Introduction
Oral squamous cell carcinoma (OSCC) is a common
malignancy that affects 300,000 individuals per year worldwide
(1). A number of etiologic factors
have been implicated in the development of OSCCs, such as the use
of tobacco, alcohol or betel nut chewing, human papillomavirus
infection, and the presence of incompatible prosthetic materials.
Tobacco is a major risk factor for the development of OSCC and
overall risk of OSCC among smokers is 7–10 times higher than that
of non-smokers (2–4).
The primary treatment for management of OSCC is
surgical intervention. Despite considerable advances in the
treatment of OSCC over the past two decades, overall disease
outcomes have only modestly improved (5). Local tumor recurrence affects
approximately 60% of patients and metastasis develops in 15–25%
(6). The prevention and management
of this disease is likely to greatly benefit from the
identification of molecular markers and targets (7,8).
However, little is known about the molecular basis
of OSCC compared with other malignancies. Molecular alterations in
a number of oncogenes and tumor suppressor genes (TSGs) associated
with the development of OSCC may be significant clues with which to
address these problems (9,10).
Inactivation of TSG is considered to be associated
with carcinogenesis, and alterations in TSGs are accepted to be
critical events in the multi-step process leading toward the
development of cancer. Loss of chromosome 3p22-24 is a common early
genetic event in OSCC (11).
Specific regions of arm harbor candidate tumor suppressor genes
including FHIT and RSSF1A. It is generally accepted
that the transformation of normal tissue into malignant tissue
follows an accumulation of genetic changes in the TSGs and
oncogenes (9). High throughput
investigation into the molecular characteristics of OSCC has mainly
utilized microarray technology to search for gene expression
profiles associated with disease and disease outcome.
In the present study, microarray technology was
applied to screen novel TSGs in OSCC patients, and we selected
candidate gene CRISP3. CRISP3 DNA copy number was evaluated
by real-time QPCR in 5 OSCC-derived cell lines and 60 primary OSCC
samples. Human CRISP3 is also likely to be involved in the
pathogenesis of prostate cancer, where CRISP3 expression is
significantly upregulated (12).
CRISP3 is secreted and can be detected in human tissue
fluids including saliva, sweat, blood and seminal plasma, rendering
it an ideal candidate biomarker for pathophysiological conditions,
including ectopic pregnancy (12–14).
Ye et al reported the expression of CRISP3 in OSCC
using microarray technology (15).
Therefore, the purpose of the present study was to assess the role
of CRISP3 in OSCC.
Materials and methods
Cells
The 5 human OSCC-derived cell lines used in this
study were SAS, Ca9-22, KON, HSC2, and HSC4 (Human Science Research
Resources Bank, Osaka, Japan). SAS was from male tongue, Ca9-22
from male gingiva, KON from male oral floor, HSC2 from male mouth
and HSC4 from male tongue. The cell lines were maintained at 37°C
(humidified atmosphere 5% CO2/95% air) in 150×200 mm
tissue culture dishes (Nunc, Roskilde, Denmark) and cultured in
Dulbecco’s modified Eagle’s medium F-12 HAM (Sigma, St. Louis, MO,
USA) with 10% fetal bovine serum (Sigma) plus 50 U/ml penicillin
and streptomycin.
Normal oral keratinocyte (NOKs) strains from two
patients who had undergone dental surgery served as the controls,
and the patients provided written informed consent prior to the
start of the study. The normal oral specimens were washed in
Dulbecco’s phosphate-buffered saline (PBS) (Sigma) and then placed
overnight in 0.25% trypsin-EDTA solution (Sigma) at 4°C. After the
epithelial tissue was separated from the connective tissue, it was
disaggregated by incubation in 0.25% trypsin-EDTA solution for 15
min with gentle pipetting at 37°C. Isolated epithelial cells were
then seeded into Collagen I Cellware 60-mm dish biocoat cell
environments (Becton Dickinson Labware, Bedford, MA, USA) and
cultured in Keratinocyte Basal Medium-2 (Cambrex, Walkersville, MD,
USA) with 0.4% bovine pituitary extract, 0.1% human epidermal
growth factor, 0.1% insulin, 0.1% hydrocortisone, 0.1% transferrin,
0.1% epinephrine and 0.1% GA-1000 (Cambrex) (16).
Patient characteristics
A total of 60 patients with OSCC were included in
the present study. Surgical resection of primary tumors and
marginal normal tissues from all patients was performed at the
Hospital of Chimei, Tainan, Taiwan, and the Hospital of Tokyo
Dental College, Chiba, Japan, between July 1999 and September 2009.
Written informed consent was obtained from all patients and the
study approved by the ethics committees of the Hospital of Chimei
and the Tokyo Dental College, ethical clearance number 205.
Informed consent was obtained from each patient prior to surgical
resection.
Staging of tumors was performed according to the
International Union Against Cancer TNM staging system (17). Cervical LNM during the 12-month
follow-up period was evaluated by computed tomography and magnetic
resonance imaging. In case of a positive signal, metastasis was
further confirmed by histopathological examination of the resected
tissues. Patients not exhibiting any cervical LNM for 12 months
after surgery were considered metastasis-free. Patients with
distant metastasis at the time of clinical examination or those
receiving preventive radiotherapy or chemotherapy were excluded
from this study. Detailed patient characteristics are shown in
Table I.
 | Table IClinical characteristics of 60 OSCC
patients. |
Table I
Clinical characteristics of 60 OSCC
patients.
| Case | Gender | Age | Ethnic group | Tumor site | T | N | Stage | pN | Tobacco | Alcohol |
|---|
| 1 | M | 43 | Taiwanese | Buccal mucosa | 3 | 0 | III | − | + | + |
| 2 | M | 66 | Taiwanese | Lower gingival | 2 | 0 | II | − | + | + |
| 3 | M | 31 | Taiwanese | Tongue | 2 | 0 | II | − | + | + |
| 4 | M | 55 | Taiwanese | Buccal mucosa | 1 | 0 | I | − | + | + |
| 5 | M | 37 | Taiwanese | Tongue | 3 | 0 | III | − | + | + |
| 6 | M | 58 | Taiwanese | Buccal mucosa | 1 | 0 | I | − | + | + |
| 7 | F | 71 | Taiwanese | Upper gingival | 2 | 0 | II | − | − | − |
| 8 | M | 43 | Taiwanese | Oral floor | 2 | 0 | II | − | + | + |
| 9 | M | 39 | Taiwanese | Buccal mucosa | 2 | 0 | II | − | + | + |
| 10 | M | 56 | Taiwanese | Tongue | 1 | 0 | I | − | + | + |
| 11 | M | 49 | Taiwanese | Buccal mucosa | 3 | 0 | III | − | + | + |
| 12 | M | 72 | Taiwanese | Tongue | 1 | 1 | III | + | + | + |
| 13 | M | 51 | Taiwanese | Lower gingival | 4 | 0 | IV | − | + | + |
| 14 | M | 55 | Taiwanese | Tongue | 2 | 2 | IV | + | − | − |
| 15 | M | 59 | Taiwanese | Buccal mucosa | 3 | 1 | III | + | − | − |
| 16 | M | 54 | Taiwanese | Buccal mucosa | 1 | 0 | I | − | + | + |
| 17 | M | 68 | Taiwanese | Buccal mucosa | 2 | 0 | II | − | + | − |
| 18 | M | 33 | Japanese | Tongue | 2 | 1 | III | + | − | + |
| 19 | M | 55 | Japanese | Oral floor | 3 | 2c | IV | + | + | + |
| 20 | M | 74 | Japanese | Tongue | 2 | 1 | III | − | + | + |
| 21 | M | 54 | Japanese | Buccal mucosa | 4 | 2c | IV | + | − | + |
| 22 | M | 64 | Japanese | Lower gingival | 4 | 2a | IV | + | − | + |
| 23 | M | 53 | Japanese | Tongue | 2 | 1 | III | + | + | − |
| 24 | F | 70 | Japanese | Buccal mucosa | 3 | 1 | III | + | − | − |
| 25 | M | 44 | Japanese | Tongue | 1 | 0 | I | − | − | + |
| 26 | F | 50 | Japanese | Upper gingival | 1 | 0 | I | − | − | + |
| 27 | M | 36 | Japanese | Upper gingival | 2 | 0 | II | − | − | + |
| 28 | F | 37 | Japanese | Tongue | 2 | 2c | IV | − | − | − |
| 29 | M | 47 | Japanese | Tongue | 2 | 0 | II | + | − | + |
| 30 | M | 43 | Japanese | Tongue | 1 | 0 | I | − | − | + |
| 31 | M | 57 | Japanese | Tongue | 2 | 2b | IV | − | + | + |
| 32 | M | 52 | Japanese | Tongue | 1 | 0 | I | − | + | + |
| 33 | F | 58 | Japanese | Tongue | 2 | 0 | II | − | − | − |
| 34 | M | 59 | Japanese | Upper gingival | 3 | 1 | III | − | − | + |
| 35 | M | 62 | Japanese | Tongue | 1 | 0 | I | − | + | + |
| 36 | F | 70 | Japanese | Upper gingival | 2 | 1 | III | − | − | − |
| 37 | M | 82 | Japanese | Upper gingival | 3 | 0 | III | − | − | + |
| 38 | F | 69 | Japanese | Buccal mucosa | 2 | 0 | II | − | − | − |
| 39 | M | 80 | Japanese | Lower gingival | 2 | 2b | IV | + | − | − |
| 40 | M | 48 | Japanese | Tongue | 1 | 0 | I | − | − | + |
| 41 | M | 70 | Japanese | Lower gingival | 2 | 1 | III | − | + | + |
| 42 | M | 49 | Japanese | Lower gingival | 2 | 2b | IV | − | − | − |
| 43 | M | 85 | Japanese | Tongue | 2 | 1 | III | − | − | − |
| 44 | M | 71 | Japanese | Lower gingival | 4 | 1 | IV | − | − | + |
| 45 | F | 73 | Japanese | Tongue | 1 | 0 | I | − | − | − |
| 46 | F | 58 | Japanese | Lower gingival | 3 | 0 | III | − | − | − |
| 47 | M | 41 | Japanese | Tongue | 3 | 2b | IV | − | + | + |
| 48 | M | 58 | Japanese | Tongue | 1 | 1 | III | + | + | + |
| 49 | F | 66 | Japanese | Buccal mucosa | 2 | 1 | III | + | − | − |
| 50 | M | 63 | Japanese | Tongue | 1 | 0 | I | − | + | + |
| 51 | F | 60 | Japanese | Lower gingival | 2 | 0 | II | − | − | − |
| 52 | M | 52 | Japanese | Tongue | 2 | 0 | II | − | + | + |
| 53 | F | 72 | Japanese | Upper gingival | 2 | 2a | IV | − | − | − |
| 54 | F | 63 | Japanese | Lower gingival | 4 | 2b | IV | − | − | − |
| 55 | F | 66 | Japanese | Buccal mucosa | 1 | 0 | I | − | − | − |
| 56 | M | 66 | Japanese | Oral floor | 4 | 2c | IV | + | + | + |
| 57 | M | 66 | Japanese | Lower gingival | 4 | 2b | IV | − | − | + |
| 58 | M | 76 | Japanese | Oral floor | 2 | 1 | III | + | − | + |
| 59 | M | 77 | Japanese | Oral floor | 2 | 1 | III | − | − | + |
| 60 | F | 69 | Japanese | Lower gingival | 4 | 2b | IV | + | − | − |
Primary tumor samples
Resected primary tumor tissues were divided into two
sections. Of these, one section was frozen immediately in liquid
nitrogen and stored at −80°C. The other was fixed in 10% formalin
for histopathological examination. The resected marginal normal
tissues were frozen immediately in liquid nitrogen and stored at
−80°C.
Microarray gene expression profiling
A total of 3 OSCC patients (2 tongue and 1 oral
floor patient) were subjected to whole-genome analysis using
microarray technology to determine the TSGs.
Total RNA was extracted from 3 OSCC patients using
the Qiagen RNeasy mini kit and the SuperScript double-stranded cDNA
synthesis kit (Invitrogen) was used to generate cDNA according to
the manufacturer’s instructions. Cy3 labeling of ds-cDNA was
performed overnight using the NimbleGen One-Color DNA labeling
kit.
Cy3-labeled ds-cDNA (4 μg) was hybridized to the
Homo sapiens 4×72 K gene expression array (Roche NimbleGen)
representing 24,000 protein-coding genes, according to the
manufacturer’s instructions. The mRNA expression data were analyzed
using NimbleScan software version 2.4, which applied quintile
normalization (18), and expression
values were obtained using the Robust Multi-Chip Average algorithm
as described by Irizarry et al (19). Expressional alterations of 2-fold
across the two biological repeats were considered significant.
DNA copy number analysis by real-time
QPCR
DNA from the frozen 60 tumor and marginal normal
tissues was extracted using the QIAamp tissue kit (Qiagen, Hilden,
Germany) according to the manufacturer’s instructions.
Real-time QPCR was performed using 60 tumor and
marginal normal tissue samples with SYBR-Green I fluorescence
detection on a LightCycler (Roche Diagnostics, Basel, Switzerland).
Oligonucleotide primers for real-time QPCR were designed using
Primer 3 software (Whitehead Institute for Biomedical Research),
and uniqueness in the human genome was confirmed using a BLAST
search. The primer set specific for CRISP3 was forward:
5′-ATCAGGCTGCATC CCAATAC-3′, and reverse: 5′-AACACCAAATCCCCACA
GAA-3′. The 20-μl reaction mixture consisted of 10 μl 2X iQ
SYBR-Green Supermix (Bio-Rad Laboratories, Hercules, CA, USA), 10
ng genomic DNA, and 800 nM of each PCR primer. The reaction mixture
was loaded into glass capillary tubes and submitted to an initial
denaturation at 95°C for 10 min, followed by 45 cycles of
amplification at 95°C for 10 sec for denaturation, 58°C for 10 sec
for annealing, and 72°C for 15 sec for extension, with a
temperature slope of 20°C/sec, performed in the LightCycler. The
crossing point for each amplification curve was determined by the
second derivative maximum method. The copy numbers are presented as
the log ratio of each target locus in tumor normalization to
internal reference loci (GAPDH) and relative to the normal
DNA. The primer set specific to GAPDH was forward:
5′-CCACTAGGCGCTCACTGTTCT-3′, and reverse: 5′-GCG
AACTCACCCGTTGACT-3′. DNA copy number loss was determined as <1.2
(20). The data were analyzed as
the mean ± SD of three independent experiments with samples in
triplicate.
Statistical analysis
The association between copy number loss and any
clinical findings were assessed by the Fisher’s exact test. The
association between the DNA copy number of tumor and normal tissues
was assessed by the unpaired U test (SAS Institute, Cary, NC, USA).
P-<0.05 was considered to indicate statistical significance.
Results
Clinicopathological findings
This study included 60 patients who had undergone
surgical resection of primary OSCC. The patients, 45 males and 15
females, had average ages of 56.7 years for the males (range 31–85)
and 63.1 years for the females (range 37–73). Regarding patient
ethnicity,17 patients of Taiwanese and 43 of Japanese ethnicity
were included in this study. The tumor sites were as follows: 23
patients, tongue; 13, buccal mucosa; 12, lower gingiva; 7, upper
gingiva; and 5, oral floor. The T classifications, which indicate
the sizes of the primary clinical tumors, were as follows: 15
patients with T1, 27 with T2, 10 with T3 and 8 with T4. The
classifications by TNM stage were: 13 patients with Stage I, 12
with Stage II, 19 with Stage III, and 16 with Stage IV. A total of
18 of the 60 patients had histopathologically-confirmed cervical
lymph node metastasis (LNM) at the time of diagnosis or during the
12-month follow-up period (LNM present).
Microarray analysis
Three OSCC patients were subjected to microarray
analysis to screen for TSGs in this population. As in previous
studies, we know that there are 17 upregulated genes in OSCC,
comprising matrix metallopeptidase 1 MMP1, MMP10,
MMP3, MMP12, PTHLH, INHBA,
LAMC2, IL8, KRT17, COL1A2, IF16,
ISG15, PLAU, GREM1, MMP9, IFI44
and CXCL1, and that there are 18 downregulated genes in
OSCC, comprising KRT4, MAL, CRNN, SCEL,
CRISP3, SPINK5, CLLA4, ADH1B,
P11, TGM3, RHCG, PPP1R3C,
CEACAM7, HPGD, CFD, ABCA8, CLU
and CYP3A5 (15,21). In this study, we found 2 upregulated
genes, MMP1 and MMP3, and 11 downregulated genes,
CRISP3, SCGB3A1, AGR2, PIP,
C20orf114, TFF1, STATH, AZGP1,
MUC7, DMBT1 and LOC389429, in all three
patients. The detected genes of up- and down-regulation are shown
in Table II. CRISP3 was
selected as it is currently unknown as to whether this gene is
associated with OSCC.
 | Table IIList of aberrantly expressed
genes. |
Table II
List of aberrantly expressed
genes.
| Gene symbol | Gene name | Location |
|---|
| Upregulated
genes |
| MMP1 | Matrix
metallopeptidase 1 | 11q22 |
| MMP3 | Matrix
metallopeptidase 3 | 11q22 |
| Downregulated
genes |
| STATH | Statherin | 4q11-q13 |
| MUC7 | Mucin 7 | 4q13-q21 |
| SCGB3A1 | Secretoglobin,
family 3A, member 1 | 5q35-qter |
| CRISP3 | Cysteine-rich
secretory protein 3 | 6p12.3 |
|
LOC389429 | Hypothetical
LOC389429 | 6q21 |
| AGR2 | Anterior gradient
homolog 2 | 7p21.3 |
| AZGP1 | α-2-glycoprotein 1,
zinc-binding | 7q22.1 |
| PIP | Prolactin-induced
protein | 7q34 |
| DMBT1 | Deleted in
malignant brain tumors 1 | 10q26.13 |
|
C20orf114 | Chr. 20 open
reading frame 114 | 20q11.21 |
| TFF1 | Trefoil factor
1 | 21q22.3 |
Assessment of CRISP3 by real-time
QPCR
To evaluate microarray data at CRISP3,
real-time QPCR was performed using non-amplified genomic DNA as a
template. Initially, PCR primer sets located in CRISP3 were
successfully designed to meet the criteria for reliable
quantification. One PCR primer set was designed in a housekeeping
gene, GAPDH, located on chromosome 12p, as a reference for
normalization. Real-time QPCR analysis was performed with the
above-mentioned primer sets using genomic DNA of 5 OSCC cell lines
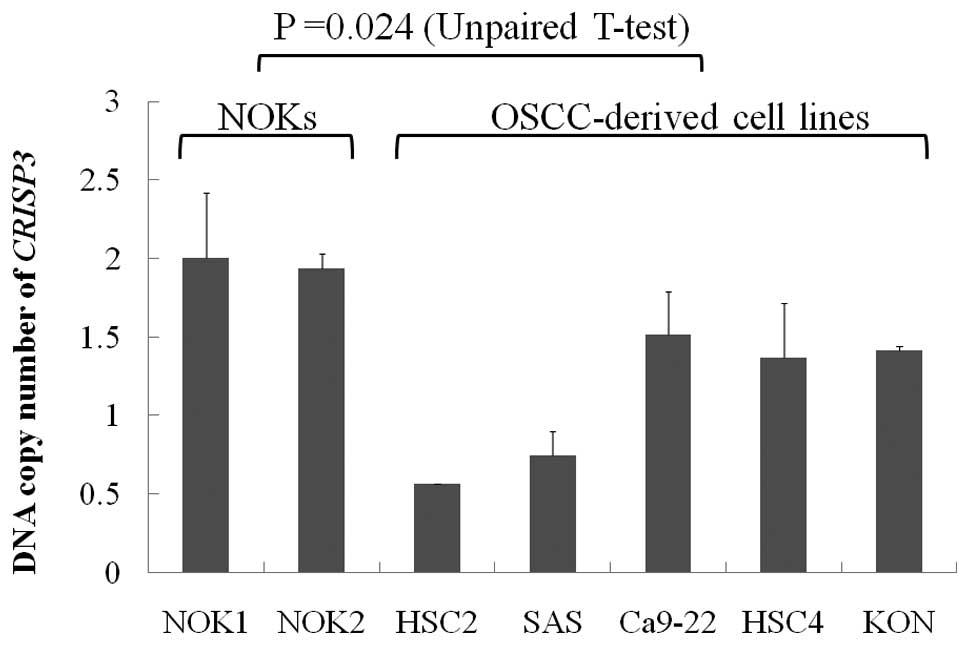
and 60 OSCC patients. DNA copy number loss of CRISP3 was
observed in 2 of the 5 OSCC-derived cell lines (SAS and HSC2). The
copy number of CRISP3 was significantly reduced in
OSCC-derived cell lines compared with NOKs (P=0.024, Unpaired
U-test: Fig. 1). The copy number
loss of CRISP3 was observed in 24 (40%) of the 60 patient
specimens. We evaluated statistically significant differences in
all clinical characteristics pertaining to the CRISP3 copy
number between OSCCs and normal tissues. No statistically
significant differences were noted. Moreover, no statistically
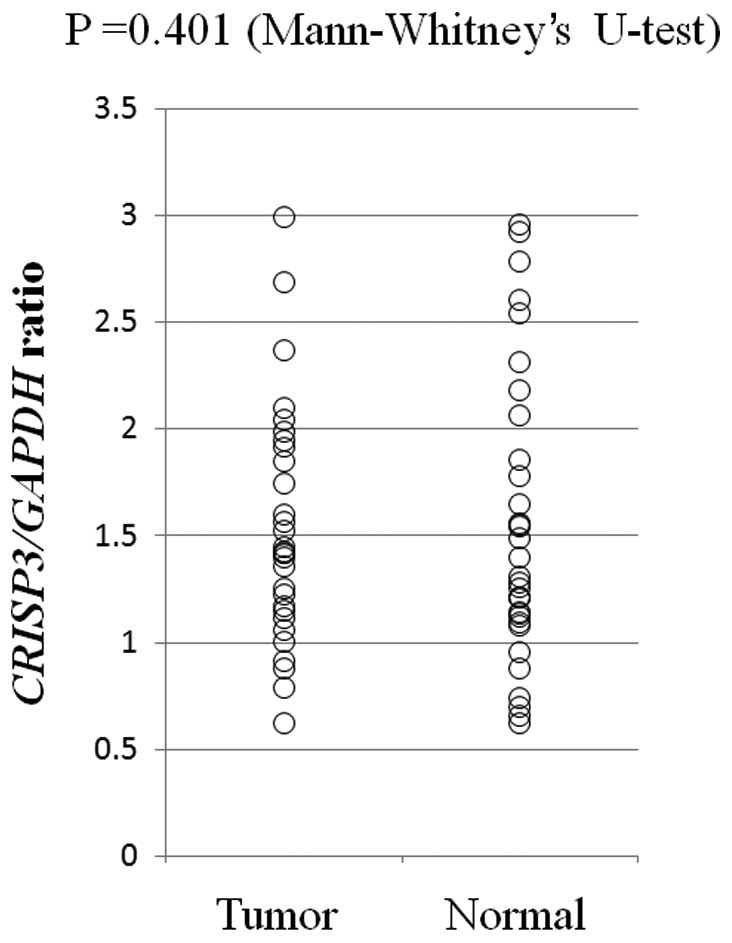
significant differences were observed in the CRISP3 copy
number between OSCC tumor tissues and normal tissues in the 60 OSCC
patients (P=0.401, Mann-Whitney’s U-test; Fig. 2).
Clinicopathological findings and
statistical analysis
We compared our results with the clinicopathological
findings for each tumor. The copy number loss of CRISP3 was
observed in 24 (40%) of the 60 patients. The Fisher’s exact test
was performed to evaluate the significance of correlations between
copy number loss of CRISP3 and clinicopathological findings
(Table III). A significant
statistical correlation between the copy number loss and gender and
T classification was observed. The copy number loss of
CRISP3 was detected in early stage lesions; and the copy
number loss of CRISP3 tended to be higher in early clinical
stages. Moreover, no statistically significant correlation was
found between the copy number loss of CRISP3 and other
clinicopathological findings such as age, ethnicity, lymph node
metastasis, tobacco and alcohol associated with the tumor
samples.
 | Table IIICorrelation between the DNA copy
numbers of CRISP3 and clinical characteristics in OSCCs. |
Table III
Correlation between the DNA copy
numbers of CRISP3 and clinical characteristics in OSCCs.
| Loss | Normal | P-value |
|---|
| Gender |
| Male | 13 | 32 | 0.005 |
| Female | 11 | 4 | |
| Age |
| <65 | 17 | 21 | 0.104 |
| ≥65 | 15 | 7 | |
| Ethnic group |
| Taiwanese | 6 | 11 | 0.773 |
| Japanese | 18 | 25 | |
| Tumor site |
| Tongue | 6 | 17 | 0.491 |
| Buccal mucosa | 6 | 7 | |
| Lower
gingival | 6 | 6 | |
| Upper
gingival | 4 | 3 | |
| Oral floor | 2 | 3 | |
| T
classification |
| T1/T2 | 21 | 21 | 0.021 |
| T3/T4 | 3 | 15 | |
| LNM |
| Present | 6 | 12 | 0.573 |
| Absent | 18 | 24 | |
| Stage |
| I | 5 | 8 | 0.111 |
| II | 8 | 4 | |
| III | 6 | 13 | |
| IV | 5 | 11 | |
| Tobacco |
| Yes | 7 | 19 | 0.11 |
| No | 17 | 17 | |
| Alcohol |
| Yes | 12 | 27 | 0.058 |
| No | 12 | 9 | |
Discussion
OSCC is often associated with loss of eating and
speech function, disfigurement and psychological distress. The
development of OSCC is strongly associated with smoking and
excessive alcohol consumption (22). The prevention and management of this
disease is likely to benefit from the identification of molecular
markers and targets (7,8).
Recently, the development of tools for measuring
gene expression and copy numbers across the entire genome has
revolutionized our ability to characterize cancers at the molecular
level. Cytogenetic analyses in conjunction with molecular genetics
analyses by a number of research groups have shown an accumulation
of genetic abnormalities during the development and/or progression
of OSCC (23). In this study, we
identified 11 downregulated genes, CRISP3, SCGB3A1,
AGR2, PIP, C20orf114, TFF1,
STATH, AZGP1, MUC7, DMBT1 and
LOC389429, and 2 up-regulation genes, MMP1 and
MMP3, using microarray technology. CRISP3 was
selected due to the fact that it is unknown as to whether
CRISP3 is associated with OSCC. However, to the best of our
knowledge no previous OSCC studies have identified CRISP3 as
we observed in the current study. Our findings indicate that DNA
copy number loss of CRISP3 is involved in carcino-genesis in
OSCC patients.
Little is known about the function of the mammalian
CRISPs; however, CRISP1 and CRISP2 are known to be
involved in various steps in reproduction (24). The C-terminal domain of
CRISP2 has been shown to interact with calcium channels
(25) and to bind a kinase present
in the acrosome of mouse sperm (26). Less is known about human CRISP3.
CRISP3, which is also known as specific granule protein of 28
kDa (SGP28), belongs to a family of CRISPs characterized by their
size (220–230 amino acids), their secretory properties and a
content of 16 highly conserved cysteine residues, which form an
intra-molecular disulphide bond (27). Apart from its ability to bind A1BG
in serum, Udby et al have shown that CRISP3 forms
similar complexes with one of the three major proteins secreted
from the prostate in seminal plasma, β-microseminoprotein (28). Human CRISP3 is also likely to
be involved in the pathogenesis of prostate cancer, where
CRISP3 expression is significantly upregulated (12). However, to the best of our knowledge
no previous reports have identified the CRISP3 gene in
OSCC.
Table II lists
other candidate genes that may be associated with carcinogenesis in
OSCC. These genes exhibit correlations with various types of
carcinoma excluding C20orf114, STATH, and
LOC389429. SCGB3A1 (HIN1) is a TSG that is highly
expressed in a number of epithelial tissues, including the breast,
lung, trachea, pancreas, prostrate and salivary gland. Inactivation
of SCGB3A1 expression by promoter methylation is frequent in many
types of epithelial carcinoma and carcinoma in situ,
including breast, lung and nasopharyngeal carcinoma (29,30).
AGR2 is a putative member of the protein disulfide isomerase
family and was first identified as a homolog of the Xenopus
laevis gene XAG-2. AGR2 was down-regulated in gastric
tumor tissue compared to the control (31). AGR2 has previously been found
to be one of several genes that encode secreted proteins showing an
increased expression in prostate cancer cells compared to normal
prostatic epithelium (32). We
observed that the MMP1 gene is up-regulated in OSCC.
MMP1 is located on chromosome 11q22.3 and belongs to the MMP
family, which is responsible for the degradation of extracellular
matrix components. There is clear evidence indicating that
MMP1 is involved in various cell and tumour events,
including cancer-cell development, growth, proliferation,
apoptosis, invasion and metastasis, as well as angiogenesis and
immune surveillance (33–36).
Our results suggest that the CRISP3 gene is a
novel TSG particular to OSCC, and inactivation of the CRISP3
gene may play one or more roles in the carcinogenesis of OSCCs. In
the present study, we applied whole-genome analysis of TSGs in
specimens from 3 patients with OSCC by microarray techno-logy. A
significant statistical correlation was observed between the DNA
copy number of CRISP3 and T-classification and gender. No
significant correlation was found between DNA copy number loss of
CRISP3 and age, ethnic group, tumor site, lymph node
metastasis, tumor stage, tobacco or alcohol. In this population, 15
patients were habitual consumers of tobacco, alcohol and/or betel
nuts. We compared these 15 patients with 22 patients who did not
consume tobacco, alcohol or betel nuts. No significant correlation
was found between the DNA copy number loss of CRISP3 and
tobacco, alcohol and/or betel nut consumption. These results
indicate that inactivation of the CRISP3 is an early event
in OSCC, since T1/T2 classification is correlated with DNA copy
number loss of CRISP3 rather than T3/T4 classification. We
were not able to apply the functional analysis of the CRISP3
gene. Further studies employing techniques including
immunoblotting, immunofluorescence, and immunohistochemistry are
required to clarify the function of this gene in the development
and progression of OSCC. Furthermore, a study with a larger patient
series is requred to validate these results, in order that more
appropriate treatment modalities can be offered to OSCC patients in
Taiwan, Japan, and worldwide.
Acknowledgements
We thank Dr Homare Kawachi for his help with
collecting OSCC samples and supplying the clinical data for the
preparation of this manuscript.
References
|
1
|
Sudbo J: Novel management of oral cancer:
a paradigm of predictive oncology. Clin Med Res. 2:233–242. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Macfarlane GJ, Zheng T, Marshall JR, Boyle
P, et al: Alcohol, tobacco, diet and the risk of oral cancer: a
pooled analysis of three case-control studies. Oral Oncol.
31:181–187. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mashberg A, Boffetta P, Winkelman R and
Garfinkel L: Tobacco smoking, alcohol drinking, and cancer of the
oral cavity and oropharynx among U.S. veterans. Cancer.
72:1369–1375. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Warnakulasuriya S, Sutherland G and Scully
C: Tobacco, oral cancer, and treatment of dependence. Oral Oncol.
41:244–260. 2005. View Article : Google Scholar
|
|
5
|
Ries LAG, Harkins D, Krapcho M, et al:
Cancer Statistics Review 1975–2003. National Cancer Institute;
2006
|
|
6
|
Genden EM, Ferlito A, Bradley PJ, Rinaldo
A and Scully C: Neck disease and distant metastases. Oral Oncol.
39:207–212. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sabichi AL, Demierre MF, Hawk ET, Lerman
CE and Lippman SM: Frontiers in cancer prevention research. Cancer
Res. 63:5649–5655. 2003.
|
|
8
|
Spafford MF, Koch WM, Reed AL, et al:
Detection of head and neck squamous cell carcinoma among exfoliated
oral mucosal cells by microsatellite analysis. Clin Cancer Res.
7:607–612. 2001.PubMed/NCBI
|
|
9
|
Fearon ER and Vogelstein B: A genetic
model for colorectal tumorigenesis. Cell. 61:759–767. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Marshall CJ: Tumor suppressor genes. Cell.
64:313–326. 1991. View Article : Google Scholar
|
|
11
|
Garnis C, Baldwin C, Zhang L, Rosin MP and
Lam WL: Use of complete coverage array comparative genomic
hybridization to define copy number alterations on chromosome 3p in
oral squamous cell carcinomas. Cancer Res. 63:8582–8585.
2003.PubMed/NCBI
|
|
12
|
Bjartell A, Johansson R, Björk T, et al:
Immunohistochemical detection of cysteine-rich secretory protein 3
in tissue and in serum from men with cancer or benign enlargement
of the prostate gland. Prostate. 66:591–603. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Horne AW, Duncan WC, King AE, et al:
Endometrial cysteine-rich secretory protein 3 is inhibited by human
chorionic gonadotrophin, and is increased in the decidua of tubal
ectopic pregnancy. Mol Hum Reprod. 15:287–294. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Udby L, Cowland JB, Johnsen AH, Sørensen
OE, Borregaard N and Kjeldsen L: An ELISA for SGP28/CRISP-3, a
cysteine-rich secretory protein in human neutrophils, plasma, and
exocrine secretions. J Immunol Methods. 263:43–55. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ye H, Yu T, Temam S, et al: Transcriptomic
dissection of tongue squamous cell carcinoma. BMC Genomics. 6:9–69.
2008.
|
|
16
|
Kato H, Uzawa K, Onda T, et al:
Down-regulation of 1D- myo-inositol 1,4,5-trisphosphate 3-kinase A
protein expression in oral squamous cell carcinoma. Int J Oncol.
28:873–881. 2006.PubMed/NCBI
|
|
17
|
UICC. TNM classification of malignant
tumours. Fifth edition. pp. 20–24. 1997
|
|
18
|
Bolstad BM, Irizarry RA, Astrand M and
Speed TP: A comparison of normalization methods for high density
oligonucleotide array data based on variance and bias.
Bioinformatics. 19:185–193. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Irizarry RA, Hobbs B, Collin F, et al:
Exploration, normalization, and summaries of high density
oligonucleotide array probe level data. Biostatistics. 4:249–264.
2003. View Article : Google Scholar
|
|
20
|
Kuroiwa T, Yamamoto N, Onda T and
Shibahara T: Expression of the FAM5C in tongue squamous cell
carcinoma. Oncol Rep. 22:1005–1011. 2009.PubMed/NCBI
|
|
21
|
Xu C, Liu Y, Wang P, et al: Integrative
analysis of DNA copy number and gene expression in metastatic oral
squamous cell carcinoma identifies genes associated with poor
survival. Mol Cancer. 9:143–148. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
La Vecchia C: Epidemiology and prevention
of oral cancer. Oral Oncol. 33:302–312. 1997.
|
|
23
|
Martin CL, Reshmi SC, Ried T, et al:
Chromosomal imbalances in oral squamous cell carcinoma: examination
of 31 cell lines and review of the literature. Oral Oncol.
44:369–382. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cohen DJ, Busso D, Da Ros V, et al:
Participation of cysteine-rich secretory proteins (CRISP) in
mammalian sperm-egg interaction. Int J Dev Biol. 52:737–742. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gibbs GM, Scanlon MJ, Swarbrick J, et al:
The cysteine-rich secretory protein domain of Tpx-1 is related to
ion channel toxins and regulates ryanodine receptor Ca2+
signaling. J Biol Chem. 281:4156–4163. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gibbs GM, Bianco DM, Jamsai D, et al:
Cysteine-rich secretory protein 2 binds to mitogenactivated protein
kinase 11 in mouse sperm. Biol Reprod. 77:108–114. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gibbs GM and O’Bryan MK: Cysteine rich
secretory proteins in reproduction and venom. Soc Reprod Fertil.
65:261–267. 2007.PubMed/NCBI
|
|
28
|
Udby L, Lundwall Å, Johnsen AH, et al:
β-microseminoprotein binds CRISP-3 in human seminal plasma. Biochem
Biophys Res Commun. 333:555–561. 2005.
|
|
29
|
Guo M, Ren J, Brock MV, Herman JG and
Carraway HE: Promoter methylation of HIN-1 in the progression to
esophageal squamous cancer. Epigenetics. 3:336–341. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Castro M, Grau L, Puerta P, et al:
Multiplexed methylation profiles of tumor suppressor genes and
clinical outcome in lung cancer. J Transl Med. 8:86–96. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bai Z, Ye Y, Liang B, et al:
Proteomics-based identification of a group of apoptosis-related
proteins and biomarkers in gastric cancer. Int J Oncol. 38:375–383.
2011.PubMed/NCBI
|
|
32
|
Maresh EL, Mah V, Alavi M, et al:
Differential expression of anterior gradient gene AGR2 in prostate
cancer. BMC Cancer. 13:680–687. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Brinckerhoff CE and Matrisian LM: Matrix
metalloproteinases: a tail of a frog that became a prince. Nat Rev
Mol Cell Biol. 3:207–214. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nagase H and Woessner JF Jr: Matrix
metalloproteinases. J Biol Chem. 274:21491–21494. 1999. View Article : Google Scholar
|
|
36
|
Ala-aho R and Kahari VM: Collagenases in
cancer. Biochimie. 87:273–286. 2005. View Article : Google Scholar
|