human anti-transforming growth
factor-beta (TGFβ) monoclonal antibody in patients with advanced
malignant melanoma or renal cell carcinoma. PLoS One. 9:e903532014.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Gunderson AJ, Yamazaki T, McCarty K, Fox
N, Phillips M, Alice A, Blair T, Whiteford M, O'Brien D, Ahmad R,
et al: TGFβ suppresses CD8+ T cell expression of CXCR3 and tumor
trafficking. Nat Commun. 11:17492020. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Abdallah N, Kim S, Ayash L, Klimecki S,
Ventimiglia M, Alavi A, Ratanatharathorn V, Uberti J and Deol A:
Does use of biosimilar G-CSF change plerixafor utilization during
stem cell mobilization for autologous stem cell transplant? Bone
Marrow Transplant. 55:1655–1657. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Hecht JR, Oberoi A, Cabanas EG, Chon HJ,
Digklia A, Rottey S, Jimenez MM, Chaney M, Hippenmeyer J, Lawrence
T, et al: Phase Ib/II trial of talimogene laherparepvec alone and
with pembrolizumab in advanced solid tumors with liver metastases
and hepatocellular carcinoma. Oncologist. 30:oyae2032025.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Raman S, Pless M, Cubillo A, Calvo A,
Hecht R, Liu C, Chan E, Chesney J and Prat A: 3:36 PM Abstract No.
375 Early safety from a phase 1, multicenter, open-label clinical
trial of talimogene laherparepvec (T-VEC) injected into liver
tumors. JVIR. 29:S1612018. View Article : Google Scholar
|
|
89
|
Heo J, Reid T, Ruo L, Breitbach C, Rose S,
Bloomston M, Cho M, Lim HY, Chung HC, Kim CW, et al: Randomized
dose-finding clinical trial of oncolytic immunotherapeutic vaccinia
JX-594 in liver cancer. Nat Med. 19:329–336. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Abou-Alfa GK, Galle PR, Chao Y, Erinjeri
J, Heo J, Borad MJ, Luca A, Burke J, Pelusio A, Agathon D, et al:
PHOCUS: A phase 3, randomized, open-label study of sequential
treatment with pexa-vec (JX-594) and sorafenib in patients with
advanced hepatocellular carcinoma. Liver Cancer. 13:248–264. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Butterfield LH, Ribas A, Dissette VB, Lee
Y, Yang JQ, De la Rocha P, Duran SD, Hernandez J, Seja E, Potter
DM, et al: A phase I/II trial testing immunization of
hepatocellular carcinoma patients with dendritic cells pulsed with
four alpha-fetoprotein peptides. Clin Cancer Res. 12:2817–2825.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Sawada Y, Yoshikawa T, Nobuoka D,
Shirakawa H, Kuronuma T, Motomura Y, Mizuno S, Ishii H, Nakachi K,
Konishi M, et al: Phase I trial of a glypican-3-derived peptide
vaccine for advanced hepatocellular carcinoma: immunologic evidence
and potential for improving overall survival. Clin Cancer Res.
18:3686–3696. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Ikeda M, Okusaka T, Ohno I, Mitsunaga S,
Kondo S, Ueno H, Morizane C, Gemmoto K, Suna H, Ushida Y and Furuse
J: Phase I studies of peptide vaccine cocktails derived from GPC3,
WDRPUH and NEIL3 for advanced hepatocellular carcinoma.
Immunotherapy. 13:371–385. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Kwak J, Nguyen H, Camai A, Huffman G,
Mekvanich S, Kenney N, Zhu X, Randolph TW and Houghton AM: CXCR1/2
antagonism inhibits neutrophil function and not recruitment in
cancer. Oncoimmunology. 13:23846742024. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Kim Y, Kim G, Kim S, Cho B, Kim SY, Do EJ,
Bae DJ, Kim S, Kweon MN, Song JS, et al: Fecal microbiota
transplantation combined with anti-PD-1 inhibitor for unresectable
or metastatic solid cancers refractory to anti-PD-1 inhibitor. Cell
Host Microbe. 32:1380–1393.e9. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Song C, Zhang J, Wen R, Li Q, Zhou J, Liu
X, Wu Z, Lv Y and Wu R: Improved anti-hepatocellular carcinoma
effect by enhanced Co-delivery of Tim-3 siRNA and sorafenib via
multiple pH triggered drug-eluting nanoparticles. Mater Today Bio.
16:1003502022. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Choukér A, Thiel M, Lukashev D, Ward J,
Kaufmann I, Apasov S, Sitkovsky MV and Ohta A: Critical role of
hypoxia and A2A adenosine receptors in liver tissue-protecting
physiological anti-inflammatory pathway. Mol Med. 14:116–123. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Srivastava A, Moufarrij S, Hadley M,
Chisholm S, Lopez-Acevedo M, Villagra A and Chiappinelli K:
Abstract 1395: HDAC6 and DNMT inhibition affect immunogenicity of
ovarian cancer cells: A rationale for combining epigenetic and
immune therapy in ovarian cancer. Cancer Res. 78 (Suppl
13):13952018. View Article : Google Scholar
|
|
99
|
Chen H, Li Z, Qiu L, Dong X, Chen G, Shi
Y, Cai L, Liu W, Ye H, Zhou Y, et al: Personalized neoantigen
vaccine combined with PD-1 blockade increases CD8+ tissue-resident
memory T-cell infiltration in preclinical hepatocellular carcinoma
models. J Immunother Cancer. 10:e0043892022. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Shi D, Shi Y, Kaseb AO, Qi X, Zhang Y, Chi
J, Lu Q, Gao H, Jiang H, Wang H, et al: Chimeric antigen
receptor-glypican-3 T-cell therapy for advanced hepatocellular
carcinoma: Results of phase I trials. Clin Cancer Res.
26:3979–3989. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Chattopadhyay S, Hazra R, Mallick A, Gayen
S and Roy S: A review exploring the fusion of oncolytic viruses and
cancer immunotherapy: An innovative strategy in the realm of cancer
treatment. Biochim Biophys Acta Rev Cancer. 1879:1891102024.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Teng CF, Wang T, Shih FY, Shyu WC and Jeng
LB: Therapeutic efficacy of dendritic cell vaccine combined with
programmed death 1 inhibitor for hepatocellular carcinoma. J
Gastroenterol Hepatol. 36:1988–1996. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Lee EHJ, Murad JP, Christian L, Gibson J,
Yamaguchi Y, Cullen C, Gumber D, Park AK, Young C, Monroy I, et al:
Antigen-dependent IL-12 signaling in CAR T cells promotes regional
to systemic disease targeting. Nat Commun. 14:47372023. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Zhu W, Peng Y, Wang L, Hong Y, Jiang X, Li
Q, Liu H, Huang L, Wu J, Celis E, et al: Identification of
α-fetoprotein-specific T-cell receptors for hepatocellular
carcinoma immunotherapy. Hepatology. 68:574–589. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Topper MJ, Vaz M, Marrone KA, Brahmer JR
and Baylin SB: The emerging role of epigenetic therapeutics in
immuno-oncology. Nat Rev Clin Oncol. 17:75–90. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Yang W, Feng Y, Zhou J, Cheung O, Cao J,
Wang J, Tang W, Tu Y, Xu L, Wu F, et al: A selective HDAC8
inhibitor potentiates antitumor immunity and efficacy of immune
checkpoint blockade in hepatocellular carcinoma. Sci Transl Med.
13:eaaz68042021. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Yau T, Park JW, Finn RS, Cheng AL,
Mathurin P, Edeline J, Kudo M, Harding JJ, Merle P, Rosmorduc O, et
al: Nivolumab versus sorafenib in advanced hepatocellular carcinoma
(CheckMate 459): A randomised, multicentre, open-label, phase 3
trial. Lancet Oncol. 23:77–90. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Finn RS, Ryoo BY, Merle P, Kudo M,
Bouattour M, Lim HY, Breder V, Edeline J, Chao Y, Ogasawara S, et
al: Pembrolizumab as second-line therapy in patients with advanced
hepatocellular carcinoma in KEYNOTE-240: A randomized,
double-blind, phase III trial. J Clin Oncol. 38:193–202. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Cheng X, Li J, Feng L, Feng S, Wu X and Li
Y: The role of hypoxia-related genes in TACE-refractory
hepatocellular carcinoma: Exploration of prognosis, immunological
characteristics and drug resistance based on onco-multi-OMICS
approach. Front Pharmacol. 13:10110332022. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Trommer M, Yeo SY, Persigehl T, Bunck A,
Grüll H, Schlaak M, Theurich S, von Bergwelt-Baildon M,
Morgenthaler J, Herter JM, et al: Abscopal effects in
radio-immunotherapy-response analysis of metastatic cancer patients
with progressive disease under anti-PD-1 immune checkpoint
inhibition. Front Pharmacol. 10:5112019. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Steffin D, Ghatwai N, Montalbano A, Rathi
P, Courtney AN, Arnett AB, Fleurence J, Sweidan R, Wang T, Zhang H,
et al: Interleukin-15-armored GPC3-CAR T cells for patients with
solid cancers. Res Sq. 3:rs.3.rs–4103623. 2024.
|
|
112
|
Luo X, Cui H, Cai L, Zhu W, Yang WC,
Patrick M, Zhu S, Huang J, Yao X, Yao Y, et al: Selection of a
clinical lead TCR targeting alpha-fetoprotein-positive liver cancer
based on a balance of risk and benefit. Front Immunol. 11:6232020.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Lee JH, Tak WY, Lee Y, Heo MK, Song JS,
Kim HY, Park SY, Bae SH, Lee JH, Heo J, et al: Adjuvant
immunotherapy with autologous dendritic cells for hepatocellular
carcinoma, randomized phase II study. Oncoimmunology.
6:e13283352017. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Adachi Y, Kamiyama H, Ichikawa K, Ozawa Y,
Fukushima S, Satoshi G, Yamaguchi S, Matsuki M, Miyano SW, Yokoi A,
et al: Abstract 6637: Inhibition of FGFR signaling by lenvatinib
activates tumor interferon gamma signaling pathway and potentiates
antitumor activity of anti-PD-1 antibody. Cancer Res. 80:66372020.
View Article : Google Scholar
|
|
115
|
Leslie J, Mackey JBG, Jamieson T,
Ramon-Gil E, Drake TM, Fercoq F, Clark W, Gilroy K, Hedley A, Nixon
C, et al: CXCR2 inhibition enables NASH-HCC immunotherapy. Gut.
71:2093–2106. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Sun C, Wang B and Hao S: Adenosine-A2A
receptor pathway in cancer immunotherapy. Front Immunol.
13:8372302022. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Angell H and Galon J: From the immune
contexture to the immunoscore: The role of prognostic and
predictive immune markers in cancer. Curr Opin Immunol. 25:261–267.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Paik J: Nivolumab plus relatlimab: First
approval. Drugs. 82:925–931. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Finn RS, Ryoo BY, Hsu CH, Li D, Burgoyne
AM, Cotter C, Badhrinarayanan S, Wang Y, Yin A, Edubilli TR, et al:
Tiragolumab in combination with atezolizumab and bevacizumab in
patients with unresectable, locally advanced or metastatic
hepatocellular carcinoma (MORPHEUS-Liver): A randomised,
open-label, phase 1b-2, study. Lancet Oncol. 26:214–226. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Kelley RK, Oliver JW, Hazra S, Benzaghou
F, Yau T, Cheng AL and Rimassa L: Cabozantinib in combination with
atezolizumab versus sorafenib in treatment-naive advanced
hepatocellular carcinoma: COSMIC-312 Phase III study design. Future
Oncol. 16:1525–1536. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Gutteridge TP, Kelly AB and Laurens KR:
Increased likelihood of distressing and functionally impairing
psychotic-like experiences among children with co-occurring
internalising and externalising problems. Schizophr Res.
252:225–230. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Exposito MJ, Akce M, Alvarez J, Assenat E,
Balart L, Baron A, Decaens T, Heurgue-Berlot A, Martin A, Paik S,
et al: Abstract No. 526 CheckMate-9DX: phase 3, randomized,
double-blind study of adjuvant nivolumab vs placebo for patients
with hepatocellular carcinoma (HCC) at high risk of recurrence
after curative resection or ablation. J Vasc Interv Radiol. 30
(Suppl 1):S227–S228. 2019. View Article : Google Scholar
|
|
123
|
Du JS, Hsu SH and Wang SN: The current and
prospective adjuvant therapies for hepatocellular carcinoma.
Cancers (Basel). 16:14222024. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Knox J, Cheng A, Cleary S, Galle P, Kokudo
N, Lencioni R and Fan J: A phase 3 study of durvalumab with or
without bevacizumab as adjuvant therapy in patients with
hepatocellular carcinoma at high risk of recurrence after curative
hepatic resection or ablation: EMERALD-2. Ann Oncol. 30:iv59–iv60.
2019. View Article : Google Scholar
|
|
125
|
Xia X, Li X, Feng G, Zheng C, Liang H and
Zhou G: Intra-arterial interleukin-12 gene delivery combined with
chemoembolization: Anti-tumor effect in a rabbit hepatocellular
carcinoma (HCC) model. Acta Radiol. 54:684–689. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Karapetyan L, Luke JJ and Davar D:
Toll-Like receptor 9 agonists in cancer. Onco Targets Ther.
13:10039–10060. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Katz SC, Burga RA, McCormack E, Wang LJ,
Mooring W, Point GR, Khare PD, Thorn M, Ma Q, Stainken BF, et al:
Phase I hepatic immunotherapy for metastases study of
intra-arterial chimeric antigen receptor-modified T-cell therapy
for CEA+ liver metastases. Clin Cancer Res. 21:3149–3159. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Reynolds K, Thomas M and Dougan M:
Diagnosis and management of hepatitis in patients on checkpoint
blockade. Oncologist. 23:991–997. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
De Martin E, Fulgenzi CAM, Celsa C,
Laurent-Bellue A, Torkpour A, Lombardi P, D'Alessio A and Pinato
DJ: Immune checkpoint inhibitors and the liver: balancing
therapeutic benefit and adverse events. Gut. 74:1165–1177. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Andrews MC, Duong CPM, Gopalakrishnan V,
Iebba V, Chen WS, Derosa L, Khan MAW, Cogdill AP, White MG, Wong
MC, et al: Gut microbiota signatures are associated with toxicity
to combined CTLA-4 and PD-1 blockade. Nat Med. 27:1432–1441. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Zhou G, Zhang N, Meng K and Pan F:
Interaction between gut microbiota and immune checkpoint
inhibitor-related colitis. Front Immunol. 13:10016232022.
View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Strouse J, Chan KK, Baccile R, He G,
Louden DKN, Giurcanu M, Singh A, Rieth J, Abdel-Wahab N, Katsumoto
TR, et al: Impact of steroid-sparing immunosuppressive agents on
tumor outcome in the context of cancer immunotherapy with highlight
on melanoma: A systematic literature review and meta-analysis.
Front Immunol. 15:14994782024. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Syed S, Hines J, Baccile R, Rouhani S and
Reid P: Studying outcomes after steroid-sparing immunosuppressive
agent vs. steroid-only treatment for immune-related adverse events
in non-small-cell lung cancer (NSCLC) and melanoma: A retrospective
case-control study. Cancers (Basel). 16:18922024. View Article : Google Scholar : PubMed/NCBI
|
















