Introduction
Gastric cancer is one of the most common causes of
cancer-related mortality and is responsible for approximately one
million fatalities each year (1).
Generally a fatal malignancy, gastric cancer is particularly
prevalent in East Asian countries such as Korea, China and Japan
(2–5). Along with gastric cancer,
cholangiocarcinoma causes worldwide concern due to its destructive
malignancy. Cholangiocarcinoma has a five-year survival rate of
<5%. The majority of patient fatalities occur within 12 months
and 60–70% of patients have a virtually inoperable condition at the
time of diagnosis (6,7). In attempts to identify a cure for such
cancers, researchers often explore the effects of transforming
growth factor-β1 (TGF-β1). TGF-β is a pleiotropic cytokine and a
versatile polypeptide. It is involved in a wide range of cellular
and genetic activities, including cell proliferation, cell growth
inhibition, cell morphology transformation, cell signaling pathway
participation and the activation of various types of genes and
proteins (8–10). Among the numerous isoforms of TGF-β
(TGF-β1, TGF-β2 and TGF-β3), TGF-β1 is widely known for its
inhibitory growth effect in angiogenesis and fibrogenesis (11–13).
TGF-β1 inhibits the growth of nonneoplastic epithelial cells by
regulating molecules related to the G1 and S phases of the cell
cycle. More specifically, inhibition occurs through upregulation of
mito-inhibitors including p15, p21 and p27, and through
downregulation of mito-activators including cyclins and cyclin
dependent kinases (cdks) (14–17).
As cdks dictate cell cycle progression and p27 inhibits cdk
activity by preventing the transition from G1 to S phase in the
cell cycle (18), the down- and
upregulation of these two factors by TGF-β1 effectively inhibits
uncontrolled cell growth. Through this signaling pathway, TGF-β1
plays a crucial role in initiating cell arrest and fibrosis in
cancer cells (19–22). In the present study, we aimed to
identify whether TGF-β1 can function as an antitumor agent in two
cancer cell lines; cholangiocarcinoma and gastric cancer. The
downregulation of cdk4 and upregulation of p27 was investigated
through a number of different methods including cell proliferation
assay, bicinchoninic acid (BCA) assay and western blot
analysis.
Materials and methods
Cells and culture conditions
Recombinant human TGF-β1 was provided by R&D
Systems, Inc. (Minneapolis, MN, USA) and was derived from a Chinese
hamster ovary cell line with the structure of a disulfide-linked
homodimer. The human cholangiocarcinoma cell line (SUN-1196) and
human gastric cancer cell line (AGS) were both obtained from the
Korean Cell Line Bank (Seoul National University College of
Medicine, Seoul, Korea). All cell lines were grown in RPMI-1640
medium (Thermo, Waltham, MA, USA) and were supplemented with
glucose, 10% fetal bovine serum (FBS) and 1%
penicillin/streptomycin. The cells were grown at 37°C and 5%
CO2 in an incubator.
The study was approved by the Ethics Committee of
Kangbuk Samsung Hospital, Sungkyun-kwan University School of
Medicine, Seoul, Korea.
Cell proliferation assay
Cell viability was measured by Cell Viability
Reagent (Invitrogen, Grand Island, NY, USA). Cholangiocarcinoma
cells (SUN-1196) and gastric cancer cells (AGS) were cultured with
recombinant human TGF-β1 at concentrations of 0, 0.5, 5, 25 and 50
ng/ml for 24 h. PrestoBlue Cell Viability Reagent solution was
added to each well, followed by incubation for 2 h. The cell
absorbance values were measured with ELISA (Bio-Rad, Hercules, CA,
USA) at a wavelength of 570 nm.
Western blot analysis
The protein was extracted from cultured cells using
PRO-PREP for Cell/Tissue Protein Extraction Solution (Intron
Biotechnology, Sungnam, Korea) and protein concentration was
determined by BCA Protein Assay kit (Thermo). For western blot
analysis, protein samples (20 μg) were subjected to sodium
dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) and
then transferred to polyvinylidene difluoride (PVDF) membranes. The
membranes were incubated with primary antibodies to cdk4 (34 kDa,
1:2000, rabbit polyclonal; Abcam, Cambridge, UK), p27 (27 kDa,
1:2500, mouse monoclonal; BD Biosciences, Franklin Lakes, NJ, USA)
and actin (42 kDa, 1:5000, mouse monoclonal; Abcam). The specific
protein was detected by enhanced chemiluminescence (ECL),
horseradish peroxidase (HRP) developing agents (AbFrontier, Anyang,
Korea) and autoradiography film (GE Healthcare, Amersham, Bucks,
UK), while band quantitation was performed with Geliance 600
(PerkinElmer, Waltham, MA, USA).
Results
Absorbance values of AGS gastric cancer
cell lines increases within a certain range of TGF-β1
concentrations in a cell proliferation assay
The present study sought to determine changes in
absorbance values due to changes in TGF-β1 concentration by
performing a cell proliferation assay on the AGS cancer cell line.
The study aimed to detect patterns in the absorbance value changes
that could help identify the effect of increasing TGF-β1
concentration on the number of cancer cells remaining following
TGF-β1 treatment. Since calculated absorbance values are based on
the amount of light that remains after being absorbed by cancer
cells, the values are good indicators of the number of remaining
cancer cells following TGF-β1 treatment. The results are shown in
Table I. The untreated (0 ng/ml
TGF-β1) AGS cancer cell line displayed an absorbance value of
0.698, which is the average value obtained from two successive
trials. The AGS cancer cell line treated with 0.5 ng/ml TGF-β1
displayed a higher absorbance value of 0.724. Cells treated with 5
ng/ml TGF-β1 had an absorbance value of 0.980, demonstrating an
increase from the previous concentration. The AGS cancer cell line
treated with 25 and 50 ng/ml TGF-β1 displayed lower absorbance
values, thereby discontinuing the pattern exhibited by the cell
proliferation assay. From this result, TGF-β1 may potentially
regulate gastric cancer metastasis within the concentration range
of 0–5 ng/ml.
 | Table I.Cell proliferation assay for AGS
cells. |
Table I.
Cell proliferation assay for AGS
cells.
| AGS absorbance
|
|---|
| TGF-β1 concentration
(ng/ml) | 1st trial | 2nd trial | Average | Standard
deviation |
|---|
| 0 | 0.697 | 0.699 | 0.698 | 0.001 |
| 0.5 | 0.714 | 0.734 | 0.724 | 0.014 |
| 5.0 | 0.911 | 1.049 | 0.98 | 0.098 |
| 25 | 0.7 | 0.834 | 0.767 | 0.095 |
| 50 | 0.706 | 0.728 | 0.717 | 0.016 |
TGF-β1 exerts an antitumor effect on AGS
cancer cell lines through cdk4 and p27 pathways
Following the cell proliferation assay, western blot
analysis was performed to track the specific pathways through which
TGF-β1 exerts its anti-proliferative effect on AGS cancer cell
lines. Three antibodies (cdk4, p27 and actin) were subjected to
western blot analysis. Their respective protein bands in each
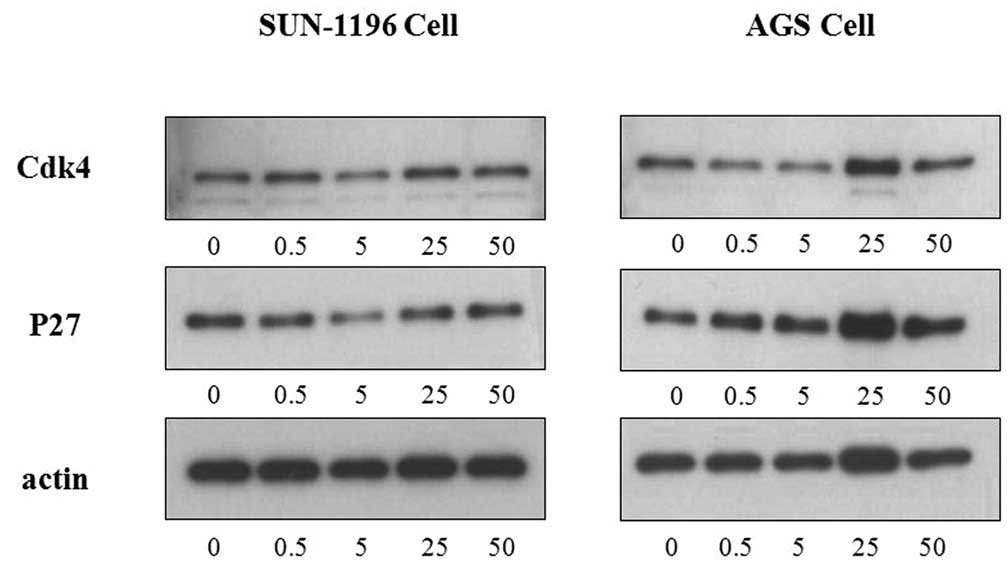
TGF-β1 concentration are reproduced in Fig. 1. From Fig. 1, a generally decreasing thickness
pattern is evident for cdk4 protein bands, whereas an increasing
thickness pattern is demonstrated in p27 protein bands within a
certain range. This result is similar to that of the cell
proliferation assay performed on AGS cancer cell lines. To ensure
uniformity of the western blot analysis, another antibody (actin)
was used. The actin protein band thickness indicated whether the
values obtained for other antibodies were reliable, as the first
three actin bands have consistent thickness for AGS cancer cell
lines. From the pattern exhibited by protein bands alone, TGF-β1
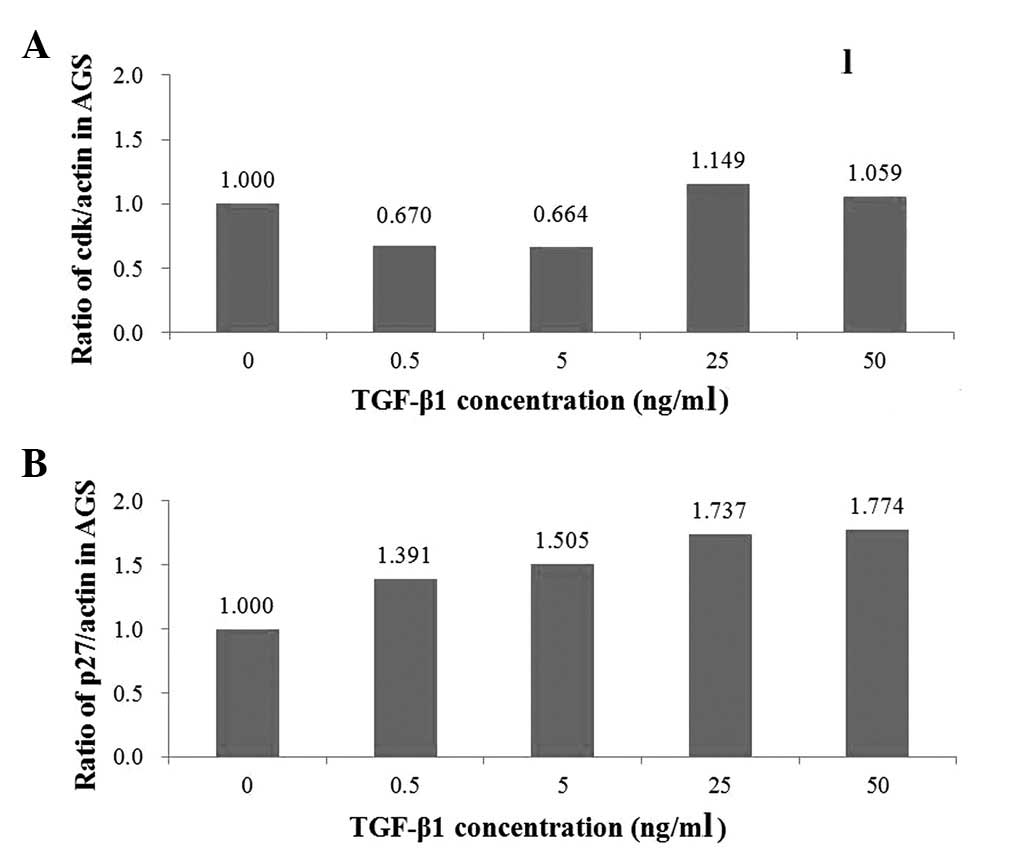
appears to function through two pathways. In Fig. 2A, cdk4 values decreased from 1.000
to 0.670 and then to 0.664, with increasing TGF-β1 concentrations
of 0, 0.5 and 5 ng/ml, respectively. From 25 ng/ml TGF-β1, cdk4
values began to increase. In contrast with cdk4, which decreased
with increasing TGF-β1 concentrations, p27 increased from 1.000 to
1.391 and then to 1.505 with increasing TGF-β1 concentrations of 0,
0.5 and 5 ng/ml (Fig. 2B). Also
dissimilar to cdk4, p27 values demonstrated consistent increases,
including at 25 and 50 ng/ml of TGF-β1, where p27 values were 1.737
and 1.774, respectively.
Absorbance values of SUN-1196
cholangiocarcinoma cell lines increase within a certain range of
TGF-β1 concentrations in the cell proliferation assay
Absorbance values of SUN-1196 cholangiocarcinoma
cell lines were measured with a cell proliferation assay. The
change in absorbance values displayed increasing and decreasing
patterns within certain ranges of TGF-β1 concentrations. As shown
in Table II, SUN-1196 cancer cell
lines treated with 0, 0.5 and 5 ng/ml TGF-β1 displayed a decreasing
pattern of absorbance values; 0.643, 0.613 and 0.609, respectively.
Cells treated with 25 and 50 ng/ml TGF-β1 displayed an increasing
pattern of absorbance values; 0.626 and 0.697, respectively. This
result reveals a potential antineoplastic effect of TGF-β1 on
cholangiocarcinoma cells within a 5–50 ng/ml concentration
range.
 | Table II.Cell proliferation assay for SUN-1196
cells. |
Table II.
Cell proliferation assay for SUN-1196
cells.
| SUN-1196 absorbance
|
|---|
| TGF-β1 concentration
(ng/ml) | 1st trial | 2nd trial | Average | Standard
deviation |
|---|
| 0 | 0.645 | 0.641 | 0.643 | 0.003 |
| 0.5 | 0.611 | 0.615 | 0.613 | 0.003 |
| 5 | 0.604 | 0.613 | 0.609 | 0.006 |
| 25 | 0.617 | 0.635 | 0.626 | 0.013 |
| 50 | 0.675 | 0.718 | 0.697 | 0.030 |
TGF-β1 exerts an antitumor effect on
SUN-1196 cholangiocarcinoma cancer cell lines through the p27 (and
not the cdk4) pathway
The influence of TGF-β1 on SUN-1196
cholangiocarcinoma cancer cell lines was observed by western blot
analysis of three antibodies; cdk4, p27 and actin. As is evident in
Fig. 1, cdk4 protein bands had
indiscriminate thicknesses with increasing TGF-β1 concentrations,
while p27 protein bands varied in thickness according to a pattern
within certain TGF-β1 concentration ranges. The actin protein bands
had uniform thickness, indicating that the results for other
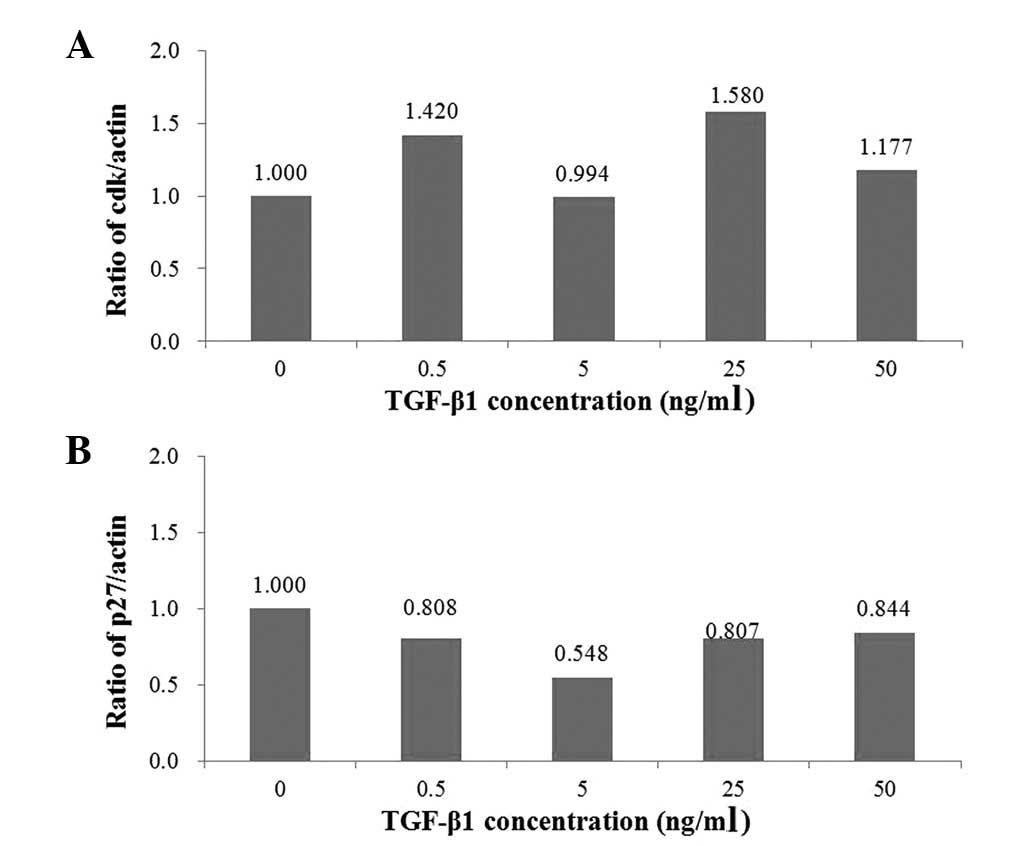
antibody protein bands are reliable. As shown in Fig. 3A, cdk4 values (1.000, 1.420, 0.994,
1.580 and 1.177) varied without any distinct patterns. However,
Fig. 3B reveals that p27 values
decreased from 1.000 to 0.808 and then to 0.548 with increasing
TGF-β1 concentrations of 0, 0.5 and 5 ng/ml. The p27 values also
increased from 0.548 to 0.807 and then to 0.844 with increasing
TGF-β1 concentrations of 5, 25 and 50 ng/ml.
Discussion
Within a certain concentration range, TGF-β1 was
revealed to play an antitumor role in two types of cancer; gastric
cancer and cholangiocarcinoma. According to the cell proliferation
assay results for AGS cancer cell lines, the absorbance values of
AGS cells treated with 0, 0.5 and 5 ng/ml TGF-β1 consistently
increased. The pattern of increasing absorbance indicates that the
number of AGS cells decreased with increasing TGF-β1 concentration,
as absorbance is measured by detecting the amount of light
remaining after being partially absorbed by cells. A higher
absorbance value indicates that the machine detected a greater
amount of light and the cells absorbed less light, demonstrating
that fewer cells are present. Since the absorbance value was
highest in AGS cells treated with 5 ng/ml TGF-β1, the smallest
number of AGS cells remained in that concentration. This result
demonstrated the antitumor role of TGF-β1 within a specific
concentration range. In short, TGF-β1 plays an antitumor role in
gastric cancer cell lines when its concentration is between 0 and 5
ng/ml. The western blot analysis results confirmed the
aforementioned analysis of the cell proliferation assay and
explained the specific pathways through which TGF-β1 exerts an
anti-neoplastic effect on gastric cancer cells. The unitless cdk4
and p27 in Fig. 2A and B indicate
the quantity of each antibody detected by film detection technique
in the western blot analysis. As shown in Fig. 2A, the quantity of cdk4 decreased
with an increasing TGF-β1 concentration between 0 and 5 ng/ml. As
shown in Fig. 2B, the quantity of
p27 increased with increasing TGF-β1 concentration between 0 and 50
ng/ml. These two results confirmed our hypothesis that TGF-β1
exerts an antitumor effect on gastric cancer cell lines through the
downregulation of cdks (cdk4) and the upregulation of p27. The
western blot analysis results were also concordant with the cell
proliferation assay results, which suggested that TGF-β1 plays an
antitumor role in AGS when its concentration is between 0 and 5
ng/ml. The present study demonstrated that TGF-β1 exerts an
antitumor effect on gastric cancer cells through two pathways, cdk4
and p27, by downregulating cdk4 and by upregulating p27. Bhayal
et al revealed that TGF-β1 may be a risk factor of genetic
susceptibility to gastric cancer in the south Indian population
(23). Yuan et al
demonstrated that TGF-β1 plays an antitumor role in gastric cancer
rather than inducing gastric cancer cells to escape human
immunological surveillance (24–26).
Our results are concordant with those of Yuan et al(24).
As a result of the SUN-1196 cell proliferation
assay, another distinct pattern of increasing absorbance data was
identified within the TGF-β1 concentration range of 5 to 50 ng/ml,
which is different from the range found in AGS gastric cancer cell
lines. A lower absorbance value indicates that the machine detected
less light, thus the SUN-1196 cells absorbed more light. A greater
number of SUN-1196 cells remained when retreated with 5 ng/ml
TGF-β1, and this number decreased with increasing TGF-β1
concentrations of 5, 25 and 50 ng/ml, as indicated in Table II. These results suggest that TGF-β1
has an antitumor and anti-proliferative effect on
cholangiocarcinoma cells when its concentration ranges from 5 to 50
ng/ml. Fig. 3A and B reaffirmed the
anti-neoplastic influence of TGF-β1 on cholangiocarcinoma cells and
revealed the pathway through which the antitumor effect is exerted.
As with the AGS cell western blot analysis results, the cdk4 and
p27 values in Fig. 3 refer to the
quantity of antibodies indicated by the thickness of protein bands
in Fig. 1. Due the lack of a
pattern in the distribution of cdk4 values with increasing TGF-β1
concentration (Fig. 3A), we
concluded that TGF-β1 does not follow the cdk4 pathway and so does
not downregulate cdk4 as was hypothesized previously. However,
Fig. 3B revealed a clear pattern of
increase in the quantity of p27 within a range of 5–50 ng/ml. This
pattern confirmed that TGF-β1 exerts an anti-cancer influence via
the p27 pathway as was hypothesized. This result was also
concordant with the cell proliferation assay, which suggested that
TGF-β1 is at its most effective as an anti-cancer agent at the
concentration range of 5–50 ng/ml. In brief, TGF-β1 has an
antitumor effect on cholangiocarcinoma cells through the p27
pathway, but not through the cdk4 pathway. Zen et al
demonstrated that TGF-β1 did not influence the cell-proliferative
activities of three cultured human intrahepatic cholangiocarcinoma
(ICC) cells (27). However, the
present study revealed that when its concentration is between 5 and
50 ng/ml, TGF-β1 has an anti-proliferative effect on
cholangiocarcinoma cells. Zen et al also demonstrated that
cyclin D1 is key for ICC cells attaining TGF-B1 resistance.
However, the present study has demonstrated (by western blot
analysis) that through upregulation of p27, TGF-β1 is capable of
deterring cholangiocarcinoma proliferation, thus nullifying the
resistance of cholangiocarcinoma to TGF-β1 (27). Furthermore, Shimizu et al
revealed that TGF-β1 stimulation in ICC results in cellular
proliferation. The present study demonstrated that TGF-β1
stimulation in cholangiocarcinoma resulted in downregulation of
cellular proliferation when TGF-β1 concentration was between 5 and
50 ng/ml (28).
In conclusion, certain concentrations of TGF-β1 play
antitumor roles in gastric cancer through the downregulation of
cdk4 and the upregulation of p27. These TGF-β1 concentrations also
have antitumor roles for cholangiocarcinoma through the
upregulation of p27. These results bring us a step closer to
finding a cure for cholangiocarcinoma and gastric cancer. In future
studies, we intend to increase the number of antibodies for western
blot analysis, the types of cancer cell being tested and the
concentrations of TGF-β1. Additionally, PCR will be included to
refine our conclusions. With these additions, we may be able to
produce more significant results that may further enhance the
effort to find a novel cure for cancers.
Acknowledgements
This study was supported by Medical
Research Funds from the Kangbuk Samsung Hospital.
References
|
1.
|
World Health Organization: Cancer: Fact
sheet N297, February 2009. http://www.who.int/mediacentre/factsheets/fs297/en/.
Accessed March 24, 2011.
|
|
2.
|
Murray CJ and Lopez AD: Mortality by cause
for eight regions of the world: Global Burden of Disease Study.
Lancet. 349:1269–1276. 1997. View Article : Google Scholar
|
|
3.
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar
|
|
4.
|
Crew KD and Neugut AI: Epidemiology of
gastric cancer. World J Gastroenterol. 12:354–362. 2006.
|
|
5.
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
6.
|
Okada T, Sawada T and Kubota K: Rapamycin
inhibits growth of cholangiocarcinoma cells.
Hepatogastroenterology. 56:6–10. 2009.
|
|
7.
|
Shabby Y and El-Serage HB: The
epidemiology of cholangiocarcinoma. Semin Liver Dis. 24:115–125.
2004. View Article : Google Scholar
|
|
8.
|
Massagué J: The transforming growth
factor-beta family. Annu Rev Cell Biol. 6:597–641. 1990.
|
|
9.
|
Moses HL, Yang EY and Pietenpol JA: TGF-β
stimulation and inhibition of cell proliferation: new mechanistic
insights. Cell. 63:245–247. 1990.
|
|
10.
|
Massagué J, Blain SW and Lo RS: TGF-β
signaling in growth control, cancer, and heritable disorders. Cell.
103:295–309. 2000.
|
|
11.
|
Derynck R, Akhurst RJ and Balmain A:
TGF-beta signaling in tumor suppression and cancer progression. Nat
Genet. 29:117–129. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Löhr M, Schmidt C, Ringel J, et al:
Transforming growth factor-beta1 induces desmoplasia in an
experimental model of human pancreatic carcinoma. Cancer Res.
61:550–555. 2001.PubMed/NCBI
|
|
13.
|
Bellone G, Carbone A, Tibaudi D, et al:
Differential expression of transforming growth factors-beta1,
-beta2 and –beta3 in human colon carcinoma. Eur J Cancer.
37:224–233. 2001.
|
|
14.
|
Ko TC, Sheng HM, Reisman D, Thompson EA
and Beauchamp RD: Transforming growth factor-beta1 inhibits cyclin
D1 expression in intestinal epithelial cells. Oncogene. 10:177–184.
1995.PubMed/NCBI
|
|
15.
|
Gene Y and Weinberg RA: Transforming
growth factor beta effects on expression of G1 cyclins and
cyclin-dependent protein kinases. Proc Natl Acad Sci USA.
90:10315–10319. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Ravitz MJ and Wenner CE: Cyclin-dependent
kinase regulation during G1 phase and cell cycle regulation by
TGF-beta. Adv Cancer Res. 71:165–207. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Carneiro C, Alvarez CV, Zalvide J, Vidal A
and Domínguez F: TGF-beta1 actions on FRTL-5 cells provide a model
for the physiological regulation of thyroid growth. Oncogene.
16:1455–1465. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Luo J, Chen YJ, Wang WY and Zou SQ: Effect
of mutant p27(kip1) gene on human cholangiocarcinoma cell line,
QBC(939). World J Gastroenterol. 14:5344–5348. 2008. View Article : Google Scholar
|
|
19.
|
Czaja MJ, Weiner FR, Flanders KC, et al:
In vitro and in vivo association of transforming
growth factor-beta1 with hepatic fibrosis. J Cell Biol.
108:2477–2482. 1989. View Article : Google Scholar
|
|
20.
|
Bissell DM, Wang SS, Jarnagin WR and Roll
FJ: Cell-specific expression of transforming growth factor-beta in
rat liver. Evidence for autocrine regulation of hepatocyte
proliferation. J Clin Invest. 96:447–455. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Ichikawa T, Zhang YQ, Kogure K, et al:
Transforming growth factor beta and activin tonically inhibit DNA
synthesis in the rat liver. Hepatology. 34:918–925. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Yata Y, Gotwals P, Koteliansky V and Rokey
DC: Dose-dependent inhibition of hepatic fibrosis in mice by a
TGF-beta soluble receptor: implications for antifibrotic therapy.
Hepatology. 35:1022–1030. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Bhayal AC, Prabhakar B, Pandu K, et al:
Role of transforming growth factor-β1 -509 C/T promoter
polymorphism in gastric cancer in south Indian population. Tumor
Biol. 32:1049–1053. 2011.
|
|
24.
|
Yuan XL, Chen L, Zhang TT, et al: Gastric
cancer cells induce human CD4+Foxp3+ regulatory T cells through the
production of TGF-β1. World J Gastroenterol. 17:2019–2027.
2011.
|
|
25.
|
Shevach EM: Mechanisms of foxp3+ T
regulatory cell-mediated suppression. Immunity. 30:636–645.
2009.
|
|
26.
|
von Boehmer H: Mechanisms of suppression
by suppressor T cells. Nat Immunol. 6:338–344. 2005.PubMed/NCBI
|
|
27.
|
Zen Y, Harada K, Sasaki M, et al:
Intrahepatic cholangiocarcinoma escapes from growth inhibitory
effect of transforming growth factor-beta1 by overexpression of
cyclin D1. Lab Invest. 85:572–581. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Shimizu T, Yokomuro S, Mizuguchi Y, et al:
Effect of transforming growth factor-beta1 on human intrahepatic
cholangiocarcinoma cell growth. World J Gastroenterol.
12:6316–6324. 2006.PubMed/NCBI
|