Introduction
Aromatase inhibitors (AI) are standard agents in
adjuvant hormone therapy for postmenopausal breast cancer patients
(1–3). Since 2005, the American Society of
Clinical Oncology Technology Assessment has recommended initial AI
or AI administered after treatment with tamoxifen for
postmenopausal breast cancer (4).
However, as yet no standardized therapy is available for metastatic
breast cancer in patients with AI-resistant breast cancer (5–9). Thus,
the development of non-cross-resistant endocrine agents is crucial,
particularly since AI has increasingly been used in the adjuvant
setting.
Toremifene (TOR) is a selective estrogen receptor
(ER) modulators (SERM). The efficacy in the adjuvant setting has
been equivalent to tamoxifen (TAM) (10–13).
The standard dose of TOR is 40 mg administered orally once a day.
Moreover, high-dose TOR of 120 mg once a day has been approved in
Japan. High-dose TOR has the property of competing with estrogen at
the ER site, as well as suppressing insulin-like growth factor
I-dependent growth (14) and
angiogenesis (15). High-dose TOR
is effective in TAM-resistant breast cancer and has been used for
secondary endocrine therapy (16).
We therefore conducted a prospective, multicenter phase II study of
high-dose TOR in the first-line treatment of metastatic breast
cancer following aromatase inhibitor adjuvant therapy.
Patients and methods
Study design and ethics
This was an open-label multicenter phase II study
conducted at 8 centers in Japan (International Clinical Trials
Registry No.: UMIN000000489). This study was conducted in
accordance with the Declaration of Helsinki and Japanese Ethical
Guideline of Clinical Research. The protocol was reviewed and
approved by the institutional review board of each participating
institution. Written informed consent was obtained from the
patients prior to the study.
Eligibility
Patients were included if they met the following
eligibility criteria: women who had undergone surgery for
histologically confirmed primary invasive breast cancer that was
positive for estrogen receptor (ER) and progesterone receptor
(PgR), or both; had received adjuvant AI postoperatively for >1
year including switching after TAM and relapse during the treatment
or within 12 months of completion of adjuvant therapy; for
postmenopausal women, menopause in this study was defined as: age
>60 years, age >45 years with amenorrhea for ≥2 years without
hysterectomy or bilateral ovariectomy; measurable disease by
Response Evaluation Criteria in Solid Tumors; no prior therapy for
metastatic disease; Eastern Cooperative Oncology Group performance
status of 0–1; life expectancy of >12 weeks; and adequate organ
function at the time of enrollment. Pre- or postoperative
chemotherapy was allowed. Patients were excluded if they had any of
the following conditions: invasive cancer in other organs for which
treatment was not completed within 5 years; brain metastasis;
bilateral breast cancer; male breast cancer; severe drug allergy;
uncontrollable complications; and psychological disease.
Treatment
Patients were treated with 120 mg toremifene
(Fareston®, Nippon Kayaku Co., Ltd, Tokyo, Japan) once a
day orally. Treatment was administered until tumor progression or
unacceptable adverse events occurred.
Clinical response assessment
Radiological evaluation was scheduled at least every
3 months using computed tomography or magnetic resonance imaging.
Clinical response was evaluated according to the Response
Evaluation Criteria in Solid Tumors (RECIST) guidelines (17). Efficacy was judged by the clinicians
at each facility.
Toxicity assessment
The severity of adverse events (AEs) was evaluated
using the National Cancer Institute Common Toxicity Criteria
(NCI-CTC) version 3.0. Patients were monitored for clinical and
laboratory toxic effects at least every 12 weeks. Drug-related AEs
were monitored during the study period.
Endpoints and statistical analysis
The primary endpoint was objective response rate
(ORR). The secondary endpoints were clinical benefit (CB) rate,
progression-free survival (PFS) and toxicity. A complete response
(CR) was defined as the complete disappearance of the measurable
lesions; a partial response (PR) as a decrease by 30% or more in
the sum of the longest diameters (LDs) of measurable lesions;
progressive disease (PD) as an increase of 20% or more in the sum
of the LDs of measurable lesions; and long-lasting stable disease
(long SD) as stable disease (SD) in the size of measurable lesions
for 24 weeks or longer. ORR was defined as the sum of the
frequencies of CR and PR, and the CB rate as the sum of the
frequencies of CR, PR, and long SD. For analysis of primary
efficacy, we expected that approximately 20% of the patients would
achieve clinical response, and the acceptable lower limit of
response rate was estimated to be 5%. The necessary number of
patients was calculated to be 40 under the conditions of the
one-sided test (α=0.05, β=0.10). All treated patients were analyzed
for safety. The case records were reviewed carefully and judged by
the Clinical Trial Office, Nagoya University, Japan.
Statistical analysis
Statistical analysis was performed using SAS
software, version 9.1.3, Service Pack 4 (SAS Institute Japan,
Ltd.). Survival rates were calculated by the Kaplan-Meier method,
and statistical significance was evaluated using the log-rank test.
The relationship between response and duration of AI was assessed
by using Spearman’s rank correlation coefficient. P<0.05 was
considered to be statistically significant.
Results
Patient characteristics
A total of 13 patients were enrolled from 8 centers
between January 2006 and August 2010. The patient characteristics
are shown in Table I. All patients
had received postoperative adjuvant AI for >1 year and relapsed
during the treatment. They had no prior therapy for metastasis. The
mean age was 63.6 years (range 52–75). A total of 8 patients
(61.5%) had undergone chemotherapy. Of these, 5 patients (38.5%)
were administered an anthracycline-containing regimen and taxane,
respectively, 2 patients (15.4%) had only an
anthracycline-containing regimen, and 1 patient (7.7%) was treated
with an anthracycline-containing regimen, taxane and trastuzumab,
respectively. With regard to adjuvant AI, 12 patients (92.3%) had
received anastrozole (Arimidex®, AstraZenaca KK, Osaka,
Japan) and 1 patient (7.7%) had received exemestane
(Aromacin®, Pfizer Japan Inc., Tokyo, Japan). A total of
12 patients (92.3%) had received AI as initial hormone therapy, and
1 patient (7.7%) switched hormone therapy after TAM. The mean
duration of AI therapy prior to recurrence was 33.2 months (range
14–52).
 | Table IBaseline characteristics of patients
(n=13). |
Table I
Baseline characteristics of patients
(n=13).
| Characteristic | Patients |
|---|
| Median age, years
(range) | 63.6 (52–75) |
| Primary stage |
| I | 2 |
| II | 2 |
| III | 9 |
| Hormone receptor |
| ER (+) and PgR
(+) | 3 |
| ER (+) and PgR
(−) | 10 |
| ER (−) and PgR
(+) | 0 |
| ER (−) and PgR
(−) | 0 |
| HER2
overexpression |
| 0 | 6 |
| 1 | 4 |
| 2 | 0 |
| 3 | 3 |
| Disease sites |
| Lymph nodes, soft
tissue | 7 |
| Chest wall | 1 |
| Lung | 2 |
| Liver | 3 |
| Duration of AI for
adjuvant therapy (years) |
| <1 | 0 |
| 1–2 | 3 |
| 2–3 | 5 |
| 3–4 | 3 |
| 4–5 | 2 |
| <5 | 0 |
Clinical response and safety profiles of the 13
patients were used for this analysis. This study was prematurely
terminated due to low accrual recruitment as a result of the
paucity of cases with only first-line treatment of patients with
metastatic breast cancer resistant to the adjuvant aromatase
inhibitor.
Clinical responses
Patients were evaluable for assessment of response.
ORR was 7.7% (1/13) [95% CI, 0.2–36.0%] with 0% CR and 7.7% PR. A
total of 7 patients (53.8%) had SD, 5 of whom were long SD, and 5
patients (38.5%) experienced progressive disease (PD). The CB rate
was 46.2% (6/13) [95% CI, 19.2–74.9%]. The patient characteristics
according to the responses are shown in Tables II and III. The responders were HER2-negative.
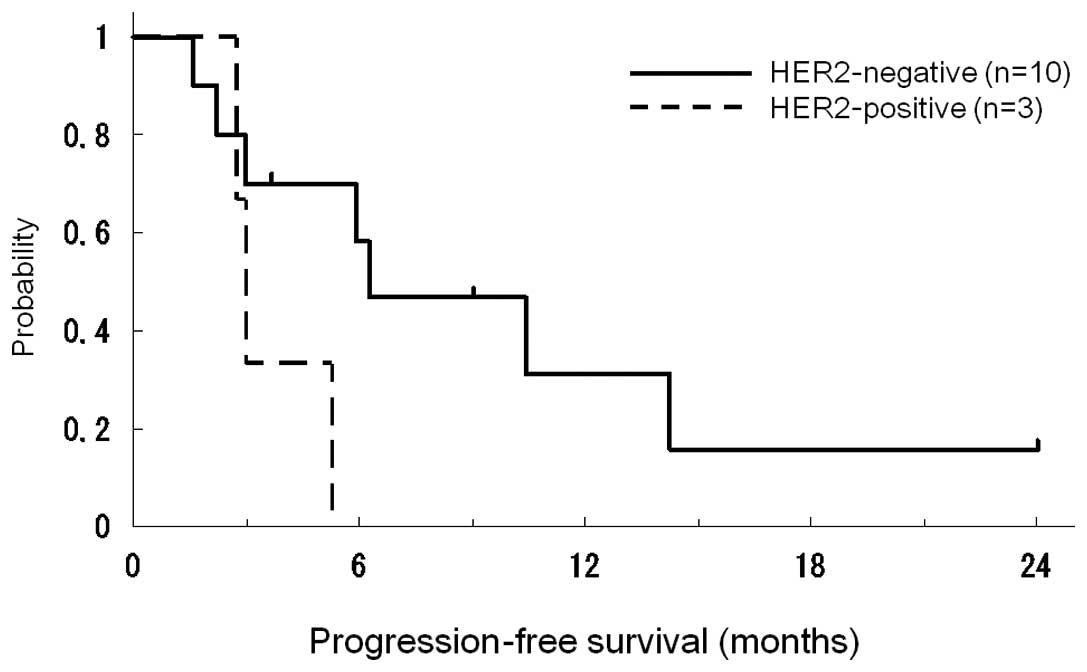
Median times in PFS curves according to HER2 status are shown in
Fig. 1. The median time to PFS was
5.9 months (range 1.7–24.0). Patients with HER2-positive disease
had marginally poorer PFS (p=0.08). The clinical responses
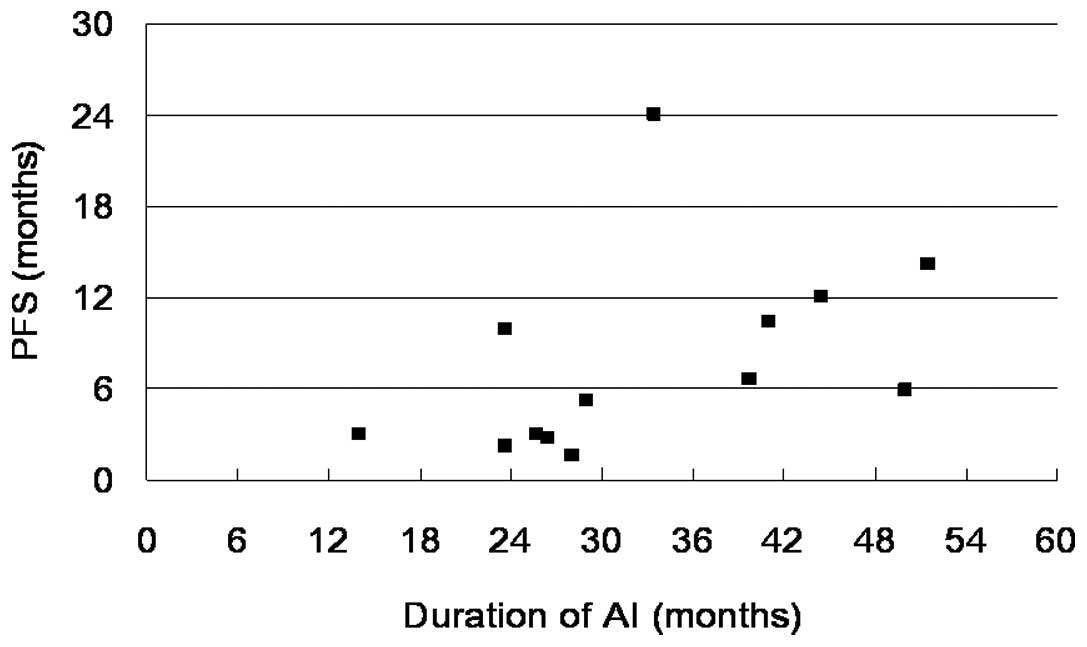
according to the duration of AI are shown in Fig. 2. Patients with PD exhibited a
relatively short duration of AI treatment compared with the
responders, who had a longer period of AI treatment (p=0.02).
 | Table IIPatients responding to high-dose
toremifene therapy. |
Table II
Patients responding to high-dose
toremifene therapy.
| Age | Response | ER/PgR | HER2 | Duration of AI
(months) | Disease sites | Time to progression
(months) |
|---|
| 74 | Long SD | +/− | 0 | 33.5 | Chest wall | 24.0 |
| 58 | PR | +/− | 0 | 41.0 | Liver | 10.4 |
| 75 | Long SD | +/− | 0 | 51.5 | Lung | 14.2 |
| 52 | Long SD | +/− | 0 | 23.7 | Liver | 9.9 |
| 65 | Long SD | +/− | 0 | 44.5 | Lymph node | 9.0 |
| 66 | Long SD | +/− | 1 | 39.8 | Lung | 6.7 |
 | Table IIIPatients not responding to high-dose
toremifene therapy. |
Table III
Patients not responding to high-dose
toremifene therapy.
| Age | Initial response | ER/PgR | HER2 | Duration of AI
(months) | Disease sites |
|---|
| 68 | PD | +/+ | 3 | 26.5 | Lymph nodes |
| 65 | PD | +/− | 1 | 14.0 | Lymph nodes |
| 55 | PD | +/− | 1 | 23.7 | Liver |
| 52 | PD | +/− | 3 | 25.7 | Lymph nodes |
| 75 | PD | +/+ | 1 | 28.0 | Lymph nodes |
Safety and tolerability
Adverse events were evaluated in 13 patients, and
are shown in Table IV. No serious
adverse events were observed. Patients received >80% of planned
treatment with TOR 120 mg. No patients required premature
discontinuation. In 1 case, hepatic dysfunction of Grade 3 was
observed, but was cured without interruption. No cases of
thromboembolism were reported.
 | Table IVAdverse events (n=13)a. |
Table IV
Adverse events (n=13)a.
| Events | n | Grade (n) |
|---|
| AST, sGOT | 3 | 3 (1), 1 (2) |
| ALT, sGPT | 3 | 3 (1), 1 (2) |
|
Hypertriglyceridemia | 2 | 3 (1), 1 (1) |
| Alkaline
phosphatase | 1 | 1 (1) |
| GGT (γGTP) | 1 | 1 (1) |
|
Hypercholesteremia | 1 | 1 (1) |
| Leukocytes | 1 | 1 (1) |
| Anorexia | 1 | 1 (1) |
| Creatinine | 1 | 1 (1) |
| Hypocalcemia | 1 | 1 (1) |
Discussion
Despite improvements in adjuvant endocrine therapy,
numerous patients harboring hormone-responsive tumors relapse and
require endocrine therapy in advanced disease. Although AIs are
standard agents for adjuvant hormone therapy for postmenopausal
breast cancer patients, treatment options are required for
metastatic breast cancer in patients with AI-resistant metastatic
breast cancer. In the most up-to-date guidelines, AI therapy has
been recommended at a certain point during adjuvant treatment as
up-front therapy or in sequential treatment following tamoxifen
(18). However, no randomized data
are available to date regarding the efficacy of estrogens or SERMs
among patients failing treatment with an AI. Thus, more data
regarding clinical efficacy and toxicity are required to support
endocrine therapy as a reasonable choice in this setting (9).
Our phase II study, albeit of a limited number of
patients, indicates that high-dose toremifene is effective and safe
as a first-line treatment for patients with adjuvant AI-resistant
metastatic breast cancer. In published studies regarding
AI-resistant metastatic breast cancer, ORR and CB of TAM treatment
after anastrozole failure (n=95) were 7.4 and 56.8%, respectively
(5). ORR and CB of exemestane
regarding non-steroidal AI failure (n=105) were 4.8 and 20.0%,
respectively (6). ORR and CB of
fulvestrant, an ER antagonist, after treatment with a
third-generation AI (n=77) were 14.3 and 35%, respectively
(7). Another study group revealed
that the CB of fulvestrant after AI treatment was 30%, and
indicated that any prior response to an AI did not appear to offer
predictive benefit with fulvestrant (8).
The efficacy of high-dose TOR following AI failure
has been evaluated in only a small number of clinical studies. In a
larger retrospective study in which the efficacy of 120 mg TOR was
analyzed with AI-failure cases (n=80), ORR and CB were 15 and 45%,
respectively (19). In cases where
tamoxifen preceded AI, high-dose TOR was effective for
tamoxifen-resistant breast cancer (20–22).
The majority of the patients in that study as well as ours
tolerated the side effects and experienced a more favorable quality
of life during treatment.
In our limited number of patients, high-dose TOR was
ineffective on HER2-positive patients or those who had undergone a
relatively short period of AI treatment. Recently, trastuzumab plus
anastrozole exhibited improved outcomes for patients with
HER2/hormone receptor-co-positive metastatic breast cancer compared
with anastrozole alone (23). In
the case of HER2/hormone receptor-co-positive patients, use of a
hormone receptor blockade alone may not suffice for inhibition of
cancer growth. Endocrine resistance while receiving AIs may be due
to enhanced signal transduction pathways, such as HER2 and
ras/raf/mitogen-activated protein kinase (24). Further investigation using a larger
cohort is required for a more precise predictive biomarker
analysis.
In conclusion, high-dose TOR is effective for
AI-resistant metastatic breast cancer with favorable toxicity, and
may be considered as a treatment option. This is the first report
on the efficacy of high-dose TOR therapy for metastatic breast
cancer in patients with AI-resistant metastatic breast cancer. A
phase III study is required to determine the most favorable
treatment following AI failure.
Acknowledgements
This study was presented in part at the primary
therapy of early breast cancer 12th International Conference, St.
Gallen, Switzerland, 16–19, March 2011.
Abbreviations:
|
AI
|
aromatase inhibitors
|
|
TAM
|
tamoxifen
|
|
TOR
|
toremifene
|
|
SERM
|
selective estrogen receptor
modulators
|
References
|
1
|
Baum M, Budzar AU, Cuzick J, Forbes J,
Houghton JH, Klijn JG and Sahmoud T: Anastrozole alone or in
combination with tamoxifen versus tamoxifen alone for adjuvant
treatment of postmenopausal women with early breast cancer: first
results of the ATAC randomised trial. Lancet. 359:2131–2139. 2002.
View Article : Google Scholar
|
|
2
|
Howell A, Cuzick J, Baum M, Buzdar A,
Dowsett M, Forbes JF, Hoctin-Boes G, Houghton J, Locker GY and
Tobias JS: Results of the ATAC (Arimidex, Tamoxifen, Alone or in
Combination) trial after completion of 5 years’ adjuvant treatment
for breast cancer. Lancet. 365:60–62. 2005.
|
|
3
|
Thurlimann B, Keshaviah A, Coates AS,
Mouridsen H, Mauriac L, Forbes JF, Paridaens R, Castiglione-Gertsch
M, Gelber RD, Rabaglio M, et al: A comparison of letrozole and
tamoxifen in postmenopausal women with early breast cancer. N Engl
J Med. 353:2747–2757. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Winer EP, Hudis C, Burstein HJ, Wolff AC,
Pritchard KI, Ingle JN, Chlebowski RT, Gelber R, Edge SB, Gralow J,
et al: American Society of Clinical Oncology technology assessment
on the use of aromatase inhibitors as adjuvant therapy for
postmenopausal women with hormone receptor-positive breast cancer:
status report 2004. J Clin Oncol. 23:619–629. 2005. View Article : Google Scholar
|
|
5
|
Thurlimann B, Robertson JF, Nabholtz JM,
Buzdar A and Bonneterre J: Efficacy of tamoxifen following
anastrozole (‘Arimidex’) compared with anastrozole following
tamoxifen as first-line treatment for advanced breast cancer in
postmenopausal women. Eur J Cancer. 39:2310–2317. 2003.
|
|
6
|
Lonning PE, Bajetta E, Murray R,
Tubiana-Hulin M, Eisenberg PD, Mickiewicz E, Celio L, Pitt P, Mita
M, Aaronson NK, et al: Activity of exemestane in metastatic breast
cancer after failure of nonsteroidal aromatase inhibitors: a phase
II trial. J Clin Oncol. 18:2234–2244. 2000.PubMed/NCBI
|
|
7
|
Ingle JN, Suman VJ, Rowland KM,
Mirchandani D, Bernath AM, Camoriano JK, Fishkin PA, Nikcevich DA
and Perez EA: Fulvestrant in women with advanced breast cancer
after progression on prior aromatase inhibitor therapy: North
Central Cancer Treatment Group Trial N0032. J Clin Oncol.
24:1052–1056. 2006. View Article : Google Scholar
|
|
8
|
Perey L, Paridaens R, Hawle H, Zaman K,
Nole F, Wildiers H, Fiche M, Dietrich D, Clement P, Koberle D, et
al: Clinical benefit of fulvestrant in postmenopausal women with
advanced breast cancer and primary or acquired resistance to
aromatase inhibitors: final results of phase II Swiss Group for
Clinical Cancer Research Trial (SAKK 21/00). Ann Oncol. 18:64–69.
2007. View Article : Google Scholar
|
|
9
|
Lonning PE: Additive endocrine therapy for
advanced breast cancer – back to the future. Acta Oncol.
48:1092–1101. 2009.
|
|
10
|
Lewis JD, Chagpar AB, Shaughnessy EA,
Nurko J, McMasters K and Edwards MJ: Excellent outcomes with
adjuvant toremifene or tamoxifen in early stage breast cancer.
Cancer. 116:2307–2315. 2010.PubMed/NCBI
|
|
11
|
Pyrhonen S, Ellmen J, Vuorinen J,
Gershanovich M, Tominaga T, Kaufmann M and Hayes DF: Meta-analysis
of trials comparing toremifene with tamoxifen and factors
predicting outcome of antiestrogen therapy in postmenopausal women
with breast cancer. Breast Cancer Res Treat. 56:133–143. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gams R: Phase III trials of toremifene vs
tamoxifen. Oncology (Williston Park). 11:23–28. 1997.PubMed/NCBI
|
|
13
|
Holli K, Valavaara R, Blanco G, Kataja V,
Hietanen P, Flander M, Pukkala E and Joensuu H: Safety and efficacy
results of a randomized trial comparing adjuvant toremifene and
tamoxifen in postmenopausal patients with node-positive breast
cancer. Finnish Breast Cancer Group. J Clin Oncol. 18:3487–3494.
2000.
|
|
14
|
Iino Y, Takai Y, Ando T, Sugamata N,
Maemura M, Takeo T, Ohwada S and Morishita Y: Effect of toremifene
on the growth, hormone receptors and insulin-like growth factor-1
of hormone-dependent MCF-7 tumors in athymic mice. Cancer Chemother
Pharmacol. 32:353–358. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ruohola JK, Valve EM, Karkkainen MJ,
Joukov V, Alitalo K and Harkonen PL: Vascular endothelial growth
factors are differentially regulated by steroid hormones and
antiestrogens in breast cancer cells. Mol Cell Endocrinol.
149:29–40. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stenbygaard LE, Herrstedt J, Thomsen JF,
Svendsen KR, Engelholm SA and Dombernowsky P: Toremifene and
tamoxifen in advanced breast cancer – a double-blind cross-over
trial. Breast Cancer Res Treat. 25:57–63. 1993.
|
|
17
|
Therasse P, Arbuck SG, Eisenhauer EA,
Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van
Oosterom AT, Christian MC and Gwyther SG: New guidelines to
evaluate the response to treatment in solid tumors. European
Organization for Research and Treatment of Cancer, National Cancer
Institute of the United States, National Cancer Institute of
Canada. J Natl Cancer Inst. 92:205–216. 2000. View Article : Google Scholar
|
|
18
|
Burstein HJ, Prestrud AA, Seidenfeld J,
Anderson H, Buchholz TA, Davidson NE, Gelmon KE, Giordano SH, Hudis
CA, Malin J, et al: American Society of Clinical Oncology clinical
practice guideline: update on adjuvant endocrine therapy for women
with hormone receptor-positive breast cancer. J Clin Oncol.
28:3784–3796. 2010. View Article : Google Scholar
|
|
19
|
Yamamoto Y, Masuda N, Ohtake T, Yamashita
H, Saji S, Kimijima I, Kasahara Y, Ishikawa T, Sawaki M, Hozumi Y
and Iwase H: Clinical usefulness of high-dose toremifene in
patients relapsed on treatment with an aromatase inhibitor. Breast
Cancer. 17:254–260. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jonsson PE, Malmberg M, Bergljung L,
Ingvar C, Ericsson M, Ryden S, Nilsson I and Terje IJ: Phase II
study of high dose toremifene in advanced breast cancer progressing
during tamoxifene treatment. Anticancer Res. 11:873–875.
1991.PubMed/NCBI
|
|
21
|
Vogel CL, Shemano I, Schoenfelder J, Gams
RA and Green MR: Multicenter phase II efficacy trial of toremifene
in tamoxifen-refractory patients with advanced breast cancer. J
Clin Oncol. 11:345–350. 1993.PubMed/NCBI
|
|
22
|
Pyrhonen S, Valavaara R, Vuorinen J and
Hajba A: High dose toremifene in advanced breast cancer resistant
to or relapsed during tamoxifen treatment. Breast Cancer Res Treat.
29:223–228. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kaufman B, Mackey JR, Clemens MR, Bapsy
PP, Vaid A, Wardley A, Tjulandin S, Jahn M, Lehle M, Feyereislova
A, et al: Trastuzumab plus anastrozole versus anastrozole alone for
the treatment of postmenopausal women with human epidermal growth
factor receptor 2-positive, hormone receptor-positive metastatic
breast cancer: results from the randomized phase III TAnDEM study.
J Clin Oncol. 27:5529–5537. 2009. View Article : Google Scholar
|
|
24
|
Kato S, Endoh H, Masuhiro Y, Kitamoto T,
Uchiyama S, Sasaki H, Masushige S, Gotoh Y, Nishida E, Kawashima H,
et al: Activation of the estrogen receptor through phosphorylation
by mitogen-activated protein kinase. Science. 270:1491–1494. 1995.
View Article : Google Scholar : PubMed/NCBI
|