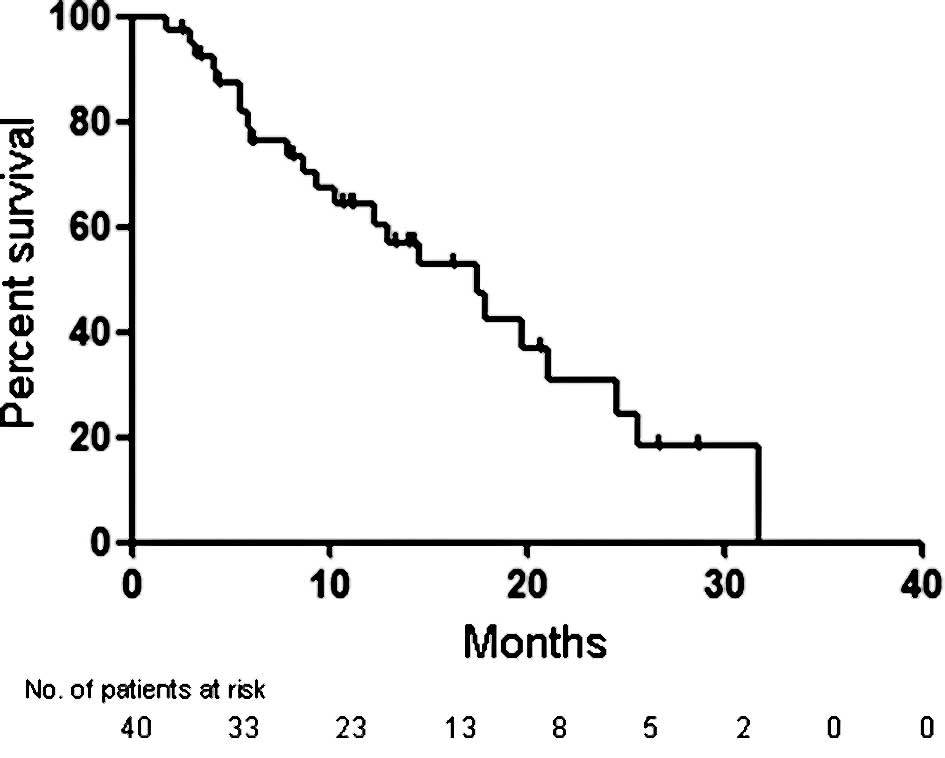
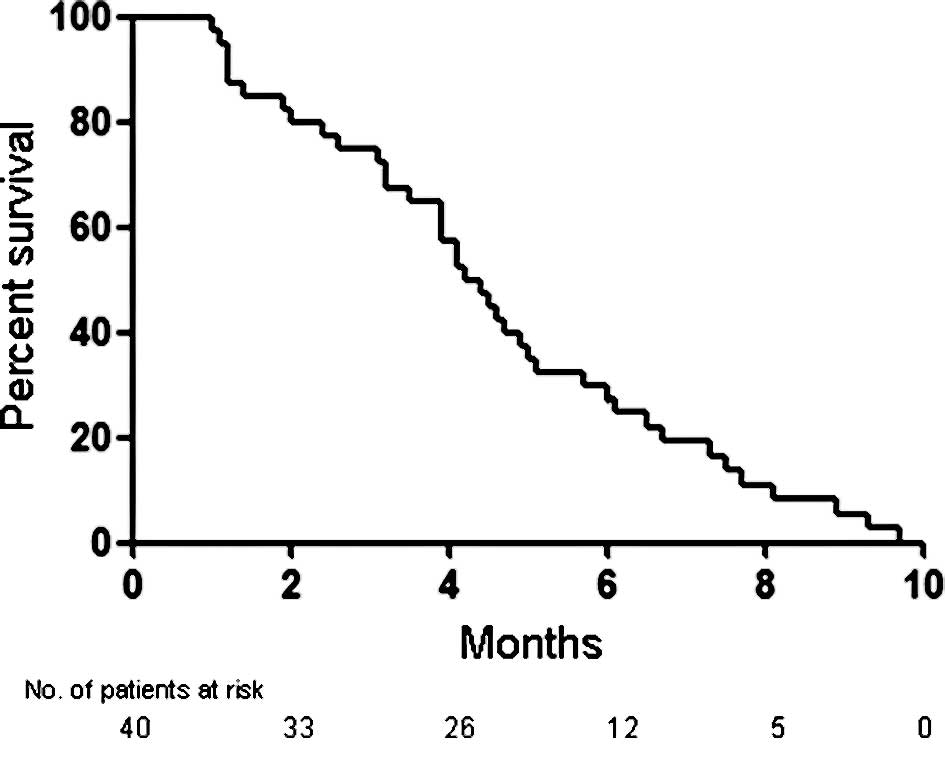
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics. CA Cancer J Clin. 59:225–249. 2009.
|
|
2
|
Shirasaka T, Shimamato Y, Ohshimo H,
Yamaguchi M, Kato T, Yonekura K and Fukushima M: Development of a
novel form of an oral 5-fluorouracil derivative (S-1) directed to
the potentiation of the tumor selective cytotoxicity of
5-fluorouracil by two biochemical modulators. Anticancer Drugs.
7:548–557. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Saif MW, Syrigos KN and Katirtzoglou NA:
S-1: a promising new oral fluoropyrimidine derivative. Expert Opin
Investig Drugs. 18:335–348. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shirasaka T, Yamamitsu S, Tsuji A and
Taguchi T: Conceptual changes in cancer chemotherapy: from an oral
fluoropyrimidine prodrug, UFT, to a novel oral fluoropyrimidine
prodrug, S-1, and low-dose FP therapy in Japan. Invest New Drugs.
18:315–329. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Van Groeningen CJ, Peters GJ, Schornagel
JH, Gall H, Noordhuis P, de Vries MJ, Turner SL, Swart MS, Pinedo
HM, Hanauske AR and Giaccone G: Phase I clinical and
pharmacokinetic study of oral S-1 in patients with advanced solid
tumors. J Clin Oncol. 18:2772–2779. 2000.
|
|
6
|
Yamada Y, Hamaguchi T, Goto M, Muro K,
Matsumura Y, Shimada Y, Shirao K and Nagayama S: Plasma
concentrations of 5-fluorouracil and F-beta-alanine following oral
administration of S-1, a dihydropyrimidine dehydrogenase inhibitory
fluoropyrimidine, as compared with protracted venous infusion of
5-fluorouracil. Br J Cancer. 89:816–820. 2003. View Article : Google Scholar
|
|
7
|
Kawahara M, Furuse K, Segawa Y, Yoshimori
K, Matsui K, Kudoh S, Hasegawa K and Niitani H: Phase II study of
S-1, a novel oral fluorouracil, in advanced non-small-cell lung
cancer. Br J Cancer. 85:939–943. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ichinose Y, Yoshimori K, Sakai H, Nakai Y,
Sugiura T, Kawahara M and Niitani H: S-1 plus cisplatin combination
chemotherapy in patients with advanced non-small cell lung cancer:
a multi-institutional phase II trial. Clin Cancer Res.
10:7860–7864. 2004. View Article : Google Scholar
|
|
9
|
Ozawa Y, Inui N, Naitoh T, Yasuda K,
Nagayama M, Shirai T, Suganuma H, Fujii M, Nakamura H, Suda T and
Chida K: Phase II study of combination chemotherapy with S-1 and
weekly cisplatin in patients with previously untreated advanced
non-small cell lung cancer. Lung Cancer. 63:68–71. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tamura K, Okamoto I, Ozaki T, Kashii T,
Takeda K, Kobayashi M, Matsui K, Shibata T, Kurata T, Nakagawa K
and Fukuoka M: Phase I/II study of S-1 plus carboplatin in patients
with advanced non-small cell lung cancer. Eur J Cancer.
45:2132–2137. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Okamoto I, Nishimura T, Miyazaki M,
Yoshioka H, Kubo A, Takeda K, Ebi N, Sugawara S, Katakami N,
Fukuoka M and Nakagawa K: Phase II study of combination therapy
with S-1 and irinotecan for advanced non-small cell lung cancer:
West Japan Thoracic Oncology Group 3505. Clin Cancer Res.
14:5250–5254. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schiller JH, Harrington D, Belani CP,
Langer C, Sandler A, Krook J, Zhu J and Johnson DH: Comparison of
four chemotherapy regimens for advanced non-small-cell lung cancer.
N Engl J Med. 346:92–98. 2002. View Article : Google Scholar
|
|
13
|
Kelly K, Crowley J, Bunn PA Jr, Presant
CA, Grevstad PK, Moinpour CM, Ramsey SD, Wozniak AJ, Weiss GR,
Moore DF, Israel VK, Livingston RB and Gandara DR: Randomized phase
III trial of paclitaxel plus carboplatin versus vinorelbine plus
cisplatin in the treatment of patients with advanced non-small-cell
lung cancer: a Southwest Oncology Group trial. J Clin Oncol.
19:3210–3218. 2001.PubMed/NCBI
|
|
14
|
Shepherd FA, Dancey J, Ramlaun R, Mattson
K, Gralla R, O'Rourke M, Levitan N, Gressot L, Vincent M, Burkes R,
Coughlin S, Kim Y and Berille J: Prospective randomized trial of
docetaxel versus best supportive care in patients with
non-small-cell lung cancer previously treated with platinum-based
chemotherapy. J Clin Oncol. 18:2095–2103. 2000.
|
|
15
|
Shepherd FA, Pereira JR, Ciuleanu T, Tan
EH, Hirsh V, Thongprasert S, Campos D, Maoleekoonpiroj S, Smylie M,
Martins R, van Kooten M, Dediu M, Findlay B, Tu D, Johnston D,
Bezjak A, Clark G, Santabárbara P and Seymour L: Erlotinib in
previously treated non-small cell lung cancer. N Engl J Med.
353:123–132. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Scagliotti GV, Parikh P, von Pawel J,
Biesma B, Vansteenkiste J, Manegold C, Serwatowski P, Gatzemeier U,
Digumarti R, Zukin M, Lee JS, Mellemgaard A, Park K, Patil S,
Rolski J, Goksel T, de Marinis F, Simms L, Sugarman KP and Gandara
D: Phase III study comparing cisplatin plus gemcitabine with
cisplatin plus pemetrexed in chemotherapy-naïve patients with
advanced-stage non-small cell lung cancer. J Clin Oncol.
26:3543–3551. 2008.PubMed/NCBI
|
|
17
|
Sandler A, Gray R, Perry MC, Brahmer J,
Schiller JH, Dowlati A, Lilenbaum R and Johnson DH:
Paclitaxel-carboplatin alone or with bevacizumab for non-small cell
lung cancer. N Engl J Med. 355:2542–2550. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ishikawa T, Sekiguchi F, Fukase Y, Sawada
N and Ishitsuka H: Positive correlation between the efficacy of
capecitabine and doxifluridine and the ratio of thymidine
phosphorylase to dihydropyrimidine dehydrogenase activities in
tumors in human cancer xenografts. Cancer Res. 58:685–690.
1998.
|
|
19
|
Chujo M, Miura T, Kawano Y, Miyawaki M,
Imakiire T, Hayashita Y and Kawahara K: Thymidine phosphorylase
levels and dihydropyrimidine dehydrogenase levels in non-small cell
lung cancer tissues. Oncol Rep. 16:777–780. 2006.PubMed/NCBI
|