Introduction
Lung cancer is the leading cause of mortality
worldwide, and non-small cell lung cancer (NSCLC) accounts for
approximately 80% of all cases of lung cancer (1). The majority of NSCLC patients present
with advanced disease at the time of diagnosis, and treatment of
these patients with intensive chemotherapy does not prevent
recurrence. Therefore, most NSCLC patients become candidates for
palliative chemotherapy or radiotherapy.
Advances in chemotherapeutic agents led to new
chemotherapy strategies for NSCLC. Pemetrexed, a multitargeted
antifolate, revealed both efficacy and tolerability as an active
therapeutic agent for NSCLC patients in two Phase III trials
(2,3). Integration analysis of the two Phase
III trials indicated that the survival benefit of pemetrexed
therapy was observed only in non-squamous histology (4). This outcome revealed a new treatment
strategy of selecting the chemotherapeutic agent in accordance with
the histology.
Epidermal growth factor receptor (EGFR) is a
promising target for anticancer therapy in various tumors. In
NSCLC, newly developed EGFR-targeted anticancer agents include
EGFR-tyrosine kinase inhibitors (EGFR-TKIs), such as gefitinib and
erlotinib. EGFR-TKIs inhibit intracellular signals for the
proliferation and survival of cancer cells and have shown efficacy
in clinical practice. In 2004, two pivotal reports demonstrated
that sensitivity to EGFR-TKI therapy is significantly associated
with somatic mutations in the tyrosine kinase domain of the EGFR
gene at exons 19 and 21 (5,6). These active EGFR mutations and
clinical characteristics, including female gender, Asian ethnicity,
adenocarcinoma histology, and never or light smoker, are now
established as useful biomarkers for predicting the efficacy of
EGFR-TKIs (7–9).
Docetaxel is well established as the first agent
selected for previously treated advanced NSCLC patients. FDA
approval of docetaxel was based on two Phase III trials, TAX317 and
TAX320 (10,11). These pivotal studies demonstrated
favorable survival rates compared with best supportive care alone
or other single-agent therapies. No chemotherapeutic agents have
shown a survival benefit comparable to docetaxel for unselected
NSCLC recurrence patients. Biomarkers, such as Class III β-tubulin
expression and mRNA, are suggested to predict the efficacy of
docetaxel. However, these methods are not used in the clinic
(12,13).
To identify biomarkers that may actually be used to
predict the efficacy of docetaxel, we investigated the potential of
NSCLC histology and the favorable therapeutic effect of EGFR-TKIs
as predictive markers for second-line docetaxel therapy in our
institution.
Patients and methods
Patients
A total of 454 consecutive NSCLC patients treated
with docetaxel at the Shizuoka Cancer Center, Japan, between April
2002 and April 2009 were retrospectively reviewed. The patients
included in this study were 193 males (81%) and 46 females (19%),
with a median age of 63 years. The study included patients with
histologically or cytologically proven NSCLC who had previously
been treated with docetaxel monotherapy, following a previous
regimen of platinum doublet therapy. The study protocol was
reviewed and approved by the Institutional Review Board of the
Shizuoka Cancer Center.
Collection of data and response
evaluation
Demographic data were collected from the patients
with regard to gender, age, ECOG performance status (PS), clinical
stage, histology, and history of smoking as of the date that
docetaxel therapy started. Docetaxel was administered every 3 weeks
as a 1-h intravenous infusion of 60 mg/m2. Tumor
response was assessed as complete response (CR), partial response
(PR), stable disease (SD) or progressive disease (PD) in accordance
with the World Health Organization criteria (14). The data cut-off date was March 30,
2010.
Subgroup classification
Subgroup analyses were performed according to
histology and the clinical benefit of EGFR-TKIs following docetaxel
therapy. The histological subtypes were classified into a squamous
cell carcinoma group and a non-squamous cell carcinoma group, which
included adenocarcinoma, large-cell carcinoma and other NSCLCs not
otherwise specified. Moreover, to assess the relativity of the EGFR
mutation status and the efficacy of docetaxel therapy, we collected
data on the EGFR gene mutation status. However, only a small number
of patients could be assessed for EGFR gene mutation. It was
previously reported that the clinical benefit of EGFR-TKIs is a
useful marker for predicting active EGFR mutations (15). We considered the clinical benefit of
EGFR-TKIs as a surrogate marker of active EGFR mutations for
assessing the EGFR mutation status in practical data, and performed
an analysis. The objective clinical benefit from treatment with
EGFR-TKIs was defined as: documented PR or CR, or durable (>180
days) clinical benefit with SD after initiation of EGFR-TKIs
(15). The patients were classified
into two groups according to the clinical benefit of EGFR-TKIs
after docetaxel therapy: patients who achieved clinical benefit
from EGFR-TKIs were classified as the responder group (EGFR-R
group) and patients who did not achieve clinical benefit from
EGFR-TKIs or who were not administered EGFR-TKIs were classified as
the other group (EGFR-OTH group).
Statistical analysis
The comparison of clinical characteri stics and
response rate was performed using Pearson’s χ2 test,
two-sided Fisher’s exact test and Wilcoxon’s test, as appropriate.
Overall survival time was calculated as the number of months from
the date of docetaxel administration until the date the patient
succumbed. The progression-free survival (PFS) time was the period
from the date of docetaxel administration until the date of
progression or death, whichever occurred first. The Kaplan-Meier
method was used to calculate the median duration of overall
survival and PFS, and the log-rank test was used to compare the two
curves. The Cox proportional hazard regression test was used to
determine univariate and multivariate hazard ratios of PFS and
overall survival for docetaxel therapy. The prespecified prognostic
factors (gender, age, performance status and clinical stage) were
included in the multivariate analysis. Two-sided p-values of
<0.05 were considered to be statistically significant. Data were
analyzed using JMP 8 statistical software for Windows (SAS
Institute Inc., Cary, NC, USA).
Results
Patient demographics and clinical
characteristics
In total, 239 patients with advanced NSCLC were
treated with docetaxel as a second-line therapy following the
failure of platinum-based chemotherapy in our institute. The
patient characteristics are shown in Table I. Among the patients, 193 were male
(81%) and 46 were female (19%). The median age was 63 years, and 13
patients (13%) had a performance status of 2. Fifty-nine (25%)
patients had squamous cell carcinoma histology and 180 (75%) had
non-squamous histology. The majority of eligible patients were
current or former smokers (83%). When classified according to
histology, no significant difference was found among the
characteristics in each group. Of the 239 patients, 96 (40%)
received EGFR-TKIs after docetaxel therapy and 32 (13%) achieved
clinical benefit from EGFR-TKIs. When classified according to the
therapeutic effect of EGFR-TKIs, a significantly lower rate of male
individuals and current or former smokers was observed in the
EGFR-R group.
 | Table IPatient characteristics at
administration of docetaxel therapy. |
Table I
Patient characteristics at
administration of docetaxel therapy.
| | Histology | | Therapeutic effect of
EGFR-TKIs | |
|---|
| |
| |
| |
|---|
| Total | Sq | Non-sq | | EGFR-R | EGFR-OTH | |
|---|
|
|
| |
| |
|---|
| (n=239) | (n=59) | (n=180) | | (n=32) | (n=207) | |
|---|
| No. (%) | No. (%) | No. (%) | p-value | No. (%) | No. (%) | p-value |
|---|
| Gender |
| Male | 193 (81) | 51 (56) | 142 (79) | 0.201 | 18 (56) | 175 (85) | <0.001 |
| Female | 46 (19) | 8 (14) | 38 (21) | | 14 (44) | 32 (15) | |
| Age |
| Median (range) | 63 (23–82) | 63 (23–82) | 63.5 (45–77) | 0.391 | 64 (23–79) | 60.5 (45–82) | 0.233 |
| ECOG performance
status |
| 0 | 67 (28) | 18 (30) | 49 (27) | 0.177 | 8 (25) | 59 (28) | 0.157 |
| 1 | 142 (59) | 30 (51) | 112 (62) | | 23 (72) | 119 (58) | |
| 2 | 30 (13) | 11 (19) | 19 (11) | | 1 (3) | 29 (14) | |
| Stage |
| IIIB | 34 (14) | 7 (12) | 27 (15) | 0.549 | 1 (3) | 33 (16) | 0.057 |
| VI | 205 (86) | 52 (88) | 153 (85) | | 31 (97) | 174 (84) | |
| Histology |
| Adenocarcinoma | 163 (68) | 0 (0) | 163 (91) | <0.001 | 29 (91) | 134 (65) | 0.002 |
| Squamous | 59 (25) | 59 (100) | 0 (0) | | 1 (3) | 58 (28) | |
| Lar | 9 (4) | 0 (0) | 9 (5) | | 0 (0) | 9 (4) | |
| Other | 8 (3) | 0 (0) | 8 (4) | | 2 (6) | 6 (3) | |
| Smoking history
(smoker >10 pack/year) | 199 (83) | 133 (82) | 66 (87) | 0.311 | 19 (53) | 180 (89) | <0.001 |
Efficacy of docetaxel according to
histological type
A summary of the efficacy data for docetaxel therapy
is shown in Table II. The median
number of cycles of docetaxel in all patients was 2, and ranged
from 1 to 31. The objective response to docetaxel was obtained in
21 of the 239 patients [8.8%; 95% confidence interval (CI):
5.8–13.0%]. The median PFS, median overall survival time (MST) and
one-year survival rate of all patients were 7.8 weeks, 9.1 months
and 39.8%, respectively. In the category of histological grouping,
a significantly higher response rate was achieved in the
non-squamous group compared to the squamous group (11.1 vs. 1.7%,
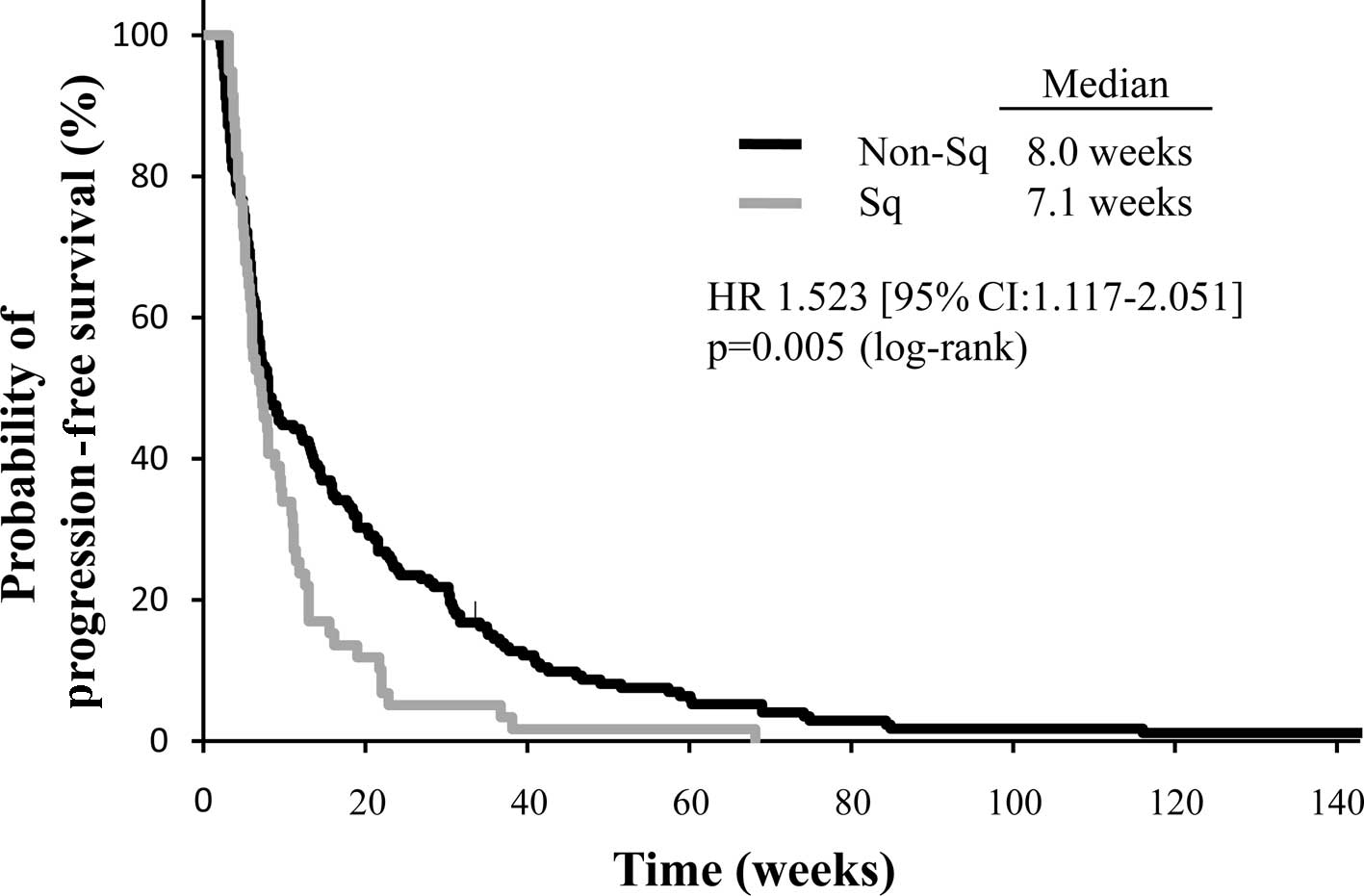
respectively; p=0.031). The median PFS was significantly shorter in
the squamous group compared with the non-squamous group: 7.1 vs.
8.0 weeks (HR, 1.523; 95% CI, 1.117–2.051; p=0.005) (Fig. 1). Similarly, survival time was
significantly shorter in the squamous group than the non-squamous
group; the MST and one-year survival rate were 8.7 months and 28.5%
vs. 9.3 months and 43.2%, respectively (HR, 1.463; 95% CI,
1.053–2.003; p=0.019).
 | Table IISummary of efficacy of docetaxel
therapy according to histology or therapeutic effect of
EGFR-TKIs. |
Table II
Summary of efficacy of docetaxel
therapy according to histology or therapeutic effect of
EGFR-TKIs.
| | Histology | Therapeutic effect of
EGFR-TKIs |
|---|
| |
|
|
|---|
| Total (n=239) | Sq (n=59) | Non-sq (n=180) | p-value | EGFR-R (n=32) | EGFR-OTH
(n=207) | p-value |
|---|
| Number of cycles,
median (range) | 2 (1–31) | 2 (1–31) | 2 (1–8) | 0.064 | 4 (1–15) | 2 (1–31) | <0.001 |
| Response to
docetaxel therapy, no. (%) | | | | | | | |
| CR | 0 (0) | 0 (0) | 0 (0) | 0.084 | 0 (0) | 0 (0) | <0.001 |
| PR | 21 (8.8) | 1 (1.7) | 20 (11.1) | | 10 (31.2) | 11 (5.3) | |
| SD | 89 (37.2) | 22 (37.3) | 67 (37.2) | | 15 (46.9) | 74 (35.7) | |
| PD | 120 (50.2) | 32 (54.2) | 88 (48.9) | | 7 (21.9) | 113 (54.6) | |
| NE | 9 (3.8) | 4 (6.8) | 5 (2.8) | | 0 (0) | 9 (4.4) | |
| Response rate (95%
CI) | 8.8 (5.8–13.0) | 1.7 (0.3–9.0) | 11.1
(7.3–16.5) | 0.031 | 31.2
(17.9–48.5) | 5.3 (3.0–9.3) | <0.001 |
| Median PFS, weeks
(95% CI) | 7.8 (6.7–9.0) | 7.1 (5.7–8.8) | 8.0 (6.7–12.1) | 0.005 | 21.0
(12.1–27.8) | 7.1 (6.4–7.8) | 0.027 |
| MST, months (95%
CI) | 9.1 (7.6–10.8) | 8.7 (6.9–10.2) | 9.3 (7.6–11.9) | 0.019 | 31.0
(25.2–42.9) | 7.6 (6.4–8.9) | <0.001 |
| One year survival,
% | 39.8 | 28.5 | 43.2 | | 87.5 | 31.6 | |
Evaluation of association between
EGFR-TKI therapeutic effect and docetaxel efficacy
After the failure of docetaxel therapy, 91
non-squamous patients were treated with EGFR-TKIs. Twenty-eight
patients (30%) demonstrated a partial response and 4 patients (4%)
achieved clinical benefit with long-term disease stabilization.
These 32 patients were included in the EGFR-R group. Sixty patients
(66%) did not respond to EGFR-TKIs and 4 patients were not
evaluable, and along with the patients who were not administered
EGFR-TKIs, the 207 patients were included in the EGFR-OTH group.
The EGFR-R group was compared with the EGFR-OTH group for
differences in efficacy of docetaxel therapy. The response rate of
second-line docetaxel therapy in the EGFR-R group was significantly
superior to that in the EGFR-OTH group (31.2 vs. 5.3%,
respectively; p<0.001) (Table
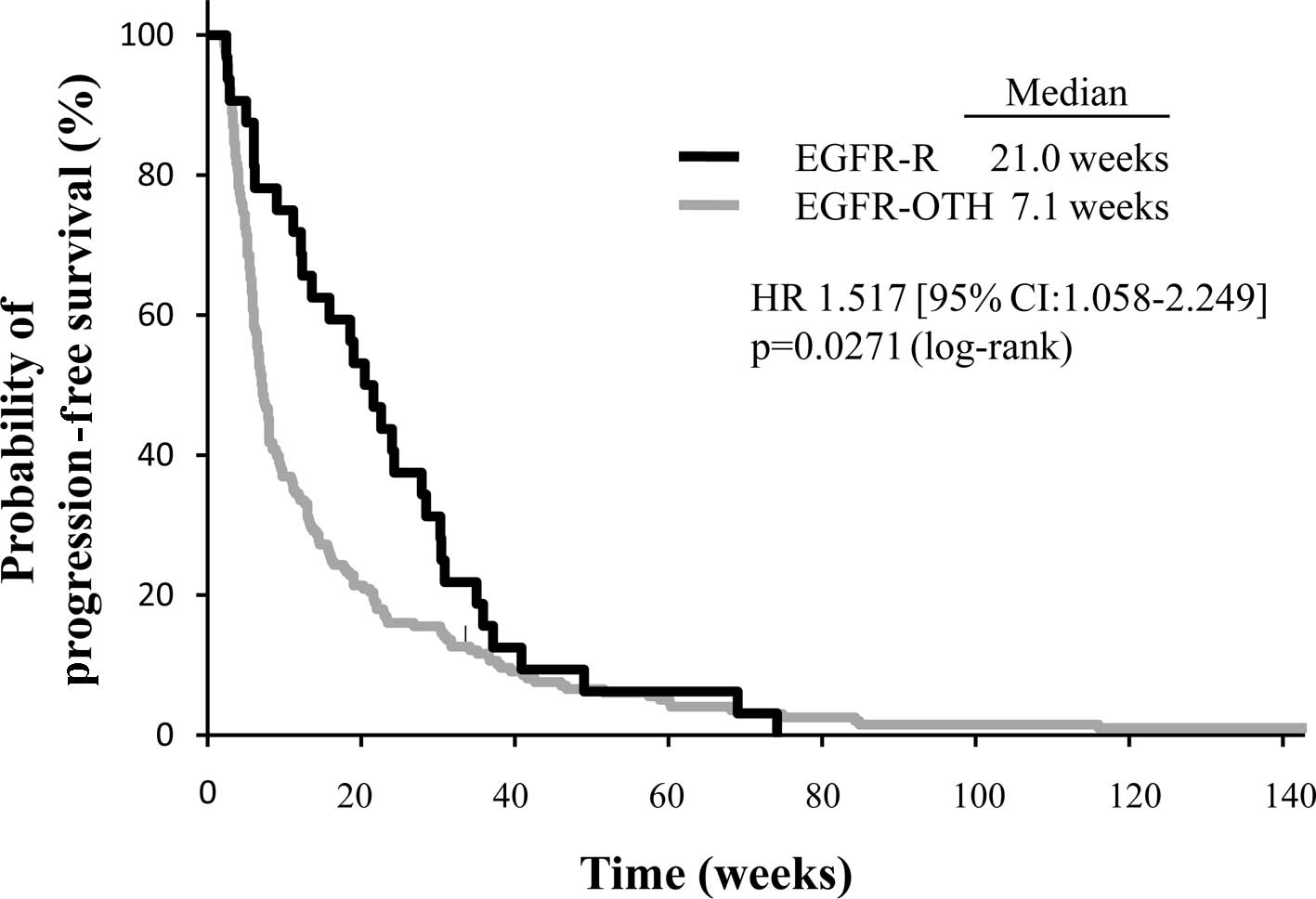
II). In addition, the median PFS in the EGFR-R group was
significantly longer than that in the EGFR-OTH group (21.0 vs. 7.1
weeks, respectively; p=0.027) (Fig.
2). Overall survival time was markedly prolonged in the EGFR-R
group compared to the EGFR-OTH group (31.0 vs. 7.6 months,
respectively; p<0.001).
Univariate and multivariate analysis of
progression-free survival in second-line docetaxel therapy
The results of the univariate and multivariate
analysis for determining the predictive factor for PFS of
second-line docetaxel therapy are shown in Table III. The univariate analysis
revealed that histology, PS and clinical efficacy of EGFR-TKIs were
significant predictive factors. In previously reported multivariate
analyses using other prognostic covariates, the clinical efficacy
of EGFR-TKIs remained a significant predictive factor for PFS of
second-line docetaxel therapy (HR 1.484, 95% CI, 1.006–2.252;
p=0.0464) (Table III).
 | Table IIIUnivariate and multivariate analysis
for progression-free survival in second-line docetaxel therapy
(n=239). |
Table III
Univariate and multivariate analysis
for progression-free survival in second-line docetaxel therapy
(n=239).
| Factors | | | Univariate | Multivariate |
|---|
| | |
|
|
|---|
| Total | PFS (weeks) | HR | 95% CI | p-value | HR | 95% CI | p-value |
|---|
| Gender |
| Female | 193 | 7.2 | 0.901 | 0.658–1.260 | 0.5351 | 0.789 | 0.563–1.127 | 0.1893 |
| Male | 46 | 8.0 | | | | | | |
| Age |
| <70 | 176 | 7.2 | 0.816 | 0.605–1.086 | 0.1660 | 0.912 | 0.669–1.229 | 0.5538 |
| >70 | 63 | 8.0 | | | | | | |
| Histology |
| Non-squamous | 180 | 8.0 | 1.523 | 1.117–2.051 | 0.0084 | 1.377 | 0.995–1.882 | 0.0533 |
| Squamous | 59 | 7.1 | | | | | | |
| PS |
| 0–1 | 209 | 8.0 | 1.739 | 1.157–2.522 | 0.0088 | 1.482 | 0.968–2.198 | 0.0688 |
| 2 | 30 | 6.0 | | | | | | |
| Stage |
| IIIB | 37 | 11.8 | 1.314 | 0.933–1.905 | 0.1206 | 1.264 | 0.884–1.855 | 0.2027 |
| IV | 202 | 7.0 | | | | | | |
| EGFR-TKI
therapy |
| Responder | 32 | 21.0 | 1.517 | 1.058–2.249 | 0.0222 | 1.484 | 1.006–2.252 | 0.0464 |
| Other | 207 | 7.1 | | | | | | |
Discussion
This is the first report to demonstrate the efficacy
of docetaxel therapy for previously treated NSCLC patients
according to NSCLC histology and the therapeutic effect of
EGFR-TKIs. In the non-squamous patient group, the response rate and
survival benefit of docetaxel therapy were significantly superior
compared to those in the squamous group. This result is similar to
the previously reported difference in efficacy of pemetrexed
therapy according to histology. Another finding of our analysis was
that the efficacy of docetaxel is associated with sensitivity to
EGFR-TKIs following docetaxel administration. Patients who achieved
a clinical benefit from EGFR-TKIs revealed a significantly higher
response rate and longer progression-free survival time with
second-line docetaxel therapy. These results suggest that the
efficacy of docetaxel therapy may be predicted by patient
characteristics, including histology and the therapeutic effect of
EGFR-TKIs.
Docetaxel is the most frequently investigated agent
for previously treated NSCLC patients, but the difference in its
efficacy according to histology was not observed in earlier large
randomized studies. In this study, the efficacy of docetaxel was
found to be significantly superior for patients with non-squamous
cell carcinoma histology. This discrepancy may simply be attributed
to the retrospective analysis in a single institution. Another
possible explanation is that the administration dose for docetaxel
is lower in Japan compared to that in the USA and Europe. In Japan,
the recommended dose for docetaxel was determined as 60
mg/m2 every three weeks due to the similar efficacy
shown with this dose in two Phase II studies (16,17).
Another noteworthy finding is the relationship
between the therapeutic effect of EGFR-TKIs and docetaxel efficacy
in the second-line setting. A number of large Phase III trials and
retrospective analyses suggested that patients with active EGFR
mutations are sensitive to cytotoxic agents. In the V15–32 trial,
the response to docetaxel differed significantly by EGFR mutation
status (active mutation vs. wild-type; 46 vs. 0%, respectively) in
a small subset analysis (18). The
IPASS study also showed similar indications in first-line
chemotherapy; the combination therapy of carboplatin plus
paclitaxel had relatively favorable efficacy for patients with the
active EGFR mutation compared to EGFR wild-type patients (active
mutation vs. wild type; 47.3 vs. 23.5%, respectively). In addition,
three retrospective analyses reported that the efficacy of
chemotherapy by cytotoxic agents tended to be high in patients with
active EGFR mutations (19–21). These data suggest that the EGFR gene
mutation would be a useful predictive biomarker for treatment with
cytotoxic agents. The clinical benefit of EGFR-TKIs suggests that
it is a useful method for predicting active EGFR mutations
(15). Therefore, the results of
our analysis suggest that active EGFR mutation is a predictive
factor for the efficacy of docetaxel treatment. The EGFR gene
mutation is one of the major causes of oncogenic addiction. If the
biological features of cancer cells are significantly affected by
the addicted oncogene, there is a possibility that the
effectiveness of chemotherapy may differ according to the EGFR gene
mutation. However, basic data for explaining this hypothesis are
not currently available, and further molecular biological studies
are required.
In the univariate and multivariate analysis,
sensitivity to EGFR-TKIs was extracted as a predictive factor for
the efficacy of docetaxel. On the other hand, histological type was
extracted as a significant factor in the univariate analysis;
however, it did not remain a significant factor in the
multi-variate analysis with other covariates. A global Phase III
study revealed that the efficacy of EGFR-TKI is high for
adenocarcinoma histology. Adenocarcinoma histology and sensitivity
to EGFR-TKIs are strong confounding factors, which is why it was
not extracted as an independent predictive factor in the
multivariate analysis. In contrast, results of this study showed
that the efficacy of docetaxel is markedly high in patients with
clinical benefit from EGFR-TKIs. There is a possibility that
sensitivity to docetaxel in adenocarcinoma patients depends on the
sensitivity to docetaxel in the EGFR-R group. It is well known that
there are differences in the frequency of active EGFR mutations
among different ethnic groups, i.e., this frequency is extremely
high in Asian patients with lung adenocarcinoma compared with
American patients (22). This fact
may be another reason for the difference in efficacy of docetaxel
according to histology not being observed in the large randomized
Phase III study in the USA, which was observed in this analysis of
patients in a Japanese institute.
The major limitation of the present study is that it
is based on a retrospective analysis of patients in a single
institute, thus there may be a potential bias with regard to
patient selection and follow-up procedure. Furthermore, the
analysis of EGFR status is based solely on the therapeutic effect
of EGFR-TKI. We were able to examine the EGFR gene mutation status
for only 21 patients; active EGFR mutation was observed in 6 cases
and wild-type in 15 cases. No association was determined between
the efficacy of docetaxel and active EGFR mutations due to lack of
computing power for the statistical analysis (data not shown).
There are two reasons for the difficulty experienced in examining a
sufficient number of EGFR gene mutations for statistical analysis.
First, the EGFR gene mutation had not been examined in clinical
practice for most of the target period. Second, assessment of the
EGFR gene mutation by polymerase chain reaction and direct
sequencing methods requires proper preservation of tumor tissue.
Hindrances in the assessment of patient EGFR mutation status are
often experienced due to economic constraints or difficulty in
collecting tumor specimens in clinical practice. Therefore, we
frequently use the patient’s background data such as smoking
history and adenocarcinoma histology as predictive factors for
EGFR-TKI efficacy; the so-called IPASS population. Further analysis
is necessary to show the association of the EGFR mutation status
and the efficacy of docetaxel therapy, such as through prospective
trials based on EGFR mutation status. However, our results support
the significant hypothesis that the effectiveness of chemotherapy
differs according to the EGFR gene mutation status. Therefore, EGFR
gene mutation status, which has already been established as a
predictive marker for chemotherapy by cytotoxic agents, may be
useful for predicting the efficacy of docetaxel.
In this analysis, we presented two major results.
First, the efficacy of docetaxel is superior in non-squamous
patients; and second, it produces favorable results in patients who
achieved clinical benefit from EGFR-TKIs. This retrospective
analysis is the first to suggest that the efficacy of docetaxel
differs according to the histology and therapeutic effect of
EGFR-TKIs. These findings provide physicians with a crucial basis
for selecting agents for second-line therapy.
In conclusion, this retrospective study suggests
that non-squamous histology and the favorable therapeutic effect of
EGFR-TKIs are useful markers for predicting the efficacy of
docetaxel as a second-line therapy for NSCLC. Confirmation of these
observations requires further investigation through prospective
clinical trials and basic molecular biology analyses.
Acknowledgements
The authors express their appreciation to the staff
of the Department of Pathology at the Shizuoka Cancer Center for
their histological diagnosis and technical assistance in the
analysis of EGFR mutations.
References
|
1
|
Carney DN and Hansen HH: Non-small-cell
lung cancer - stalemate or progress? N Engl J Med. 343:1261–1262.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hanna N, Shepherd FA, Fossella FV, et al:
Randomized Phase III trial of pemetrexed versus docetaxel in
patients with non-small-cell lung cancer previously treated with
chemotherapy. J Clin Oncol. 22:1589–1597. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Scagliotti GV, Parikh P, von Pawel J, et
al: Phase III study comparing cisplatin plus gemcitabine with
cisplatin plus pemetrexed in chemotherapy-naive patients with
advanced-stage non-small-cell lung cancer. J Clin Oncol.
26:3543–3551. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Scagliotti G, Hanna N, Fossella F, et al:
The differential efficacy of pemetrexed according to NSCLC
histology: a review of two Phase III studies. Oncologist.
14:253–263. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lynch TJ, Bell DW, Sordella R, et al:
Activating mutations in the epidermal growth factor receptor
underlying responsiveness of non-small-cell lung cancer to
gefitinib. N Engl J Med. 350:2129–2139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Paez JG, Janne PA, Lee JC, et al: EGFR
mutations in lung cancer: correlation with clinical response to
gefitinib therapy. Science. 304:1497–1500. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mok TS, Wu YL, Thongprasert S, et al:
Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N
Engl J Med. 361:947–957. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mitsudomi T, Morita S, Yatabe Y, et al:
Gefitinib versus cisplatin plus docetaxel in patients with
non-small-cell lung cancer harbouring mutations of the epidermal
growth factor receptor (WJTOG3405): an open label, randomised phase
3 trial. Lancet Oncol. 11:121–128. 2010. View Article : Google Scholar
|
|
9
|
Maemondo M, Inoue A, Kobayashi K, et al:
Gefitinib or chemotherapy for non-small-cell lung cancer with
mutated EGFR. N Engl J Med. 362:2380–2388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shepherd FA, Dancey J, Ramlau R, et al:
Prospective randomized trial of docetaxel versus best supportive
care in patients with non-small-cell lung cancer previously treated
with platinum-based chemotherapy. J Clin Oncol. 18:2095–2103.
2000.
|
|
11
|
Fossella FV, DeVore R, Kerr RN, et al:
Randomized phase III trial of docetaxel versus vinorelbine or
ifosfamide in patients with advanced non-small-cell lung cancer
previously treated with platinum-containing chemotherapy regimens.
The TAX 320 Non-Small Cell Lung Cancer Study Group. J Clin Oncol.
18:2354–2362. 2000.
|
|
12
|
Hayashi Y, Kuriyama H, Umezu H, et al:
Class III β-tubulin expression in tumor cells is correlated with
resistance to docetaxel in patients with completely resected
non-small-cell lung cancer. Intern Med. 48:203–208. 2009.
|
|
13
|
Wang LF, Yin HT, Qian XP, et al: β-Tubulin
III mRNA expression and docetaxel sensitivity in non-small cell
lung cancer. Clin Invest Med. 32:E2782009.
|
|
14
|
Miller AB, Hoogstraten B, Staquet M and
Winkler A: Reporting results of cancer treatment. Cancer.
47:207–214. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jackman D, Pao W, Riely GJ, et al:
Clinical definition of acquired resistance to epidermal growth
factor receptor tyrosine kinase inhibitors in non-small-cell lung
cancer. J Clin Oncol. 28:357–360. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Onoshi T, Watanabe K, Furuse K, et al:
Late phase II trial of RP56976 (docetaxel) in patients with
non-small-cell lung cancer. Gan To Kagaku Ryoho. 22:59–65.
1995.PubMed/NCBI
|
|
17
|
Yokoyama A, Kurita Y, Watanabe K, et al:
Early phase II clinical study of RP56976 (docetaxel) in patients
with primary pulmonary cancer: Docetaxel Cooperative Study Group
for Lung Cancer. Gan To Kagaku Ryoho. 21:2609–2616. 1994.PubMed/NCBI
|
|
18
|
Maruyama R, Nishiwaki Y, Tamura T, et al:
Phase III study, V-15-32, of gefitinib versus docetaxel in
previously treated Japanese patients with non-small-cell lung
cancer. J Clin Oncol. 26:4244–4252. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee KH, Han SW, Hwang PG, et al: Epidermal
growth factor receptor mutations and response to chemotherapy in
patients with non-small-cell lung cancer. Jpn J Clin Oncol.
36:344–350. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hotta K, Kiura K, Toyooka S, et al:
Clinical significance of epidermal growth factor receptor gene
mutations on treatment outcome after first-line cytotoxic
chemotherapy in Japanese patients with non-small cell lung cancer.
J Thorac Oncol. 2:632–637. 2007. View Article : Google Scholar
|
|
21
|
Wu SG, Yang CH, Yu CJ, et al: Good
response to pemetrexed in patients of lung adenocarcinoma with
epidermal growth factor receptor (EGFR) mutations. Lung Cancer.
72:333–339. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rudin CM, Avila-Tang E, Harris CC, et al:
Lung cancer in never smokers: molecular profiles and therapeutic
implications. Clin Cancer Res. 15:5646–5661. 2009. View Article : Google Scholar : PubMed/NCBI
|