Introduction
Breast cancer is a common malignancy and a leading
cause of death in women throughout the the world. Radiotherapy is
considered crucial treatment for most common types of cancer and is
usually used in conjunction with chemotherapy, hormone therapy or
surgery. Radiation is known to activate multiple signaling
pathways, causing cancer cells to become inactivated and resulting
in diverse types of stress responses, including apoptosis, cell
cycle arrest, senescence and gene induction. However, a large
number of tumors fail to respond to radiotherapy as they become
less sensitive or more resistant to radiation after consecutive
treatments. Various studies on the molecular mechanisms of
resistance to radiotherapy have been carried out. However,
obstacles related to overcoming this resistance remain to be
solved. Therefore, identification of the radiation-responsive genes
may aid to better understand the molecular mechanisms involved in
the response of tumors to radiation and, ultimately, improve
radiotherapy.
A number of aspects of the initial response to
radiation-induced DNA damage have been extensively analyzed via p53
and other DNA damage checkpoint responses (1,2). For
example, the tumor suppressor gene TP53 (p53) plays a
significant role in the cellular response to radiation-induced
stress (3–5). In cells carrying a non-functional
p53, cell-cycle arrest and DNA repair cannot occur. This
increases the level of genomic instability and allows tumor growth
despite the exposure to radiation (4–6). In
addition, a number of radiation-responsive genes are identified
through different approaches. Various stress-responsive effector
genes have been known to be inducible by radiation (7–11). The
major effector genes identified in the radiation-induced response
include RAF1, CDKNIA (p21), GADD45A (GADD45), 14-3-3 σ (a
member of the YWHA family), BAX, TNFRSF (Fas/APO1),
TNFRSF 10B (KILLER/DR5), PIG, THBS1 (TSP1),
IGFBP3 and DIR1. These radiation-responsive effector
genes and their control factors play key roles in the cellular
response to radiation-induced stress by modulating cell cycle
checkpoints, apoptosis and DNA repair, which enhance cell survival
(3,8–10,12–16).
Changes in the mRNA expression levels were reported by cDNA
microarray data targeting radiation-responsive genes in human
breast cancer cells (17–21). In addition, Kis et al
identified radiation-responsive genes using a microarray analysis
in primary human fibroblasts (22).
These authors detected approximately 200 ionizing radiation (IR)
responsive genes at the transcriptional level, of which 30 (28 up-
and 2 downregulated) responded to radiation in all investigated
cells and 20 were grouped according to function: DNA damage
response (GADD45A, BTG2, PCNA and IER5), regulation of the cell
cycle and cell proliferation (CDKN1A, PPM1D, SERTAD1, PLK2, PLK3
and CYR61), programmed cell death (BBC3 and TP53INP1), signaling
pathways (SH2D2A, SLIC1, GDF15, and THSD1), and other functions
(SEL10, FDXR, CYP26B1 and OR11A1) (22). However, the mRNA expression profiles
did not match their protein expression profiles as the
radiation-responsive genes appeared to respond differently at the
transcriptional and protein levels. Moreover, the molecular
mechanisms inactivating tumor cells in response to radiation have
yet to be elucidated, although several mechanisms are known to be
involved in this process. We, therefore, aimed to identify the
radiation-responsive genes at the protein level using
two-dimensional polyacrylamide gel electrophoresis (2D-PAGE) and
matrix assisted laser desorption/ionization time of flight-mass
spectrometry (MALDI-TOF-MS) in MCF-7 human breast cancer cells.
Tools such as 2D-PAGE and MALDI-TOF-MS have been widely used for
the study of cancer proteomics, as noted in several other studies
(23–25).
In this study, a global analysis of the protein
expression pattern was performed using 2D-PAGE and MALDI-TOF-MS to
identify radiation-responsive proteins in MCF-7 breast cancer
cells.
Materials and methods
Condition of the MCF-7 cell culture and
treatment of ionizing radiation (IR)
The MCF-7 human breast cancer cell line was
purchased from the American Type Culture Collection (ATCC,
Manassas, VA, USA). MCF-7 cells were cultured at 37°C in a
humidified atmosphere composed of 95% air and 5% CO2 in
Dulbecco’s modified Eagle’s medium (DMEM) (WelGENE Inc., Daegu-si,
Korea). This medium was supplemented with 10% fetal bovine serum
(Gibco BRL, Seoul, Korea) and 1% antibiotic-antimycotic (Gibco
BRL). To induce an IR response, the MCF-7 cells were irradiated
with γ-rays with a 137Cs γ-ray source (Atomic Energy of
Canada, Ltd., Ontario, Canada). The cells were harvested after the
indicated time of incubation at 37°C. Cell viability was assessed
by a trypan blue exclusion test.
Protein extraction
Cells were washed with cold phosphate-buffered
saline (PBS) and centrifuged at 3,000 rpm, 4°C for 3 min. The
centrifuged cells were resuspended with lysis buffer [500 mM HEPES
(pH 8.5), 4% CHAPS, 8 M urea, 1 μg/ml aprotinin, 100 μg/ml PMSF,
and 2.4 mg/ml DTT], sonicated for 10 sec (5X) on ice, and then
centrifuged at 13,000 rpm, at 4°C for 10 min. The concentrations of
the protein samples were determined using a modified Bradford
protein assay (Bio-Rad, Hercules, CA, USA).
2D-PAGE, gel scanning and image
analysis
The first dimensional isoelectric focusing (IEF) was
performed on precast 18 cm immobilized pH 3.0–10.0 gradient (IPG)
strips (Amersham Pharmacia Biotech) at 20°C using a commercial
flatbed electrophoresis system (IPGphor; Amersham Pharmacia
Biotech). Proteins of 500 μg were mixed with a rehydration buffer
containing 8 M urea, 2% (w/v) CHAPS, 2.4 mg/ml DTT, 2% (v/v) IPG
buffer, and a trace of bromophenol blue as a tracking dye. The
mixtures were loaded onto an IPG strip, followed by 12 h of active
rehydration at 50 V and 50 μA, which was ramped to 500 V and 50 μA
over a period of 10 min. It was then maitained at 5000 V and 50 μA
for 1 h. At the end of the first dimension run (80 kV•h), the IPG
strips were immediately loaded onto 11% SDS-PAGE gels and held in
place with 0.5% agarose dissolved in a SDS-PAGE running buffer. In
the second dimension, SDS-PAGE was performed for separation without
a stacking gel at 150 V, and 20 mA for 20 h per gel in a
SDS-electrophoresis buffer (25 mM Tris, 92 mM glycine and 0.1%
SDS). After electrophoresis separation, the gels were stained using
Bio-Rad Silver Stain kit (catalog no. 161-0443). The stained 2-D
gels were scanned on a Las-3000 (Fuji Photo Film Co.) using 2-D
software PDQuest (Bio-Rad). The different gel patterns were then
automatically matched to each other and the quantities of the
matched spots in the different gels were compared. The molecular
masses and pI values were calculated with the PDQuest software
using the selected pI and Mr standard proteins.
Matrix-assisted laser
desorption/ionization-time of flight-mass spectrometry
(MALDI-TOF-MS)
Proteins from the gels were identified by
MALDI-TOF-MS. After tryptic in-gel digestion of 2D-PAGE resolved
proteins, samples for MALDI peptide mass mapping were prepared, as
previously described (26). Mass
spectra were obtained using a Voyager-DE STR MALDI-TOF mass
spectrometer (Applied Biosystems, Cambridge, MA, USA). The proteins
were identified according to their tryptic peptide mass fingerprint
after a database search was performed on MS-Fit, which is
accessible over the World Wide Web at http://prospector.ucsf.edu/. MS-Fit performed a rapid
database search by comparing experimentally determined masses from
the proteolytic digestion of proteins with peptide database masses
calculated from the NCBlnr protein database and Swiss-Prot
accession numbers.
Cell cycle analysis by
fluorescence-activated cell sorting (FACS)
For a FACS analysis, cells were harvested at the
indicated time points, washed twice in ice-cold PBS, and fixed by
resuspending them in absolute ethanol for 30 min. The fixed cells
were centrifuged at 1,500 rpm for 5 min and washed twice with cold
PBS. The cell pellets were resuspended in 0.5 ml of PBS containing
50 μg/ml propidium iodide (Sigma-Aldrich Chemical Co., Korea), 10%
sodium citrate (Sigma), 100 μg/ml RNase (Invitrogen, Korea), and
0.001% NP40 (Sigma). Following their incubation at 37°C for 30 min
in the dark, the samples were analyzed by a FACScan flow cytometer
(Becton-Dickinson FACScan, Sunnyvale, CA, USA) equipped with the
CellQuest 3.2 software (Becton-Dickinson).
Results
G2 cell cycle arrest induced by IR in
MCF-7 cells
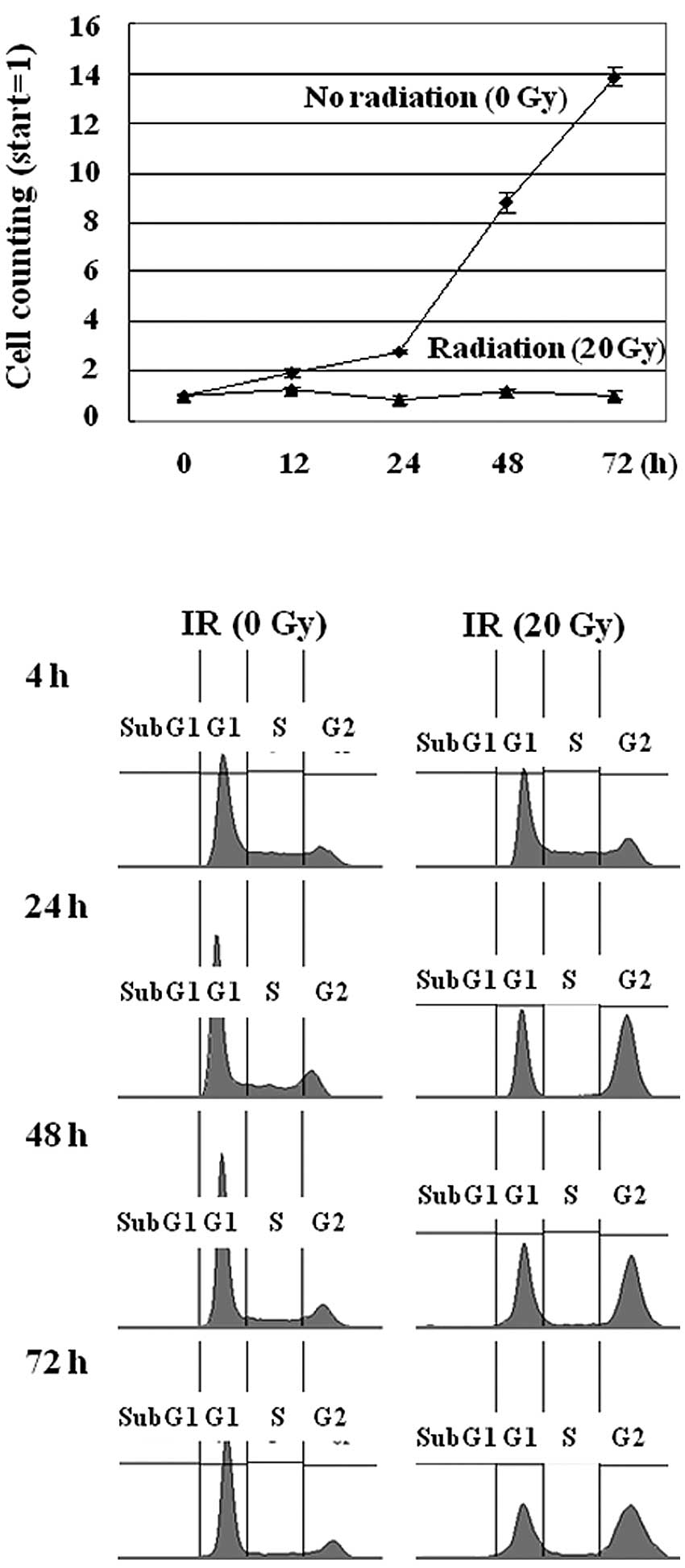
MCF-7 cells were irradiated with different doses of
γ-radiation of 1, 5, 10, or 20 Gy, in which the cell growth was
repressed in the cells exposed to 20 Gy of radiation, as shown in
Fig. 1A. The IR treatment of the
MCF-7 cells did not affect cell viability, as assessed by the
trypan blue exclusion test (data not shown). Instead, the cell
growth was repressed due to cell cycle arrest at the G2 phase, as
revealed in the FACS analysis (Fig.
1B). This result suggests that IR-irradiated MCF-7 cells
undergo cell cycle arrest rather than apoptosis. These data are
consistent with previous studies in which IR induced cell cycle
arrest but failed to activate the mitochondrial death pathway in
MCF-7 cells (27). Thus, to
establish the genes responsible for this phenotype we aimed to
identify the radiation-responsive proteins in MCF-7 cells.
Identification of radiation-responsive
genes in MCF-7 cells
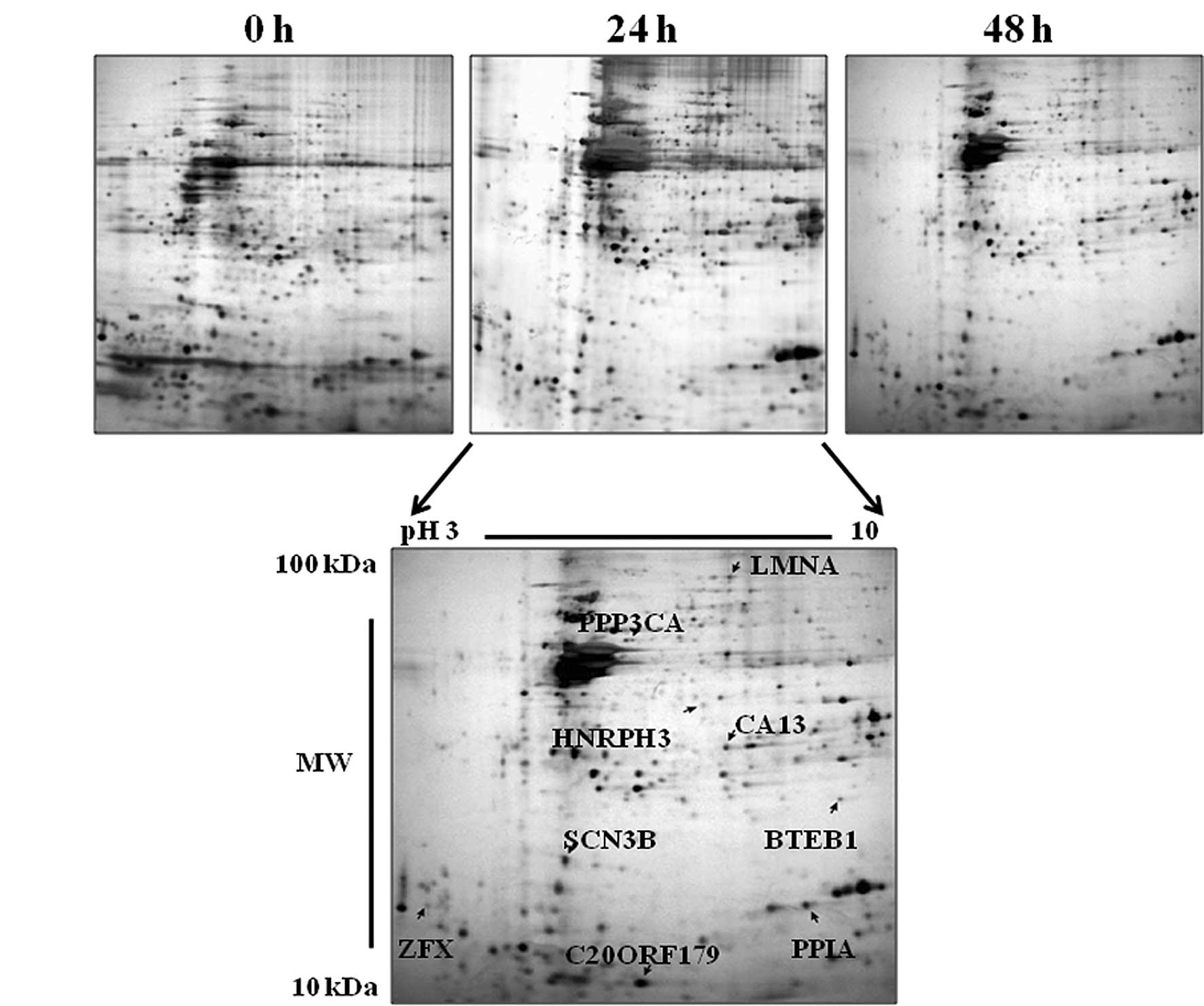
To obtain information on the expression profile of
radiation-induced and reduced protein, MCF-7 breast cancer cells
were irradiated with 20 Gy of γ-rays and harvested after incubation
for 0, 4, 12, 24 or 48 h. We then observed differentially expressed
proteins using 2D-PAGE and MALDI-TOF-MS as described in Materials
and methods. Proteins with approximately 1,000–1,200 spots in each
gel were visualized by silver staining and detected by PDQuest
2D-image-analysis software (Bio-Rad). After the spot detection, the
gels were matched to each other, with the aid of the so-called
landmark function, and approximately 730 spots in total were
matched in all gels analyzed according to the PDQuest software. For
internal standards, 9 spots were chosen randomly to calculate the
deviation of the spot position and identified by MALDI-TOF-MS.
These identified proteins included LMNA, PPP3CA, HNRPH3, CA13,
BTEB1, SCN3B, ZFX, PPIA and C20ORF179 (Fig. 2 and Table I), and were used for the generation
of a relevant pI and Mr scale for the entire pattern.
 | Table IStandard proteins identified by
MALDI-TOF-MS. |
Table I
Standard proteins identified by
MALDI-TOF-MS.
| ANa | Gene | Protein | pI/Mw (Da) |
|---|
| P02545 | LMNA | Lamin A/C | 6.6/74140 |
| Q08209 | PPP3CA | Serine/threonine
protein phosphatase
2B catalytic subunit, α isoform | 5.6/58688 |
| P31942 | HNRPH3 | Heterogeneous
nuclear ribonucleoprotein H3 | 6.4/36927 |
| Q8N1Q1 | CA13 | Carbonic anhydrase
XIII | 6.5/29443 |
| Q13886 | BTEB1 | Transcription
factor BTEB1 | 8.8/27235 |
| Q9NY72 | SCN3B | Sodium channel β-3
subunit | 4.7/24703 |
| 20977872b | ZFX | X-linked zinc
finger protein | 3.9/18903 |
| P05092 | PPIA | Peptidyl-prolyl
cis-trans isomerase A | 7.7/18013 |
| Q9H503 |
C20ORF179 | Hypothetical
BAF-like protein C20orf179 | 5.5/10309 |
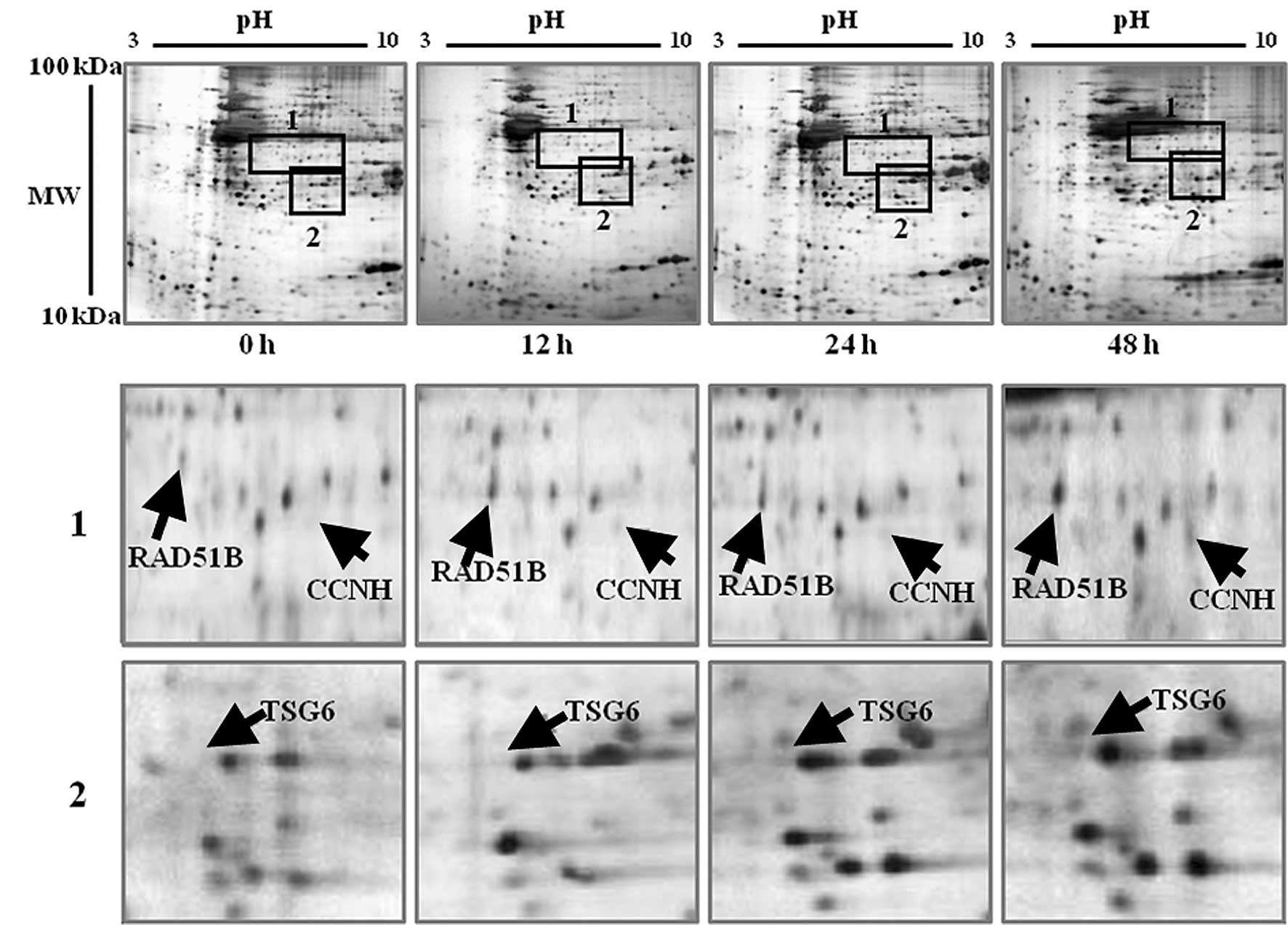
When the expression patterns of proteins were
compared to the control gel, numerous spots were found to differ
greatly from one another. Among them, 11 spots were found to be
significantly different. One set of proteins was upregulated in
response to IR in the MCF-7 cells. These proteins included RAD51B,
CCNH, TSG6, GH2, RGS17, BAK1, IGFBP1 and CASP14 (Table II). Fig. 3 shows the regions containing
upregulated proteins, which are marked by black rectangles and are
magnified on the lower gels. All of these proteins showed a similar
pattern in terms of the protein expression level. The expression
level of each protein started to increase after exposure to γ-rays
(Fig. 3A-C). Most of these proteins
are known to be involved in the cellular processes of cell cycle
control, apoptosis, DNA repair, cell proliferation, and other
functions.
 | Table IIDifferentially expressed proteins in
response to IR in MCF-7 cells. |
Table II
Differentially expressed proteins in
response to IR in MCF-7 cells.
| Class | ANa | Gene | Protein | pI/Mw (Da)b |
|---|
| Class I
(Upregulated proteins in response to IR in MCF-7 cells) |
| O15315 | RAD51B | DNA repair protein
RAD51 homolog 2 (R51H2) | 5.8/38257 |
| P51946 | CCNH | Cyclin H | 6.7/37644 |
| P98066 | TSG6 | Tumor necrosis
factor-inducible protein TSG-6 | 6.5/31232 |
| P01242 | GH2 | Growth hormone
variant | 7.6/25000 |
| Q9UGC6 | RGS17 | Regulator of
G-protein signaling 17 | 5.6/24360 |
| Q16611 | BAK1 | Bcl-2 homologous
antagonist/killer | 5.7/23409 |
| P08833 | IGFBP1 | Insulin-like growth
factor binding protein 1 | 5.1/27904 |
| P31944 | CASP14 | Caspase-14 | 5.4/27680 |
| Class II
(Downregulated proteins in response to IR in MCF-7 cells) |
| O75973 | C1QRF | C1q-related
factor | 5.3/26453 |
| Q9NRY7 | PLSCR2 | Phospholipid
scramblase 2 | 5.5/25523 |
| Q9UHV2 |
p34SEI-1 | cycline-dependent
kinase 4 (cdk4)-binding protein | 4.3/24674 |
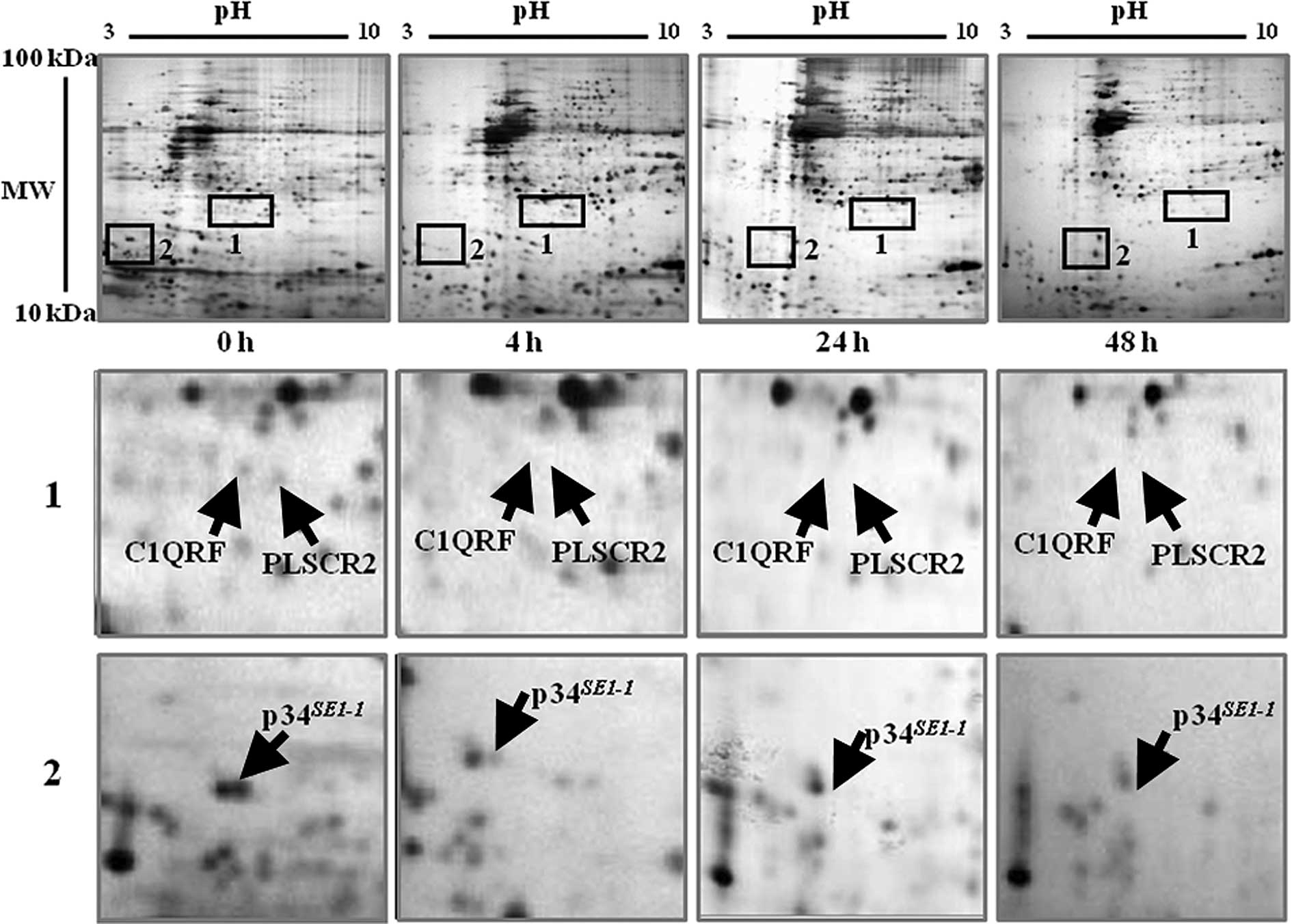
In another set, the expression levels of three
proteins were downregulated following exposure to γ-rays. They
include C1QRF, PLSCR2 and p34SE1-1. These
proteins are marked by black rectangles on the gels and are
magnified on the lower gels (Fig.
4). In the percentage volume of the spots compared between 0
and 48 h using the PDQuest software, a considerable amount of each
protein was detected in the control gel (0 h). The C1QRF and PLSCR2
proteins were found only at 0 h and p34SE1-1 was
detected at 0 and 4 h. However, their expression levels started to
decrease from 12 h and were significantly downregulated after 48 h
(Fig. 4).
Discussion
In this study, we observed up- and downregulated
proteins in the MCF-7 breast cancer cell line. The proteins
maintained at an elevated level in the radiation-derived MCF-7
cells are correlated to cell cycle control, apoptosis, DNA repair,
and cell proliferation. The genes responsible for this elevation
include: RAD51B, which encodes a DNA repair protein RAD51
homolog 2, which is involved in the homologous recombination repair
(HRR) pathway of double-stranded DNA breaks arising during DNA
replication or induced by DNA-damaging agents (28). CCNH encodes cyclin H, which
regulates CDK7, the catalytic subunit of the CDK-activating kinase
(CAK) enzymatic complex. It is involved in cell cycle control and
RNA transcription by RNA polymerase II. Its expression and activity
are constant throughout the cell cycle (29). TSG6 encodes the tumor
necrosis factor-inducible protein TSG-6. This gene may be involved
in cell-cell and cell-matrix interactions during inflammation and
tumorigenesis (30). GH2
encodes a growth hormone variant that plays an important role in
growth control and stimulates the proliferation of human MCF-7
mammary carcinoma cells by activating the MAPK signaling pathway
(31). RGS17 encodes a
regulator of G-protein signaling 17 that inhibits signal
transduction by increasing the GTPase activity of G protein α
subunits thereby driving them into their inactive GDP-bound form.
RGS17 plays important roles in T-cell proliferation and IL-2
production and RGS17 deficiency leads to impaired T-cell activation
(32). BAK1 encodes a Bcl-2
homologous antagonist/killer that has been reported to be regulated
by p53 (33). It induces cell death
and accelerated apoptosis (34–36).
IGFBP1 encodes insulin-like growth factor binding protein 1.
IGFBP1 is expressed in the breast both in vitro and in
vivo (37,38). The upregulation of IGFBP1 is
associated with the malignant transformation of breast tissue
(38). CASP14 encodes caspase-14
which is involved in the death receptor and granzyme B apoptotic
pathways. It may function as a downstream signal transducer of cell
death (39).
On the other hand, protein expression levels for
C1QRF, PLSCR2 and p34SE1-1 were decreased. The
genes responsible in this case include: C1QRF, which encodes
a C1q-related factor containing one C1q domain that is a component
of the complement pathway, and is involved in tumor cytotoxicity
(40). Moreover, PLSCR2
encodes phospholipid scramblase 2, which plays a central role in
the recognition of apoptotic and injured cells by the
reticuloendothelial system (41).
p34SE1-1 encodes a transcriptional regulator
interacting with PHD-bromodomain 1 (Trip-Br1), which renders the
activity of cyclin D1/CDK4 resistant to the inhibitory effects of
p16INK4a. In addition, p16INK4a specifically
binds and inhibits CDK4, the partner kinase of the D1 cyclin
strongly implicated in the phosphorylation of pRb and thereby in
G1/S control (42–44). The decreased expression of the
p34SE1-1 protein may be related to the induction
of cell cycle arrest at the G2 phase in MCF-7 cells.
In conclusion, our study may aid in better
understanding the molecular mechanism that responds to radiation in
cancer cells. Additionally, our findings may contribute to the
development of more effective ways of combining radiation therapy
with other systemic therapies.
Abbreviations:
References
|
1
|
El-Deiry WS: The role of p53 in
chemosensitivity and radio-sensitivity. Oncogene. 22:7486–7495.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Iliakis G, Wang Y, Guan J and Wang H: DNA
damage checkpoint control in cells exposed to ionizing radiation.
Oncogene. 22:5834–5847. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Amundson SA, Myers TG and Fornace Jr:
Roles for p53 in growth arrest and apoptosis: Putting on the brakes
after genotoxic stress. Oncogene. 17:3287–3299. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fornace AJ Jr, Amundson SA, Bittner M,
Myers TG, Meltzer P, Weinsten JN and Trent J: The complexity of
radiation stress reponse: analysis by informatics and functional
genomics approaches. Gene Expr. 7:387–400. 1999.PubMed/NCBI
|
|
5
|
King TC, Estalilla OC and Safran H: Role
of p53 and p16 gene alterations in determining response to
concurrent paclitaxel and radiation in solid tumor. Semin Radiat
Oncol. 9:4–11. 1999.PubMed/NCBI
|
|
6
|
Lu X and Lane DP: Differential induction
of transcriptionally active p53 following UV or ionizing
irradiation: defects in chromosome instability syndromes? Cell.
75:765–778. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Keyse SM: The induction of gene expression
in mammalian cells by radiation. Semin Cancer Biol. 4:119–128.
1993.PubMed/NCBI
|
|
8
|
Iliakis G: Cell cycle regulation in
irradiated and nonirradiated cells. Semin Oncol. 24:602–615.
1997.PubMed/NCBI
|
|
9
|
Eckardt Schupp F and Klaus C: Radiation
inducible DNA repair processes in eukaryotes. Biochimie.
81:161–171. 1999.PubMed/NCBI
|
|
10
|
Maity A, McKenna WG and Muschel RJ: The
molecular basis for cell cycle delays following ionizing radiation:
a review. Radiother Oncol. 31:1–13. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Forrester HB, Vidair CA, Albright N, Ling
CC and Dewey WC: Using computerized video time lapse for
quantifying cell death of Xirradiated rat embryo cells transfected
with cmyc or cHaras. Cancer Res. 59:931–939. 1999.PubMed/NCBI
|
|
12
|
Kasid U, Suy S, Dent P, Ray S, Whiteside
TL and Sturgill TW: Activation of Raf by ionizing radiation.
Nature. 382:813–816. 1996. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Waldman T, Lengauer C, Kinzler KW and
Vogelstein B: Uncoupling of S phase and mitosis induced by
anticancer agents in cells lacking p21. Nature. 381:713–716. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
El-Deiry WS: Regulation of p53 downstream
genes. Semin Cancer Biol. 8:345–357. 1998. View Article : Google Scholar
|
|
15
|
Robson T, Joiner MC, Wilson GD, McCullough
W, Price ME, Logan I, Jones H, McKeown SR and Hirst DG: A novel
human stress response-related gene with a potential role in induced
radio-resistance. Radiat Res. 152:451–461. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lowe SW, Schmitt EM, Smith SW, Osborne BA
and Jacks T: p53 is required for radiation-induced apoptosis in
mouse thymocytes. Nature. 362:847–849. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Honda M, Kaneko S, Kawai H, Shirota Y and
Kobayashi K: Differential gene expression between chronic hepatitis
B and C hepatic lesion. Gastroenterology. 120:955–966. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Okabe H, Satoh S, Kato T, Kitahara O,
Yanagawa R, Yamaoka Y, Tsunoda T, Furukawa Y and Nakamura Y:
Genome-wide analysis of gene expression in human hepatocellular
carcinomas using cDNA microarray: identification of genes involved
in viral carcinogenesis and tumor progression. Cancer Res.
61:2129–2137. 2001.
|
|
19
|
Shirota Y, Kaneko S, Honda M, Kawai HF and
Kobayshi K: Identification of differently expressed genes in
hepatocellular carcinoma with cDNA microarrays. Hepatology.
33:832–840. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Takeo S, Arai H, Kusano N, Harada T, et
al: Examination of oncogene amplification by genomic DNA microarray
in hepatocellular carcinomas comparison with comparative genomic
hybridization analysis. Cancer Genet Cytogenet. 130:127–132. 2001.
View Article : Google Scholar
|
|
21
|
Xu XR, Huang J, Xu ZG, et al: Insight into
hepatocellular carcinogenesis at transcriptome level by comparing
gene expression profiles of hepatocellular carcinoma with those of
corresponding noncancerous liver. Proc Natl Acad Sci USA.
98:15089–15094. 2001. View Article : Google Scholar
|
|
22
|
Kis E, Szatmari T, Keszei M, Farkas R,
Esik O, Lumniczky K, Falus A and Safrany G: Microarray analysis of
radiation response genes in primary human fibroblasts. Int J Radiat
Oncol Biol Phys. 66:1506–1514. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hondermarck H, Vercoutter-Edouart A-S,
Revillion F, Lemoine J, El-Yazidi-Belkoura I, Nurcombe V and Peyrat
JP: Proteomics of breast cancer for marker discovery and signal
pathway profiling. Proteomics. 1:1216–1232. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Oh JM, Brichory F, Puravs E, Kuick R, Wood
C, Rouillard JM, Tra J, Kardia S, Beer D and Hanash S: A database
of protein expression in lung cancer. Proteomics. 1:1303–1319.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Stulik J, Hernychova L, Porkertova S,
Knižek J, Macela A, Bures J, Jandik P, Langridge JI and Jungblut
PR: Proteome study of colorectal carcinogenesis. Electrophoresis.
22:3019–3025. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim W, Oe LS, Kim JS, Ryu YH, Byeon JY,
Kim HJ, Kim YI, Heo JS, Park YM and Jung G: Comparison of proteome
between hepatitis B virus- and hepatitis C virus-associated
hepatocellular carcinoma. Clin Cancer Res. 9:5493–5500.
2003.PubMed/NCBI
|
|
27
|
Janicke RU, Engels IH, Dunkern T, Kaina B,
Schulze-Osthoff K and Porter AG: Ionizing radiation but not
anticancer drugs causes cell cycle arrest and failure to activate
the mitochondrial death pathway in MCF-7 breast carcinoma cells.
Oncogene. 20:5043–5053. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Albala JS, Thelen MP, Prange C, Fan W,
Christensen M, Thompson LH and Lennon GG: Identification of a novel
human RAD51 homolog, RAD51B. Genomics. 46:476–479. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jin K, Nagayama T, Chen J, Stetler AR,
Kawaguchi K, Simon RP and Graham SH: Molecular cloning of a cell
cycle regulation gene cyclin H from ischemic rat brain: expression
in neurons after global cerebral ischemia. J Neurochem.
73:1598–1608. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee TH, Wisniewski HG and Vilcek J: A
novel secretory tumor necrosis factor-inducible protein (TSG-6) is
a member of the family of hyaluronate binding proteins, closely
related to the adhesion receptor CD44. J Cell Biol. 116:545–557.
1992. View Article : Google Scholar
|
|
31
|
Kaulsay KK, Mertani HC, Tornell J, Morel
G, Lee KO and Lobie PE: Autocrine stimulation of human mammary
carcinoma cell proliferation by human growth hormone. Exp Cell Res.
250:35–50. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Oliveira-dos-Santos AJ, Matsumoto G, Snow
BE, et al: Regulation of T cell activation, anxiety, and male
aggression by RGS2. Proc Natl Acad Sci USA. 97:12272–12277. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pearson AS, Spitz FR, Swisher SG, Kataoka
M, Sarkiss MG, Meyn RE, McDonnell TJ, Cristiano RJ and Roth JA: Up-
regulation of the proapoptotic mediators Bax and Bak after
adenovirus-mediated p53 gene transfer in lung cancer cells. Clin
Cancer Res. 6:887–890. 2000.PubMed/NCBI
|
|
34
|
Chittenden T, Harrington EA, O’Connor R,
Flemington C, Lutz RJ, Evan GI and Guild BC: Induction of apoptosis
by the Bcl-2 homologue Bak. Nature. 374:733–736. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Farrow SN, White JH, Martinou I, Raven T,
Pun KT, Grinham CJ, Martinou JC and Brown R: Cloning of a bcl-2
homo-logue by interaction with adenovirus E1B 19K. Nature.
374:731–733. 1995. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kiefer MC, Brauer MJ, Powers VC, Wu JJ,
Umansky SR, Tomei LD and Barr PJ: Modulation of apoptosis by the
widely distributed Bcl-2 homologue Bak. Nature. 374:736–739. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Clemmons DR, Camacho-Hubner C, Coronado E
and Osborne CK: Insulin-like growth factor binding protein
secretion by breast carcinoma cell lines: correlation with estrogen
receptor status. Endocrinology. 127:2679–2686. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pekonen F, Nyman T, Ilvesmöki V and
Partanen S: Insulin-like growth factor binding proteins in human
breast cancer tissue. Cancer Res. 52:5204–5207. 1992.PubMed/NCBI
|
|
39
|
Pistritto G, Jost M, Srinivasula SM, Baffa
R, Poyet JL, Kari C, Lazebnik Y, Rodeck U and Alnemri ES:
Expression and transcriptional regulation of caspase-14 in simple
and complex epithelia. Cell Death Differ. 9:995–1006. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jurianz K, Ziegler S, Garcia-Schüler H,
Kraus S, Bohana-Kashtan O, Fishelson Z and Kirschfink M: Complement
resistance of tumor cells: basal and induced mechanisms. Mol
Immunol. 36:929–939. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sugimoto M, Nakamura T, Ohtani N, Hampson
L, Hampson IN, Shimamoto A, Furuichi Y, Okumura K, Niwa S, Taya Y
and Hara E: Regulation of CDK4 activity by a novel CDK4-binding
protein, p34 (SEI-1). Genes Dev. 13:3027–3033. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Herbert BR, Sanchez J-C, Bini L, Wilkins
MR, Williams KL, Appel RD and Hochstrasser DF: Proteome Research:
New Frontiers in Functional Genomics. Springer; Berlin: pp. 13–33.
1997
|
|
43
|
Sherr CJ and Roberts JM: CDK inhibitors:
positive and negative regulators of G1-phase progression. Genes
Dev. 13:1501–1512. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bartek J, Bartkova J and Lukas J: The
retinoblastoma protein pathway in cell cycle control and cancer.
Exp Cell Res. 237:1–6. 1997. View Article : Google Scholar : PubMed/NCBI
|