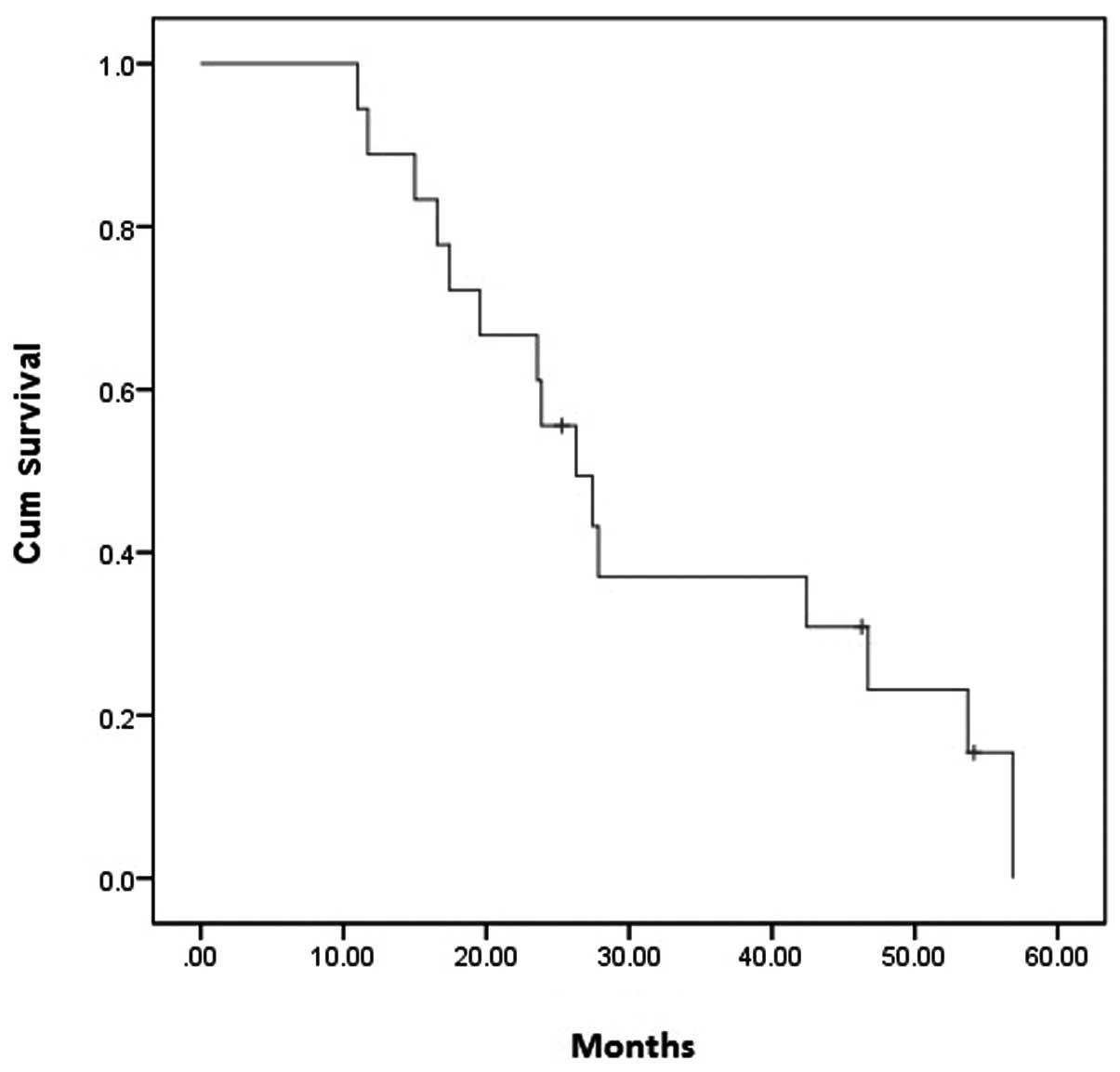
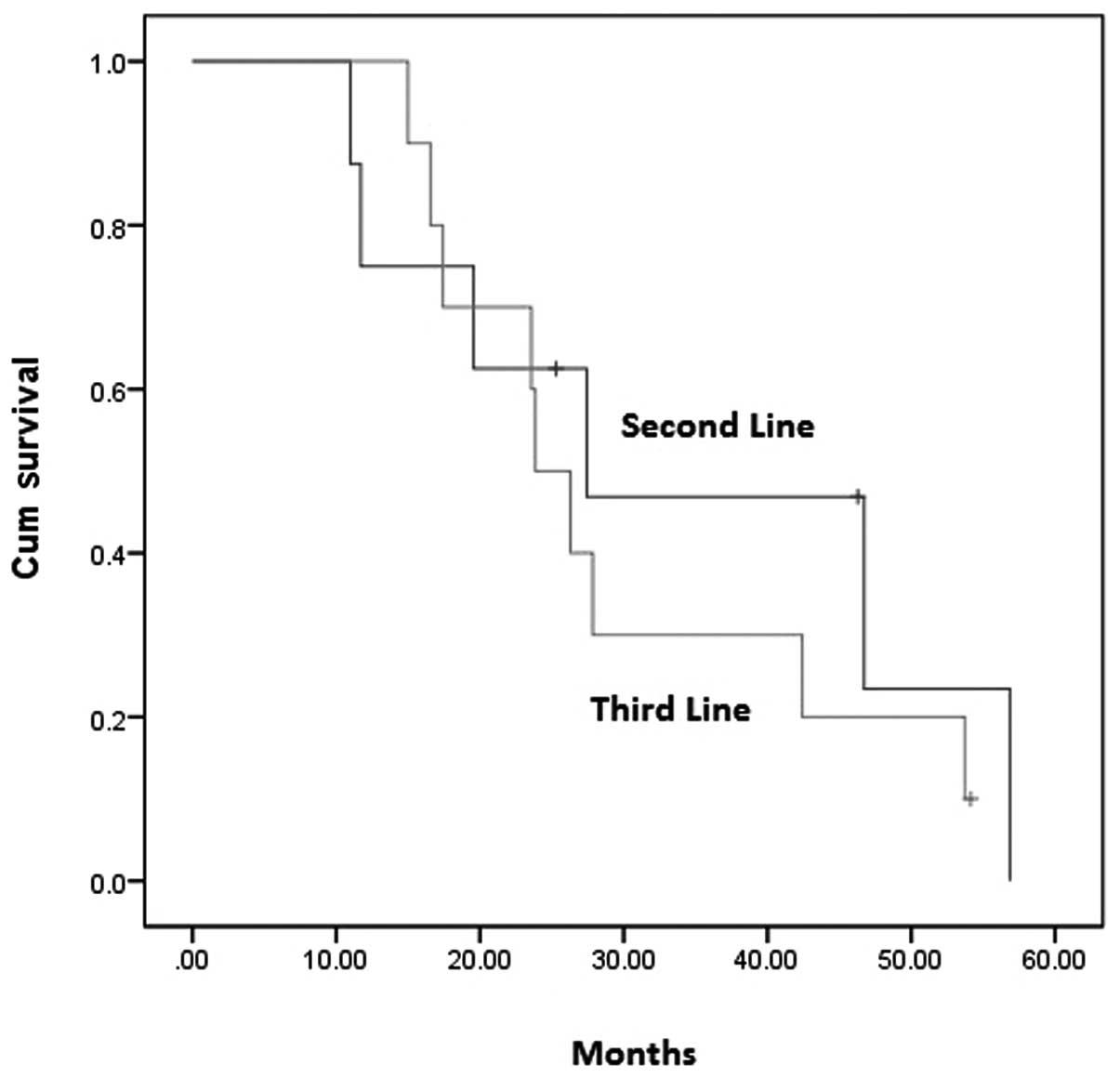
|
1.
|
de Gramont A, Figer A, Seymour M, et al:
Leucovorin and fluorouracil with or without oxaliplatin as
first-line treatment in advanced colorectal cancer. J Clin Oncol.
18:2938–2947. 2000.
|
|
2.
|
Douillard JY, Cunningham D, Roth AD, et
al: Irinotecan combined with fluorouracil compared with
fluorouracil alone as first-line treatment for metastatic
colorectal cancer: a multicentre randomised trial. Lancet.
355:1041–1047. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Goldberg RM, Sargent DJ, Morton RF, et al:
A randomized controlled trial of fluorouracil plus leucovorin,
irinotecan, and oxaliplatin combinations in patients with
previously untreated metastatic colorectal cancer. J Clin Oncol.
22:23–30. 2004. View Article : Google Scholar
|
|
4.
|
Saltz LB, Cox JV, Blanke C, et al:
Irinotecan plus fluorouracil and leucovorin for metastatic
colorectal cancer. N Engl J Med. 343:905–914. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Cassidy J, Clarke S, Díaz-Rubio E, et al:
Randomized phase III study of capecitabine plus oxaliplatin
compared with fluorouracil/folinic acid plus oxaliplatin as
first-line therapy for metastatic colorectal cancer. J Clin Oncol.
26:2006–2012. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Hurwitz H, Fehrenbacher L, Novotny W, et
al: Bevacizumab plus irinotecan, fluorouracil, and leucovorin for
metastatic colorectal cancer. N Engl J Med. 350:2335–2342. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Saltz LB, Clarke S, Díaz-Rubio E, et al:
Bevacizumab in combination with oxaliplatin-based chemotherapy as
first-line therapy in metastatic colorectal cancer: a randomized
phase III study. J Clin Oncol. 26:2013–2019. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Giantonio BJ, Catalano PJ, Meropol NJ, et
al: Bevacizumab in combination with oxaliplatin, fluorouracil, and
leucovorin (FOLFOX4) for previously treated metastatic colorectal
cancer: results from the Eastern Cooperative Oncology Group Study
E3200. J Clin Oncol. 25:1539–1544. 2007. View Article : Google Scholar
|
|
9.
|
Van Cutsem E, Köhne CH, Hitre E, et al:
Cetuximab and chemo-therapy as initial treatment for metastatic
colorectal cancer. N Engl J Med. 360:1408–1417. 2009.PubMed/NCBI
|
|
10.
|
Cunningham D, Humblet Y, Siena S, et al:
Cetuximab mono-therapy and cetuximab plus irinotecan in
irinotecan-refractory metastatic colorectal cancer. N Engl J Med.
351:337–345. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Jonker DJ, O’Callaghan CJ, Karapetis CS,
et al: Cetuximab for the treatment of colorectal cancer. N Engl J
Med. 357:2040–2048. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Bokemeyer C, Bondarenko I, Hartmann JT, et
al: Efficacy according to biomarker status of cetuximab plus
FOLFOX-4 as first-line treatment for metastatic colorectal cancer:
the OPUS study. Annals of Oncology. 22:1535–1546. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Grothey A, Sargent D, Goldberg RM and
Schmoll HJ: Survival of patients with advanced colorectal cancer
improves with the availability of fluorouracil-leucovorin,
irinotecan, and oxaliplatin in the course of treatment. J Clin
Oncol. 22:1209–1214. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Tournigand C, André T, Achille E, et al:
FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced
colorectal cancer: a randomized GERCOR study. J Clin Oncol.
22:229–237. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Tol J, Koopman M, Cats A, et al:
Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal
cancer. N Engl J Med. 360:563–572. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Hecht JR, Mitchell E, Chidiac T, et al: A
randomized phase IIIB trial of chemotherapy, bevacizumab, and
panitumumab compared with chemotherapy and bevacizumab alone for
metastatic colorectal cancer. J Clin Oncol. 27:672–680. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Norguet E, Dahan L, Gaudart J, Gasmi M,
Ouafik Lh and Seitz JF: Cetuximab after bevacizumab in metastatic
colorectal cancer: Is it the best sequence? Dig Liver Dis.
43:917–919. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Bianco R, Rosa R, Damiano V, et al:
Vascular endothelial growth factor receptor-1 contributes to
resistance to anti-epidermal growth factor receptor drugs in human
cancer cells. Clin Cancer Res. 14:5069–5080. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Naumov GN, Nilsson MB, Cascone T, et al:
Combined vascular endothelial growth factor receptor and epidermal
growth factor receptor (EGFR) blockade inhibits tumor growth in
xenograft models of EGFR inhibitor resistance. Clin Cancer Res.
15:3484–3494. 2009. View Article : Google Scholar
|
|
20.
|
Viloria-Petit A, Crombet T, Jothy S, et
al: Acquired resistance to the antitumor effect of epidermal growth
factor receptor-blocking antibodies in vivo. Cancer Res.
61:5090–5101. 2001.PubMed/NCBI
|
|
21.
|
Patt YZ, Lee FC, Liebmann JE, et al:
Capecitabine plus 3-weekly irinotecan (XELIRI regimen) as
first-line chemotherapy for metastatic colorectal cancer: phase II
trial results. Am J Clin Oncol. 30:350–357. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Cassidy J, Twelves C, Van Cutsem E, et al:
First-line oral capecitabine therapy in metastatic colorectal
cancer: a favorable safety profile compared with intravenous
5-fluorouracil/leucovorin. Annals of Oncology. 13:566–575. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Eisenhauer EA, Therasse P, Bogaerts J, et
al: New response evaluation criteria in solid tumours: Revised
RECIST guideline (version 1.1). Eur J Cancer. 45:228–247. 2009.
View Article : Google Scholar
|
|
24.
|
Kozloff M, Yood MU, Berlin J, et al:
Clinical outcomes associated with bevacizumab-containing treatment
of metastatic colorectal cancer: the BRiTE observational cohort
study. Oncologist. 14:862–870. 2009. View Article : Google Scholar
|
|
25.
|
Van Cutsem E, Rivera F, Berry S, et al:
Safety and efficacy of first-line bevacizumab with FOLFOX, XELOX,
FOLFIRI and fluoropyrimidines in metastatic colorectal cancer: the
BEAT study. Ann Oncol. 20:1842–1847. 2009.PubMed/NCBI
|
|
26.
|
Trotti A, Colevas AD, Setser A, et al:
CTCAE v3.0: development of a comprehensive grading system for the
adverse effects of cancer treatment. Semin Radiat Oncol.
13:176–181. 2003. View Article : Google Scholar
|
|
27.
|
Emmanouilides C, Pegram M, Robinson R,
Hecht R, Kabbinavar F and Isacoff W: Anti-VEGF antibody bevacizumab
(Avastin) with 5FU/LV as third line treatment for colorectal
cancer. Tech Coloproctol. 8:s50–52. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Kang B, Kim T, Lee JL, et al: Bevacizumab
plus FOLFIRI or FOLFOX as third-line or later treatment in patients
with meta-static colorectal cancer after failure of 5-fluorouracil,
irinotecan, and oxaliplatin: a retrospective analysis. Med Oncol.
26:32–37. 2009. View Article : Google Scholar
|
|
29.
|
Chen HX, Mooney M, Boron M, et al: Phase
II multicenter trial of bevacizumab plus fluorouracil and
leucovorin in patients with advanced refractory colorectal caner:
an NCI Treatment Referral Center Trial TRC-0301. J Clin Oncol.
24:3354–3360. 2006. View Article : Google Scholar
|
|
30.
|
Vincenzi B, Santini D, Russo A, et al:
Bevacizumab in association with de Gramont 5-fluorouracil/folinic
acid in patients with oxaliplatin-, irinotecan-, and
cetuximab-refractory colorectal cancer: a single-center phase 2
trial. Cancer. 115:4849–4856. 2009. View Article : Google Scholar
|