Introduction
Papillary thyroid carcinoma (PTC) is the most
prominent malignancy of the thyroid gland, accounting for more than
80% of all thyroid cancers. The majority of PTC patients have a
favorable prognosis with the appropriate treatment, but a poor
prognosis, such as recurrence, metastasis or even death, is noted
in other patients. Once metastasis occurs, the therapeutic effect
of PTC shows a marked decrease. Therefore, agents are required to
predict the metastatic potential of the carcinoma. Such agents may
be utilized as clinical guidance for the further treatment and
post-operative management of patients. It was reported that PTC
exhibiting infiltration of adjacent tissues was likely to recur and
lead to a poor prognosis (1). These
malignant biological behaviors result from aberrant genetic
alterations and protein expression in cancer cells. Thus, advanced
understanding of the molecular events associated with PTC
pathogenesis may lead to the confirmation of more effective PTC
prognostic markers, which would aid in the prediction of the
metastatic potential of the carcinoma.
V-raf murine sarcoma viral oncogene homolog B1
(BRAF) gene encodes a serine/threonine protein kinase, BRAF
protein, as a key member of the RAS/RAF/MEK/ERK/MAPK pathway
(2). Previous studies showed that
the BRAF mutation (V600E) played a role in the molecular
carcinogenesis of PTC by aberrant activation of the MAPK pathway
(3,4), whereas the association of BRAF
mutation with prognosis of the metastatic potential was
controversial (5–8). Certain studies showed that the
variations of BRAF protein expression levels were independent of
the V600E mutation in thyroid carcinomas and the carcinomas were
induced by mutation or protein overexpression of the gene,
respectively. (9) To the best of
our knowledge, little information is available on BRAF expression
with regard to the metastatic potential of PTC. Unrestrained cell
proliferation is a fundamental biological behavior of carcinomas,
which may be regarded as a prognostic marker. Proliferating cell
nuclear antigen (PCNA) was originally identified as a protein
expressing in the nuclei during the DNA synthesis phase and is
deemed to be a marker for cell proliferation (10). The overexpression of PCNA was
observed in various malignant carcinomas, since carcinomas always
exhibit uncontrolled proliferation (11,12).
Cvejic et al reported that PCNA expression was significantly
up-regulated according to the increasing malignancy of thyroid
carcinomas (13). The association
of the proliferation status with the metastatic potential of PTC
required investigation.
The mismatch repair (MMR) system is primarily
responsible for correcting DNA base-base mismatches and base
insertion/deletion error during DNA replication, thereby preventing
mutagenesis and maintaining genomic stability (14). The expression of MMR genes generally
maintains a high level in labile or cancer cells that exhibit
unrestrained proliferation due to the increasing rate of base
mismatching during the replication process of cell proliferation.
As stable cells, the thyroid cells are inactive with only minimal
replicative activity. Base-base mismatches in the cells rarely
occur and the MMR gene expression often maintains a low level in
normal thyroid cells. The hMSH2 gene is the first identified MMR
gene. Deficiency of the hMSH2 gene results in the incidence of
numerous carcinomas (15). On the
other hand, the protein overexpression of the gene was detected in
certain carcinomas (16). However,
the function status and expression level of hMSH2 in PTC remains
controversial.
In this study, the protein expression of BRAF, PCNA
and hMSH2 in PTC, with and without lymph node metastasis was
detected. Moreover, these agents served as novel markers in the
prediction of the metastatic potential of PTC.
Materials and methods
Patients and tissue samples
A total of 70 PTC and 29 nodular goiter (NG) tissues
were used in this study. The tissues were obtained from patients
who underwent surgery between 2006 and 2007 at certain hospitals in
Dalian, China. Among the 70 PTC tissues, 21 cases presented with
lymph node metastasis (LNM group) and 49 cases were without lymph
node metastasis (non-LNM group); 12 male and 58 female cases with a
mean ± SD age at diagnosis of 50.0±14.4 years (range 19–87). The
formalin-fixed paraffin-embedded tissue sections were used
following the approval of the Ethics Committee of Dalian Medical
University, after informed consent was obtained from the patients,
and the histological diagnoses of the samples were independently
established according to the World Health Organization
classification by two certified pathologists.
Immunohistochemistry
The specimens were fixed in 10% neutral-buffered
formalin and embedded in serial 3-mm paraffin blocks. Sections
(4-μm) were prepared for immunohistochemistry (IHC). Monoclonal
antibodies were used as follows: BRAF: F-7, 1:500 dilution (Santa
Cruz); hMSH2: FE 11, 1:250 dilution (Zymed Lab Inc., San Francisco,
CA, USA); and PCNA: PC10, 1:200 dilution (NeoMarkers). IHC was
performed according to the manufacturer's instructions. Briefly,
the slides were deparaffinized with xylene and rehydrated through
graded ethanol to distilled water. Endogenous peroxidase was
quenched in 3% H2O2. The sections were rinsed
with phosphate-buffered saline (PBS), processed for antigen
retrieval with 10 mM citrate buffer (pH 6.0) and allowed to cool
naturally at room temperature. Then, 10% normal horse serum was
used to block non-specific binding. The sections were incubated
with primary antibodies at 4°C overnight, secondary antibodies at
37°C for 30 min and streptavidin peroxidase at 37°C for 30 min. The
reaction was visualized using
3,3′-diaminobenzidine-tetrahydrochloride (DAB) as chromogen and was
stopped by the addition of water after 5 min. The sections were
counterstained with hematoxylin. Primary antibodies were replaced
by PBS in the negative control. The detailed protocol of IHC was
performed as described by Li et al (16).
Assessment of staining and statistical
data
The immunoreactivity of BRAF and hMSH2 was evaluated
as: (−), <10% of parenchymal cells showed positive
immunoreactivity; and (+), ≥10% showed positive immunoreactivity.
The immunoreactivity of PCNA was evaluated as: (−), <20% of
parenchymal cells showed positive immunoreactivity; and (+), ≥20%
showed positive immunoreactivity. The staining results were
independently evaluated by two investigators.
Statistical comparisons of data were performed by
Student's t-test, Chi-square test and Spearman's correlation
analysis on SPSS 13.0 software. P<0.05 was considered to be
statistically significant.
Results
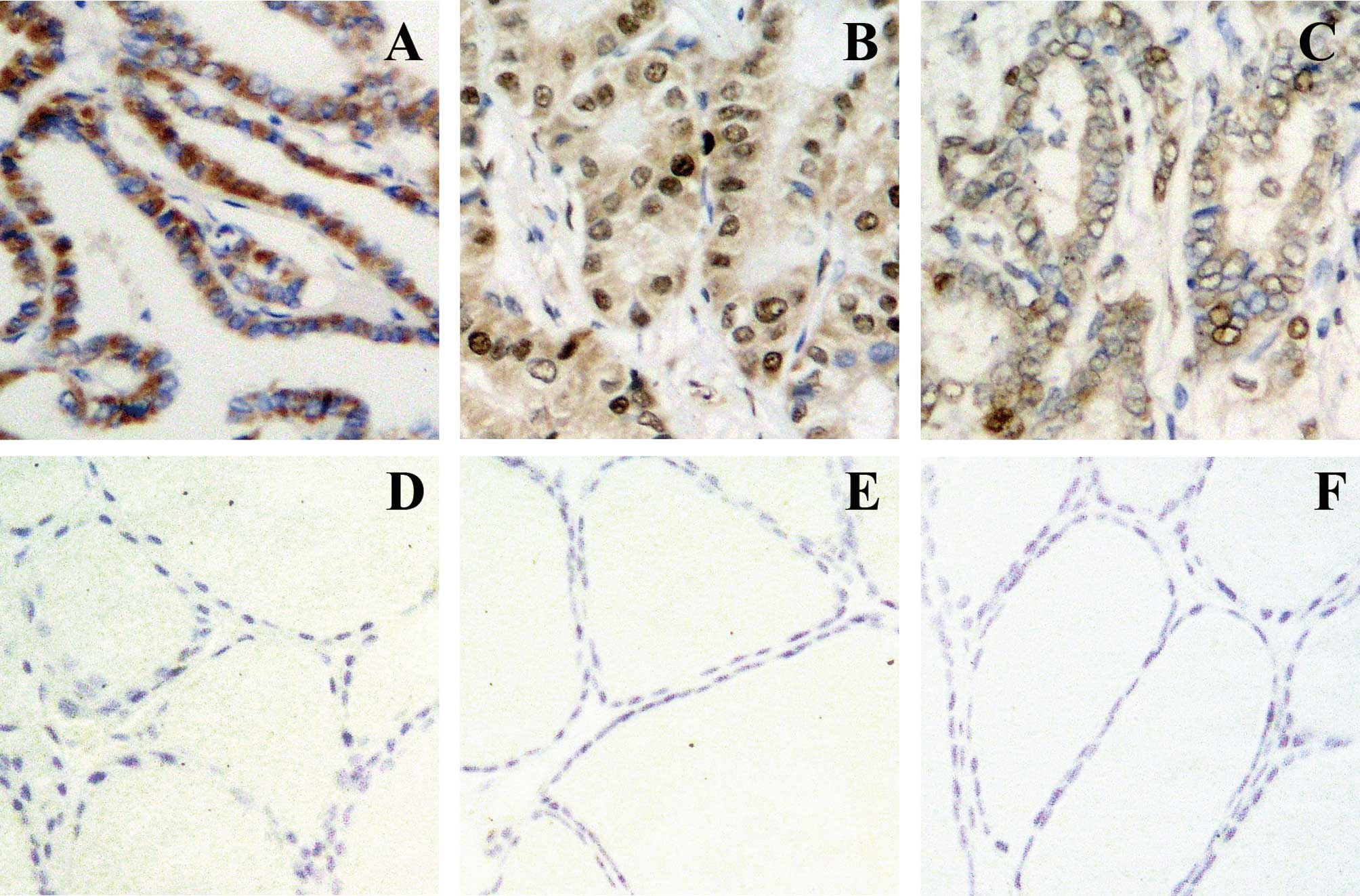
The immunoreactivity of BRAF was localized in the
cytoplasm and those of PCNA and hMSH2 were localized in the nuclei
(Fig. 1). Variations in the
positive rates of BRAF, PCNA and hMSH2 expression between PTC and
NG are shown in Table I. The
positive rate of BRAF, PCNA and hMSH2 expression in PTCs was
significantly higher than that in NGs (P=0.000, P=0.000 and
P=0.003, respectively). The immunoreactions of BRAF, PCNA and hMSH2
were not detected in NGs.
 | Table IVariations in BRAF, PCNA and hMSH2
expression between PTC and NG. |
Table I
Variations in BRAF, PCNA and hMSH2
expression between PTC and NG.
| No. | BRAF | PCNA | hMSH2 |
|---|
| |
|
|
|
|---|
| | + | P-value | + | P-value | + | P-value |
|---|
| PTC, no. (%) | 70 | 42 (60.0) | 0.000 | 28 (40.0) | 0.000 | 18 (25.7) | 0.003 |
| NG, no. (%) | 29 | 0 (0.0) | 0.000 | 0 (0.0) | 0.000 | 0 (0.0) | 0.003 |
The correlations of BRAF, hMSH2 and PCNA expression
with the clinicopathological characteristics of the PTC patients
are shown in Table II. The
positive rate of BRAF expression in the LNM group was significantly
higher compared to that in the non-LNM group (P=0.019). No
correlation of hMSH2 and PCNA expression with the LNM status of the
PTC patients was found.
 | Table IICorrelations of BRAF, PCNA and hMSH2
expression with the clinicopathological characteristics of PTC
patients. |
Table II
Correlations of BRAF, PCNA and hMSH2
expression with the clinicopathological characteristics of PTC
patients.
| | BRAF | PCNA | hMSH2 |
|---|
| |
|
|
|
|---|
| No. | + | P-value | + | P-value | + | P-value |
|---|
| Age (no.) | 70 | 24 | NS | 28 | NS | 18 | NS |
| Mean ± SD | 50.0±14.4 | 51.4±16.1 | | 48.3±18.3 | | 51.4±16.9 | |
| Gender, no. (%) | | | NS | | NS | | NS |
| Male | 12 | 6 (50.0) | | 5 (41.7) | | 2 (16.7) | |
| Female | 58 | 36 (62.1) | | 23 (39.7) | | 16 (27.6) | |
| LNM, no. (%) | | | 0.019 | | NS | | NS |
| Present | 21 | 17 (81.0) | | 10 (47.6) | | 5 (23.8) | |
| Absent | 49 | 25 (51.0) | | 18 (36.7) | | 13 (26.5) | |
The age at diagnosis of PTC patients with LNM
(56.0±14.7 years) was found to be significantly older than that
without LNM (47.4±13.7 years) (P=0.021). No correlation between the
LNM status and gender of the PTC patients was noted.
Correlations of the BRAF expression with hMSH2 and
PCNA expression is shown in Table
III. The positive rate of BRAF expression significantly
correlated with that of PCNA and hMSH2 expression (P=0.000 and
P=0.019, respectively).
 | Table IIICorrelations of BRAF expression with
hMSH2 and PCNA expression. |
Table III
Correlations of BRAF expression with
hMSH2 and PCNA expression.
| PCNA | hMSH2 |
|---|
|
|
|
|---|
| + | − | P-value | + | − | P-value |
|---|
| BRAF | | | 0.000 | | | 0.019 |
| Positive | 24 | 18 | | 15 | 27 | |
| Negative | 4 | 24 | | 3 | 25 | |
Discussion
PTC derives from the follicular epithelium and is
the most common subtype of thyroid cancer. Although many PTC
patients have favorable outcomes, a poor, or even fatal, prognosis
is noted in other patients. In general, elderly individuals often
receive a worse prognosis. In our study, metastasis was correlated
with age, indicating that metastasis was common in elderly
patients.
Certain cancer cells that are able to survive the
journey to another area of the body, metastasize. In other words,
the potential for metastasis and prognosis of PTC patients, is
determined by the biological behaviors of the carcinoma, the
alterations of which rely on the variations of gene and dysfunction
or the aberrant activation of vital proteins. Therefore, the
aberrant expression of certain proteins may be a marker for
malignant molecular events in cells, aiding in the prediction of
biological behavior as a metastatic potential of PTC.
BRAF is a key activator of the MAPK pathway, which
regulates cell survival, differentiation, apoptosis and
proliferation. BRAF protein expresses with various levels according
to the type of tissue. In this study, the positive rate of BRAF
expression in PTC was significantly higher than that in NG.
Similarly, among the PTC tissues, the positive rate was
significantly higher in the LNM group than in the non-LNM group.
Previous investigations suggested that the gene copy number gain
involves one mechanism causing overexpression of the BRAF protein
and is a new mechanism of BRAF activation in thyroid carcinomas
(17). We hypothesize that the gene
copy number gain occurs in the carcinogenesis of PTC, abnormally
up-regulating the BRAF expression and deteriorating cell biological
behaviors, leading to cell metastasis. It appears that the high
level of BRAF expression was a particular phenotype feature of the
metastatic potential in PTC. Furthermore, a method routinely used
in numerous laboratories with simpler procedures than DNA mutation
detection was used to identify the BRAF protein.
PCNA encodes an auxiliary protein of DNA polymerase
δ. PCNA expression in the S phase of the cell cycle is used to
evaluate the proliferative activity of cells, whereas normal
thyroid cells rarely proliferate as stable cells; thus, the PCNA
expression maintains a low level. Certain studies showed that PCNA
expression levels varied according to the heterogeneity of thyroid
carcinomas (13). Our data showed
that PCNA expression was higher in PTCs compared to that of NGs and
was concomitant with the BRAF protein in PTC. Consequently, PTC
cells with BRAF overexpression expressed proliferation.
hMSH2, a member of the MMR system, is responsible
for genomic stability. Normal thyroid cells have been found to
maintain a low level of hMSH2 expression, which was higher in
thyroid carcinomas. In our study, the expression of hMSH2 in PTC
was markedly higher than that in NG and was concomitant with that
of BRAF in PTC. Previous studies suggested that BRAF mutations were
rarely attributable to the defection of hMSH2 in carcinomas
(18). Nevertheless, as regards
protein levels, an indirect relationship exists between BRAF and
hMSH2. The correlation of BRAF to hMSH2 expression is shown by the
fact that BRAF expression was up-regulated to carry out its
molecular functions and induce thyroid cells to proliferate
excessively, followed by the up-regulation of hMSH2 expression in
order to repair the increased mismatching errors during cell
proliferation. Therefore, up-regulation of the hMSH2 expression may
indicate the increasing proliferative capacity of cells.
In conclusion, BRAF, PCNA and hMSH2 overexpression
was a molecular event of PTC tumorigenesis. Elderly patients with
BRAF overexpression are a high-risk group for PTC metastasis. Our
data on BRAF, PCNA and hMSH2 expression showed that BRAF activated
the MAPK downstream pathway independently, inducing the
unrestrained proliferation of cells with the presence of PCNA
overexpression and increasing mismatched errors indirectly during
frequent cell proliferation with the presence of hMSH2
overexpression. Although the specific mechanism for these
relationships has yet to be elucidated, the detection of BRAF
expression may aid in the prediction of the metastatic potential of
PTC.
Acknowledgements
We thank Shihua Cheng and Liying Guo of the First
Affiliated Hospital of Dalian Medical University for the sample
collection and technical assistance.
References
|
1
|
Mai KT, Perkins DG, Yazdi HM, Commons AS,
Thomas J and Meban S: Infiltrating papillary thyroid carcinoma:
review of 134 cases of papillary carcinoma. Arch Pathol Lab Med.
122:166–171. 1998.PubMed/NCBI
|
|
2
|
Michaloglou C, Vredeveld LC, Mooi WJ and
Peeper DS: BRAF (E600) in benign and malignant human tumours.
Oncogene. 27:877–895. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cohen Y, Xing M, Mambo E, Guo Z, Wu G,
Trink B, Beller U, Westra WH, Ladenson PW and Sidransky D: BRAF
mutation in papillary thyroid carcinoma. J Natl Cancer Inst.
95:625–627. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dhomen N and Marais R: New insight into
BRAF mutations in cancer. Curr Opin Genet Dev. 17:31–39. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kebebew E, Weng J, Bauer J, Ranvier G,
Clark OH, Duh QY, Shibru D, Bastian B and Griffin A: The prevalence
and prognostic value of BRAF mutation in thyroid cancer. Ann Surg.
246:466–470. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu RT, Chen YJ, Chou FF, Li CL, Wu WL,
Tsai PC, Huang CC and Cheng JT: No correlation between BRAFV600E
mutation and clinicopathological features of papillary thyroid
carcinomas in Taiwan. Clin Endocrinol. 63:461–466. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ito Y, Yoshida H, Maruo R, et al: BRAF
mutation in papillary thyroid carcinoma in a Japanese population:
its lack of correlation with high-risk clinicopathological features
and disease-free survival of patients. Endocr J. 56:89–97. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lassalle S, Hofman V, Ilie M, Butori C,
Bozec A, Santini J, Vielh P and Hofman P: Clinical impact of the
detection of BRAF mutations in thyroid pathology: potential
usefulness as diagnostic, prognostic and theragnostic applications.
Curr Med Chem. 17:1839–1850. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kondo T, Nakazawa T, Murata S, Kurebayashi
J, Ezzat S, Asa SL and Katoh R: Enhanced B-Raf protein expression
is independent of V600E mutant status in thyroid carcinomas. Hum
Pathol. 38:1810–1818. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Baserga R: Growth regulation of the PCNA
gene. J Cell Sci. 98:433–436. 1991.
|
|
11
|
Leonardi E, Girlando S, Serio G, Mauri FA,
Perrone G, Scampini S, Dalla Palma P and Barbareschi M: PCNA and
Ki67 expression in breast carcinoma: correlations with clinical and
biological variables. J Clin Pathol. 45:416–419. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
El-kott AF, El-baz MA and Mokhtar AA:
Proliferating cell nuclear antigen (PCNA) overexpression and
microvessel density predict survival in the urinary bladder
carcinoma. Int Urol Nephrol. 38:237–242. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cvejic D, Selemetjev S, Savin S, Paunovic
I and Tatic S: Changes in the balance between proliferation and
apoptosis during the progression of malignancy in thyroid tumours.
Eur J Histochem. 53:65–71. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jun SH, Kim TG and Ban C: DNA mismatch
repair system. Classical and fresh roles. FEBS J. 273:1609–1619.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Plevová P, Krepelová A, Papezová M, et al:
Immunohistochemical detection of the hMLH1 and hMSH2 proteins in
hereditary non-polyposis colon cancer and sporadic colon cancer.
Neoplasma. 51:275–284. 2004.PubMed/NCBI
|
|
16
|
Li M, Liu L, Wang Z, Wang L, Liu Z, Xu G
and Lu S: Overexpression of hMSH2 and hMLH1 protein in certain
gastric cancers and their surrounding mucosae. Oncol Rep.
19:401–406. 2008.PubMed/NCBI
|
|
17
|
Ciampi R, Zhu Z and Nikiforov YE: BRAF
copy number gains in thyroid tumors detected by fluorescence in
situ hybridization. Endocr Pathol. 16:99–105. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang L, Cunningham JM, Winters JL,
Guenther JC, French AJ, Boardman LA, Burgart LJ, McDonnell SK,
Schaid DJ and Thibodeau SN: BRAF mutations in colon cancer are not
likely attributable to defective DNA mismatch repair. Cancer Res.
63:5209–5212. 2003.PubMed/NCBI
|