Introduction
The metabolic abnormalities of prostate cancer cells
have yet to be adequately investigated (1,2).
Results of our previous studies showed that relative specific amino
acid dependency is one of the metabolic abnormalities of human
prostate cancer cells. A significant effect of specific amino acid
restriction is the induction of apoptosis in invasive,
androgen-independent DU145 and PC3 prostate cancer cells, but not
in normal human prostate epithelial cells (HPrE) or in
non-invasive, androgen-dependent LNCaP prostate cancer cells
(3,4). Moreover, selective amino acid
restriction targets the mitochondria of DU145 and PC3 cells. For
example, the restriction of tyrosine and phenylalanine (Tyr/Phe),
glutamine (Gln) or methionine (Met) inhibits ATP synthesis of the
two cell lines (3). Additionally,
the restriction of Tyr/Phe or Met in DU145 and Met in PC3 cells
reduces the mitochondrial membrane potential (5).
Mitochondria trigger cell death in various ways,
including the disruption of electron transport and energy
metabolism, the release/activation of proteins that mediate
apoptosis and the alteration of cellular reduction/oxidation
(redox) potential (6). Specific
amino acid restriction was previously found to alter the
mitochondrial distribution of various apoptosis-related proteins,
such as apoptosis-inducing factor, Bak and Bcl-XL, in DU145 cells
(5). The present study was designed
to determine whether specific amino acid restriction alters glucose
metabolism and cellular redox status, and whether these alterations
are related to mitochondrial DNA damage in DU145 and PC3 cells
restricted for specific amino acids.
Materials and methods
Cell culture
Two human prostate cancer cell lines, DU145 and PC3,
were maintained in suitable media (DMEM for DU145 and RPMI-1640 for
PC3) with 10% fetal bovine serum (Equitech-Bio, Inc., Kerrville,
TX, USA). HPrE cells were cultured in PrEBM Basal Medium with PrEGM
SingleQuot kit supplement and growth factors (Lonza Walkersville
Inc., Walkersville, MD, USA). The cells were initially cultured in
complete medium until they achieved 30–40% confluence prior to
changing to the amino acid-depleted medium. For these experiments,
cells were cultured in media depleted of individual amino acids
(Met or Gln) or two combined amino acids (Tyr/Phe). The amino
acid-deprived media were custom-manufactured by Life Technologies
(Grand Island, NY, USA), as previously described (3,7). The
experiments were repeated at least three times with similar
results.
Determination of glucose consumption and
lactate production
Cells (10,000) were seeded in 12-well plates and
cultured in complete or respective amino acid-free medium for 4
days. The glucose or lactate concentrations in the culture medium
and cell numbers (Beckman Coulter Vi-Cell XR counter, Brea, CA,
USA) were determined daily. Glucose consumption and lactate
production rates were normalized to the cell number.
Mitochondrial pyruvate dehydrogenase
activity
Pyruvate dehydrogenase (PDH) catalyzes the reaction
that converts pyruvate to acetyl-CoA, which then enters the citric
acid cycle (TCA). Thus, this enzyme links glycolysis and the TCA
cycle. The cells were cultured in an amino acid-deprived medium for
4 days. The attached cells were collected for mitochondria
isolation on days 2, 3 and 4 (Mitochondrial DNA Isolation kit;
Biovision, Mountain View, CA, USA) and to determine PDH enzyme
activity (MitoProfile microplate assay kit for PDH activity;
MitoSciences Inc., Eugene, OR, USA). The isolation and assay
procedures were performed according to the manufacturer’s
instructions and the results were normalized to the mitochondrial
protein content.
Measurement of reactive oxygen
species
Intracellular reactive oxygen species (ROS),
including hydrogen peroxide and hydroxyl radicals, was detected
with 5-(and-6)-chloromethyl-2′,7′-dichorodihydro-fluorescein
diacetate, acetyl ester (Molecular Probes Inc., Eugene, OR, USA)
staining and analyzed by flow cytometry. Briefly, 5 μM of this
compound was added into the culture medium 30 min prior to cell
harvesting. The cells were then washed and resuspended in PBS. The
fluorescence intensity of 10,000 events was analyzed with a FACScan
flow cytometer (BD Biosciences, San Jose, CA, USA).
Determination of cellular nicotinamide
adenine dinucleotide and nicotinamide adenine dinucleotide
phosphate
Nicotinamide adenine dinucleotide (NAD)/NADH and
nicotinamide adenine dinucleotide phosphate (NADP)/NADPH ratios
were determined with NAD/NADH and NADP/NADPH quantification kits
(Biovision) according to the manufacturer’s instructions.
Glutathione peroxidase activity
Glutathione peroxidase (GPx) catalyzes the reduction
of hydroperoxides, including hydrogen peroxide, by reduced
glutathione (GSH) and protects the cell from oxidative damage. The
enzyme activity during restriction of Tyr/Phe, Gln and Met was
measured. The cells were cultured in specific amino acid-deprived
medium for 4 days. The attached cells were collected on days 2, 3
and 4 and analyzed for GPx enzyme activity (Glutathione Peroxidase
assay kit; Cayman Chemical Company, Ann Arbor, MI, USA). The
results were normalized to the cellular protein content as
determined by the Bio-Rad protein assay (Bio-Rad Laboratories Inc.,
Hercules, CA, USA).
Mitochondrial superoxide dismutase
activity
Superoxide dismutase (SOD) catalyzes the conversion
of superoxide anion into hydrogen peroxide and oxygen. The cells
were treated with specific amino acid-deprived medium for 4 days
and the attached cells were collected on days 2, 3 and 4. The
mitochondria were isolated (Mitochondrial DNA isolation kit;
Biovision) and manganese (Mn) SOD enzyme activity was determined
(SOD-560: A colorimetric assay kit for SOD activity; Applied
Bioanalytical Labs, Sarasota, FL, USA). The isolation and assay
procedures were performed according to the manufacturer’s
instructions. The results were normalized to the mitochondrial
protein content as determined by the Bio-Rad protein assay (Bio-Rad
Laboratories Inc.).
Measurement of reduced, oxidized and
total glutathione
Glutathione is a signficant intracellular
low-molecular weight thiol that plays a critical role in cellular
defense against oxidative stress. Cells were cultured in amino
acid-deprived medium for 4 days and the attached cells were
collected to determine the amount of reduced glutathione (GSH),
oxidized glutathione (GSSG) and total glutathione (Glutathione
assay kit; Biovision) according to the manufacturer’s instructions.
The results were normalized to the cellular protein content as
determined by the Bio-Rad protein assay (Bio-Rad Laboratories
Inc.).
Measurement of mitochondrial DNA
damage
Apurinic/apyrimidinic (AP) sites are one of the
major types of DNA lesions formed during the course of base
excision and repair of oxidized or alkylated bases. The level of AP
sites is a good indicator of DNA damage. Mitochondrial DNA (mtDNA)
was isolated (Mitochondrial DNA isolation kit; Biovision) and
quantified (Quant-It PicoGreen dsDNA reagent and kits; Molecular
Probes Inc.). For each sample, 0.5 μg mtDNA in triplicate was used
to determine the density of AP sites. MtDNA AP site density was
measured using a DNA damage quantification kit (Biovision). The
procedures were performed according to the manufacturer’s
instructions.
Statistical analysis
The data in the figures are the means ± SE from
three separate experiments. The differences in the means were
compared using one-way ANOVA. The curves were compared using
two-way ANOVA. Multiple comparisons were performed by the least
squares means method.
Results
Specific amino acid restriction differentially
modulates glucose metabolism, cellular redox enzyme and the
glutathione status in DU145 and PC3 prostate cancer cells. The data
also indicate a correlation between the metabolic alterations and
mitochondrial DNA damage in the two cell lines.
Amino acid restriction differentially
modulates glucose consumption and lactate production in DU145 and
PC3 cells
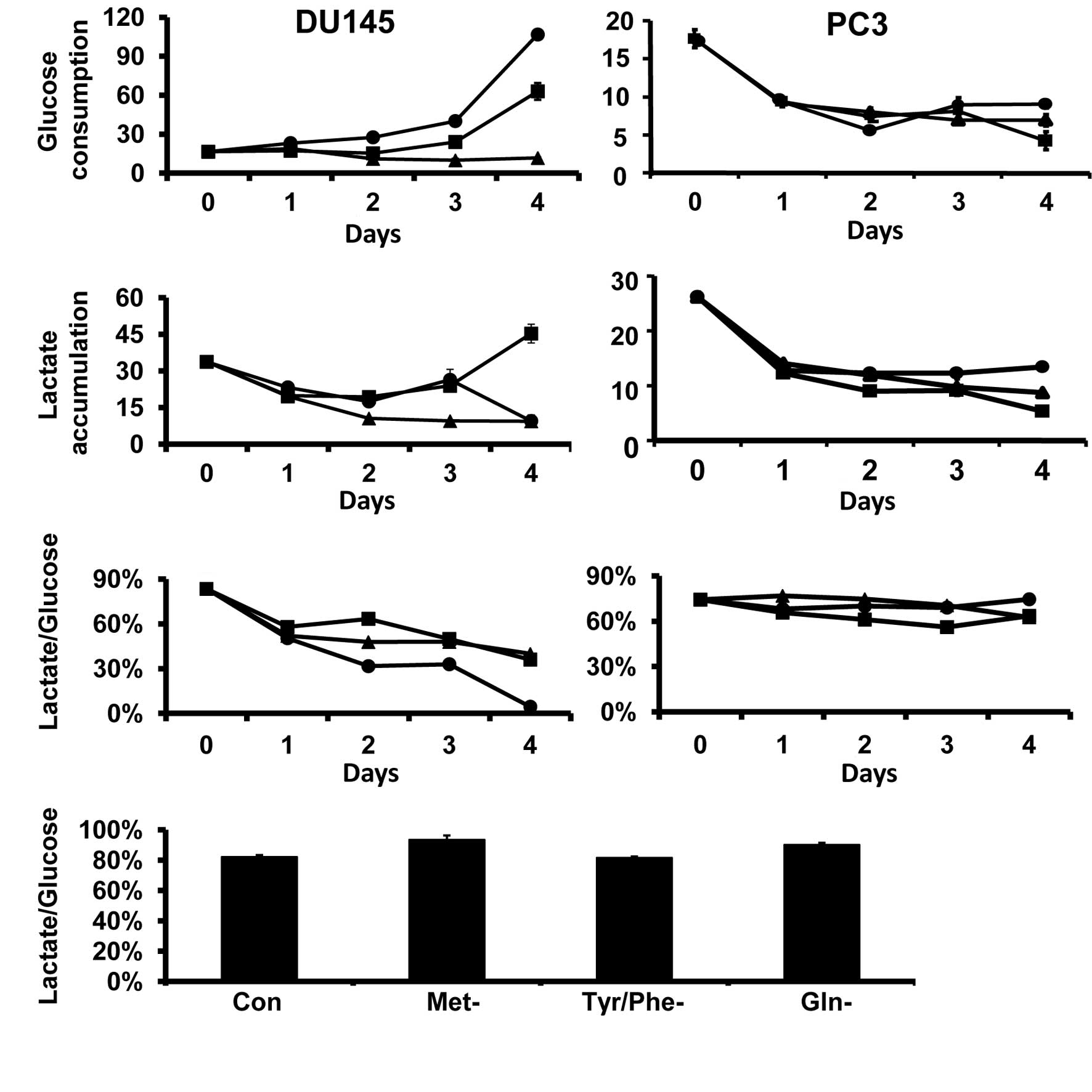
Fig. 1 shows the
effects of amino acid restriction on glucose consumption and
lactate production in DU145 and PC3 cells. Glucose consumption and
lactate production did not change with the period of time that
DU145 and PC3 cells were cultured in amino acid complete media. In
DU145 cells, Gln and Met restriction increased glucose consumption,
but glucose consumption was not significantly altered by the
Tyr/Phe restriction (Fig. 1A).
Lactate production was reduced by the Tyr/Phe restriction from days
1 to 4 of restriction. Lactate production in Gln- and Met-deprived
cells was also reduced during the first 2 days of the restriction,
but then returned to control levels on day 3. However, lactate
production was decreased in Gln-deprived cells and increased in
Met-deprived cells on day 4 (Fig.
1B). The lactate production/glucose consumption ratio was
reduced in DU145 cells by all specific amino acid restrictions at
all time points (Fig 1C).
The specific amino acid restrictions reduced glucose
consumption and lactate production in PC3 cells (Fig. 1A and B). The lactate
production/glucose consumption ratio was not altered by the amino
acid restrictions (Fig. 1C).
Compared to DU145 and PC3 prostate cancer cells, the
specific amino restrictions did not alter the glucose consumption
or lactate production in HPrE cells (data not shown). This lack of
alteration is due to the fact that the lactate production/glucose
consumption ratios did not differ in cells grown in amino acid
complete medium (Fig. 1D).
PDH is the rate-determining enzyme for the entry of
carbohydrate-derived acetyl units into oxidative metabolism (TCA
cycle). PDH activity is negatively regulated by ATP and NADH.
Cellular ATP is reduced in DU145 and PC3 cells during selective
amino acid restriction (5), and the
relative amount of NADH is also decreased by restriction. The
mitochondrial activity of PDH was examined since NADH is a cofactor
involved in PDH-mediated catalysis. In DU145 cells, the
restrictions initially elevated PDH activity on day 2 of
restriction, with the greatest increase in activity in
Gln-restricted cells (Fig. 2A). The
activity of PDH was not altered in PC3 cells by amino acid
restriction (Fig. 2A).
 | Figure 2(A) Selective restriction of amino
acids differentially modulated mitochondrial pyruvate dehydrogenase
(PDH) activity in DU145 and PC3 cells. Data are expressed as the
ratio of PDH activity in the amino acid-restricted cells compared
to cells cultured in complete media (mean ± SE). In DU145, the
restrictions elevated PDH activity compared to the control after
2–3 days of restriction (p<0.05). The activity of PDH was not
significantly altered in PC3 cells by amino acid restriction
compared to the control (p>0.05). (B) Cellular nicotinamide
adenine dinucleotide (NAD)/NADH ratio. Data are expressed as the
ratio of NAD/NADH in amino acid-deprived cells as compared to
control. In DU145 cells, glutamine (Gln) (day 3) and methionine
(Met) (days 3 and 4) restrictions elevated the NAD/NADH ratio
(p<0.05). No ratio for Gln-deprived cells was noted on day 4,
since NADH was undetectable on this day. Tyrosine and phenylalanine
(Tyr/Phe) restriction did not alter the nicotinamide adenine
dinucleotide phosphate (NADP)/NADPH ratio. In PC3 cells, the
NAD/NADH ratio was only elevated on day 4 (p<0.05) and neither
Tyr/Phe nor Met restriction altered the ratio. (C) Cellular
NADP/NADPH ratio. In DU145 cells, Gln restriction increased the
ratio on day 4 (p<0.05). The ratio was not altered by
Met-restriction and was reduced by Tyr/Phe restriction from days 2
to 3. In PC3 cells, the ratio was not significantly altered by the
amino restrictions, with the exception of Met restriction, where
the ratio was reduced on days 3 and 4 (p<0.05) compared to day
0. ■, Met restriction; ▴, Tyr/Phe restriction; ●, Gln
restriction. |
Amino acid restriction differentially
modulates reduction/oxidation status in DU145 and PC3 cells
The NAD/NADH ratio increased in DU145 cells on days
3 and 4 in Gln- and Met-restricted cells (Fig. 2B). No data are available for day 4
for Gln-restricted cells, since NADH was not detectable on this
day. The NADP/NADPH ratio also increased in Gln-restricted cells,
reaching a maximum on day 4 (Fig.
2C). The NADP/NADPH ratio was reduced during Tyr/Phe
restriction throughout the experimental period (Fig. 2C), but was not changed by Met
restriction.
In PC3 cells, the NAD/NADH ratio gradually increased
to a maximum at 4 days during Gln restriction. The ratio was not
significantly altered during Tyr/Phe or Met restriction (Fig. 2B). Moreover, the restrictions did
not alter the NADP/NADPH ratio (Fig.
2C) in these cells. These results show that selective amino
acid restriction differentially alters the cellular redox status in
PC3 and DU145 cells.
Amino acid restriction differentially
modulates mitochondrial antioxidant enzymes and glutathione in
DU145 and PC3 cells
ROS is mainly generated by the redox centers in the
mitochondrial electron transport chain (8,9). In
DU145 cells, all the specific amino acid restrictions increased the
amount of ROS. In PC3 cells, only Met restriction increased the
amount of ROS (Fig. 3A).
 | Figure 3(A) Restriction of amino acids
increased reactive oxygen species (ROS) in DU145 and PC3 cells. The
cells were cultured in complete or amino acid-deprived media for 3
days and stained with dichorodihydro-fluorescein diacetate, acetyl
ester. The x-axis shows the density of fluorescence. The y-axis
shows the cell count. Peak shifts to the right are the cell
population whose ROS increased. Tyr/Phe, Tyr/Phe-deprived; Met,
Met-deprived, Gln, Gln-deprived. (B) Selective restriction of amino
acids differentially increased cellular glutathione peroxidase
(GPx) activity in DU145 and PC3 cells. GPx activity was expressed
as nmol/min/mg protein. In DU145, the restrictions progressively
increased GPx activity compared to day 0 (p<0.05). The increase
was greatest in the glutamine (Gln)- and methionine (Met)-deprived
cells compared to tyrosine and phenylalanine (Tyr/Phe)-deprived
cells (p<0.001). GPx activity also progressively increased in
PC3 cells (p<0.05); however, the increase was most pronounced in
Met-deprived cells compared to Gln- or Tyr/Phe-restricted cells
(p<0.00). (C) Selective restriction of amino acids
differentially modulated mitochondrial superoxide dismutase (SOD)
activity in DU145 and PC3 cells. SOD activity was expressed as U/μg
mitochondrial protein. In DU145 cells, Tyr/Phe restriction
increased mitochondrial SOD activity by 3-fold on day 2 of the
restriction (p<0.001). The activity gradually returned to
control levels by day 4. Met restriction slightly reduced SOD
activity on day 4 (p<0.05) compared to day 0, and Gln
restriction did not alter SOD activity. In PC3 cells, only Tyr/Phe
restriction increased mitochondrial SOD activity and the difference
was significant on days 3 to 4 (p<0.05). (D) Selective
restriction of amino acids decreased the ratio of reduced
glutathione (GSH) to total glutathione. The cells were cultured in
complete or respective amino acid-deprived medium for 4 days and
then the amount of GSH and total glutathione was determined. The
amino acid deprivations reduced GSH in the two cell lines
(*p<0.05 compared to Con). In PC3, a marked decrease
was induced by Met restriction (#p<0.05 compared to
the other amino acid restriction groups). Con, cells cultured in
complete medium; ■, Met restriction; ▴, Tyr/Phe restriction; ●, Gln
restriction. |
The clearance of ROS is closely regulated by the
enzymes MnSOD and GPx in the mitochondria (8–13). The
low molecular weight thiol, GSH, plays a key role in the cellular
defense against oxidative stress (9,14). The
enzymatic action of GPx requires reduced GSH (9). Since cellular ROS is elevated in DU145
and PC3 cells, the activity of mitochondrial GPx and SOD and the
amount of GSH in the two cell lines was examined. In DU145 and PC3
cells, the amino acid restrictions significantly increased
mitochondrial GPx activity (Fig.
3B). In DU145 cells, the greatest increase was observed in Gln-
and Met-restricted cells (Fig. 3B).
In PC3 cells, the greatest increase was found in Met-restricted
cells (Fig. 3B).
Tyr/Phe restriction increased mitochondrial SOD
activity on day 2 in DU145 cells and the activity returned to
control levels on day 4 (Fig. 3C).
By day 4, SOD activity was lower than the control in the
Met-restricted cells. Gln restriction did not alter mitochondrial
SOD activity in DU145 cells (Fig.
3C). In PC3 cells, SOD activity was not significantly altered
by Gln or Met restriction; however, it progressively increased in
Tyr/Phe-restricted cells over time (Fig. 3C). These results show that specific
amino acid restriction differentially altered mitochondrial SOD and
GPx activity in PC3 and DU145 cells.
Specific amino acid restriction reduced cellular
GSH/GSSG in DU145 and PC3 cells (Fig.
3D). In DU145 cells, this ratio was similarly reduced by the
amino acid restrictions. However, in the PC3 cells, the degree of
reduction in GSH/GSSG in Met-restricted cells was 2-fold greater
than that in Tyr/Phe- and Gln-restricted cells (Fig. 3D). Collectively, the results in
Fig. 3 indicate that specific amino
acid restriction differentially modulates the cellular antioxidant
system in DU145 and PC3 cells.
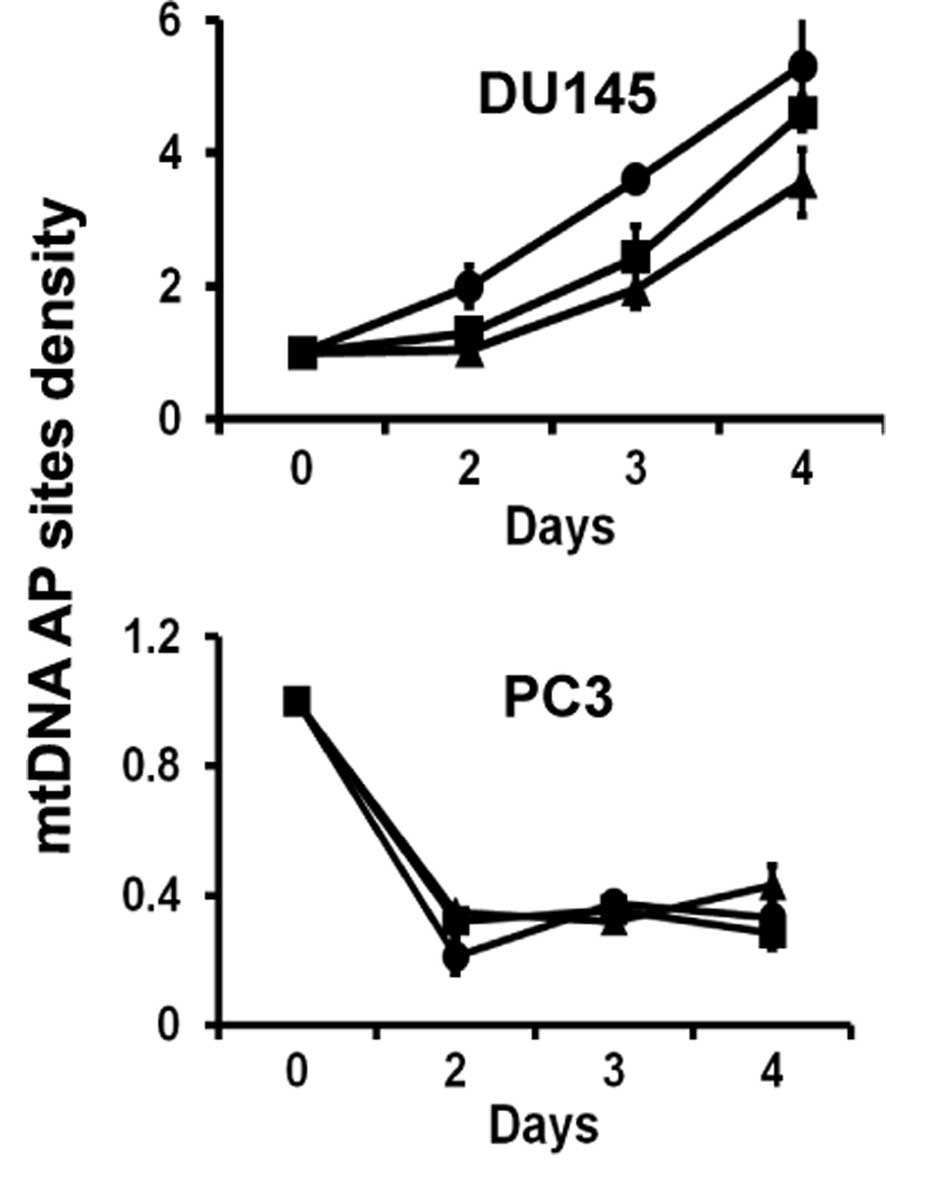
Amino acid restriction induces
mitochondrial DNA damage in DU145 cells
The abnormal accumulation of ROS in cells could
result in oxidative damage to mitochondrial nucleic acids.
Mitochondrial DNA damage was observed in all amino acid-restricted
DU145 cells (Fig. 4). Specific
amino acid restriction decreased mitochondrial DNA damage in PC3
cells (Fig. 4).
Discussion
In previous studies, we found that relative specific
amino acid dependency is one of the metabolic abnormalities of
human prostate cancer cells that regulates cell proliferation and
survival (3). These studies showed
that selective amino acid restriction promotes mitochondrial outer
membrane permeabilization and reduces intracellular ATP in DU145
and PC3 prostate cancer cells (5).
Results of the present study showed that specific amino acid
restriction differentially modulates cellular metabolism in the two
cell lines (15), including glucose
metabolism, cellular redox status and antioxidant systems. The
increased glycolytic activity (Warburg effect) of cancer cells
enhances the proliferation and resistance of the cells to apoptosis
(16). We previously found that the
addition of glucose or pyruvate differentially reduces or postpones
the apoptotic effect of specific amino acid restriction in DU145
and PC3 cells (15).
In the present study, glucose metabolism was found
to be differentially modulated by the specific restriction of amino
acids in the two cell lines. For example, Gln and Met restriction
increased glucose consumption in DU145 cells, but was not altered
in Tyr/Phe-restricted cells (Fig.
1A). In DU145, the lactate production/glucose consumption ratio
was reduced by the examined amino acid restrictions (Fig. 1C). Since the lactate
production/glucose consumption ratio expresses the proportion of
glycolysis relative to total glucose utilization, these findings
suggest that selective amino acid restriction accelerates TCA cycle
activity. PDH was the rate-determining enzyme for the entry of
carbohydrate-derived acetyl units into oxidative metabolism, and
the specific amino acid restrictions elevated the activity of this
enzyme (Fig. 2A). These findings
further support the hypothesis that the specific amino acid
restriction accelerates the TCA cycle in DU145 cells. During the
initial phase of specific amino acid restriction, a new energy
balance is established in the cell that favors survival. However,
this balance changes over time in such a manner that the continued
pressure of amino acid restriction results in cell death, as
previously described (3).
Since a variety of amino acids enter the TCA cycle
in a variety of manners, the restriction of each amino acid may
have different effects on the cycle. For example, Gln is a
significant energy source in general and particularly for malignant
cells. It is normally metabolized by L-glutamate dehydrogenase to
α-ketoglutarate, a substrate in the TCA cycle. Gln restriction is
likely to reduce the availability of α-ketoglutarate in the TCA
cycle, leading to a compensatory accelerated conversion of pyruvate
into acetyl-CoA, the primary component of the TCA cycle. This
possibility is consistent with the results in Fig. 3A, where Gln restriction elicited a
rapid increase of PDH activity in DU145 cells.
Compared to DU145 cells, the amino acid restrictions
reduced glucose consumption and lactate production in PC3 cells
(Fig. 1A and B). The lactate
production/glucose consumption ratio and PDH activity were not
altered by the specific amino acid restrictions (Figs. 1C and 3A). The results indicate that the amino
acid restrictions uniformly decreased glucose metabolism in PC3
cells. Therefore, during the initial phase of amino acid
restriction, the cells adapted by gradually decreasing glucose
metabolism, thereby establishing a new energy balance that favored
cell survival.
The accelerated TCA cycle of DU145 cells by amino
acid restriction alters the cellular redox status. This is
expressed by fluctuations in the cellular NAD/NADH and NADP/NADPH
ratios and in the amount of ROS (Fig.
2 and 3). The alterations
initially stimulated two antioxidant enzymes, SOD and GPx, to rid
the cell of ROS (Fig. 3B and C).
However, continued exposure to specific amino acid restriction
resulted in the inability of the cells to compensate effectively
and they subsequently died (3,5,15).
Additional evidence to support this finding is that GSH
availability was limited (Fig. 3D)
and mitochondrial DNA was damaged (Fig.
4) in addition to the damage induced in mitochondrial membranes
associated with the induction of apoptosis (3,5,15).
Compared to DU145, PC3 cells appear to have stronger
antioxidant activity, since mitochondrial DNA is not damaged and
the NAD/NADH and NADP/NADPH ratios are not altered to the same
degree as the ratios in DU145 cells. Although all of the amino acid
restrictions decrease GSH, Met-restricted cells showed the greatest
decrease in GSH and in the accumulation of ROS (Fig. 3A and D). These data along with our
previous finding may demonstrate why only Met restriction induces
apoptosis in PC3 cells (3,5,15).
In conclusion, findings of the present study show
that specific amino acid restriction has a wide-spread effect on
the metabolism of DU145 and PC3 prostate cancer cells, and it
differentially modulates glucose metabolism and cellular redox
status. Restriction of any amino acid causes metabolic stress in
cells. The gradual reduction in intracellular amino acid levels
during restriction is the proximal event that triggers signaling
events to inhibit cell proliferation and induce cell death in
prostate cancer cells (3,5). The data obtained in this study
correlate the metabolic alterations in DU145 and PC3 cells to
mitochondrial damage and induction of apoptosis. However,
additional metabolomic studies in these prostate cancer cells are
required to further define which specific metabolic reactions are
directly responsible for damaging mitochondria in the two prostate
cancer cell lines during selective amino acid restriction. Since
mitochondrial defects exist in cancer and mtDNA mutations in
prostate cancer play a key role in cellular metabolism and
behaviors (17,18), this study provides a strong
rationale for conducting additional studies to determine the manner
in which selective amino acid restriction modulates specific
mitochondrial functions in this type of cancer. Consequently, the
mechanisms associated with specific amino acid dependency of
prostate cancer cells may be elucidated, resulting in the
development of novel therapeutic approaches.
Acknowledgements
This study was supported by funds from NIH/NCI R01
CA101035 (to G.G.M.).
References
|
1
|
Tenniswood M: Apoptosis, tumour invasion
and prostate cancer. Br J Urol. 79:27–34. 1997. View Article : Google Scholar
|
|
2
|
Denmeade SR, Isaacs JT, August JT, et al:
Activation of programmed (apoptotic) cell death for the treatment
of prostate cancer. Advances in Pharmacology. 35. Academic Press,
Inc.; San Diego, CA: pp. 281–305. 1996, View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fu YM, Yu ZX, Li YQ, et al: Specific amino
acid dependency regulates invasiveness and viability of
androgen-independent prostate cancer cells. Nutr Cancer. 45:60–73.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fu YM, Yu ZX, Lin H, Fu X and Meadows GG:
Selective amino acid restriction differentially affects the
motility and directionality of DU145 and PC3 prostate cancer cells.
J Cell Physiol. 217:184–193. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fu YM, Zhang H, Ding M, et al: Selective
amino acid restriction targets mitochondria to induce apoptosis of
androgen-independent prostate cancer cells. J Cell Physiol.
209:522–534. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Green DR and Reed JC: Mitochondria and
apoptosis. Science. 281:1309–1312. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fu YM, Yu ZX, Pelayo BA, et al: Focal
adhesion kinase-dependent apoptosis of melanoma induced by tyrosine
and phenylalanine deficiency. Cancer Res. 59:758–765.
1999.PubMed/NCBI
|
|
8
|
Nemoto S, Takeda K, Yu ZX, et al: Role for
mitochondrial oxidants as regulators of cellular metabolism. Mol
Cell Biol. 20:7311–7318. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Balaban RS, Nemoto S and Finkel T:
Mitochondria, oxidants, and aging. Cell. 120:483–495. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Arbiser JL: Molecular regulation of
angiogenesis and tumorigenesis by signal transduction pathways:
evidence of predictable and reproducible patterns of synergy in
diverse neoplasms. Semin Cancer Biol. 14:81–91. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wallace DC: Mitochondrial diseases in man
and mouse. Science. 283:1482–1488. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Galluzzi L, Larochette N, Zamzami N, et
al: Mitochondria as therapeutic targets for cancer chemotherapy.
Oncogene. 25:4812–4830. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Matoba S, Kang JG, Patino WD, et al: p53
regulates mitochondrial respiration. Science. 312:1650–1653. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Benlloch M, Mena S, Ferrer P, et al: Bcl-2
and Mn-SOD antisense oligodeoxynucleotides and a glutamine-enriched
diet facilitate elimination of highly resistant B16 melanoma cells
by tumor necrosis factor-alpha and chemotherapy. J Biol Chem.
281:69–79. 2006. View Article : Google Scholar
|
|
15
|
Fu YM, Lin H and Liu X: Cell death of
prostate cancer cells by specific amino acid restriction depends on
alterations of glucose metabolism. J Cell Physiol. 224:491–500.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Weber G: Ordered biochemical program of
gene expression in cancer cells. Biochemistry. 66:1164–1173.
2001.PubMed/NCBI
|
|
17
|
Petros JA, Baumann AK, Ruiz-Pesini E, et
al: mtDNA mutations increase tumorigenicity in prostate cancer.
Proc Natl Acad Sci USA. 102:719–724. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Carew JS and Huang P: Mitochondrial
defects in cancer. Mol Cancer. 1:92002. View Article : Google Scholar
|