Introduction
Over the past decade, individualized therapeutic
strategies have been developed in the treatment of colorectal
cancer (CRC). These strategies utilize a number of novel,
molecularly targeted agents, yielding an improvement in survival
(1–12). Despite an increase in our knowledge
of molecular pathways, 40–50% of metastatic CRC patients continue
to experience no beneficial effect from such new strategies. This
effect may be due to the numerous steps involved in the
pharmacokinetics of anticancer drugs and various in vivo
factors affecting sensitivity to anticancer drugs (13). Testing the chemosensitivity of
cancer cells to a particular drug is therefore crucial in
establishing which drug is appropriate.
As a key drug in the treatment of CRC, it is crucial
to identify responders/non-responders to 5-fluorouracil (5-FU).
Previously, we investigated the sensitivity of cancer cells to 5-FU
in CRC patients using the collagen gel droplet-embedded
culture-drug sensitivity test (CD-DST). Multiple drug
concentrations and contact durations, and the area under the
concentration curve (AUC) and growth inhibition rate (IR) were
combined to produce an AUC-IR curve, which was approximated to the
logarithmic curve. Moreover, the individualized AUCIR50
and AUC value, which gives 50% growth inhibition, were calculated
using the AUC-IR curve (14,15).
This study aimed to identify responders/non-responders to 5-FU
based on the individual AUCIR50 obtained with the CD-DST
in order to establish individualized chemotherapy for CRC
patients.
Materials and methods
Patients
Surgically resected specimens of primary tumors were
obtained from 101 colorectal cancer patients between January 2002
and April 2010. Resection was performed without pre-operative
chemotherapy. Informed consent for measuring drug sensitivity was
obtained from the patients.
Methods
CD-DST was applied to tumor tissue excised from the
primary surgical specimens. The specimen was washed five times with
50 ml saline solution, followed by five times with 50 ml antibiotic
fluid containing 1.0 mg/ml piperacillin and 0.5 mg/ml kanamycin.
The transport bottle contained 1.0 mg/ml piperacillin, 0.5 mg/ml
kanamycin and 2.5 μg/ml amphotericin B. Tumor sensitivity to 5-FU
was evaluated using CD-DST, performed as described by Kobayashi
et al (16). Tissue (1 g)
was treated with dispersion enzyme cocktail (EZ; Kurabo Industries
Ltd., Japan) for 2 h. Dispersed cell suspensions were inoculated
into pre-culture media in collagen-coated flasks overnight. Viable
tumor cells were then recovered by 0.05% collagenase treatment.
Recovered cells were embedded in 30-μl collagen gel droplets.
Embedded cells were cultivated in culture media containing 5-FU at
0.2 μg/ml for 3 h, 1 μg/ml for 3 h, 10 μg/ml for 3 h, 0.2 μg/ml for
24 h, 1 μg/ml for 24 h, 10 μg/ml for 24 h, 0.2 μg/ml for 120 h, 1
μg/ml for 120 h or 10 μg/ml for 120 h. The 5-FU-containing media
were removed and the cells were cultured for 7 days in serum-free
culture media (PCM-2; Kurabo Industries Ltd.) to prevent growth of
fibroblasts. Viable cells were stained with neutral red solution
and counted by the imaging colorimetric quantification method. The
surviving cell number ratio between the drug-treated and control
groups was calculated. Where a growth rate in excess of 0.8 was
observed or ≥4 culture conditions were recorded, the case was
considered successful.
Following conversion of the drug concentrations and
contact time to AUC, the AUC-IR curve was plotted against the
growth inhibition rate and the individual AUCIR50, i.e.,
the AUC value that indicates 50% growth inhibition, was calculated
from the AUC-IR regression curve. The approximate expression of the
cumulative distribution of the individual AUCIR50 was
obtained using the non-linear least squares method. The transition
of the approximate expression with an increase in the number of
patients was evaluated.
Statistical analysis
Correlations between the cumulative distribution of
the individual AUCIR50 and the approximate expression of
the cumulative distribution of the individual AUCIR50
were analyzed by linear regression analysis. The statistical tests
were carried out using the SPSS package (version II for Windows).
P<0.05 was considered to be statistically significant.
Results
Table I shows the
patient characteristics of the 101 patients enroled in this study.
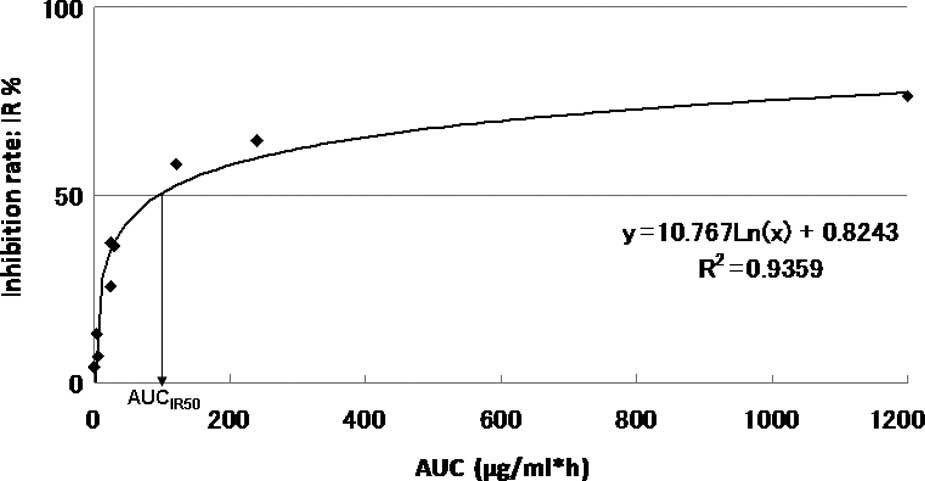
The AUC-IR curve of a representative patient is shown in Fig. 1.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| No. of patients | 101 |
| Mean age, years
(range) | 65.2 (30–85) |
| Gender
(male/female) | 45/56 |
| Histological
type |
| Well-differentiated
carcinoma | 15 |
| Moderately
differentiated carcinoma | 73 |
| Poorly
differentiated carcinoma | 4 |
| Mucinous
carcinoma | 9 |
| Dukes’ stage
(A/B/C/D) | 8/42/33/18 |
| Colon/rectum | 80/21 |
The approximate expression and correlation
coefficients were y=10.767Ln(x) + 0.8243, (R2=0.9359)
for AUC and 96.3 μg × h/ml [y=10.767Ln(x) + 0.8243,
R2=0.9359] for the individual AUCIR50 value
calculated from the regression curve in this patient. The curve
between the AUC and the growth IR approximated a logarithmic curve
(R2=0.655–0.999). The individual AUCIR50
value was calculated from the AUC-IR curve (AUCIR50 =
8.1 μg − 98.8 g × h/ml) for all 101 patients.
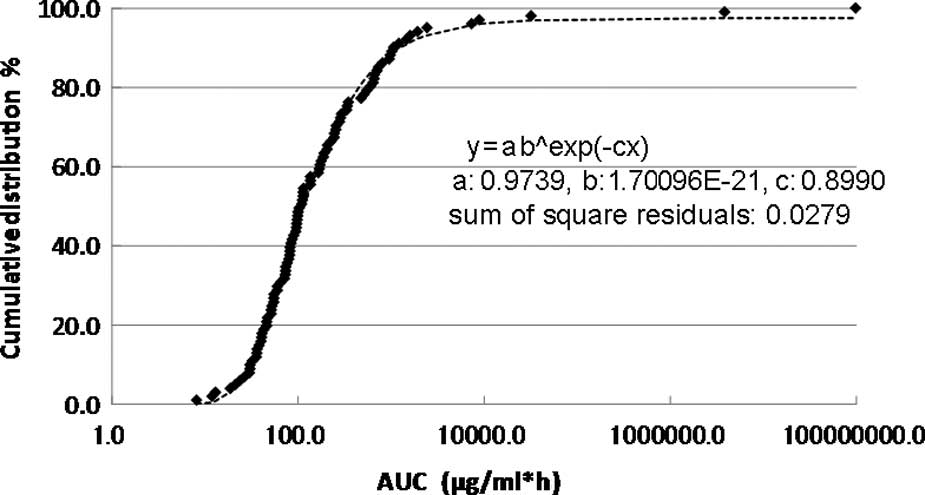
The cumulative distribution of the individual
AUCIR50 in the 101 patients is shown in Fig. 2. The cumulative distribution of the
individual AUCIR50 was regressed over the sigmoid curve
(logarithmic scale). The approximate expression was almost exactly
y=ab^exp(−cx) (a=0.9739, b=1.7096E-21, c=0.8990 and the sum of
square residuals, 0.0279).
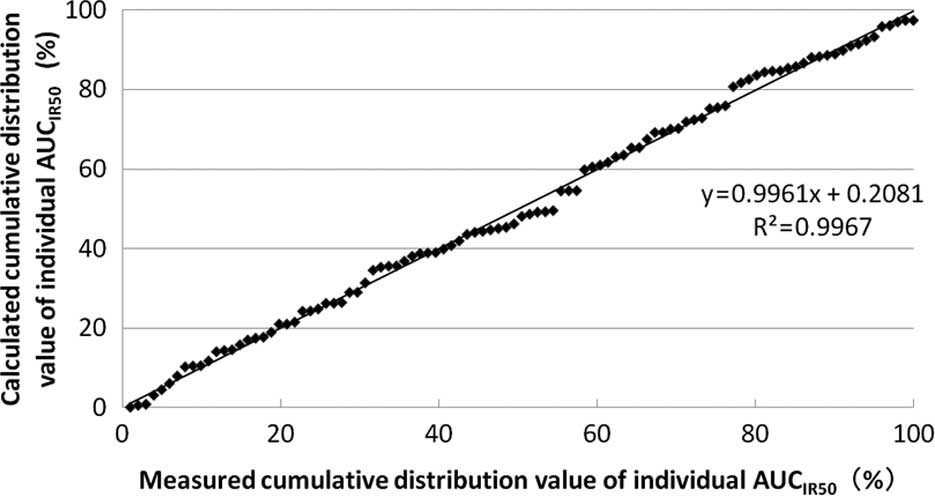
The correlation between the measured and the
calculated cumulative distribution values of the individual
AUCIR50 from the approximate expression is shown in
Fig. 3. The approximate expression
and correlation coefficients were y=0.9961x + 0.2081
(R2=0.9967) using linear regression analysis. Linear
regression with an increase in the number of patients is shown in
Table II.
 | Table IILinear regression with an increase in
the number of patients. |
Table II
Linear regression with an increase in
the number of patients.
| No. of patients | Linear
regression | R2 | P-value |
|---|
| 30 | y=0.9667x +
0.0179 | 0.9712 | 0.993 |
| 40 | y=0.9717x +
0.0162 | 0.9824 | 0.979 |
| 50 | y=0.9836x +
0.0093 | 0.9894 | 0.987 |
| 60 | y=0.9918x +
0.0042 | 0.9918 | 1.000 |
| 70 | y=0.9916x +
0.0048 | 0.9945 | 0.992 |
| 80 | y=1.0005x −
0.0011 | 0.9954 | 0.985 |
| 90 | y=0.9974x +
0.0012 | 0.9965 | 0.997 |
| 101 | y=0.9961x +
0.0021 | 0.9967 | 0.998 |
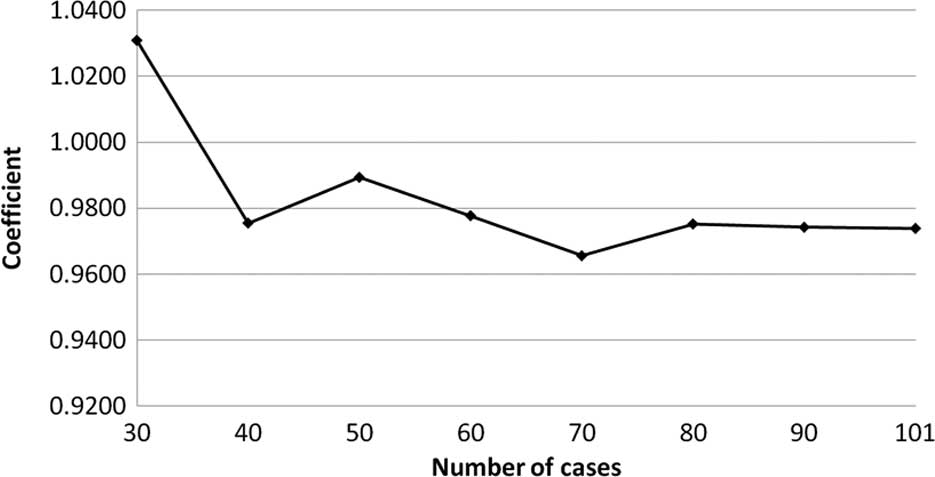
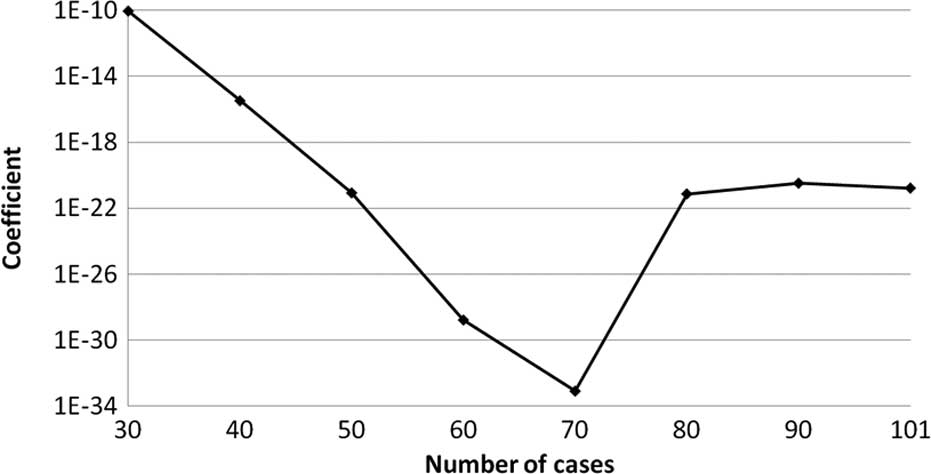
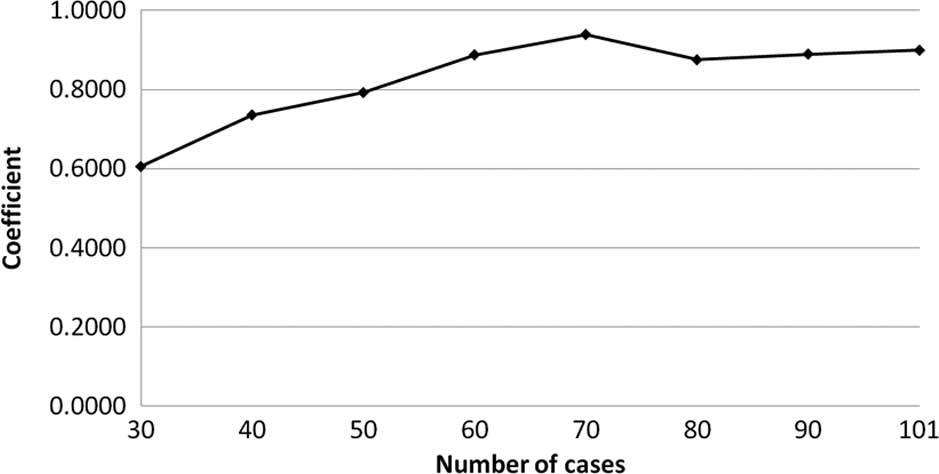
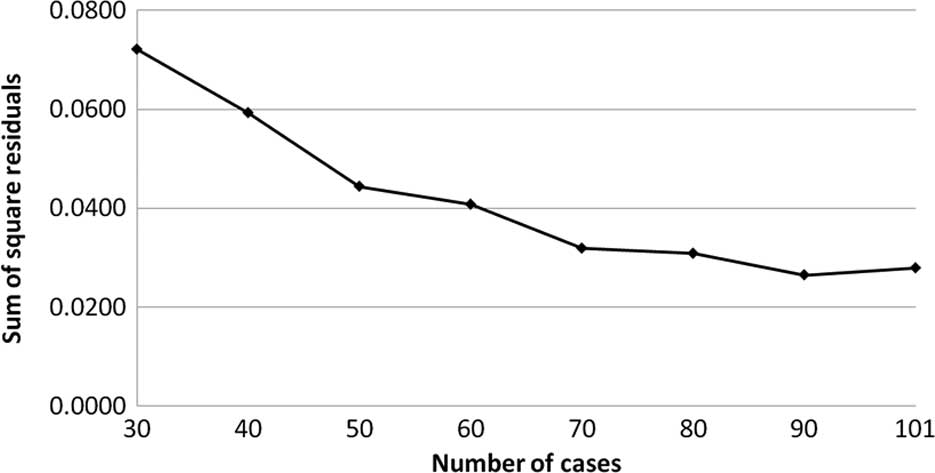
The transition of the coefficients (a, b and c) and
the sum of the square residuals in the approximate expression
[y=ab^exp(−cx)] with an increase in the number of patients are
shown in Figs. 4, 5, 6 and
7, respectively. In the 80 cases
examined, no notable change was observed in the regression curve
with an increase in the number of patients.
Discussion
Advances in the cytotoxic treatment of CRC have been
noted over the past decade. Moreover, the introduction of novel
molecularly targeted agents has yielded improvements in
progression-free and overall survival in metastatic CRC patients
(1–12). Despite these improvements in the
treatment of CRC and increased knowledge regarding the molecular
pathways involved, approximately half of all CRC patients have yet
to receive any benefit from such progress, and selecting patients
likely to prove responsive to a particular drug remains a
challenge. Although a number of novel molecular predictive and
prognostic markers have been identified (13), more remain to be determined, as
numerous steps are involved in the pharmacokinetics of anticancer
drugs, especially with regard to absorption, metabolism and
excretion. Numerous in vivo factors affect sensitivity to an
anticancer drug, including activation enzymes, degradation enzymes,
membrane transport proteins, DNA repair enzymes and the target
protein in the cancer cell. These factors are regulated by
individual genes. In other words, the behavior of the cancer is
affected by the body as a whole. Thus, tumor and cell function,
enzymatic activity, amount of enzymes, mRNA expression and, gene
polymorphisms and mutations are involved in affecting the
occurrence of cancer. Therefore, testing for chemosensitivity to a
particular drug is crucial in distinguishing potential responders
from non-responders.
The majority of chemotherapy regimens for CRC
incorporate 5-FU, both in an adjuvant and palliative setting. 5-FU
therefore is the key drug used in the treatment of CRC. The
single-agent response rate to 5-FU varies between 20 and 25% in
patients with advanced-stage CRC (17). No other anticancer agent surpasses
5-FU in terms of single-agent response rate. Therefore, assessing
its antitumor effect in each individual CRC patient is crucial.
Previously, we investigated cancer cell sensitivity to 5-FU in CRC
patients using CD-DST under multiple drug concentrations and
contact durations. Moreover, AUC and IR were combined to produce an
AUC-IR curve, which was approximated to the logarithmic curve
(14). We also reported that the
growth inhibition rate calculated from the AUC-IR curve and the
actual growth inhibition rate at an AUC of 48 μg × h/ml were
identical. Based on these results, we proposed that the in
vitro antitumor effect of 5-FU was dependent on the AUC in
colorectal cancer, and that the AUC-IR curve was reliable (15). Moreover, we calculated the
individualized AUCIR50, the AUC value which gives 50%
growth inhibition, using the AUC-IR curve (18).
In this study, we evaluated the distribution of the
individual AUCIR50 in order to establish individualized
chemotherapy in CRC patients, focusing, in particular, on the
transition of the approximate expression with an increase in the
number of patients. The cumulative distribution of the individual
AUCIR50 in 101 patients was regressed over the sigmoid
curve (logarithmic scale). The approximate expression was almost
exactly y=ab^exp(−cx) (a=0.9739, b=1.7096E-21, c=0.8990 and the sum
of square residuals, 0.0279). The approximate expression and
correlation coefficients between the measured and calculated
cumulative distribution values of the individual AUCIR50
were y=0.9961x + 0.2081 (R2=0.9967) using linear
regression analysis. No notable change was observed in the
transition of the coefficients (a, b and c) or the sum of the
square residuals in the approximate expression [y=ab^exp(−cx)] with
an increase in the number of patients. In particular, in over 80
cases the approximate expression [y=ab^exp(−cx)] remained almost
identical. These results indicate that the regression curve may
serve as the standard curve for describing responders to 5-FU among
CRC patients. From this standard curve, we were able to ascertain
that non-responders accounted for approximately 5% of all patients
and, thus, determine the responders.
In individualized 5-FU-based chemotherapy for CRC,
it is crucial to identify the non-responders and classify
responders as good or intermediate by applying the standard curve
to each patient. In palliative chemotherapy, irinotecan molecularly
targeted drugs or irinotecan with molecularly targeted drugs are
recommended for non-responders to 5-FU, whereas oxaliplatin plus
5-FU and leucovorin (FOLFOX) ± molecularly targeted drugs or
irinotecan plus 5-FU and leucovorin (FOLFIRI) ± molecularly
targeted drugs are recommended for responders (1–12). In
adjuvant chemotherapy, good responders are suitable candidates for
shorter-term, standard 5-FU-based chemotherapy, i.e., oral 5-FU or
FOLFOX. Nevertheless, intermediate responders are also suitable
candidates for longer-term or more intensive 5-FU-based
chemotherapy, i.e., FOLFOX with molecularly targeted drugs.
However, administration of molecularly targeted drugs in adjuvant
chemotherapy remains to be investigated (19–21).
Selection of adjuvant chemotherapy is challenging in non-responders
to 5-FU. Single-response rates to irinotecan and oxaliplatin are
relatively low; thus, close follow-up is required, and remains to
be investigated.
More recently, numerous adjuvant chemotherapies have
been assessed (22,23). These adjuvant chemotherapy regimens
have incorporated 5-FU, indicating the importance of evaluating the
response to this drug. In the present study, a standard curve was
generated to identify responders to 5-FU among CRC patients. From
this standard curve, we were able to ascertain that non-responders
accounted for approximately 5% of all patients. Moreover, we were
able to identify responders as good or intermediate candidates. We
believe that this standard curve offers a useful tool in
identifying responders to 5-FU among CRC patients, enabling the
establishment of individualized chemotherapy in CRC patients.
In Japan, the medical cost of CRC treatment,
particularly in unresectable or recurring cases, is not
cost-effective. Consequently, many patients are unable to receive
such treatment due to the financial outlay involved. Therefore, our
approach to identifying patients likely to be responsive to such
treatment offers a viable solution.
References
|
1
|
De Gramont A, Figer A, Seymour M, et al:
Leucovorin and fluorouracil with or without oxaliplatin as
first-line treatment in advanced colorectal cancer. J Clin Oncol.
18:2938–2947. 2000.
|
|
2
|
Goldberg RM, Sargent DJ, Morton RF, et al:
A randomized controlled trial of fluorouracil plus leucovorin,
irinotecan, and oxaliplatin combinations in patients with
previously untreated metastatic colorectal cancer. J Clin Oncol.
22:23–30. 2004. View Article : Google Scholar
|
|
3
|
Rothenberg ML, Oza AM, Bigelow RH, et al:
Superiority of oxaliplatin and fluorouracil-leucovorin compared
with either therapy alone in patients with progressive colorectal
cancer after irinotecan and fluorouracil-leucovorin: interim
results of a phase III trial. J Clin Oncol. 21:2059–2069. 2003.
View Article : Google Scholar
|
|
4
|
Douillard JY, Cunningham D, Roth AD, et
al: Irinotecan combined with fluorouracil compared with
fluorouracil alone as first-line treatment for metastatic
colorectal cancer: a multicentre randomised trial. Lancet.
355:1041–1047. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tournigand C, André T, Achille E, et al:
FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced
colorectal cancer: a randomized GERCOR study. J Clin Oncol.
22:229–237. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hochster HS, Hart LL, Ramanathan RK, et
al: Safety and efficacy of oxaliplatin and fluoropyrimidine
regimens with or without bevacizumab as first-line treatment of
metastatic colorectal cancer: results of the TREE Study. J Clin
Oncol. 21:3523–3529. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Saltz LB, Clarke S, Díaz-Rubio E, et al:
Bevacizumab in combination with oxaliplatin-based chemotherapy as
first-line therapy in metastatic colorectal cancer: a randomized
phase III study. J Clin Oncol. 26:2013–2019. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Giantonio BJ, Catalano PJ, Meropol NJ, et
al: Bevacizumab in combination with oxaliplatin, fluorouracil, and
leucovorin (FOLFOX4) for previously treated metastatic colorectal
cancer: results from the Eastern Cooperative Oncology Group Study
E3200. J Clin Oncol. 25:1539–4154. 2007. View Article : Google Scholar
|
|
9
|
Bokemeyer C, Bondarenko I, Makhson A, et
al: Fluorouracil, leucovorin, and oxaliplatin with and without
cetuximab in the first-line treatment of metastatic colorectal
cancer. J Clin Oncol. 27:663–671. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Maughan TS, Adams R, Smith CG, et al:
Identification of potentially responsive subsets when cetuximab is
added to oxaliplatin-fluoropyrimidine chemotherapy (CT) in
first-line advanced colorectal cancer (aCRC). J Clin Oncol.
28(Suppl 5): abs. 3502. 2010.
|
|
11
|
Fuchs CS, Marshall J, Mitchell E, et al:
Randomized, controlled trial of irinotecan plus infusional, bolus,
or oral fluoropyri-midines in first-line treatment of metastatic
colorectal cancer: results from the BICC-C Study. J Clin Oncol.
25:4779–4786. 2007. View Article : Google Scholar
|
|
12
|
Van Cutsem E, Köhne CH, Hitre E, et al:
Cetuximab and chemotherapy as initial treatment for metastatic
colorectal cancer. N Eng J Med. 360:1408–1417. 2009.PubMed/NCBI
|
|
13
|
Winder T and Lenz HJ: Molecular predictive
and prognostic marker in colon cancer. Cancer Treat Rev.
36:550–556. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ochiai T, Nishimura K, Noguchi H, Kitajima
M, Tsuruoka Y and Takahashi Y: Evaluation of 5-fluorouracil
applicability by multi-point collagen gel droplet embedded drug
sensitivity test. Oncol Rep. 14:201–205. 2005.PubMed/NCBI
|
|
15
|
Ochiai T, Nishimura K, Noguchi H, et al:
Evaluation of 5-fluorouracil applicability by the collagen gel
droplet embedded drug sensitivity test with area under the curve
analysis. Anticancer Drugs. 18:17–21. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kobayashi H, Higashiyama M, Minamigawa K,
et al: Examination of in vitro chemosensitivity test using collagen
gel droplet culture method with colorimetric endpoint
quantification. Jpn J Cancer Res. 92:203–210. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gill S, Loprinzi CL, Sargent DJ, et al:
Pooled analysis of fluorouracil-based adjuvant therapy for stage II
and III colon cancer: who benefits and by how much? J Clin Oncol.
22:1797–1806. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ochiai T, Nishimura K, Watanabe T, et al:
Evaluation of the individual 50% inhibitory area under the
concentration curve of 5-fluorouracil based on the collagen gel
droplet embedded culture drug sensitivity test in colorectal
cancer. Mol Med Rep. 2:405–409. 2009.
|
|
19
|
André T, Boni C, Mounedji-Boudiaf L, et
al: Multicenter international study of
oxaliplatin/5-fluorouracil/leucovorin in the adjuvant treatment of
colon cancer (MOSAIC) investigators. N Eng J Med. 350:2343–2351.
2004.
|
|
20
|
André T, Boni C, Navarro M, et al:
Improved overall survival with oxaliplatin, fluorouracil, and
leucovorin as adjuvant treatment in stage II or III colon cancer in
the MOSAIC trial. J Clin Oncol. 27:3109–3116. 2009.PubMed/NCBI
|
|
21
|
Wolmark N, Yothers G, O’Connell MJ, et al:
A phase III trial comparing mFOLFOX6 to mFOLFOX6 plus bevacizumab
in stage II or III carcinoma of the colon: results of NSABP
Protocol C-08. J Clin Oncol. 27(Suppl 18): abs. LBA4. 2009.
|
|
22
|
Alberts SR, Sargent DJ, Smyrk TC, et al:
Adjuvant mFOLFOX6 with or without cetuximab (Cmab) in KRAS
wild-type (WT) patients (pts) with resected stage III colon cancer
(CC): results from NCCTG Intergroup Phase III Trial NO 147. J Clin
Oncol. 28(Suppl 18): abs. CRA3507. 2010.
|
|
23
|
Goldberg RM, Sargent DJ, Thibodeau SN, et
al: Adjuvant mFOLFOX6 plus or minus cetuximab (Cmab) in patients
(pts) with KRAS mutant (m) resected stage III colon cancer (CC):
NCCTG Intergroup Phase III Trial N0147. J Clin Oncol. 28(Suppl 15):
abs. 3508. 2010.
|