Introduction
Invasion and metastasis are the two key
characteristics of malignant tumors, and the direct cause of death
in patients with tumors. Tumor prognosis is closely correlated to
the invasive and metastatic potential of tumor cells (1). Invasion and metastasis of tumor cells
form a complex, multi-step, multi-link cascade and reactive process
including local invasion, tumor cell infiltration into blood
vessels and tumor cell adhesion. The tumor cells damage the
basement membrane and degrade the extracellular matrix during this
process to achieve tumor invasion and metastasis (2). Matrix metalloproteinase-3 (MMP-3) and
the tissue inhibitor of metalloproteinase-3 (TIMP-3) are the two
crucial in vivo enzymes present during extracellular matrix
synthesis and regulation of the degradation metabolism balance that
are closely correlated to tumor cell invasion and metastasis
(3).
In the present study, immunohistochemistry was used
to determine the expression of MMP-3 and TIMP-3 in the gastric
cancer tissue of 44 patients with gastric cancer in order to
investigate the role of MMP-3 and TIMP-3 in the occurrence and
development of gastric cancer. Transmission electron microscopy
(TEM) was used to observe lymphocytes and tumor cells in gastric
cancer tissue to further investigate the relationship between
MMP-3, TIMP-3 and clinical stages.
Materials and methods
Clinical information
A total of 44 gastric cancer patients who had
received surgery at the Yancheng First People’s Hospital, China,
between January 2009 and December 2010 were selected, all of whom
were pathologically diagnosed as gastric cancer following surgery.
None of the patients had received any treatment for gastric cancer
prior to surgery. According to the invasion depth of gastric
cancer, the patients were staged as follows: 18 cases in the early
and medium stages (early-stage group, cancer tissue limited to the
mucosa and submucosa, with or without lymph node metastasis)
comprising 13 males and 5 females aged between 32 and 62 years,
mean age 50.2±7.3 years; and 26 cases in the advanced stage
(advanced-stage group, cancer tissue invading into the muscular
layer) comprising 16 males and 10 females aged between 42 and 64
years, mean age 52.9±6.9 years. There was no statistically
significant difference in age and gender distribution between the
two groups (P>0.05).
Experimental methods
Immunohistochemistry method
MMP-3 and TIMP-3 streptavidin-biotin complex
immunohistochemistry kits were purchased from American Santa Cruz
Company. Immunohistochemistry was applied using the S-P method and
the laboratory operations were performed according to the kit
instructions. The gastric cancer tissues were fixed, embedded and
conventionally sliced with PBS buffer solution formalin solution,
then dewaxed to water and treated as follows: 3%
H2O2 was used to block endogenous peroxidase
and was repaired using a microwave in 0.01 mol/l citrate buffer (pH
6.0) for 30 min and sealed with normal goat serum A. The primary
and secondary antibodies and horseradish acid were successively
added to indicate streptomycin working solution (MMP-3 and TIMP-3
antibodies were diluted and incubated at 1:100 and stored overnight
at 4°C). The solutions were then colored with DAB, re-stained with
hematoxylin and sealed with neutral gum. PBS was used as a blank
control instead of the primary and secondary antibodies. MMP-3 and
TIMP-3 immunohistochemical staining slices were observed, and 10
fields of vision were randomly selected under high magnification
(x40) for each slice, with 20 randomly tested positive cancer cells
for each field. A total of 200 positive cells were tested for each
sample. The average gray values of the positive area were tested,
where the strength of immunohistochemistry was positively
proportional with the gray values, higher positive material
corresponding to deeper dyeing and greater gray value, otherwise
smaller.
Transmission electron microscope (TEM)
method
The gastric cancer tissue samples were cut into
1-mm3 slices and placed into 3% glutaraldehyde phosphate
buffer (pH 7.4) and fixed for 2–4 h. The samples were then re-fixed
with 1% osmic acid and gradually dehydrated with alcohol. They were
then embedded with epoxy resin 812, sliced with LKB IV
ultramicrotome and double-stained with uranyl acetate-lead citrate.
The samples were then observed using a JEM-1220 transmission
electron microscope.
Statistical analysis
SPSS 13.0 software was used to process data; the
metrological data were expressed as the mean ± standard deviation
(χ̄+s). The independent samples t-test was used to compare the
MMP-3 and TIMP-3 expression in early and advanced gastric cancer
tissue, as well as the MMP-3:TIMP-3 ratio. The above-mentioned
hypothesis test was a two-sided test, with a test level (α) of
0.05. P<0.05 was considered to be statistically significant.
Results
Expression of MMP-3 and TIMP-3
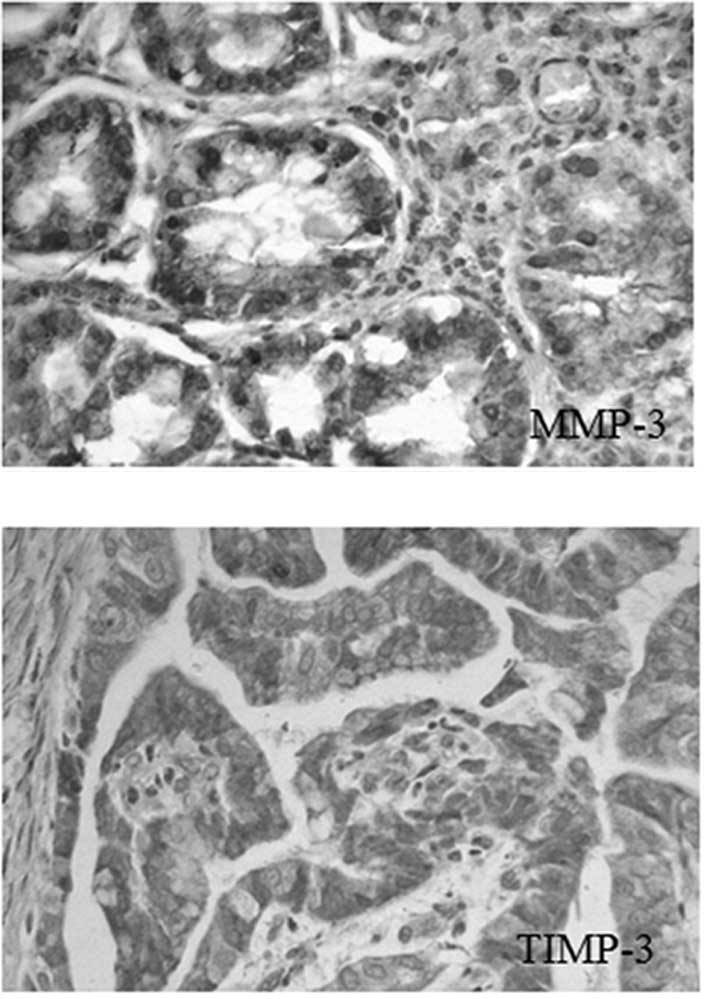
MMP-3 was mainly expressed in the cytoplasm of
cancer cells, in brownish-yellow granules. The granules with a
positive reaction of TIMP-3 were mainly expressed in the cytoplasm
of cancer cells, as shown in Fig.
1.
Comparison of MMP-3 and TIMP-3
Table I shows a
significantly decreased MMP-3 expression and increased TIMP-3
expression in gastric cancer tissue during the early stage,
compared with that in the advanced stage. The MMP-3/ratio was
therefore significantly decreased (P<0.05).
 | Table IThe average gray values of MMP-3 and
TIMP-3 in gastric cancer tissue during early and advanced
stages. |
Table I
The average gray values of MMP-3 and
TIMP-3 in gastric cancer tissue during early and advanced
stages.
| Groups | n | MMP-3 | TIMP-3 | MMP-3/TIMP-3 |
|---|
| Early-stage | 18 | 63.32±18.23 | 122.20±16.07 | 0.53±0.16 |
| Advanced-stage | 26 | 99.87±22.24 | 69.41±17.87 | 1.53±0.53 |
| t | | 5.757 | 10.032 | 7.769 |
| P-value | | 0.001 | 0.001 | 0.001 |
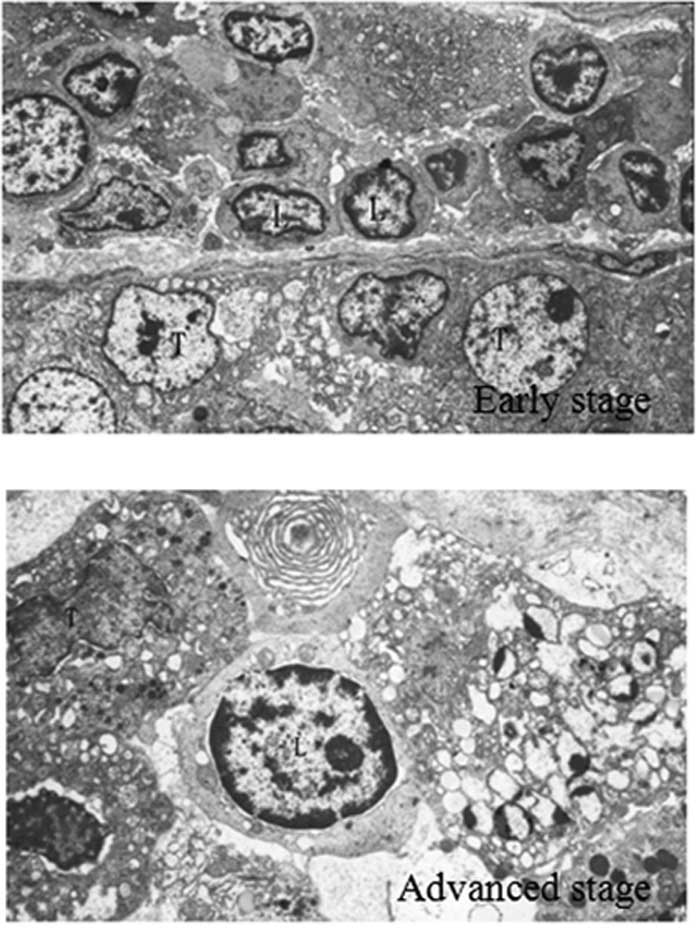
Lymphocytes and tumor cells in gastric
cancer tissue
During the early stage of gastric cancer, a
substantial lymphocyte invasion was observed in the peripheral
tissues. The lymphocytes were arranged in clusters along the
basement membrane close to each other, with moderately integrated
basement membranes. Cancer cells were observed in the nests, with
an irregular nucleus, and visible vacuolar degeneration. During the
advanced stage of gastric cancer, the basement membrane was
markedly damaged, with less lymphocyte infiltration compared to
that during the earlier stage. However, the number of tumor cells
penetrating the basement membrane increased, as shown in Fig. 2.
Discussion
Gastric cancer is one of the most common malignant
tumors, representing the second and fourth most common malignancies
in males and females, respectively, worldwide (4). In China, gastric cancer is the most
common malignant tumor, with an average annual mortality rate of
25.53 per 10 million deaths. Thus, gastric cancer is a clear threat
to human health. Consequently, the identification of new prevention
and treatment measures to improve the diagnosis and treatment
levels of gastric cancer in China is crucial.
The extracellular matrix (ECM) and basement membrane
of the tissue are natural barriers preventing tumor invasion and
metastasis. Degraded ECM and loss of integrity of the basement
membrane lead to tumor invasion and metastasis (5). In vivo, matrix
metalloproteinases (MMPs) are the key proteolytic enzymes by which
the ECM and basement membrane are degraded, thereby promoting
cancer cell invasion into the surrounding tissue (6). MMP-3 is generated by connective
interstitial cells, fibroblasts, capillary endothelial cells,
macrophages and tumor cells (7),
with extensive substrates, and is capable of degrading the basement
membrane, proteoglycans, laminin, fibronectin, and II, III, IV, V
and VI collagen (8). The unique
function of MMP-3 is to activate other types of MMP, such as MMP-2
and MMP-9 (9). The activity of
MMP-3 is specifically inhibited by TIMP-3 (10). TIMP-3 is a new member of the TIMP
family, mainly generated by the mesoblastema. TIMP-3 possesses
additional regulation sites for MMPs and is capable of promoting
the proliferation and transformation of non-transformed cells.
Moreover, it is capable of developing covalent binding with MMP
enzyme precursors or active enzymes, so as to inhibit the
activation and activity of MMP enzyme precursors (11).
Studies on tissues of nasopharyngeal carcinoma
(12), cervical cancer (13), breast cancer (14), lung cancer (15) and colon cancer (16) have shown that MMP-3 and TIMP-3
expression is correlated to the invasion and metastasis of cancer
cells. This study has shown that TIMP-3 expression in the gastric
tissues of the early-stage group was significantly higher than that
of the advanced-stage group, whereas MMP-3 expression and the
MMP-3/TIMP-3 ratio were significantly lower than the advanced-stage
group. The TEM images demonstrated increased lymphocytes and
inconspicuous tumor cells penetrating the basement membrane in the
gastric cancer tissue of the early-stage group, and decreased
lymphocytes and obvious tumor cells penetrating the basement
membrane in the advanced-stage group. This observation indicates
that MMP-3 is capable of degrading ECM and causing marked damage to
the basement membrane: The cancer cells are therefore capable of
infiltrating and growing to distant locations along the basement
membrane matrix defects and matrix space. On the other hand, the
lymphocytes surrounding the cancer area increase during the
advanced stage, with decreased defense capacity. Therefore, a large
number of cancer cells are capable of permeating the basement
membrane and causing invasion and metastasis.
In conclusion, MMP-3 and TIMP-3 are closely
correlated to the occurrence and development of gastric cancer.
However, studies regarding the relationship between the imbalance
of MMP-3 and TMIP-3 expression and the occurrence and development
of gastric cancer are in the initial stage. Further studies
regarding this relationship are therefore required.
References
|
1
|
Bi J, Lau SH, Hu L, et al: Downregulation
of ZIP kinase is associated with tumor invasion, metastasis and
poor prognosis in gastric cancer. Int J Cancer. 124:1587–1593.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zheng L, Pu J, Jiang G, Weng M, He J, Mei
H, Hou X and Tong Q: Abnormal expression of early growth response 1
in gastric cancer: association with tumor invasion, metastasis and
heparanase transcription. Pathol Int. 60:268–277. 2010. View Article : Google Scholar
|
|
3
|
Stetler-Stevenson WG: The role of matrix
metalloproteinases in tumor invasion, metastasis, and angiogenesis.
Surg Oncol Clin N Am. 10:383–392. 2001.PubMed/NCBI
|
|
4
|
Lawson JD, Sicklick JK and Fanta PT:
Gastric cancer. Curr Probl Cancer. 35:97–127. 2011. View Article : Google Scholar
|
|
5
|
Watanabe H: Extracellular matrix –
regulation of cancer invasion and metastasis [J]. Gan To Kagaku
Ryoho. 37:2058–2061. 2010.
|
|
6
|
Gialeli C, Theocharis AD and Karamanos NK:
Roles of matrix metalloproteinases in cancer progression and their
pharmacological targeting. FEBS J. 278:16–27. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jin X, Yagi M, Akiyama N, Hirosaki T,
Higashi S, Lin CY, Dickson RB, Kitamura H and Miyazaki K:
Matriptase activates stromelysin (MMP-3) and promotes tumor growth
and angiogenesis. Cancer Sci. 97:1327–1334. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zinzindohoué F, Lecomte T, Ferraz JM,
Houllier AM, Cugnenc PH, Berger A, Blons H and Laurent-Puig P:
Prognostic significance of MMP-1 and MMP-3 functional promoter
polymorphisms in colorectal cancer. Clin Cancer Res. 11:594–599.
2005.PubMed/NCBI
|
|
9
|
Fiedorczyk M, Klimiuk PA, Sierakowski S,
Domyslawska l and Chwiecko J: Correlations between serum matrix
metalloproteinase (MMP-1, MMP-3, MMP-9, MMP-13) concentrations and
markers of disease activity in early rheumatoid arthritis. Przegl
Lek. 62:1321–1324. 2005.
|
|
10
|
Pietruszewska W, Kobos J, Gryczyński M,
Durko T and Bojanowska-Pożniak K: Analysis of TIMP-1, TIMP-2 and
TIMP-3 expression as a prognostic factor of laryngeal cancer
progression. Otolaryngol Pol. 62:380–387. 2008.PubMed/NCBI
|
|
11
|
Lee MH, Atkinson S and Murphy G:
Identification of the extracellular matrix (ECM) binding motifs of
tissue inhibitor of metalloproteinases (TIMP)-3 and effective
transfer to TIMP-1. J Biol Chem. 282:6887–6898. 2007.PubMed/NCBI
|
|
12
|
Li YH, Shao JY, Li S, Zou BY, Huang HQ and
Guan ZZ: Clinical significance of quantitative analysis of serum
VEGF, CD44s, and MMP-3 protein in nasopharyngeal carcinoma. Ai
Zheng. 23:1060–1064. 2004.PubMed/NCBI
|
|
13
|
Argüello-Ramírez J, Pérez-Cárdenas E,
Delgado-Chávez R, Solorza-Luna G, Villa-Trevińo S and
Arenas-Huertero F: Matrix metalloproteinases-2, -3, and -9 secreted
by explants of benign and malignant lesions of the uterine cervix.
Int J Gynecol Cancer. 14:330–340. 2004.PubMed/NCBI
|
|
14
|
Mylona E, Magkou C, Giannopoulou I,
Agrogiannis G, Markaki S, Keramopoulos A and Nakopoulou L:
Expression of tissue inhibitor of matrix metalloproteinases
(TIMP)-3 protein in invasive breast carcinoma: relation to tumor
phenotype and clinical outcome. Breast Cancer Res. 8:R572006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mino N, Takenaka K, Sonobe M, Miyahara R,
Yanagihara K, Otake Y, Wada H and Tanaka F: Expression of tissue
inhibitor of metalloproteinase-3 (TIMP-3) and its prognostic
significance in resected non-small cell lung cancer. J Surg Oncol.
95:250–257. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Işlekel H, Oktay G, Terzi C, Canda AE,
Fűzűn M and Kűpelioğlu A: Matrix metalloproteinase-9,-3 and tissue
inhibitor of matrix metalloproteinase-1 in colorectal cancer:
relationship to clinicopathological variables. Cell Biochem Funct.
25:433–441. 2007.PubMed/NCBI
|