Introduction
Broders was a pioneer in the classification of oral
squamous cell carcinoma. The considerable variation in the
histological aspect of this condition led to the development of a
number of systems for the histological grading of malignancy. These
systems were designed to allow for an interpretation of the
aggressiveness of the tumor (1). In
1920, Broders (2) used cell
pleomorphism and keratin production as parameters for the
assessment of cell differentiation and the number of mitoses for
the assessment of tumor growth. In 1941, these parameters were
correlated to tumor prognosis (3).
Following this period, other systems were used in the histological
grading of malignant tumors (Table
I) (4–7). In 1998, Bryne et al (8) suggested that only the front of the
tumor invasion should be assessed using the degree of
keratinization, nuclear pleomorphism, invasion pattern and
inflammatory infiltrate as parameters. Based on this system, it has
been suggested that cells at the front of the tumor invasion
exhibit different molecular characteristics to those at the surface
areas of the tumor, and that interactions between the tumor and
host in this region are ‘crucial to the dissemination of the
neoplasm’, making this an essential region for the determination of
prognosis.
 | Table IParameters used in the various systems
for the histological grading of malignancy. |
Table I
Parameters used in the various systems
for the histological grading of malignancy.
| Authors | Year | Parameters | Refs. |
|---|
| Wahi | 1971 | Keratinization of
individual cells, formation of horn pearls, presence of
intercellular bridges, number and aspect of mitosis figures, degree
of cell and nuclear pleomorphism and presence of multi-nucleated
giant cells. | (4) |
| Jakobsson et
al | 1973 | Neoplastic structure,
keratinization, nuclear pleomorphism, number of mitoses, mode and
stage of invasion, vascular invasion and lymphoplasmocyte
inflammatory infiltrate. | (5) |
| Crissman, Gluckman,
Cummings | 1984 | Degree of
keratinization, nuclear pleomorphism, number of mitoses, invasion
pattern, inflammatory infiltrate and vascular invasion. | (6) |
| Anneroth, Batsakis,
Luna | 1987 | Degree of
keratinization, cell pleomorphism, number of mitoses, invasion
pattern, stage of invasion and lymphoplasmocyte inflammatory
infiltrate. | (7) |
Kurokawa et al (1) studied the relationship between the
scores obtained using Bryne’s method and the prognosis of patients
with squamous cell carcinoma of the tongue. Results showed that
patients with a score below 8 had a more positive prognosis,
whereas those with a score of 11 or higher had a poorer prognosis.
According to Costa et al (9), the histological grading of the deepest
sections of the tumor directly affect patient survival due to the
fact that tumor cells in these sites are undifferentiated. The
authors found a predominantly discrete inflammatory infiltrate in
cases of aggressive squamous cell carcinoma when correlated with
the development of metastasis in cervical lymph nodes (TNM II/IV).
Da Silveira et al (10)
found that positive CD8 and CD25 inflammatory cells had a greater
involvement in tumors of the lower lip and found that the anti-ζ
transmembrane molecule, which is responsible for translating the
bonding of the antigen to the extracellular receptor in activating
signals to the interior of the cell, was more pronounced in cases
without metastasis, regardless of the anatomic site. The author
states that the inflammatory infiltrate exerted a certain impact on
the aggressiveness of squamous cell carcinoma of the tongue and
lower lip when assessed using Bryne’s method. To a certain extent,
this effect may be a contributing factor to the controversy
regarding the use of this parameter as a morphological indicator of
aggressiveness, as the vast majority (80%) of the samples exhibited
an inflammatory infiltrate ranging from moderate to intense.
Human tumors activate CD4 or CD8 lymphocytes,
depending on the processing pathway to trigger the immune response.
Moreover, control of the tumor depends on the magnitude of the
initial immune response and the capacity to sustain this response
for a prolonged period of time (11). The main anti-tumor defense mechanism
is the death of tumor cells by CD8 T lymphocytes, also known as
cytotoxic T lymphocytes, which are capable of identifying and
killing potentially malignant cells that express peptides derived
from mutant cell proteins or oncogenic viral proteins associated
with MHC class I. The involvement of CD4 T lymphocytes appears to
be related to the production of TNF by macrophages and INFγ by the
Th1 population. Moreover, these lymphocytes may be responsible for
the increase in the expression of MHC-I by tumor cells, resulting
in the sensitization of CD8 T lymphocytes and consequent lysis of
the tumor cells by the perforin/granzyme system or through the
bonding of Fas in the tumor cells with Fas-L (CD95) from the
lymphocytes. Therefore, the suppression of CD4 Th1 lymphocytes has
been shown to result in tumor progression (12). However, the immunosuppressive
property of CD4 T cells was associated with a low survival rate
among patients with ovarian cancer (13). On the other hand, certain authors do
not support the hypothesis that the infiltration of CD4 T cells
alone is responsible for this lower survival rate, but that the
effect of the modulation of CD4 over the benefits of CD8 depends on
the number of regulating T cells in the CD4 population (14).
Active suppression by regulating cells is one of the
various mechanisms used to keep the immune response under control,
in particular CD4+ CD25+ T cells
(Treg), which constitutively express CTLA-4, GITR and
Forkhead Box P3 (FOXP3) molecules (15). Attention has been given to the study
of FOXP3, which establishes and maintains the genetic program of
Treg and acts as a negative regulator of the activation
of T cells and possibly as a transcriptional effector of
anti-inflammatory cytokine programs (16).
Chronic inflammation as a risk factor for cancer was
first conceived by Virchow in the early 19th century and reported
by Weitzman and Gordon in the association of various chronic
inflammatory diseases, including irritable bowel syndrome, atrophic
gastritis, chronic colecistitis and reflux esophagitis, with the
development of cancer (17). The
advent of molecular biology has enabled a number of studies to
address the relationship between the tumor and peritumoral stroma,
with its evident contribution to tumor growth, invasion and
metastasis (18–24). Subsequently, it has become easier to
suggest the involvement of cytokines and inflammatory cells in
tumor development and progression as compared to an anti-tumor
response from the host, as proposed in a number of other studies
(5,7,8).
The aim of the present study was to assess the
suppressant role of the inflammatory infiltrate in oral
carcinogenesis through the immunohistochemical expression of CD8
and FOXP3 and to discuss how representative this expression is, as
well as other parameters considered to be of prognostic value.
Materials and methods
A total of 20 cases of oral epithelial dysplasia and
40 cases of oral squamous cell carcinoma were randomly selected
from the archives of the Pathological Anatomy Service of the
Federal University of Sergipe and the Pathological Anatomy
Laboratory of the Oral Pathology Sector of the Federal University
of Rio Grande do Norte, in Brazil. The study received approval from
the Research Ethics Committee of the latter institution (process
no. 233/2007).
The hematoxylin and eosin-stained slides were viewed
under a light microscope and examined in a double-blind manner by
two histopathologists. The criteria of the World Health
Organization were used for the histological grading of the
dysplasia (25). For carcinoma
cases, a method was developed for the present study using
well-established parameters in the literature, based on Wahi
(4). The proposed method considered
the following parameters: i) type of invasion, which reveals a
greater or lesser speed in the growth and/or invasion, ii) maturity
of the epithelial cells, iii) presence of epithelial masses between
the lining of the epithelium or ulcerated tissue surface and the
invasive front, and iv) dysmorphism of the epithelial masses,
revealing the incapacity of normal differentiation among these
cells. A binary numeral system, using the digits 0 and 1, was
adopted to characterize each of these parameters (Table II). After analysis of all possible
combinations, the cases were grouped and classified as stage I
(low-grade) or stage II (high-grade) (Table III) to enable the comparative
study of these stages with Bryne’s grading system (8) and CD8 and FOXP3 expression.
 | Table IIParameters assessed for the definition
of stage of oral squamous cell carcinoma. |
Table II
Parameters assessed for the definition
of stage of oral squamous cell carcinoma.
| Invasive front | Epithelial
masses |
|---|
|
|
|
|---|
| Score | Type | Maturity | Presence | Dysmorphism |
|---|
| 0 | Masses or
trabecules | Mature | Absent | Low |
| 1 | Nests or strings | Immature | Present | High |
 | Table IIIStages of oral squamous cell carcinoma
according to the binary numeral system for each of the
parameters. |
Table III
Stages of oral squamous cell carcinoma
according to the binary numeral system for each of the
parameters.
| Situation | Type of invasion | Maturity | Presence of
masses | Dysmorphism | Stage |
|---|
| 1 | 0 | 0 | 0 | 0 | I |
| 2 | 0 | 0 | 0 | 1 | I |
| 3 | 0 | 0 | 1 | 0 | I |
| 4 | 0 | 0 | 1 | 1 | I |
| 5 | 0 | 1 | 0 | 0 | I |
| 6 | 0 | 1 | 0 | 1 | I |
| 7 | 0 | 1 | 1 | 0 | I |
| 8 | 0 | 1 | 1 | 1 | II |
| 9 | 1 | 0 | 0 | 0 | II |
| 10 | 1 | 0 | 0 | 1 | II |
| 11 | 1 | 0 | 1 | 0 | II |
| 12 | 1 | 0 | 1 | 1 | II |
| 13 | 1 | 1 | 0 | 0 | II |
| 14 | 1 | 1 | 0 | 1 | II |
| 15 | 1 | 1 | 1 | 0 | II |
| 16 | 1 | 1 | 1 | 1 | II |
To pair the scores of Bryne’s grading system with
those obtained in the binary method, the cases with scores of 4–8
and 3–6 were considered as stage I (low-grade), whereas cases with
scores of 9–16 and 7–12 were considered as stage II (high-grade) in
Bryne’s classification. Bryne’s grading system for the invasive
front was analyzed with and without the inflammatory response
parameter. The κ test was used to measure the correlation between
the method proposed in this study and Bryne’s grading system.
Immunohistochemical analysis was performed for
assessment of the expression of anti-CD8 and anti-FOXP3 in cases of
dysplasia and carcinoma. Paraffin-embedded specimens were cut (3
μm) and the slices were mounted on glass slides prepared with an
organosilane-based adhesive (3-aminopropyltriethoxysilane, Sigma
Chemical Co, St. Louis, MO, USA). Antibodies directed against the
proteins studied were then maintained overnight, along with the
Dako CD8/144B clone at a dilution of 1:200 for 60 min, and the
FOXP3 (H190) clone (Santa Cruz Biotechnology, Santa Cruz, CA, USA)
at a dilution of 1:100. Antigen recovery was performed with
Tris-EDTA at pH 9.0, using the SABC and Envision-HRP methods. For
the negative controls, the primary antibodies were omitted. For the
positive controls, a previously tested periapical granuloma was
used. Following the classification of the cases of dysplasia and
carcinoma, a semi-quantitative analysis was performed on the CD8
and FOXP3 expression, with reactions of a brown coloration
considered positive, regardless of intensity. The percentage of
positive cells was calculated for each case, classifying those with
<5% labeled cells as low expression, those with 5–50% labeled
cells as moderate expression and those with >50% labeled cells
as high expression, following the criteria proposed by Abbas et
al (26).
The statistical analysis was performed using the
SPSS 13.0 program for Windows. The χ2 test and Fisher’s
exact test were employed to determine associations in the
distribution of the data between the two stages of the carcinoma
obtained by the binary numeral system and the parameters used in
this assessment. The non-parametric Mann-Whitney test was used to
determine the hypothesis of equality in CD8 and FOXP3 expression
with regard to the types of lesion and histological grade of
carcinoma. The Friedman ANOVA test was used for comparison between
the grades of dysplasia in relation to CD8 and FOXP3 expression.
The Spearman’s rank correlation coefficient was used to investigate
the correlation between inflammatory infiltrate intensity and
positivity for CD8 and FOXP3. P<0.05 was considered to be
statistically significant.
Results
Of the 20 cases of dysplasia, 45% were classified as
mild, 15% as moderate and 40% as severe. In most cases, the
inflammatory infiltrate was moderate. In relation to oral squamous
cell carcinoma, the results demonstrated good replicability of the
binary method compared to Bryne’s grading system (k=0.590;
p<0.0001), even when the inflammatory response parameter was
excluded (k=0.688; p<0.0001). A total of 42.5% of the oral
squamous cell carcinomas were classified as low-grade (stage I) and
57.5% as high-grade (stage II), according to the histological
grading system developed. A total of 64.7% of the low-grade cases
revealed intense inflammatory infiltrate, in contrast to 34.8% of
the high-grade cases. The descriptive analysis of the dysplasia and
oral squamous cell carcinoma is shown in Table IV.
 | Table IVDescriptive analysis of the dysplasia
and oral squamous cell carcinoma cases. |
Table IV
Descriptive analysis of the dysplasia
and oral squamous cell carcinoma cases.
| Lesions | No. of cases | Relative frequency
(%) |
|---|
| Dysplasia
(n=20) |
| Grade |
| Mild | 9 | 45 |
| Moderate | 3 | 15 |
| Severe | 8 | 40 |
| Inflammatory
infiltrate |
| Mild | 5 | 25 |
| Moderate | 10 | 50 |
| Intense | 5 | 25 |
| Carcinoma
(n=40) |
| Invasive
front |
| Type |
| Masses or
trabecules (score 0) | 24 | 60 |
| Nests or strings
(score I) | 16 | 40 |
| Maturity |
| Mature (score
0) | 23 | 57.5 |
| Immature (score
I) | 17 | 42.5 |
| Epithelial
masses |
| Presence |
| Absent (score
0) | 13 | 32.5 |
| Present (score
I) | 27 | 67.5 |
| Dysmorphism |
| Low (score
0) | 16 | 40 |
| High (score
I) | 24 | 60 |
| Stage |
| I | 17 | 42.5 |
| II | 23 | 57.5 |
| Inflammatory
infiltrate (stage I) |
| Mild | 2 | 11.8 |
| Moderate | 4 | 23.5 |
| Intense | 11 | 64.7 |
| Inflammatory
infiltrate (stage II) |
| Mild | 9 | 39.1 |
| Moderate | 5 | 21.8 |
| Intense | 9 | 39.1 |
The stages of the oral squamous cell carcinomas were
significantly associated with the type of invasive front, cell
maturity and the presence of masses between the invasion front and
surface of the lesion, as well as the dysmorphism of the cells
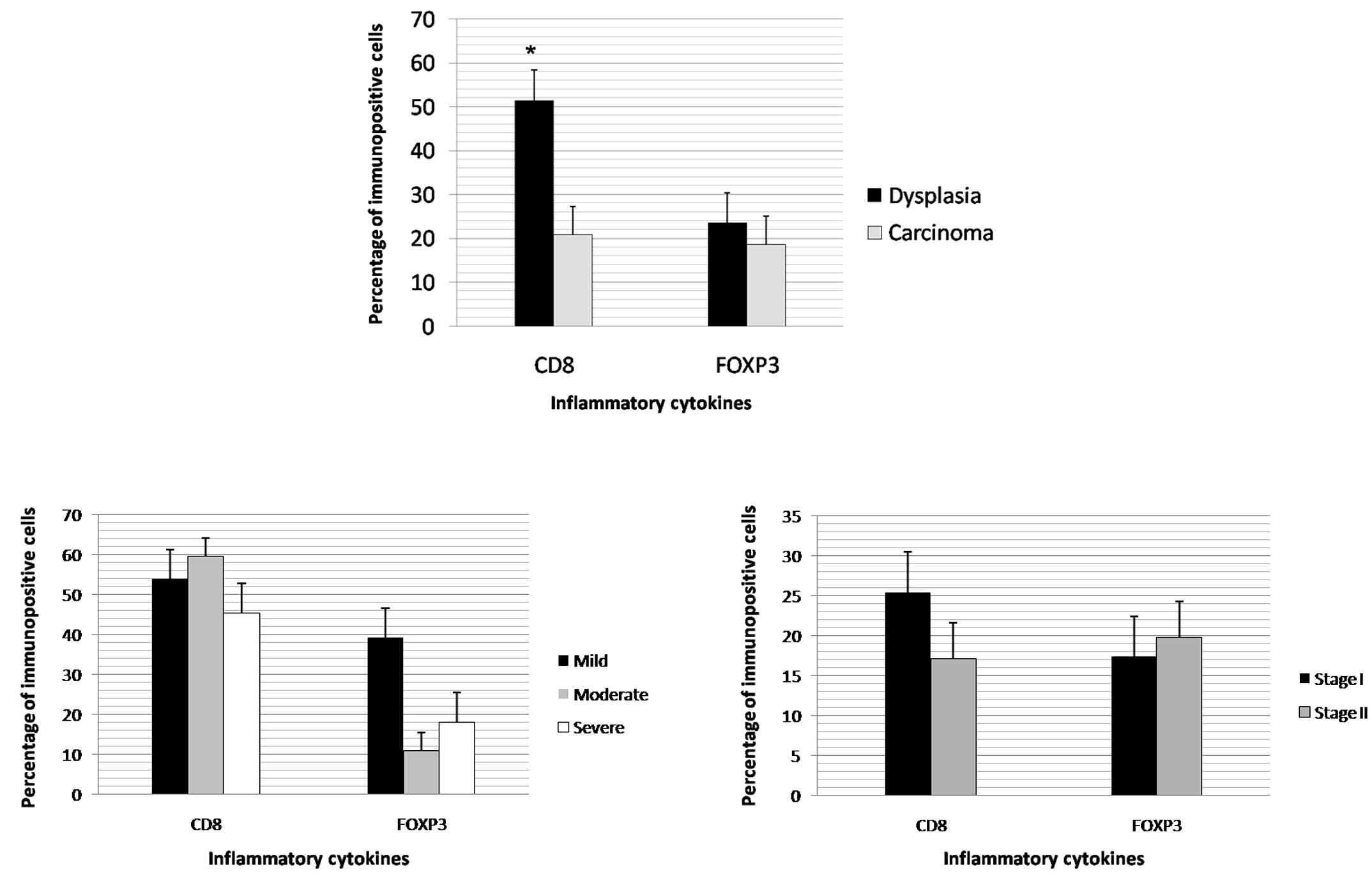
(Table V). CD8 expression was found
to be significantly higher in the dysplasia cases (Fig. 1A). No differences were noted in the
CD8 and FOXP3 expression levels between the grades of dysplasia and
carcinoma (Figs. 1B and 1C). In the
carcinoma cases, inflammatory infiltrate intensity had a direct,
significant correlation with CD8 (Table VI).
 | Table VClassification of oral squamous cell
carcinoma according to the parameters proposed for this study. |
Table V
Classification of oral squamous cell
carcinoma according to the parameters proposed for this study.
| Histological grade
of malignancy | |
|---|
|
| |
|---|
| Parameters | Stage I (low-grade)
no. (%) | Stage II
(high-grade) no. (%) | P-value |
|---|
| Invasive front |
| Type |
| Masses or
trabecules | 17 (100) | 7 (30.5) | <0.05a |
| Nests or
strings | 0 (0) | 16 (69.5) | |
| Maturity |
| Mature | 15 (88.2) | 7 (30.5) | <0.05a |
| Immature | 2 (11.8) | 16 (69.5) | |
| Epithelial
masses |
| Presence |
| Absent | 9 (53.0) | 4 (17.4) | <0.05a |
| Present | 8 (47) | 19 (82.6) | |
| Dysmorphism |
| Low | 10 (62.5) | 6 (25.0) | <0.05a |
| High | 6 (37.5) | 18 (75.0) | |
 | Table VICorrelation between inflammatory
infiltrate intensity and positivity for CD8 and FOXP3
expression. |
Table VI
Correlation between inflammatory
infiltrate intensity and positivity for CD8 and FOXP3
expression.
| Inflammatory
infiltrate intensity |
|---|
|
|
|---|
| Dysplasia
(n=20) | Carcinoma
(n=40) |
|---|
|
|
|
|---|
| Markers | Mild | Mod. no. (%) | Int. | Total | r | P-value | Mild | Mod. no. (%) | Int. | Total | r | P-value |
|---|
| CD8 |
| <5% | 2(40) | 1 (10) | 1 (20) | 4 (20) | | | 11 (100) | 6 (60) | 10 (50) | 27 (67.5) | | |
| 5–50% | 2 (40) | 2 (20) | 0 (0) | 4 (20) | 0.391 | N.S. | 0 (0) | 1 (10) | 2 (10) | 3 (7.5) | 0.365 | <0.05a |
| >50% | 1 (20) | 7 (70) | 4 (80) | 12 (60) | | | 0 (0) | 2 (20) | 8 (40) | 10 (25.0) | | |
| FOXP3 |
| <5% | 3 (60) | 6 (60) | 2 (40) | 11 (55) | | | 7 (63.6) | 6 (60) | 11 (57.9) | 24 (60.0) | | |
| 5–50% | 1 (20) | 2 (20) | 2 (40) | 5 (25) | 0.109 | N.S. | 2 (18.2) | 3 (30) | 6 (31.6) | 11 (27.5) | 0.018 | N.S. |
| >50% | 1 (20) | 2 (20) | 1 (20) | 4 (20) | | | 2 (18.2) | 1 (10) | 2 (10.5) | 5 (12.5) | | |
Discussion
The considerable variation in the histological
aspect of oral epidermoid carcinoma has led, to the development of
a number of systems for the histological grading of malignancy
designed to allow an interpretation of the aggressiveness of the
tumor. However, all of the systems, with the exception of Wahi’s
classification, considered the inflammatory infiltrate as a
parameter of aggressiveness. In the grading system developed for
the present study, four established parameters were considered
(type of invasion, maturity, presence of epithelial masses and
dysmorphism), and the results of this new type of assessment were
then compared with Bryne’s grading system, obtaining good
replicability between the two methods.
In relation to the analysis of the invasive front,
it was observed that when the invasion occurred in the form of
nests or strings, the tumor had a high degree of aggressiveness,
whereas when the invasion occurred in masses or trabecules, the
tumor was less aggressive. In the assessment of cell maturity,
there was a predominance of mature cells at the invasive front in
stage I cases, whereas immature cells predominated in stage II
cases. However, immaturity cannot be used for the assessment of
cell pleomorphism, as suggested in Bryne’s grading system, since it
is manifested in the course of the differentiation process. In
Bryne’s method, this evaluation is incoherent, since it is
performed only at the invasive front, where most cells are
immature.
While the type of invasion associated with cell
maturity in the invasion front demonstrates the speed of tumor
growth, the presence of tumor masses between the lining of the
epithelium or ulcerated surface and the invasive front denote the
existence of a ‘time’ necessary for these cells to undergo
differentiation and demonstrate (through dysmorphism) whether or
not this differentiation was successful, relying on its end
product, keratin, for this assessment. Therefore, superficial
masses may not represent the site in which the main reactions led
by inflammatory cytokines occur, but the site where the result of
these masses is observed.
In cases where the tumor is in a phase of
proliferation, the majority of cells at the invasive front are
immature. This is contrary to the process observed when growth is
slow, where keratin production and accumulation occurs, thereby
giving volume to surface masses. Thus, the molecular
characteristics may differ in areas of proliferation, which would
be expected even in normal epithelium, with greater intensity at
the invasive front of highly proliferative tumors that are made up
almost exclusively of immature cells. In tumors with slow growth,
either atypical or well-differentiated, it is difficult to
determine this difference in the labeling pattern between the
masses and the invasive front; the difference is observed between
the two types of tumors. Thus, the analysis of the invasive front
is as crucial in the assessment of this growth potential as the
assessment of the masses in relation to their differentiation
capacity, both of which are significant in the assessment of
carcinoma behavior.
A number of authors have stated that the presence of
inflammatory infiltrate is common in tumors of the oral cavity
(27). In the present study,
inflammatory infiltrate was present in all cases of dysplasia and
carcinoma, but was more intense in the latter. An anti-tumor
defense role has been attributed to inflammatory infiltrate by
authors from Jakobsson et al (5) to Bryne et al (8). However, recent studies, including that
carried out by Da Silveira et al (10), warn of the possibility of this
infiltrate exerting another role, or of not even exerting an
anti-tumor defense role at all, termed ‘immunological evasion’. It
may be suggested that the absence of inflammatory infiltrate in the
initial phase of development of oral carcinoma facilitates the
action of the aggressor, which may have autonomy for progression,
thereby triggering a reaction, albeit small, from the host, which
is immediately enlisted by the tumor cells to work in their favor.
This is in agreement with the 81.8% of high-grade carcinomas
detected among the 11 cases that exhibited mild inflammatory
infiltrate in the present study, and is also in agreement with the
findings described by Costa et al (9). It is well known that a lack of
response from the host, whether or not it is due to an absence or
inadequacy of an inflammatory response, may be related to
aggressiveness; however, the opposite is not valid. Therefore, the
intensity of pure and simple inflammation cannot be used as a
parameter for prognosis, as it may take on different roles in the
various stages of carcinogenesis. It is crucial to understand what
its presence indicates and the manner in which it acts on tumor
transformation, progression and invasion. However, findings by
Bryne et al (8) are somewhat
valid regarding the significance of the interaction between the
inflammatory infiltrate (regardless of its role) and the tumor
cells at the invasive front, although the molecular differences in
the area of the front of the tumor invasion may depend on the speed
of the growth and vice versa.
Comparative studies of dysplasia and carcinoma have
demonstrated significant molecular alterations, which do not occur
between the histological grades of carcinoma (26). In the present study, a greater
expression of CD8 was detected in the cases of dysplasia than in
those of carcinoma, suggesting an initially protective function of
the inflammatory infiltrate. However, the maintenance of the
stimulus and greater or lesser expression of other inflammatory
cytokines may favor transformation, followed by invasion. Although
the carcinoma cases exhibited a more intense inflammatory reaction
and an increase in CD8 expression associated with this intensity,
CD8 expression remained far lower than in the cases of dysplasia.
Further studies are required to assess the roles of this and other
inflammatory cytokines in carcinogenesis and the possibility of
using these cytokines in antitumor therapy.
In conclusion, we suggested that intensity of the
inflammatory infiltrate should not be used as a parameter in the
prognostic assessment of oral squamous cell carcinoma, as it
exercises different functions in the various stages of
carcinogenesis. The histological grading of malignancy is a
reliable prognostic indicator, particularly when linked to
immunohistochemical analysis. The proposed histological grading of
malignancy has greater prognostic value due to the fact that it
does not use the intensity of the inflammatory infiltrate as a
parameter, and the binary system reduces subjectivity in the
evaluation. The anti-tumor reaction exerted by CD8 T lymphocytes
occurs with greater frequency in cases of dysplasia, suggesting
that a high concentration of this cell population slows down the
transformation and invasion process. Moreover, although the intense
inflammatory infiltrate has a positive correlation to CD8, the
anti-tumor defense action is minimal, as only 32.5% of oral
squamous cell carcinomas exhibited a greater than 5% labeling.
References
|
1
|
Kurokawa H, Zhang M, Matsumoto S,
Yamashita Y, et al: The high prognostic value of the histologic
grade at the deep invasive front of tongue squamous cell carcinoma.
J Oral Pathol Med. 34:329–333. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Broders AC: Squamous-cell epithelioma of
the lip: a study of five hundred and thirty-seven cases. J Am Med
Assoc. 6:656–664. 1920. View Article : Google Scholar
|
|
3
|
Broders AC: The microscopic grading of
cancer. Surg Clin North Am. 21:947–961. 1941.
|
|
4
|
Wahi PM: Tipos histológicos de tumores
orales y orofaringeos. Ginebra: Organización Mundial de la Salud;
1971
|
|
5
|
Jakobsson PA, Eneroth GM, Killander D,
Moberger G and Artenson B: Histologic classification and grading of
malignancy in carcinoma of the larynx (a pilot study). Acta Radiol
Ther Phys Biol. 12:1–8. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Crissman JD, Liu WY, Gluckman JL and
Cummings G: Prognostic value of histopathologic parameters in
squamous cell carcinoma of the oropharynx. Cancer. 54:2995–3001.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Anneroth G, Batsakis JG and Luna M: Review
of the literature and a recommended system of malignancy grading of
squamous cell carcinoma. Scand J Dent Res. 95:222–249.
1987.PubMed/NCBI
|
|
8
|
Bryne M: Is the invasive front of an oral
carcinoma the most important area for prognostication? Oral
diseases. 4:70–77. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Costa Ade L, Araújo RF Júnior and Ramos
CC: Correlation between TNM classification and malignancy
histological feature of oral squamous cell carcinoma. Braz J
Otorhinolaryngol. 71:181–187. 2005.PubMed/NCBI
|
|
10
|
Da Silveira EJ, Miguel MC, Lima KC, de
Freitas RA, de Morais ML and Queiroz LM: Analysis of local immunity
in squamous cell carcinoma of the tongue and lower lip. Exp Mol
Pathol. 88:171–175. 2010.PubMed/NCBI
|
|
11
|
Sabel MS, Hess SD, Egilmez NK, Conway TF
Jr, Chen FA and Bankert RB: CTLA-4 blockade augments human T
lymphocyte-mediated suppression of lung tumor xenografts in SCID
mice. Cancer Immunol Immunother. 54:944–952. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chang MC, Chiang CP, Lin CL, Lee JJ, Hahn
LJ and Jeng JH: Cell-mediated immunity and head and neck cancer:
with special emphasis on betel quid chewing habit. Oral Oncol.
41:757–775. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Curiel TJ, Coukos G, Zou L, et al:
Specific recruitment of regulatory T cells in ovarian carcinoma
fosters immune privilege and predicts survival. Nat Med.
10:942–949. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sato E, Olson SH, Ahn J, et al:
Intraepithelial CD8+ tumor-infiltrating lymphocytes and
a high CD8+/regulatory T cell ratio are associated with
favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA.
102:18538–18543. 2005.
|
|
15
|
Fontenot JD, Gavin MA and Rudensky AY:
FoxP3 programs the development and function of
CD4+CD25+ regulatory T cells. Nat Immunol.
4:330–336. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fontenot JD, Rasmussen JP, Williams LM,
Dooley JL, Farr AG and Rudensky AY: Regulatory T cell lineage
specification by the forkhead transcription factor FoxP3. Immunity.
22:329–341. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Weitzman SA and Gordon LI: Inflammation
and cancer: Role of phagocyte-generated oxidants in carcinogenesis.
Blood. 76:655–663. 1990.PubMed/NCBI
|
|
18
|
Balkwill F and Mantovani A: Inflammation
and cancer: back to Virchow? The Lancet. 85:473–483. 2001.
|
|
19
|
Coussens LM and Werb Z: Inflammatory cells
and cancer think different! J Exp Med. 193:23–26. 2001.
|
|
20
|
O’Byrne KJ and Dalgleish AG: Chronic
immune activation and inflammation as the cause of malignancy. Br J
Cancer. 85:473–483. 2001.
|
|
21
|
Yan L, Zucker S and Toole BP: Roles of the
multifunctional glycoprotein, emmprin (basigin; CD 147), in tumour
progression. Thromb Haemost. 93:199–204. 2005.PubMed/NCBI
|
|
22
|
Mignona MD, Fedele S, Lo Russo L, Lo Muzio
L and Bucci E: Immune activation and chronic inflammation as the
cause of malignancy in oral lichen planus: is there any evidence?
Oral Oncol. 40:120–130. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Aggarwal BB, Shishodia S, Sandur SK,
Pandey MK and Sethi G: Inflammation and cancer: How hot is the
link? Biochem Pharmacol. 72:1605–1621. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vigneswaran N, Beckers S and Waigel S:
Increased EMMPRIN (CD 147) expression during oral carcinogenesis.
Exp Mol Pathol. 80:147–159. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Warnakulasuriya S, Reibel J, Bouquot J and
Dabelsteen E: Oral epithelial dysplasia classification systems:
predictive value, utility, weaknesses and scope for improvement. J
Oral Pathol Med. 37:127–133. 2008. View Article : Google Scholar
|
|
26
|
Abbas NF, Labib El-Sharkawy S, Abbas EA
and Abdel Monem El-Shaer M: Immunohistochemical study of p53 and
angiogenesis in benign and preneoplastic oral lesions and oral
squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radio
Oral Endod. 103:385–390. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hoffmann TK, Bier H and Whiteside TL:
Targeting the immune system: novel therapeutic approaches in
squamous cell carcinoma of the head and neck. Cancer Immunol
Immunother. 53:1055–1067. 2004. View Article : Google Scholar : PubMed/NCBI
|