Introduction
A growing body of evidence has shown that a small
subpopulation of stem-like cancer cells within the tumor bed may be
the major cause of tumor progression and relapse following extreme
therapeutic treatment (1,2). These cells are known as cancer
stem-like cells (CSCs), cancer-initiating cells or
tumor-propagating cells, since they form the small group of tumor
cells capable of inducing tumors and self-renewal in the same
manner as normal stem cells. After first being identified in
leukemia (3), CSCs have also been
identified in almost all solid tumors, including brain (4), breast (5), colon (6), lung (7) and other tissues (8). These cells are regarded as being
responsible for resistance to chemo- and radiotherapy, tumor
angiogenesis and tumor metastasis (2). CSCs have been reported to be enriched
by treatment with chemotherapeutic agents and irradiation (9,10).
Therefore, targeting CSCs may be a useful therapeutic strategy for
reducing the risk of tumor relapse following therapy. However, a
critical approach to eradicate these cells has not been
developed.
CSCs also persist in established cell lines. In
serum-free media with growth factors (GFs), such as epidermal
growth factor (EGF), basic fibroblast growth factor (bFGF) and
platelet-derived growth factor (PDGF), the CSC population may be
enriched from serum-cultured cells as a side population or sphere
type (11,12). The standard culture condition for
maintaining CSCs in vitro uses media (DMEM/F12 with B27
and/or N2 supplements and growth factors such as EGF and bFGF) in
the absence of serum, although the subtle ingredients vary
depending on the cell type. EGF signaling has been reported to act
through the EGF receptor (EGFR) and is crucial for the maintenance
of stemness in glioma stem cells (13). In contrast, bFGF is known to be a
major mitogen for neural stem cells (NSCs) (14). In the C6 glioma cell line model,
both PDGF and bFGF are required to increase the side population and
form tumor spheres (15). However,
glioblastoma stem cells may be grown without the exogenous addition
of GFs by autocrine factors (16,17).
In the present study, we aimed to determine whether
CSCs from established cell lines may be enriched as tumor spheres
more efficiently without the GFs, EGF and bFGF, as compared with
the GFs.
Materials and methods
Reagents
Gefitinib and AG1478 were obtained from Calbiochem
(La Jolla, CA, USA). Antibodies against EGFR were obtained from
Cell Signaling Biotechnology (Denvers, MA, USA). Neutralizing
antibodies against EGF and bFGF were purchased from Upstate (Lake
Placid, NY, USA). Recombinant EGF and bFGF were obtained from
Peprotech (Rocky Hill, NJ, USA).
Cell culture and sphere-forming
assay
Human breast cancer cell line (MCF7), glioma cell
line (U87) and non-small cell lung cancer cell line (A549) were
cultured in Dulbecco's modified Eagle's medium (DMEM) and
RPMI-1640, respectively, supplemented with 10% fetal bovine serum
(FBS; JR Scientific, Inc., Woodland, CA, USA) and 1%
penicillin/streptomycin and were maintained at 37˚C in a 5%
CO2 incubator.
For the sphere-forming assay, a single cell
suspension from trypsinization was cultured in ultra low cluster
6-well plates (Corning, Corning, NY, USA) with DMEM/F12 (Cellgro,
Manassas, VA, USA) in the presence or absence of GFs (10 ng/ml each
of EGF and bFGF) without serum at a density of 1×103
cells/ml. After 10 days, spheres were attached by adding FBS (10%),
stained with Diff-Quick solution (Sysmex, Kobe, Japan), and
counted.
Antibody array
An analysis of conditioned media (CM) using an
antibody array kit was conducted. CM was collected from the MCF7
cells cultured in the presence or absence of FBS with or without
GFs for 48 h and was concentrated with Centrifugal Filter Units
(Millipore Corporation, Billerica, MA, USA). Five micrograms of
each sample were subjected to antibody array for the detection of
secreted proteins using the human angiogenesis antibody array kit
(R&D Systems, Minneapolis, MN, USA). Antibody array membranes
were visualized by enhanced chemiluminescence (Amersham, Arlington
Heights, IL, USA) according to the manufacturer's instructions.
Western blot analysis
Cells were lysed in TNN buffer [50 mM Tris-HCl (pH
7.4), 100 mM NaCl, 5 mM EDTA, 0.5% Nonidet P-40 and protease
inhibitor cocktail tablet (Roche, Indianapolis, IN, USA)] and
protein content was determined by Bio-Rad protein assay (Bio-Rad,
Hercules, CA, USA). An aliquot (30–50 μg protein/lane) of the total
protein was separated by SDS-PAGE and electrotransferred to the
nitrocellulose membrane (Millipore Corporation) for 2 h at 80
volts. The membrane was blocked with 5% skimmed milk in TBST [20
mmol/l Tris-HCl (pH 7.6), 137 mmol/l NaCl, and 0.01% Tween-20] for
1 h at room temperature followed by incubation with the primary
antibody overnight at 4˚C. After extensive washing with TBST the
membrane was probed with a secondary antibody conjugated with
horseradish peroxidase for 1 h at room temperature. After washing
five times with TBST, membranes were visualized by enhanced
chemiluminescence (Amersham, Arlington Heights, IL, USA) according
to the manufacturer's instructions.
Statistical analysis
Statistical analysis was performed using an
independent samples t-test. Differences were considered
statistically significant at p<0.05.
Results
GF-free culture is more efficient for the
formation of spheres in cancer cell lines than GF-containing
culture
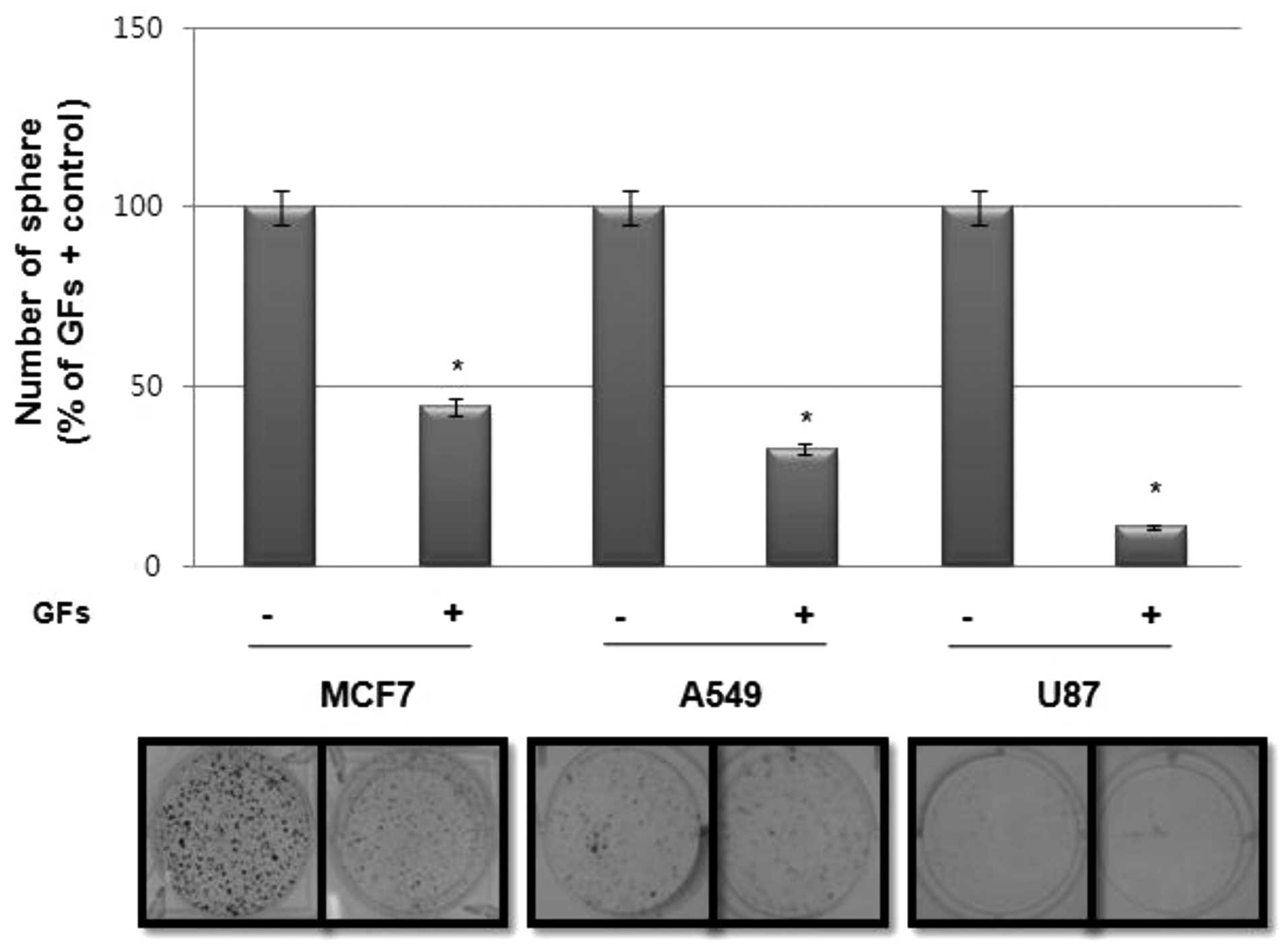
Since sphere-forming activity is regarded as a
surrogate marker for the self-renewal activity of CSCs in
vitro, we investigated the effect of GFs, particularly EGF and
bFGF, on sphere formation in various tumor cell lines. Notably, as
shown in Fig. 1, sphere formation
in cancer cell lines, including MCF7, A549 and U87, was much higher
in cultures without GFs than those with GFs.
GF-free culture enhances EGF secretion
and EGFR expression in MCF7 and A549 cells
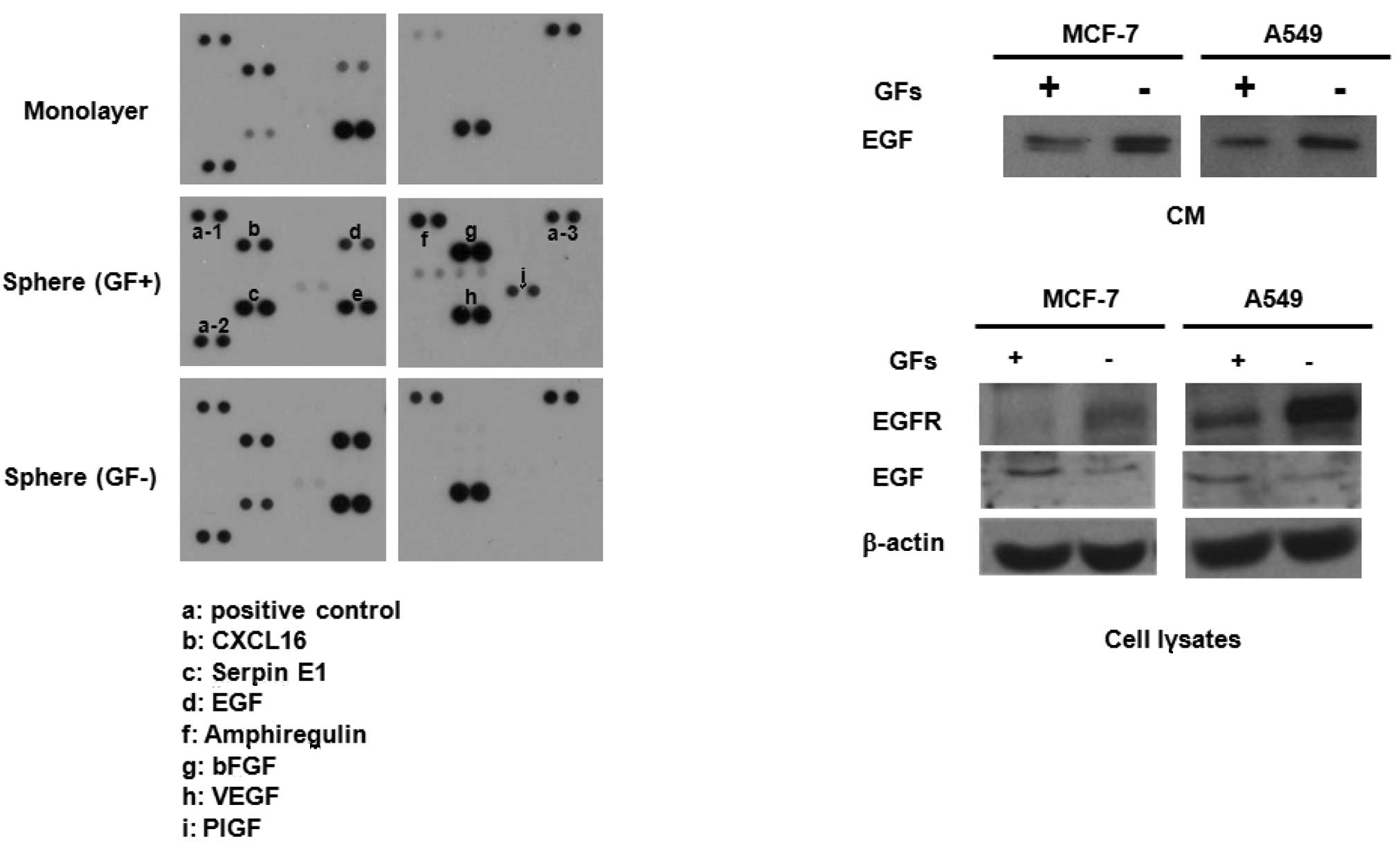
To investigate the possible mechanism involved in
these events, we collected CM from monolayer cultures,
GF-containing sphere cultures and GF-free sphere cultures of MCF7
cells, and then obtained blots using an antibody array kit. As
shown in Fig. 2A, sphere-cultured
CM revealed a high secretion of cytokines, including serpin E1,
amphiregulin, EGF and placental growth factor (PIGF). Notably, EGF
secretion was higher in GF-free CM than in GF-containing CM.
Western blot analysis also confirmed the elevated secretion of EGF
in CM from GF-free cultures compared with that of GF-containing
cultures of MCF7 and A549 cell lines (Fig. 2B, upper panel). Notably, EGFR
expression was also greatly enhanced in the cell lysates of GF-free
cultures compared with those from GF-containing cultures of MCF7
and A549 cells (Fig. 2B, lower
panel). Taken together, the higher sphere-forming activity in cell
lines cultured under GF-free conditions may be due to the high
secretion of EGF and expression of EGFR followed by autocrine
activation of the EGF/EGFR signaling cascade.
Blockade of EGFR with pharmacological
inhibitors and neutralization of EGF suppresses sphere-forming
activity in MCF7 and A549 cells
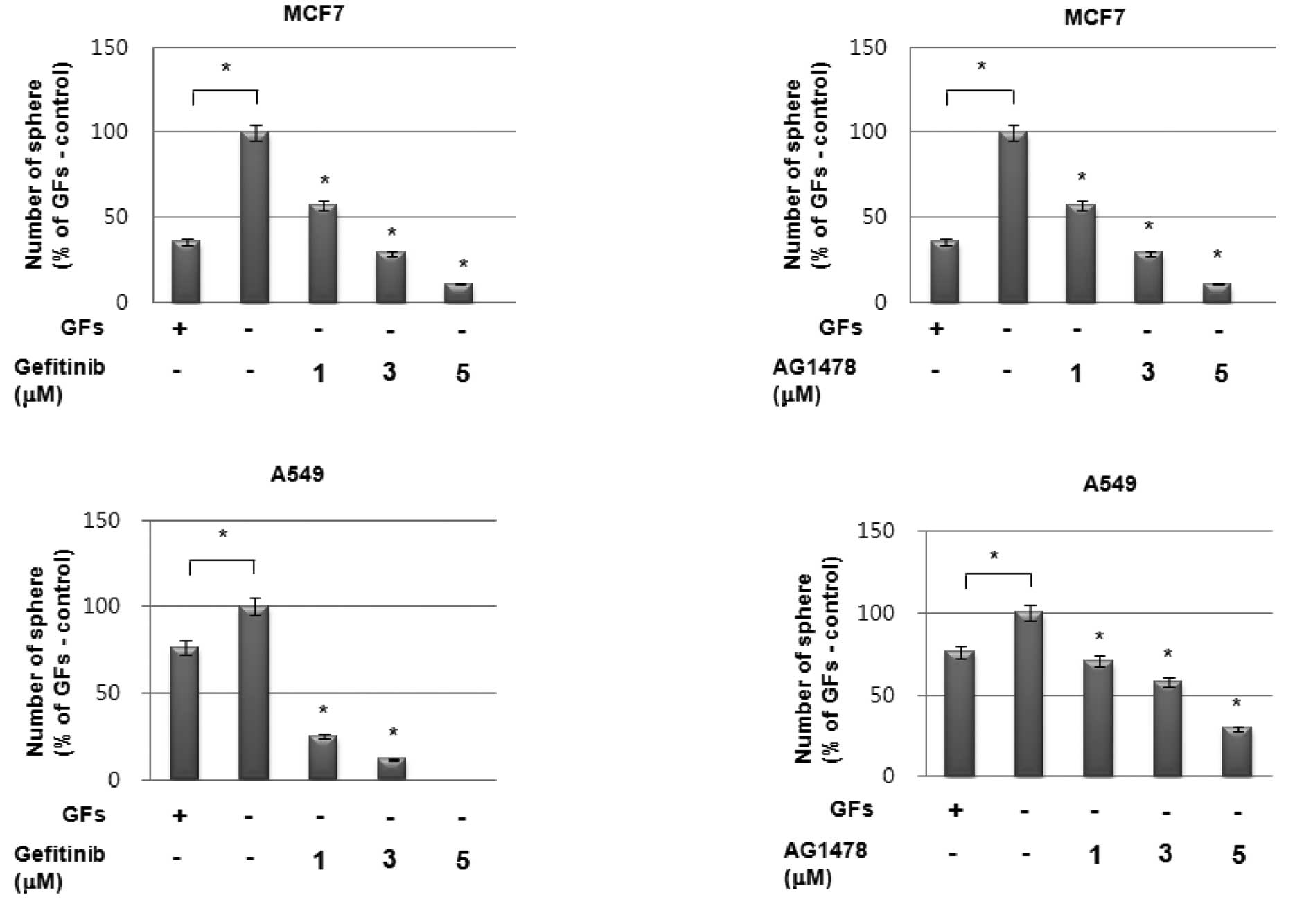
To investigate the possible role of enhanced EGF
secretion followed by EGFR activation on increased sphere formation
under GF-free conditions, we treated cells with the EGFR-targeted
inhibitors gefitinib and AG1478. As shown in Fig. 3A and B, pretreatment with
EGFR-specific inhibitors significantly suppressed sphere formation
by MCF7 and A549 cells in a dose-dependent manner under GF-free
sphere culture conditions. Additionally, treatment with a
neutralizing antibody against EGF also demonstrated the same
pattern of results as EGFR inhibitors in these cells (Fig. 3C). By contrast, treatment with a
neutralizing antibody against bFGF even enhanced sphere formation
in these cells (Fig. 3D).
Therefore, these data strongly suggest that the high sphere-forming
activity of the cell lines under GF-free culture conditions is
caused by the enhanced activation of the EGF/EGFR signaling
cascade.
bFGF suppresses EGFR expression in MCF7
and A549 cells
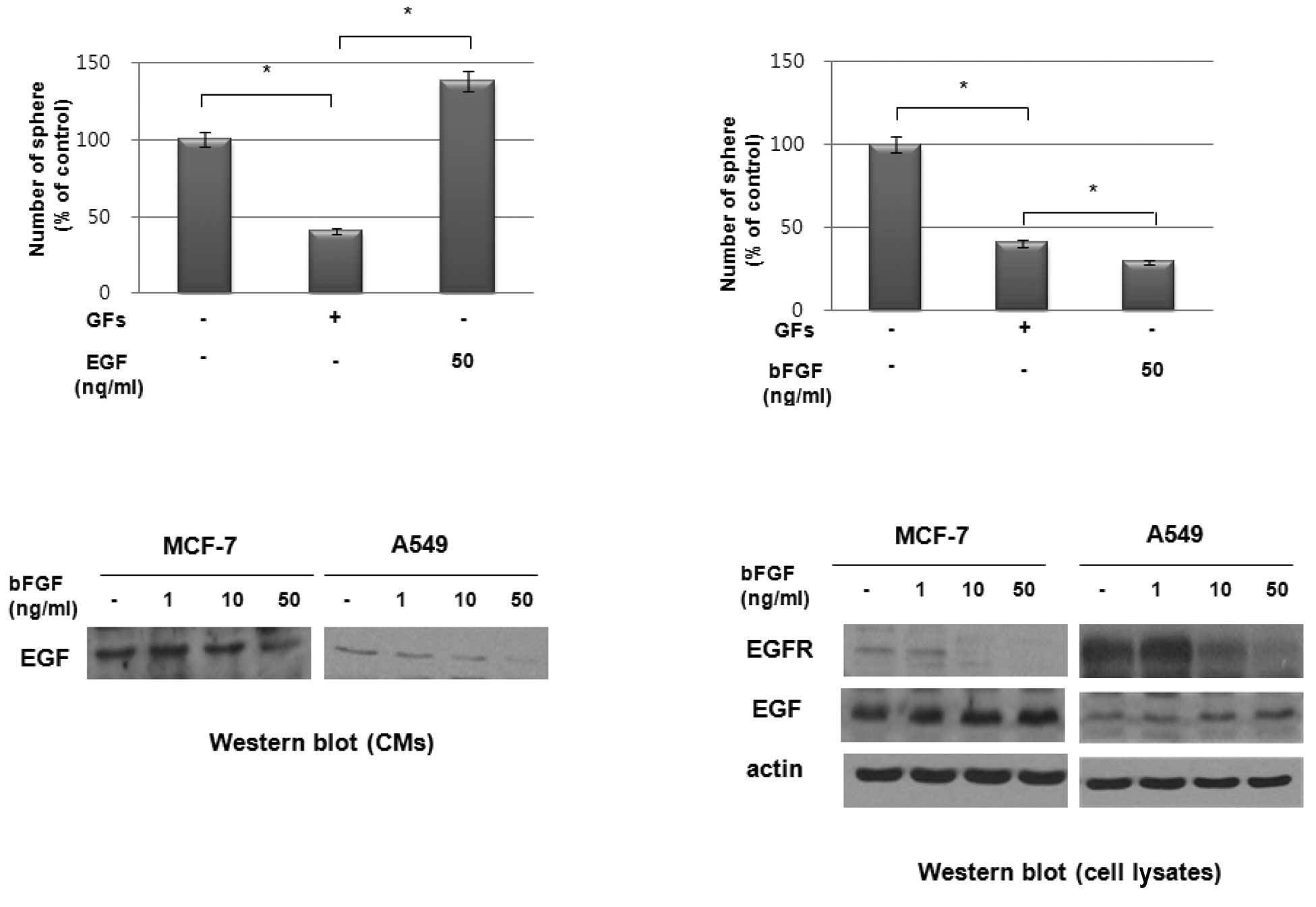
We investigated the effect of exogenous EGF and bFGF
on sphere formation by these cell lines. As shown in Fig. 4A, the exogenous addition of EGF
stimulated sphere formation in a dose-dependent manner in MCF7
cells. However, the addition of exogenous bFGF suppressed sphere
formation in these cells (Fig. 4B).
To determine the possible role of bFGF in suppressing sphere
formation in these cell lines, we treated MCF7 and A549 cells with
bFGF and examined the expression of EGF and EGFR. Notably, western
blot analysis revealed that bFGF inhibited the secretion of EGF
(Fig. 4C) and the expression of
EGFR (Fig. 4D) in these cells. By
contrast, EGF expression in cell lysates was increased by bFGF
treatment in a dose-dependent manner (Fig. 4D). These data therefore suggest that
the increased sphere formation under GF-free culture conditions was
due to the elimination of the effect of bFGF on the suppression of
EGFR expression under these culture conditions.
Discussion
Stem-like cancer cells are believed to be preserved
in culture with serum, and transition of these cultures into
serum-free media with GFs such as EGF and bFGF enriches these
cells. In this study, however, we found that the sphere formation
by cancer cells in CSC media was much higher under GF-free culture
conditions than under GF-containing culture conditions. The
increased sphere formation of GF-free cultured cells may be due to
the increased autocrine secretion of EGF and the enhanced
expression of EGFR compared with the GF-containing cultured
cells.
It has been reported that EGF-mediated EGFR
signaling, but not bFGF-mediated signaling, is crucial for the
maintenance of brain tumor stem cells (13). Flow cytometry has shown that the
CD133-positive population increased by EGF in a
concentration-dependent manner (13). In a similar manner, Kelly et
al indicated that in a model of brain tumor stem cell culture
without the exogenous addition of GFs, the blockade of EGFR
signaling reduced exogenous mitogen-independent sphere formation
(16). Our data also suggest that
EGF/EGFR signaling is critical for the maintenance of CSCs in tumor
cells. Since the addition of EGF further increased sphere
formation, we speculated that bFGF suppresses EGF expression
followed by secretion outside of the cells. bFGF was found to
suppress the secretion of EGF in epithelial tumor cell lines. Of
note, bFGF also inhibited the expression of EGFR in a
dose-dependent manner. Thus, the increase in CSCs from epithelial
tumors through sphere cultures is much more efficient under GF-free
culture conditions due to the inhibitory effect of bFGF on EGF
secretion and EGFR expression.
In conclusion, our data suggested that CSCs from
cell lines are enriched more efficiently without GFs than with GFs,
since the addition of bFGF suppressed EGF/EGFR signaling. The
results also showed that the autocrine secretion of EGF is more
prominent in GF-free CM than in GF-containing CM. The blockade of
EGF action with neutralizing antibody EGF, or the inhibition of
EGFR by pharmacological inhibitors significantly reduced tumor
sphere formation, whereas bFGF neutralizing antibody enhanced
sphere formation. The exogenous addition of EGF further stimulated
sphere formation, whereas bFGF suppressed this event. bFGF also
suppressed EGFR expression and EGF secretion. Our findings suggest
the that autocrine secretion of GFs, including EGF, may sustain
CSCs effectively, and that bFGF may have a negative effect on tumor
sphere formation by suppressing EGFR expression in sphere culture
conditions. Therefore, GF-free culture promotes tumor sphere
formation more than GF-containing culture in epithelial tumor cell
lines. However, the critical mechanism of action of bFGF should be
defined through further investigation.
Acknowledgements
This study was supported by a grant from the Nuclear
Research and Development Program of the Korea Science and
Engineering Foundation funded by the Korean government (MEST).
References
|
1
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jordan CT, Guzman ML and Noble M: Cancer
stem cells. N Engl J Med. 355:1253–1261. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lapidot T, Sirard C, Vormoor J, Murdoch B,
Hong T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA and
Dick JE: A cell initiating human acute myeloid leukemia after
transplantation into SCID mice. Nature. 367:645–648. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Singh SK, Hawkins C, Clarke ID, Squire JA,
Bayani J, Hide T, Henkelman RM, Cusimano MD and Dirks PB:
Identification of human brain tumour initiating cells. Nature.
432:396–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ricci-Vitiani L, Lombardi DG, Pilozzi E,
Biffoni M, Todaro M, Peschle C and De Maria R: Identification and
expansion of human colon-cancer-initiating cells. Nature.
445:111–115. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Eramo A, Lotti F, Sette G, Pilozzi E,
Biffoni A, Di Virgilio A, Conticello C, Ruco L, Peschle C and De
Maria R: Identification and expansion of the tumorigenic lung
cancer stem cell population. Cell Death Differ. 15:504–514. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Visvader JE and Lindeman GJ: Cancer stem
cells in solid tumours: accumulating evidence and unresolved
questions. Nat Rev Cancer. 8:755–768. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bao S, Wu Q, McLendon RE, Hao Y, Shi Q,
Hjelmeland AB, Dewhirst MW, Bigner DD and Rich JN: Glioma stem
cells promote radioresistance by preferential activation of the DNA
damage response. Nature. 444:756–760. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee HE, Kim JH, Kim YJ, Choi SY, Kim SW,
Kang E, Chung IY, Kim IA, Kim EJ, Choi Y, Ryu HS and Park SY: An
increase in cancer stem cell population after primary systemic
therapy is a poor prognostic factor in breast cancer. Br J Cancer.
104:1730–1738. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Setoguchi T, Taga T and Kondo T: Cancer
stem cells persist in many cancer cell lines. Cell Cycle.
3:414–415. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Patrawala L, Calhoun T,
Schneider-Broussard R, Zhou J, Claypool K and Tang DG: Side
population is enriched in tumorigenic, stem-like cancer cells,
whereas ABCG2+ and ABCG2- cancer cells are similarly tumorigenic.
Cancer Res. 65:6207–6219. 2005.
|
|
13
|
Soeda A, Inagaki A, Oka N, Ikegame Y, Aoki
H, Yoshimura S, Nakashima S, Kunisada T and Iwama T: Epidermal
growth factor plays a crucial role in mitogenic regulation of human
brain tumor stem cells. J Biol Chem. 283:10958–10966. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kitchens DL, Snyder EY and Gottlieb DI:
FGF and EGF are mitogens for immortalized neural progenitors. J
Neurobiol. 25:797–807. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kondo T, Setoguchi T and Taga T:
Persistence of a small subpopulation of cancer stem-like cells in
the C6 glioma cell line. Proc Natl Acad Sci USA. 101:781–786. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kelly JJ, Stechishin O, Chojnacki A, Lun
X, Sun B, Senger DL, Forsyth P, Auer RN, Dunn JF, Cairncross JG,
Parney IF and Weiss S: Proliferation of human glioblastoma stem
cells occurs independently of exogenous mitogens. Stem Cells.
27:1722–1733. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li G, Chen Z, Hu YD, Wei H, Li D, Ji H and
Wang DL: Autocrine factors sustain glioblastoma stem cell
self-renewal. Oncol Rep. 21:419–424. 2009.PubMed/NCBI
|