Introduction
Hepatocellular carcinoma (HCC) is the fifth most
common cancer in the world and one of the most prevalent
malignancies in Asia (1). Current
curative treatment options include surgical resection and liver
transplantation. Liver transplantation is effective only in the
early stages of disease. Surgery is possible in few patients and
curative in only a small percentage, due to recurrence following
the surgery (2). Besides exploring
effective therapeutic methods, investigators are attempting to find
biomarkers to predict tumor recurrence. The NY-ESO-1 antigen was
originally found in esophageal cancer by serological recombinant
cDNA expression cloning (SEREX) and belongs to the cancer/testis
antigen (CTA) family (3). NY-ESO-1
expression is restricted to testicular germ cells in normal adult
tissues and is found in numerous malignancies including malignant
melanoma, hepatoma, breast and lung cancer. The NY-ESO-1 protein is
known to be markedly immunogenic for numerous advanced and
metastatic types of cancer (4,5).
NY-ESO-1 is a potential biomarker for the prediction of tumor
recurrence and treatment outcomes in a variety of tumors, including
gastrointestinal stromal tumors (6)
and cutaneous melanoma (7).
Although a number of studies showed inconsistently
that NY-ESO-1 is positive in certain HCCs (8–16), two
studies have demonstrated that NY-ESO-1 correlates with the
metastasis of HCC (11,12). However, the role of NY-ESO-1 in the
prognosis of HCC following surgery remains unclear. The present
study used immunohistochemistry (IHC) to evaluate the NY-ESO-1
expression in tissues from 120 HCC patients, the correlation
between the patients’ clinical parameters and recurrence-free
survival (RFS). In addition, an NY-ESO-1 overexpressing cell line
was used to explore the effect of NY-ESO-1 on cell behavior.
Materials and methods
Patients and specimens
Between August 2004 and March 2008, 120 consecutive
patients who underwent curative resection or liver transplantation
with no microscopic evidence of residual tumor were included in
this study. All 120 patients had histologically proven HCC. The
inclusion criteria for the study were: i) the absence of
extrahepatic metastasis; ii) curative resection defined as
histological evidence of the complete removal of HCC tumors; and
iii) no additional therapies or multi-modality treatment for HCC
until the development of recurrence. Patients diagnosed with a
mixed tumor containing HCC and cholangiocarcinoma components were
excluded from the study.
Follow-up
Patients were regularly followed up at the
outpatient clinic (every 1-3 months). The endpoint was recurrence
or November 30, 2009. The clinical profiles were age, gender,
HBsAg, Child-Pugh classification, surgery (liver transplantation
and curative resection), primary HCC lesion [timing of diagnosis,
size, number, gross major vessel invasion (portal vein), tumor
distribution and histological differentiation], and tumor relapse
(time). The study conformed to the tenets of the Declaration of
Helsinki and informed consent was obtained from all patients prior
to the study.
Assessment
Patients were monitored by serum α-fetoprotein
(AFP), abdomen ultrasound and chest X-ray every 1 to 6 months,
according to the postoperative time. Tumor recurrences were
confirmed by computed tomography (CT), and, if necessary, magnetic
resonance imaging (MRI). RFS was calculated from the date of
surgery until the date of recurrence.
Immunohistochemistry (IHC)
Tumor samples were retrieved from the archives of
the Department of Pathology at YouAn Hospital, Beijing, China.
Serial formalin-fixed, paraffin-embedded tumor samples were
deparaffinized in xylene and rehydrated in a series of graded
alcohols and distilled water. Endogenous peroxidase activity was
quenched by 3% H2O2. Prior to immunostaining,
sections were incubated for 12 min in citrate buffer (pH 6.0) in a
microwave oven at 99°C to enhance the immunoreactivity. Following
blocking, the sections were incubated at 4°C overnight with mouse
monoclonal NY-ESO-1 antibody (E978 clone; Invitrogen, Carlsbad, CA,
USA). Detection was carried out using the
streptavidin-biotin-peroxidase method with a PV-9000 kit according
to the manufacturer’s instructions. The DAB system was employed as
a chromogen. Normal adult testis with intact spermatogenesis served
as positive controls. Slides were counterstained with hematoxylin
and mounted. Normal liver tissues served as negative controls.
Immunoreactivity of tumor cells was graded based on the amount of
immunopositive tumor cells as follows: +++, >67% of cells
stained; ++, 33–67%; +, 5–33%; focal <5% and −, no staining
cells. Focal staining was also considered as negative.
Cell line and construction of
NY-ESO-1-expressing plasmid
To determine the role of NY-ESO-1 in HCC, we
established stably transfected NY-ESO-1-overexpressing HepG2 cells
(ESO-HepG2) and evaluated their proliferation and migration
behaviors. Briefly, HepG2 (HCC) cells were obtained from the
American Type Culture Collection and cultured in DMEM supplemented
with antibiotics/antimycotics and 10% fetal bovine serum (FBS). The
NY-ESO-1 cDNA encoding NY-ESO-1 was amplified by PCR from mRNA and
subcloned into a pcDNA3.1 expression vector (Invitrogen) to form
plasmid-designated pcDNA3.1-ESO.
Transfection and selection of stably
transfected ESO-HepG2 cells
Subconfluent HepG2 cells (70–80%) were transfected
with 2 μg of pcDNA3.1-ESO and pcDNA3.1 using Lipofectamine 2000
(Invitrogen) according to the manufacturer’s instructions. For
stable transfection, the cells were exposed to 400 μg/ml G418
(Invitrogen) after 1 day of transfection. Four weeks later, the
cells were plated at a lower density in DMEM with 250 μg/ml G418
and 10% FBS in 96-well plates until a single colony was formed.
Single cloned cells designated as ESO-HepG2 cells were isolated and
grown. RT-PCR was used to test NY-ESO-1 expression.
Cell cycle and proliferation
analysis
Cells were diluted and seeded at
6×105/ml, 1×105/ml and 2.5×104/ml
in ACEA’s x96 microtiter plates in 100 μl of culture medium. Cell
proliferation was continuously monitored every 15 min using the
xCELLigence for a period of 24–72 h via calculation of a ‘cell
index’ (to reflect the surface area covered by the cells) for each
plate (Roche, Indianapolis, IN, USA) (17). Following synchronization, cells were
collected, fixed and stained with propidium iodide. The cell cycle
distribution was determined using a BD FACSCalibur flow cytometer
and Cell Quest software.
Cell migration assay
Cells were seeded at 6×105/ml in 100 μl
of serum-free medium in the upper chamber with an 8 μm pore size,
and the lower chamber contained 10% serum. Cell migration was
continuously monitored every 15 min using the xCELLigence for a
period of 24 h via calculation of a ‘cell index’ (to reflect the
surface area covered by the cells) for each plate (Roche).
Statistical analysis
The χ2 test (including Fisher’s exact
test) and independent sample t-test were employed for comparison
between groups. A RFS curve was plotted using the Kaplan-Meier
method. A statistical comparison of the RFS was performed using the
log-rank test. A multivariate analysis using the Cox proportional
hazard model was used to identify the independent risk factors for
tumor recurrence. P<0.05 was considered to be statistically
significant. SPSS 11.5 was used for all analyses.
Results
Baseline characteristics of the
patients
Of the 120 HCC patients, 79 were successfully
followed up, 21 refused to cooperate and 20 were lost to follow-up.
The median follow-up period was 19 months (range 1.0–61.0). Given
the possibility of selection bias, we compared the baseline
characteristics of the 79 and 41 cases, as shown in Table I. No significant difference was
found in any of the indices, such as age, gender, HBV infection,
serum AFP, Child-Pugh Score and the pathologic parameters including
tumor size and number, portal vein thrombosis and histological
differentiation. Thus, the data from the 79 patients represent the
entire cohort. Of the 79 patients, 11 succumbed to other diseases.
As there was no evidence of tumor relapse, these patients were also
included in the analysis.
 | Table IThe baseline characteristics of 79
cases with follow-up and 41 cases without follow-up. |
Table I
The baseline characteristics of 79
cases with follow-up and 41 cases without follow-up.
| 120 cases | | |
|---|
|
| | |
|---|
| Clinical
parameters | 79 cases (%) | 41 cases (%) | χ2 | P-value |
|---|
| NY-ESO-1 expression
(positive) | 19.0 | 31.7 | 2.441 | 0.118 |
| Gender (male) | 82.3 | 78.0 | 0.312 | 0.557 |
| Age <52 years | 45.6 | 41.5 | 0.185 | 0.667 |
| Tumor size ≥5 cm | 51.9 | 56.1 | 0.191 | 0.662 |
| Tumor number (120)
multinodular | 40.5 | 34.1 | 0.462 | 0.497 |
| Portal vein
thrombosis (with) | 24.4 | 36.6 | 1.968 | 0.161 |
| Abnormal serum
AFP | 78.1 | 84.2 | 0.591 | 0.442 |
| Transplantation
surgery | 58.2 | 56.1 | 0.050 | 0.823 |
| HBsAg-positive | 75.0 | 71.8 | 0.137 | 0.711 |
| Child-Pugh Score | | | 0.072 | 0.964 |
| A | 61.4 | 60.7 | | |
| B | 12.3 | 14.3 | | |
| C | 26.3 | 25.0 | | |
| Histological
differentiation | | | 1.990 | 0.370 |
| Well | 52.8 | 53.8 | | |
| Moderately | 30.6 | 38.5 | | |
| Low | 16.6 | 7.7 | | |
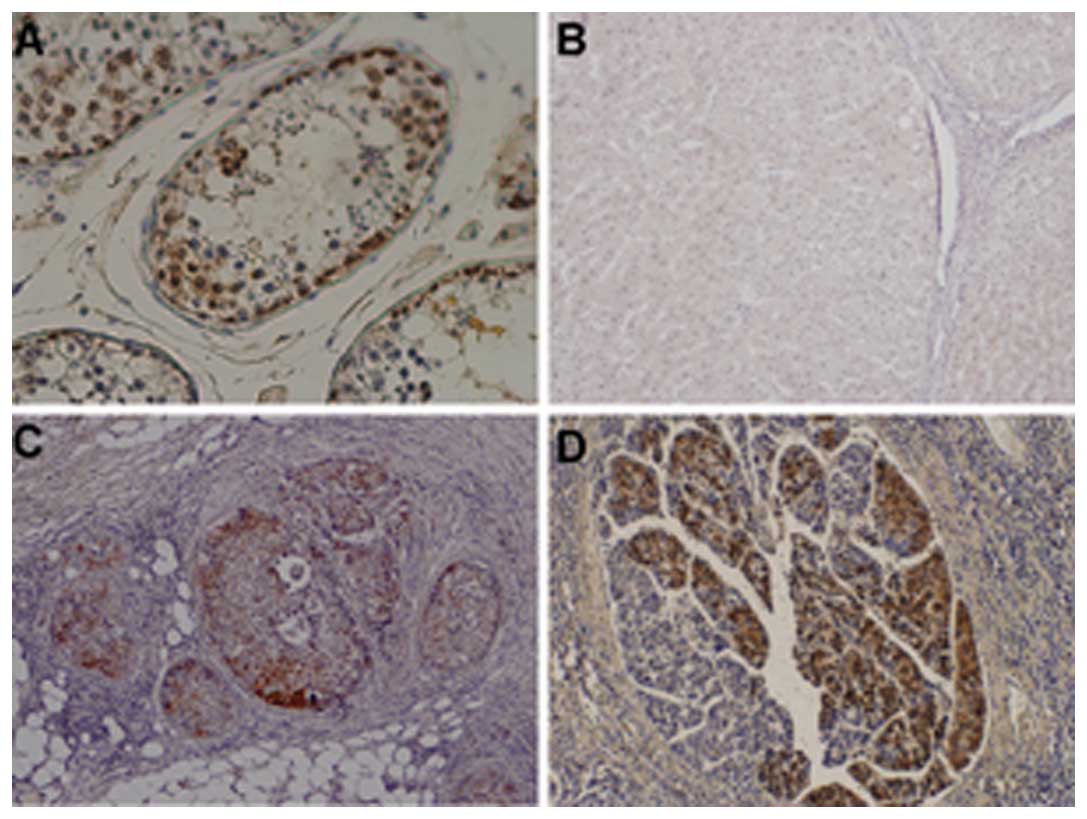
NY-ESO-1 expression in HCCs
NY-ESO-1 staining was positive in the testis
epithelial cells (Fig. 1A), but not
in normal liver tissue (Fig. 1B). A
total of 28 out of 120 HCC specimens were positive (23.3%). Among
them, one was graded as +++, 7 as ++ and 20 as + (Fig. 1C and D ). NY-ESO-1 was located
predominantly in the cytoplasm (Fig.
1C), although the nucleus was also stained in a few cases
(Fig. 1D). Notably, even in the
same specimen, certain areas were positive, whereas other areas
were negative. No staining was observed in the tissue adjacent to
HCC.
Correlation between NY-ESO-1 expression
and recurrence in HCC
In 13 out of 38 tumor recurrence patients NY-ESO-1
was found to be positive (34.2%, Table
II), whereas in 2 of 30 non-recurrence cases NY-ESO-1 was
positive (6.7%). The difference was statistically significant
(P=0.007). Of the 68 patients who completed follow-up, the median
RFS was 19 months. Regarding NY-ESO-1-positive patients, it was
found that in 73.3% of them the RFS was <19 months, and in 26.7%
it was ≥19 months. On the other hand, in the NY-ESO-1-negative
patients, 41.5% had an RFS <19 months, while in 58.5% it was ≥19
months (p=0.027)
 | Table IIUnivariate analysis of factors
affecting recurrence of HCC. |
Table II
Univariate analysis of factors
affecting recurrence of HCC.
| Recurrence (68
cases) | | |
|---|
|
| | |
|---|
| Clinical
parameters | 30 cases No (%) | 38 cases Yes (%) | χ2 | P-value |
|---|
| NY-ESO-1 expression
(positive) | 6.7 | 34.2 | 7.398 | 0.007 |
| Gender (male) | 73.3 | 86.8 | 1.979 | 0.160 |
| Age <52 years | 60.0 | 52.6 | 0.369 | 0.543 |
| Tumor size ≥5 cm | 53.3 | 55.3 | 0.025 | 0.874 |
| Tumor number (120)
multinodular | 36.7 | 36.8 | 0.000 | 0.988 |
| Portal vein
thrombosis (with) | 24.1 | 21.1 | 0.090 | 0.764 |
| Abnormal serum
AFP | 73.1 | 83.8 | 1.069 | 0.301 |
| Transplantation
surgery | 36.8 | 63.2 | 10.719 | 0.001 |
| HBsAg-positive | 72.4 | 80.6 | 0.600 | 0.439 |
| Child-Pugh
Score | | | 12.600 | 0.002 |
| A | 38.1 | 86.2 | | |
| B | 19.0 | 3.4 | | |
| C | 42.9 | 10.4 | | |
| Histological
differentiation | | | 6.440 | 0.040 |
| Well | 65.6 | 36.4 | | |
| Moderately | 17.2 | 45.5 | | |
| Low | 17.2 | 18.2 | | |
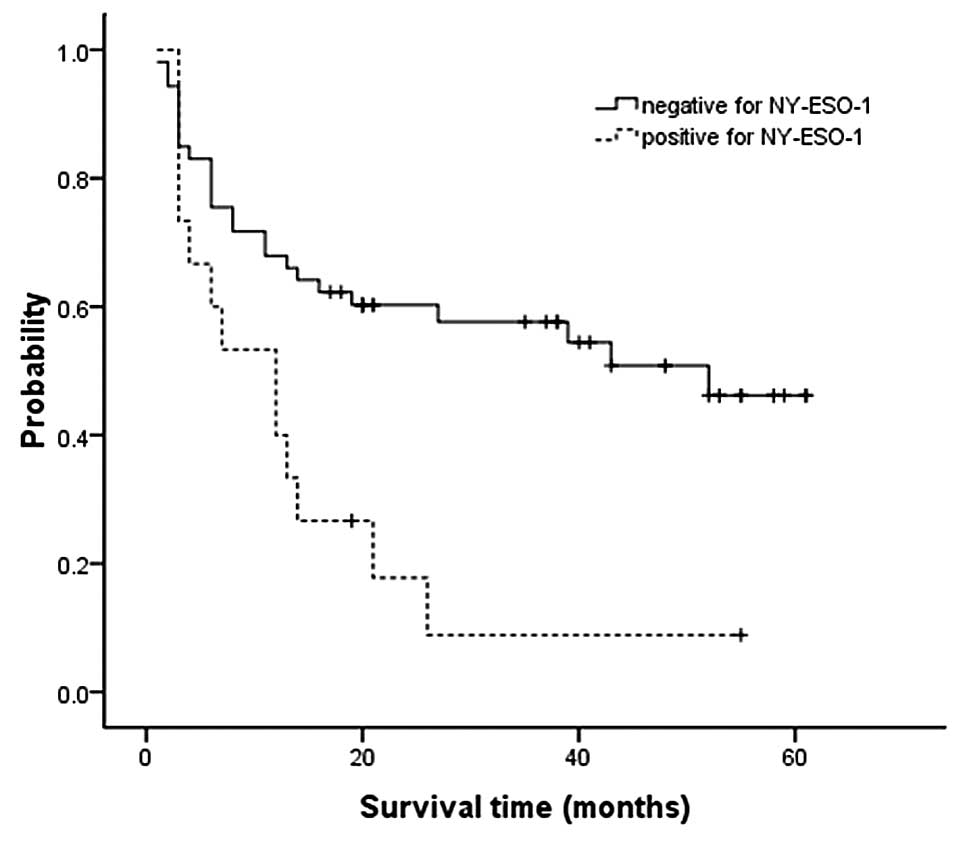
Using the Kaplan-Meier method, we observed that the
median RFS was 12 months in NY-ESO-1-positive patients, whereas in
NY-ESO-1-negative patients the RFS was estimated to be 40–50 months
(the endpoint blocked the continuation of the follow-up, log-rank
test, P=0.003, Fig. 2).
Various clinicopathological parameters were also
evaluated for their association with HCC recurrence (Table II). A total of 63.2% patients who
were NY-ESO-1-positive relapsed following liver transplantation
compared with 36.8% of those who were NY-ESO-1-negative
(χ2 test, P =0.033). The total recurrence rate in
NY-ESO-1-positive patients was 86.2%, while this rate was 38.1% in
NY-ESO-1-negative cases (P=0.002). Additionally, 36.4% of
NY-ESO-1-positive cases were well-differentiated, whereas in
NY-ESO-1-negative cases this rate was 65.6% (χ2 test,
P=0.040). Other parameters, such as age, gender, HBV infection,
serum AFP, tumor size and number and portal vein thrombosis had no
role in prognosis.
Multivariate analysis demonstrated that the type of
surgery (P<0.001) and NY-ESO-1 expression (P=0.022) were
independent predictors for the recurrence of HCC following curative
surgery (Table III). When using
the forward method and backward method, we found the same
results.
 | Table IIIMultivariate analysis of factors
affecting recurrence of HCC. |
Table III
Multivariate analysis of factors
affecting recurrence of HCC.
| Clinical
parameters | B | HR (95% CI) | P-value |
|---|
| NY-ESO-1 (positive
vs. negative) | 0.980 | 2.664
(1.150–6.169) | 0.022 |
| Surgery (resection
vs. transplantation) | 2.256 | 9.549
(3.214–28.370) | <0.001 |
| Age | −0.052 | 0.938
(0.898–0.980) | 0.004 |
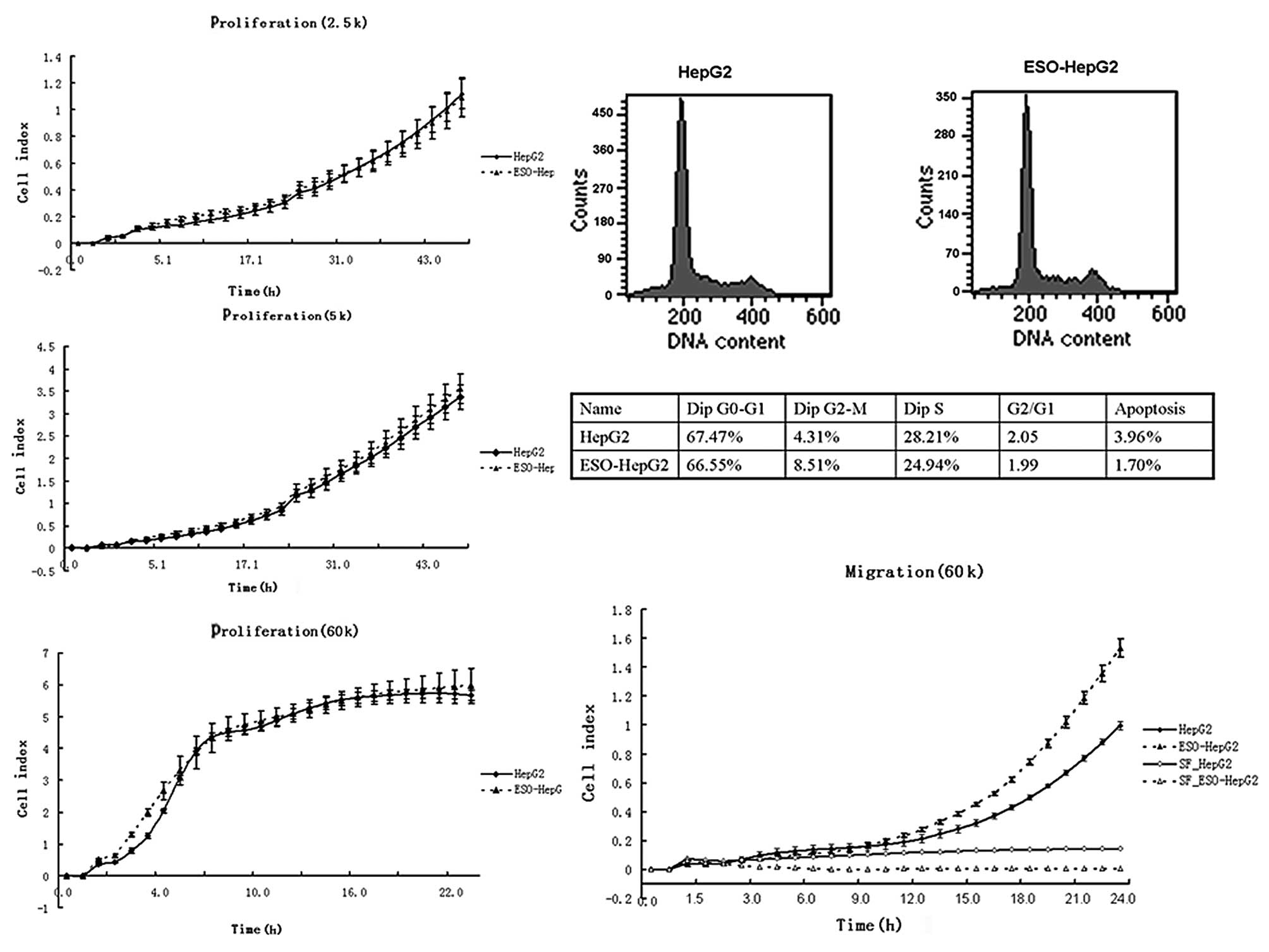
NY-ESO-1 modulates the migration but not
proliferation of HepG2 cells
The cell growth curves of HepG2 cells were
comparable to those of ESO-HepG2 cells even when cells were seeded
to the E-plate with three different densities (Fig. 3A-C). Flow cytometry showed that the
cell cycle distributions of these cells were similar (Fig. 3D). ESO-HepG2 cells migrated more
compared with HepG2 cells (P=0.002, Fig. 3E).
Discussion
To the best of our knowledge, the present study is
the first to demonstrate that NY-ESO-1 is an independent prognostic
factor in HCC, and that the mechanism may involve the enhancement
of cancer cell migration.
NY-ESO-1 belongs to the CTA family. It was found in
a variety of malignant tumors, such as melanoma, prostate, breast
and lung cancer. Furthermore, NY-ESO-1 is demonstrated to be a
prognostic marker in certain tumors, including malignant melanoma
and gastrointestinal tumors. However, the correlation between
NY-ESO-1 and HCC remains unclear. Previous studies showed that the
NY-ESO-1-positive rate is from 0–43.9% in HCC patients (8–16).
Certain studies demonstrated that NY-ESO-1 enhanced metastasis in
HCC. However, the mechanism remains unclear. There is no detailed
study on the predictive value of NY-ESO-1 on HCC recurrence. The
present study found that the NY-ESO-1-positive rate in this cohort
is 23.3%. NY-ESO-1 is a promising antigen for HCC-specific
immunotherapy given that it is the most immunogenic CT antigen
identified thus far and induces tumor-specific humoral and cellular
immune responses to NY-ESO-1 in HCC (10). Although international clinical
trials of NY-ESO-1 protein vaccine therapy for multiple types of
cancer including malignant melanoma, non small cell lung, ovarian,
breast and bladder cancer were conduted, the potential
immunotherapy in HCC remains to be evaluated. Understanding the
NY-ESO-1 protein expression in HCC is the first step. The finding
that NY-ESO-1 is expressed with relatively high frequency in HCC
patients may be of clinical significance.
Besides the positive rates, we have two new
findings: one is that NY-ESO-1 is a prognostic marker in HCC.
NY-ESO-1-positive patients are 7.28 (odds ratio) times more likely
to relapse compared with NY-ESO-1-negative cases following surgery.
It appears that the higher the NY-ESO-1 expression, the more likely
the recurrence. Another finding is that NY-ESO-1-positive patients
have a shorter RFS than that of NY-ESO-1-negative patients. Our
data are consistent with those of Perez et al in
gastrointestinal stromal tumors (18). Univariate and multivariate analysis
clearly indicated that patients with NY-ESO-1-positive tumors have
a shorter RFS, which is in accordance with the results of the
majority of the previous studies showing that NY-ESO-1 expression
may be a biomarker of poor prognosis in numerous types of cancer.
For example, the expression of NY-ESO-1 protein has been reported
to be correlated with the metastasis of HCC (12). In non-small cell lung cancer,
NY-ESO-1 expression significantly increased with the advancement of
disease stage in the TNM classification, particularly that related
to lymph node metastasis (19). In
addition, NY-ESO-1 is more frequently expressed in metastatic than
in primary malignant melanoma and its expression is associated with
advanced stage (20). The point to
be considered is the manner in which NY-ESO-1 increases tumor
recurrence and reduces RFS. Two ways in which this can be achieved
are possible: one is that NY-ESO-1 enhances tumor cell migration
and, therefore, metastasis; another is that NY-ESO-1 stimulates
tumor cell growth. Zhou et al found that the larger the HCC,
the higher the NY-ESO-1 expression (21). Other authors found no correlation
between NY-ESO-1 expression and HCC size (11,12).
To clarify the mechanism of NY-ESO-1 and HCC behavior, we
transfected HepG2 cells with pcDNA3.1-ESO. We found that
over-expression of NY-ESO-1 enhanced cell migration, but not
proliferation.
In conclusion, the present study confirmed NY-ESO-1
expression in a certain number of HCC patients, and showed that
NY-ESO-1 is a promising target for immunotherapy based on its
relatively high frequency expression in HCC. NY-ESO-1 may be a
potential biomarker for early recurrence and, therefore, a shorter
RFS following surgery. Moreover, NY-ESO-1 enhanced tumor cell
migration and, therefore, metastasis.
Acknowledgements
We thank Dr Jan J. Melenhorst at the National
Institute of Health for reading and editing the manuscript. We
thank our colleague XinQiu Yu (Branch of Liver Surgery, Beijing
YouAn Hospital) for help during HCC case collection. This study was
supported by the Beijing Natural Science Foundation (7092044-J.L),
the National High Technology Research and Development Program of
China (No. 2007AA02Z151) and (2009zx10004-309).
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
2
|
Venook AP: Treatment of hepatocellular
carcinoma: too many options? J Clin Oncol. 12:1323–1334.
1994.PubMed/NCBI
|
|
3
|
Chen YT, Scanlan MJ, Sahin U, et al: A
testicular antigen aberrantly expressed in human cancers detected
by autologous antibody screening. Proc Natl Acad Sci USA.
94:1914–1918. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen YT, Gure AO, Tsang S, et al:
Identification of multiple cancer/testis antigens by allogeneic
antibody screening of a melanoma cell line library. Proc Natl Acad
Sci USA. 95:6919–6923. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang RF, Johnston SL, Zeng G, Topalian SL,
Schwartzentruber DJ and Rosenberg SA: A breast and melanoma-shared
tumor antigen: T cell responses to antigenic peptides translated
from different open reading frames. J Immunol. 161:3598–3606.
1998.PubMed/NCBI
|
|
6
|
Perez D, Hauswirth F, Jager D, et al:
Protein expression of cancer testis antigens predicts tumor
recurrence and treatment response to imatinib in gastrointestinal
stromal tumors. Int J Cancer. 128:2947–2952. 2011. View Article : Google Scholar
|
|
7
|
Svobodova S, Browning J, Macgregor D, et
al: Cancer-testis antigen expression in primary cutaneous melanoma
has independent prognostic value comparable to that of Breslow
thickness, ulceration and mitotic rate. Eur J Cancer. 47:460–469.
2010. View Article : Google Scholar
|
|
8
|
Chen CH, Chen GJ, Lee HS, et al:
Expressions of cancer-testis antigens in human hepatocellular
carcinomas. Cancer Lett. 164:189–195. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Luo G, Huang S, Xie X, et al: Expression
of cancer-testis genes in human hepatocellular carcinomas. Cancer
Immun. 2:112002.PubMed/NCBI
|
|
10
|
Korangy F, Ormandy LA, Bleck JS, et al:
Spontaneous tumor-specific humoral and cellular immune responses to
NY-ESO-1 in hepatocellular carcinoma. Clin Cancer Res.
10:4332–4341. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Peng JR, Chen HS, Mou DC, et al:
Expression of cancer/testis (CT) antigens in Chinese hepatocellular
carcinoma and its correlation with clinical parameters. Cancer
Lett. 219:223–232. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang WM, Xiao G, Xie D, Zhang M, Guo AL
and Wen JM: Correlation of NY-ESO-1 gene and protein expression to
metastasis and clinicopathologic features of hepatocellular
carcinoma. Ai Zheng. 24:622–626. 2005.PubMed/NCBI
|
|
13
|
Zhang WM, Xiao G, Zhang M, Guo AL, Dong Y
and Wen JM: Expression of NY-ESO-1 and LAGE-1 cancer-testis
antigens in hepatocellular carcinoma. Zhonghua Bing Li Xue Za Zhi.
34:202–205. 2005.PubMed/NCBI
|
|
14
|
Nakamura S, Nouso K, Noguchi Y, et al:
Expression and immunogenicity of NY-ESO-1 in hepatocellular
carcinoma. J Gastroenterol Hepatol. 21:1281–1285. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lu Y, Wu LQ, Lu ZH, Wang XJ and Yang JY:
Expression of SSX-1 and NY-ESO-1 mRNA in tumor tissues and its
corresponding peripheral blood expression in patients with
hepatocellular carcinoma. Chin Med J (Engl). 120:1042–1046.
2007.PubMed/NCBI
|
|
16
|
Wang XY, Chen HS, Luo S, Zhang HH, Fei R
and Cai J: Comparisons for detecting NY-ESO-1 mRNA expression
levels in hepatocellular carcinoma tissues. Oncol Rep. 21:713–719.
2009.PubMed/NCBI
|
|
17
|
Abassi YA, Jackson JA, Zhu J, O’Connell J,
Wang X and Xu X: Label-free, real-time monitoring of IgE-mediated
mast cell activation on microelectronic cell sensor arrays. J
Immunol Methods. 292:195–205. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Perez D, Herrmann T, Jungbluth AA, et al:
Cancer testis antigen expression in gastrointestinal stromal
tumors: new markers for early recurrence. Int J Cancer.
123:1551–1555. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Konishi J, Toyooka S, Aoe M, et al: The
relationship between NY-ESO-1 mRNA expression and
clinicopathological features in non-small cell lung cancer. Oncol
Rep. 11:1063–1067. 2004.PubMed/NCBI
|
|
20
|
Velazquez EF, Jungbluth AA, Yancovitz M,
et al: Expression of the cancer/testis antigen NY-ESO-1 in primary
and metastatic malignant melanoma (MM)--correlation with prognostic
factors. Cancer Immun. 7:112007.PubMed/NCBI
|
|
21
|
Zhou JX, Li Y, Chen SX and Deng AM:
Expression and prognostic significance of cancer-testis antigens
(CTA) in intrahepatic cholagiocarcinoma. J Exp Clin Cancer Res.
30:22011. View Article : Google Scholar : PubMed/NCBI
|