Introduction
Genital human papillomavirus (HPV) is the most
common virus of sexually transmitted infections. In the 1970s, it
was suggested that the possible role of HPV in cancer be
investigated (1). It was recently
established that HPV is crucial in human carcinogenesis. HPV causes
infected epithelial cells in mucous membranes and skin to become
abnormal. More than 40 HPV genotypes are able to infect the genital
organs of females and males, including the vulva, vagina, cervix
and penis (2,3). Although HPV-DNA is detected in
numerous cervical cancer tissues, the presence of HPV may be
insufficient by itself to establish full malignant transformation.
Other factors should therefore be considered in the carcinogenic
process. The synergistic action of HPV associated with poor
hygienic condition may be required for malignant transformation.
Epidemiological evidence suggests that numerous individuals are
infected with HPV, although only a small percentage progress to
being classified as malignant over a period of years, often decades
(4,5).
The causal relationship between chronic inflammation
and cancer is widely accepted. Numerous investigators have
identified nuclear factor-κB (NF-κB) as a key modulator in driving
chronic inflammation to neoplastic cells. This transcriptional
factor is indispensable in the malignant progression of
transforming cells within the various inflammatory conditions
containing a network of signaling molecules. The NF-κB
transcription factor family in mammals comprises the proteins, RelA
(p65), RelB, c-Rel, p105/p50 (NF-κB1) and p100/p52 (NF-κB2). These
proteins form homo- and heterodimeric complexes through their
conserved prototypical Rel homology domain. NF-κB plays a critical
role in the diverse cellular processes associated with
proliferation, cell death and development. Experimental evidence
showing specific mechanisms by which NF-κB affects cancer
initiation, promotion and progression has been reported (6,7). The
expression and function of numerous cytokines, chemokines, growth
factors and survival factors are NF-κB-dependent. NF-κB activation
plays a role in a variety of processes correlated to transformation
and oncogenesis (8).
Initially, we attempted to detect HPV genotypes
employing PCR and DNA sequences as in our previous studies
(9–11), but the HPV-DNA could not be
extracted from the paraffin-embedded tissues. Therefore, in
situ hybridization (ISH) was used to confirm the presence of
HPV in cervical cancer. Cervical cancer is the most common cancer
found among females in sub-Saharan Africa. This study aimed to
determine the relationship between HPV infection and NF-κB in
cervical cancer in Western Kenya. This report also investigated HPV
in penile cancer from the same study area (12). To the best of our knowledge, the
relationship between cervical and penile cancer associated with HPV
in the same area using tissue materials has yet to be reported.
Materials and methods
Tissue specimens
The biopsy materials studied were obtained from 62
cervical cancer cases from specimens that had been submitted for
pathologic diagnosis to the Department of Histopathology, Rift
Valley Provincial General Hospital from various hospitals in the
western part of Kenya (Western, Nyanza and Rift Valley provinces,
1983–2000). This was a retrospective study and the specimens used
were archival. This investigation was authorized by the Government
of Kenya (research permit No. OP.13/001/8C224/36). The specimens
were fixed in 10% formalin, embedded in paraffin and examined
histologically and by ISH. Histological analysis was carried out
using 3.5 μm sections of tissue stained with H&E. Parallel
sections were prepared for ISH. Findings from the cervical cancer
were compared to those of the penile cancer in a previous study
(12).
In situ hybridization
The paraffin-embedded tissue specimens were cut into
3.5 μm sections and collected on silane-coated glass slides. For
the detection of HPV-DNA, an HPV screening detection kit (Kreatech
Diagnostics Co., Amsterdam, The Netherlands) was used. Pan HPV-DNA
probe, ISH-positive control probe, ISH-negative control probe and
HPV-positive control slides (supplied with the kit) were examined.
The detection kit used a digoxigenin-labeled pan HPV-encoded DNA
probe, which is composed of a mixture of HPV 6, 11, 16, 18, 31 and
33. ISH-negative control is a digoxigenin-labeled DNA probe,
derived from plasmid DNA (pSP) and does not contain any sequences
of human or viral origin. The steps involved in the ISH procedure
using the HPV screening detection kit are: following hybridization
with the probes, alkaline phosphatase-conjugated antibody against
digoxigenin was applied to the sections. The localization of
HPV-DNA was detected using a NBT/BCIP substrate and observed under
a light microscope.
Immunohistochemistry of NF-κB
Sections of 3.5 μm were placed on silane-coated
glass slides. The sections were deparaffinized to remove the
embedded medium and dehydrated. The slides were then boiled in 0.01
mol/l citrate buffer, pH 7.0, at 98°C for 40 min for antigen
retrieval and cooled at room temperature for 30 min. After the
slides had been rinsed in 0.01 M phosphate-buffered saline (PBS),
pH 7.4, the endogenous peroxidase activity was blocked with 3%
H2O2 and absolute methanol for 10 min. The
tissue sections were covered with 1:50 dilution of mouse monoclonal
anti-human NF-κB antibody (Cell Signaling Technology Inc., Beverly,
MA, USA) or control serum at 37°C for 3 h. After being washed with
PBS, the sections were covered with the peroxidase-labeled dextran
polymer (Dako, Carpinteria, CA, USA) at 37°C for 40 min and rinsed
in PBS. Target antigenic sites on the sections were demonstrated by
reacting with a chromogen of 0.05% 3,3′-diaminobenzidine
tetrahydrochloride in 0.05 M Tris-HCl buffer and 0.01% hydrogen
peroxide for 10 min. The sections were then counterstained with
methyl green for 10 min, dehydrated in ethanol, cleared in xylene
and mounted.
Statistical analysis
Statistical analysis was performed for possible
relationships between the HPV infection and nuclear and/or
cytoplasmic expression of NF-κB.
Results
Clinicopathological findings
The results of the comparison between cervical and
penile cancer are shown in Table I.
Variations were found in the clinical data between the two types of
cancer. The age at the initial diagnosis of the 62 cervical cancer
patients ranged from 18 to 73 years (mean 50.1). The earliest age
of onset and mean age of cervical cancer were 18 and 50.1,
respectively. The cervical carcinomas were investigated
histologically, and divided into 54 keratinizing and 8
non-keratinizing squamous cell carcinomas.
 | Table IComparison between cervical and penile
cancer in Western Kenya. |
Table I
Comparison between cervical and penile
cancer in Western Kenya.
| Cervical cancer | Penile cancer |
|---|
| Earliest age of onset
(years) | 18.0 | 35.0 |
| Mean age (years) | 50.1 | 59.6 |
| Rate of HPV
infection | 45.2% | 68.2% |
| NF-κB expression in
HPV infection case | 96.4% | 100.0% |
| NF-κB expression in
HPV non-infection case | 52.9% | 28.6% |
Detection of HPV-DNA and NF-κB
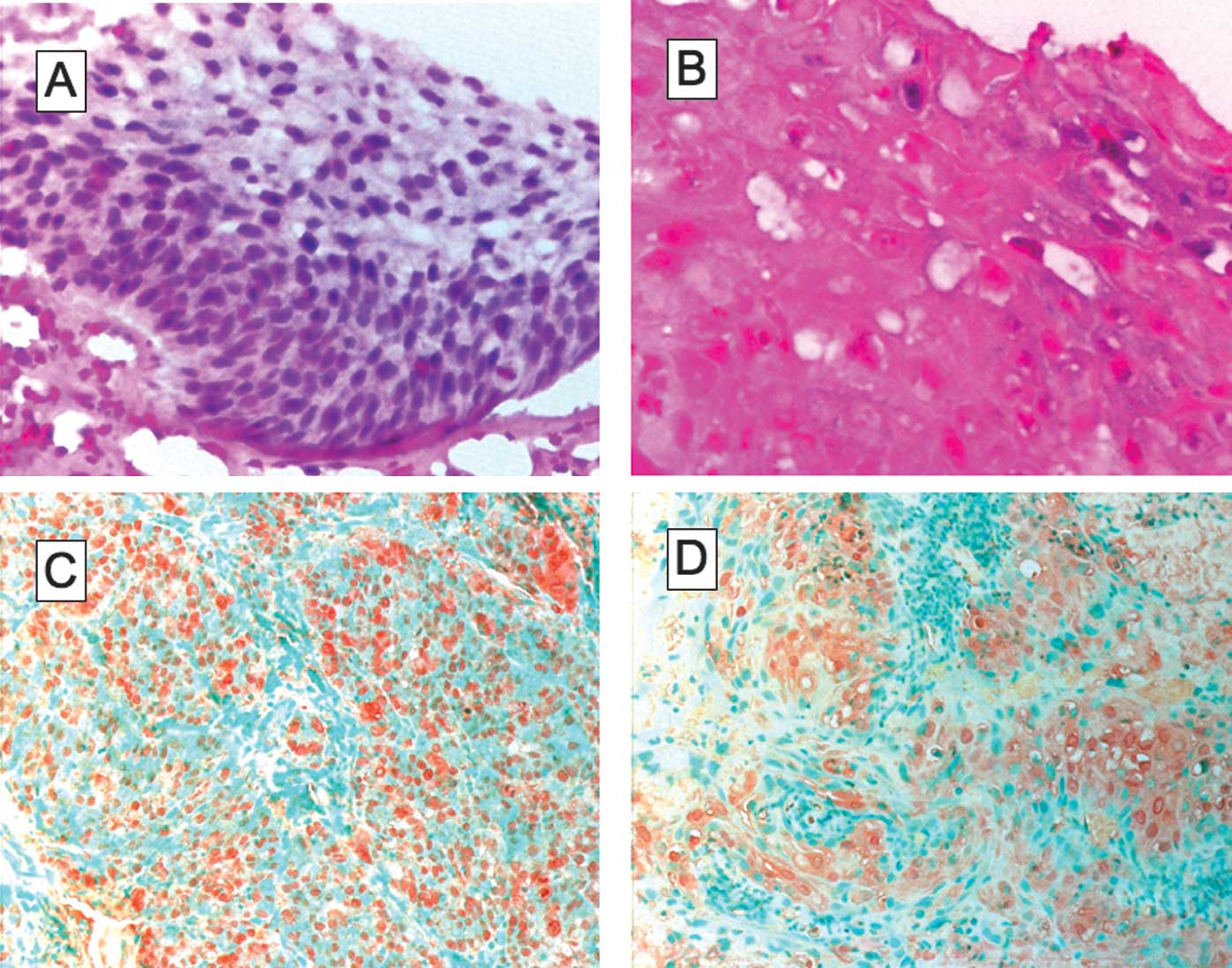
ISH showed that 28 cases (45.2%) were
HPV-DNA-positive (Fig. 1A and B).
This frequency was lower than in penile cancer (68.2%) according to
a previous study (12). Of the 54
cases of keratinizing and 8 of non-keratinizing squamous cell
carcinomas, 24 (44.4%) and 4 (50%) were infected with HPV,
respectively.
Immunohistochemical analysis for NF-κB was performed
on all 62 specimens. Of the 28 HPV-positive cases, 18 (64.3%) were
NF-κB-positive in the nucleus (Fig.
1C) and 19 (67.9%) were NF-κB-positive in the cytoplasm
(Fig. 1D). Additionally, NF-κB was
detected in the nucleus and/or the cytoplasm in 27 cases (96.4%).
Of the 34 HPV-negative cases, NF-κB localization in the nucleus,
cytoplasm and nucleus and/or cytoplasm was observed in 14 (41.2%),
14 (41.2%) and 18 cases (52.9%), respectively (Table II).
 | Table IICorrelation of NF-κB expression with
HPV infection in cervical cancer. |
Table II
Correlation of NF-κB expression with
HPV infection in cervical cancer.
| NF-κB expression | HPV-DNA | p-value |
|---|
|
| |
|---|
| Positive (n=28) | Negative (n=34) | |
|---|
| Positive in nucleus
(n=32) | 18 | 14 | |
| Negative in nucleus
(n=30) | 10 | 20 | 0.0700 |
| Positive in cytoplasm
(n=33) | 19 | 14 | |
| Negative in cytoplasm
(n=29) | 9 | 20 | 0.0653 |
| Positive in N and/or
C (n=45) | 27 | 18 | |
| Negative in N and/or
C (n=17) | 1 | 16 | 0.0001 |
Statistical analysis
The Fisher’s exact probability test showed that the
nuclear and/or cytoplasmic expression of NF-κB was correlated with
HPV infection (p<0.01) (Table
II).
Discussion
Between 2000 and 2004, cervical cancer was the
thirteenth most common cancer in the United States, with an
incidence rate of 808 per 100,000 individuals (13). Cervical cancer is the second most
common cancer among females worldwide, with an estimated 493,000
new cases and 274,000 deaths in 2002 (14). Globally, 83% of the cases of this
cancer occur in developing areas, including sub-Saharan Africa,
Melanesia, Latin America and the Caribbean, South-Central Asia and
Southeast Asia (14). While the
causal relationship between HPV infection and squamous cell
carcinoma of the genital tract is well established (15), the role of HPV in the development of
the malignancy has yet to be elucidated. However, mounting evidence
shows the involvement of HPV types in the process of oncogenesis.
Genital HPV infection is the cause of this most common sexually
transmitted disease, thus, the risk factor for HPV infection is
increased by sexual behaviors. The highest prevalence of HPV
infection is noted in sexually active adolescent and young adults.
HPV infection is a key factor in cervical oncogenesis. However,
high-risk HPV is a necessary but insufficient cause of cervical
cancer, which develops in a multi-step manner from precursor
lesions.
The overall prevalence of any type of HPV in the
general populations of sub-Saharan Africa for females with normal
cytology is 21.8% (16). Yamada
et al (17) reported that
the overall prevalence of HPV infection in the uterine cervix
associated with abnormalities was 27% in Nairobi, Kenya. The
prevalence of HPV-16 and/or HPV-18 among invasive cervical cancer
case ranges from 43.7% in Senegal to 90.2% in Ethiopia (16). The overall prevalence average of
HPV16/18 among the invasive cervical cancer cases is 69.2% in
sub-Saharan Africa (16). The
overall prevalence of HPV-DNA in cervical cancer in this study
using ISH was 45.2%, which is relatively similar to the 43.7% in
Senegal and 44.4% in Guinea (16).
This variability may depend not only on the different methods of
HPV detection used, but also on the geographic variation in HPV
distribution. Our studies showed that both cervical and penile
cancers were associated with HPV infection. Notably, the prevalence
of cervical cancer with HPV (45.2%) was lower than penile cancer
with HPV (68.2%) in surgical specimens in Kenya (12). The earliest age of onset for
cervical cancer cases was 18 years, while for penile cancer cases
it was 35 years (12). The mean age
for cervical cancer cases was 50.1 and for penile cancer cases 59.1
years (12). Variations in the
synergistic action of HPV between genders may occur, associated
with multiple epidemiological co-factors, such as life environment,
genital hygiene, sexual habits and cultural practices. To clarify
these discrepancies, further research is required.
NF-κB comprises of a family of transcription factors
that modulate signaling pathways to inhibit apoptosis, growth
factors and cell cycle regulatory proteins. NF-κB activation
promotes cell survival and growth, and therefore plays a critical
role in inflammation-based and cancer progression. This
transcription factor is indispensable for the malignant progression
of transforming cells in the environment, including network
signaling molecules mediated by various inflammatory cells. The
expression of numerous cytokines, chemokines, growth factors and
survival factors is NF-κB-dependent. NF-κB activation is modulated
for HPV (18–23). In this study, NF-κB was detected in
the nucleus and/or cytoplasm in 96.4% of the HPV-positive cases. On
the other hand, NF-κB was detected in the nucleus and/or cytoplasm
in only 52.9% of the HPV-negative cases. We demonstrated the
correlation between HPV infection and nuclear and/or cytoplasmic
NF-κB expression using the Fisher’s exact probability test. HPV
infection was considered to activate NF-κB resulting in cell
transformation (11,12,24).
The integration of HPV-DNA in the host cell DNA
involves cancer formation and development. The HPV viral oncogenes
E7 and E6 are the main contributors to the development of
HPV-induced cancers, probably due to integration of the viral genes
in the host cell genome. E7- and E6-induced genetic instability
leads to the activation of oncogenes and the inactivation of tumor
suppressor genes. Inactivation of tumor suppressors p53 and pRb is
a common event in the carcinogenesis of human cells. Notably, p53
and pRb genes are mutated in various types of human cancer.
Overexpression of viral E6 and E7 oncogenes reacts with the tumor
suppressor gene products p53 and pRb proteins in the host cells,
resulting in an induced cell immortalization, transformation and
oncogenesis due to their interference with the cell cycle and
apoptosis control (25). HPV E6 and
E7 oncogenes are key regulatory proteins inside host cells and are
associated with the transcriptional activity of NF-κB (19). Therefore, activation of NF-κB by
viral oncogenes may be the mechanism of tumor formation in cervical
cancer.
Acknowledgements
We are grateful to the Kenya Government for the
permission to study the Kenyan materials.
References
|
1
|
Hausen H: Papillomaviruses in the
causation of human cancers – a brief histological account.
Virology. 44:219–224. 2009.
|
|
2
|
Senba M, Mori N and Wada A: Oncogenesis of
human papilomavirus (HPV). DNA Tumor Virus. Tao HE: Nova Science
Publishers Inc.; New York: pp. 75–102. 2009
|
|
3
|
Senba M, Mori N and Wada A: Oncogenesis
and the link between inflammation and cancer due to human
papillomavirus (HPV) infection, and the development of vaccine
control strategies. Cancer Res J. 2:307–338. 2009.
|
|
4
|
Smith JS, Herrero R, Bosetti C, et al:
Herpes simplex virus-2 as a human papillomavirus cofactor in the
etiology of invasive cervical cancer. J Natl Cancer Inst.
94:1604–1613. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Smith JS, Munoz N, Herrero R, et al:
Evidence for Chlamydia trachomatis as a human papillomavirus
cofactor in the etiology of invasive cervical cancer in Brazil and
the Philippines. J Infect Dis. 185:324–331. 2002.
|
|
6
|
Karin M and Greten FR: NF-κB: linking
inflammation and immunity to cancer development and progression.
Nat Rev Immunol. 5:749–759. 2005.
|
|
7
|
Karin M: Nuclear factor-κB in cancer
development and progression. Nature. 441:749–759. 2006.
|
|
8
|
Kiriakidis S, Andreakos E, Monaco C, et
al: VEGF expression in human macrophages is NF-κB-dependent:
studies using adenoviruses expressing the endogenous NF-κB
inhibitor IκBα and kinase-defective form of the IκB kinase 2. J
Cell Sci. 116:665–674. 2003.
|
|
9
|
Senba M, Kumatori A, Fujita S, et al: The
prevalence of human papillomavirus genotypes in penile cancers from
northern Thailand. J Med Virol. 78:1341–1346. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fujita S, Senba M, Kumatori A, et al:
Human papillomavirus infection in oral verrucous carcinoma:
genotyping analysis and inverse correlation with p53 expression.
Pathobiology. 75:257–264. 2008. View Article : Google Scholar
|
|
11
|
Senba M, Mori N, Wada A, et al: Human
papillomavirus genotypes in penile cancers from Japanese patients
and NF-κB activation. Oncol Lett. 1:267–272. 2010.PubMed/NCBI
|
|
12
|
Senba M, Buziba N, Mori N, et al:
Detection of human papillomavirus and cellular regulators
p16INK4a, p53, and NF-κB in penile cancer cases in
Kenya. Acta Virol. 53:43–48. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Espey DK, Wu XC, Swan J, et al: Annual
report to the nation on the status of cancer, 1975–2004, featuring
cancer in American Indians and Alaska natives. Cancer.
110:2119–2152. 2007.
|
|
14
|
Parkin DM and Bray F: The burden of
HPV-related cancers. Vaccine. 24(Suppl 3): 11–25. 2006. View Article : Google Scholar
|
|
15
|
Bryan JT, Taddeo F, Skulsky D, et al:
Detection of specific human papillomavirus types in
paraffin-embedded sections of cervical carcinomas. J Med Virol.
78:117–124. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Louie KS, Sanjose S and Mayaud P:
Epidemiology and prevention of human papillomavirus and cervical
cancer in sub-Sahara Africa: a comprehensive review. Trop Med Int
Health. 14:1287–1302. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yamada R, Sasagawa T, Kirumbi LW, et al:
Human papillomavirus infection and cervical abnormalities in
Nairobi, Kenya, an area with a high prevalence of human
immunodeficiency virus infection. J Med Virol. 80:847–855. 2008.
View Article : Google Scholar
|
|
18
|
Nees M, Geoghegan JM, Hyman T, et al:
Papillomavirus type 16 oncogenes downregulate expression of
interferon-responsive genes and upregulate proliferation-associated
and NF-κB-responsive genes in cervical keratinocytes. J Virol.
75:4283–4296. 2001.PubMed/NCBI
|
|
19
|
Spitkovsky D, Hehner SP, Hofmann TG, et
al: The human papillomavirus oncoprotein E7 attenuates NF-κB
activation by targeting IκB kinase complex. J Biol Chem.
277:25576–25582. 2002.PubMed/NCBI
|
|
20
|
Havard L, Delvenne P, Frare P, et al:
Differential production of cytokines and activation of NF-κB in HPV
transformed keratinocytes. Virology. 298:271–285. 2002.
|
|
21
|
Havard L, Rahmouni S, Boniver J, et al:
High levels of p105 (NF-κB1) and p100 (NF-κB2) proteins in HPV
16-transformed keratinocytes. Virology. 331:357–366. 2005.
|
|
22
|
Mishra A, Bharti AC, Varghese P, et al:
Differential expression and activation of NF-κB family proteins
during oral carcinogenesis: role of high risk human papillomavirus
infection. Int J Cancer. 119:2840–2850. 2006.
|
|
23
|
James MA, Lee JH and Klingelhutz AJ: Human
papillomavirus type 16 E6 activates NF-κB, induces cIAP-2
expression, and protects against apoptosis in a PDZ binding
motif-dependent manner. J Virol. 80:5301–5307. 2006.
|
|
24
|
Senba M, Mori N, Fujita S, et al:
Relationship among human papillomavirus infection,
p16INK4a, p53 and NF-κB activation in penile cancer from
northern Thailand. Oncol Lett. 1:599–603. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Munger K, Baldwin A, Edwards KM, et al:
Mechanisms of human papillomavirus-induced oncogenesis. J Virol.
78:11451–11460. 2004. View Article : Google Scholar : PubMed/NCBI
|