Introduction
Lung cancer is the leading cause of
malignancy-related death worldwide, with a mortality rate of 80–90%
(1). Among patients with lung
cancer, 85% are diagnosed as having non-small cell lung cancer
(NSCLC). Despite efforts, innovations, and progress in the
diagnosis and treatment of these patients, patient survival at 5
years after diagnosis is only 15% (1). Therefore, novel therapeutic strategies
to improve the outcome of patients with NSCLC are urgently
required.
S-1 is an oral anticancer fluoropyrimidine agent
comprising the 5-fluorouracil (5-FU) prodrug tegafur and two enzyme
inhibitors, 5-chloro-2, 4-dihydroxypyridine (CDHP) and potassium
oxonate (2,3). Since CDHP inhibits dihydropyrimidine
dehydrogenase activity and potassium oxonate suppresses pyrimidine
phosphoribosyl transferase activity, oral S-1 administration
generates a higher concentration of 5-FU than a protracted
intravenous injection of 5-FU, without increasing the incidence of
adverse events in the gastrointestinal tract (4–6). In a
phase II trial, involving monotherapy with S-1 at 80
mg/m2/day for 28 days followed by a 2-week rest period
in chemotherapy-naïve patients with advanced NSCLC, the overall
response rate (RR) was 22.0% and the median survival time (MST) was
10.2 months (7). Moreover, two
phase II trials of S-1 plus cisplatin (CDDP) for advanced NSCLC
(stage IIIB, without any indication for radiotherapy, or stage IV)
yielded RRs of 32.7–47% and MSTs of 11–16 months with a mild
toxicity profile (8,9). Recent evidence has indicated favorable
efficacy of S-1 in combination with chemotherapeutic agents, with
the exception of CDDP, for advanced NSCLC (10,11).
Although the results suggest the efficacy of combinations of S-1
with other chemotherapeutic agents, the therapeutic efficacy and
safety of combination chemotherapy with S-1 plus CDDP in patients
with advanced NSCLC have yet to be elucidated.
In the present study, a phase II study of
combination chemotherapy with S-1 (40 mg/m2 twice a day
on days 1–21 followed by a 2-week rest) and CDDP (60
mg/m2 on day 8, every 5 weeks) was conducted in patients
with advanced NSCLC, and the efficacy and safety of this regimen
were determined. The MST of 17.9 months occurred over a longer
period of time, and the incidence of adverse events was lower than
that for standard NSCLC chemotherapy. Furthermore, an overall RR in
patients with squamous cell carcinoma was statistically superior
compared with that in patients with adenocarcinoma or other types
of NSCLC. These findings suggest that combination chemotherapy with
S-1 plus CDDP is a promising therapeutic candidate for patients
with advanced NSCLC, particularly squamous cell carcinoma.
Patients and methods
Patient eligibility
Patients were eligible for this phase II trial in
the event that they were either cytologically or histologically
confirmed to have NSCLC; were in stage IIIB, without any indication
of radical thoracic radiotherapy, or stage IV; had measurable
disease; received no prior treatment; were in the age range of
20–74 years; had an Eastern Cooperative Oncology Group (ECOG)
performance status of 0 or 1; and had a projected life expectancy
of at least 3 months. Other eligibility criteria for organ function
included: leukocyte count 4000–12,000/μl, absolute granulocyte
count ≥2000/μl, platelet count ≥100,000/μl, hemoglobin level ≥9
g/dl, serum bilirubin level ≤1.5 mg/dl, serum aspartate
aminotransferase and alanine amino transferase levels ≤100 IU/l,
alkaline phosphatase level of twice the upper limit or less, normal
creatinine level, creatinine clearance rate of ≥60 ml/min, and
arterial oxygen partial pressure of ≥60 Torr. Since S-1 is in
tablet form, patients were required to be able to swallow. For
staging, all 44 patients underwent a computed tomography scan of
the thorax, including upper abdomen, and either a brain computed
tomography scan or magnetic resonance imaging of the brain. A
radioisotopic bone scan was also performed in the majority of the
patients.
Exclusion criteria included pregnancy or serious
concomitant disease, concomitant malignancy, pleural effusion
requiring treatment, or symptomatic cerebral involvement. Written
informed consent was obtained from all patients, and the protocol
was approved by the Institutional Review Board of each of the
participating institutions. On enrollment to the study, the
eligibility of the patients was reviewed by the Central
Administration Office at the Department of Respiratory Medicine and
Rheumatology, University of Tokushima, Japan.
Treatment schedule
Oral administration of S-1 at 40 mg/m2
occurred twice a day, after meals, on days 1–21. The actual dose of
S-1 administration was selected as follows: in a patient with a
body surface area (BSA) <1.25 m2, 40 mg twice a day;
BSA of 1.25 m2 but <1.5 m2, 50 mg twice a
day; and BSA ≥1.5 m2, 60 mg twice a day. CDDP (60
mg/m2) was administered intravenously on day 8 when
patients were hydrated by infusion of at least 2,500 ml.
Administration of an antiemetic agent was permitted at the
discretion of each patient's physician. The treatment regimen was
repeated every 5 weeks until disease progression or unacceptable
toxicity occurred. A leukocyte count of ≥3000/μl, an absolute
granulocyte count of ≥1500/μl, a platelet count of ≥100,000/μl and
the entry eligibility criteria regarding organ functions had to be
achieved in order for the following cycle to commence. If these
criteria were satisfied 4 weeks after day 1 of each cycle of
chemotherapy, administration of the following cycle was allowed.
The doses of S-1 were adjusted according to the degree of
hematologic and non-hematologic toxicity. The dose was reduced by
one level (20 mg per day) in patients with evidence of grade 4
hematologic toxicity or grade ≥3 non-hematologic toxicity during
any cycle of administration. If recovery from such toxicities was
confirmed at a reduced dose, administration at the reduced dose was
continued. If a rest period of ≥3 weeks was required, the patient
was withdrawn from the study.
Evaluation of response and toxicity
Eligible patients who received at least one course
of S-1 plus CDDP were considered assessable for response and
toxicity. Chest X-ray, complete blood count, and blood chemistry
studies were repeated at least once a week. The response was
assessed based on the computed tomography scan findings that had
been used initially to define the extent of the tumor. The response
was evaluated at the end of each treatment cycle in accordance with
the Response Evaluation Criteria in Solid Tumors version 1.0.
Adverse events were graded according to the National Cancer
Institute Common Toxicity Criteria version 3.0.
Statistical analysis
The primary end point was RR; secondary end points
included progression-free survival (PFS), overall survival and
adverse events. PFS was defined as the time from registration to
progression or death from any cause. Overall survival was defined
as the time from registration to death from any cause or when the
patient was last known to be alive. The minimum number of patients
to be enrolled in this study was defined as 34, with the assumption
that the 95% CI would be 30% under conditions giving an α error of
0.025 (one-sided) and a β error of 0.2, assuming an anticipated RR
of 50%. Consequently, 40 patients were included to allow for
patient dropout. The median PFS and MST were estimated by the
Kaplan-Meier method of univariate analysis. The differences between
categorized groups were compared by the one-way ANOVA test. All
statistical tests were two-sided, and p<0.05 was considered to
be statistically significant.
Results
Patient population
Between July 2005 and November 2008, 44 patients
with advanced NSCLC from 7 institutions were enrolled in this
study. Of the 44 patients, 40 were assessable for efficacy and
safety. Two patients were excluded since they declined entry to the
study after enrollment. Two other patients were considered not to
be assessable as they were unable to receive any CDDP due to high
fever and massive pleural effusion, respectively, after S-1
treatment had commenced. The clinical characteristics of the 40
assessable patients are shown in Table
I. Patients included 32 males (80%) and 8 females (20%). The
median age was 64 years (range 27–74). The patients had an ECOG
performance status of 0 (12/40, 30%) or 1 (28/40, 70%). A total of
13 patients (32.5%) had stage IIIB and 27 (67.5%) had stage IV
NSCLC. The predominant histological type was adenocarcinoma (55%),
followed by squamous cell carcinoma (22.5%) and other types of
NSCLC (22.5%).
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| No. of patients | 40 |
| Age (years) |
| Median (range) | 64 (27–74) |
| Gender |
| Male | 32 (80.0%) |
| Female | 8 (20.0%) |
| Performance status
(ECOG) |
| 0 | 12 (30.0%) |
| 1 | 28 (70.0%) |
| Stage |
| IIIB | 13 (32.5%) |
| IV | 27 (67.5%) |
| Histology |
| Adenocarcinoma | 22 (55.0%) |
| Squamous cell
carcinoma | 9 (22.5%) |
| Others | 9 (22.5%) |
Response and survival
No complete response (CR) was observed in the 40
patients, whereas 7 patients showed a partial response (PR),
yielding an overall RR of 17.5% (95% CI 5.2–29.8). Stable disease
(SD) was observed in 25 patients (62.5%). Thus, the disease control
rate (PR+SD) was 80% (95% CI 67.1–92.9). Eight patients (20%)
showed progressive disease (PD). Table
II shows patient characteristics in relation to response. No
statistically significant differences were noted in the RRs among
gender and stages. Notably, the overall RR in patients with
squamous cell carcinoma (55.5%) was statistically superior compared
with the RR in patients with adenocarcinoma or other types of NSCLC
(9.1%, p=0.012; 0.0%, p=0.009, respectively). The MST of the 40
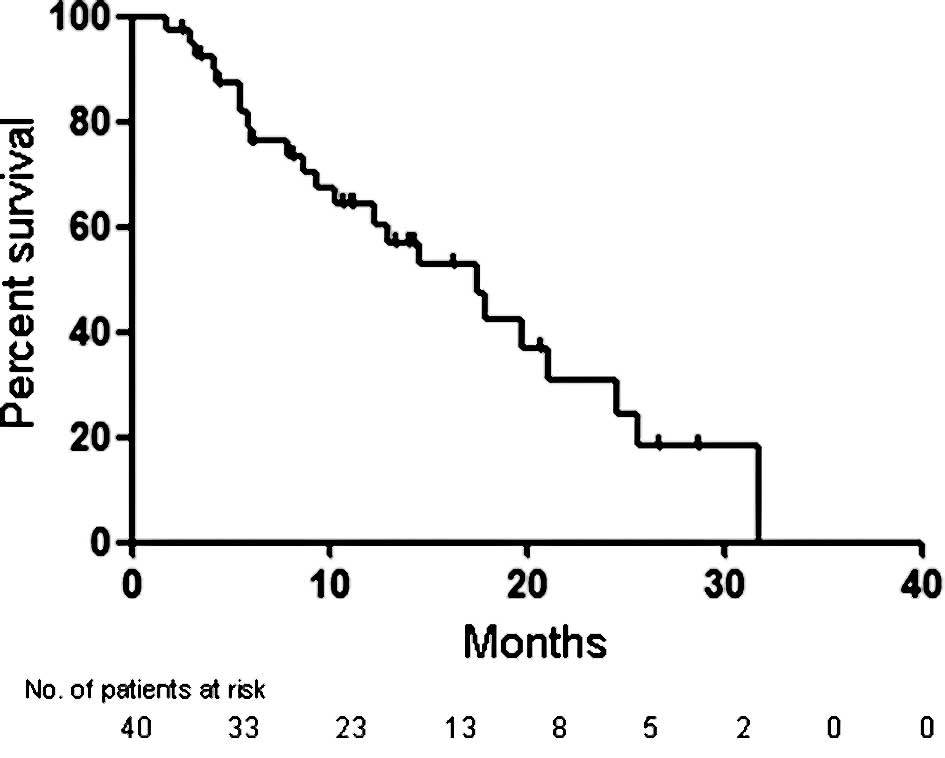
assessable patients was 17.9 months (95% CI 15.0–20.8), and the 1-
and 2-year survival rates were 63.3 and 27.3%, respectively
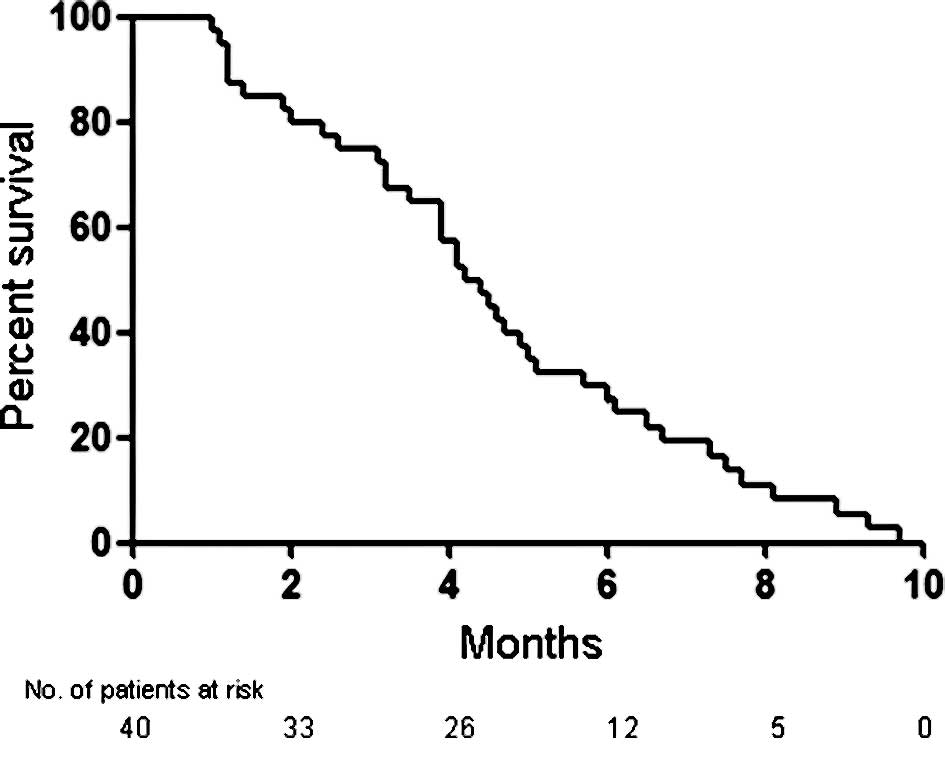
(Fig. 1). As shown in Fig. 2, the median PFS was 4.3 months (95%
CI 3.4–4.9). Exploratory analyses of the survival according to
histological subtypes showed no significant differences in the MST
and median PFS. The MSTs and median PFSs of patients with squamous
cell carcinoma, adenocarcinoma and other types of NSCLC were 21.1
and 6.5 months, 17.9 and 4 months, and 14.5 and 4.2 months,
respectively.
 | Table IIPatient characteristics in relation to
the response. |
Table II
Patient characteristics in relation to
the response.
| | Response | |
|---|
| |
| |
|---|
| Characteristics | No. of patients | CR | PR | SD | PD | Response rate
(%) |
|---|
| No. of patients | 40 | 0 | 7 | 25 | 8 | 17.5 |
| Gender |
| Male | 32 | 0 | 5 | 19 | 8 | 15.6 |
| Female | 8 | 0 | 2 | 6 | 0 | 25.0 |
| Stage |
| IIIB | 13 | 0 | 2 | 8 | 3 | 15.4 |
| IV | 27 | 0 | 5 | 17 | 5 | 18.5 |
| Histology |
|
Adenocarcinoma | 22 | 0 | 2 | 15 | 5 | 9.1 |
| Squamous cell
carcinoma | 9 | 0 | 5 | 3 | 1 | 55.5 |
| Others | 9 | 0 | 0 | 7 | 2 | 0.0 |
Adverse events
Table III shows
the major adverse events in the 40 assessable patients during the
entire treatment period. The hematological adverse events reaching
grades 3–4 were anemia (15%), leukocytopenia (7.5%), neutropenia
(5%) and thrombocytopenia (2.5%). Febrile neutropenia was observed
in only one patient (2.5%). Grade 3 non-hematologic adverse events
were anorexia (12.5%), nausea/vomiting (7.5%), infection (5%),
general fatigue (2.5%) and gastric ulcer (2.5%). No cases of grade
4 toxicity occurred. Moreover, no instance of irreversible toxicity
or treatment-related death was noted.
 | Table IIIHematologic and non-hematologic
toxicities. |
Table III
Hematologic and non-hematologic
toxicities.
| Adverse events | Grade | Grade 3–4 (%) |
|---|
|
|---|
| 2 | 3 | 4 |
| Leukocytopenia | 7 | 3 | 0 | 7.5 |
| Neutropenia | 6 | 2 | 0 | 5.0 |
| Anemia | 8 | 4 | 2 | 15.0 |
|
Thrombocytopenia | 5 | 0 | 1 | 2.5 |
| Liver disorder | 4 | 0 | 0 | 0.0 |
| Bilirubin | 1 | 0 | 0 | 0.0 |
| Creatinine | 2 | 0 | 0 | 0.0 |
| Hyponatremia | 0 | 1 | 0 | 2.5 |
| Infection | 0 | 2 | 0 | 5.0 |
| Anorexia | 6 | 5 | 0 | 12.5 |
|
Nausea/vomiting | 7 | 3 | 0 | 7.5 |
| General
fatigue | 4 | 1 | 0 | 2.5 |
| Gastric ulcer | 0 | 1 | 0 | 2.5 |
| Diarrhea | 2 | 0 | 0 | 0.0 |
| Constipation | 1 | 0 | 0 | 0.0 |
| Stomatitis | 1 | 0 | 0 | 0.0 |
| Desquamation | 1 | 0 | 0 | 0.0 |
| Fever | 1 | 0 | 0 | 0.0 |
Compliance
The median number of cycles administered was 3, with
a range of 1–9 treatment cycles (1 cycle, 7 patients; 2 cycles, 9
patients; 3 cycles, 8 patients; 4 cycles, 12 patients; ≥5 cycles, 4
patients). The reasons for administration of only one cycle of
treatment were PD in 5 patients and adverse events in 2 patients. A
total of 122 cycles were administered to the 40 patients. Seven
patients required temporary or permanent cessation in S-1 during
the treatment courses due to adverse events, including
myelosuppression in 4 patients, pulmonary toxicity in 2, and
gastrointestinal toxicity in 1 patient. No dose reduction of S-1
was required in any of the 40 assessable patients. CDDP
administration was disregarded in 4 patients due to
myelosuppression, and the dose of CDDP was reduced in 1 patient due
to gastrointestinal toxicity.
Discussion
The present study indicates that combination
chemotherapy with S-1 plus CDDP may constitute an efficacious and
well-tolerated therapeutic option for patients with treatment-naïve
advanced NSCLC. The MST of 17.9 months occurred over a longer
period of time, and the incidence of adverse events was lower than
the values for the standard NSCLC chemotherapy. Consequently,
combination chemotherapy with S-1 plus CDDP is a promising
therapeutic candidate for patients with advanced NSCLC.
A phase II trial of combination chemotherapy with
S-1 at 80 mg/m2/day for 21 days and CDDP at 60
mg/m2 on day 8 yielded a RR of 47.3% and a MST of 11
months (8). Ozawa et al also
reported a phase II study of combination chemotherapy with S-1 at
80 mg/m2/day for 21 days and weekly CDDP at 25
mg/m2/week on days 7, 14 and 21, which yielded a RR of
23.1% and a MST of 13.4 months (9).
Compared with the reported RRs and MSTs of 17–28% and 7–9 months,
respectively, for standard platinum-doublet chemotherapy regimens
in patients with advanced NSCLC (12,13),
the combination of S-1 with CDDP appears to be encouraging. In the
present study, combination chemotherapy with S-1 (80
mg/m2/day, days 1–21) and CDDP (60 mg/m2 on
day 8, every 5 weeks) yielded a potentially longer MST (17.9
months) than that for the abovementioned studies, despite the
relatively low RR (17.5%). The modest improvement in survival
observed in this study as compared with previous studies may be
affected by various factors including the high rate of SD (62.5%),
which extended the disease control rate to 80.0%, or the exclusion
of patients with a performance status of 2. In addition, second-
and/or third-line chemotherapy treatments may prolong the survival
of patients with advanced NSCLC, as previously reported (14,15).
In this study, 37 patients (92.5%) received second-line
chemotherapy, involving platinum-based doublet chemotherapy,
non-platinum-doublet chemotherapy, single non-platinum antitumor
agent and epidermal growth factor receptor tyrosine kinase
inhibitors in 16 (43.2%), 7 (18.9%), 9 (24.3%) and 5 (13.5%)
patients, respectively. Among these 37 patients, 35 were assessable
for response. PR and SD were observed in 4 (11.4%) and 21 (60.0%)
patients, respectively, yielding a disease control rate (PR+SD) of
71.4%. Although a much longer MST (17.5 months) was observed in the
present study, the median PFS (4.3 months) was comparable to or
tended to be shorter than that in previous studies (10,11),
indicating that additional chemotherapy treatments following the
failure of first-line combination treatment with S-1 and CDDP may
have a favorable survival effect.
Accumulating evidence has shown that the
histological types of NSCLC affect the clinical outcome of patients
treated with anticancer drugs (16,17). A
phase III study showed a significant survival difference in favor
of CDDP/pemetrexed compared with CDDP/gemcitabine in patients with
adenocarcinoma and large-cell carcinoma but not in those with
squamous cell carcinoma (16).
Consequently, pemetrexed is approved for use in combination with
CDDP for first-line treatment in patients with advanced
non-squamous NSCLC. Moreover, serious hemorrhagic events have been
reported to occur more frequently among patients with squamous cell
carcinoma treated by bevacizumab (17). Therefore, patients with advanced
squamous cell carcinoma have been routinely excluded from
bevacizumab treatment, while the addition of bevacizumab to
carboplatin/paclitaxel in the treatment of patients with
non-squamous NSCLC has a significant survival benefit. These
findings indicate the disadvantages of NSCLC patients with squamous
histology, since they cannot benefit from these drugs and have
fewer treatment options than those with non-squamous histology.
Notably, in the present study, a significantly higher RR was
observed in patients with squamous cell carcinoma (55.5%) than in
those with adenocarcinoma (9.1%) or other types of NSCLC (0.0%).
Given the small sample size, these data should be interpreted with
caution. It is noteworthy, however, that combination chemotherapy
with S-1 plus CDDP may confer treatment benefit on lung cancer
patients with squamous cell histology. One potential explanation
for this outcome may relate to thymidine phosphorylase (TP) and
dihydropyrimidine dehydrogenase (DPD) expression levels in NSCLC
histological types. The ratio of TP to DPD is considered to be a
useful predictor of the efficacy of chemotherapy with 5-FU, since
TP converts 5′-deoxy-5-fluorouridine to 5-FU and DPD inactivates
5-FU in tumor tissue (18).
Moreover, the ratio of TP to DPD in NSCLC tissue was reported to be
higher in squamous cell carcinoma than in adenocarcinoma (19). These findings indicate that
chemotherapy with 5-FU is potentially more effective in lung cancer
patients with squamous cell carcinoma than in those with
adenocarcinoma. To the best of our knowledge, this is the first
study to show the significant treatment benefit of this combination
in lung cancer patients with squamous cell histology as compared to
non-squamous NSCLC. Further prospective randomized controlled
clinical trials are required to confirm these results.
In platinum-doublet chemotherapies, which are the
standard first-line regimen for advanced NSCLC, grade 3/4
neutropenia and gastrointestinal toxicity have been reported in
57–76 and 4–35% of patients, respectively (12,13).
By contrast, combination chemotherapy with S-1 plus CDDP is
reportedly less toxic than standard platinum-doublet regimens.
Findings of phase II studies of combination chemotherapy with S-1
and CDDP have shown grade 3/4 hematologic and non-hematologic
toxicities in 22–35 and 4–13% of patients, respectively (8,9). In
the present study, mild toxicity profiles were noted for
combination chemotherapy with S-1 plus CDDP, consistent with
previous reports (8,9). With regards to hematologic adverse
events, grade 3/4 leukocytopenia, neutropenia, anemia and
thrombocytopenia were observed in 3 (7.5%), 2 (5%), 6 (15%) and 1
(2.5%) patients, respectively. Only 1 patient (2.5%) developed
febrile neutropenia. Regarding non-hematologic adverse events,
grade 3 infection, anorexia, nausea/vomiting, general fatigue and
gastric ulcer were observed in 2 (5%), 5 (12.5%), 3 (7.5%), 1
(2.5%) and 1 (2.5%) patients, respectively. No grade 4 level
toxicity occurred, and no instance of irreversible toxicity or
treatment-related death was noted. The results indicate the
efficacy and safety of combination chemotherapy with S-1 and CDDP
for advanced NSCLC.
In conclusion, we investigated the efficacy and
safety of a combination regimen of S-1 with CDDP for the treatment
of chemotherapy-naïve patients with advanced NSCLC. Results showed
a potentially long MST with comparatively low toxicity, indicating
that this regimen is a potentially useful alternative therapeutic
strategy for patients with advanced NSCLC, particularly squamous
cell carcinoma.
Acknowledgements
This study was supported in part by a Grant-in-aid
for Cancer Research from the Ministry of Education, Science, Sports
and Culture of Japan, and the Ministry of Health and Welfare of
Japan.
Abbreviations:
|
NSCLC
|
non-small cell lung cancer
|
|
5-FU
|
5-fluorouracil
|
|
CDHP
|
5-chloro-2,4-dihydroxypyridine
|
|
RR
|
response rate
|
|
MST
|
median survival time
|
|
CDDP
|
cisplatin
|
|
ECOG
|
Eastern Cooperative Oncology Group
|
|
BSA
|
body surface area
|
|
PFS
|
progression-free survival
|
|
95% CI
|
95% confidence interval
|
|
PR
|
partial response
|
|
SD
|
stable disease
|
|
PD
|
progressive disease
|
|
TP
|
thymidine phosphorylase
|
|
DPD
|
dihydropyrimidine dehydrogenase
|
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics. CA Cancer J Clin. 59:225–249. 2009.
|
|
2
|
Shirasaka T, Shimamato Y, Ohshimo H,
Yamaguchi M, Kato T, Yonekura K and Fukushima M: Development of a
novel form of an oral 5-fluorouracil derivative (S-1) directed to
the potentiation of the tumor selective cytotoxicity of
5-fluorouracil by two biochemical modulators. Anticancer Drugs.
7:548–557. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Saif MW, Syrigos KN and Katirtzoglou NA:
S-1: a promising new oral fluoropyrimidine derivative. Expert Opin
Investig Drugs. 18:335–348. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shirasaka T, Yamamitsu S, Tsuji A and
Taguchi T: Conceptual changes in cancer chemotherapy: from an oral
fluoropyrimidine prodrug, UFT, to a novel oral fluoropyrimidine
prodrug, S-1, and low-dose FP therapy in Japan. Invest New Drugs.
18:315–329. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Van Groeningen CJ, Peters GJ, Schornagel
JH, Gall H, Noordhuis P, de Vries MJ, Turner SL, Swart MS, Pinedo
HM, Hanauske AR and Giaccone G: Phase I clinical and
pharmacokinetic study of oral S-1 in patients with advanced solid
tumors. J Clin Oncol. 18:2772–2779. 2000.
|
|
6
|
Yamada Y, Hamaguchi T, Goto M, Muro K,
Matsumura Y, Shimada Y, Shirao K and Nagayama S: Plasma
concentrations of 5-fluorouracil and F-beta-alanine following oral
administration of S-1, a dihydropyrimidine dehydrogenase inhibitory
fluoropyrimidine, as compared with protracted venous infusion of
5-fluorouracil. Br J Cancer. 89:816–820. 2003. View Article : Google Scholar
|
|
7
|
Kawahara M, Furuse K, Segawa Y, Yoshimori
K, Matsui K, Kudoh S, Hasegawa K and Niitani H: Phase II study of
S-1, a novel oral fluorouracil, in advanced non-small-cell lung
cancer. Br J Cancer. 85:939–943. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ichinose Y, Yoshimori K, Sakai H, Nakai Y,
Sugiura T, Kawahara M and Niitani H: S-1 plus cisplatin combination
chemotherapy in patients with advanced non-small cell lung cancer:
a multi-institutional phase II trial. Clin Cancer Res.
10:7860–7864. 2004. View Article : Google Scholar
|
|
9
|
Ozawa Y, Inui N, Naitoh T, Yasuda K,
Nagayama M, Shirai T, Suganuma H, Fujii M, Nakamura H, Suda T and
Chida K: Phase II study of combination chemotherapy with S-1 and
weekly cisplatin in patients with previously untreated advanced
non-small cell lung cancer. Lung Cancer. 63:68–71. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tamura K, Okamoto I, Ozaki T, Kashii T,
Takeda K, Kobayashi M, Matsui K, Shibata T, Kurata T, Nakagawa K
and Fukuoka M: Phase I/II study of S-1 plus carboplatin in patients
with advanced non-small cell lung cancer. Eur J Cancer.
45:2132–2137. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Okamoto I, Nishimura T, Miyazaki M,
Yoshioka H, Kubo A, Takeda K, Ebi N, Sugawara S, Katakami N,
Fukuoka M and Nakagawa K: Phase II study of combination therapy
with S-1 and irinotecan for advanced non-small cell lung cancer:
West Japan Thoracic Oncology Group 3505. Clin Cancer Res.
14:5250–5254. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schiller JH, Harrington D, Belani CP,
Langer C, Sandler A, Krook J, Zhu J and Johnson DH: Comparison of
four chemotherapy regimens for advanced non-small-cell lung cancer.
N Engl J Med. 346:92–98. 2002. View Article : Google Scholar
|
|
13
|
Kelly K, Crowley J, Bunn PA Jr, Presant
CA, Grevstad PK, Moinpour CM, Ramsey SD, Wozniak AJ, Weiss GR,
Moore DF, Israel VK, Livingston RB and Gandara DR: Randomized phase
III trial of paclitaxel plus carboplatin versus vinorelbine plus
cisplatin in the treatment of patients with advanced non-small-cell
lung cancer: a Southwest Oncology Group trial. J Clin Oncol.
19:3210–3218. 2001.PubMed/NCBI
|
|
14
|
Shepherd FA, Dancey J, Ramlaun R, Mattson
K, Gralla R, O'Rourke M, Levitan N, Gressot L, Vincent M, Burkes R,
Coughlin S, Kim Y and Berille J: Prospective randomized trial of
docetaxel versus best supportive care in patients with
non-small-cell lung cancer previously treated with platinum-based
chemotherapy. J Clin Oncol. 18:2095–2103. 2000.
|
|
15
|
Shepherd FA, Pereira JR, Ciuleanu T, Tan
EH, Hirsh V, Thongprasert S, Campos D, Maoleekoonpiroj S, Smylie M,
Martins R, van Kooten M, Dediu M, Findlay B, Tu D, Johnston D,
Bezjak A, Clark G, Santabárbara P and Seymour L: Erlotinib in
previously treated non-small cell lung cancer. N Engl J Med.
353:123–132. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Scagliotti GV, Parikh P, von Pawel J,
Biesma B, Vansteenkiste J, Manegold C, Serwatowski P, Gatzemeier U,
Digumarti R, Zukin M, Lee JS, Mellemgaard A, Park K, Patil S,
Rolski J, Goksel T, de Marinis F, Simms L, Sugarman KP and Gandara
D: Phase III study comparing cisplatin plus gemcitabine with
cisplatin plus pemetrexed in chemotherapy-naïve patients with
advanced-stage non-small cell lung cancer. J Clin Oncol.
26:3543–3551. 2008.PubMed/NCBI
|
|
17
|
Sandler A, Gray R, Perry MC, Brahmer J,
Schiller JH, Dowlati A, Lilenbaum R and Johnson DH:
Paclitaxel-carboplatin alone or with bevacizumab for non-small cell
lung cancer. N Engl J Med. 355:2542–2550. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ishikawa T, Sekiguchi F, Fukase Y, Sawada
N and Ishitsuka H: Positive correlation between the efficacy of
capecitabine and doxifluridine and the ratio of thymidine
phosphorylase to dihydropyrimidine dehydrogenase activities in
tumors in human cancer xenografts. Cancer Res. 58:685–690.
1998.
|
|
19
|
Chujo M, Miura T, Kawano Y, Miyawaki M,
Imakiire T, Hayashita Y and Kawahara K: Thymidine phosphorylase
levels and dihydropyrimidine dehydrogenase levels in non-small cell
lung cancer tissues. Oncol Rep. 16:777–780. 2006.PubMed/NCBI
|