Introduction
Ulcerative colitis (UC) is a chronic inflammatory
bowel disease (IBD) primarily affecting the colon which is
characterized by mucosal inflammation. Its incidence is gradually
increasing worldwide, with rates in China reaching 11.6 per 100,000
individuals (1,2). If left untreated UC can progress to
colorectal cancer or result in systemic complications (3). The pathogenesis of UC involves immune
dysregulation, excessive production of pro-inflammatory cytokines
and gut microbiota imbalance (4).
Current first-line therapies include aminosalicylates, such as
mesalazine, corticosteroids and immunomodulators. However, up to
50% of patients experience treatment-related adverse events, such
as glucose intolerance and hepatotoxicity (5), highlighting the need for safer and
more effective therapeutic alternatives. Natural products have
therefore attracted growing attention as potential candidates
(6,7).
The intestinal barrier serves an important role in
the pathogenesis of UC. Disruption of its structural and functional
integrity contributes to increased intestinal permeability,
microbial translocation and sustained immune activation. Growing
evidence suggests that enhancing intestinal barrier function
represents a promising therapeutic strategy for UC (8–14).
Among the signaling mechanisms implicated, the Janus kinase 2
(JAK2)/signal transducer and activator of transcription 3 (STAT3)
pathway has been extensively studied. Hyperactivation of this
pathway is a hallmark of UC, contributing to both inflammation and
barrier dysfunction (15,16). The preliminary data of the present
study (data not shown), together with prior reports (17,18),
have suggested that naringin, a natural flavonoid, may exert
anti-inflammatory effects through modulation of kinase signaling
pathways. The present study therefore focused on the JAK2/STAT3
pathway to elucidate the mechanism underlying its protective
activity.
Citrus aurantium L., a member of the Rutaceae
family also known as bitter orange, is widely consumed in China as
both a food and traditional medicine, owing to its
anti-inflammatory properties (18). Previous studies have demonstrated
that Citrus aurantium L. alleviates trintrobenzene sulfonic
acid (TNBS)-induced IBD in rats, while its major flavonoid naringin
reduces the expression of inducible nitric oxide synthase (iNOS),
cytochrome c oxidase subunit 2 (COX-2) and TNF-α (17,18).
Naringin has also been shown to ameliorate clinical symptoms in
murine models of IBD (17,18). Given that the JAK2/STAT3 pathway is
hyperactivated in UC and promotes inflammation (15,16),
its pharmacological inhibition has emerged as a promising
therapeutic approach (19–21). However, the role of naringin in
modulating UC through this pathway remains undefined. The present
study aimed to investigate the effects of naringin on sodium
sulfate dextran (DSS)-induced colitis and its influence on the
JAK2/STAT3 signaling pathway.
Materials and methods
Materials
Naringin (cat. no. B21594-1g; Shanghai Yuanye
Bio-Technology Co., Ltd.) and fluorescein isothiocyanate dextrose
4000 (FD-4; molecular weight, 3,000-5,000 kDa; cat. no. FD4-250MG)
were purchased from Sigma Chemical Co. Mesalazine (cat. no.
20230605) was obtained from Heilongjiang Tianhong Pharmaceutical
Co., Ltd. DSS (molecular weight, 36,000-50,000 kDa; cat. no.
02160110-CF) was purchased from MP Biomedicals, LLC. Caco-2 cells
were obtained from Cell Resource Center, Peking Union Medical
College. Cell culture reagents (HyClone™; Cytiva)
included modified Eagle's medium (MEM; cat. no. SH30024.01), fetal
bovine serum (FBS; cat. no. SH30071.03) and penicillin-streptomycin
(cat. no. SV30010).
Animals
Healthy male BALB-c mice (aged 6–8 weeks; 18–22 g)
were obtained from Sbeifu (Beijing) Biotechnology Co., Ltd.
(certificate of conformity no. 110324210106095731). Mice were
housed in cages with controlled temperature (22±2°C), relative
humidity (40–60%) and a 12-h light/dark cycle throughout the study.
All mice were fed a standard diet (crude protein 16%, crude fat 4%,
crude fiber 12% and ash 8%) and had free access to water. Animal
experiments were approved by the Animal Welfare Committee of Hebei
North University (Zhangjiakou, China; approval no. HBNV20240223003)
and performed according to institutional guidelines.
DSS-induced colitis mice
A total of 32 mice were randomly divided into four
groups, each consisting of eight mice: The normal, control,
mesalazine control and naringin groups. Intragastric administration
volumes were 0.3 ml per 10 g body weight. Mesalazine and naringin
were suspended in 5% acacia gum (cat. no. V900768-500G;
MilliporeSigma; Merck KgaA) and the final doses were 0.2 g/kg and
40 mg/kg, respectively. The dose of 40 mg/kg naringin was selected
based on previous studies demonstrating efficacy in rodent models
of colitis (17,18). Similarly, the mesalazine dose of
0.2 g/kg is a standard dose used in DSS murine models (22,23).
Except for the normal group, all the other groups were freely
administered 3% DSS (dissolved in drinking water) to establish the
UC mouse model, which was administered alongside other treatments
(22,23). The naringin and mesalazine groups
received their respective drug suspensions (in 5% acacia gum) by
oral gavage once daily for 10 days. The normal and control groups
received an equivalent volume of the vehicle (5% acacia gum
solution) on the same schedule. Diet and hydration, body movement,
body weight, diarrhea incidence and bloody stool were recorded
daily throughout the present study.
Disease activity index (DAI) and
analysis of colon injury
Body weight, stool character and incidence of bloody
stools were recorded daily after models were established. The DAI
was determined using a previously established scoring system
(Table I) (24). On day 11, mice were anesthetized
via inhaled isoflurane (induction, 4%; maintenance, 1.5–2% in
oxygen) delivered by a precision vaporizer for final blood
collection from the retro-orbital plexus. A terminal blood sample
not exceeding 10% of total blood volume (0.6–0.8 ml) was collected.
Following this, pentobarbital sodium (40 mg/kg) was administered by
intraperitoneal injection to ensure a surgical plane of anesthesia,
confirmed by the loss of pedal reflex, before euthanasia was
performed via cervical dislocation. This approach guaranteed the
absence of pain or distress throughout the procedure. Death was
verified by the cessation of cardiac and respiratory activity. All
animal procedures were strictly performed in accordance with the
approved animal protocol. The colon segment was resected to
evaluate the macroscopic colon injury, which was scored according
to the method reported in the literature (Table II) (18). Routine hematoxylin and
eosin-stained colon sections, according to previously described
morphological criteria and observed colon tissue damage, were
assessed blindly by two investigators according to a modified
histological grading scale, which considered both inflammatory cell
infiltration and tissue damage (Table III) (18). Any discrepant scores were resolved
through a joint re-evaluation of the slide under a multi-head
microscope to reach a consensus. If a consensus could not be
reached, a third experienced pathologist was consulted for a final
decision.
 | Table I.Evaluation of DAI scores. |
Table I.
Evaluation of DAI scores.
| DAI score | Weight loss, % | Stool
consistency | Occult or gross
bleeding |
|---|
| 0 | None | None | None |
| 1 | 1-5 | Loose | Hemoccult
positive |
| 2 | 5-10 | Loose | Hemoccult
positive |
| 3 | 10-15 | Diarrhea | Gross bleeding |
| 4 | >15 | Diarrhea | Gross bleeding |
 | Table II.Evaluation of macroscopic scores. |
Table II.
Evaluation of macroscopic scores.
| Colon damage | Score |
|---|
| No damage | 0 |
| Hyperemia with
ulcers | 1 |
| Hyperemia and wall
thickening without ulcers | 2 |
| One ulceration site
without wall thickening | 3 |
| Two or more
ulceration sites | 4 |
| 0.5 cm extension of
inflammation or major damage | 5 |
| 1 cm extension of
inflammation or severe damage | 6-10 |
 | Table III.Evaluation of histological
scores. |
Table III.
Evaluation of histological
scores.
| A, Inflammatory
cell infiltration |
|---|
|
|---|
| Pathological
characteristic | Score |
|---|
| No
infiltration | 0 |
| Increased number of
inflammatory cells in the lamina propria | 1 |
| Inflammatory cells
extending into the submucosa | 2 |
| Transmural
inflammatory cell infiltration | 3 |
|
| B, Tissue
damage |
|
| Pathological
characteristic | Score |
|
| No mucosal
damage | 0 |
| Discrete epithelial
lesions | 1 |
| Erosion or focal
ulcerations | 2 |
| Severe mucosal
damage with extensive ulceration extending into the bowel wall | 3 |
Western blot analysis for JAK2/STAT3
signaling pathway-related proteins from colon tissues
To determine the protective effect of naringin,
western blot analysis was performed. Colon tissues were homogenized
in RIPA buffer (cat. no. P0013B; Beyotime Biotechnology)
supplemented with 1% (v/v) Protease and Phosphatase Inhibitor
Cocktail (cat. no. P1050; Beyotime Biotechnology). Protein
concentrations were measured using Cytation 5 (BioTek; Agilent
Technologies, Inc.) using a bicinchoninic acid (BCA) protein assay
kit (cat. no. PC0020; Beijing Solarbio Science & Technology
Co., Ltd.). A total of 60 µg protein were mixed with 4X loading
dye, as well as Laemmli buffer and 2-mercapto ethanol, and were
heated at 95°C for 5 min. The proteins were resolved by 8–12%
SDS-PAGE and transferred to immunoblot PVDF membranes (cat. no.
IPVH00010; Merck KGaA). The membranes were blocked with 5% non-fat
dry milk in Tris-buffered saline containing 0.1% Tween-20 (TBST)
for 2 h at room temperature, then incubated at 4°C overnight with
primary antibodies against IL-6 (1:1,000; cat. no. Bs-0782R;
BIOSS), phosphorylated (p-)JAK2 (1:1,000; cat. no. bs-2485R;
BIOSS), JAK2 (1:1,000; cat. no. bs-0908R; BIOSS), p-STAT3 (1:1,000;
cat. no. bs-1658R; BIOSS), STAT3 (1:1,000; cat. no. bs-55208R;
BIOSS), occludin (1:1,000; cat. no. A2601; ABclonal Biotech Co.,
Ltd.), zona occludens-1 (ZO-1; 1:1,000; cat. no. A0659; ABclonal
Biotech Co., Ltd.) and β-actin (1:1,000; cat. no. ab8227; Abcam).
The membranes were washed with TBST three times for 10 min each and
incubated with a horseradish peroxidase (HRP)-labeled secondary
goat anti-rabbit (1:5,000; cat. no. ab6721; Abcam) antibodies for 1
h at room temperature. After washing three times with TBST, bands
were detected using ECL Plus Ultra-Sensitive Substrate (cat. no.
PE0010; Beijing Solarbio Science & Technology Co., Ltd.) and
visualized using the ChemiDoc MP Imaging System (Bio-Rad
Laboratories, Inc.) after washing three times. The band intensities
were semi-quantified using ImageJ v1.53 gel analysis software
(National Institutes of Health).
IL-6-induced Caco-2 barrier injury
model
Caco-2 cells were cultured in MEM supplemented with
20% FBS, penicillin (100 units/ml), streptomycin (100 µg/ml),
L-glutamine (4.5 mg/ml) and glucose (4.5 mg/ml), and incubated at
37°C in a humidified atmosphere containing 5% CO2. The
medium was refreshed every other day. A total of 2×106
cells/ml Caco-2 cells were then seeded into a 24-well plate and
incubated overnight at 37°C. The following day, the medium was
changed to a new medium containing 20 ng/ml IL-6 (cat. no. P05231;
Boster Biological Technology) and cells were incubated for 45 min
at 37°C. This resulted in the formation of mucosal barrier damage
in the Caco-2 cells.
Measurement of transepithelial
electrical resistance (TEER) and FD-4 permeability
As described in previously published protocols
(18) with slight modification,
1.5×105 cells/ml Caco-2 cells were seeded in Transwell
cell culture chambers (6.5 mm diameter inserts; 3.0 mm pore size;
Costar; Corning, Inc.), and the growth medium was changed every 2
days. A monolayer of Caco-2 cells was formed after cells were
cultured for 10 days. Subsequently, cells were incubated with or
without IL-6 (20 ng/ml) for 45 min at 37°C and incubated with
naringin (40 µmol/l for 2 h at 37°C. The cell culture was replaced
with serum-free medium and incubated for another 30 min at 37°C
before the sample TEER (Ω/cm2) was assessed.
After the detection of TEER, 100 ml of 1 mg/ml FD-4
was added into the Transwell upper chambers before the cells were
incubated in 37°C for 30 min. After that, 100 ml medium from the
lower chamber of the Transwell was added into a black well to
detect the fluorescence content at an excitation wavelength of 480
nm and emission wavelength of 520 nm using SpectraMax®
M5 (Molecular Devices, LLC) as reported in a previous study
(25).
Immunofluorescence assay
Cells were fixed with 4% paraformaldehyde (cat. no.
BL539A; Beijing Lanjieke Technology Co., Ltd.) at room temperature
for 30 min. After discarding the fixative, the cells were washed
with Dulbecco's phosphate-buffered saline and blocked with 5%
bovine serum albumin sealing solution at room temperature for 2 h.
For immunofluorescence experiments, cells were incubated with the
primary antibodies ZO-1 (cat. no. A0659; ABclonal Biotech Co.,
Ltd.) and occludin (cat. no. A2601; ABclonal Biotech Co., Ltd.) at
1:200 dilution overnight at 4°C after blocking. Cells were then
washed with PBS containing 0.1% Tween-20 and subsequently incubated
with goat anti-rabbit-Alexa Fluor 488 (1:500; cat. no. 4412S; CST
Biological Reagents Co., Ltd.) and goat anti-rabbit-Alexa Fluor 647
(1:500; cat. no. 4414S, CST Biological Reagents Co., Ltd.)
fluorescent secondary antibodies at room temperature for 2 h in the
absence of light. Finally, cells were counter-stained with DAPI
(cat. no. D1306; Thermo Fisher Scientific, Inc.) for 10 min, and
the images were collected and analyzed using a laser confocal
microscope (FV3000; Olympus Corporation).
Small interfering RNA (siRNA)
transfection and western blot analysis in Caco-2 cells
siRNA targeting STAT3 (siSTAT3) and negative control
siRNA (siNC) were purchased from Suzhou GenePharma Co., Ltd. The
sequences were as follows: siSTAT3, sense
5′-CCCGGAAAUUUAACAUUCUTT-3′, antisense 5′-AGAAUGUUAAAUUUCCGGGTT-3′;
and siNC, sense 5′-UUCUCCGAACGUGUCACGUTT-3′, antisense
5′-ACGUGACACGUUCGGAGAATT-3′. Caco-2 cells were transfected with
siRNA using Lipofectamine® 3000 reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. Briefly, Caco-2 cells were seeded at a density of
2×106 cells/well in a 6-well plate. In each well, 100
pmol siRNA was complexed with Lipofectamine 3000 reagent. The
transfection mixture was incubated with the cells for 6 h at 37°C,
after which it was replaced with fresh complete medium. A total of
24 h after transfection, the transfected cells were treated with
IL-6 (20 ng/ml) for 45 min at 37°C, followed by naringin (40
µmol/l) treatment for 2 h at 37°C, prior to protein extraction. For
protein detection, Caco-2 cells were lysed using ice-cold RIPA
buffer (Thermo Fisher Scientific, Inc.) and centrifuged 12,000 × g
for 5 min at 4°C. Protein concentrations were determined using a
BCA assay (Beyotime Biotechnology). Total proteins (20 mg/lane)
were subjected to electrophoresis in a 12% polyacrylamide gel and
transferred onto a PVDF membrane. Subsequently, the membrane was
blocked at 37°C for 1 h with 5% non-fat milk in TBS, with 0.05%
Tween-20. Membranes were subsequently incubated separately with
primary antibodies for p-JAK2 (1:1,000; cat. no. bs-2485R; BIOSS),
JAK2 (1:1,000; cat. no. bs-0908R; BIOSS), p-STAT3 (1:1,000; cat.
no. bs-1658R; BIOSS), STAT3 (1:1,000; cat. no. bs-55208R; BIOSS),
occludin (1:1,000; cat. no. A2601; ABclonal Biotech Co., Ltd.),
ZO-1 (1:1,000; cat. no. A0659; ABclonal Biotech Co., Ltd.) and
β-actin (1:1,000; cat. no. ab8227; Abcam), and then with
HRP-conjugated goat anti-rabbit IgG secondary antibody (1:5,000;
cat. no. ab6721; Abcam). Signals were detected by enhanced
chemiluminescence (Thermo Fisher Scientific, Inc.). Western blot
images were semi-quantified using ImageJ v1.53 software, Inc.).
Relative band intensities were normalized to β-actin.
Statistical analysis
Experiments were performed with multiple independent
replicates as indicated in the figure legends (typically, n=8 for
in vivo studies and n=3 for in vitro assays). Data
are expressed as mean ± SEM. Statistical analyses were performed
using SPSS v11.0 (SPSS, Inc.). One-way ANOVA followed by Tukey's
post-hoc test was applied. P<0.05 was considered to indicate a
statistically significant difference.
Results
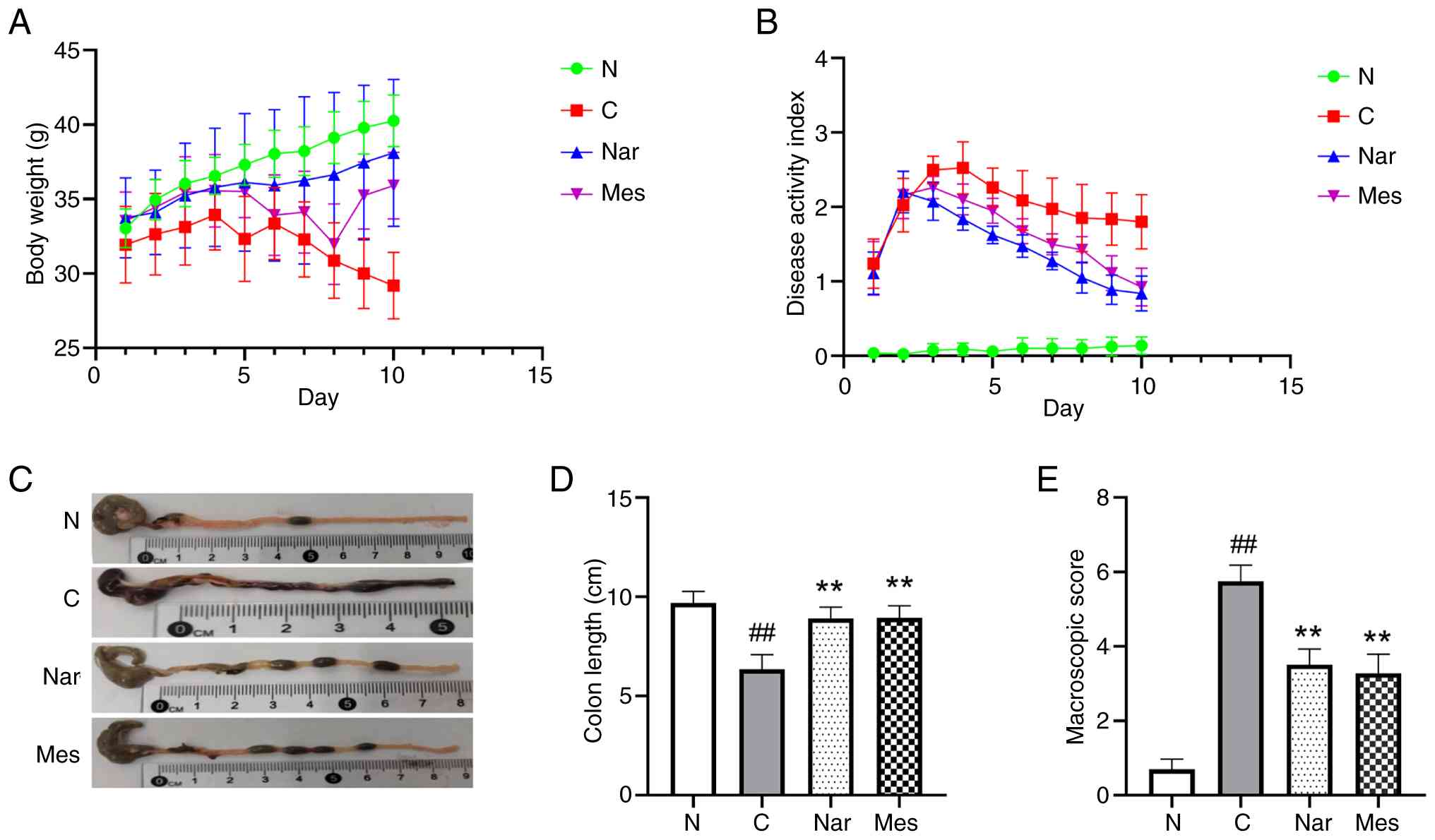
Naringin ameliorates clinical symptoms
of UC in DSS-induced mice
To evaluate whether oral administration of naringin
could ameliorate intestinal damage in UC mice, the present study
established a DSS-induced colitis model and assessed clinical and
pathological indicators, including body weight, diarrhea,
bloody-stool incidence colon length and macroscopic injury.
Compared with the normal group, DSS-treated mice in the control
group exhibited a notable reduction in body weight (Fig. 1A) and a marked increase in DAI
(Fig. 1B). In addition, DSS
administration caused significant colon shortening (P<0.01;
Fig. 1C and D) and a significant
increase in macroscopic injury score (P<0.01; Fig. 1E). Notably, naringin treatment
effectively alleviated these pathological changes (P<0.01),
indicating its protective effect against DSS-induced UC. The
positive control drug, mesalazine, showed a comparable protective
efficacy in ameliorating these clinical symptoms (Fig. 1A-E).
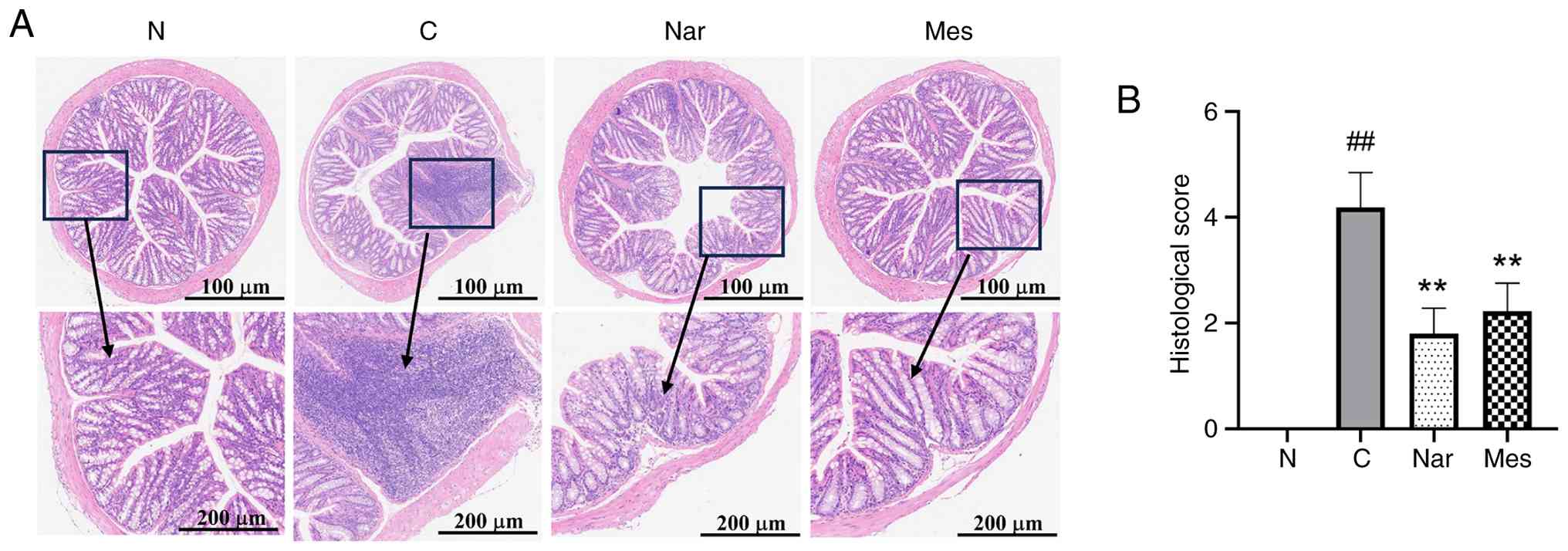
Naringin suppresses histological
injury in DSS-induced colitis in mice
The present study subsequently evaluated the effects
of naringin on colorectal histopathology in mice with DSS-induced
UC (Fig. 2A). Compared with in the
normal group, DSS-treated mice exhibited severe mucosal damage,
including epithelial disruption, erosion, crypt abscesses and
extensive inflammatory cell infiltration (primarily neutrophils and
lymphocytes). Both naringin and mesalazine treatments markedly
ameliorated these pathological features, preserving mucosal and
crypt architecture.
Pathological scoring further supported these
observations (P<0.01; Fig. 2B).
Compared with the normal group, DSS treatment significantly
increased histological scores. Treatment with either naringin or
mesalazine significantly reduced these scores compared with the DSS
group.
Naringin repairs intestinal mucosa and
decreases JAK2/STAT3 signaling pathway-related protein expression
in DSS-induced colitis in mice
The JAK2/STAT3 signaling pathway is abnormally
activated in patients with UC and is known to exacerbate colonic
inflammation and compromise mucosal barrier function (26,27).
Consistent with this, western blot analysis revealed a significant
upregulation of p-JAK2 and p-STAT3, as well as significantly
elevated IL-6 expression, in DSS-induced mice compared with the
normal group. Notably, treatment with naringin or mesalazine
significantly suppressed the activation of JAK2/STAT3, as evidenced
by reduced p-JAK2 and p-STAT3 levels, and significantly reduced
IL-6 expression, showing a clear difference compared with the model
group (P<0.05; Fig. 3B-H).
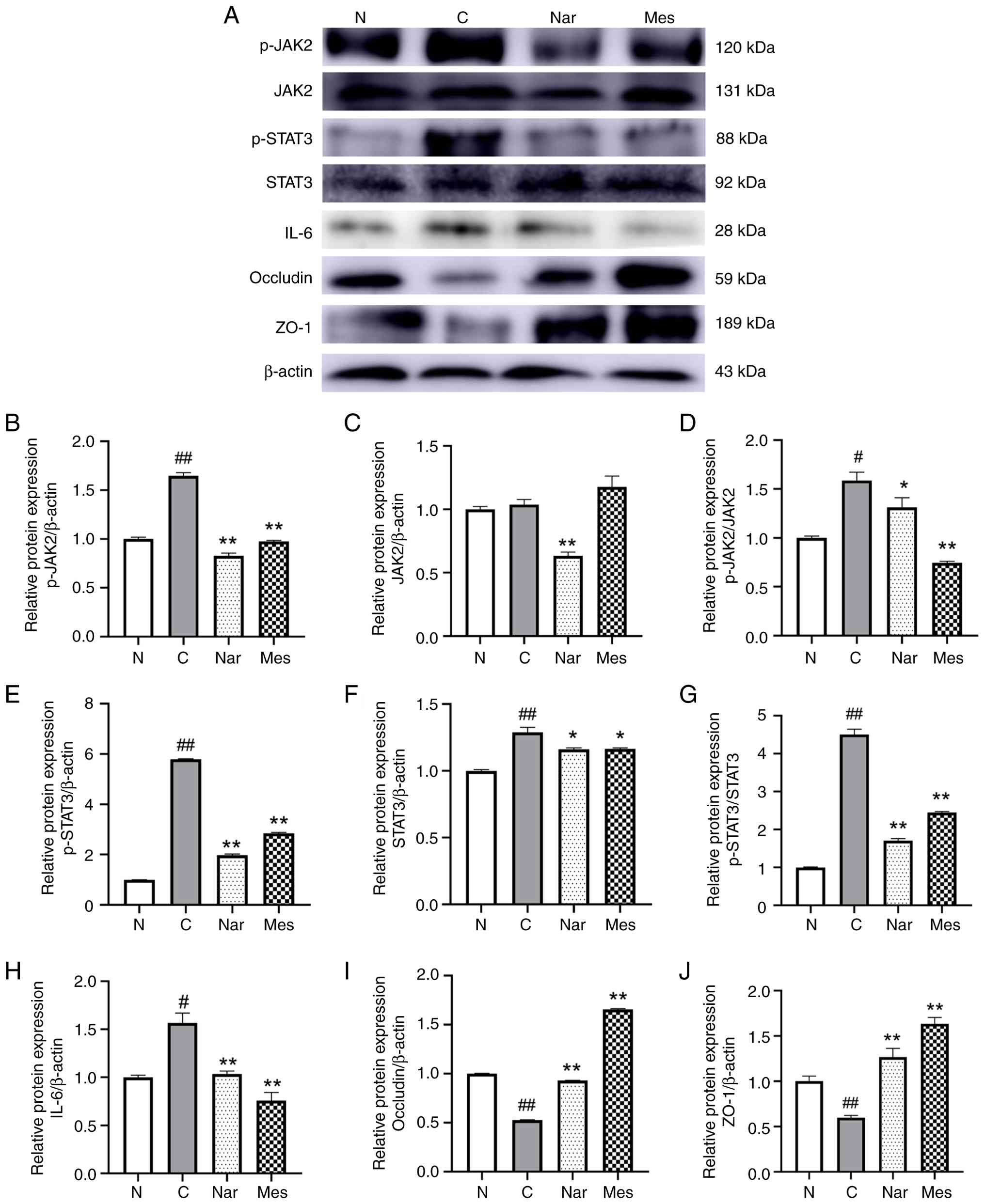 | Figure 3.Naringin inhibits JAK2/STAT3
signaling and restores tight junction proteins in dextran sulfate
sodium-induced colitis. (A) Representative western blotting images
of p-JAK2, JAK2, p-STAT3, STAT3, IL-6, occludin, ZO-1 and β-actin
expression in colon tissues. Densitometric semi-quantification of
the relative protein expression levels of (B) p-JAK2/β-actin, (C)
JAK2/β-actin, (D) p-JAK2/JAK2, (E) p-STAT3/β-actin, (F)
STAT3/β-actin, (G) p-STAT3/STAT3, (H) IL-6/β-actin, (I)
occludin/β-actin and (J) ZO-1/β-actin. Data are presented as mean ±
SEM. n=3. *P<0.05 and **P<0.01 vs. control group;
#P<0.05 and ##P<0.01 vs. normal group.
One-way ANOVA followed by Tukey's post-hoc test was applied. N,
normal group; C, control group; Nar, naringin group; Mes,
mesalazine group; p-, phosphorylated; JAK2, Janus kinase 2; STAT3,
signal transducer and activator of transcription 3; IL-6, ZO-1,
zona occludens-1. |
Tight junction proteins are important structural
components of the intestinal mucosal barrier and are closely linked
to the pathogenesis of UC. Among these, ZO-1 and occludin serve
important roles in maintaining epithelial integrity (28,29).
To further investigate the protective effects of naringin, the
present study assessed their expression in colonic tissues.
Naringin treatment significantly increased the expression of both
ZO-1 and occludin in UC mice, with levels significantly higher than
those in the DSS model group (P<0.01; Fig. 3I and J). Similarly, mesalazine
treatment significantly upregulated the expression of these tight
junction proteins compared with the DSS group. As expected, the
expression levels of ZO-1 and occludin in the DSS control group
were significantly lower than those in the normal group.
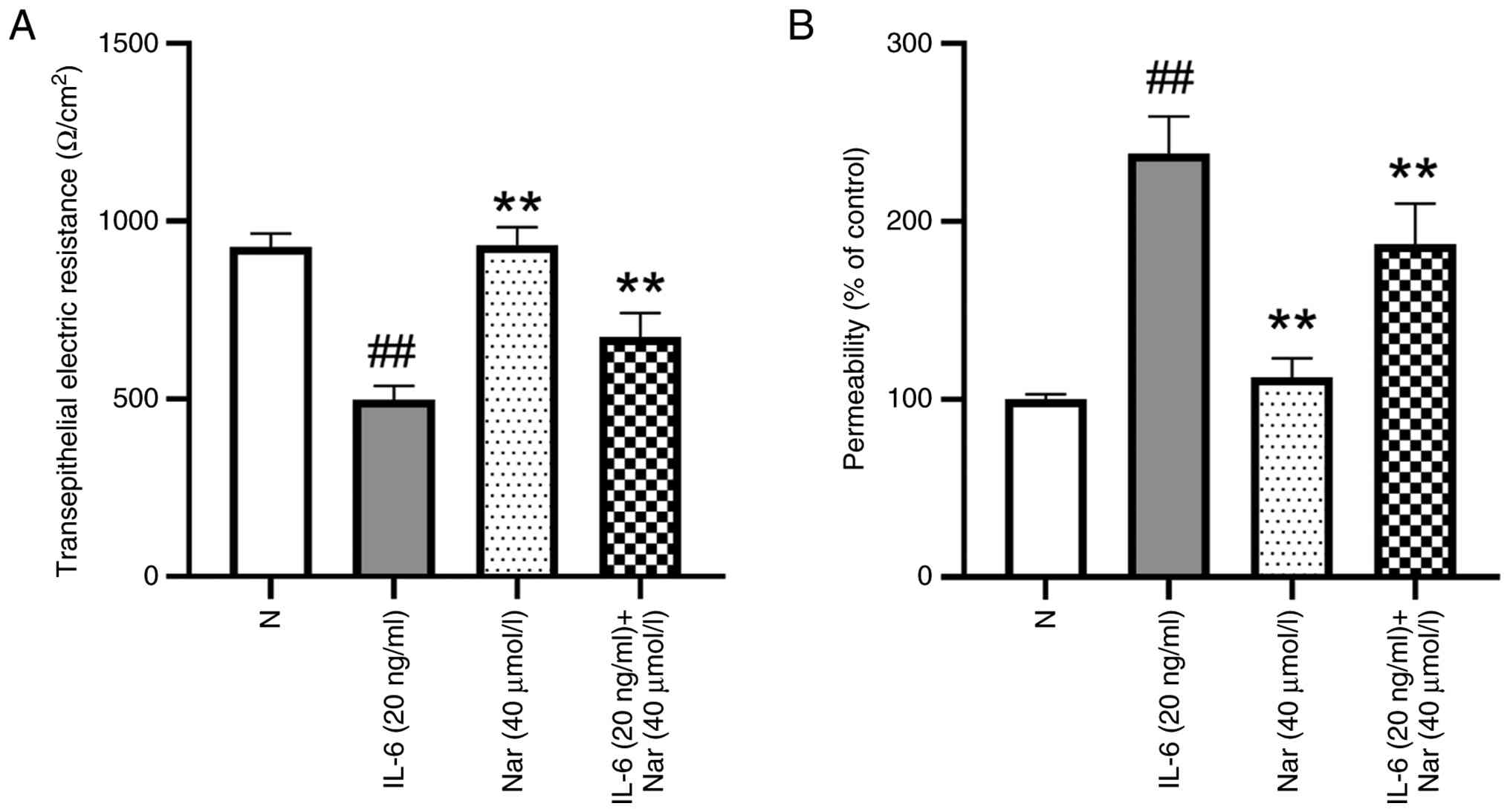
Naringin repairs IL-6-induced barrier
injury in Caco-2 cells
The intestinal mucosal barrier is an important
component of host defense, and its disruption in patients with UC
leads to impaired barrier function and pathological immune
activation. A decrease in TEER is widely recognized as an indicator
of barrier dysfunction (30,31).
To evaluate the protective effect of naringin, the present study
established an IL-6-induced barrier injury model in Caco-2
monolayers. After 10 days of culture, confluent Caco-2 cells formed
a resistant monolayer with a baseline TEER of 927.9
Ω/cm2. IL-6 stimulation caused a significant decrease in
TEER, providing evidence of impaired barrier integrity. Treatment
of IL-6 stimulated cells with naringin significantly restored TEER
values compared with the IL-6 group, whereas naringin alone did not
alter TEER in unstimulated cells compared with the normal group
(P<0.01; Fig. 4A).
To further assess barrier permeability, FD-4 was
added to the upper chamber of Transwell inserts. In IL-6-treated
cells, FD-4 transport into the lower chamber increased 2.3-fold
compared with the normal group, indicating elevated paracellular
permeability. Naringin co-treatment significantly reduced FD-4 flux
compared with the IL-6 group (P<0.01; Fig. 4B). Together, these results
demonstrate that naringin effectively repairs IL-6-induced barrier
damage in Caco-2 cells.
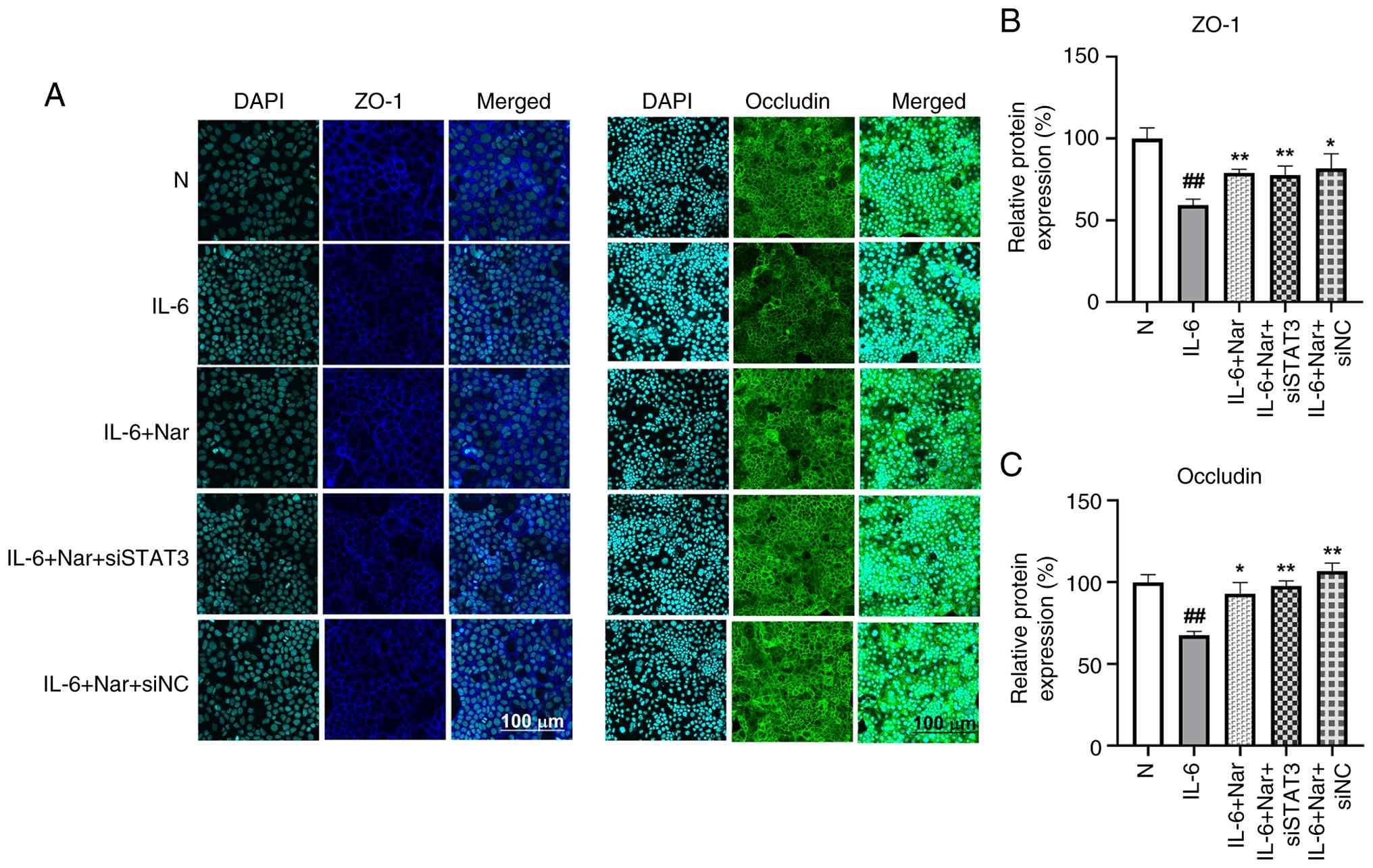
Naringin enhances fluorescent
expression of tight junction proteins in Caco-2 cells
A previous study has shown that naringin can enhance
the mRNA expression of tight junction proteins ZO-1 and occludin in
Caco-2/RAW264.7 co-culture systems (18). To further clarify whether the
mucosal protective effect of naringin was related to the JAK2/STAT3
signaling pathway, the present study used IL-6 to induce mucosal
damage in cells and used STAT3 gene silencing to examine the
mechanism of action of naringin. Tight junction protein expression
was assessed using immunofluorescence (Fig. 5), and JAK2/STAT3 pathway activation
was evaluated using western blot analysis (Fig. 6).
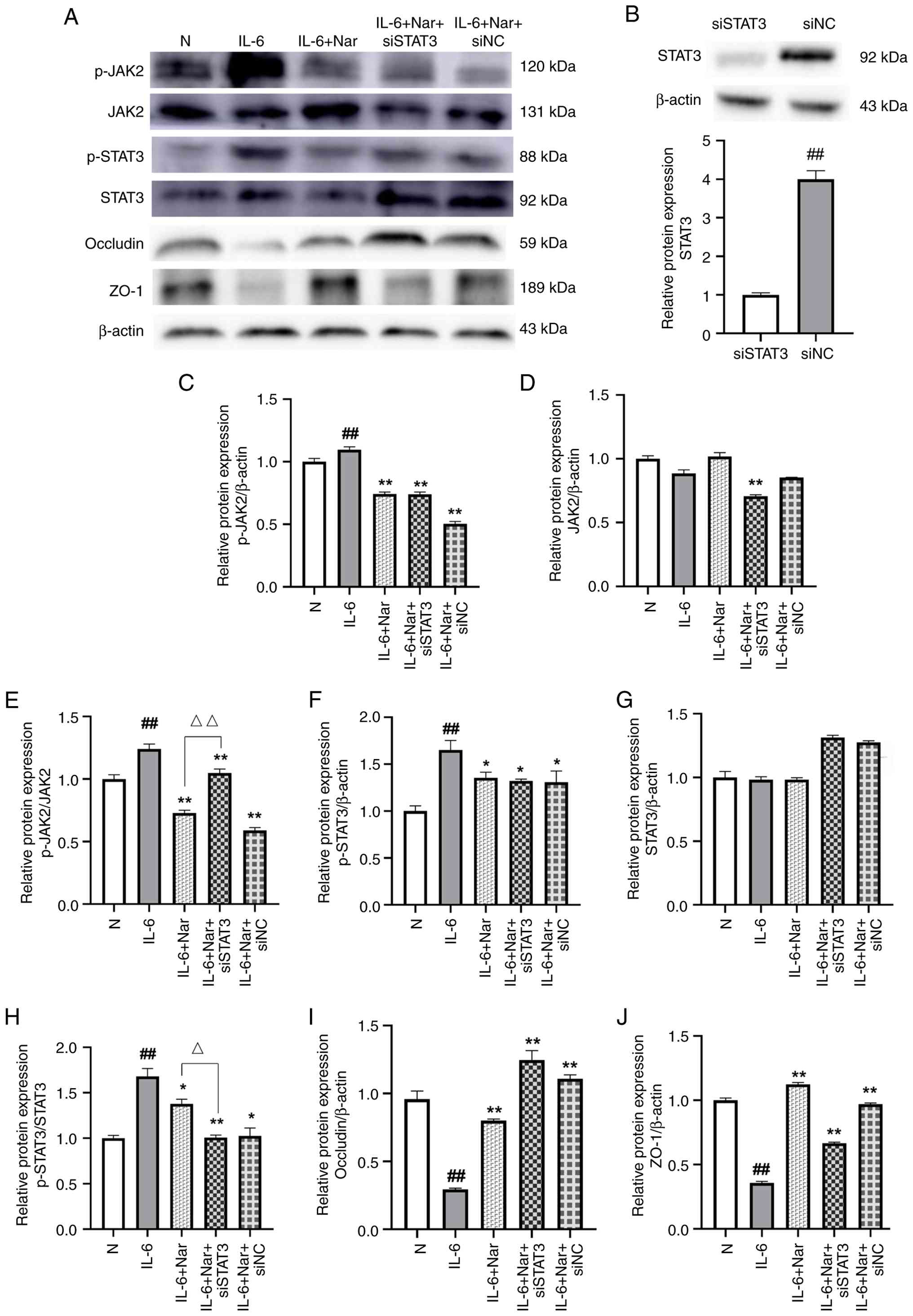 | Figure 6.Naringin suppresses JAK2/STAT3
activation in IL-6-stimulated Caco-2 cells with STAT3 silencing.
(A) Western blot analysis of p-JAK2, JAK2, p-STAT3, STAT3, occludin
and ZO-1 in Caco-2 cells under indicated treatments. (B) Validation
of STAT3 knockdown efficiency: Relative STAT3 expression in cells
transfected with siSTAT3 vs. siNC. ##P<0.01 vs. siNC.
Densitometric semi-quantification of relative protein expression
levels of (C) p-JAK2/β-actin, (D) JAK2/β-actin, (E) p-JAK2/JAK2,
(F) p-STAT3/β-actin, (G) STAT3/β-actin, (H) p-STAT3/ STAT3, (I)
occludin/β-actin, (J) ZO-1/β-actin. Data are presented as mean ±
SEM. n=3. *P<0.05 and **P<0.01 vs. IL-6 group;
##P<0.01 vs. normal group; ΔP<0.05 and
ΔΔP<0.01 IL-6 + Nar group vs. IL-6 + Nar + siSTAT3
group. One-way ANOVA followed by Tukey's post-hoc test was applied.
N, normal group; Nar, naringin group; Mes, mesalazine group; p-,
phosphorylated; JAK2, Janus kinase 2; STAT3, signal transducer and
activator of transcription 3; ZO-1, zona occludens-1; si, small
interfering RNA; NC, negative control. |
Immunofluorescence analysis revealed that in the
normal group, ZO-1 (blue) and occludin (green) displayed strong,
continuous fluorescence signals along cell-cell junctions,
reflecting intact tight junctions. IL-6 stimulation significantly
reduced the fluorescence intensity of both proteins compared with
the normal group (P<0.01), indicating barrier disruption.
Naringin treatment significantly alleviated this reduction,
restoring ZO-1 (P<0.01) and occludin (P<0.05) expression at
cell borders. Notably, the combination of STAT3 silencing and
naringin treatment (IL-6 + Nar + siSTAT3 group) also significantly
restored the fluorescence intensities of ZO-1 and occludin compared
with those in the IL-6 group (P<0.01), to a level comparable
with that achieved by naringin alone (Fig. 5). This supports the conclusion that
naringin-induced protection of tight junctions is mediated, at
least in part, through the inhibition of JAK2/STAT3 signaling
(Fig. 5).
Western blot analysis showed that IL-6 stimulation
significantly increased the expression of p-JAK2 (P<0.01;
Fig. 6C and E) and p-STAT3
(P<0.01; Fig. 6F and H) in
Caco-2 cells compared with the normal group, while JAK2 and STAT3
levels remained largely unchanged (P>0.05; Fig. 6D and G). Transfection with siSTAT3
alone significantly reduced total STAT3 protein levels compared
with siNC transfection (P<0.01; Fig. 6B), confirming knockdown efficiency.
Naringin treatment significantly reduced the expression of p-JAK2
and p-STAT3 relative to the IL-6 group (P<0.05; Fig. 6C, E, F, H). The present study
subsequently examined the effect of STAT3 silencing in the context
of IL-6 and naringin treatment. Compared with in the IL-6 group,
the combination treatment of naringin and STAT3 siRNA (IL-6 + Nar +
siSTAT3) led to a significant reduction in both p-JAK2 (P<0.01;
Fig. 6C and E) and p-STAT3 levels
(P<0.05; Fig. 6F and H).
However, when compared specifically to the IL-6 +
Nar group, the combination treatment (IL-6 + Nar + siSTAT3)
significantly decreased p-STAT3 levels (P<0.05; Fig. 6H). By contrast, p-JAK2 levels were
significantly increased in the IL-6 + Nar + siSTAT3 group relative
to the IL-6 + Nar group (P<0.01; Fig. 6E). An increase in total STAT3
protein was observed in both siRNA co-treatment groups (IL-6 + Nar
+ siSTAT3 and IL-6 + Nar + siNC) compared with that in the normal,
IL-6 and IL-6 + Nar groups (P>0.05; Fig. 6G), which may represent a
compensatory cellular response. Despite this increase in total
STAT3, JAK2/STAT3 pathway activity (i.e., the phosphorylation
levels of JAK2 and STAT3, semi-quantified as p-JAK2/JAK2 and
p-STAT3/STAT3 ratios) in the IL-6 + Nar + siSTAT3 group remained
significantly suppressed compared with in the IL-6 group
(P<0.01; Fig. 6E and H).
Furthermore, analysis of tight junction proteins showed that
naringin treatment (IL-6 + Nar) increased the expression of both
occludin and ZO-1 compared with in the IL-6 group (P<0.01;
Fig. 6I and J). Notably, the
combination treatment with STAT3 silencing (IL-6 + Nar + siSTAT3)
differentially affected the two proteins: It further enhanced
occludin expression but reduced ZO-1 expression relative to the
IL-6 + Nar group. This suggests a complex regulatory role of STAT3
in modulating distinct tight junction components.
Discussion
UC is a chronic, non-specific inflammatory disease
of the gastrointestinal tract that primarily affects the colon and
rectum, and is commonly characterized by abdominal pain, diarrhea
and bloody stool (32,33). Current pharmacological
interventions, such as sulfonamides, corticosteroids and
immunosuppressive agents, can alleviate UC symptoms; however, high
recurrence rates and limited long-term efficacy remain notable
clinical challenges (34,35). Consequently, the development of
effective strategies for treating and preventing UC has attracted
considerable attention globally (34,36).
Citrus aurantium L., dried immature orange, possesses both
nutritional and medicinal value, with flavonoids representing its
principal bioactive constituents. Naringin, the dominant flavonoid,
exhibits multiple pharmacological activities (17,37).
Previous studies have shown that naringin suppresses jejunal
contractions, alleviates intestinal spasms (19), enhances the repair of TNBS-induced
intestinal mucosal barrier damage in experimental mice,
downregulates inflammatory factors such as iNOS, COX-2 and TNF-α,
and preserves cellular permeability and integrity (18) in IBD models. These findings suggest
that naringin may exert protective effects in UC, thereby
supporting its clinical potential as a food-derived therapeutic
agent.
In experimental UC models, the DAI provides a
comprehensive measure of disease severity by integrating weight
loss, stool consistency and presence of blood (38,39).
Colon shortening is another hallmark of DSS-induced UC and serves
as a reliable morphological indicator of disease severity (33,40).
In the present study, DSS administration markedly increased DAI
scores and significantly reduced colon length compared with the
normal group. Naringin treatment ameliorated these parameters,
indicating a protective effect. Histopathological analysis further
revealed that naringin alleviated epithelial necrosis, goblet cell
loss, crypt destruction and inflammatory infiltration in
DSS-treated mice.
Mechanistically, the present study demonstrated that
naringin protected against UC by inhibiting the JAK2/STAT3
signaling pathway and enhancing intestinal barrier integrity. Tight
junction proteins such as ZO-1 and occludin are important for
barrier function, and their disruption leads to increased
permeability, microbial translocation and sustained inflammation
(8–11). The data of the present study showed
that naringin upregulated ZO-1 and occludin expression while
suppressing phosphorylation of JAK2 and STAT3 in colon tissue and
IL-6-stimulated Caco-2 cells. These findings suggest that naringin
restores intestinal barrier integrity through JAK2/STAT3
inhibition.
The JAK2/STAT3 signaling pathway serves a dual role
in UC. In inflamed intestinal tissues of patients with UC, this
pathway is excessively activated and serves as a key driver of
disease progression. However, in healthy intestinal epithelial
cells, the activation of STAT3 can promote the repair of the
intestinal mucosal barrier (15,23,41).
The present study focused on UC mouse intestinal tissue and Caco-2
cells, which are fundamentally different from intestinal epithelial
cells; thus, the JAK2/STAT3 signaling pathway observed in Caco-2
primarily reflected its role in promoting inflammatory reactions.
This interpretation was consistent with multiple previous reports
(22,42,43).
ZO-1 and occludin are important proteins in the tight junction
structures of the intestinal barrier, and their expression and
distribution are usually used to assess the function of the
intestinal barrier in the colon (12–14).
The present data demonstrated that naringin upregulated ZO-1 and
occludin expression while suppressing phosphorylation of JAK2 and
STAT3, highlighting its potential as a dietary intervention for UC.
Future studies are warranted to explore the efficacy of the
clinical translation of naringin.
JAK2 is an important tyrosine protein kinase that
serves an important role in the inflammatory cascade of UC, while
STAT3 is a key transcription factor involved in cell proliferation,
differentiation, apoptosis and the immune and inflammatory response
(44). Accumulating evidence
indicates that activation of the JAK2/STAT3 pathway exacerbates UC
symptoms (45,46). In the present study, IL-6 was
selected to induce mucosal barrier damage and inflammatory
responses in Caco-2 cells, as it is a primary activator of the
JAK2/STAT3 pathway (15,45). Although lipopolysaccharide is
commonly used to model inflammation, it primarily activates
Toll-like receptor 4/NF-κB signaling; therefore, IL-6 was more
appropriate for studying JAK2/STAT3-specific effects. Furthermore,
IL-6 is elevated in patients with UC and contributes to barrier
dysfunction. By silencing the STAT3 gene the protective effect of
naringin on this pathway was explored. IL-6 binds to its soluble
receptor, forms a complex with membrane-bound glycoprotein 130,
activates JAK2, induces STAT3 phosphorylation and activates
downstream transcription factors, regulating the expression of
inflammatory cytokines and thus mediating the occurrence of
inflammatory responses (15).
The results of the present study showed that
naringin significantly reduced the levels of p-JAK2 and p-STAT3 in
the colon tissue of UC mice and inhibited JAK2/STAT3 activation in
IL-6-stimulated Caco-2 cells. The enhanced suppression of pathway
activation in the IL-6 + Nar + siSTAT3 group compared with the IL-6
group confirmed the involvement of the JAK2/STAT3 pathway in
mediating the effects of naringin. Although the combination
treatment (IL-6 + Nar + siSTAT3) reduced p-STAT3 compared with that
in the IL-6 + Nar group, it did not lead to a further reduction in
p-JAK2 levels, indicating a complex interaction. Nevertheless, the
significant reduction in both p-JAK2 and p-STAT3 in the IL-6 + Nar
+ siSTAT3 group compared with the IL-6 group underscores that the
therapeutic action of naringin is mediated, at least in part,
through the suppression of JAK2/STAT3 signaling. Notably, in the
siRNA-treated groups (IL-6 + Nar + siSTAT3 and IL-6 + Nar + siNC)
under IL-6 stimulation, an increase in total STAT3 protein was
observed, which may represent a compensatory cellular response to
STAT3 knockdown. Furthermore, despite this increase in total STAT3
protein, the combination treatment (IL-6 + Nar + siSTAT3) still
achieved notable suppression of JAK2/STAT3 phosphorylation compared
with the IL-6 group. This effective uncoupling of pathway
activation from total STAT3 protein levels underscored that the
therapeutic benefit of naringin was achieved through suppressing
the activation of the JAK2/STAT3 pathway.
Laser confocal microscopy revealed that IL-6
stimulation significantly decreased ZO-1 and occludin expression in
Caco-2 cells, whereas naringin treatment significantly restored
their expression. This restorative effect persisted even after
STAT3 silencing, reinforcing the conclusion that naringin enhances
tight junction protein expression primarily through JAK2/STAT3
inhibition, thereby repairing the intestinal mucosal barrier and
exerting therapeutic effects in UC.
Inflammatory responses were evaluated indirectly
through histological scoring of inflammatory cell infiltration and
directly through activation assessment of the JAK2/STAT3 pathway,
which functions upstream of multiple pro-inflammatory cytokines.
However, direct measurement of cytokine levels, such as those of
TNF-α and IL-1β, would provide additional validation. The effects
of naringin on other inflammatory factors require further
elucidation in subsequent studies.
Although JAK2 inhibition would provide more
comprehensive mechanistic insights, experimental focus was placed
on STAT3 due to resource constraints. Previous investigations on
Citrus aurantium (17,18)
have demonstrated inhibitory effects on JAK2 downstream pathways,
including key inflammatory factors such as TNF-α and NF-κB. Given
that naringin represents a major active component of Citrus
aurantium (47), these
findings provide biological plausibility for the approach in the
present study. The direct targeting of JAK2 by naringin requires
further investigation in future studies. Additionally, while
naringin may potentially interact with other inflammatory pathways
such as the NF-κB, MAPK or NACHT, LRR and PYD domains-containing
protein 3 pathways, the present experimental design specifically
focused on the JAK2/STAT3 axis based on prior evidence from
Citrus aurantium (17,18).
These limitations indicate important directions for future
research. Studies employing molecular docking or surface plasmon
resonance could strengthen mechanistic claims and are recommended
for subsequent investigations. Further studies are warranted to
explore additional mechanisms and validate direct molecular targets
of naringin with the ultimate goal of comprehensively elucidating
its therapeutic potential for UC.
In summary, integrated in vitro and in
vivo evidence indicates that naringin, a flavonoid from the
edible fruit Citrus aurantium L., significantly ameliorates
DSS-induced UC in mice. Its therapeutic effects are mediated
through suppression of the JAK2/STAT3 signaling pathway, leading to
both anti-inflammatory effects and enhanced tight junction protein
expression. These findings support the potential of naringin as a
food-based intervention for UC and provide a molecular basis for
further mechanistic investigation. Although the JAK2/STAT3 pathway
serves a notable role in UC pathogenesis, the precise mechanisms
underlying naringin-induced modulation of this pathway require
further elucidation.
Acknowledgements
Not applicable.
Funding
The present research was supported by the Hebei Youth Fund for
Science and Technology Research in Colleges and Universities (grant
no. QN2022014).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
WH was responsible for funding acquisition,
experimental design, conceptualization, writing the original draft
and supervision. YaW contributed to the experimental design,
assisted in the interpretation of data and critically revised the
manuscript for important intellectual content. MW was contributed
towards writing the original draft, reviewing and editing the
manuscript, formal analysis and data curation. YA contributed to
writing the original draft, reviewing and editing the manuscript,
formal analysis and data curation. YL contributed towards
experimental design and formal analysis. YiW was responsible for
validation and data curation. CW contributed towards validation and
formal analysis. MW and YA confirm the authenticity of all the raw
data. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Animal experiments were approved by the Animal
Welfare Committee of Hebei North University (approval no.
HBNV20240223003) and performed according to institutional
guidelines.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
JAK2
|
Janus kinase 2
|
|
STAT3
|
signal transducer and activator of
transcription 3
|
|
UC
|
ulcerative colitis
|
References
|
1
|
Gros B and Kaplan GG: Ulcerative colitis
in adults: A review. JAMA. 330:951–965. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Segal JP, LeBlanc JF and Hart AL:
Ulcerative colitis: An update. Clin Med (Lond). 21:135–139. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pabla BS and Schwartz DA: Assessing
severity of disease in patients with ulcerative colitis.
Gastroenterol Clin North Am. 49:671–688. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wangchuk P, Yeshi K and Loukas A:
Ulcerative colitis: Clinical biomarkers, therapeutic targets, and
emerging treatments. Trends Pharmacol Sci. 45:892–903. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee SD: Health maintenance in ulcerative
colitis. Gastroenterol Clin North Am. 49:xv–xvi. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cheng WW, Liu BH, Hou XT, Meng H, Wang D,
Zhang CH, Yuan S and Zhang QG: Natural products on inflammatory
bowel disease: Role of gut microbes. Am J Chin Med. 52:1275–1301.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zobeiri M, Momtaz S, Parvizi F, Tewari D,
Farzaei MH and Nabavi SM: Targeting mitogen-activated protein
kinases by natural products: A novel therapeutic approach for
inflammatory bowel diseases. Curr Pharm Biotechnol. 21:1342–1353.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li YY, Wang XJ, Su YL, Wang Q, Huang SW,
Pan ZF, Chen YP, Liang JJ, Zhang ML, Xie XQ, et al: Baicalein
ameliorates ulcerative colitis by improving intestinal epithelial
barrier via AhR/IL-22 pathway in ILC3s. Acta Pharmacol Sin.
43:1495–1507. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu X, Wei S, Chen M, Li J, Wei Y, Zhang J
and Dong W: P2RY13 exacerbates intestinal inflammation by damaging
the intestinal mucosal barrier via activating IL-6/STAT3 pathway.
Int J Biol Sci. 18:5056–5069. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang X, Xie X, Li Y, Xie X, Huang S, Pan
S, Zou Y, Pan Z, Wang Q, Chen J, et al: Quercetin ameliorates
ulcerative colitis by activating aryl hydrocarbon receptor to
improve intestinal barrier integrity. Phytother Res. 38:253–264.
2024. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Niu C, Hu XL, Yuan ZW, Xiao Y, Ji P, Wei
YM and Hua YL: Pulsatilla decoction improves DSS-induced colitis
via modulation of fecal-bacteria-related short-chain fatty acids
and intestinal barrier integrity. J Ethnopharmacol. 300:1157412023.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zong Y, Meng J, Mao T, Han Q, Zhang P and
Shi L: Repairing the intestinal mucosal barrier of traditional
Chinese medicine for ulcerative colitis: A review. Front Pharmacol.
14:12734072023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guo H, Guo H, Xie Y, Chen Y, Lu C, Yang Z,
Zhu Y, Ouyang Y, Zhang Y and Wang X: Mo3Se4
nanoparticle with ROS scavenging and multi-enzyme activity for the
treatment of DSS-induced colitis in mice. Redox Biol.
56:1024412022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang Y, Zhang J, Zhang B, Lu M, Ma J, Liu
Z, Huang J, Ma J, Yang X, Wang F and Tang X: Modified Gegen Qinlian
decoction ameliorated ulcerative colitis by attenuating
inflammation and oxidative stress and enhancing intestinal barrier
function in vivo and in vitro. J Ethnopharmacol. 313:1165382023.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao Y, Luan H, Jiang H, Xu Y, Wu X, Zhang
Y and Li R: Gegen Qinlian decoction relieved DSS-induced ulcerative
colitis in mice by modulating Th17/Treg cell homeostasis via
suppressing IL-6/JAK2/STAT3 signaling. Phytomedicine.
84:1535192021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Y, Lai W, Zheng X, Li K, Zhang Y,
Pang X, Gao J and Lou Z: Linderae Radix extract attenuates
ulcerative colitis by inhibiting the JAK/STAT signaling pathway.
Phytomedicine. 132:1558682024. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
He W, Liu M, Li Y, Yu H, Wang D, Chen Q,
Chen Y, Zhang Y and Wang T: Flavonoids from Citrus aurantium
ameliorate TNBS-induced ulcerative colitis through protecting
colonic mucus layer integrity. Eur J Pharmacol. 857:1724562019.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
He W, Li Y, Liu M, Yu H, Chen Q, Chen Y,
Ruan J, Ding Z, Zhang Y and Wang T: Citrus aurantium L. and
its flavonoids regulate TNBS-induced inflammatory bowel disease
through anti-inflammation and suppressing isolated jejunum
contraction. Int J Mol Sci. 19:30572018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu Q, Liu Y, Liang J, Dai A, Du B, Xi X,
Jin L and Guo Y: Baricitinib relieves DSS-induced ulcerative
colitis in mice by suppressing the NF-κB and JAK2/STAT3 signalling
pathways. Inflammopharmacology. 32:849–861. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Puppala ER, Aochenlar SL, Shantanu PA,
Ahmed S, Jannu AK, Jala A, Yalamarthi SS, Borkar RM, Tripathi DM
and Naidu VGM: Perillyl alcohol attenuates chronic restraint stress
aggravated dextran sulfate sodium-induced ulcerative colitis by
modulating TLR4/NF-κB and JAK2/STAT3 signaling pathways.
Phytomedicine. 106:1544152022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang NN, Yang JW, Ye Y, Huang J, Wang L,
Wang Y, Su XT, Lin Y, Yu FT, Ma SM, et al: Electroacupuncture
ameliorates intestinal inflammation by activating α7nAChR-mediated
JAK2/STAT3 signaling pathway in postoperative ileus. Theranostics.
11:4078–4089. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
He Z, Liu J and Liu Y: Daphnetin
attenuates intestinal inflammation, oxidative stress, and apoptosis
in ulcerative colitis via inhibiting REG3A-dependent JAK2/STAT3
signaling pathway. Environ Toxicol. 38:2132–2142. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wen J, Yang Y, Li L, Xie J, Yang J, Zhang
F, Duan L, Hao J, Tong Y and He Y: Magnoflorine alleviates dextran
sulfate sodium-induced ulcerative colitis via inhibiting JAK2/STAT3
signaling pathway. Phytother Res. 38:4592–4613. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Duan B, Hu Q, Ding F, Huang F, Wang W, Yin
N, Liu Z, Zhang S, He D and Lu Q: The effect and mechanism of
Huangqin-Baishao herb pair in the treatment of dextran sulfate
sodium-induced ulcerative colitis. Heliyon. 9:e230822023.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang J, Zhang C, Guo C and Li X: Chitosan
ameliorates DSS-induced ulcerative colitis mice by enhancing
intestinal barrier function and improving microflora. Int J Mol
Sci. 20:57512019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bao X, Tang Y, Lv Y, Fu S, Yang L, Chen Y,
Zhou M, Zhu B, Ding Z and Zhou F: Tetrastigma hemsleyanum
polysaccharide ameliorated ulcerative colitis by remodeling
intestinal mucosal barrier function via regulating the
SOCS1/JAK2/STAT3 pathway. Int Immunopharmacol. 137:1124042024.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Deng B, Wang K, He H, Xu M, Li J, He P,
Liu Y, Ma J, Zhang J and Dong W: Biochanin A mitigates colitis by
inhibiting ferroptosis-mediated intestinal barrier dysfunction,
oxidative stress, and inflammation via the JAK2/STAT3 signaling
pathway. Phytomedicine. 141:1566992025. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ouyang F, Li B, Wang Y, Xu L, Li D, Li F
and Sun-Waterhouse D: Attenuation of palmitic acid-induced
intestinal epithelial barrier dysfunction by 6-shogaol in Caco-2
cells: The role of MiR-216a-5p/TLR4/NF-κB axis. Metabolites.
12:10282022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Qu Y, Li X, Xu F, Zhao S, Wu X, Wang Y and
Xie J: Kaempferol alleviates murine experimental colitis by
restoring gut microbiota and inhibiting the LPS-TLR4-NF-κB axis.
Front Immunol. 12:6798972021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu XX, Huang XL, Chen RR, Li T, Ye HJ, Xie
W, Huang ZM and Cao GZ: Paeoniflorin prevents intestinal barrier
disruption and inhibits lipopolysaccharide (LPS)-induced
inflammation in Caco-2 cell monolayers. Inflammation. 42:2215–2225.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Spalinger MR, Sayoc-Becerra A, Santos AN,
Shawki A, Canale V, Krishnan M, Niechcial A, Obialo N, Scharl M, Li
J, et al: PTPN2 regulates interactions between macrophages and
intestinal epithelial cells to promote intestinal barrier function.
Gastroenterology. 159:1763–1777.e14. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xiong T, Zheng X, Zhang K, Wu H, Dong Y,
Zhou F, Cheng B, Li L, Xu W, Su J, et al: Ganluyin ameliorates
DSS-induced ulcerative colitis by inhibiting the enteric-origin
LPS/TLR4/NF-κB pathway. J Ethnopharmacol. 289:1150012022.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yuan SN, Wang MX, Han JL, Feng CY, Wang M,
Wang M, Sun JY, Li NY, Simal-Gandara J and Liu C: Improved colonic
inflammation by nervonic acid via inhibition of NF-κB signaling
pathway of DSS-induced colitis mice. Phytomedicine. 112:1547022023.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wu Y, Jha R, Li A, Liu H, Zhang Z, Zhang
C, Zhai Q and Zhang J: Probiotics (Lactobacillus plantarum HNU082)
supplementation relieves ulcerative colitis by affecting intestinal
barrier functions, immunity-related gene expression, gut
microbiota, and metabolic pathways in mice. Microbiol Spectr.
10:e01651222022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jeong DY, Kim S, Son MJ, Son CY, Kim JY,
Kronbichler A, Lee KH and Shin JI: Induction and maintenance
treatment of inflammatory bowel disease: A comprehensive review.
Autoimmun Rev. 18:439–454. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang M, Fu R, Xu D, Chen Y, Yue S, Zhang S
and Tang Y: Traditional Chinese Medicine: A promising strategy to
regulate the imbalance of bacterial flora, impaired intestinal
barrier and immune function attributed to ulcerative colitis
through intestinal microecology. J Ethnopharmacol. 318:1168792024.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Alam F, Mohammadin K, Shafique Z, Amjad ST
and Asad MHHB: Citrus flavonoids as potential therapeutic agents: A
review. Phytother Res. 36:1417–1441. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mirsepasi-Lauridsen HC: Therapy used to
promote disease remission targeting gut dysbiosis, in UC patients
with active disease. J Clin Med. 11:74722022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wu Y, Ran L, Yang Y, Gao X, Peng M, Liu S,
Sun L, Wan J, Wang Y, Yang K, et al: Deferasirox alleviates
DSS-induced ulcerative colitis in mice by inhibiting ferroptosis
and improving intestinal microbiota. Life Sci. 314:1213122023.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ruan Y, Zhu X, Shen J, Chen H and Zhou G:
Mechanism of Nicotiflorin in San-Ye-Qing rhizome for
anti-inflammatory effect in ulcerative colitis. Phytomedicine.
129:1555642024. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tang L, Liu Y, Tao H, Feng W, Ren C, Shu
Y, Luo R and Wang X: Combination of Youhua Kuijie Prescription and
sulfasalazine can alleviate experimental colitis via
IL-6/JAK2/STAT3 pathway. Front Pharmacol. 15:14375032024.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shalaby M, Abdelaziz RR, Ghoneim HA and
Suddek GM: Imatinib mitigates experimentally-induced ulcerative
colitis: Possible contribution of NF-kB/JAK2/STAT3/COX2 signaling
pathway. Life Sci. 321:1215962023. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yu T, Li Z, Xu L, Yang M and Zhou X:
Anti-inflammation effect of Qingchang suppository in ulcerative
colitis through JAK2/STAT3 signaling pathway in vitro and in vivo.
J Ethnopharmacol. 266:1134422021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jiang M, Zhong G, Zhu Y, Wang L, He Y, Sun
Q, Wu X, You X, Gao S, Tang D and Wang D: Retardant effect of
dihydroartemisinin on ulcerative colitis in a JAK2/STAT3-dependent
manner. Acta Biochim Biophys Sin (Shanghai). 53:1113–1123. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang X, Huang S, Zhang M, Su Y, Pan Z,
Liang J, Xie X, Wang Q, Chen J, Zhou L and Luo X: Gegen Qinlian
decoction activates AhR/IL-22 to repair intestinal barrier by
modulating gut microbiota-related tryptophan metabolism in
ulcerative colitis mice. J Ethnopharmacol. 302:1159192023.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Voshagh Q, Anoshiravani A, Karimpour A,
Goodarzi G, Tehrani SS, Tabatabaei-Malazy O and Panahi G:
Investigating the association between the tissue expression of
miRNA-101, JAK2/STAT3 with TNF-α, IL-6, IL-1β, and IL-10 cytokines
in the ulcerative colitis patients. Immun Inflamm Dis.
12:e12242024. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Miles EA and Calder PC: Effects of citrus
fruit juices and their bioactive components on inflammation and
immunity: A narrative review. Front Immunol. 12:7126082021.
View Article : Google Scholar : PubMed/NCBI
|