Introduction
Colorectal cancer (CRC) is the second most prevalent
cancer and the third leading cause of cancer deaths worldwide
(1). Oxaliplatin (L-OHP)-based
chemotherapeutic regimens have proven to be effective in the
prevention and treatment of tumor recurrence and metastasis in CRC.
However, drug toxicity remains to be clarified in the clinic
(2). Efforts have focused on
incorporating cytotoxic and molecularly targeted agents into
chemotherapy regimens to decrease toxicity and increase efficacy
(3–5).
The mammalian target of rapamycin (mTOR), an
intracellular protein with a central role in the synthesis of key
cellular proteins, has emerged as a significant target for
anticancer therapy in various types of tumor.
Phosphatidylinositol-3-kinase (PI3K)/Akt/mTOR pathway has been
reported to be constitutively overexpressed in malignant tumors
including CRC (6–7). Rapamycin (RAPA) is the special
inhibitor of mTOR, which has demonstrated anti-tumor effects in a
variety of malignancies in preclinical and clinical studies. In
addition to being used as a monotherapy, RAPA and its analogs have
been shown to enhance the efficacy of a number of cytotoxic
chemotherapeutic agents in various types of cancer (8–11).
Studies have shown that the anti-tumor mechanism of RAPA includes
both apoptosis and autophagy, and its role in the fate of cancer
cells remains controversial (12).
The interaction between RAPA and L-OHP has yet to be clarified as
the role of apoptosis and autophagy in this combination is unknown.
The present study aimed to investigate whether RAPA increased the
anti-tumor efficacy of L-OHP and its potential biological
mechanism.
Materials and methods
Cell culture
The HCT116 human colon cancer cell line was
generously provided by Professor Morito Monden of Osaka University,
Japan. Cells were grown in monolayer culture in Dulbecco’s modified
Eagle’s medium (DMEM, Sigma), supplemented with 10% fetal bovine
serum, 100 U/ml penicillin, and 100 μg/ml streptomycin in 5%
CO2 at 37°C. Cells in the exponential growth phase were
used in all of the experiments.
Reagents and antibodies
RAPA was obtained from Calbiochem (Gibbstown, NJ,
USA) and L-OHP was from Sigma (St. Louis, MO, USA). All antibodies
were obtained from Cell Signaling Technology.
MTT assay
Cell proliferation was measured using the
3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT)
assay. In brief, cells were seeded into 96-well plates at a density
of 3.5×103 cells/well (100 μl), and incubated for 24 h
for sufficient attachment before drug exposure. After 48 h
treatment with varying doses of single drug or combined therapy, 20
μl of MTT (Sigma) solution (5 mg/ml) were added to each well and
the plates were incubated at 37°C for another 4 h. The medium was
then replaced by 200 μl DMSO. Absorbance at 490 nM was measured
using a 96-well format plate reader. Wells containing only DMEM and
MTT were used as controls. Cell viability was determined based on
the mitochondrial conversion of MTT to yellowish formazine. Each
experiment was performed using six replicated wells for each drug
concentration and carried out independently three times.
Dose-effect analyses
The IC50 value was determined on the
basis of dose-response curves from the MTT assay. The combined
effect of L-OHP and RAPA was analyzed by calculating the
combination index (CI) based on the Chou-Talalay method (13). When CI <1, the effect was
synergistic and when CI=1, the effect was additive. The effect was
antagonistic when CI >1. IC50 and CI were calculated
using CalcuSyn software (Biosoft, Cambridge, UK).
Annexin V staining
Apoptosis was detected using the Annexin
V-fluorescein isothiocyanate (FITC) apoptosis kit (BD Bioscience,
Rockville, MD, USA) in accordance with the manufacturer’s
instructions. In brief, cells (5×105) were seeded and
cultured in 10-cm dishes for 24 h. Cells treated for 48 h with
L-OHP, RAPA alone or in combination were collected. Untreated cells
were also collected as controls. The cells were resuspended in 500
μl of binding buffer, with 5 μl of Annexin V-FITC and then 5 μl of
propidium iodide was (PI) added. The cells were then incubated at
room temperature for 5 min in the dark and analyzed for Annexin
V-FITC binding by flow cytometry (FACSort) using a fluorescein
isothiocyanate (FITC) signal detector (FL1) and a PI signal
detector (FL2). The cells in the FITC-positive and PI-negative
fraction were regarded as apoptotic cells. Each experiment was
repeated three times and reproducible results were obtained.
Western blotting
Western blotting was performed as previously
described (14). Briefly, cells
plated at a density of 3×105/ml in 6-well plates were
exposed to L-OHP, RAPA or a combination of the two drugs for 48 h
prior to harvest. Following centrifugation and sonication, cell
extracts were clarified at 12,000 rpm for 10 min at 4°C. Protein
concentrations were measured using a BCA assay. Protein samples (30
μg), diluted with SDS sample buffer, were separated by 10%
polyacrylamide gel electrophoresis, followed by electroblotting on
a polyvinylidene difluoride membrane. After blocking in 5% non-fat
dry milk, the membrane was incubated with cleaved poly (ADP-ribose)
polymerase (PARP) antibody (1:1,000) for 1 h at room temperature.
Equal loading of the protein samples was confirmed by parallel
Western blots for β-actin (1:1,000). The protein bands were
detected using the ECL detection system (Pierce, Rockford, IL,
USA). Each experiment was repeated three times.
Acridine orange staining
For fluorescence microscope examination, cells were
seeded on glass coverslips in 24-well plates at a concentration of
1×104 cells/well. Tumor cells were treated using a
single drug or a combination of both for 48 h and then vital
staining with acridine orange was performed as previously described
(15). Briefly, the treated tumor
cells were stained with acridine orange at a final concentration of
1 μg/ml for 15 min. Samples were examined under a
fluorescence microscope. Images were obtained using a Nikon ECLIPSE
80i fluorescence microscope (exciter filter: BP 450–490 nm; barrier
filter: LP 520 nm).
Statistical analysis
Data are expressed as the means ± SD and were
analyzed using the Student’s t-test. P<0.05 was considered to be
statistically significant.
Results
Effect of L-OHP and RAPA on cell
proliferation
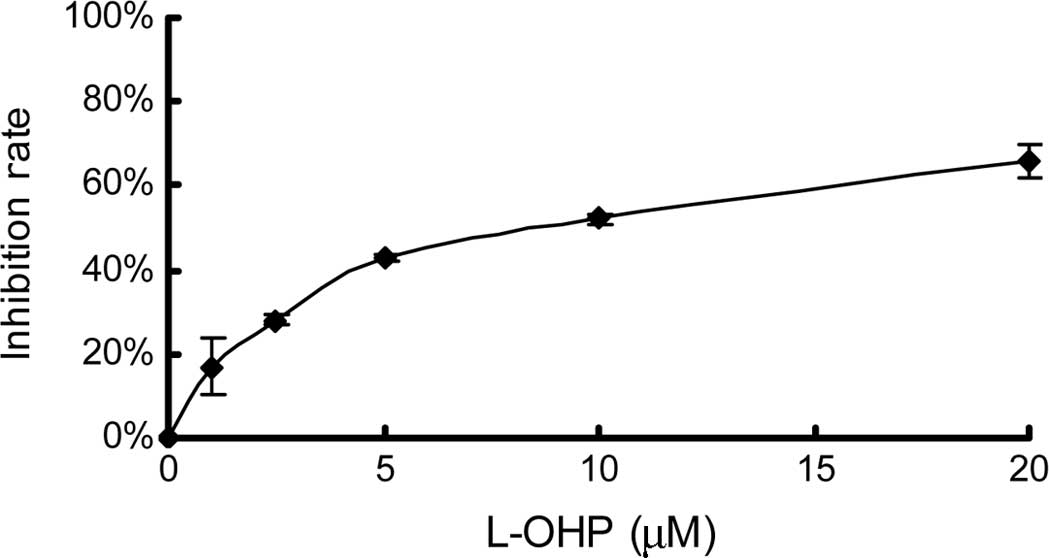
First, single drug-induced growth inhibition was
measured using an MTT assay. The concentrations of the drugs were
1, 2.5, 5, 10 and 20 μM for L-OHP and 10, 50, 100, 200 and 400 nM
for RAPA. After 48 h of treatment, an MTT assay was performed. As
shown in Fig. 1, the effect of both
L-OHP and RAPA was dose-dependent. The IC50 values of
L-OHP and RAPA, calculated using CalcuSyn 2.0 software, in the
HCT116 colon cancer cell line were 8.35±0.78 μM (r=0.99) and
223.44±38.10 nM (r=0.94), respectively.
Combination effect and interaction
between low-dose L-OHP and RAPA
To test whether RAPA enhanced the anti-tumor
efficacy of low-dose L-OHP, concentrations below the
IC50 values of each drug were selected based on the
growth inhibitory curve. The doses and their inhibitory efficacy
used for the combination analysis were: 1 (17%), 2.5 (28%) and 5 μM
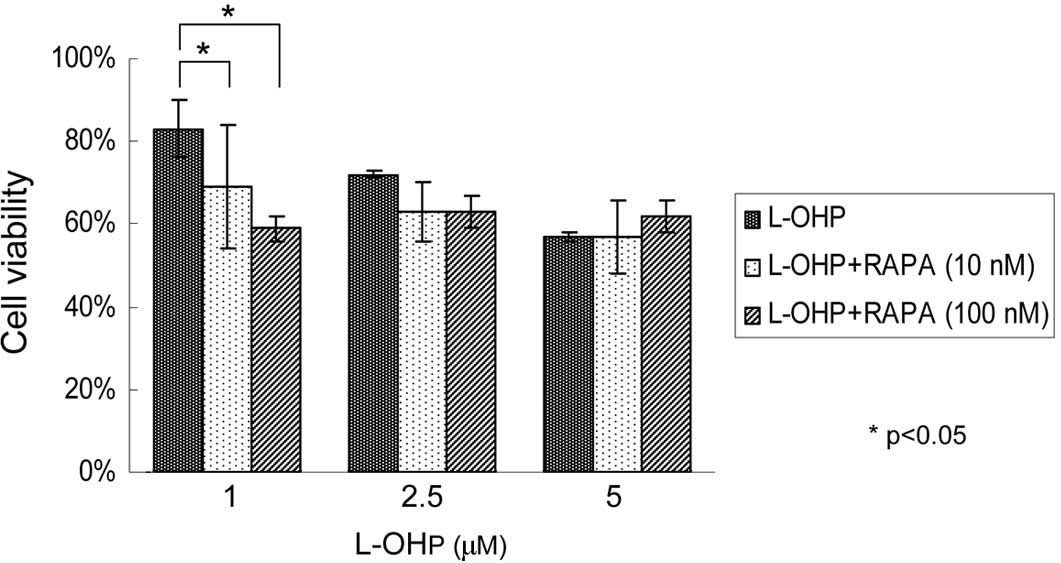
(43%) in L-OHP, and 10 (17%) or 100 nM (34%) in RAPA. Fig. 2 shows that compared with 83% cells
surviving after treatment with L-OHP at a concentration of 1 μM for
48 h, the percentage of viable cells decreased to 69 and 59% when
the same dose of L-OHP was used in combination with RAPA at a
concentration of 10 or 100 nM (p<0.05). To elucidate the
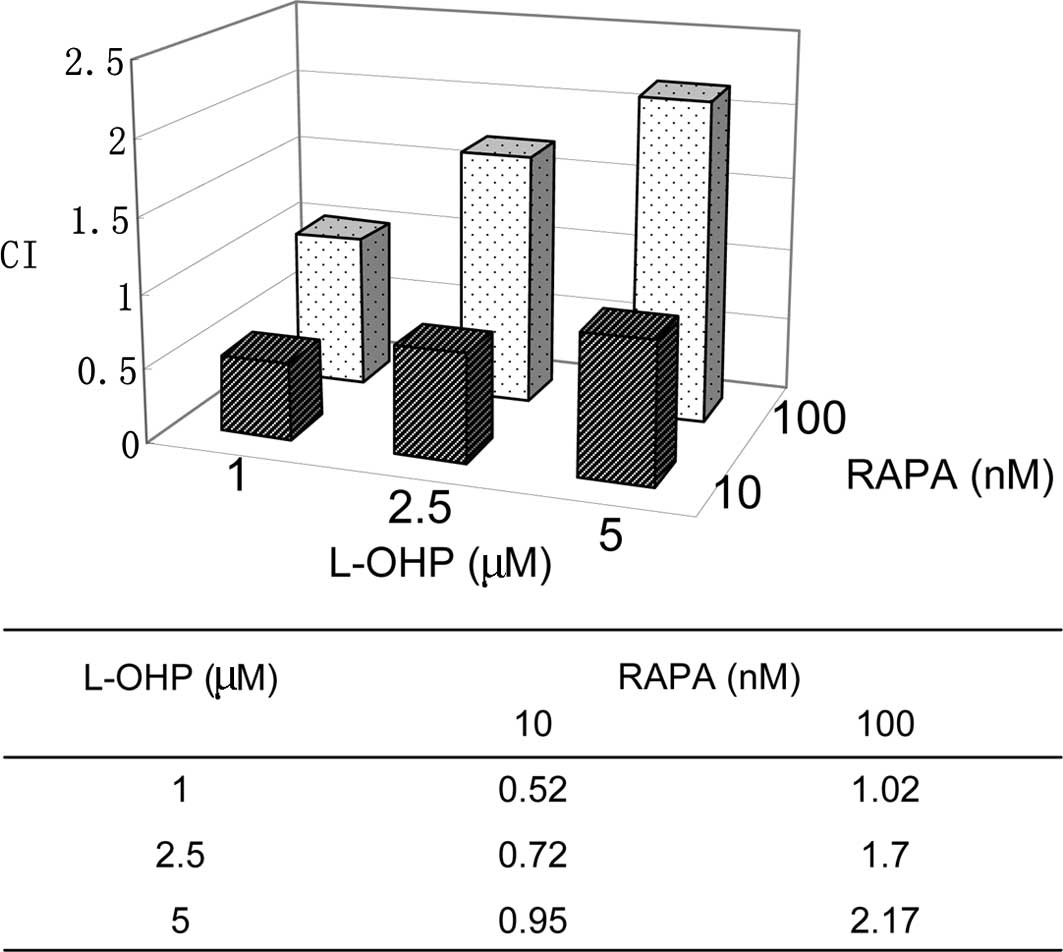
interaction between the combinations, CI was calculated using
CalcuSyn 2.0 software. As shown in Fig.
3, the CI values increased with the higher concentrations of
L-OHP. CI was ≤1 when a 1 to 5 μM dose of L-OHP was combined with
RAPA at a dose of 10 nM, suggesting synergistic or additive
effects. CI was ≥1 when 100 nM RAPA was used with a low dose of
L-OHP, indicating an additive to antagonistic effect. To further
characterize the mechanisms of the synergy of RAPA and low-dose
L-OHP combination, the combination of L-OHP at a dose of 1 μM and
RAPA of 10 nM was selected for further study.
Effect of combination therapy on
apoptosis and autophagy
To investigate the potential mechanisms of synergy
involved in low-dose combination therapy, Annexin V/PI flow
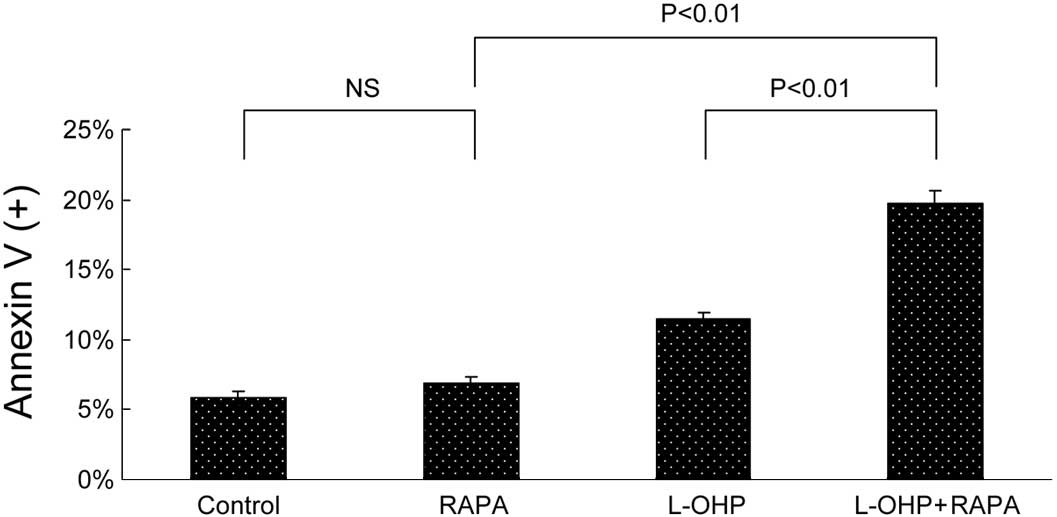
cytometry was performed first to test apoptosis. As shown in
Fig. 4A, no significant difference
was found in the percentage of Annexin V-positive cells between the
control and RAPA groups (5.81 vs. 6.89%, p=0.09). The combination
therapy induced 19.76% Annexin V-positive cells, which was higher
than that of L-OHP (11.45%, p<0.01) or RAPA (6.89%, p<0.01)
alone. Our results further confirmed the effect of combined therapy
on apoptosis by testing PARP cleavage using Western immunoblotting.
As shown in Fig. 4B, after 48 h
treatment, RAPA induced minimal PARP cleavage, whereas the
combination therapy showed the highest cleaved PARP expression.
Finally, acridine orange staining was used to determine
morphological changes in both apoptosis and autophagy. Autophagy is
the process of isolating cytoplasmic proteins in the lytic
component and is characterized by the formation and promotion of
acidic vesicular organelles (AVO). Under a fluorescence microscope
examination, the lysosomotropic agent acridine orange accumulating
in acidic intracellular compartments appears green at low
concentrations and red at high concentrations. As shown in Fig. 4C, typical bright red AVO was
detected only in RAPA-treated cells. In the L-OHP and combination
groups, green condensation of chromatin in apoptotic bodies, a
hallmark of apoptosis, was revealed.
Discussion
In this study, we found that low-dose combination of
L-OHP and RAPA synergistically inhibited HCT116 colon cancer cell
growth by enhanced apoptosis in a human HCT116 colon cancer cell
line. This inhibition suggested that the low-dose combination of
L-OHP and RAPA is a promising effective target therapy for CRC.
Developing new therapeutic approaches with limited
side-effects and favorable efficacy is crucial to cancer research.
Although the side-effects of L-OHP are minimal compared with other
platinum analogs, L-OHP-induced neurotoxicity often resulting in
treatment delay or even cessation is the main reason of treatment
failure. A recent prospective study by Park et al showed
that L-OHP dose reduction or cessation as a result of neurotoxicity
was required in 40% of a series of CRC patients (16). However, the pathophysiological
mechanisms remain unclear and attempts to prevent neurotoxicity
have been unsuccessful. Under such conditions, the synergetic
effect of a low-dose combination of L-OHP (1 μM) and RAPA (10 nM)
may provide alternative treatment modalities.
Results of this study showed that at a low
concentration of 10 vs. 100 nM RAPA demonstrated a synergistic
interaction with low-dose L-OHP. At the concentration of 10 nM RAPA
was found not to induce significant apoptosis, consistent with its
cytostatic effect on cancer cell lines, as reported recently
(17–19). This result indicated the
significance of using a combination of low-dose RAPA with L-OHP in
the clinic, in that the combined therapy led to less toxicity and
better tolerability. In a study testing a RAPA and 17-AAG
combination in myeloma, Francis et al found that there was
no significant difference in the synergistic effect whether RAPA
was used at a concentration of 20 or 100 nM (CI: 0.056 vs. 0.046)
(20).
As with our report, the results of some studies have
shown that RAPA increased chemosensitivity when combined with
low-dose cytotoxic agents. In MCF-7 and MDA-MB-468 breast cancer
cell lines, Mondesire et al found that low concentrations of
carboplatin (0.01 μg/ml) or paclitaxel (0.1 μg/ml)
alone did not induce a significant increase in apoptosis. When
these drugs were combined with RAPA (10 nM), a marked increase in
the percentage of Annexin V-positive cells was observed, indicative
of induction of apoptosis (21). In
their study on human endometrial cancer cell lines, Shafer and
Bae-Jump et al demonstrated that even low doses of RAPA (1
nM) in combination with low doses of paclitaxel (0.01–0.1 μM) or
cisplatin (0.01 μM) resulted in a strong synergistic effect by
enhanced apoptosis (22–23).
Furthermore, our data suggested that the possible
mechanism by which RAPA increased chemosensitivity was due to
enhanced apoptosis as compared to the induction of autophagy. This
observation is consistent with recent studies in breast cancer and
myeloma (21–23). Autophagy or ‘self-eating’ is
frequently activated in tumor cells treated with chemotherapy or
irradiation, particularly by mTOR inhibitors. However, whether
autophagy is a survival mechanism or whether it contributes to cell
death remains controversial. However, the consensus is that the
role of autophagy is cell type- and tumor type-specific (24). Our results suggest that autophagy
induced by low-dose RAPA was not involved in L-OHP-induced
apoptosis.
Our data demonstrate that the interaction between
the two agents was dose-dependent. An obvious trend was evident
from synergy to antagonism as drug concentration increased,
indicating that complicated mechanisms were involved. Although
further studies should be conducted on the effect of the
combination therapy on the mTOR signal pathway to elucidate the
interaction effect, the combination of low-dose L-OHP and RAPA
appears to be a potential therapeutic strategy for CRC and should
be investigated in vivo.
Acknowledgements
This study was funded by grants from the National
Natural Science Foundation of China (No. 30700997) to Dr Xueying Lu
and from the Heilongjiang overseas fund (No. LC06C27) to Dr Jinyu
Gu.
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
2
|
McWhinney SR, Goldberg RM and McLeod HL:
Platinum neurotoxicity pharmacogenetics. Mol Cancer Ther. 8:10–16.
2009. View Article : Google Scholar
|
|
3
|
Gerber DE and Choy H: Cetuximab in
combination therapy: from bench to clinic. Cancer Metastasis Rev.
29:171–180. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rodriguez J, Zarate R, Bandres E, Viudez
A, Chopitea A, García-Foncillas J and Gil-Bazo I: Combining
chemotherapy and targeted therapies in metastatic colorectal
cancer. World J Gastroenterol. 28:5867–5876. 2007. View Article : Google Scholar
|
|
5
|
Wadlow RC and Ryan DP: The role of
targeted agents in preoperative chemoradiation for rectal cancer.
Cancer. 116:3537–3548. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Johnson SM, Gulhati P, Rampy BA, Han Y,
Rychahou PG, Doan HQ, Weiss HL and Evers BM: Novel expression
patterns of PI3K/Akt/mTOR signaling pathway components in
colorectal cancer. J Am Coll Surg. 210:767–776. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Silvestris N, Tommasi S, Petriella D,
Santini D, Fistola E, Russo A, Numico G, Tonini G, Maiello E and
Colucci G: The dark side of the moon: the PI3K/PTEN/AKT pathway in
colo-rectal carcinoma. Oncology. 77(Suppl 1): 69–74. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Merimsky O, Gorzalczany Y and
Sagi-Eisenberg R: Molecular impacts of rapamycin-based drug
combinations: combining rapamycin with gemcitabine or imatinib
mesylate (Gleevec) in a human leiomyosarcoma model. Int J Oncol.
31:225–232. 2007.
|
|
9
|
Pencreach E, Guérin E, Nicolet C,
Lelong-Rebel I, Voegeli AC, Oudet P, Larsen AK, Gaub MP and Guenot
D: Marked activity of irinotecan and rapamycin combination toward
colon cancer cells in vivo and in vitro is mediated through
cooperative modulation of the mammalian target of
rapamycin/hypoxia-inducible factor-1alpha axis. Clin Cancer Res.
15:1297–1307. 2009. View Article : Google Scholar
|
|
10
|
Perotti A, Locatelli A, Sessa C, Hess D,
Viganò L, Capri G, Maur M, Cerny T, Cresta S, Rojo F, Albanell J,
Marsoni S, Corradino I, Berk L, Rivera VM, Haluska F and Gianni L:
Phase IB study of the mTOR inhibitor ridaforolimus with
capecitabine. J Clin Oncol. 28:4554–4561. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Houghton PJ, Morton CL, Gorlick R, Lock
RB, Carol H, Reynolds CP, Kang MH, Maris JM, Keir ST, Kolb EA, Wu
J, Wozniak AW, Billups CA, Rubinstein L and Smith MA: Stage 2
combination testing of rapamycin with cytotoxic agents by the
Pediatric Preclinical Testing Program. Mol Cancer Ther. 9:101–112.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Eisenberg-Lerner A, Bialik S, Simon HU and
Kimchi A: Life and death partners: apoptosis, autophagy and the
cross-talk between them. Cell Death Differ. 16:966–975. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Raje N, Kumar S, Hideshima T, Ishitsuka K,
Chauhan D, Mitsiades C, Podar K, Le Gouill S, Richardson P, Munshi
NC, Stirling DI, Antin JH and Anderson KC: Combination of the mTOR
inhibitor rapamycin and CC-5013 has synergistic activity in
multiple myeloma. Blood. 104:4188–4193. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gu J, Yamamoto H, Lu X, Ngan CY, Tsujino
T, Konishi K, Takemasa I, Ikeda M, Nagata H, Hashimoto S, Matsuzaki
T, Sekimoto M, Takagi A and Monden M: Low-dose oxaliplatin enhances
the antitumor efficacy of paclitaxel in human gastric cancer cell
lines. Digestion. 74:19–27. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Paglin S, Hollister T, Delohery T, Hackett
N, McMahill M, Sphicas E, Domingo D and Yahalom J: A novel response
of cancer cells to radiation involves autophagy and formation of
acidic vesicles. Cancer Res. 61:439–444. 2001.PubMed/NCBI
|
|
16
|
Park SB, Goldstein D, Lin CS, Krishnan AV,
Friedlander ML and Kiernan MC: Acute abnormalities of sensory nerve
function associated with oxaliplatin-induced neurotoxicity. J Clin
Oncol. 27:1243–1249. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nagata Y, Takahashi A, Ohnishi K, Ota I,
Ohnishi T, Tojo T and Taniguchi S: Effect of rapamycin, an mTOR
inhibitor, on radiation sensitivity of lung cancer cells having
different p53 gene status. Int J Oncol. 37:1001–1010. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shigematsu H, Yoshida K, Sanada Y, Osada
S, Takahashi T, Wada Y, Konishi K, Okada M and Fukushima M:
Rapamycin enhances chemotherapy-induced cytotoxicity by inhibiting
the expressions of TS and ERK in gastric cancer cells. Int J
Cancer. 126:2716–2725. 2010.PubMed/NCBI
|
|
19
|
Fung AS, Wu L and Tannock IF: Concurrent
and sequential administration of chemotherapy and the Mammalian
target of rapamycin inhibitor temsirolimus in human cancer cells
and xenografts. Clin Cancer Res. 15:5389–5395. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Francis LK, Alsayed Y, Leleu X, Jia X,
Singha UK, Anderson J, Timm M, Ngo H, Lu G, Huston A, Ehrlich LA,
Dimmock E, Lentzsch S, Hideshima T, Roodman GD, Anderson KC and
Ghobrial IM: Combination mammalian target of rapamycin inhibitor
rapamycin and HSP90 inhibitor
17-allylamino-17-demethoxygeldanamycin has synergistic activity in
multiple myeloma. Clin Cancer Res. 12:6826–6835. 2006. View Article : Google Scholar
|
|
21
|
Mondesire WH, Jian W, Zhang H, Ensor J,
Hung MC, Mills GB and Meric-Bernstam F: Targeting mammalian target
of rapamycin synergistically enhances chemotherapy induced
cytotoxicity in breast cancer cells. Clin Cancer Res. 10:7031–7042.
2004. View Article : Google Scholar
|
|
22
|
Shafer A, Zhou C, Gehrig PA, Boggess JF
and Bae-Jump VL: Rapamycin potentiates the effects of paclitaxel in
endometrial cancer cells through inhibition of cell proliferation
and induction of apoptosis. Int J Cancer. 126:1144–1154.
2010.PubMed/NCBI
|
|
23
|
Bae-Jump VL, Zhou C, Boggess JF and Gehrig
PA: Synergistic effect of rapamycin and cisplatin in endometrial
cancer cells. Cancer. 115:3887–3896. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kondo Y and Kondo S: Autophagy and cancer
therapy. Autophagy. 2:85–90. 2006. View Article : Google Scholar
|