Introduction
Taxol or paclitaxel is a mitotic inhibitor used in
cancer chemotherapy. It was first isolated from the bark of the
Pacific yew tree, Taxus brevifolia, and termed ‘Taxol’ in
1967. Taxol functions by interfering with normal microtubule
breakdown during cell division (1).
Taxol has been shown to induce programmed cell death (apoptosis) in
cancer cells by binding to an apoptosis-stopping protein called
Bcl-2 (B-cell leukemia 2), thereby arresting its action.
One common characteristic of most cancer cells is
their rapid rate of cell division. To accommodate this rapid cell
division, the cytoskeleton of a cell undergoes extensive
restructuring. Taxol is an effective treatment for aggressive
cancers as it adversely affects the process of cell division by
preventing this restructuring (2).
Cancer cells are also destroyed by the aforementioned anti-Bcl-2
mechanism. Other cells are affected adversely. However, cancer
cells divide more rapidly than non-cancer cells, rendering them
more susceptible to Taxol treatment.
Taxol is one of the most active cancer
chemotherapeutic agents. It is effective against a variety of human
tumors including ovarian, breast and non-small-cell lung tumors, as
well as head and neck carcinomas (1,3–6).
However, its effectiveness is often limited due to resistance to
Taxol, observed in many tumors (7).
This study examined whether this resistance could be reduced.
Acetyl-CoA carboxylase (ACC) is a biotin-dependent
enzyme that catalyzes the irreversible carboxylation of acetyl-CoA
to produce malonyl-CoA. ACC plays a role in the regulation of the
fatty mechanism. When the enzyme is active, malonyl-CoA is
produced. Malonyl-CoA inhibits the transfer of the fatty acyl group
from acyl CoA to carnitine with carnitine acyltransferase, which
inhibits the β-oxidation of the fatty acid in mitochondria
(8). The activity of ACC is
regulated by the reversible phosphorylation.
ACC activity is inhibited when it is phosphorylated.
The phosphorylation takes place when hormones, such as glucagon or
epinephrine, bind to the receptors or the energy status of the cell
is low, leading to the activation of AMPK (9). The presence of fatty acid inhibits the
activities of the enzyme. When insulin binds to its cell receptors,
it activates a phosphatase to dephosphorylate the enzyme thereby
enhancing ACC activity (10).
Recent studies have shown ACC to be an enzyme that plays a crucial
role in de novo fatty acid biosynthesis and lipogenesis
(11), and is essential for cancer
cell survival (12). Inhibition of
ACC induced the apoptosis of cancer cells (13,14).
This study investigated whether ACC is involved in
Taxol-induced cell death in ovarian cancer cells and examined the
cell signaling pathways associated with ACC inhibition. Results
showed for the first time that Taxol induced ACC inhibition in
ovarian cancer cells and that EGFR and AMPK are involved in ACC
signaling. Consequently, ACC is considered to be a new molecular
target of Taxol.
Materials and methods
Chemicals and reagents
PD153035, AG1478 and compound C were obtained from
Calbiochem (San Diego, CA, USA). Goat anti-rabbit IgG-HRP and goat
anti-mouse IgG-HRP antibodies were purchased from Santa Cruz
Biotechnology (Santa Cruz, CA, USA). Monoclonal mouse anti-β-actin
was obtained from Sigma (St. Louis, MO, USA). Anti-phospho-ACC
(Ser79) and cleaved caspase-3 (Asp175) were obtained from Cell
Signaling Technology (Beverly, MA, USA).
Cell culture
Human ovarian cancer cells (CaOV3 cells) were
maintained in DMEM medium (Sigma) supplemented with 10% fetal
bovine serum (FBS), penicillin/streptomycin (1:100, Sigma) and 4 mM
L-glutamine, in a CO2 incubator at 37°C. For Western
blotting, cells were seeded in 6-well plates at a density of
0.2×106 cells/ml with fresh complete culture medium.
Western blot analysis
As previously described (15–18),
treated and untreated cultured cells were washed with cold PBS and
harvested by scraping into 150 μl of RIPA buffer with protease
inhibitors. Proteins (20–40 μg) were separated by SDS-PAGE and
transfered onto PVDF membranes (Millipore, Bedford, MA, USA). After
blocking with 10% milk in Tris-buffered saline (TBS), membranes
were incubated with specific antibodies in dilution buffer (2%
bovine serum albumin in TBS) overnight at 4°C followed by
horseradish peroxidase-conjugated anti-rabbit or anti-mouse IgG at
appropriate dilutions at room temperature for 1 h. Antibody binding
was detected using an ECL detection system from Amersham
Biosciences (Piscataway, NJ, USA) according to the manufacturer’s
instructions and visualized by fluorography with Hyperfilm.
Cell viability assay (MTT dye assay)
Cell viability was measured by the
3-[4,5-dimethylthylthiazol-2-yl]-2,5 diphenyl- tetrazolium bromide
(MTT) method. Briefly, cells were collected and seeded in 96-well
plates at a density of 2×105 cells/cm2. After
incubation for 24 h, cells were exposed to fresh medium containing
reagents at 37°C. After incubation for a certain period, 20 μl of
MTT tetrazolium (Sigma) salt dissolved in Hank’s balanced salt
solution at a concentration of 5 mg/ml was added to each well and
incubated in a CO2 incubator for 4 h. The medium was
then aspirated from each well and 150 μl of DMSO (Sigma) was added
to dissolve formazan crystals. The absorbance of each well was
obtained using a Dynatech MR5000 plate reader at a test wavelength
of 490 nm with a reference wavelength of 630 nm.
Results
Taxol induces ACC inhibition in CaOV3
ovarian cancer cells
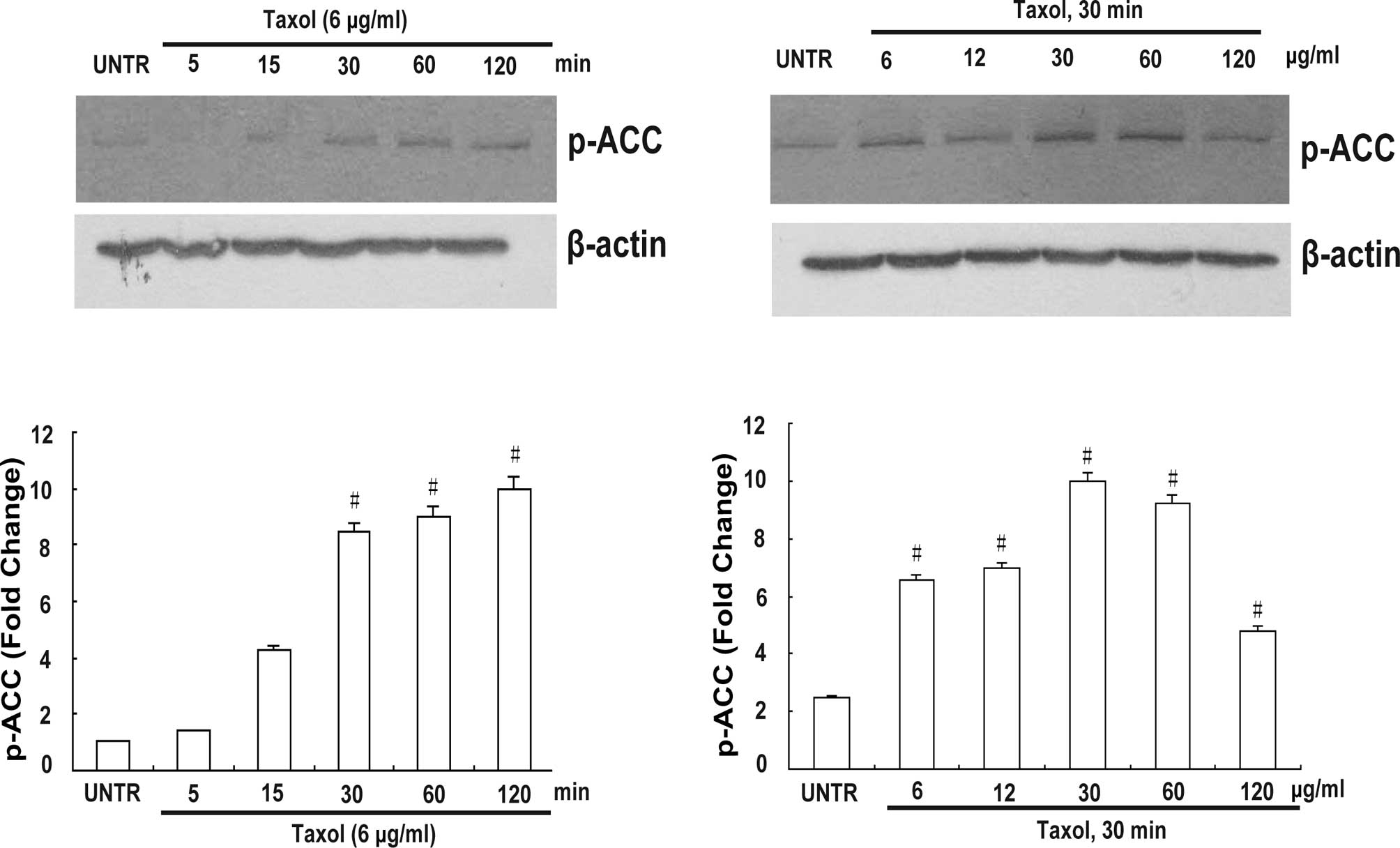
This study examined whether Taxol induces ACC
inhibition in human ovarian cancer cells. Cells were treated with 6
μg/ml Taxol at different time points (5, 15, 30, 60 and 120 min) or
with Taxol at different concentrations (6, 12, 30, 60 and 120
μg/ml) for 30 min. Cell lysates were then analyzed for phospho-ACC
by Western blotting as described above. As shown in Fig. 1, Taxol induced ACC inhibition in a
time- and concentration-dependent manner. ACC inhibition was
observed within 30 min and peaked at 120 min (Fig. 1A and B). Taxol at concentrations of
6, 12 and 30 μg/ml significantly induced ACC inhibition (Fig. 1C and D).
Effect of selective ACC inhibitor
5-(tetradecyloxy)-2-furoic acid (TOFA) mediates Taxol-induced ACC
inhibition
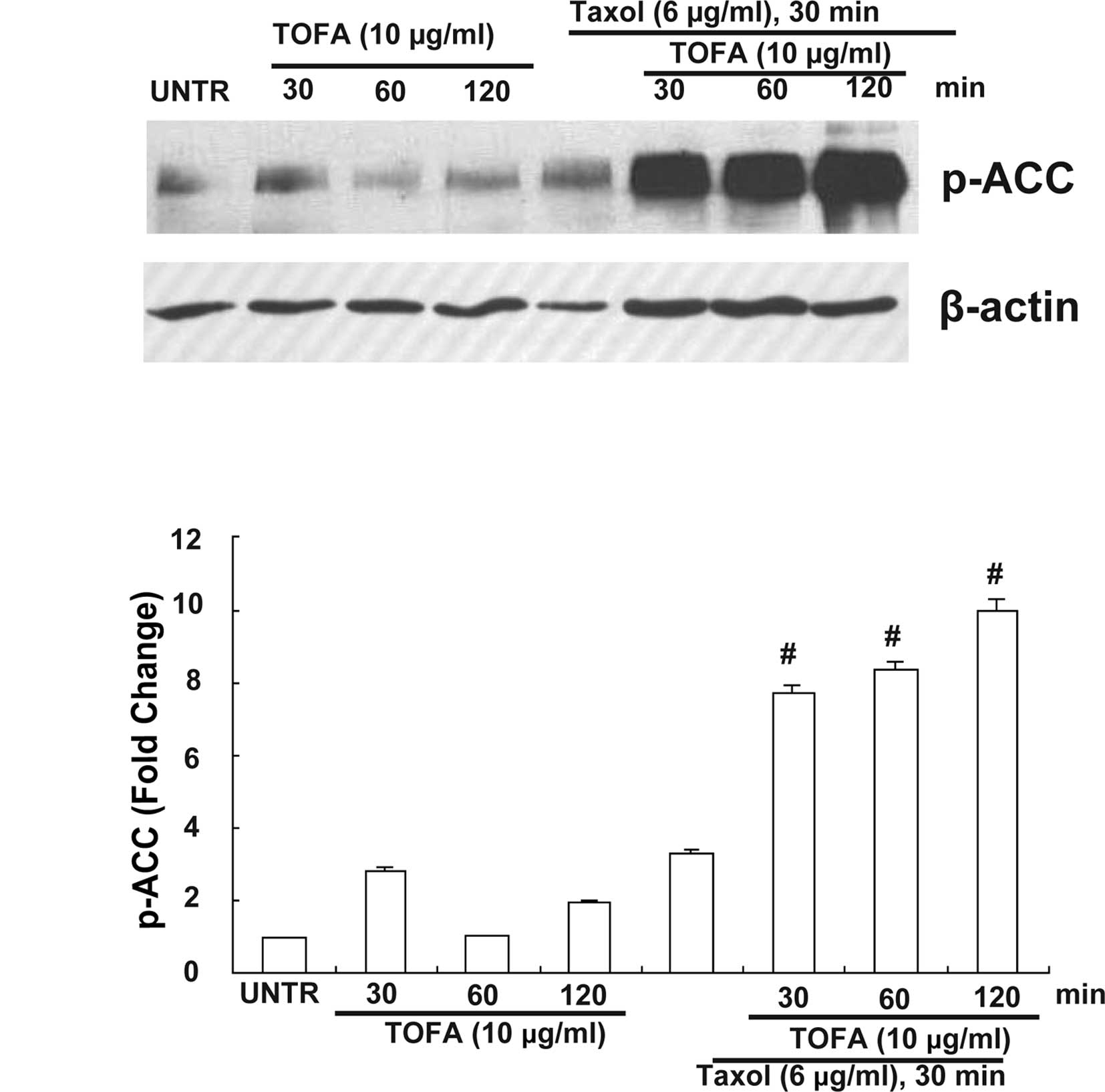
We examined whether TOFA affects ACC signaling in
the presence of Taxol. Taxol-induced ACC phosphorylation was
enhanced by pretreatment with TOFA (10 μg/ml) for 30, 60 or 120
min. As shown in Fig. 2, TOFA
enhanced ACC phosphorylation in a time-dependent manner in the
presence of Taxol.
Effect of selective AMPK inhibitor
compound C and selective AMPK activator AICAR on Taxol-induced ACC
inhibition
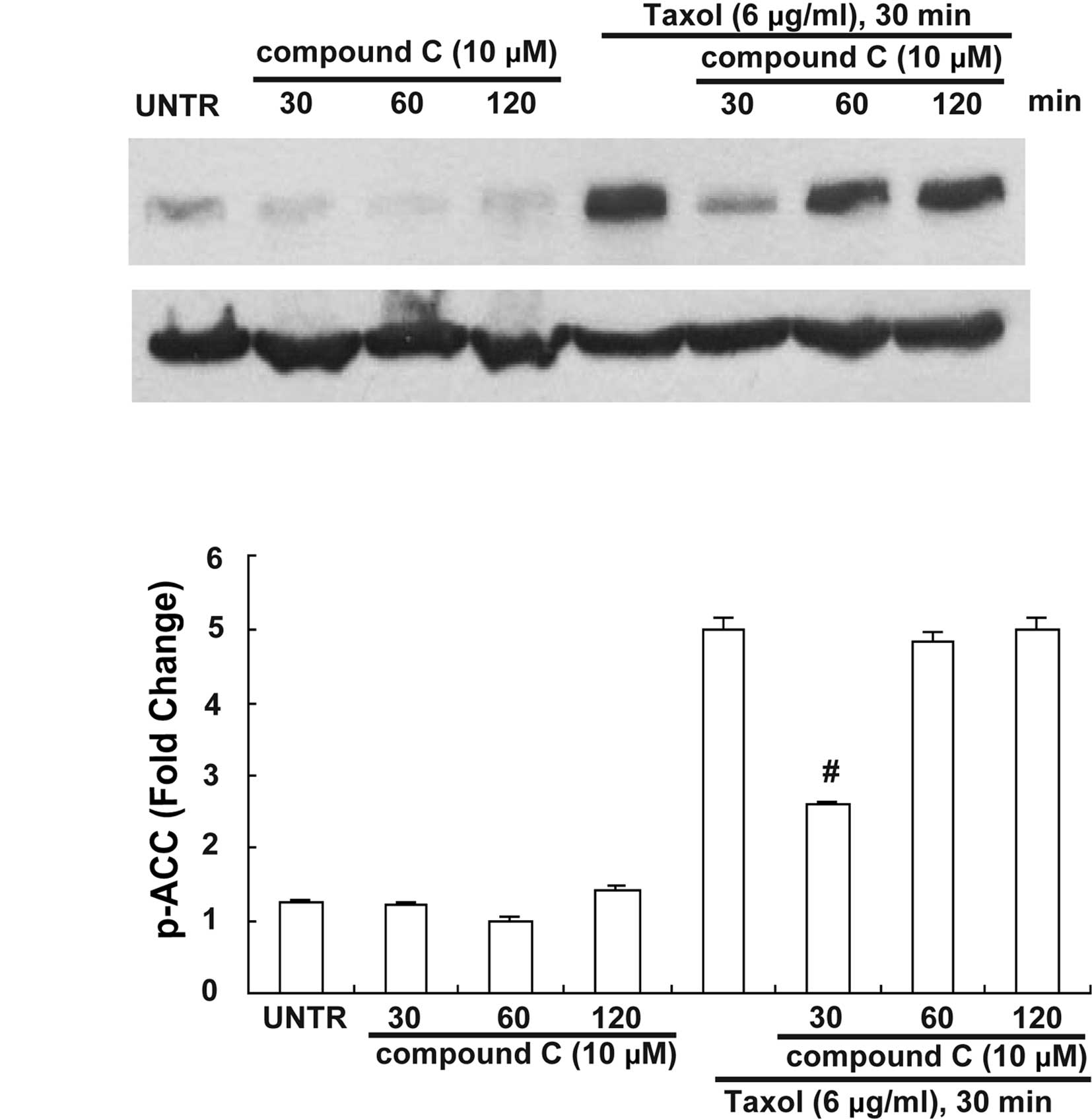
AMPK is a significant up-stream signal of ACC, and
Taxol activates AMPK in human ovarian cancer cells (19). To investigate the manner in which
AMPK affects ACC inhibition in response to Taxol treatment, AMPK
inhibitor compound C and activator AICAR were used. Our data showed
that pretreatment with AMPK inhibitor compound C at 10 μM for 30
min but not 60 or 120 min blocked Taxol-induced ACC phosphorylation
(Fig. 3), whereas Taxol-induced ACC
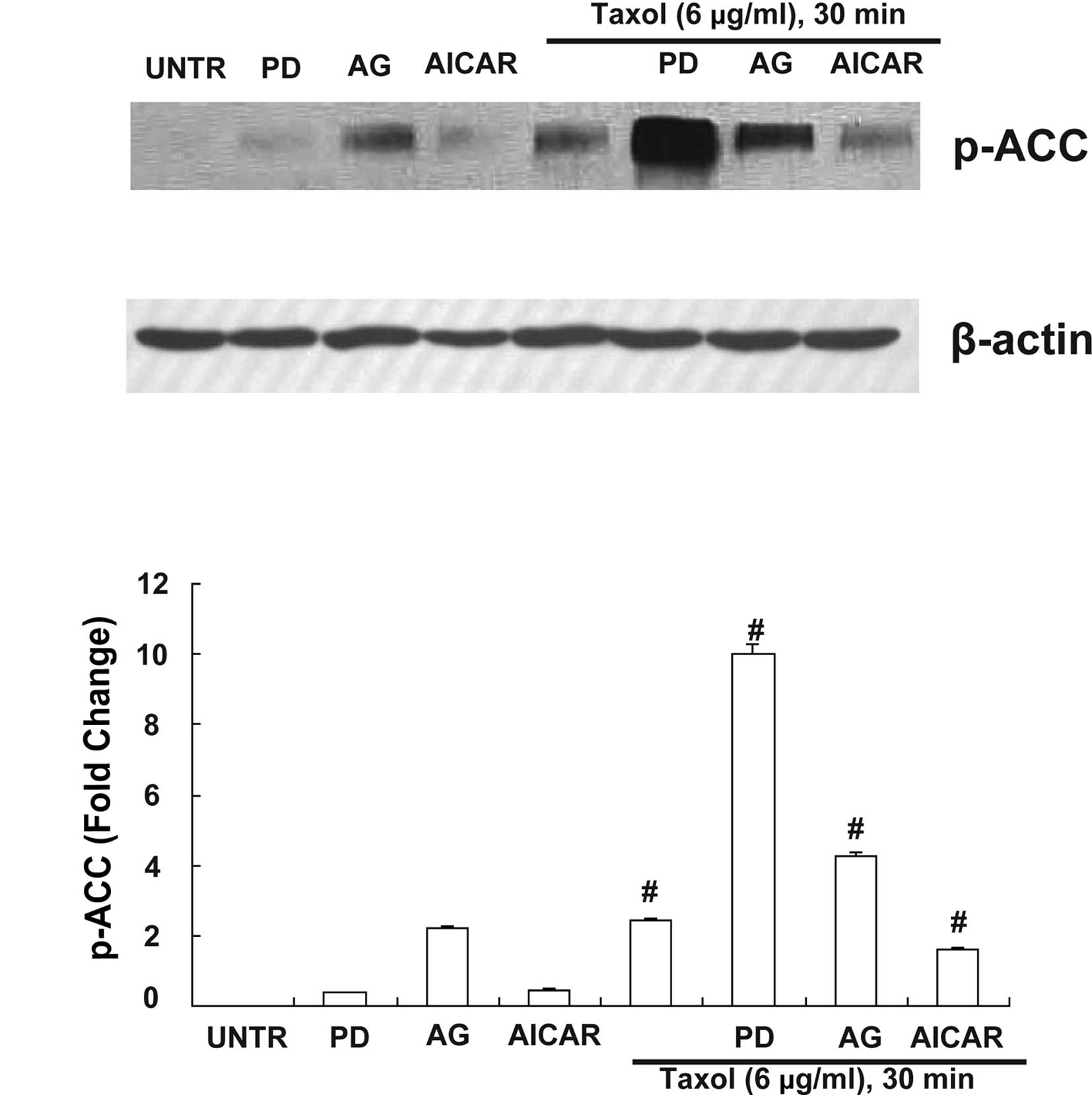
phosphorylation was enhanced by pretreatment with AICAR at 1 mM for
30 min (Fig. 4).
PD (selective EGFR inhibitor) and AG
(selective EGFR inhibitor) mediates Taxol-induced ACC
activation
Numerous studies have shown that over-expression of
EGFR is attributed to ovarian cancer cell resistance to
Taxol-induced cell death (18,20).
Since EGFR is a significant upstream signal of ACC and EGFR is also
activated by Taxol in human ovarian cancer cells (18), the hypothesis that PD and AG were
involved in ACC signaling in the presence of Taxol was examined. PD
was shown to mediate ACC inhibition, since pretreatment with PD 1
μM for 30 min enhanced Taxol-induced ACC phosphorylation (Fig. 4). AG also mediated ACC inhibition,
since pretreatment with AG 1 μM for 30 min enhanced Taxol-induced
ACC phosphorylation (Fig. 4).
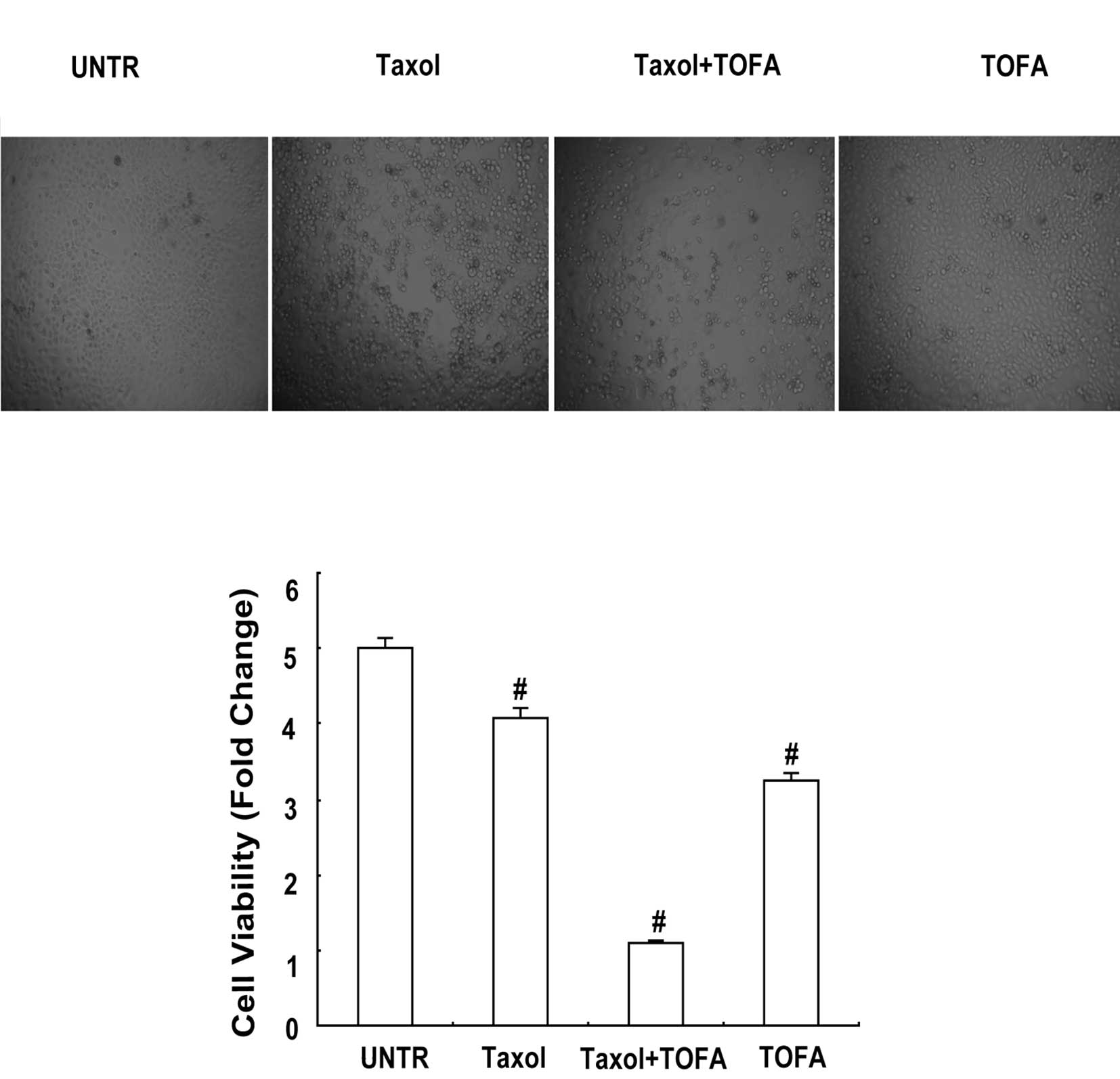
TOFA alleviates Taxol-induced cell
death
TOFA was found to induce CaOV3 cell death. MTT dye
assay was applied to investigate the anti-tumor effect of ACC
inhibitor in CaOV3 cells. Taxol (6 μg/ml) and incubation for 24 h
reduced cell viability by 81.47% (Fig.
5B). Cell viability was decreased to 64.70% by 10 μg/ml of TOFA
and incubation for 24 h (Fig. 5B).
Furthermore, cell viability was decreased to 21.65% when both Taxol
(6 μg/ml) and TOFA (10 μg/ml) were administered, followed by
incubation for 24 h (Fig. 5B). As
shown in Fig. 5A, the difference
was evident.
Discussion
Ovarian cancer is the most lethal cancer of the
female reproductive system. Its high patient mortality rate can
partly be attributed to a lack of early detection and screening.
Despite good initial responses to chemotherapy, side effects and
drug resistance are notable disadvantages of current
chemotherapeutic agents. Subsequently, drugs with low toxicity and
high efficiency have been in high demand.
Taxol is one such agent and has been administered to
thousands of ovarian cancer patients. Taxol has been shown to
inhibit transformation, proliferation and tumor metastasis.
Carcinogenesis is a multistep process in which numerous biochemical
pathways and hundreds of molecules are deregulated. These events
include growth factors and their receptors, cytokines, enzymes and
genes regulating apoptosis and proliferation.
Taxol has been shown to target a number of the
molecules involved in carcinogenesis, including transcription
factors such as AMPK and similar protein kinases. Moreover, Taxol
has been found to be targeting EGFR in CaOV3 cells (18). Exploration of Taxol’s molecular
targets has aided in the understanding of its pharmacological
effects and has also provided experimental data with which its
clinical application can be guided.
In the present study, ACC was found to be another
molecular target of Taxol in CaOV3 ovarian cancer cells. ACC is
phosphorylated simultaneously by Taxol treatment, thereby
confirming ACC inhibition. p-ACC has been shown to be an
independent marker for prediction of better survival in lung
adenocarcinoma patients. Median overall survival was longer in
patients with p-ACC-positive than those with p-ACC-negative tumors
(21).
Taxol induced ACC phosphorylation in CaOV3 ovarian
cancer cells. Phospho-ACC was enhanced by pretreatment with TOFA, a
selective inhibitor of ACC. Notably, Taxol-induced cell death was
increased by pretreatment with TOFA. Apoptosis in CaOV3 cells was
even induced by TOFA administration alone. ACC is therefore a
crucial target in CaOV3 ovarian cancer cell apoptosis.
AMPK is considered to be an important component of
apoptosis, as well as a possible target of cancer control. Kim
et al showed that activation by AICAR increased apoptosis in
HT-29 colon cancer cells under capsaicin treatment. Additionally,
both the activation of AMPK and an increased expression of the
inactive form of ACC were detected in the apoptosis process of
colon cancer cells (22).
Pretreatment with Compound C, a selective AMPK
inhibitor almost abolished ACC phosphorylation by Taxol. However,
ACC phosphorylation by Taxol was enhanced by AICAR, a selective
AMPK activator. The results therefore indicate that AMPK lies
upstream of ACC, and the AMPK activator has been noted to
contribute to ACC inhibition.
In a previous study, Taxol was found to transiently
transactivate EGFR, leading to the activation of cell survival
factors, all of which are inclusively accountable for ovarian
cancer cell resistance to Taxol treatment. A combination of Taxol
with inhibitors such as PD and AG may be a more viable option for
ovarian cancer treatment (18).
ACC phosphorylation by Taxol was found to have been
enhanced following pretreatment with the selective EGFR inhibitors
PD and AG. The reason that a combination of PD and AG with Taxol is
a more feasible option for ovarian cancer treatment has been
indicated by the ACC pathway. ACC inhibition may be up-regulated by
PD and AG, which in turn increases Taxol-induced CaOV3 cancer cell
apoptosis.
In conclusion, ACC has been shown for the first time
to be a new molecular target for Taxol. Moreover, ACC inhibition
partially accounts for the cell apoptosis of Taxol-induced
cytotoxicity in ovarian cancer cells. Agents that potentially
enhance ACC inhibition and then block the cell survival process
were also investigated. Taxol-induced cell death may be reduced by
agents such as PD, AG, AICAR and TOFA. Our findings indicate that
insights into understanding the molecular mechanism of Taxol’s
effect on CaOV3 cancer cell death are required, which may offer
better clinical management of ovarian cancer.
Acknowledgements
This study was partly supported by a grant from the
National Natural Science Foundation of China (no. 30772306, WD) and
partly by a grant from NIH (P20 RR016457 from the INBRE Program of
the National Center for Research Resources, YW).
References
|
1
|
Rowinsky EK and Donehower RC: Paclitaxel
(Taxol). N Engl J Med. 332:1004–1014. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yvon AM, Wadsworth P and Jordan MA: Taxol
suppresses dynamics of individual microtubules in living human
tumor cells. Mol Biol Cell. 10:947–959. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McGuire WP, Rowinsky EK, Rosenshein NB, et
al: Taxol: a unique antineoplastic agent with significant activity
in advanced ovarian epithelial neoplasms. Ann Intern Med.
111:273–279. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liebmann JE, Cook JA, Lipschultz C, et al:
Cytotoxic studies of paclitaxel (Taxol) in human tumour cell lines.
Br J Cancer. 68:1104–1109. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Eisenhauer EA and Vermorken JB: The
taxoids. Comparative clinical pharmacology and therapeutic
potential. Drugs. 55:5–30. 1998.PubMed/NCBI
|
|
6
|
Wiseman LR and Spencer CM: Paclitaxel. An
update of its use in the treatment of metastatic breast cancer and
ovarian and other gynaecological cancers. Drugs Aging. 12:305–334.
1998.PubMed/NCBI
|
|
7
|
Sangrajrang S and Fellous A: Taxol
resistance. Chemotherapy. 46:327–334. 2000. View Article : Google Scholar
|
|
8
|
Tong L: Acetyl-coenzyme A carboxylase:
crucial metabolic enzyme and attractive target for drug discovery.
Cell Mol Life Sci. 62:1784–1803. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wakil SJ and Abu-Elheiga LA: Fatty acid
metabolism: target for metabolic syndrome. J Lipid Res.
50:S138–S143. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tong L and Harwood HJ Jr: Acetyl-coenzyme
A carboxylases: versatile targets for drug discovery. J Cell
Biochem. 99:1476–1488. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ronnebaum SM, Joseph JW, Ilkayeva O, et
al: Chronic suppression of acetyl-CoA carboxylase 1 in beta-cells
impairs insulin secretion via inhibition of glucose rather than
lipid metabolism. J Biol Chem. 283:14248–14256. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Swinnen JV, Brusselmans K and Verhoeven G:
Increased lipogenesis in cancer cells: new players, novel targets.
Curr Opin Clin Nutr Metab Care. 9:358–365. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bandyopadhyay S, Zhan R, Wang Y, et al:
Mechanism of apoptosis induced by the inhibition of fatty acid
synthase in breast cancer cells. Cancer Res. 66:5934–5940. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chajes V, Cambot M, Moreau K, et al:
Acetyl-CoA carboxylase alpha is essential to breast cancer cell
survival. Cancer Res. 66:5287–5294. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Qiu L, Zhou C, Sun Y, et al: Crosstalk
between EGFR and TrkB enhances ovarian cancer cell migration and
proliferation. Int J Oncol. 29:1003–1011. 2006.PubMed/NCBI
|
|
16
|
Qiu L, Zhou C, Sun Y, et al: Paclitaxel
and ceramide synergistically induce cell death with transient
activation of EGFR and ERK pathway in pancreatic cancer cells.
Oncol Rep. 16:907–913. 2006.PubMed/NCBI
|
|
17
|
Zhou C, Qiu L, Sun Y, et al: Inhibition of
EGFR/PI3K/AKT cell survival pathway promotes TSA’s effect on cell
death and migration in human ovarian cancer cells. Int J Oncol.
29:269–278. 2006.PubMed/NCBI
|
|
18
|
Qiu L, Di W, Jiang Q, et al: Targeted
inhibition of transient activation of the EGFR-mediated cell
survival pathway enhances paclitaxel-induced ovarian cancer cell
death. Int J Oncol. 27:1441–1448. 2005.PubMed/NCBI
|
|
19
|
Aissat N, Le Tourneau C, Ghoul A, et al:
Antiproliferative effects of rapamycin as a single agent and in
combination with carboplatin and paclitaxel in head and neck cancer
cell lines. Cancer Chemother Pharmacol. 62:305–313. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qiu L, Wang Q, Di W, et al: Transient
activation of EGFR/AKT cell survival pathway and expression of
survivin contribute to reduced sensitivity of human melanoma cells
to betulinic acid. Int J Oncol. 27:823–830. 2005.PubMed/NCBI
|
|
21
|
Conde E, Suarez-Gauthier A, Garcia-Garcia
E, et al: Specific pattern of LKB1 and phospho-acetyl-CoA
carboxylase protein immunostaining in human normal tissues and lung
carcinomas. Hum Pathol. 38:1351–1360. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim YM, Hwang JT, Kwak DW, et al:
Involvement of AMPK signaling cascade in capsaicin-induced
apoptosis of HT-29 colon cancer cells. Ann NY Acad Sci.
1095:496–503. 2007. View Article : Google Scholar : PubMed/NCBI
|