Introduction
Bladder carcinoma is a common cancer worldwide, with
an overall incidence of 340,000 new cases each year (1). In the USA, it is the second most
common malignant genitourinary disease, with an estimated 70,500
cases in 2010 (2). There were
approximately 21,400 new cases in Germany in the same year
(3). In Western countries, more
than 90% of malignant bladder neoplasms are of urothelial origin
(4). Several factors are used to
predict the clinical outcome of invasive urothelial carcinoma, with
multifocality, size, stage, grade and concurrent carcinoma in
situ (Cis) being the most common (1). No molecular parameter has been
established for routine clinical management of patients with
urothelial carcinomas (5).
Glucose transporter 1 (GLUT1) belongs to the
expanding mammalian facilitative glucose transporter family, which
currently includes 13 members. GLUT1 is an energy-independent
transport protein with a high affinity for glucose (6). It is highly expressed on endothelial
cell surfaces at the blood-brain-barrier, allowing glucose entry
into the brain (7), on erythrocytes
(8), at the blood-ocular barrier
(9) and the perineurium (10). Elevated GLUT1 mRNA levels
have been reported in cancers of the pancreas, stomach, oesophagus
and colon (11). In addition, GLUT1
protein expression is elevated in breast cancer (12), renal cell carcinoma (13), squamous cell carcinomas of the head
and neck (14) and in carcinomas of
various other sites (10). GLUT1
expression was found to be negative in normal bladder mucosa
(15) and other non-neoplastic
tissues (10). In contrast, a
positive GLUT1 expression was reported to be present in the
majority of urothelial urinary bladder carcinomas, including
urothelial carcinoma of the renal pelvis (21), with varying effects on
clinicopathological parameters (15,17–21).
Malignant cells have an accelerated metabolism with
an increased need for energy. This excess energy can be supplied by
enhanced aerobic and/or anaerobic glycolytic pathways with
increased glucose influx, which is associated with an increased
expression of GLUTs. Glucose is required not only for rapid ATP
production, but also for accumulating biomass via the generation of
precursor molecules for nucleotides, cell membranes and other
components required for cell division (6). Therefore, we expect to observe marked
increases in GLUT1 expression in non-invasive forms of carcinoma
and further increases in invasive carcinoma relative to normal
tissue.
The aim of the present study was to systematically
evaluate GLUT1 protein expression in a large collection of
urothelial carcinomas of increasing grade of malignancy. The
analysis was supplemented by determining Ki-67-positive
proliferation indices. Particular attention was paid to
non-invasive precursors of urothelial carcinoma.
Materials and methods
Patients
The collection included samples from 94 men (90%)
and 11 women (10%). The mean age of the patients was 67.8 years
(range 44–87; SD, ±9.8 years; men, 68 years; women, 69 years).
Sample collection
A total of 105 paraffin-embedded samples were
allocated from the archive of the Institute of Pathology and
Neuropathology, University Hospital of Essen, Germany. Consistent
with the concept of this study, the collection included samples of
increasing grade of malignancy: 20 cases of low- and 19 of
high-grade papillary carcinoma (pTa), 11 of urothelial Cis, 16 of
(muscle-) invasive (G2) and 20 of muscle-invasive (G3) urothelial
carcinomas, as well as 19 normal urethral mucosa from transurethral
resection specimens, which served as controls, from patients
operated due to benign prostate hyperplasia. Surgical pathological
diagnosis and grading were conducted using the 1998 ISUP/2004 WHO
criteria and classified according to the UICC Classification of
Malignant Tumours, seventh edition (22). Normal urothelium was defined as 5–7
layers of urothelial cells with regular architecture and absent
anaplasia. Papillary carcinoma was diagnosed in cases with
papillary architecture consisting of more-or-less fused and
branching papillae, minor (low-grade) or marked (high-grade)
cellular and nuclear anaplasia, loss of polarity and maturation,
thickening to more than seven layers and increased mitotic rate.
Cases of urothelial carcinoma in situ exhibited anaplasia
similar to high-grade pTa, focally not extending to the entire
urothelial thickness. Normal urothelium, pTa and Cis specimens
exhibited an intact basement membrane. In invasive carcinomas,
tumour deposits were found beyond the basement membrane within the
lamina propria or deeper.
The study was performed in accordance with the
ethical standards of the Helsinki Declaration.
Immunohistochemistry
For immunohistochemistry, 4-μm sections were
cut from paraffin-embedded tissue blocks. A rabbit polyclonal
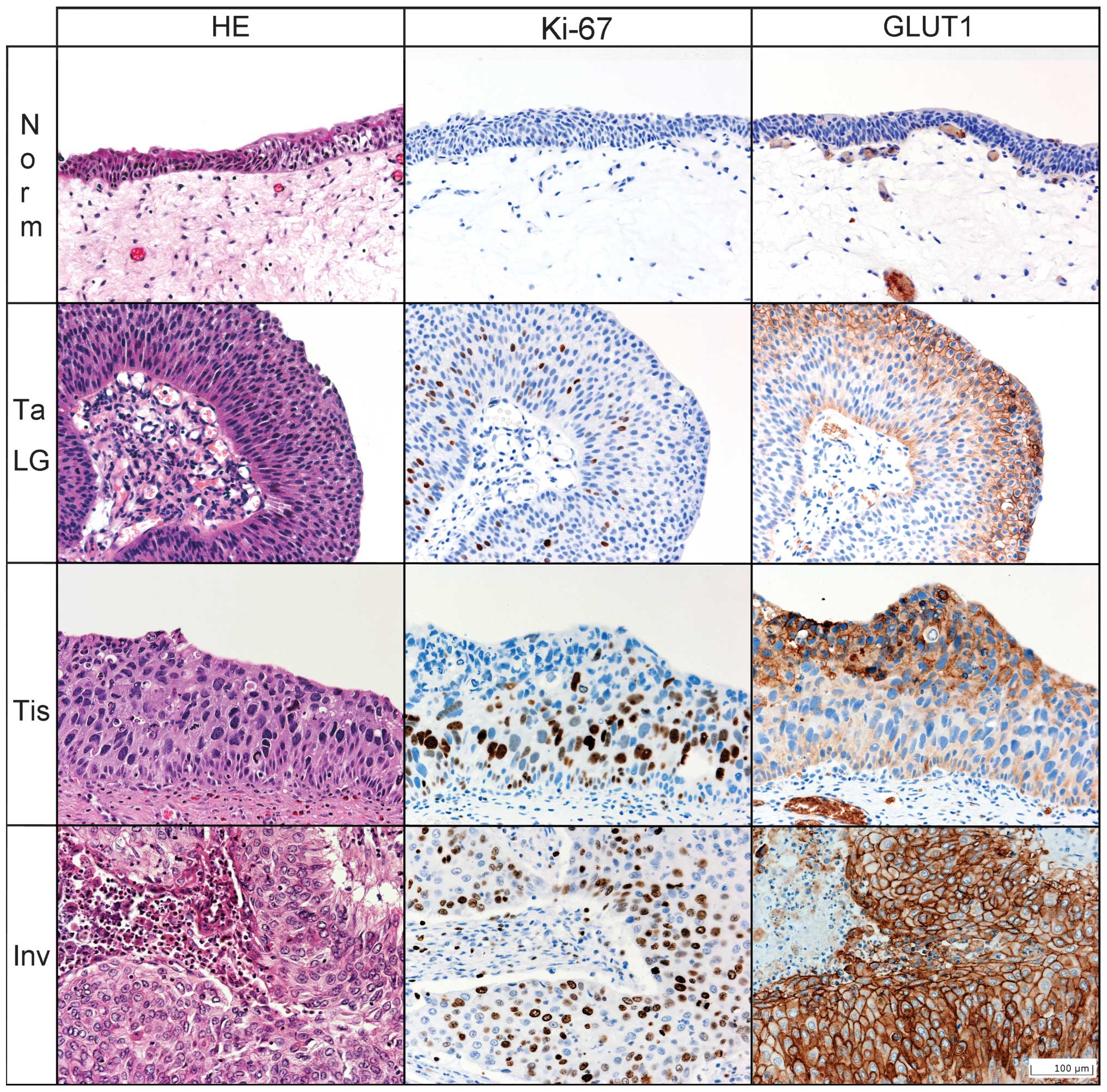
antibody against GLUT1 (1:400; IgG, Abcam, Cambridge, UK; Fig. 1) and a mouse monoclonal antibody
against Ki-67 (1:2000; IgG1, clone K-2, Zytomed Systems, Berlin,
Germany; Fig. 1) were used together
with a highly sensitive and specific polymer detection system
utilising horseradish peroxidase (ZytoChem-Plus HRP Polymer-Kit,
Zytomed Systems). Development was carried out with a permanent
brown chromogenic substrate system (Permanent AEC Kit, Zytomed
Systems). At the end of the procedure, sections were counterstained
with hematoxylin for 5 min.
Staining intensity of GLUT1 was assessed with an
immunoreactive score (IRS) by multiplying the level of staining
intensity (0 points, no staining; 1 point, weak staining; 2 points,
strong staining) by the percentage of positive tumour cells (0%, 0
points; <10%, 1 point; 11–50%, 2 points; 51–80%, 3 points;
>80%, 4 points) and scored on the following scale: negative (0
points), weak (1–2 points), moderate (3–4 points) and strong (6–8
points). GLUT1-expression was judged as positive in cases of
distinct circumferential membranous staining. Scant cytoplasmic and
staining in areas of thermal injury were not considered positive.
Erythrocytes served as an internal positive control.
The Ki-67 labelling index was determined by counting
positive nuclei in representative urothelial hot spots. In each
case, Ki-67 staining was evaluated in 100 cells. Areas showing
thermal or mechanical injury were excluded from analysis.
Statistical analysis
Statistical analyses and graphical illustrations
were performed using SPSS version 17.0 for Windows (SPSS Inc.,
Chicago, IL, USA). Univariate analysis of variance was performed
for the statistical evaluation of normally distributed continuous
parametric variables. Kruskal-Wallis one-way analysis of variance
and the Mann-Whitney U-test were used as non-parametric methods for
testing non-normally distributed variables where appropriate.
Spearman’s correlation analysis was used for ordinal variables.
P<0.05 was regarded as statistically significant.
Results
GLUT1 immunohistochemistry
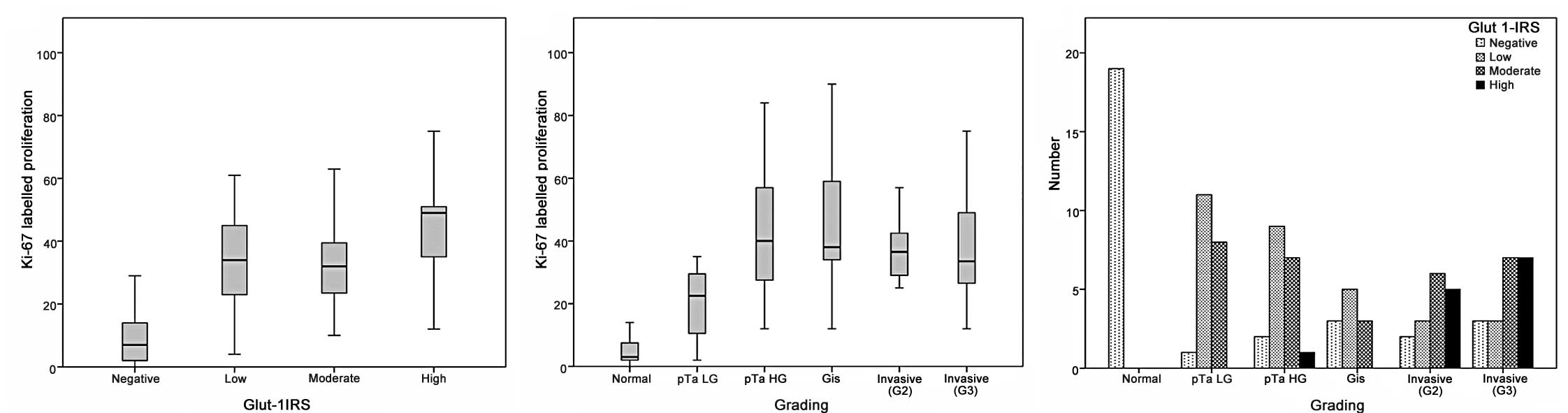
Increased GLUT1-IRS was significantly associated
with an increased grade of tumour malignancy (p<0.0001, Table I, Figs.
1 and 2C). The GLUT1-IRS was
lowest in normal urothelium and exhibited an increase in
non-invasive carcinoma types (p<0.0001; Table I). No association was found when
comparing low- vs. high-grade pTa (p=0.967), high-grade pTa vs. Cis
(p=0.287) or low-grade pTa vs. Cis (p=0.261). A further marked
increase in GLUT1-IRS was detected in the step to invasive
carcinoma (Table I). No significant
associations were found within the group of invasive carcinomas. No
correlation was noted for tumour grade (p=0.894; Table I) or stage (p=0.295; Table II).
 | Table IDistribution of Ki-67 counts and
GLUT1-IRS according to stage and grade. |
Table I
Distribution of Ki-67 counts and
GLUT1-IRS according to stage and grade.
| | | Ki-67 | GLUT1-IRS |
|---|
| | |
|
|
|---|
| Grade | N (%) | (SE) | P | P | Neg. (%) | Low (%) | Mod. (%) | High (%) | P | P |
|---|
| Non- invasive | Normal | 19 (18.1) | 5 (1) | <0.0001 | | 19 (100) | 0 (0) | 0 (0) | 0 (0) | <0.0001 | |
| pTa LG | 20 (19.0) | 21 (2) | | | 1 (5) | 11 (55) | 8 (40) | 0 (0) | | |
| pTa HG | 19 (18.1) | 43 (4) | | | 2 (11) | 9 (47) | 7 (37) | 1 (5) | | |
| Cis | 11 (10.5) | 45 (7) | | 0.227 | 3 (27) | 5 (45) | 3 (27) | 0 (0) | | <0.0001 |
| Invasive | G2 | 16 (15.2) | 36 (3) | 0.805 | | 2 (13) | 3 (19) | 6 (38) | 5 (31) | 0.894 | |
| G3 | 20 (19.0) | 38 (4) | | | 3 (15) | 3 (15) | 7 (35) | 7 (35) | | |
| Overall | | 105 (100) | 30 (2) | | | 30 (29) | 31 (30) | 31 (30) | 13 (12) | | |
 | Table IIGLUT1-IRS in correlation with Ki-67
proliferative index and distribution of Ki-67 counts and GLUT1-IRS
according to invasive carcinoma stage. |
Table II
GLUT1-IRS in correlation with Ki-67
proliferative index and distribution of Ki-67 counts and GLUT1-IRS
according to invasive carcinoma stage.
| GLUT1 -IRS | N (%) | Ki-67 (SE) | P | Stage | N (%) | Ki-67 (SE) | P | GLUT1-IRS | P |
|---|
|
|---|
| Neg. | Low (%) | Mod. (%) | High (%) |
|---|
| Negative | 30 (28.6) | 13 (3) | <0.0001 | pT1 | 1 (3) | 40 | 0.445 | 0 | 1 (100) | 0 (0) | 0 (0) | 0.295 |
| Low | 31 (29.5) | 35 (3) | | pT2 | 14 (39) | 37 (5) | | 3 (21%) | 4 (29) | 4 (29) | 3 (21) | |
| Moderate | 31 (29.5) | 36 (4) | | pT3 | 17 (47) | 40 (4) | | 0 | 0 (0) | 8 (47) | 9 (53) | |
| High | 13 (12.4) | 43 (5) | | pT4 | 4 (11) | 27 (5) | | 2 (50%) | 1 (25) | 1 (25) | 0 (0) | |
| Overall | 105 (100) | 30 (2) | | | 36 (100) | 37 (3) | | | | | | |
Ki-67 immunohistochemistry
The GLUT1-IRS was significantly correlated with the
Ki-67-labelled proliferative fraction (p<0.0001; Table II, Fig.
2A).
The mean number of Ki-67 positive cells was
significantly correlated with the increasing grade of malignancy in
non-invasive carcinoma (p<0.0001; Table I, Fig.
2B). However, no statistical difference was found between
high-grade pTa and Cis (p=0.832). A slight decrease in the mean
number of Ki-67-positive cells was noted from Cis to invasive
carcinoma, irrespective of tumour grade (p=0.805; Table I). No correlation was found between
Ki-67 staining and invasive tumour stage (p=0.445; Table II).
Discussion
As in other types of cancer (6,10–14),
GLUT1 protein expression was found to be elevated in urothelial
carcinomas (15,19–21).
However, findings regarding the association between GLUT1 protein
expression and pathological parameters have been inconsistent
(19–21).
In our study, we systematically evaluated GLUT1
protein expression in the largest collection of malignant
urothelial neoplasms of increasing grade of malignancy thus far.
The mean GLUT1-IRS and the mean proliferative index, estimated by
Ki-67 staining, were shown to be strongly associated with an
increasing grade of malignancy, from normal urothelium to
non-invasive and invasive carcinoma (p<0.0001; Table I). The GLUT1-IRS and the Ki-67
proliferative index were also significantly correlated
(p<0.0001; Table II).
The background of enhanced GLUT1 protein expression
in urothelial neoplasms of rising malignant potential may be
attributable to the increased metabolism of abnormal cells. In
general, these cells require greater amounts of glucose not only
for energy (ATP) generation, but also for the accumulation of
biomass via the generation of precursor molecules for nucleotides,
cell membranes and other components required for cell division
(6). This correlation is also
supported by the parallel increase of the mean proliferative index
in non-invasive carcinoma (p<0.0001; Table I). Additionally, the increase in
both GLUT1-IRS and Ki-67 index in non-invasive carcinoma occurs in
parallel with known essential genetic alterations. Whereas FGFR3
and Ha-ras mutations play significant roles in the development of
low-grade non-invasive carcinoma, a combination of p53/pRb, p107,
and PTEN mutations and deficiencies are crucial alterations
underlying high-grade urothelial carcinomas (23). These genetic changes allow cells
with damaged DNA to bypass anti-proliferative and pro-apoptotic
mechanisms (23).
When carcinoma cells have acquired the capacity for
invasiveness, for example, at a certain level of anaplasia, genetic
instability and loss of anti-tumoural inhibitors, no further
acceleration of cell metabolism (glucose consumption) can be
achieved. In this study, this effect may explain the failure of the
GLUT1-IRS and Ki-67 indices to discriminate between tumour grades
or stages following invasion. The significance of GLUT1 protein
accumulation in this study appears to be as a marker of hypoxia, as
the membranous GLUT1 antibody reaction was strongest in areas close
to necrosis (Fig. 1). This effect
was previously described and elucidated by Hoskin et al
(19).
In the diagnostic setting, GLUT1
immunohistochemistry may aid the discrimination of neoplastic from
non-neoplastic tissues, especially in cases of flat non-invasive
urothelial lesions vs. normal urothelium and papillary carcinoma
vs. papilloma. Normal urothelium consistently exhibited no GLUT1
staining (15), whereas the
majority of Cis cases in the present study exhibited GLUT1
immunoreactivity. Urothelial papilloma also lacked GLUT1
immunoreactivity (16), whereas
low- and high-grade pTa were, with certain exceptions,
GLUT1-positive. In ambiguous cases, GLUT1 immunohistochemistry may
aid in differential diagnosis in conjunction with
histomorphological features and an immunohistochemical panel of
CK20, Ki-67 and p53 (24).
In the clinical setting, increased glucose uptake
may be visualised in malignant tumours using positron emission
tomography (PET) and intravenously administered
18F-labelled 2-fluoro-2-deoxy-D-glucose (FDG) (6). Despite the excretion of FDG through
the urinary tract, PET is useful for the detection of urothelial
carcinomas of the urinary bladder when used in combination with CT
imaging (25). The sensitivity of
this examination may also be increased by diminishing excreted FDG
activity by diuresis with furosemid or other agents (25). As in other malignant tumours
(26–27), GLUT1 protein expression may be
correlated with increased PET signal strength in urothelial bladder
cancer, thus reflecting augmented glucose uptake and
utilisation.
In conclusion, we have used a standardised scoring
system to demonstrate that the GLUT1 protein expression pattern is
correlated with increasing malignant potential. In addition, we
have discussed possible links to underlying genetic alterations and
diagnostic implications.
References
|
1
|
Lopez-Beltran A and Sauter G: Infiltrating
urothelial carcinoma. World Health Organization Classification of
Tumours – Tumours of the Urinary System and Male Genital Organs.
Eble N: IARC Press; Lyon: pp. 93–109. 2004
|
|
2
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
Statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar
|
|
3
|
Husmann G, Kaatsch P, Katalinic A, Bertz
J, Haberland J, Kraywinkel K and Wolf U: 3.17 Bladder. Cancer in
Germany 2005/2006 Incidence and Trends. Robert Koch Institute (ed.)
and Association of Population-based Cancer Registries in Germany
(ed). Robert-Koch-Institut; Berlin: pp. 84–87. 2010
|
|
4
|
Boyle P and Levin B: Bladder cancer. World
Health Organization-World Cancer Report 2008. Boyle P and Levin B:
IARC Press; Lyon: pp. 444–449. 2008
|
|
5
|
Kaufman DS, Shipley WU and Feldman AS:
Bladder cancer. Lancet. 374:239–249. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Macheda ML, Rogers S and Best JD:
Molecular and cellular regulation of glucose transporter (GLUT)
proteins in cancer. J Cell Physiol. 202:654–662. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Agus DB, Gambhir SS, Pardridge WM,
Spielholz C, Baselga J, Vera JC and Golde DW: Vitamin C crosses the
blood-brain barrier in the oxidized form through the glucose
transporters. J Clin Invest. 100:2842–2848. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gould GW and Holman GD: The glucose
transporter family: structure, function and tissue-specific
expression. Biochem J. 295:329–341. 1993.PubMed/NCBI
|
|
9
|
Harik SI, Kalaria RN, Whitney PM,
Andersson L, Lundahl P, Ledbetter SR and Perry G: Glucose
transporters are abundant in cells with ‘occluding’ junctions at
the blood-eye barriers. Proc Natl Acad Sci USA. 87:4261–4264.
1990.
|
|
10
|
Younes M, Lechago LV, Somoano JR, Mosharaf
M and Lechago J: Wide expression of the human erythrocyte glucose
transporter Glut1 in human cancers. Cancer Res. 56:1164–1167.
1996.PubMed/NCBI
|
|
11
|
Yamamoto T, Seino Y, Fukumoto H, Koh G,
Yano H, Inagaki N, Yamada Y, Inoue K, Manabe T and Imura H:
Over-expression of facilitative glucose transporter genes in human
cancer. Biochem Biophys Res Commun. 170:223–230. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brown RS and Wahl RL: Overexpression of
Glut-1 glucose transporter in human breast cancer. An
immunohistochemical study. Cancer. 72:2979–2985. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nagase Y, Takata K, Moriyama N, Aso Y,
Murakami T and Hirano H: Immunohistochemical localization of
glucose transporters in human renal cell carcinoma. J Urol.
153:798–801. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mellanen P, Minn H, Grenman R and Harkonen
P: Expression of glucose transporters in head-and-neck tumors. Int
J Cancer. 56:622–629. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chang S, Lee S, Lee C, Kim JI and Kim Y:
Expression of the human erythrocyte glucose transporter in
transitional cell carcinoma of the bladder. Urology. 55:448–452.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee JH, Kim YW and Chang SG: Glucose
transporter-1 expression in urothelial papilloma of the bladder.
Urol Int. 74:268–271. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
O’Keeffe MB, Devlin AH, Burns AJ, Gardiner
TA, Logan ID, Hirst DG and McKeown SR: Investigation of pericytes,
hypoxia, and vascularity in bladder tumors: association with
clinical outcomes. Oncol Res. 17:93–101. 2008.PubMed/NCBI
|
|
18
|
Zhou JT, Cai ZM, Li NC and Na YQ:
Expression of hypoxia inducible factor-1alpha and glucose
transporter protein 1 in renal and bladder cancers and the clinical
significance thereof. Zhonghua Yi Xue Za Zhi. 86:1970–1974.
2006.PubMed/NCBI
|
|
19
|
Hoskin PJ, Sibtain A, Daley FM and Wilson
GD: GLUT1 and CAIX as intrinsic markers of hypoxia in bladder
cancer: relationship with vascularity and proliferation as
predictors of outcome of ARCON. Br J Cancer. 89:1290–1297. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Visca P, Sebastiani V, Pizer ES, Botti C,
de Carli P, Filippi S, Monaco S and Alo PL: Immunohistochemical
expression and prognostic significance of FAS and GLUT1 in bladder
carcinoma. Anticancer Res. 23:335–339. 2003.PubMed/NCBI
|
|
21
|
Younes M, Juarez D, Lechago LV and Lerner
SP: Glut 1 expression in transitional cell carcinoma of the urinary
bladder is associated with poor patient survival. Anticancer Res.
21:575–578. 2001.PubMed/NCBI
|
|
22
|
Cheng L, Montironi R, Davidson DD and
Lopez-Beltran A: Staging and reporting of urothelial carcinoma of
the urinary bladder. Mod Pathol. 22:S70–S95. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu XR: Biology of urothelial
tumorigenesis: insights from genetically engineered mice. Cancer
Metastasis Rev. 28:281–290. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lindemann-Docter K and Knuchel R: Update
on urothelial carcinoma histopathology. Pathologe. 29:331–338.
2008.PubMed/NCBI
|
|
25
|
Patil VV, Wang ZJ, Sollitto RA, Chuang KW,
Konety BR, Hawkins RA and Coakley FV: 18F-FDG PET/CT of
transitional cell carcinoma. AJR Am J Roentgenol. 193:W497–W504.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hiyoshi Y, Watanabe M, Imamura Y, Nagai Y,
Baba Y, Yoshida N, Toyama E, Hayashi N and Baba H: The relationship
between the glucose transporter type 1 expression and
F-fluorodeoxyglucose uptake in esophageal squamous cell carcinoma.
Oncology. 76:286–292. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Usuda K, Sagawa M, Aikawa H, Ueno M,
Tanaka M, Machida Y, Zhao XT, Ueda Y, Higashi K and Sakuma T:
Correlation between glucose transporter-1 expression and
(18)F-fluoro-2-deoxyglucose uptake on positron emission tomography
in lung cancer. Gen Thorac Cardiovasc Surg. 58:405–410. 2010.
View Article : Google Scholar : PubMed/NCBI
|