Introduction
Intrahepatic cholangiocarcinomas (ICCs) arise from
the epithelial cells lining the intrahepatic biliary tree.
Macroscopically, ICCs are divided into three types; the
mass-forming (MF), the periductal-infiltrating (PI) and the
intraductal growth (IG) types (1–3).
MF-type ICCs form masses in the hepatic parenchyma, while the
PI-type grow along bile ducts and the IG-type demonstrate
intraductal polypoid or papillary growth.
ICCs are further classified into hilar and
peripheral ICCs. Hilar ICCs evolve from the lining cholangiocytes
of the large collecting bile ducts in the hilum or the peribiliary
glands around them, and their histological characteristics are
similar to those of extrahepatic cholangiocarcinomas (4). On the other hand, peripheral ICCs
develop from the lining epithelial cells of small collecting bile
ducts, interlobular bile ducts, bile ductules or canals of Hering
(3,4). Peripheral ICCs arising from
interlobular bile ducts, bile ductules or canals of Hering are
usually MF-type ICCs, whereas ICCs arising from the large
collecting bile ducts are MF-, PI- or IG-type (3,4).
Hepatic progenitor cells are thought to be capable
of differentiating into hepatocytes and cholangiocytes, which
reside in the most peripheral branches of the biliary tree; i.e.,
the bile ductules and canals of Hering (5). Therefore, MF-type peripheral ICCs,
which arise from bile ductules and canals of Hering, may express
hepatocyte markers. In addition, cholangiocytes have been shown to
be capable of transforming into hepatocytes, suggesting that
hepatocyte markers are expressed by certain hilar and peripheral
ICCs (6). Various studies reported
the rare expression of hepatocyte markers such as α-fetoprotein
(AFP) or HepPar-1 antigen in ICCs (7–10).
Furthermore, D'Errico et al (11) reported that 4 of 6 peripheral ICCs
expressed albumin mRNA. Therefore, in the present study, the
expression of hepatocyte markers in 14 MF-type peripheral and 14
PI-type hilar ICCs was examined. Arginase-1 was used as a
hepatocyte marker in addition to AFP and HepPar-1 antigen, since
arginase-1 has been reported to be a more sensitive hepatocyte
marker than HepPar-1 (12).
Materials and methods
Subjects
Specimens of 14 hilar and 14 peripheral ICCs were
used for this study. The specimens were obtained from liver tumors
resected at Meiwa General Hospital, Nippon Steel Hirohata Hospital,
and the hospital attached to Hyogo College of Medicine, Japan,
between 1988 and 2010. Written consent was obtained from each
patient prior to surgery, and anonymous usage of tissue samples for
pathological studies was permitted. The number of male and female
patients with hilar ICCs and peripheral ICCs were 5 and 9, and 10
and 4, respectively.
Samples
The surgically obtained tumors were fixed in 10%
0.01 M phosphate-buffered formalin (pH 7.4) and cut through the
largest area; several samples, including those with the largest
area, were prepared and embedded in paraffin. Sections (5 μm) of
these samples were used for H&E staining, periodic acid Schiff
reaction (PAS) staining and immunohistochemical analysis.
Immunohistochemistry
The sources of antibodies and their dilutions were
as follows: anti-human HepPar-1 antigen mouse monoclonal antibody
(OHC1E5) (25-fold dilution; Dako Japan, Tokyo, Japan), anti-human
cytokeratin (CK)-7 mouse monoclonal antibody (OV-TL12/30) (100-fold
dilution; Dako Japan), anti-human CK-19 mouse monoclonal antibody
(RCK108) (100-fold dilution; Dako Japan), anti-human AFP rabbit
polyclonal antibody (100-fold dilution; Dako Japan), anti-human
neural cell adhesion molecule (N-CAM) mouse monoclonal antibody
(1B6) (pre-diluted; Nichirei Bioscience, Tokyo, Japan) and
anti-human arginase-1 rabbit polyclonal antibody (500-fold
dilution; Sigma-Aldrich Japan, Tokyo, Japan). The antibodies were
diluted with 0.01 M phosphate-buffered saline (PBS) (pH 7.4)
containing 1% bovine serum albumin (BSA).
The antigen retrieval procedure for the
immunohistochemical analysis was: autoclave treatment at 121°C for
5 min in a target retrieval solution (pH 9.0) (Dako Japan) for
CK-19, autoclave treatment at 121°C for 5 min in a target retrieval
solution (Dako Japan) for HepPar-1 antigen, CK-7, AFP and N-CAM;
boiling in a citrate buffer (pH 6.0) (Mitsubishi Chemical Medicine
Corporation, Tokyo, Japan) at 85–90°C for 3 min and cooling at room
temperature for arginase-1.
To block the internal peroxidase activity and
non-specific binding of the primary antibodies, sections were
treated with 0.35% hydrogen peroxide in methanol at room
temperature for 15 min and with PBS containing 1% BSA and 0.1%
Tween-20 at room temperature for 30 min, respectively.
Immunohistochemical staining was carried out using an Envision™+
dual link system (Dako Japan) with a 3,3′-diaminobenzidine (DAB)
solution (Nichirei Bioscience). Immunostaining was graded according
to the proportion of positive cells (p): -, p<1%; 1+,
1%≤p<5%; 2+, 5%≤p<10%; 3+, 10%≤p<40%; 4+, 40%≤p<70%;
5+, p≥70%.
Double immunostaining of CK-7 and
HepPar-1 antigen
Sections were first immunostained for CK-7 as
described above. These sections were then treated in an autoclave
at 121°C for 5 min in a target retrieval solution (Dako Japan) for
antigen retrieval and denaturation of the attached antibody and
secondary antibody-conjugated horseradish peroxidase, and treated
with PBS containing 1% BSA and 0.1% Tween-20 at room temperature
for 30 min to block any non-specific binding of the anti-HepPar-1
antigen antibody. The sections were then divided into two groups;
one group was incubated with the anti-HepPar-1 antigen antibody,
whereas the other group was not. The antibody bound to the HepPer-1
antigen was detected using a Histofine® Simple Stain AP
(multi) kit (Nichirei Bioscience) with alkaline phosphatase- and
secondary antibody-conjugated polymer, and a new fuchsin substrate
solution (Nichirei Bioscience) to which levamisole (Dako Japan) was
added to block the internal alkaline peroxidase activity.
Statistical analysis
The immunohistochemical staining was analyzed by the
Chi-square test using the StatMate III software program for Windows
(ATMS, Tokyo, Japan). P<0.05 was considered to be statistically
significant.
Results
Table I shows the
macroscopic type and major histology of the 14 hilar and 14
peripheral ICCs examined in the study, and the results of the PAS
and immunohistochemical staining of these ICCs. All hilar ICCs were
PI-type, growing along the intrahepatic large bile ducts, and all
peripheral ICCs were MF-type. The histological classification was
performed according to Nakajima et al (13). The major histology of ICCs was well-
or moderately differentiated tubular adenocarcinoma, poorly
differentiated adenocarcinoma or papillary adenocarcinoma. Among
the 14 hilar ICCs, only one demonstrated the major histology of
poorly differentiated adenocarinoma, while among the 14 peripheral
ICCs, 7 demonstrated poorly differentiated adenocarcinoma. PAS
staining revealed that all hilar and peripheral ICCs produced
mucus, but that less mucus was produced by peripheral ICCs than by
hilar ICCs.
 | Table IMacroscopic type, major histology, PAS
staining and immunohistochemical staining of the hilar and
peripheral intra-hepatic cholangiocarcinomas. |
Table I
Macroscopic type, major histology, PAS
staining and immunohistochemical staining of the hilar and
peripheral intra-hepatic cholangiocarcinomas.
| A, Hilar intrahepatic
cholangiocarcinoma. |
|---|
|
|---|
| No. | Macroscopic type | Major histology | PAS | CK-7 | CK-19 | AFP | HepPar-1 | Arginase-1 | N-CAM |
|---|
| 1 | PI | Pap | (+) | 5+ | 5+ | – | – | 3+ | – |
| 2 | PI | Por | (+) Focal | 5+ | 5+ | – | – | – | – |
| 3 | PI | Tub1 | (+) | 5+ | 5+ | – | – | – | – |
| 4 | PI | Tub1 | (+) | 5+ | 5+ | – | – | – | – |
| 5 | PI | Tub1 | (+) | 5+ | 5+ | – | – | 1+ | – |
| 6 | PI | Tub1 | (+) | 5+ | 5+ | – | – | 1+ | – |
| 7 | PI | Tub1 | (+) | 5+ | 5+ | – | 2+ | – | – |
| 8 | PI | Tub1 | (+) | 5+ | 5+ | – | – | 1+ | – |
| 9 | PI | Tub1 | (+) Focal | 4+ | 4+ | – | – | 1+ | – |
| 10 | PI | Tub1 | (+) | 5+ | 5+ | – | – | – | – |
| 11 | PI | Tub1 | (+) | 5+ | 5+ | – | – | 3+ | – |
| 12 | PI | Tub2 | (+) | 5+ | 5+ | – | 4+ | 3+ | – |
| 13 | PI | Tub2 | (+) | 5+ | 5+ | – | – | 3+ | 1+ |
| 14 | PI | Tub2 > Por | (+) | 5+ | 5+ | – | – | – | – |
|
| B, Peripheral
intrahepatic cholangiocarcinoma. |
|
| No. | Macroscopic
type | Major
histology | PAS | CK-7 | CK-19 | AFP | HepPar-1 | Arginase-1 | N-CAM |
|
| 1 | MF | Pap > Tub2 | (+) | 4+ | 4+ | 3+ | 3+ | 2+ | – |
| 2 | MF | Por | (+) | 5+ | 3+ | – | – | – | – |
| 3 | MF | Por | (+) Focal | 5+ | 5+ | – | – | 3+ | – |
| 4 | MF | Por | (+) | 5+ | 5+ | – | – | 3+ | – |
| 5 | MF | Por | (+) | 5+ | 5+ | – | – | 3+ | 1+ |
| 6 | MF | Por | (+) | 2+ | 4+ | – | – | 1+ | – |
| 7 | MF | Por | (+) | 5+ | 5+ | – | – | – | – |
| 8 | MF | Por > Tub2 | (+) Focal | 5+ | 5+ | – | – | 2+ | 5+ |
| 9 | MF | Tub1 | (+) | 5+ | 4+ | – | – | 3+ | – |
| 10 | MF | Tub1 | (+) Focal | 4+ | 5+ | – | – | 4+ | – |
| 11 | MF | Tub2 > Tub1 | (+) | 5+ | 4+ | – | – | 3+ | – |
| 12 | MF | Tub2 | (+) Focal | 5+ | 5+ | 2+ | – | 3+ | 4+ |
| 13 | MF | Tub2 | (+) Focal | 5+ | 3+ | – | – | – | – |
| 14 | MF | Tub2 | (+) | 5+ | 5+ | – | – | 1+ | – |
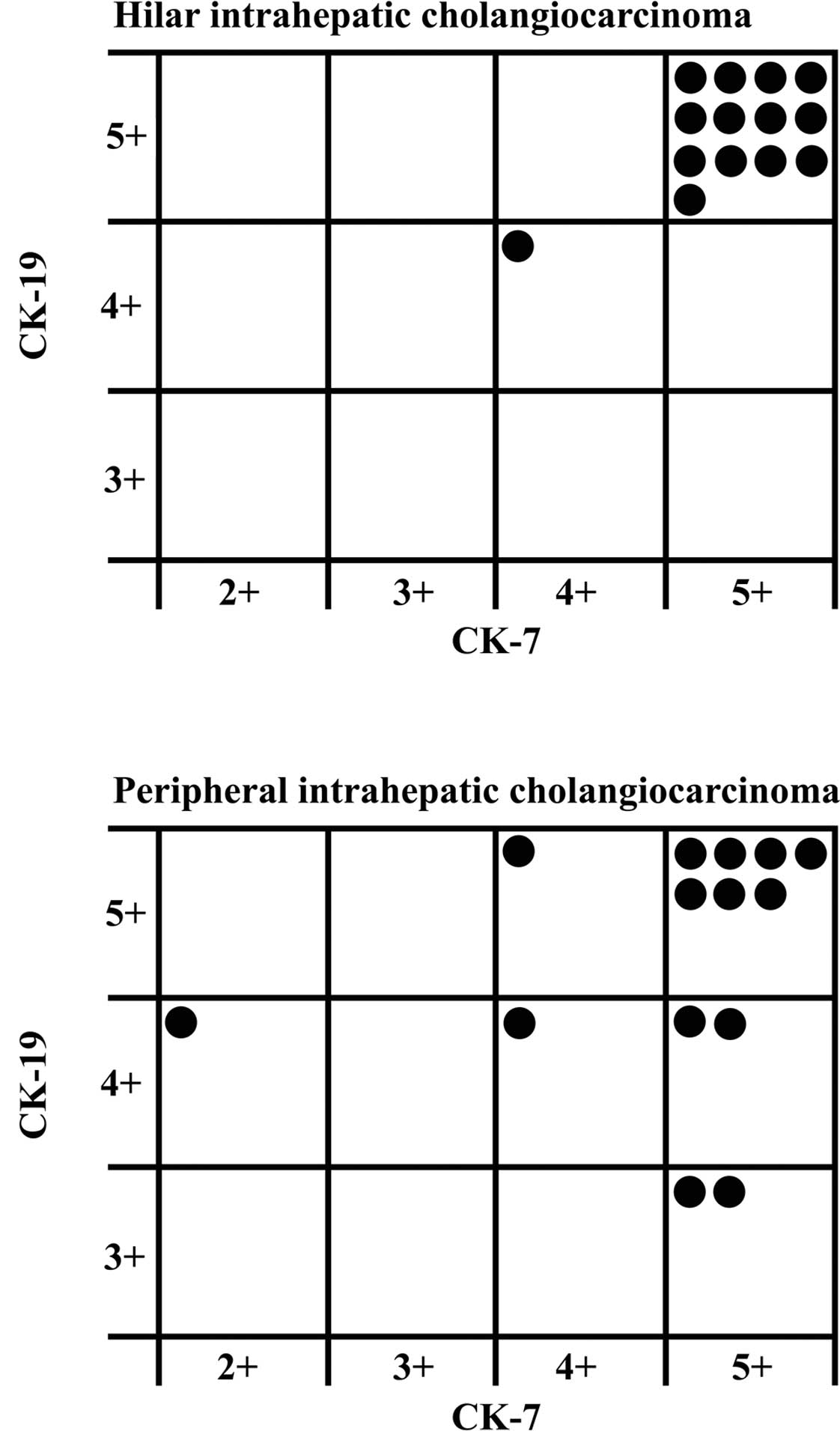
Fig. 1 shows the
grade of immunostaining for CK-19 and CK-7 in each hilar or
peripheral ICC. Grade 5 immunostaining for CK-19 and CK-7 was
observed in 13 (92.9%) of the 14 hilar ICCs. Conversely, only 7
(50%) of the 14 peripheral ICCs revealed grade 5 immunostaining for
both CK-19 and CK-7.
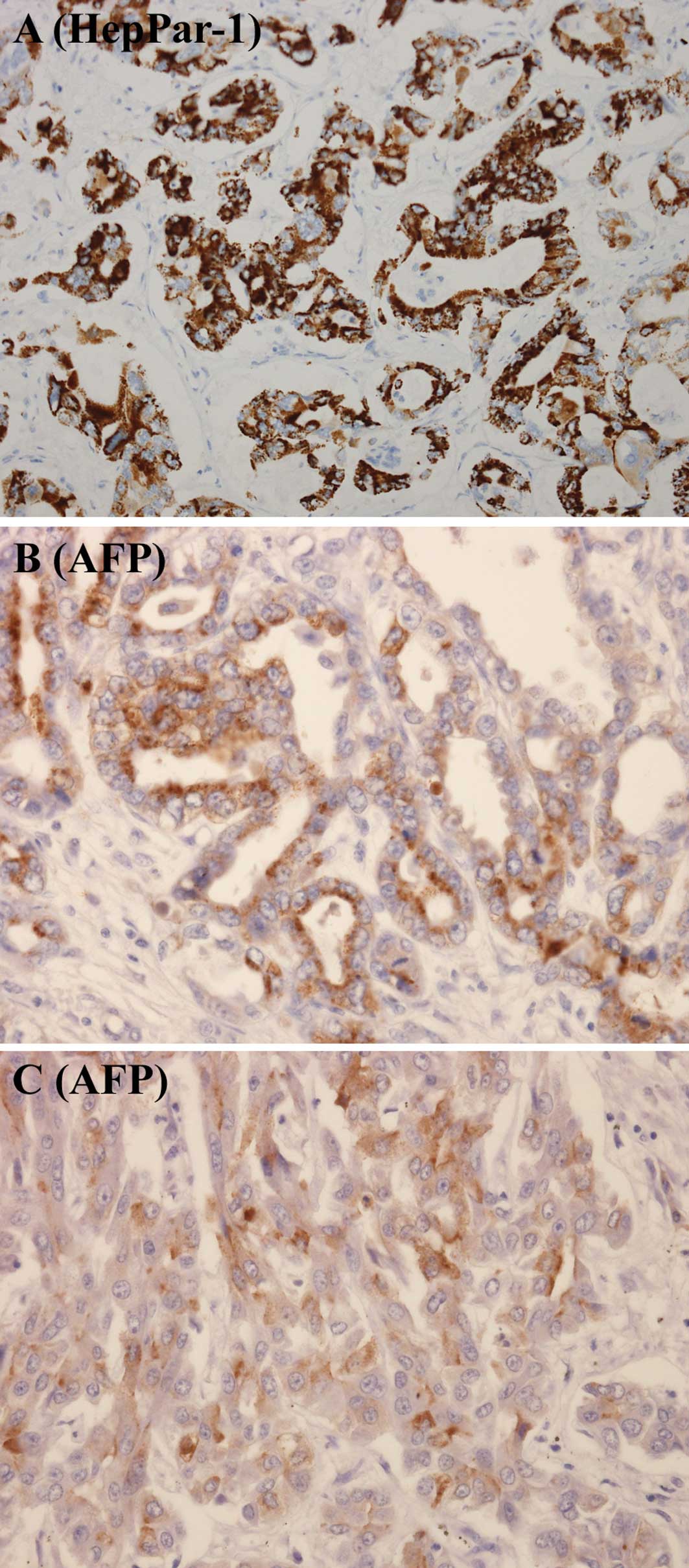
Rare hilar and peripheral ICCs expressed AFP or
HepPar-1 antigen (Table II,
Fig. 2A-C). None of the hilar ICCs
revealed positive immunostaining for AFP, and 2 of the hilar ICCs
revealed positive immunostaining for HepPar-1 antigen. Positive
immunostaining for AFP and HepPar-1 antigen was found in 2 and 1
peripheral ICCs, respectively. AFP or HepPar-1 antigen was
expressed in 2 (14.3%) and 2 (14.3%) of the hilar and peripheral
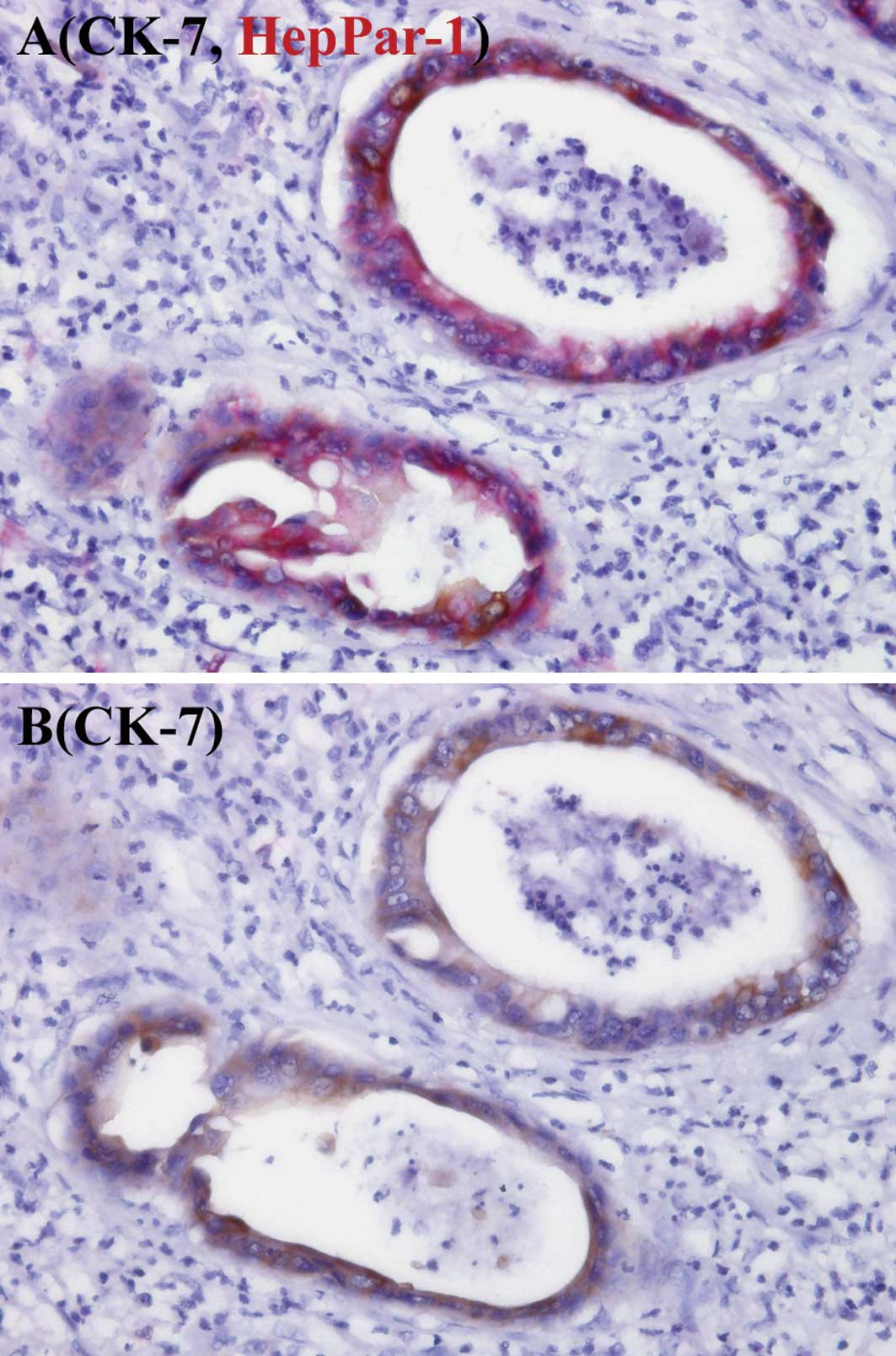
ICCs, respectively. Fig. 3 shows
the co-expression of CK-7 and HepPar-1 antigen in cancer cells.
 | Table IIExpression of AFP or HepPar-1 antigen
in 14 hilar and 14 peripheral intrahepatic cholangiocarcinomas
(ICCs). |
Table II
Expression of AFP or HepPar-1 antigen
in 14 hilar and 14 peripheral intrahepatic cholangiocarcinomas
(ICCs).
| Marker | Hilar ICCs | Peripheral
ICCs |
|---|
|
|
|
|---|
| No. (%) | Each grade | No. (%) | Each grade |
|---|
| AFP | 0 (0) | – | 2 (14.3) | 3+, 2+ |
| HepPar-1 | 2 (14.3) | 4+, 2+ | 1 (7.1) | 3+ |
| AFP or
HepPar-1 | 2 (14.3) | 4+, 2+ | 2 (14.3) | 3+, 2+ |
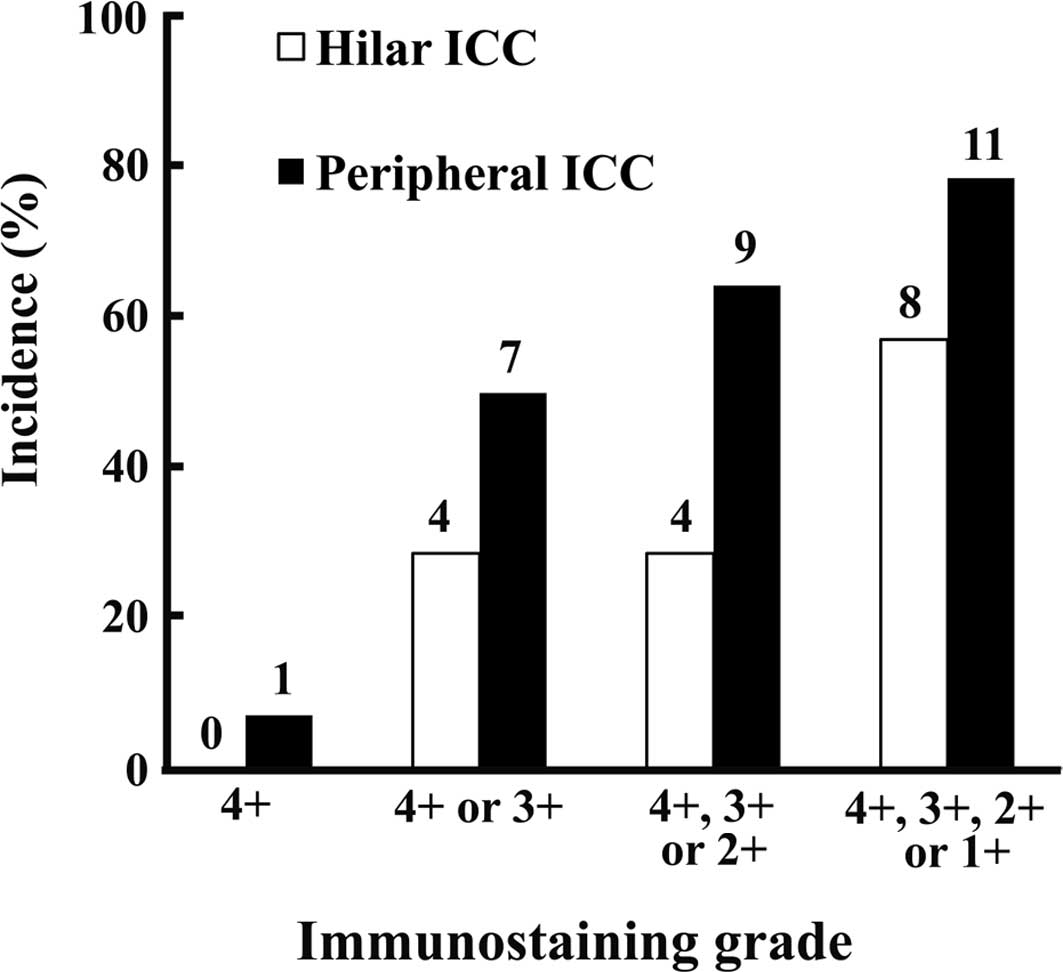
Fig. 4 shows the
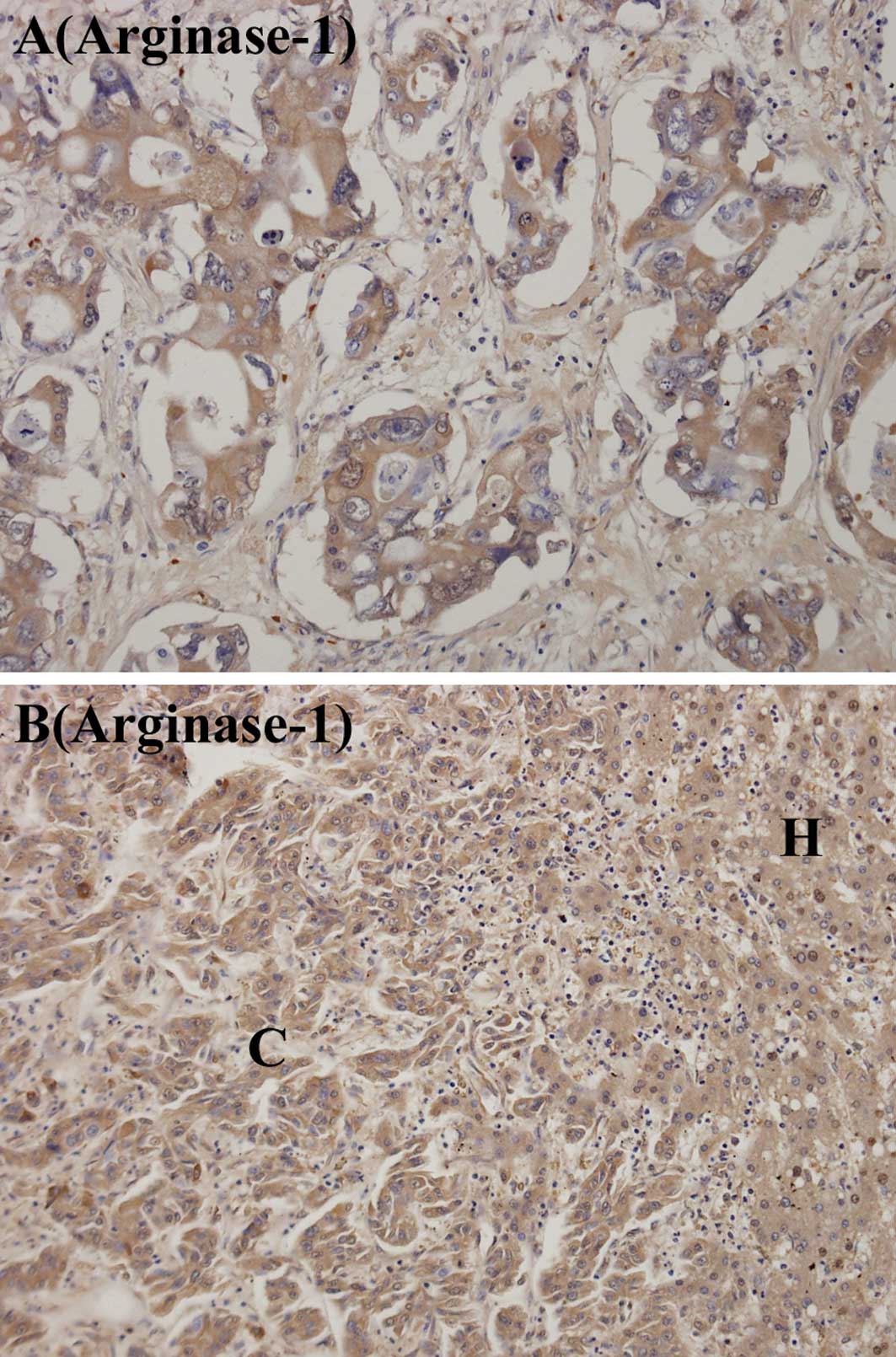
immunostaining grades for arginase-1 in the hilar and peripheral
ICCs, and Fig. 5 shows the
immuno-staining for arginase-1. Immunostaining for arginase-1 at
grades 1, 2, 3 or 4 was found in 8 (57.1%) of the hilar ICCs and 11
(78.6%) of the peripheral ICCs. Immunostaining for arginase-1 at
grades 3 or 4 was found in 4 (28.6%) of the hilar ICCs and in 7
(50%) of the peripheral ICCs. No difference was observed in the
expression of arginase-1 between peripheral ICCs showing the major
histology of poorly differentiated adenocarcinoma and those showing
the major histology of well- or moderately differentiated tubular
adenocarcinoma or papillary adenocarcinoma (Table III). All of the hilar ICCs
expressing arginase-1 (8 of the 14 hilar ICCs) showed major
histology of well- or moderately differentiated tubular
adenocarcinoma or papillary adenocarcinoma (Table I).
 | Table IIIExpression of arginase-1 in
peripheral intrahepatic cholangiocarcinomas. |
Table III
Expression of arginase-1 in
peripheral intrahepatic cholangiocarcinomas.
| Grade | Well-or moderately
differentiated tubular adenocarcinoma or papillary adenocarcinoma
(n=7) (%) | Poorly
differentiated adenocarcinoma (n=7) (%) |
|---|
| 4+ | 1 (14.3) | 0 (0) |
| 4+ or 3+ | 4 (57.1) | 3 (42.9) |
| 4+, 3+ or 2+ | 5 (71.4) | 4 (57.1) |
| 4+, 3+, 2+ or
1+ | 6 (85.7) | 5 (71.4) |
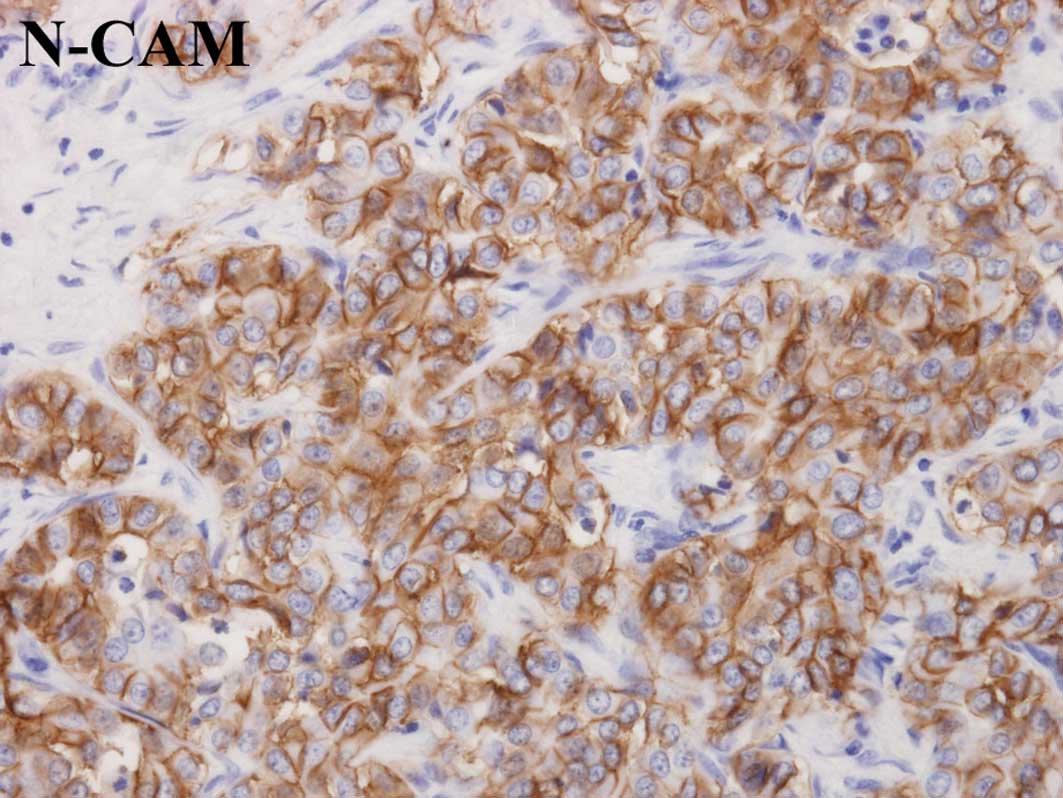
The neural cell adhesion molecule (N-CAM) has been
reported to be a possible marker of hepatic progenitor cells which
are assumed to reside in the bile ductules and canals of Hering
(5,14,15).
Therefore, the expression of N-CAM was examined in peripheral ICCs.
N-CAM was expressed in 3 of the 14 peripheral ICCs (Table I, Fig.
6). However, the expression of N-CAM was not associated with
the expression of arginase-1 (Table
IV).
 | Table IVExpression of arginase-1 in
N-CAM-positive or negative peripheral intrahepatic
cholangiocarcinomas (ICCs). |
Table IV
Expression of arginase-1 in
N-CAM-positive or negative peripheral intrahepatic
cholangiocarcinomas (ICCs).
| Expression of
N-CAM | No. of
arginase-positive ICCs |
|---|
| Positive (n=3)
(%) | 3 (100) |
| Negative (n=11)
(%) | 8 (72.7) |
Discussion
Immunostaining of grade 5 for CK-19 and CK-7 was
observed in 13 (92.9%) of the 14 hilar PI ICCs, and in 7 (50%) of
the 14 peripheral MF ICCs. Sasaki et al (16) have reported that 22.2 and 22.2% of
MF ICCs did not reveal immunostaining for CK-19 and CK-7,
respectively, in more than 10% of cancer cells. Aishima et
al (17) have reported that 16
and 10% of MF ICCs did not express CK-19 and CK-7, respectively, in
more than 10% of cancer cells. In addition, D'Errico et al
(11) have shown that all of their
hilar ICCs and ICCs originating from major bile ducts expressed
both CK-19 and CK-7, and all peripheral ICCs expressed CK-19, but
50% of peripheral ICCs did not express CK-7. Thus, it is likely
that at least certain MF ICCs express less CK-19 or CK-7 compared
to hilar PI ICCs. This difference may reflect the origin of MF ICCs
in the periphery of the intrahepatic biliary tree. In support of
this hypothesis, Guedj et al (18) reported a difference in the protein
expression profile between hilar and peripheral ICCs.
The expression of HepPar-1 antigen and AFP in ICCs
has been reported to be 0–11% (8–10,19–21)
and 0–7% (7,19–21),
respectively. In agreement with these reports, AFP or HepPar-1
antigen was expressed in only 14.3% of the hilar ICCs and in only
14.3% of the peripheral ICCs in the present study.
The reason for ICCs expressing hepatocyte markers,
such as AFP or HepPar-1 antigen, remains unclear. The double
immunostaining for CK-7 and HepPar-1 antigen revealed the presence
of cancer cells expressing both CK-7 and HepPar-1 antigen. Thus,
certain ICCs expressing hepatocyte markers may be derived from
hepatic progenitor cells capable of differentiating into
cholangiocytes and hepatocytes (14). It is also possible that cancer cells
of ICCs originating from cholangiocytes may transdifferentiate into
hepatocellular carcinoma (HCC) cells, since mouse gall bladder
epithelial cells have been demonstrated to be capable of
transdifferentiating into hepatocytes (6).
Recently, Yan et al (12) showed arginase-1 to be a more
sensitive hepatocyte marker in HCCs, particularly in poorly
differentiated HCCs, compared to HepPar-1 antigen. In that study,
HepPar-1 antigen and arginase-1 were positively stained in 46.4 and
85.7% of poorly differentiated HCCs, respectively. Arginase-1 was
shown to be positively stained in only 1 of 6 ICCs, and useful for
distinguishing a poorly differentiated HCC from an adenocarcinoma.
In the present study, ICCs were stained immunohistochemically for
arginase-1 using the same antibody and the same procedure used by
Yan et al (12). However,
our results show that 4 (28.6%) of 14 hilar ICCs, and 7 (50%) of 14
peripheral ICCs expressed arginase-1 in more than 10% of cancer
cells. Furthermore, our results demonstrate that 3 (42.9%) of 7
peripheral ICCs, exhibiting a major histology of poorly
differentiated adenocarcinoma, expressed arginase-1 in more than
10% of cancer cells. These results indicate that care should be
taken when using arginase-1 as a hepatocyte marker for
distinguishing a poorly differentiated HCC and a poorly
differentiated peripheral ICC.
A ductal reaction, hyperplasia of ductules, observed
in the cirrhotic liver, contains N-CAM-positive cells, and
intermediate hepatobilliary cells that express hepatocyte and
cholangiocyte markers (14,15). Moreover, N-CAM has been reported to
be a potential marker of hepatic progenitor cells (14,15).
However, in the present study the expression of N-CAM was not found
to be associated with the expression of arginase-1, suggesting that
the expression of arginase-1 in ICCs is independent of its
origin.
In conclusion, findings of the present study
indicate that the hepatocyte markers, AFP and HepPar-1 antigen, are
rarely but definitely expressed in hilar and peripheral ICCs, and
that another hepatocyte marker, arginase-1, is expressed at a high
frequency in hilar and peripheral ICCs, irrespective of their
histology.
Acknowledgements
The authors thank Mr. K. Kobayashi and Ms. M.
Kakihana for their valuable technical support.
References
|
1
|
Sano T, Kamiya J, Nagino M, et al:
Macroscopic classification and preoperative diagnosis of
intrahepatic cholangiocarcinoma in Japan. J Hepatobiliary Pancreat
Surg. 6:101–107. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yamasaki S: Intrahepatic
cholangiocarcinoma: macroscopic type and stage classification. J
Hepatobiliary Pancreat Surg. 10:288–291. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nakanuma Y, Sato Y, Harada K, Sasaki M, Xu
J and Ikeda H: Pathological classification of intrahepatic
cholangiocarcinoma based on a new concept. World J Hepatol.
2:419–427. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nakanuma Y, Sasaki M, Ikeda H, et al:
Pathology of peripheral intrahepatic cholangiocarcinoma with
reference to tumorigenesis. Hepatol Res. 38:325–334. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Roskams T: Liver stem cells and their
implication in hepato-cellular and cholangiocarcinoma. Oncogene.
25:3818–3822. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kuver R, Savard CE, Lee SK, Haigh WG and
Lee SP: Murine gallbladder epithelial cells can differentiate into
hepatocyte-like cells in vitro. Am J Physiol Gastrointest Liver
Physiol. 293:G944–G955. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Maeda T, Kajiyama K, Adachi E, Takenaka K,
Sugimachi K and Tsuneyoshi M: The expression of cytokeratins 7, 19,
and 20 in primary and metastatic carcinomas of the liver. Mod
Pathol. 9:901–909. 1996.PubMed/NCBI
|
|
8
|
Chu PG, Ishizawa S, Wu E and Weiss LM:
Hepatocyte antigen as a marker of hepatocellular carcinoma: an
immunohistochemical comparison to carcinoembryonic antigen, CD 10,
and alpha-fetoprotein. Am J Surg Pathol. 26:978–988. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fan Z, van de Rijn M, Montgomery K and
Rouse RV: Hep Par 1 antibody stain for the differential diagnosis
of hepatocellular carcinoma: 676 tumors tested using tissue
microarrays and conventional tissue sections. Mod Pathol.
16:137–144. 2003. View Article : Google Scholar
|
|
10
|
Mosnier J-F, Kandel C, Cazals-Hatem D, et
al: N-cadherin serves as diagnostic biomarker in intrahepatic and
perihilar cholangiocarcinomas. Mod Pathol. 22:182–190. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
D'Errico A, Baccarini P, Fiorentino M, et
al: Histogenesis of primary liver carcinomas: strengths and
weaknesses of cytokeratin profile and albumin mRNA detection. Hum
Pathol. 27:599–604. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yan BC, Gong C, Song J, et al: Arginase-1:
a new immunohistochemical marker of hepatocytes and hepatocellular
neoplasms. Am J Surg Pathol. 34:1147–1154. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nakajima T, Kondo Y, Miyazaki M and Okui
K: A histopathologic study of 102 cases of intrahepatic
cholangiocarcinoma: histologic classification and modes of
spreading. Hum Pathol. 19:1228–1234. 1988. View Article : Google Scholar
|
|
14
|
Roskams TA, Theise ND, Balabaud C, et al:
Nomenclature of the finer branches of the biliary tree: canals,
ductules, and ductular reactions in human livers. Hepatology.
39:1739–1745. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou H, Rogler LE, Teperman L, Morgan G
and Rogler CE: Identification of hepatocytic and bile ductular cell
lineages and candidate stem cells in bipolar ductular reactions in
cirrhotic human liver. Hepatology. 45:716–724. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sasaki A, Kawano K, Aramaki M, Nakashima
K, Yoshida T and Kitano S: Immunohistochemical expression of
cytokeratins in intrahepatic cholangiocarcinoma and metastatic
adenocarcinoma of the liver. J Surg Oncol. 70:103–108. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Aishima S, Asayama Y, Taguchi K, et al:
The utility of keratin 903 as a new prognostic marker in
mass-forming-type intra-hepatic cholangiocarcinoma. Mod Pathol.
15:1181–1190. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guedj N, Zhan Q, Perigny M, et al:
Comparative protein expression profiles of hilar and peripheral
hepatic cholangiocarcinomas. J Hepatol. 51:93–101. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lau SK, Prakash S, Geller SA and Alsabeh
R: Comparative immunohistochemical profile of hepatocellular
carcinoma, cholangiocarcinoma, and metastatic adenocarcinoma. Hum
Pathol. 33:1175–1181. 2002. View Article : Google Scholar
|
|
20
|
Kakar S, Muir T, Murphy LM, Lloyd RV and
Burgart LJ: Immunoreactivity of Hep Par 1 in hepatic and
extrahepatic tumors and its correlation with albumin in situ
hybridization in hepatocellular carcinoma. Am J Clin Pathol.
119:361–366. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tsuji M, Kashihara T, Terada N and Mori H:
An immunohistochemical study of hepatic atypical adenomatous
hyperplasia, hepatocellular carcinoma, and cholangiocarcinoma with
α-fetoprotein, carcinoembrionic antigen, CA19-9, epithelial
membrane antigen, and cytokeratins 18 and 19. Pathol Int.
49:310–317. 1999.PubMed/NCBI
|