Introduction
Chronic myeloid leukemia (CML) is a clonal stem cell
disease with deregulated tyrosine kinase activity of BCR-ABL.
BCR-ABL can be targeted by drugs, and various tyrosine kinase
inhibitors (TKIs) are effective in the treatment of CML. From a
genetic viewpoint, although the cascade of events following
BCR-ABL oncogene transcription that leads to CML is actively
being investigated (1,2), the manner in which ABL kinase
activity is increased by BCR at the genetic level is not
clearly understood (3–5). The promoter region that plays a key
role in the transcription of BCR-ABL remains to be
elucidated. ABL is regulated by two promoter regions, one of
which is Pa. Pa is conserved in the case of fusion with BCR,
but the methylation status of Pa does not correlate with disease
status in CML (6,7). Instead, ABL activity is thought
to be modulated by the BCR promoter (8,9), and
BCR promoter DNA methylation is a proposed mechanism for
transcription control in CML (10).
The methylation status of the BCR promoter, however, has
rarely been investigated in CML. If BCR promoter DNA
methylation actually controls transcription of the BCR-ABL
fusion gene in CML, then the methylation status of the BCR
promoter should exhibit a correlation with disease status.
Furthermore, the methylation status of BCR may also be used
as a predictive marker during tyrosine kinase inhibitor (TKI)
therapy.
Imatinib (Gleevec®; Novartis, Basel,
Switzerland) is the most commonly used TKI therapy for all phases
of CML [chronic phase (CP), accelerated phase (AP) and blast crisis
(BC)] (11). Approximately 10–15%
of CML patients treated with imatinib as first-line therapy suffer
disease progression (12).
Furthermore, imatinib is associated with secondary resistance
(13,14). For patients who fail to respond to
first-line standard dose imatinib therapy, dose-escalated imatinib
is a reasonable option, along with second generation TKIs. We
previously performed a prospective multi-center single-arm phase IV
study in which escalated doses of imatinib were administered to
Korean patients who had less than optimal response to the standard
dose imatinib (15). The present
study demonstrated considerable efficacy of dose-escalated imatinib
in CML patients, showing suboptimal response to standard dose
imatinib. Moreover, early molecular response, defined as a
reduction in the BCR-ABL/ABL ratio by more than 50% within 6
months, was found to be a surrogate marker for long-term response.
On the other hand, the BCR-ABL mutation rate was relatively
low in the suboptimal responders to imatinib, and the mutation
status did not affect the outcome of escalated dose imatinib
therapy.
Consequently, BCR promoter DNA methylation
analysis was performed i) to reveal the role of the BCR
promoter in the transcriptional control of the BCR-ABL
fusion gene, and ii) to investigate epigenetic predictive markers
for response and long-term outcome of imatinib dose escalation
treatment. For further comparison, BCR promoter DNA
methylation status was analyzed in another two groups. The second
group included patients who achieved complete cytogenetic remission
after receiving 400 mg/day imatinib (optimal responders) and the
third group were healthy controls.
Materials and methods
Study population
A total of 71 Korean patients from 19 centers in
Korea were enrolled in this study between 2005 and 2006. The
BCR promoter DNA methylation status was evaluated in three
groups of subjects. The first group comprised CP CML patients
enrolled in the imatinib dose escalation study. The study design
and results of this imatinib dose escalation trial were described
elsewhere (15). Briefly, this
open-label, single-arm, multi-center phase IV study enrolled CML
patients between 15 and 75 years of age with adequate organ
function. Patients in CP with a less than optimal response to
standard dose imatinib were included. Patients in AP or BC who
failed to achieve complete hematologic response after 3 months of
imatinib were also eligible. Those patients who experienced more
than grade 2 adverse events to the standard imatinib dose were
excluded. Imatinib was administered orally at 600 mg/day for CP
patients. Escalation to 800 mg/day imatinib was permitted for
patients in AP or BC. Patients received dose-escalated imatinib for
at least 12 months or until progressive disease or intolerable
toxicity occurred. Cytogenetic response (CyR) was assessed every 6
months. Molecular response (MR) was assessed every 3 months with
standardized BCR-ABL/ABL of a peripheral blood or bone
marrow aspirate using real-time reverse transcription quantitative
PCR. The criterion for time to treatment failure (TTFx) followed
the criterion advocated by LeukemiaNET (11). A baseline BCR-ABL gene
mutation test was performed using matrix-assisted laser
desorption/ionization time of flight mass spectrometry.
The second group, treated at Seoul National
University Hospital, comprised CML patients who achieved complete
CyR with the standard dose imatinib (300 or 400 mg/day; optimal
responders). The patients did not previously experience
dose-escalated imatinib and were required to be in complete
cytogenetic response (CCyR) at the time of blood sampling. The
duration of the standard dose imatinib was evaluated. Written
informed consent was obtained from all patients.
The third group included healthy individuals who
exhibited no evidence of any disease. Healthy controls willing to
donate a blood sample with informed consent were included. The
study of BCR promoter DNA methylation in the second and
third group of patients was approved by the Institutional Review
Board of Seoul National University Hospital.
DNA preparation for BCR promoter DNA
methylation
The methylation of the BCR promoter was
assessed in 37 CP CML patients whose samples were available at
baseline and 6 months after imatinib dose escalation. Additionally,
BCR promoter DNA methylation status was evaluated in 29
optimal responders and 39 healthy controls. Genomic DNA was
extracted from patient blood using the DNeasy® Blood and
Tissue kit (Qiagen, Hilden, Germany). Gender-matched human genomic
DNA (Promega, Madison, WI, USA) was used as reference DNA.
Extracted DNA was quantified using a NanoDrop ND-1000
spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA).
BCR promoter methylation analysis
To quantitatively measure DNA methylation of the
BCR promoter region, bisulfite PCR pyrosequencing was
performed. Bisulfite-treated DNA was used for the pyrosequencing
analysis as previously described (16). In brief, bisulfite converts cytosine
to uracil, but has no effect on methylated cytosine. PCR was
performed on the bisulfite-converted DNA for the BCR gene
[BCR-F: TTAGGTTGTGAGGTGTGAGGAAT and BCR-R (bio):
biotin-CAAAAACTACTCTCTCTAACAAAACTC]. Streptavidin-sepharose beads
(Amersham Biosciences, Uppsala, Sweden) and the Vacuum Prep Tool
(Biotage, Uppsala, Sweden) were used to purify the single-stranded
biotinylated PCR product. Sequencing primers (BCR-SP1:
ATGGAAGGTGTTTTT and BCR-SP2: TGGTGGTTTTTG ATA) were annealed
to the purified PCR product and used for a pyrosequencing reaction
using the PSQ 96HS system (Biotage). Raw data were analyzed with
the allele quantification algorithm using the software provided.
The BCR pyrosequencing assay measured the level of DNA
methylation of four CpG sites in the promoter region, and the
average methylation level was used for analysis.
Statistical analysis
Variables included for analysis in the study were
age, gender, duration of standard dose imatinib, mutation status,
cytogenetic response, molecular response, baseline BCR
promoter methylation, change in BCR promoter methylation
following 6 months of dose escalation and TTFx. Statistical
analyses of 2×2 contingency tables of categorical variables were
performed using Pearson's χ2 test or Fisher's exact
test, as appropriate. To compare serial values, a generalized
linear model with repeated measurements was used. Median durations
of TTFx were calculated using the Kaplan-Meier method, and
comparisons between the groups were made using log-rank tests. The
impact of continuous numeric variables on clinical outcome was
calculated using logistic regression and a Cox regression model. A
multivariate analysis was performed using a logistic regression
model for response and Cox regression models for TTFx. Factors with
p-values <0.1 in the univariate analysis were examined with
multivariate regression models. The statistical tests were
two-sided with significance defined as p<0.05. All analyses were
performed using SPSS for Windows version 12.0 (SPSS Inc.).
Results
Patient demographics
Characteristics of the patients enrolled in this
imatinib dose escalation study were described elsewhere (15). A total of 71 Korean patients from 19
centers were enrolled between 2005 and 2006. Among them, 64
patients were in CP CML. Table I
shows the baseline characteristics and treatment results of the 64
CP patients. Of the 64 CP CML patients, 38 (59.4%) achieved a 50%
reduction in the BCR-ABL/ABL ratio within 6 months following
dose escalation (early molecular responder; EMR). Estimated median
TTFx of the 64 patients was 27.0 months. Patients who showed a
suboptimal response to standard dose imatinib had a longer median
TTFx compared to those who showed treatment failure to standard
doses of imatinib (not achieved vs. 12.3 months, respectively,
p=0.023). EMRs achieved CCyR more frequently at 6 and 12 months
(p=0.010 and p<0.001, respectively). Similarly, TTFx of the EMR
patients was longer than that of the non-EMR patients (not achieved
vs. 11.0 months, p<0.001). The baseline BCR-ABL mutation
study showed 2 mutants and 26 wild-type patients. For the remaining
patients, either the PCR assay did not reveal sufficient cancer
cells for inclusion in the study or the patients refused to
participate in the mutation study. Specific mutations found
included H396R and F317L.
 | Table IDemographics, baseline characteristics
and treatment results of the 64 chronic myeloid leukemia
patients. |
Table I
Demographics, baseline characteristics
and treatment results of the 64 chronic myeloid leukemia
patients.
| No. of patients | Percentage | Median | Range |
|---|
| Age (years) | | | 50 | 20–71 |
| Gender | | | | |
| Male | 46 | 71.9 | | |
| Female | 18 | 21.8 | | |
| Duration of
standard dose imatinib (months) | | | 13.9 | 0.6–52.8 |
| Baseline
cytogenetics | | | | |
| Partial CyR | 29 | 45.3 | | |
| Less than partial
CyR | 35 | 54.7 | | |
| Baseline
status | | | | |
| Suboptimal
response | 19 | 29.7 | | |
| Treatment
failure | 45 | 70.3 | | |
| Mutation
status | | | | |
| Wild-type | 19 | 29.7 | | |
| Mutanta | 3 | 4.7 | | |
| Unknown | 42 | 65.6 | | |
| Cytogenetic
response at 6 months | | | | |
| Complete CyR | 16 | 25.0 | | |
| Partial CyR | 14 | 21.9 | | |
| Less than partial
CyR | 19 | 29.7 | | |
| Cytogenetic
response at 12 months | | | | |
| Complete CyR | 17 | 26.6 | | |
| Partial CyR | 11 | 17.2 | | |
| Less than partial
CyR | 14 | 21.9 | | |
| Early molecular
responder | | | | |
| Yes | 38 | 59.4 | | |
| No | 19 | 29.7 | | |
Among the 64 CP patients, 37 patients whose blood
samples were available underwent BCR promoter DNA
methylation analysis. Patient characteristics of the 37 patients in
comparison with the optimal responders and healthy donors are shown
in Table II. Median age was 50
years (range 20–71), and the median duration of treatment with the
standard dose of imatinib was 14.3 months (range 1.0–52.8).
Regarding baseline cytogenetic response, 16 patients exhibited
partial cytogenetic response (PCyR), and 21 patients exhibited less
than PCyR (sub-PCyR) while on the standard imatinib dose. When the
cytogenetic status was considered with respect to treatment
duration with the standard imatinib dose, we found that 12 patients
were in suboptimal response and 25 patients were in treatment
failure. The 2 patients with the BCR-ABL mutants were
available for BCR promoter DNA methylation analysis. The
patient with the H396R mutation experienced treatment failure at
6.13 months, whereas the patient with the F317L mutation did not
experience treatment failure for 18.7 months. The patient with the
H396R mutation was EMR, but the patient with the F317L mutation was
a non-EMR.
 | Table IIComparison between the 3 study groups
that underwent BCR promoter DNA methylation analysis. |
Table II
Comparison between the 3 study groups
that underwent BCR promoter DNA methylation analysis.
| Patients in the
dose escalation study (n=37) | Optimal responders
(n=29) | Healthy controls
(n=39) | P-value |
|---|
| Age, mean (range)
in years | 49.2 (20–71) | 44.8 (19–68) | 25.5 (20–36) | <0.001a |
| Gender | | | 0.085 | |
| Male | 27 | 20 | 20 | |
| Female | 10 | 9 | 20 | |
| Duration of
standard dose imatinib, mean (range) in months | 20.3
(1.0–52.8) | 33.9
(5.8–63.5) | NA | 0.002 |
DNA methylation of the BCR promoter was also
evaluated in the 29 optimal responders and the 39 healthy controls.
Optimal responders included 20 males and 9 females. Median age was
47 years (range 19–68). The age did not differ from the average age
of the study population (p=0.103). The median duration of treatment
with the standard dose imatinib was 29.9 months (range 5.8–63.5).
The duration of standard dose imatinib was longer in the optimal
responders compared to the suboptimal responders (p<0.001).
Healthy controls comprised 20 males and 19 females, with a median
age of 25.5 years (range 20–36). This age was significantly lower
when compared with the age of the study population (p<0.001) and
the optimal responders (p<0.001).
BCR promoter methylation analysis in
patients who received escalated dose imatinib
The mean methylation level in the patients was 47.9%
(range 31.6–61.2%) at the time of study enrollment (baseline). Age
had an inverse linear correlation with the baseline methylation
level (p=0.005), and the methylation level was significantly higher
in patients <50 years of age as compared to the older patients
(p=0.001). Baseline methylation had an inverse linear correlation
depending on the treatment with standard imatinib dose. Moreover,
baseline methylation decreased with a prolonged duration of the
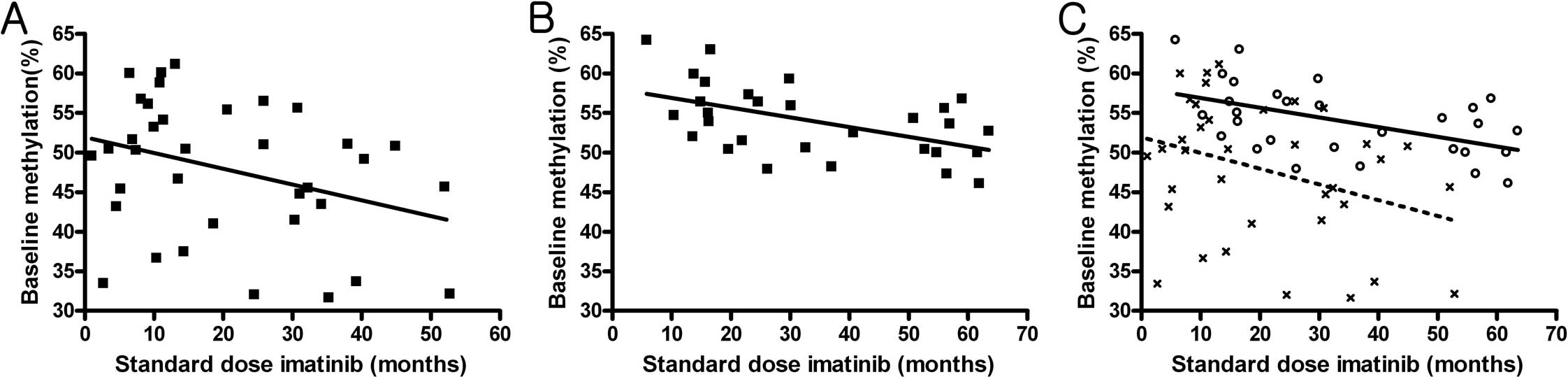
previously administered standard imatinib dose (p=0.041) (Fig. 1A). No correlation was noted between
age and the duration of standard dose imatinib. Baseline
methylation levels were significantly higher in patients who were
in PCyR at baseline compared to patients in sub-PCyR (mean level
53.3 vs. 43.8%, respectively; p=0.001). The baseline methylation
level did not predict EMR status (p=0.399). Baseline methylation
levels were inversely correlated to TTFx, with a hazard ratio (HR)
of 0.944 [95% confidence interval (CI), 0.893–0.999; p=0.044].
The mean methylation level at 6 months was 49.8%
(range 31.2–62.2%). When compared to the baseline, 21 patients
exhibited increased methylation levels (increased methylator), and
15 patients exhibited decreased methylation levels (decreased
methylator) after receiving dose-escalated imatinib for 6 months.
Methylation levels at 6 months also had an inverse linear
correlation with age (p=0.024). The methylation level at 6 months
exhibited a correlation with EMR status. In EMR patients,
methylation levels at 6 months were higher when compared with the
non-EMR patients (mean level 52.4 vs. 45.8%, respectively;
p=0.046). Finally, methylation levels at 6 months were more
strongly correlated to TTFx than baseline methylation levels with a
HR of 0.922 (95% CI, 0.873–0.975; p=0.005).
When changes in the BCR DNA methylation
levels were considered, increased methylators were predominantly
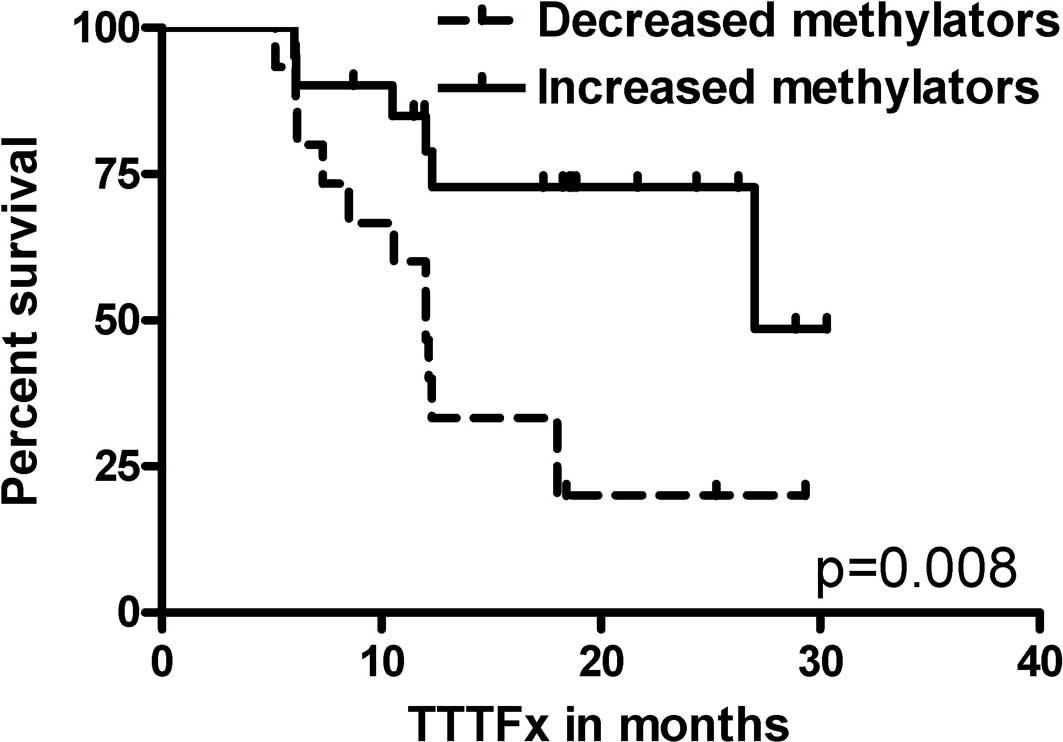
EMR patients (p=0.041). Increased methylators had a longer TTFx
compared to decreased methylators (median TTFx 27.0 vs. 12.0
months, p=0.008) (Fig. 2). The
results are noted in Table
III.
 | Table IIIBCR promoter methylation
levels according to clinical characteristics (n=37). |
Table III
BCR promoter methylation
levels according to clinical characteristics (n=37).
| Clinical
characteristics | Baseline (mean,
%) | P-value | At 6 months (mean,
%) | P-value | Change | P-value |
|---|
|
|---|
| + | − |
|---|
| Age | | 0.001 | | 0.437 | | | 0.735 |
| ≥50 | 43.7 | | 50.5 | | 11 | 7 | |
| <50 | 52.3 | | 48.0 | | 10 | 8 | |
| Gender | | 0.415 | | | | | 0.058 |
| Male | 47.2 | | | | 18 | 8 | |
| Female | 49.8 | | | | 3 | 7 | |
| Baseline FISH | | 0.001 | | 0.008 | | | 0.767 |
| PCyR | 53.3 | | 53.9 | | 9 | 6 | |
| Sub-PCyR | 43.8 | | 46.7 | | 11 | 9 | |
| Baseline
status | | 0.007 | | 0.005 | | | 1.000 |
| Suboptimal
response | 53.3 | | 54.7 | | 6 | 5 | |
| Treatment
failure | 45.3 | | 43.7 | | 15 | 10 | |
| Early molecular
responder | | 0.399 | | 0.046 | | | 0.041 |
| Yes | 48.9 | | 52.4 | | 16 | 6 | |
| No | 46.3 | | 45.8 | | 5 | 9 | |
| Time to treatment
failure (HR) | 0.944 | 0.044 | 0.922 | 0.005 | 0.287 | 1 | 0.008 |
When multivariate analysis was performed considering
the baseline cytogenetic response with duration of standard dose
imatinib, baseline BCR promoter DNA methylation levels and
changes in BCR methylation levels after 6 months, only the
increase noted in the BCR promoter DNA methylation level
following dose escalation therapy was an independent predictor for
achievement of EMR [odds ratio (OR), 0.154; p=0.022] and TTFx (HR,
0.294; p=0.015) (Table IV).
 | Table IVOdds ratio and p-value for EMR status
and TTFx in multivariate analysis. |
Table IV
Odds ratio and p-value for EMR status
and TTFx in multivariate analysis.
| EMR
achievement | TTFx |
|---|
|
|
|
|---|
| | 95% CI | | | 95% CI | |
|---|
| |
| | |
| |
|---|
|
Characteristics | OR | Low | High | P-value | HR | Low | High | P-value |
|---|
| Baseline
status | | | | | | | | 0.388 |
| Treatment
failure | 0.595 | 0.088 | 4.001 | 0.593 | 1.858 | 0.455 | 7.588 | |
| Suboptimal
response | 1 | | | | 1 | | | |
| Baseline BCR
methylation | 1.052 | 0.951 | 1.164 | 0.324 | 0.956 | 0.895 | 1.021 | 0.180 |
| Change in
BCR methylation | | | | 0.022 | | | | 0.015 |
| Increase | 6.503 | 1.314 | 32.188 | | 0.294 | 0.110 | 0.786 | |
| Decrease | 1 | | | | 1 | | | |
Thus, both baseline and 6-month BCR promoter
DNA methylation levels were higher in younger patients and in
patients who exhibited a more favorable response to the standard
imatinib dose. However, only a change in the BCR promoter
DNA methylation level following dose escalation therapy was
correlated to EMR status and TTFx.
Evaluation of BCR promoter methylation in
optimal responders and healthy controls
The mean BCR promoter DNA methylation level
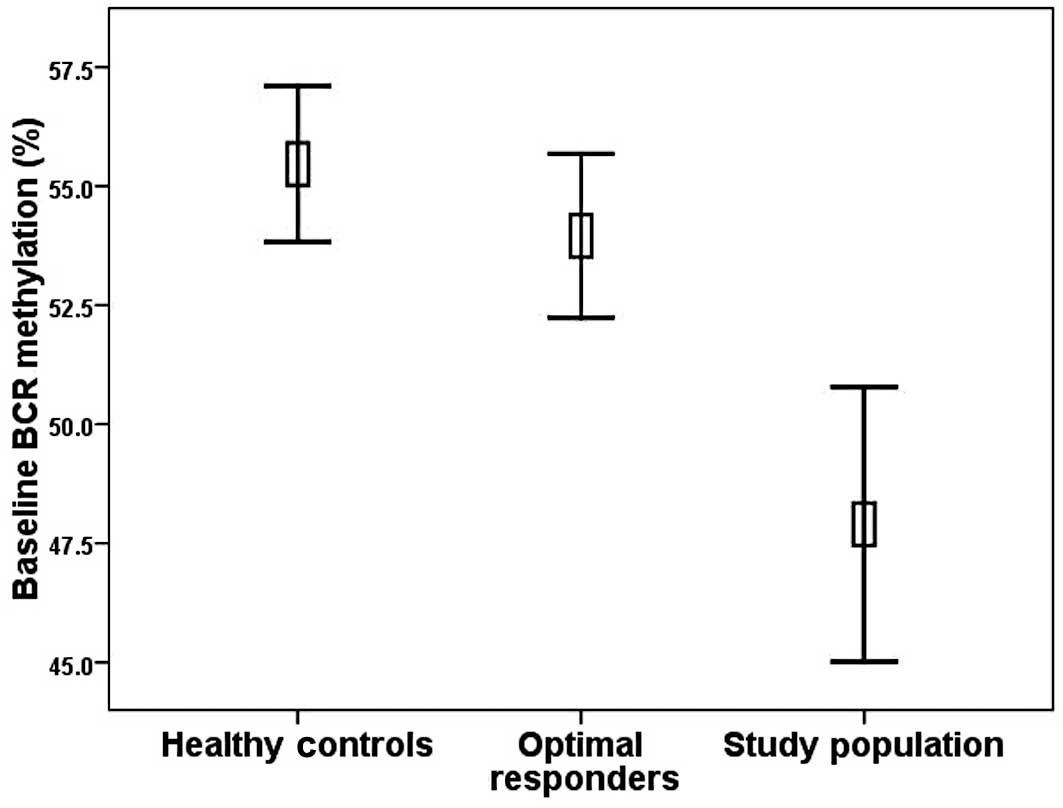
of the optimal responders was 54.0% (range 46.1–64.2%). The optimal
responders had significantly higher methylation levels when
compared with the baseline methylation levels in the study patients
(p=0.001) (Fig. 3). BCR
promoter DNA methylation again had an inverse linear correlation
with age (p=0.040) and duration of standard dose imatinib (p=0.004)
(Fig. 1B). However, the pattern of
the decrease in methylation levels in correlation with the duration
of imatinib use was significantly different between patients who
received dose-escalated imatinib and the optimal responders
(p=0.001) (Fig. 1C).
Regarding the healthy controls, the mean BCR
promoter DNA methylation level was 55.5% (40.0–62.0%). The
methylation levels of the healthy donors were significantly higher
when compared with baseline methylation levels in the study
patients (p<0.001). In contrast, no difference was noted in
methylation levels between the healthy donors and optimal
responders (Fig. 3). The
methylation levels did not correlate with age.
Thus, BCR methylation levels were higher in
the patients who were likely to have a lower disease burden and in
those with a more favorable response to imatinib. However, no
difference was found between patients with CML who had an optimal
response and those without CML.
Discussion
The main purpose of the present study was i) to
reveal the role of the BCR promoter in the transcriptional
control of the BCR-ABL fusion gene, and ii) to investigate
epigenetic predictive markers for response and long-term outcome of
imatinib treatment. For this purpose, we analyzed the clinical
implication of BCR promoter DNA methylation.
The first analysis focused on CP CML patients who
received dose-escalated imatinib treatment for suboptimal response.
In the first analysis, high baseline BCR promoter DNA
methylation levels were correlated with young age, low leukemic
burden at study enrollment and a more favorable response to the
standard imatinib dose. In contrast, BCR promoter DNA
methylation at 6 months had no correlation with age and a weaker
correlation with baseline disease burden than BCR promoter
DNA methylation at baseline. The results indicate that the
BCR promoter DNA methylation level was affected by the dose
escalation treatment. From a therapeutic viewpoint, major end
points of the analysis in this study included achievement of EMR,
defined as those patients who achieved a 50% reduction in the
BCR-ABL/ABL gene ratio within 6 months, and TTFx. We
previously showed that EMR is an ideal surrogate marker for
long-term disease control in these patients (15). For the two endpoints, the single
independent factor for favorable outcome was an increase in
BCR promoter DNA methylation following the imatinib dose
escalation treatment. The above results strongly suggest that the
BCR promoter DNA methylation status correlates well, not
only with disease status, but also with response to imatinib.
In the second analysis, we compared BCR
promoter DNA methylation among three distinct groups of subjects.
In the this second analysis, lower BCR promoter DNA
methylation levels were observed in patients who received
dose-escalated imatinib compared to the optimal responders and the
healthy controls. Although BCR promoter DNA methylation
decreased with age, no significant difference was noted between the
optimal responders and healthy controls (despite the age difference
between the two groups), indicating that disease burden is the main
factor affecting the level of BCR promoter DNA methylation.
These findings collectively suggest that BCR promoter DNA
methylation is correlated with disease status.
We measured the entire level of BCR promoter
DNA methylation. In other words, the level of BCR promoter
DNA methylation in our study included both the BCR promoter
methylation of the BCR gene without fusion and that of the
BCR-ABL fusion gene. Since we lacked information regarding
the difference between the BCR and BCR-ABL fusion
genes at the BCR promoter methylation level, it is plausible
to make two assumptions concerning the underlying mechanism of
these phenomena according to this difference.
If we assume that the BCR promoter
methylation level of the BCR-ABL fusion and BCR genes
are the same, then the phenomena suggest that the progression of
CML involves decreased methylation of the BCR promoter and
this decreased methylation in CML patients is restored upon
imatinib treatment in patients who benefit from the drug. If this
is viable, these results suggest a possible role of methylating
agents in CML.
On the other hand, if we assume that the BCR
promoter methylation level of the BCR-ABL fusion gene is
lower than that of the BCR gene, then the phenomena may be
interpreted in the followin manner: The change in BCR
promoter DNA methylation can be a simple reflection of the change
in the ratio of BCR-ABL to BCR cells or the stem cell
or population of blood cells upon treatment. Thus, high levels of
BCR promoter methylation represent normal cells whereas low
levels are CML cells, and the changes noted represent ratios of
normal to CML cells. Determination of the difference in the
BCR promoter methylation level between the BCR and
BCR-ABL fusion genes using new techniques can establish
which explanation is accurate.
Decreased BCR promoter methylation with
prolonged use of standard dose imatinib observed in the optimal
responders reveals another finding. Assuming that complete CyR at
the time of sampling of the optimal responders reflects a
negligible leukemic burden, the phenomenon suggests that BCR
promoter methylation of the BCR gene is affected by imatinib
treatment. Moreover, the significant difference in the pattern of
decrease in BCR promoter methylation levels between the
patients who received dose-escalated imatinib and the optimal
responders is another significant finding. The clinical
significance of this finding is unknown; however, it may be related
to a mechanism underlying imatinib resistance.
Finally, the mean BCR promoter DNA
methylation level was 55.5% for healthy controls. Although the
accurate normal reference of BCR promoter DNA methylation
may be different according to age or ethnicity, defining the
reference value of BCR promoter DNA methylation in the
normal population is crucial for further application of the
BCR promoter DNA methylation.
In conclusion, methylation of the BCR
promoter correlated well with disease status upon treatment with
imatinib, and an increase in BCR promoter methylation
indicated a favorable outcome. BCR promoter DNA methylation
decreased with prolonged imatinib (400 mg/day) use both in the
optimal responders and in the patients who failed to achieve
optimal responses, although the patterns of decrease were
different.
Acknowledgements
This study was supported by a grant from the Korea
Health 21 R&D Project, Ministry of Health & Welfare,
Republic of Korea (0405-BC02-0604-0004). We especially thank all
members of the Korean CML Working Party.
References
|
1
|
Goldman JM and Melo JV: BCR-ABL in chronic
myelogenous leukemia – how does it work? Acta Haematol.
119:212–217. 2008.
|
|
2
|
Ren R: Mechanisms of BCR-ABL in the
pathogenesis of chronic myelogenous leukaemia. Nat Rev Cancer.
5:172–183. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wen ST and Van Etten RA: The PAG gene
product, a stress- induced protein with antioxidant properties, is
an Abl SH3-binding protein and a physiological inhibitor of c-Abl
tyrosine kinase activity. Genes Dev. 11:2456–2467. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
McWhirter JR, Galasso DL and Wang JY: A
coiled-coil oligomerization domain of Bcr is essential for the
transforming function of Bcr-Abl oncoproteins. Mol Cell Biol.
13:7587–7595. 1993.PubMed/NCBI
|
|
5
|
Pendergast AM, Muller AJ, Havlik MH, et
al: BCR sequences essential for transformation by the BCR-ABL
oncogene bind to the ABL SH2 regulatory domain in a
non-phosphotyrosine-dependent manner. Cell. 66:161–171. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Issa JP, Kantarjian H, Mohan A, et al:
Methylation of the ABL1 promoter in chronic myelogenous leukemia:
lack of prognostic significance. Blood. 93:2075–2080.
1999.PubMed/NCBI
|
|
7
|
Sun B, Jiang G, Zaydan MA, et al: ABL1
promoter methylation can exist independently of BCR-ABL
transcription in chronic myeloid leukemia hematopoietic
progenitors. Cancer Res. 61:6931–6937. 2001.PubMed/NCBI
|
|
8
|
Zion M, Ben-Yehuda D, Avraham A, et al:
Progressive de novo DNA methylation at the bcr-abl locus in the
course of chronic myelogenous leukemia. Proc Natl Acad Sci USA.
91:10722–10726. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shtivelman E, Lifshitz B, Gale RP, et al:
Fused transcript of abl and bcr genes in chronic myelogenous
leukaemia. Nature. 315:550–554. 1985. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jiang G, Yang F, Li M, et al: Imatinib
(ST1571) provides only limited selectivity for CML cells and
treatment might be complicated by silent BCR-ABL genes. Cancer Biol
Ther. 2:103–108. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Baccarani M, Saglio G, Goldman J, et al:
Evolving concepts in the management of chronic myeloid leukemia:
recommendations from an expert panel on behalf of the European
LeukemiaNet. Blood. 108:1809–1820. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hughes T: ABL kinase inhibitor therapy for
CML: baseline assessments and response monitoring. Hematology Am
Soc Hematol Educ Program. 211–218. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hochhaus A and Hughes T: Clinical
resistance to imatinib: mechanisms and implications. Hematol Oncol
Clin North Am. 18:641–656. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Druker BJ, Guilhot F, O'Brien SG, et al:
Five-year follow-up of patients receiving imatinib for chronic
myeloid leukemia. N Engl J Med. 355:2408–2417. 2006.PubMed/NCBI
|
|
15
|
Koh Y, Kim I, Yoon SS, et al: Phase IV
study evaluating efficacy of escalated dose of imatinib in chronic
myeloid leukemia patients showing suboptimal response to standard
dose imatinib. Ann Hematol. 89:725–731. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang AS, Estecio MR, Doshi K, et al: A
simple method for estimating global DNA methylation using bisulfite
PCR of repetitive DNA elements. Nucleic Acids Res. 32:e382004.
View Article : Google Scholar : PubMed/NCBI
|