Introduction
The ileum and the right-sided colon originate from
the same embryologic midgut; however, in adults, the ileocecal
valve demarcates the colon from the ileum (1,2).
Moreover, the occurrence of ileal carcinoma is rare, but
right-sided colon carcinoma is not uncommon (3). However, cases of intestinal carcinoma
involving the terminal ileum and the right-sided colonhave rarely
encountered. Additionally, the clinicopathological variables of
such cases remain unclear. Right-sided colon carcinoma may show
microsatellite instability (MSI) associated with defects in a DNA
mismatch repair (MMR) system (3–7), but
such a relationship in ileal carcinoma is unknown. In the terminal
ileum adjacent to the colon, there is a specialized cluster of
lymphoid follicles, or Peyer's patches, contributing to antigen
sampling and pathogen entry (1,2,8).
However, the histopathological features of the carcinomatous
involvement of Peyer's patches have been poorly understood. In this
study, intestinal carcinoma involving the ileum end and the
right-sided colon (cecum and/or ascending colon) was defined as
‘colo-ileal carcinoma’ (CIC), and an attempt was made to elucidate
its clinicopathological characteristics.
Materials and methods
Materials
The surgical pathology reports of intestinal
carcinoma in 53 patients who had undergone surgical resection of
the ileum, cecum and ascending colon at the Department of
Pathology, Japan Self Defense Forces Central Hospital (1985 to
2010) and at the Division of Surgical Pathology, Mishuku Hospital
(1990 to 2010), Tokyo, Japan, were searched to identify cases of
CIC. Cases of carcinoma originating in the vermiform appendix were
excluded. A total of 16 CICs were retrieved and examined. Detailed
clinical information including patients' personal history and
family history was available in 11 cases from patient charts, none
of which included history of inflammatory bowel disease.
Our study was approved by the ethics committees of
both Japan Self Defense Forces Central hospital and Mishuku
hospital. We obtained general informed consent for further study
from patients who were included in this study.
Methods
All 16 CICs in their entirety were cut,
formalin-fixed and paraffin-embedded in 4–11 blocks. Tumors were
categorized using the World Health Organization classification of
tumors of the colon and rectum (3).
According to previously proposed models predicting a high level of
MSI (MSI-H), i.e., MsPath (5) and
PREDICT (6), tumor-infiltrating
lymphocytes, Crohn's-like lymphocytic reaction, poorly
differentiated or medullary components, peritumoral lymphocytic
reaction, increased stromal plasma cells and mucinous
adenocarcinoma components were assessed. The mucinous
adenocarcinoma component was evaluated by two methods: i) for
MsPath it was defined as at least 50% of the tumor area comprising
mucinous features (5); and ii) for
PREDICT it was scored dichotomously if CICs had mucinous features
in more than 50% of the tumor area or if there was a focal mucinous
area within a predominantly non-mucinous morphology (6). Representative sections were
immunostained with antibodies against cytokeratin 7 (CK7; OV-TL
12/30, Dako, Glostrup, Denmark), cytokeratin 20 (CK20; Ks20.8,
Dako), D2–40 (Dako), CD34 (QBEnd 10; Beckman Coulter, Marseille,
France) and MLH1 (ES05; Leica Microsystems, Newcastle, UK).
Statistical analysis was carried out using the Chi-square test,
Fisher's exact test, Mann-Whitney U-test and log-rank test, as
appropriate. A statistically significant difference was indicated
when P<0.05.
Results
Clinicopathological characteristics of
patients and CIC
The main clinicopathological findings are shown in
Table I. Patients with CICs
included 7 males and 9 females ranging in age from 26 to 85 years
(mean, 60.5). Four patients (25.0%) were diagnosed before the age
of 50 years, and four patients (25.0%) were diagnosed between the
ages of 50 and 59 years. Only two patients (Cases 14 and 16) had
synchronous colorectal and gastric carcinomas; the mother of the
youngest patient (Case 14, 26 years) had been diagnosed with
colorectal carcinoma at the age of 50 years. Sessile serrated
adenoma was found close to one CIC (Case 7). CICs ranged from 2.9
to 9.0 cm in maximal diameter (mean, 5.54 cm). One CIC (Case 15)
was restricted to the ileocecal mucosa, but the other CICs invaded
the muscularis propria or subserosa and/or pericolic tissues. Two
CICs (Cases 13 and 14) were mucinous adenocarcinoma involving
>50% of the tumor. The remaining 14 CICs showed features of
tubular adenocarcinoma, and 7 (50%) of the 14 CICs were accompanied
by focal (<50% of tumor) mucinous adenocarcinoma components.
Tumor-infiltrating lymphocytes, poorly differentiated components,
Crohn's-like lymphocytic reaction, peritumoral lymphocytic reaction
and increased stromal plasma cells were found in 6, 5, 2, 2 and 5
cases, respectively. The calculated MsPath and PREDICT scores
ranged from 1.6 to 6.6 (mean, 3.14) and from 1.6 to 7.8 (mean,
3.86), respectively. The CICs exhibited focal or diffuse CK20
expression and 7 CICs showed focal CK7 co-expression. In the
immunohistochemical examination of MHL1, we assessed a decreased
MHL1 expression in CIC compared with non-neoplastic colonic mucosa
in each case. However, in two cases, such assessment could not be
performed, as carcinoma cells and non-neoplastic mucosa were poorly
immunostained with MLH1. Only three (21.4%) of the remaining 14
CICs showed a decreased expression of MHL1.
 | Table IClinicopathological characteristics of
colo-ileal carcinomas. |
Table I
Clinicopathological characteristics of
colo-ileal carcinomas.
| Case | Age (y) | Sex | Personal history of
cancer | Family history of
cancer | Tumor size (cm) | Main
histologya | Prominent
neutrophilia | CLPP | Loss of MLH1
expression | MsPath scoreb | PREDICT scorec | Outcome |
|---|
| 1 | 74 | F | N/A | N/A | 4.5 | Ad, well | + | − | Unclear | 3.7 | 6.5 | DOC, 203 mo |
| 2 | 83 | F | None | None | 3.5 | Ad, mod | − | − | + | 1.6 | 1.6 | DOC, 5 mo |
| 3 | 79 | F | None | None | 9 | Ad, mod | + | − | − | 1.6 | 3.2 | N/A |
| 4 | 79 | M | None | None | 5 | Ad, well | − | − | Unclear | 1.6 | 1.6 | DOC, 10 mo |
| 5 | 33 | M | None | None | 6.5 | Ad, mod | + | − | − | 4.4 | 5.8 | Lost, 36 mo |
| 6 | 52 | F | None | None | 6.5 | Ad, mod | − | − | − | 2.2 | 1.6 | N/A |
| 7 | 53 | F | None | None | 4.5 | Ad, mod | − | − | − | 1.6 | 3.2 | NED, 84 mo |
| 8 | 65 | F | None | None | 6.5 | Ad, por | − | − | + | 2.2 | 1.6 | DOD, 13 mo |
| 9 | 80 | F | None | None | 5.5 | Ad, mod | + | − | − | 3.7 | 3.6 | NED, 6 mo |
| 10 | 34 | M | N/A | N/A | 6 | Ad, mod | + | − | − | 4.4 | 6.5 | N/A |
| 11 | 48 | F | N/A | N/A | 5 | Ad, mod | − | + | − | 2.3 | 2.9 | Lost, 25 mo |
| 12 | 71 | F | N/A | N/A | 7.5 | Ad, mod | + | − | − | 1.6 | 3.2 | N/A |
| 13 | 53 | M | N/A | N/A | 2.7 | Muc ad | + | − | − | 5.9 | 4.5 | Lost, 25 mo |
| 14 | 26 | M | 3 other CRCs
(synchronous) | CRC (mother, age 50
y) | 7.5 | Muc ad | + | + | + | 6.6 | 7.8 | DOD, 44 mo |
| 15 | 53 | M | None | None | 3 | Ad, well | − | − | − | 3.7 | 2.9 | NED, 94 mo |
| 16 | 85 | M | Gastric cancer
(synchronous) | None | 5.5 | Ad, mod | − | − | − | 3.7 | 5.2 | DOC, 9 mo |
Neutrophils appear to correlate with
MSI-H
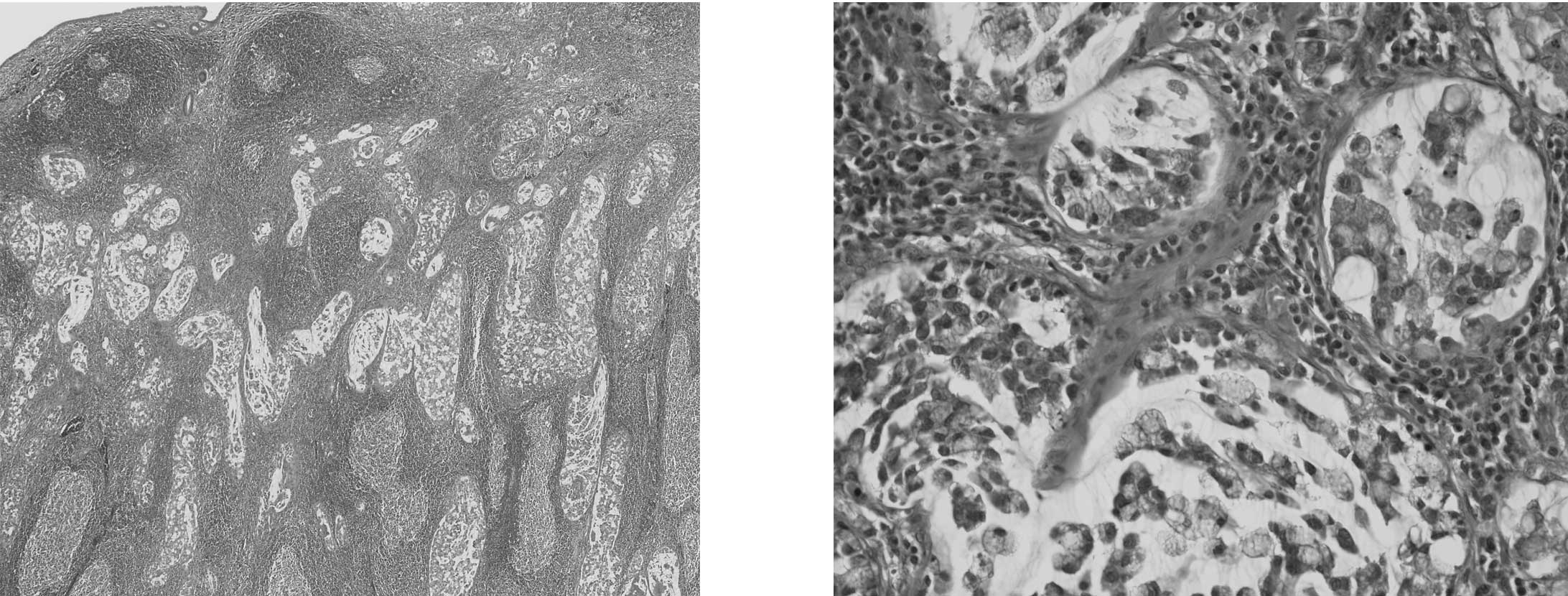
In eight (50%) of 16 CICs, prominent neutrophilic
infiltration was found in cancer tubules and the surrounding stroma
(Fig. 1), but such features were
not prominent in the non-neoplastic mucosa in each case. Of the
eight cases of prominently neutrophilic CIC and 8 cases of CIC with
non-prominent neutrophilia no significant differences were found in
patients' age (P=0.42), gender (P=1), tumor size (P=0.16),
decreased MLH1 expression (P=0.9) or patient outcome (P=0.26).
However, the MsPath and PREDICT scores in prominently neutrophilic
CICs appeared to be higher than those in CICs with non-prominent
neutrophilia. Statistical analysis revealed a significant
difference of the PREDICT score between 8 prominently neutrophilic
CICs and the remaining 8 with non-prominent neutrophilia (P=0.004),
although there was no significant difference in the total MsPath
score between them (P=0.052).
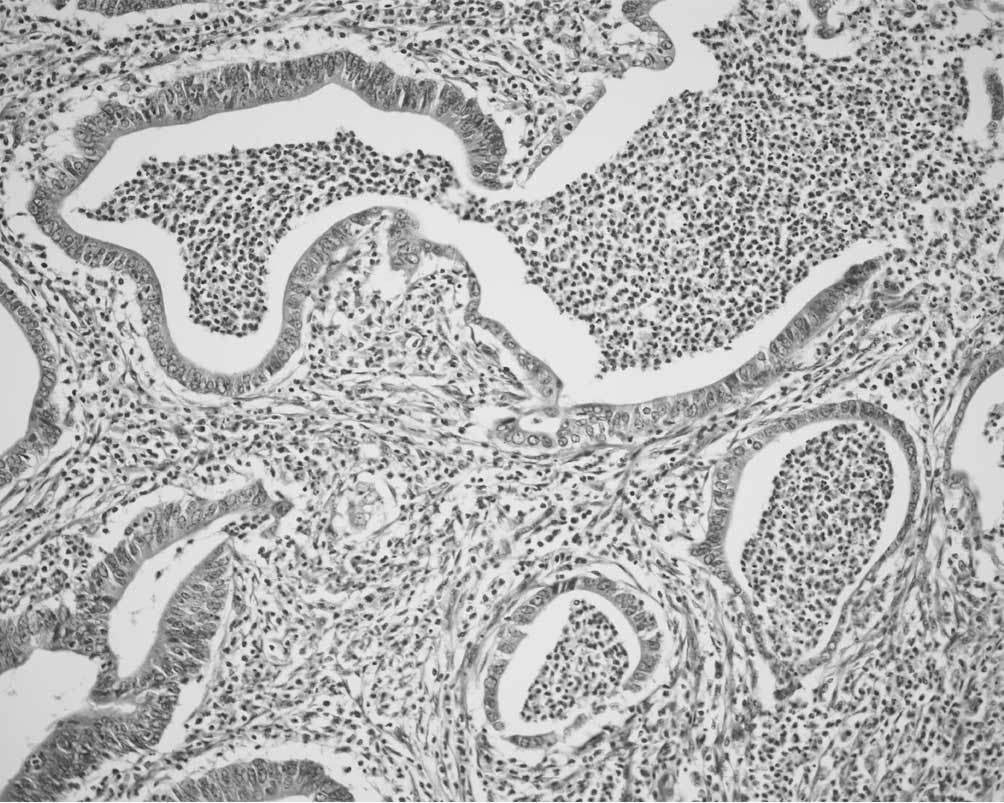
Peyer's patches were involved in CIC
In three CICs (18.8%), mucosal and/or submucosal
dilated lymphatic spaces in Peyer's patches were prominently
involved in carcinoma cell clusters (Fig. 2), suggesting cancerous lymphangiosis
in Peyer's patches (CLPP). Endothelial cells lining CLPP spaces
were almost always positive for D2–40, and occasionally showed CD34
coexpression. Three patients with CLPP-positive CICs ranged in age
from 26 to 53 years (mean, 42.3), whereas 13 patients with
CLPP-negative CICs ranged in age from 33 to 85 years (mean, 64.7);
and there was a significant difference between the two groups
(P=0.031). No significant associations were found between the
presence of CLPP and clinicopathological characteristics such as
gender (P=0.55), tumor size (P=0.31), the presence of lymph node
metastasis (P=0.53), decreased MLH1 expression (P=0.5) or patient
outcome (P=0.25). Additionally, no statistically significant
differences were found in MsPath and PREDICT scores between
them.
Discussion
In this study, all 16 CICs showed CK20 expression
with or without CK7 co-expression, which did not show whether these
CICs had originated in the right-sided colon or the ileum (9). However, histopathological features of
CICs were consistent with colon adenocarcinoma, and distinct
features indicating ileal origin were not identified. The incidence
of ileal carcinoma is known to be significantly lower than that of
colon carcinoma (3). Therefore, we
consider that almost all of the invasive CICs had originated in the
right-sided colonic mucosa and then invaded the ileum.
Slater et al noted that patients with
right-sided colon carcinomas are older than those with left-sided
colon carcinomas (10). However,
other studies have described that right-sided colon origin is one
of the major risk factors of MSI-related carcinoma, which is
capable of developing in younger patients (3–7). In
this study, the mean age of patients with CICs (60.5 years) was
younger than that previously reported (71.3 years) for patients
with right-sided colon carcinoma (10), which is attributable to the
inclusion of younger MSI-related cases. Using MsPath (5) and PREDICT (6), findings of this study showed
relatively high scores (mean MsPath score 3.14; mean PREDICT score
3.86), although there were no cases suggestive of Lynch syndrome
(3,4). However, in MsPath, only one finding of
right-sided origin can add 1.6 to the score, and the previously
recommended cut-off of 1.0 may not be specific for MSI-H (5,6). Hyde
et al reported that the specificity of MsPath without an age
limit is 38.9% (6). Moreover, they
also described that an arbitrary cut-off of 2.5 for PREDICT
resulted in sensitivity of 96.9% and specificity of 76.6% in their
validation cohort. In our series, this cut-off of 2.5 suggested
that 12 CICs are MSI-H, and the other group included 2 CICs with
decreased MLH1 immunoexpression, suggestive of MSI-H tumors.
Therefore, this study markedly suggested MSI-H status in 14 of 16
CICs (87.5%). These 14 cases also included a sessile serrated
adenoma-related case (Case 7) and the youngest patient (Case 14)
who had multiple colorectal cancers and a mother with a history of
colorectal cancer.
Previous studies have suggested that
tumor-infiltrating lymphocytes, Crohn's-like lymphoid reactions,
peritumoral lymphocytic reactions and stromal plasma cell
infiltration are MSI-related reactions (3–7).
Eosinophilia (11) and S-100
protein-positive dendritic cell infiltration (12) are also known to be good
prognosis-associated inflammatory reactions in colorectal
carcinoma. However, the clinicopathological significance of
prominent neutrophilia in CIC or colorectal carcinoma remains
unclear, although one study (13)
reported no relationship between such features and MSI-H
endometrial carcinoma. This study revealed prominent neutrophilia
in 8 CICs (8/16, 50%), which were not associated with patients'
age, gender, tumor size or prognosis. However, the PREDICT score in
prominently neutrophilic CICs was significantly higher than that in
CICs with non-prominent neutrophilia. The MsPath score in
prominently neutrophilic CICs also appeared to be higher than that
in CICs with non-prominent neutrophilia, but the difference showed
borderline significance (P=0.052). These findings suggest that
neutrophilia in CICs may be associated with MSI-H status.
In this study, CLPP was found in 18.8% of CICs. CLPP
is an aggressive; however, no significant relationship was detected
between the presence of CLPP and the presence of lymph node
metastasis and a worse prognosis. CLPP was also not associated with
gender, tumor size, decreased MLH1 expression or scores of MsPath
and PREDICT. These findings suggest that CLPP is
prognosis-independent and has non-specific features. However,
patients with CLPP-positive CICs were significantly younger than
those with CLPP-negative CICs. In 2 of 3 patients with CICs showing
CLPP, personal history was not available and there was the
possibility of a history of inflammatory bowel disease. However,
histopathological examination revealed no macroscopic or
microscopic features indicating preexisting ulcerative colitis or
Crohn's disease. Consequently, we consider that CLPP may be
associated with age-related changes of Peyer's patches, which
increase in size and number until puberty and regress steadily
thereafter (2). Peyer's patches are
composed of specialized cells or structures actively absorbing
pathogens or antigens, and play a critical role in mucosal immunity
(1,2,8). Thus,
lymphovascular flow and structures in the Peyer's patches may be
somewhat different from those in other intestinal sites. These
considerations may explain the occasional presence of non-specific
CLPP in CICs and the frequent dual expression of D2–40 and CD34 in
the endothelial cells lining the spaces around CLPP.
In conclusion, this study suggested that almost all
CICs invade the ileum secondarily with a high frequency (87.5%) of
MIS-H status. Prominent neutrophilia may be an additional
histological marker for MSI-H status. Prognosis-independent CLPP
may occasionally occur in younger patients with CICs. However, this
study included a limited number of examined cases, and more
investigations are required to confirm our conclusions and
findings.
Acknowledgements
The authors thank Drs A.O. Tadakazu, Michinori
Murayama and Kimitoshi Inoue for kindly providing the study
specimens; Haruyuki Nagasawa and Shin-ichi Katori for excellent
technical assistance; and Daniel Mrozek for editing the
manuscript.
References
|
1
|
Feldman M, Friedman LS and Brandt LJ:
Sleisenger and Fordtran's Gastrointestinal and Liver Disease. 8th
edition. Saunders/Elsevier; Philadelphia: 2006
|
|
2
|
Segal GH and Petras RE: Small Intestine.
Histology for Pathologists. 2nd edition. Sternberg SS:
Lippincott-Raven Publishers; Philadelphia: pp. 495–518. 1997
|
|
3
|
Hamilton SR and Aaltonen LA; World Health
Organization. Classification of Tumours, Pathology and Genetics,
Tumours of the Digestive System. IARC press; Lyon: 2000
|
|
4
|
Umar A, Boland CR, Terdiman JP, et al:
Revised Bethesda guidelines for hereditary nonpolyposis colorectal
cancer (Lynch syndrome) and microsatellite instability. J Natl
Cancer Inst. 96:261–268. 2004. View Article : Google Scholar
|
|
5
|
Jenkins MA, Hayashi S, O'Shea AM, et al:
Pathology features in Bethesda guidelines predict colorectal cancer
microsatellite instability: a population-based study.
Gastroenterology. 133:48–56. 2007. View Article : Google Scholar
|
|
6
|
Hyde A, Fontaine D, Stuckless S, et al: A
histology-based model for predicting microsatellite instability in
colorectal cancers. Am J Surg Pathol. 34:1820–1829. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sinicrope F, Foster NR, Sargent DJ, et al:
Model-based prediction of defective DNA mismatch repair using
clinicopathological variables in sporadic colon cancer patients.
Cancer. 116:1691–1698. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lelouard H, Henri S, De Bovis B, et al:
Pathogenic bacteria and dead cells are internalized by a unique
subset of Peyer's patch dendritic cells that express lysozyme.
Gastroenterology. 138:173–184. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Goldstein RS and Bosler DS:
Immunohistochemistry of the gastrointestinal tract, pancreas, bile
ducts, gallbladder and liver. Diagnostic Immunohistochemistry. 2nd
edition. Dabbs DJ: Churchill Livingston/Elsevier; Philadelphia: pp.
442–508. 2006, View Article : Google Scholar
|
|
10
|
Slater G, Papatestas AE, Tartter PI,
Mulvihill M and Aufses AH: Age distribution of right- and
left-sided colorectal cancers. Am J Gastroenterol. 77:63–66.
1982.PubMed/NCBI
|
|
11
|
Pretlow TP, Keith EF, Cryar AK, et al:
Eosinophil infiltration of human colonic carcinomas as a prognostic
indicator. Cancer Res. 43:2997–3000. 1983.PubMed/NCBI
|
|
12
|
Ambe K, Mori M and Enjoji M: S-100
protein-positive dendritic cells in colorectal adenocarcinomas.
Distribution and relation to the clinical prognosis. Cancer.
63:496–503. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shia J, Black D, Hummer J, Boyd J and
Soslow RA: Routinely assessed morphological features correlate with
microsatellite instability status in endometrial cancer. Hum
Pathol. 39:116–125. 2008. View Article : Google Scholar : PubMed/NCBI
|