Introduction
Pancreatic cancer is the fourth most common cause of
mortality from cancer in the USA (1). Due to its early invasion and
metastasis, this cancer is typically diagnosed at a late stage.
With a 5-year survival rate of 1–4% and a median survival period of
4–6 months, the prognosis of patients with pancreatic cancer
remains poor (2–7). Intensive molecular studies of
pancreatic cancer are key to solving these problems.
Previous studies showed that the epithelial to
mesenchymal transition (EMT) is an important process in tumor
progression and metastasis in pancreatic cancer (8,9). EMT
is characterized by the loss of epithelial characteristics and the
acquisition of a mesenchymal phenotype, which increases the
invasion and migration activity of cancer cells (10–14).
Growth factors, such as transforming growth factor β (TGF-β) and
epidermal growth factor, are currently used to induce EMT (15,16).
Mounting evidence shows that cancer stem cells
(CSCs) play a significant role in tumor growth and propagation as
they are capable of self-renewal and the production of
differentiated progeny (17–20).
Pancreatic CSCs were initially defined by their simultaneous
expression of CD44, CD24 and EpCAM (21). Compared with other pancreatic cancer
cells, CD44+CD24+ESA+ cells are
the most highly tumorigenic. Injection of as few as 100 cells
results in tumor formation in immunodeficient, non-obese,
diabetic/severe combined immunodeficient mice. Increased resistance
of CSCs to standard chemotherapy has been shown in a number of
tumor types (22–24). In their study, Mani et al
(25) reported that EMT generates
CSCs in breast cancer. In ovarian cancer, transfection with Snail
and Snail2 led to increases of a CD44+ CD117+
CSC population, which had increased resistance to chemo- and
radiotherapy (26).
In the present study, we examined the possible
association between EMT and CSCs in pancreatic cancer. We used
TGF-β to induce EMT and measured the proportion of pancreatic CSCs
by flow cytometry.
Materials and methods
Cell culture
Human pancreatic cancer cells, PANC-1, were obtained
from the Shanghai Cell Bank (Shanghai, China) and propagated in our
laboratory. All cells were cultured in Dulbecco’s modified Eagle’s
medium (DMEM; HyClone Laboratories, Inc., UT, USA) supplemented
with 10% fetal bovine serum (FBS; Gibco, Carlsbad, CA, USA) and 1%
penicillin/streptomycin.
Quantitative reverse transcription
polymerase chain reaction (qRT-PCR)
Total RNA was isolated using TRIzol reagent
(Invitrogen; Carlsbad, CA, USA), according to the manufacturer’s
instructions and using the following PCR primers: E-cadherin sense:
5′-GCGATGGCGGCATTGTA-3′, antisense: 5′-GAGAACGCATTGCCACATACA-3′;
vimentin sense: 5′-CTGAACCTGAGGGAAACTAATC-3′, antisense:
5′-GCAGAAAGGCACTTGAAAGC-3′; and β-actin sense:
5′-AGAAAATCTGGCACCACACC-3′, antisense: 5′-TAGC ACAGCCTGGATAGCAA-3′.
qRT-PCR was performed using an ABI PRISM 7000 Sequence Detection
System (Applied Biosystems; Foster City, CA, USA) with SYBR Premix
EX Taq (Takara; Dalian, China).
Western blot analysis
The protein content of cultured cells was determined
using a bicinchoninic acid (BCA) Kit (Keygen; Nanjing, China). We
resolved the protein with 10% SDS-PAGE and transferred it to
polyvinylidene difluoride membranes. The membranes were blocked
with 5% non-fat milk in Tris-buffered saline for 2 h and incubated
overnight with primary antibodies against E-cadherin (Millipore;
Bedford, MA, USA) and vimentin (Millipore). The membranes were then
washed and incubated for 2 h with horseradish peroxidase-conjugated
goat anti-rabbit or goat anti-mouse secondary antibody (Santa Cruz
Biotechnology, Inc.; Santa Cruz, CA, USA). Antibodies were detected
using an electrochemiluminescence kit (Pierce; Rockford, IL,
USA).
Flow cytometry
To identify and isolate
CD44+CD24+ and
CD44−CD24− cells, the cells were washed with
phosphate-buffered saline (PBS), removed from the culture dish with
0.25% trypsin and ethylenediaminetetraacetic acid (EDTA), and
suspended in culture medium containing 10% FBS. The cells were
stained with CD24-PE (5 μl/ml) and CD44-FITC (1 μl/ml) antibodies
(eBioscience; San Diego, CA, USA). Cell cycle analysis was
conducted using a BD FACSCalibur flow cytometer and
fluorescence-activated cell sorting (FACS) using a BD FACSAriaII
special order system.
Cell cycle assays
Cell cycle distribution was assessed by flow
cytometry. Sorted CD44+CD24+ and
CD44−CD24− cells were collected, washed with
PBS and fixed in 70% ice-cold ethanol at 4°C overnight, then
suspended in 500 ml of PBS stained with 20 μg/ml propidium iodide
and 1 mg/ml RNase.
Cell migration and invasion
For analysis of cell migration and invasion,
1×105 sorted tumor cells were seeded onto the upper side
of 24-well Transwell plates, uncoated (for migration assays) or
coated (for invasion assays) with 1 mg/ml Matrigel (BD Biosciences;
Bedford, MA, USA). The chambers were 6.5 mm in diameter with an
8-mm pore size (Corning Life Sciences; Lowell, MA, USA). DMEM (600
μl) supplemented with 10% FBS was added to the lower chamber. The
cells were incubated for 24 h at 37°C, and cells on the upper side
were then removed with cotton swabs. Migrating or invading cells on
the bottom of the membrane were stained with 0.1% crystal violet
for 30 min at 37°C. Penetrating cells were stained and counted
under a microscope.
Statistical analysis
Data were presented as the means ± standard
deviation (SD). To compare the two groups, the Student’s t-test was
performed using SPSS 13.0. P<0.05 was considered to be
statistically significant.
Results
TGF-β1 induces EMT in pancreatic cancer
cells
To determine whether cancer cells that have
undergone an EMT are enriched with cancer stem-like cells, we used
TGF-β1, which is capable of inducing EMT in epithelial cells.
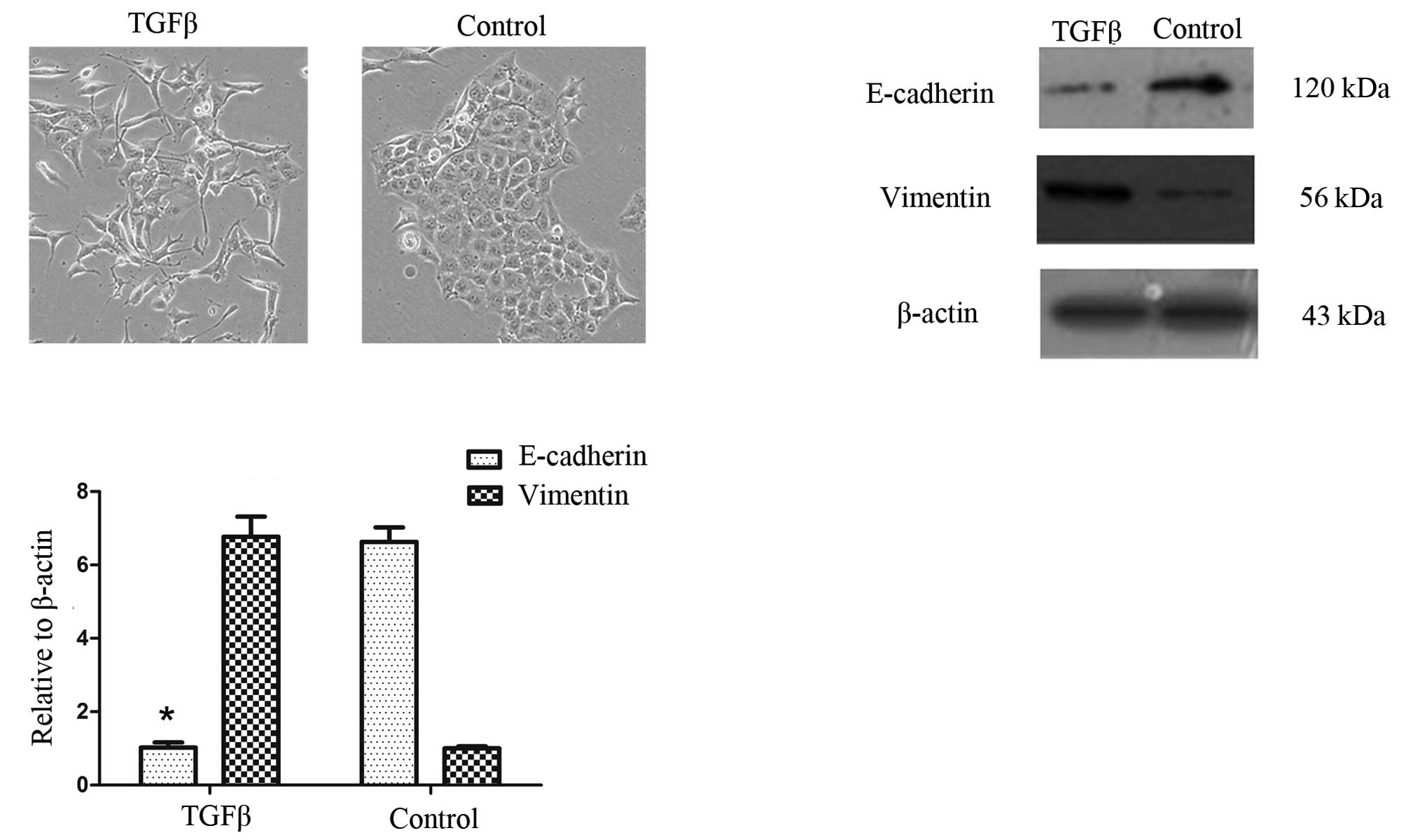
Cell morphology was assessed prior to and up to 72 h
following TGF-β1 treatment in PANC-1 cells. TGF-β1 induced EMT in
PANC-1 cells. Fig. 1A shows that,
when treated with TGF-β1 (10 ng/ml), PANC-1 cells acquire a
spindle-type morphology and that the number of cell-cell contacts
are reduced (16).
We then examined the effects of TGF-β1 on the mRNA
and protein expression of EMT-related markers in the PANC-1 cells.
As expected, TGF-β1 treatment reduced the expression of the
epithelial marker E-cadherin but increased the expression of the
mesenchymal marker vimentin (Fig. 1B
and C).
TGF-β1-induced EMT increases cancer
stem-like cells
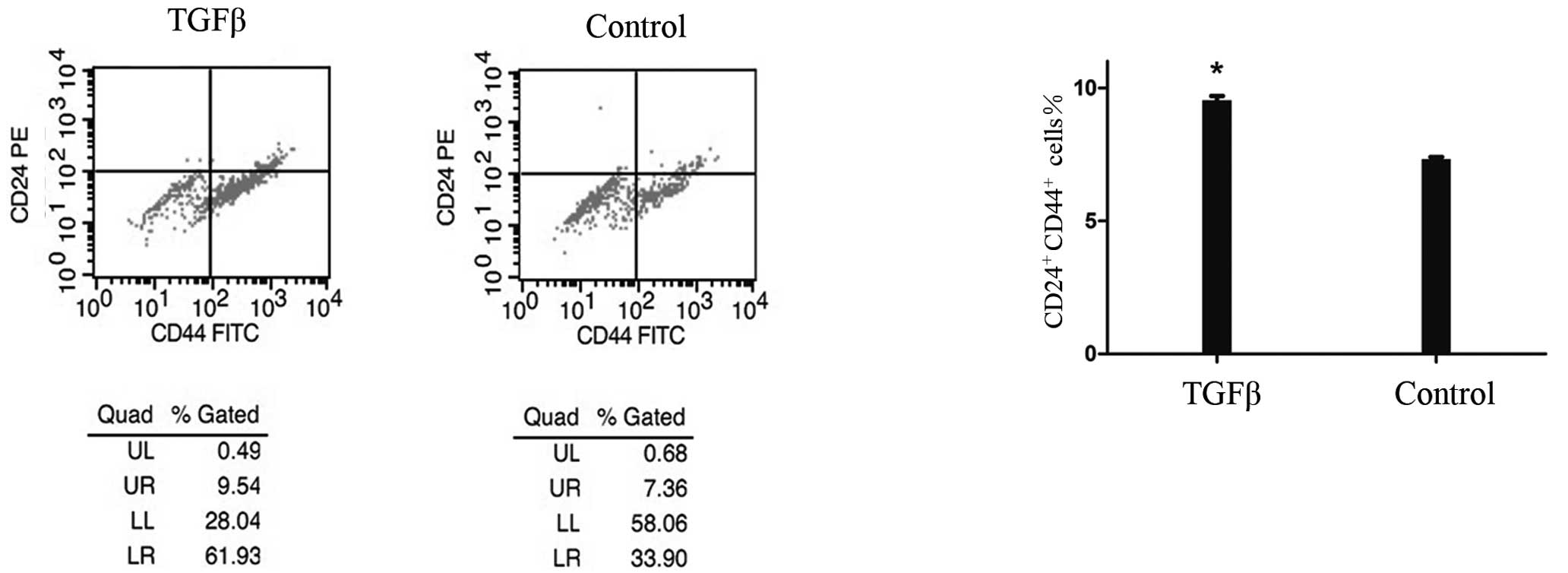
To determine the effect of TGF-β1-induced EMT on the
population of stem-like cells, we used flow cytometry to analyze
the cells prior to and following TGF-β1 treatment, based on the
expression of CD44 and CD24, two cell-surface markers whose
expression in the CD44+CD24+ configuration is
associated with pancreatic CSCs. Fig.
2 shows that the number of cancer stem-like cells was
significantly increased by 30% among 48 h-TGF-β1-treated PANC1
cells compared with the controls. This increase was time-dependent
and occurred concomitantly with morphological changes. It commenced
12 h following treatment and was maximal after 48 h. Notably, the
number of CD44+ pancreatic cancer cells was
significantly increased by 73% compared with the controls, whereas
the number of CD24+ cells was increased by 20%. The
increase in the number of CD24+ cells occurred later
than that of the CD44+ cells.
CD44+CD24+ cells
exhibit a higher degree of EMT
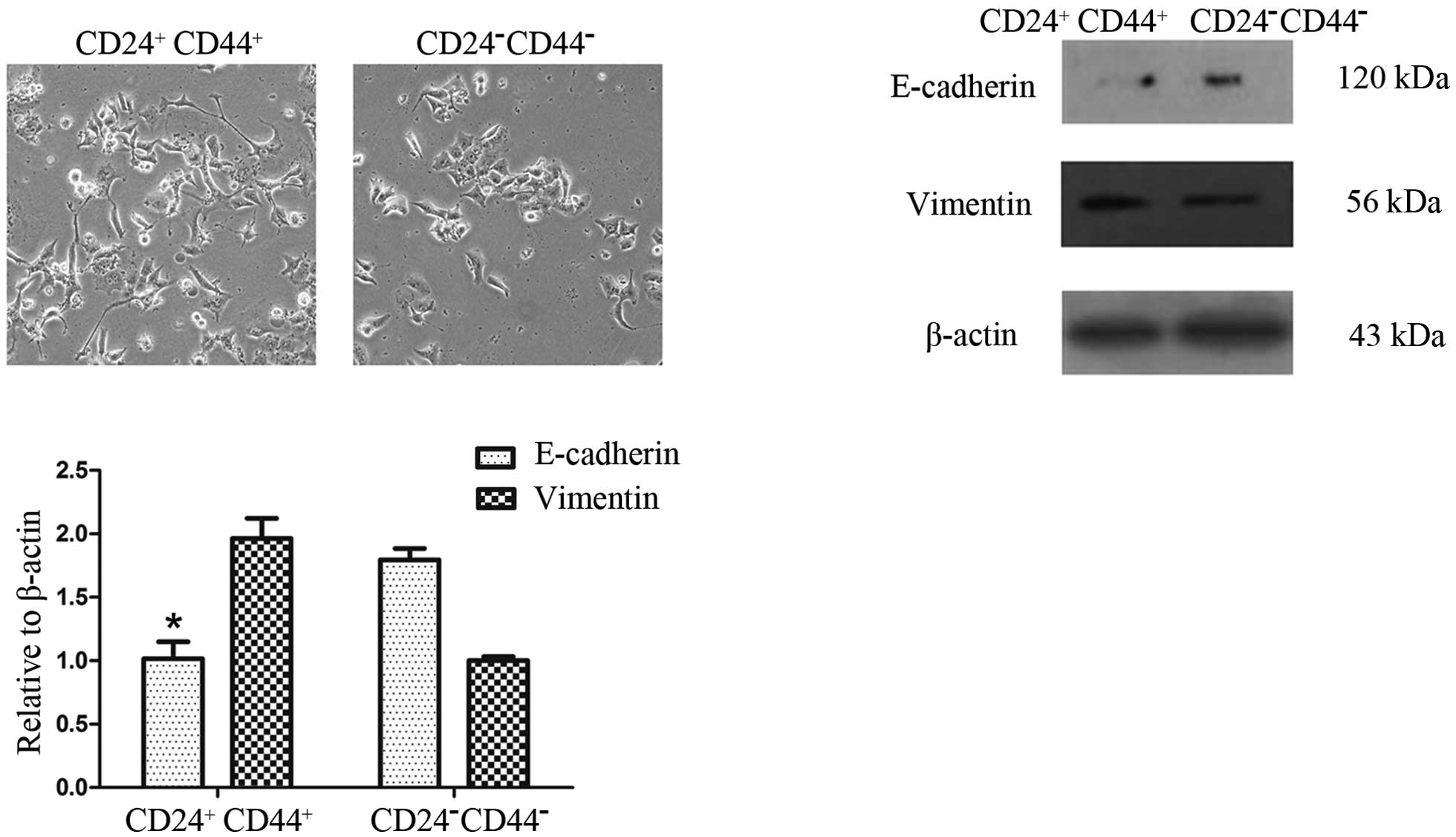
To determine whether
CD44+CD24+ cells exhibit phenotypes similar
to CD44−CD24− cells, we first sorted
CD44+CD24+ and
CD44−CD24− pancreatic cancer cells by FACS
into cell culture. The CD44+CD24+ and
CD44−CD24− cells both exhibited mesenchymal
morphology similar to that of cells that have undergone EMT.
Compared with the CD44−CD24− cells, the
CD44+CD24+ cells exhibited mesenchymal
morphology more clearly and the number of cell-cell contacts were
fewer (Fig. 3A). This difference in
phenotype was observed following 24 h of cell culture.
To determine whether the
CD44+CD24+ and
CD44−CD24− cells have varying gene expression
profiles associated with EMT, we used qRT-PCR and Western blot
analysis to measure the expression of EMT-associated markers.
Western blot analysis showed a reduced expression of the epithelial
marker E-cadherin but an increased expression of the mesenchymal
marker vimentin in CD44+CD24+ cells, compared
with the CD44−CD24− cells. These results were
confirmed by qRT-PCR analysis (Fig. 3B
and C).
Cell cycle differences between
CD44+CD24+ and
CD44−CD24− cells
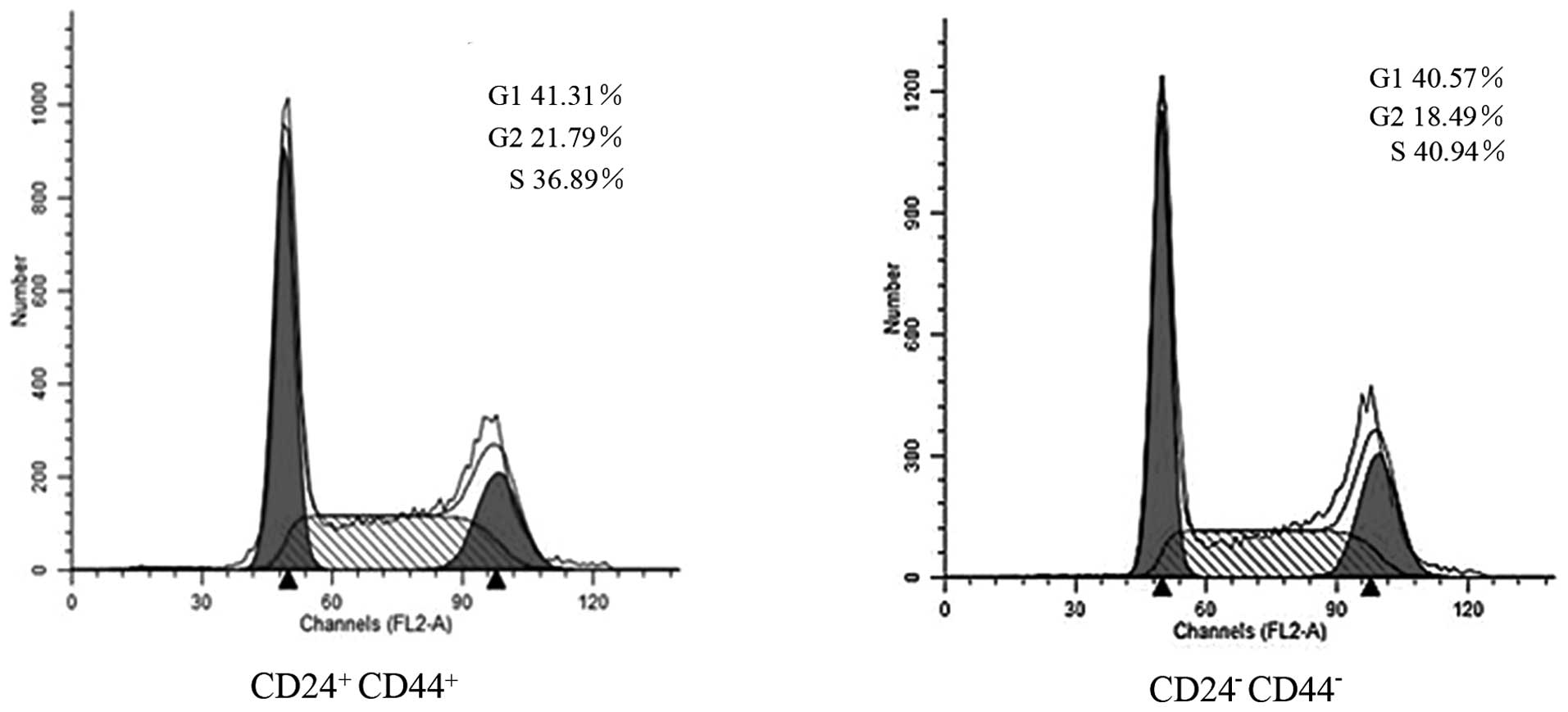
We used flow cytometry to determine whether the
differences between CD44+CD24+ and
CD44−CD24− cells sorted by FACS were a result
of differences in cell cycle distribution. Fig. 4 shows that there was no significant
accumulation of the G0/G1 phenotype among the
CD44+CD24+ cells (41.31%) compared with the
CD44−CD24− cells (40.57%).
CD44+CD24+ cells
have greater migration and invasion activity
We evaluated cell migration and invasion to assess
differences between CD44+CD24+ and
CD44−CD24− cells sorted by FACS. Fig. 5A shows that the migration ability of
the CD44+CD24+ cells was significantly
increased by 60%, compared with that of the
CD44−CD24− cells. A Matrigel invasion assay
was performed concomitantly. As shown in Fig. 5B, the invasive ability of the
CD44+CD24+ cells was significantly increased
by 70%, compared with that of the CD44−CD24−
cells. These results suggest that CD44+CD24+
cells have greater migration and invasive activity than
CD44−CD24− cells.
Discussion
The principal purpose of our study was to
investigate the connection between pancreatic cancer stem-like
cells and the EMT. The results show that: i) TGF-β1-induced EMT
increases cancer stem-like cells; ii) pancreatic cancer stem-like
cells exhibit a higher degree of EMT; and iii) pancreatic cancer
stem-like cells have greater migration and invasive activity in
vitro.
Numerous studies have suggested that TGF-β is
overexpressed in pancreatic cancer (27) and that exposure of pancreatic cancer
cells to TGF-β leads to greater motility and invasive ability
(28). We selected TGF-β1 to induce
EMT in pancreatic cancer cell lines based on the studies of
Ellenrieder et al (16), in
which five pancreatic cancer cell lines were treated with TGF-β1
and three underwent EMT. We found that the morphology of the cells
changed to spindle type and that the number of cell-cell contacts
were reduced. qRT-PCR and Western blot analysis confirmed that the
induction of EMT was successful.
Pancreatic CSCs were initially defined by their
simultaneous expression of CD44, CD24 and EpCAM (21). CSCs are capable of self-renewal and
the production of differentiated progeny. To investigate the effect
of EMT on a population of stem-like cells, we used flow cytometry
for analysis and sorting based on their expression of CD44 and
CD24. We found that the number of stem-like cells was significantly
increased in the TGF-β1-treated group compared with the controls,
and that the pancreatic stem-like cells exhibited a higher degree
of EMT than the CD44−CD24− cells, which also
have gene expression similar to that of cells that have undergone
EMT. It remains unknown as to why the
CD44−CD24− cells possess EMT features.
Notably, the increase in the number of CD24+ pancreatic
cancer cells was lower and occurred later than that of
CD44+ pancreatic cancer cells. This phenomenon has not
been reported in any previous publications and the reason remains
unclear. During cell culture, the pancreatic cancer cells underwent
EMT when the conditions altered (e.g., starving in serum-free
medium), which increased the number of stem-like cells. This
phenomenon demonstrates that EMT has a close relationship with
pancreatic cancer stem-like cells, and that the number of cancer
stem-like cells is not stable, changing under certain conditions.
Our findings are not in agreement with the current CSC
hypothesis.
We then evaluated the migration and invasive
activity of the cancer stem-like cells. To the best of our
knowledge, this is the first study to show increased migration and
invasion in pancreatic cancer stem-like cells compared with the
CD44−CD24− cells. The results may have
significant implications for the treatment of pancreatic cancer,
and explain the reason for the high mortality rate of patients with
pancreatic cancer.
In conclusion, our study demonstrates that
TGF-β1-induced EMT increases stem-like cells in pancreatic cancer
cells as pancreatic cancer stem-like cells exhibit a higher degree
of EMT than CD44−CD24− cells, which also have
gene expression profiles similar to that of cells that have
undergone EMT, and display significant migration and invasion
activity in vitro. Thus, pancreatic cancer stem-like cells
have greater migration and invasive activity. Our results also
indicate that pancreatic CSCs increase in number when the
conditions deteriorate. As a result, we assume that CSCs are
adaptable in pancreatic cancer, and that EMT is a process by which
cancer cells increase their adaptability. Therefore, findings of
the present study improve our understanding of the biological
characteristics of pancreatic CSCs and provide new insights into
EMT as an anti-cancer strategy.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (No. 30972912).
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics. CA Cancer J Clin. 59:225–249. 2009.
|
|
2
|
Ahlgren JD: Chemotherapy for pancreatic
carcinoma. Cancer. 78:654–663. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jemal A, Tiwari RC, Murray T, et al:
Cancer statistics. CA Cancer J Clin. 54:8–29. 2004.
|
|
4
|
Philip PA, Mooney M, Jaffe D, et al:
Consensus report of the National Cancer Institute clinical trials
planning meeting on pancreas cancer treatment. J Clin Oncol.
27:5660–5669. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rosenberg L: Treatment of pancreatic
cancer. Promises and problems of tamoxifen, somatostatin analogs,
and gemcitabine. Int J Pancreatol. 22:81–93. 1997.PubMed/NCBI
|
|
6
|
Rothenberg ML, Moore MJ, Cripps MC, et al:
A phase II trial of gemcitabine in patients with 5-FU-refractory
pancreas cancer. Ann Oncol. 7:347–353. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Warshaw AL and Fernandez-del CC:
Pancreatic carcinoma. N Engl J Med. 326:455–465. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nakajima S, Doi R, Toyoda E, et al:
N-cadherin expression and epithelial-mesenchymal transition in
pancreatic carcinoma. Clin Cancer Res. 10:4125–4133. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ellenrieder V, Hendler SF, Ruhland C,
Boeck W, Adler G and Gress TM: TGF-beta-induced invasiveness of
pancreatic cancer cells is mediated by matrix metalloproteinase-2
and the urokinase plasminogen activator system. Int J Cancer.
93:204–211. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kang Y and Massague J:
Epithelial-mesenchymal transitions: twist in development and
metastasis. Cell. 118:277–279. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Thiery JP, Acloque H, Huang RY, et al:
Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zeisberg M and Neilson EG: Biomarkers for
epithelial-mesenchymal transitions. J Clin Invest. 119:1429–1437.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kabashima A, Higuchi H, Takaishi H, et al:
Side population of pancreatic cancer cells predominates in
TGF-beta-mediated epithelial to mesenchymal transition and
invasion. Int J Cancer. 124:2771–2779. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ellenrieder V, Hendler SF, Boeck W, et al:
Transforming growth factor beta1 treatment leads to an
epithelial-mesenchymal transdifferentiation of pancreatic cancer
cells requiring extracellular signal-regulated kinase 2 activation.
Cancer Res. 61:4222–4228. 2001.
|
|
17
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison S and Clarke MF: Prospective identification of tumorigenic
breast cancer cells. Proc Natl Acad Sci USA. 100:3983–3988. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hermann P, Huber S, Herrler T, et al:
Distinct populations of cancer stem cells determine tumor growth
and metastatic activity in human pancreatic cancer. Cell Stem Cell.
1:313–323. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Visvader JE and Lindeman GJ: Cancer stem
cells in solid tumours: accumulating evidence and unresolved
questions. Nat Rev Cancer. 8:755–768. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li C, Heidt DG, Dalerba P, et al:
Identification of pancreatic cancer stem cells. Cancer Res.
67:1030–1037. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Costello RT, Mallet F, Gaugler B, et al:
Human acute myeloid leukemia CD34+/CD38−
progenitor cells have decreased sensitivity to chemotherapy and
Fas-induced apoptosis, reduced immunogenicity, and impaired
dendritic cell transformation capacities. Cancer Res. 60:4403–4011.
2000.PubMed/NCBI
|
|
23
|
Dean M, Fojo T and Bates S: Tumour stem
cells and drug resistance. Nat Rev Cancer. 5:275–284. 2005.
View Article : Google Scholar
|
|
24
|
Guzman ML, Swiderski CF, Howard DS, et al:
Preferential induction of apoptosis for primary human leukemic stem
cells. Proc Natl Acad Sci USA. 99:16220–16225. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mani SA, Guo W, Liao MJ, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kurrey NK, Jalgaonkar SP, Joglekar AV, et
al: Snail and slug mediate radioresistance and chemoresistance by
antagonizing p53-mediated apoptosis and acquiring a stem-like
phenotype in ovarian cancer cells. Stem Cells. 27:2059–2068. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Truty MJ and Urrutia R: Basics of TGF-beta
and pancreatic cancer. Pancreatology. 7:423–435. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ito D, Fujimoto K, Doi R, et al: Chronic
exposure of transforming growth factor beta 1 confers a more
aggressive tumor phenotype through downregulation of p21(WAF1/CIP1)
in conditionally immortalized pancreatic epithelial cells. Surgery.
136:364–374. 2004. View Article : Google Scholar
|