Introduction
Chronic myeloid leukemia (CML) is a pluripotent
hematopoietic stem cell disorder defined by expression of the
BCR/ABL fusion gene, a constitutively activated tyrosine kinase,
harbored by the Philadelphia chromosome (Ph), which is the result
of a t(9;22)(q34;q11) or a related variant translocation (1). In approximately 1% of CML patients,
bone marrow cells appear to be Ph-negative by G-banding, although
the BCR/ABL fusion gene can be identified by molecular means and
can be located by fluorescence in situ hybridization (FISH)
on chromosome 22q11, 9q34 or even a third chromosome (2–5). Cases
of Ph-negative BCR/ABL-positive CML with the chimeric gene present
on derivative chromosome der(22), as in the majority of CML cases,
or alternatively on der(9) appear
to have the same clinical and molecular characteristics as
Ph-positive patients. However, a worse prognosis associated with
the location of BCR/ABL on der(9)
was previously noted (6–9).
The biology and clinical significance of genetic
rearrangements in Ph-negative BCR/ABL-positive disease was
evaluated following initial descriptions (2–5). Two
mechanisms involved in the formation of the chimeric gene in masked
Ph-positive cells have been postulated: insertion of ABL into the
BCR region (or vice versa), or by a multiple-step model where a
classical t(9;22) is followed by translocation of the two products
and/or another autosome, thereby restoring the normal chromosome
morphology. In both instances, more than the 2 breaks associated
with classical t(9;22) are implicated (10).
This study investigated a rare CML case,
Ph-negative, with an insertion of the 3′ ABL region into the long
arm of derivative chromosome 1 and lacking the 5′ BCR region on
der(22).
Materials and methods
Case report
In July 2005, a female patient, 31 years of age,
presented for the first time with a whole blood cell count (WBC) of
35.07×109/l (72.3% neutrophils, 13% lymphocytes, 1.16%
eosinophiles, 5.7% monocytes, 4.1% basophiles and 3.6% immature
cells). The platelet count was 381×109/l, and the
hemoglobin level was 9.8 g/dl. A physical examination showed no
splenomegaly, although loss of weight was noted. Results of a
chromosome analysis using banding cytogenetics showed a karyotype
in concordance with the clinical diagnosis of CML in the chronic
phase (CP). The patient was treated with hydroxyurea (1500 mg daily
dose) for a duration of two years and eleven months. In June 2008,
the patient presented for the second time with a WBC count of
20.86×109/l (73% neutrophils, 15.2% lymphocytes, 1.2%
eosinophiles, 5.3% monocytes and 5.3% basophiles). The platelet
count was 497×109/l, and the hemoglobin level was 12.9
g/dl. Serum lactate dehydrogenase (LDH) was 1274 U/l (normal up to
460 U/l), serum alanine aminotransferase was 54 U/l (normal up to
40 U/l) and serum aspartate aminotransferase was 41 U/l (normal up
to 40 U/l). The patient was treated with hydroxyurea (1500 mg daily
dose), but was subsequently lost during follow-up.
Banding cytogenetics
Banding cytogenetics using the GTG-method was
conducted according to standard procedures (11). A total of 20 metaphases derived from
the unstimulated bone marrow of the patient were analyzed
individually. Karyotypes were described according to the
International System for Human Cytogenetic Nomenclature (12).
Fluorescence in situ hybridization
FISH using BCR/ABL dual-color dual fusion
translocation probe (Abbott Molecular/Vysis, USA) and whole
chromosome painting (wcp) probe for chromosomes 1, 9 and 22
(MetaSystems, Germany) were applied as previously described
(11). A total of 50 metaphase
spreads were analyzed, each using a fluorescence microscope (Axio
Imager.Z1 mot; Zeiss) equipped with appropriate filter sets to
discriminate between a maximum of five fluorochromes and the
counterstain DAPI (4′,6-diamino-2-phenylindole). Image capturing
and processing were carried out using an image analysis system
(MetaSystems, Altlussheim, Germany).
Results
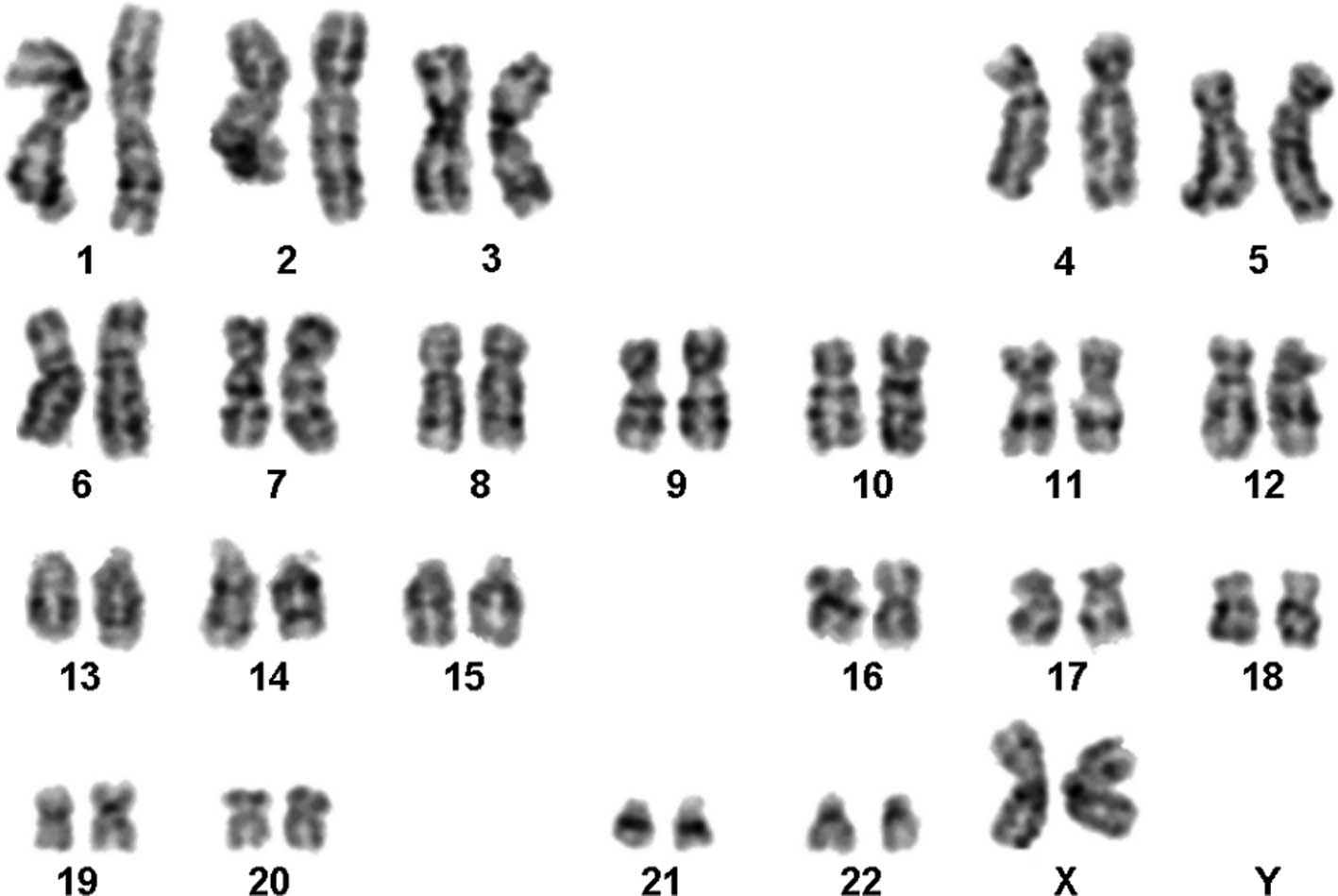
A normal karyotype 46,XX was determined in the
GTG-banding (Fig. 1) and was
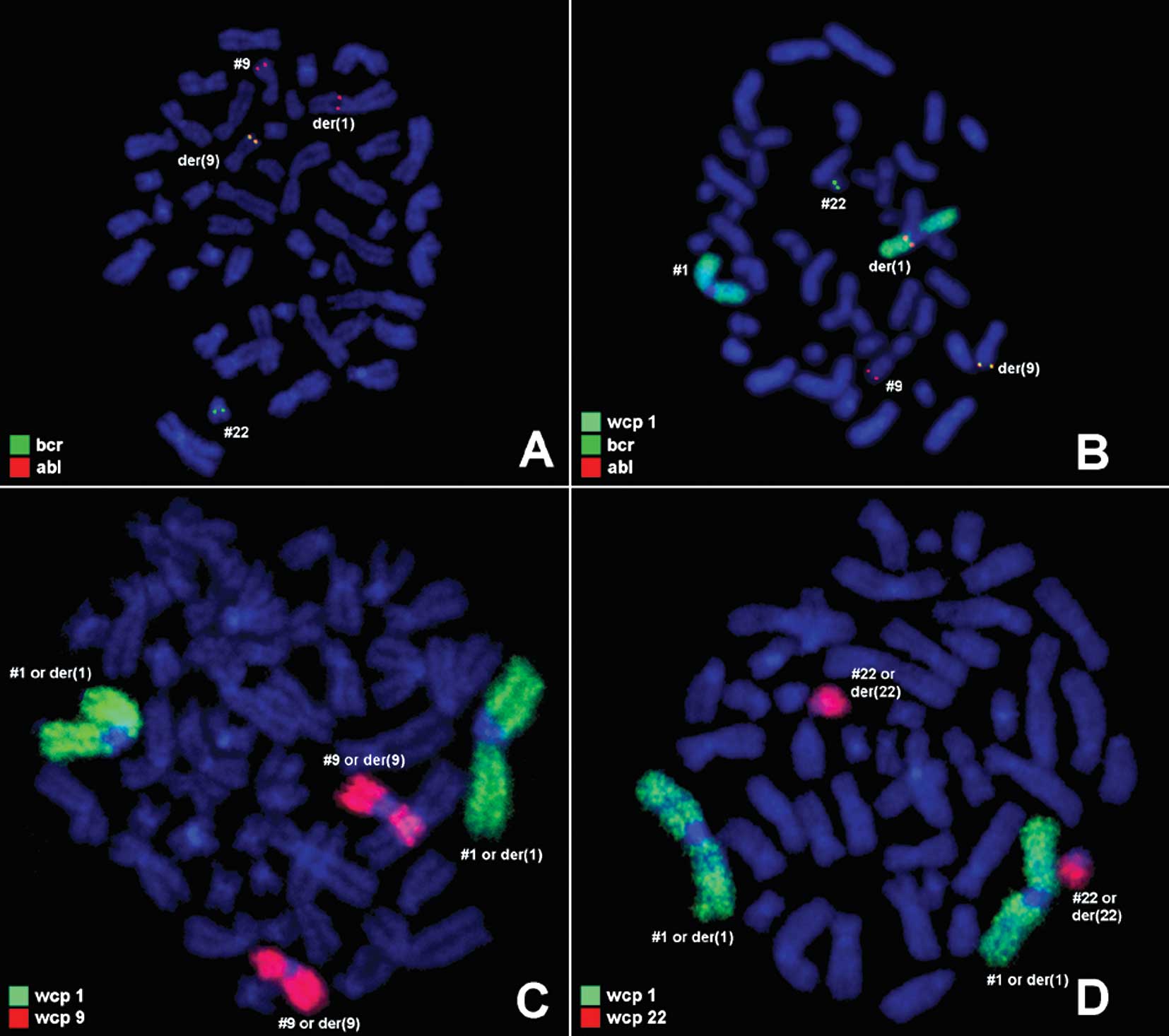
further studied by molecular cytogenetics (Fig. 2). Dual-color-FISH using probes
specific for BCR and ABL revealed that the BCR/ABL-translocation
fusion gene was absent on der(22). However, the presence of the
ABL/BCR-translocation fusion gene on der(9), insertion of the 3′ ABL region on
der(1) and lack of the 5′ BCR
region on der(22) were noted (Fig. 2A
and B). Moreover, FISH using wcp1, wcp9 and wcp22 probes did
not show a translocation between chromosome 1 and 9 or chromosome 1
and 22 (Fig. 2C and D). Thus, the
result obtained was:
46,XX,ins(1;9)(q21.3;q34q34),ins(9;22)(q34;q11q11),del(22)(q11q11).
Discussion
The present study identified one additional
chromosomal alteration, insertion of the 3′ ABL region on
der(1), in a Ph-negative
BCR/ABL-positive CML-CP case. To the best of our knowledge, the
insertion of the ABL region into der(1) has never been described in CML
(13).
According to the literature, two alternative
mechanisms were postulated to elucidate the formation of a fusion
gene in Ph-negative BCR/ABL-positive CML patients (10,14).
The first mechanism involves a one-step model where BCR/ABL results
from a simple insertion of either 3′ ABL1 into BCR or 5′ BCR into
ABL after three genomic breaks. The second mechanism is a
multiple-step model involving an initial classical t(9;22)(q34;q11)
followed by a second translocation of the two products and/or a
third chromosome, requiring a minimum of 4 genomic breaks (14).
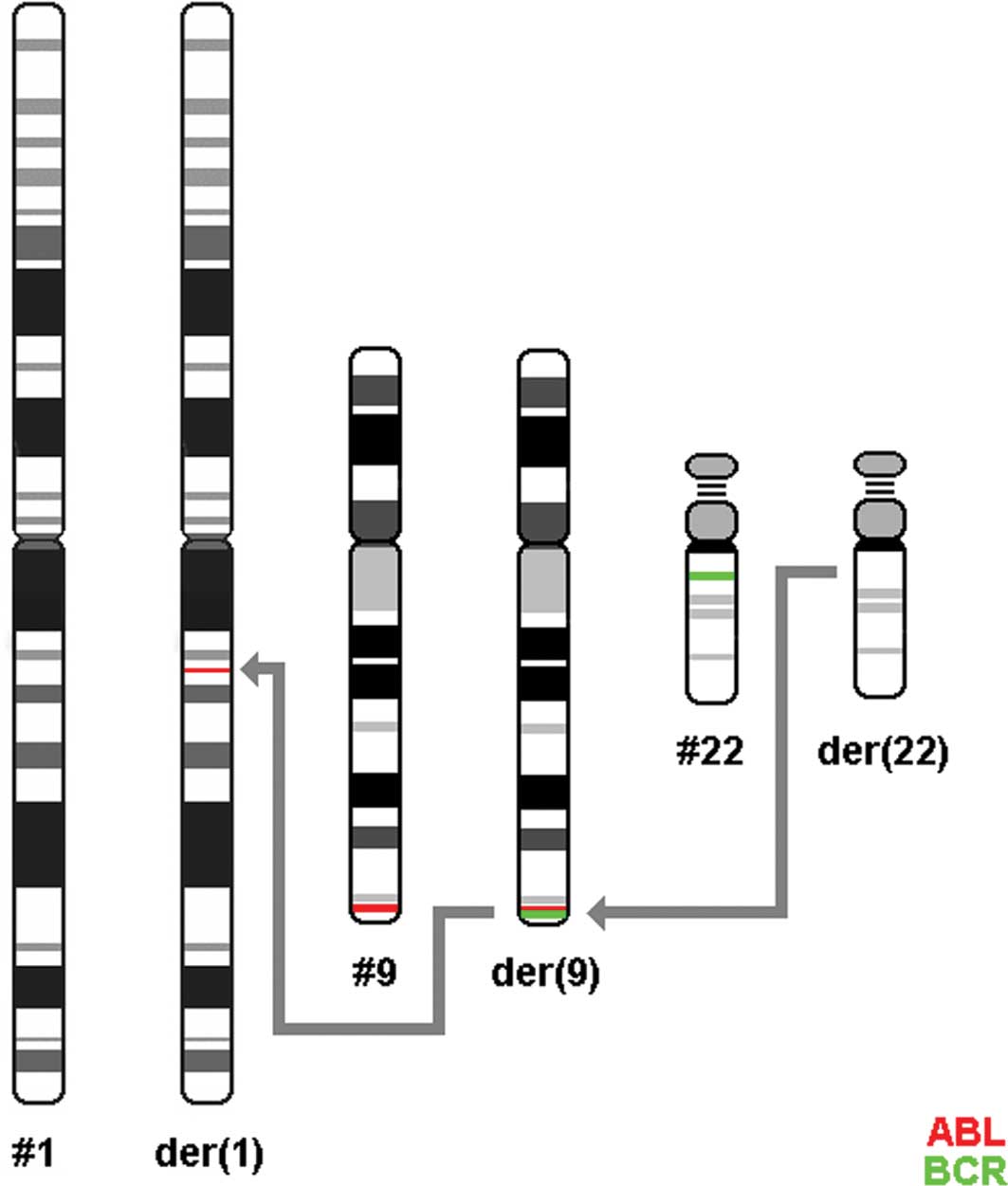
In the present case, FISH results led to the
conclusion that insertion of 5′ BCR sequences within the ABL gene
transpired while 3′ BCR sequences remained on chromosome 22
(Fig. 3). Previous studies on other
Ph-negative patients with the BCR-ABL fusion gene at 9q34 also
proposed the insertion of chromosome 22 sequences into chromosome 9
as the more likely mechanism, as opposed to one involving two
consecutive translocations. This event appears more likely as it
requires only two breaks at chromosome 22 and one at chromosome 9
instead of a total of four breaks involved in the
double-consecutive translocation (15).
A second event, insertion of 3′ ABL sequences on
der(1), possibly shifted ABL from
der(22) to the long arm of chromosome 1. The 5′ region of the BCR
gene was deleted on der(22). The patient presented with CML-CP.
Thus, the second event may be the evolution of a Ph-negative
karyotype.
In conclusion, regarding the karyotype observed in
the present case with the insertion mechanism, a minimum of five
breaks are required; one on chromosome 1, two on chromosome 9 and
two on chromosome 22, to explain the insertion and deletion.
Acknowledgements
We thank Professor I. Othman, Director General of
the Atomic Energy Commission of Syria (AECS) and Dr N. Mirali, Head
of the Molecular Biology and Biotechnology Department for their
support. This work was supported by the Syrian Atomic Energy
Commission, in part, by the Stefan-Morsch-Stiftung,
Monika-Kutzner-Stiftung and the DAAD (D/07/09624).
References
|
1
|
Melo JV and Barnes DJ: Chronic myeloid
leukaemia as a model of disease evolution in human cancer. Nat Rev
Cancer. 7:441–453. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hagemeijer A, de Klein A, Godde-Salz E,
Turc-Carel C, Smit EM, van Agthoven AJ and Grosveld GC:
Translocation of c-abl to ‘masked’ Ph in chronic myeloid leukemia.
Cancer Genet Cytogenet. 18:95–104. 1985.
|
|
3
|
Morris CM, Reeve AE, Fitzgerald PH,
Hollings PE, Beard ME and Heaton DC: Genomic diversity correlates
with clinical variation in Ph’-negative chronic myeloid leukaemia.
Nature. 320:281–283. 1986.
|
|
4
|
Hagemeijer A, Buijs A, Smit E, Janssen B,
Creemers GJ, van der Plas D and Grosveld G: Translocation of BCR to
chromosome 9: a new cytogenetic variant detected by FISH in two
Ph-negative, BCR-positive patients with chronic myeloid leukemia.
Genes Chromosomes Cancer. 8:237–245. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nacheva E, Holloway T, Brown K, Bloxham D
and Green AR: Philadelphia-negative chronic myeloid leukaemia:
detection by FISH of BCR-ABL fusion gene localized either to
chromosome 9 or chromosome 22. Br J Haematol. 87:409–412. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Aurich J, Dastugue N, Duchayne E,
Schlaifer D, Rigal-Huguet F and Caballin MR: Location of the
BCR-ABL fusion gene on the 9q34 band in two cases of Ph-positive
chronic myeloid leukemia. Genes Chromosomes Cancer. 20:148–154.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brunel V, Sainty D, Costello R,
Mozziconacci MJ, Simonetti J, Arnoulet C, Coignet L, Bouabdallah R,
Gastaut JA, Gabert J and Lafage-Pochitaloff M: Translocation of BCR
to chromosome 9 in a Philadelphia-negative chronic myeloid
leukemia. Cancer Genet Cytogenet. 85:82–84. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hsu WT, Preisler H, Szego K, Sprudzs R and
Gao XZ: The ABL/BCR fusion gene on chromosome 9 in Ph-negative
chronic myelogenous leukemia: a case for vigilance in fluorescence
in situ hybridization interpretation. Cancer Genet Cytogenet.
104:57–60. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Michalova K, Zemanova Z, Bkezinova J,
Moravcova J, Oltova A, Sobotka J, Kuglik P, Kozak T, Sindelarova L,
Jankovska M, Obomilova A, Sieglova Z, Polak J, Nadvornikova S and
Haskovec C: Location of the BCR/ABL fusion genes on both
chromosomes 9q34 in Ph-negative chronic myeloid leukemia. Leuk
Lymphoma. 43:1695–1700. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Takahashi N, Miura I, Ohshima A, Utsumi S,
Nimura T, Hashimoto K, Saito M and Miura AB: Duplication of
chromosome 9 carrying a BCR/ABL chimeric gene in Philadelphia
chromosome-negative chronic myeloid leukemia. Cancer Genet
Cytogenet. 89:166–169. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Al-achkar W, Wafa A and Nweder MS: A
complex translocation t(5;9;22) in Philadelphia cells involving the
short arm of chromosome 5 in a case of chronic myelogenous
leukemia. J Experimental Clin Cancer Res. 26:411–415.
2007.PubMed/NCBI
|
|
12
|
Shaffer L, Slovak M and Cambell L: ISCN
(2009): An International System for Human Cytogenetic Nomenclature.
S. Karger; Basel: 2009
|
|
13
|
Mitelman F, Johansson B and Mertens F:
Mitelman Database of Chromosome Aberrations in Cancer (2009).
http://cgap.nci.nih.gov/Chromosomes/Mitelman.
|
|
14
|
Morris CM, Heisterkamp N, Kennedy MA,
Fitzgerald PH and Groffen J: Ph-negative chronic myeloid leukemia:
molecular analysis of ABL insertion into M-BCR on chromosome 22.
Blood. 76:1812–1818. 1990.PubMed/NCBI
|
|
15
|
Virgili A, Brazma D, Reid AG,
Howard-Reeves J, Valgañón M, Chanalaris A, De Melo VA, Marin D,
Apperley JF, Grace C and Nacheva EP: FISH mapping of
Philadelphia-negative BCR/ABL1-positive CML. Mol Cytogenet.
1:142008. View Article : Google Scholar : PubMed/NCBI
|